Abstract
Background: Estimation of the intake of oily fish at a population level is difficult. The measurement of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in biological samples may provide a useful biomarker of intake.
Objective: We identified the most appropriate biomarkers for the assessment of habitual oily fish intake and changes in intake by elucidating the dose- and time-dependent response of EPA and DHA incorporation into various biological samples that represent roles in fatty acid transport, function, and storage.
Design: This was a double-blind, randomized, controlled intervention trial in 204 men and women that lasted 12 mo. EPA and DHA capsules were provided in a manner to reflect sporadic consumption of oily fish (ie, 1, 2, or 4 times/wk). EPA and DHA were assessed at 9 time points over 12 mo in 9 sample types (red blood cells, mononuclear cells, platelets, buccal cells, adipose tissue, plasma phosphatidylcholine, triglycerides, cholesteryl esters, and nonesterified fatty acids).
Results: A dose response (P < 0.05) was observed for EPA and DHA in all pools except for red blood cell EPA (P = 0.057). EPA and DHA measures in plasma phosphatidylcholine and platelets were best for the discrimination between different intakes (P < 0.0001). The rate of incorporation varied between sample types, with the time to maximal incorporation ranging from days (plasma phosphatidylcholine) to months (mononuclear cells) to >12 mo (adipose tissue).
Conclusions: Plasma phosphatidylcholine EPA plus DHA was identified as the most suitable biomarker of acute changes in EPA and DHA intake, and platelet and mononuclear cell EPA plus DHA were the most suitable biomarkers of habitual intake. This trial was registered at Current Controlled Trials (www.controlled-trials.com) as ISRCTN48398526.
INTRODUCTION
Dietary oily fish consumption has been shown to reduce risk of cardiovascular morbidity and mortality, some cancers, inflammatory disorders, and cognitive decline (1–6). On the basis of these benefits and those of the constituent very-long-chain (VLC)5 n−3 PUFAs (EPA and DHA), many governments and other advisory agencies have made recommendations for intakes of oily fish. In the United Kingdom, these are at least one portion/wk, along with one portion of lean fish, for the entire population with a guideline range of one to 4 portions/wk (7). However, the estimation of intakes of oily fish and VLC n−3 PUFAs from fish at a population level is difficult, and dietary reports are confounded by misreporting and underreporting as well as inaccurate and incomplete data in the fatty acid (FA) composition of foods. Dietary oily fish intake is low and infrequent in the United Kingdom (7) and United States (8), making short periods of dietary recording even less likely to accurately capture exposure.
The VLC n−3 PUFAs EPA and DHA can be readily measured in FA pools within a variety of biological sample types and offer an alternative biomarker for oily fish intake to reported dietary intake data (9, 10). After absorption from the diet, FAs can undergo one of several fates, including oxidation, conversion to other FAs, incorporation into membranes as components of phospholipids, or storage. FAs are transported between body compartments in the blood plasma either esterified as components of lipoproteins or in the nonesterified form (11, 12). Because the turnover of the FAs within each of these pools is likely to occur at different rates, they may offer options as biomarkers to reflect varying periods of dietary intake of VLC n−3 PUFAs.
Controlled intervention studies of VLC n−3 PUFA supplements have almost uniformly used a daily dosing strategy. This strategy differs from the pattern of dietary oily fish consumption that tends to be more sporadic (eg, 1 or 2 times/wk). To our knowledge, there are no controlled capsule-based intervention studies that more closely reflect the pattern of dietary consumption of VLC n−3 PUFAs from oily fish. Therefore, detailed information about how the FA composition of transport, functional, and storage pools respond to a sporadic intake of VLC n−3 PUFAs as seen with oily fish consumption is not available.
This study was designed to elucidate the dose and time response of EPA and DHA incorporation into different sample types that represent aspects of FA transport, function, and storage, when the intake of those FAs reflects the sporadic pattern of oily fish consumption. The overall aim was to identify the most appropriate biomarkers for the assessment of habitual dietary oily fish intake and changes in intake. To closely reflect dietary consumption while still maintaining control in the study, VLC n−3 PUFAs were provided in a manner to reflect oily fish portions (ie, 1, 2, or 4 times/wk).
SUBJECTS AND METHODS
Study design
The study was a double-blind, randomized, controlled intervention trial in 5 parallel groups that lasted 12 mo. Participants were recruited in Cambridge and Southampton, United Kingdom, and random assignment was stratified by age (20–39, 40–59, and 60–79 y) and sex (men and women). Participants attended the research facilities (Medical Research Council Human Nutrition Research, Cambridge, and Wellcome Trust Clinical Research Facility, Southampton) at the following 9 study time points: baseline, 1 and 2 wk, and 1, 2, 3, 6, 9, and 12 mo. All procedures were approved by the Suffolk Local Research Ethics Committee (approval 05/Q0102/181).
Subjects
Men and women aged 20–80 y who reported low or no habitual consumption of oily fish were recruited. Exclusion criteria were as follows: a diagnosis of diabetes, cancer, cardiovascular disease, or other chronic clinical conditions; untreated hypertension; concomitant prescription of anticoagulants, nonsteroidal antiinflammatory drugs, aspirin, steroids, or immunosuppressants; an allergy or intolerance to fish; the consumption of fish-oil supplements in the past 3 mo; the consumption of oily fish more than once per month; smoking; a history of substance abuse or alcoholism; pregnant, <1 y postpartum, or planning a pregnancy; recent weight change (>2 kg in the past 1 mo), planning to change dietary habits, increase physical activity, change body weight, move away from the study center locality, or take a lengthy vacation during the time of the study; and BMI (in kg/m2) <18 or >35.
Intervention
The intervention was capsule based and designed to provide amounts of EPA and DHA that would be realistically achievable at a population level by dietary modification with oily fish. To control the delivery of the intervention, all participants received six 0.75-g capsules/d as individual-day blister packs labeled with the day of the week.
The UK Scientific Advisory Committee on Nutrition defines oily fish as those with flesh high in fat, which is particularly rich in EPA and DHA (7). An average oily fish portion, defined by the UK Scientific Advisory Committee on Nutrition as 140 g, typically provides 2.8 g EPA plus DHA, with a portion (140 g) of fresh salmon containing as much as 3.5 g EPA plus DHA (1.68 g EPA and 1.82 g DHA) (7). Capsules were specifically designed and manufactured so that 6 capsules provided as much EPA plus DHA as the average oily fish portion. Six 0.75-g fish-oil capsules provided a total of 1.5 g EPA and 1.77 g DHA (ie, 3.27 g EPA plus DHA) as triglycerides (see Table 1 under “Supplemental data” in the online issue); this amount was defined in this study as equal to one portion of oily fish. To simulate habitual consumption patterns, capsules that equated to one portion of oily fish were provided on none, 1, 2, or 4 d of the week, which were designated a zero-portion or control group, a 1-portion group, a 2-portions group, or a 4-portions group. To maintain the blinding of the study design, placebo capsules were given on all remaining days of the week. Placebo oil capsules contained high oleic sunflower oil and were selected so that they would have little effect on the background pattern of FA consumption. Six 0.75-g placebo oil capsules provided 3.47 g oleic acid, 0.64 g linoleic acid, 0.2 g palmitic acid, and 0.1 g stearic acid (see Table 1 under “Supplemental data” in the online issue for details of the full composition of capsules). All participants consumed 6 capsules/d of either fish oil or placebo oil.
TABLE 1.
Characteristics of the participants at baseline1
| 0 portions | 1 portion | 2 portions | 4 portions | |
| Men/women (n) | 19/21 | 21/21 | 19/21 | 20/22 |
| Age (y) | 51.7 ± 15.12 | 49.0 ± 16.6 | 49.2 ± 14.9 | 49.6 ± 15.0 |
| Weight (kg) | 77.9 ± 16.0 | 75.8 ± 15.8 | 75.1 ± 14.0 | 71.5 ± 10.9 |
| BMI (kg/m2) | 26.2 ± 4.1 | 26.0 ± 4.2 | 25.5 ± 4.1 | 24.2 ± 3.2 |
| Body fat (%) | 23.1 ± 7.9 | 22.1 ± 7.9 | 22.0 ± 9.0 | 19.7 ± 6.5 |
| Systolic blood pressure (mm Hg) | 131 ± 16 | 126 ± 16 | 125 ± 16 | 129 ± 15 |
| Diastolic blood pressure (mm Hg) | 78 ± 9 | 75 ± 10 | 73 ± 8 | 77 ± 8 |
| Plasma PC EPA (% of total FA) | 1.1 ± 0.5 | 1.3 ± 1.0 | 1.2 ± 0.8 | 1.1 ± 0.4 |
| Plasma PC DHA (% of total FA) | 3.2 ± 1.2 | 3.8 ± 1.0 | 3.7 ± 1.4 | 3.5 ± 0.9 |
| Plasma CE EPA (% of total FA) | 1.0 ± 0.7 | 1.1 ± 0.6 | 1.0 ± 0.5 | 1.2 ± 1.0 |
| Plasma CE DHA (% of total FA) | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.6 ± 0.2 |
| Plasma NEFA EPA (% of total FA) | 0.4 ± 0.2 | 0.4 ± 0.3 | 0.4 ± 0.4 | 0.4 ± 0.3 |
| Plasma NEFA DHA (% of total FA) | 1.6 ± 0.8 | 1.8 ± 1.3 | 1.5 ± 1.8 | 1.3 ± 0.8 |
| Plasma TAG EPA (% of total FA) | 0.3 ± 0.3 | 0.4 ± 1.0 | 0.4 ± 0.8 | 0.2 ± 0.2 |
| Plasma TAG DHA (% of total FA) | 0.7 ± 0.4 | 0.9 ± 0.7 | 1.1 ± 2.0 | 0.7 ± 0.3 |
| MNC EPA (% of total FA) | 0.8 ± 0.7 | 0.8 ± 0.6 | 0.7 ± 0.6 | 0.7 ± 0.4 |
| MNC DHA (% of total FA) | 1.9 ± 0.6 | 1.9 ± 0.5 | 1.9 ± 0.5 | 1.9 ± 0.5 |
| RBC EPA (% of total FA) | 3.0 ± 4.1 | 2.2 ± 2.0 | 1.8 ± 1.5 | 1.6 ± 1.2 |
| RBC DHA (% of total FA) | 4.9 ± 1.5 | 5.3 ± 1.4 | 5.3 ± 1.3 | 5.3 ± 1.4 |
| PLAT EPA (% of total FA) | 1.0 ± 0.5 | 1.3 ± 1.0 | 1.1 ± 0.5 | 1.0 ± 0.5 |
| PLAT DHA (% of total FA) | 1.9 ± 0.5 | 2.1 ± 0.6 | 2.1 ± 0.5 | 1.9 ± 0.5 |
| BU EPA (% of total FA) | 1.3 ± 1.5 | 1.5 ± 2.2 | 2.2 ± 6.2 | 1.1 ± 1.4 |
| BU DHA (% of total FA) | 0.7 ± 0.3 | 0.8 ± 0.3 | 0.8 ± 0.3 | 0.8 ± 0.3 |
| AT EPA (% of total FA) | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| AT DHA (% of total FA) | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 |
Values are for all participants randomly assigned to intervention groups. There were no significant differences between groups (ANOVA). AT, adipose tissue; BU, buccal cell; CE, plasma cholesteryl ester; FA, fatty acid; MNC, blood mononuclear cell; NEFA, nonesterified fatty acid; PC, phosphatidylcholine; PLAT, platelet; RBC, erythrocyte (red blood cell); TAG, triglyceride.
Mean ± SD (all such values).
An additional group followed the traditional design of capsule-based intervention studies and received the equivalent amount of EPA plus DHA provided in the 2 portions of oily fish group, but which was distributed evenly over the 7 d. The results of this group are not reported here because this aspect of the trial addresses an additional research question that is beyond the scope of this article.
Dietary assessment
To monitor any changes in background diet, participants completed 3 unweighed 4-d diet diaries recording intakes as estimated portions over 3 weekdays and 1 weekend day at 3 time points during the intervention (baseline, 6 mo, and 12 mo). Dietary data were analyzed by using the in-house database Diet In, Data Out of the Medical Research Council Human Nutrition Research of the nutrient composition of foods consumed in the UK diet to calculate specific macronutrient and micronutrient intakes. This database is based on McCance and Widdowson (13) with additional data obtained from manufacturers. At each clinic visit, participants were specifically asked about the consumption of oily and white fish since their previous visit.
Sample preparation
Fasting blood samples and buccal cell (BU) samples were collected at each of the 9 time points throughout the 12-mo intervention. Abdominal subcutaneous adipose tissue samples were collected at 3 of the intervention time points (baseline, 6 mo, and 12 mo). The following samples were analyzed for FA composition at each time point: plasma phosphatidylcholine, plasma cholesteryl esters (CEs), plasma triglycerides, plasma nonesterified fatty acids (NEFAs), blood mononuclear cells (MNCs), erythrocytes (red blood cells; RBCs), platelets, BUs, and adipose tissue (AT).
Plasma was prepared from blood collected into heparin by centrifugation at 3000 rpm for 20 min at 4°C. Plasma was stored at −80°C. MNCs were isolated from heparinized blood by centrifugation on LeucoSep tubes (Greiner Bio-one) and were washed twice in phosphate-buffered saline (PBS). Platelets were isolated from citrated blood and washed twice in PBS. RBCs were isolated from heparinized blood by centrifugation and were washed with PBS. RBCs were lysed by 4 serial washes with increasingly hypotonic PBS. The final preparation of RBC membranes was resuspended in isotonic PBS. Nitrogen was bubbled through MNC, platelet, and RBC membranes for 2–3 s before storing at −80°C.
To obtain BUs, each participant was required to rinse his or her mouth twice with mineral water. Then, the participant was asked to take a mouthful of distilled water, gently chew both cheeks, and spit the residual water into a 50-mL centrifuge tube. This process was repeated 3–4 times. The sample was washed with distilled water and centrifuged twice before being bubbled with nitrogen and stored at −80°C.
An abdominal subcutaneous adipose tissue biopsy was performed with the participant lying supine on a couch. The area to be sampled was anesthetized with lignocaine. A biopsy sample was obtained by holding a skinfold with one hand and pushing a biopsy needle through a small incision site into subcutaneous abdominal fat. The biopsy needle was moved in and out of the fat with a rotating movement. The adipose tissue sample was washed with sterile saline to remove traces of blood. The sample was snap frozen in liquid nitrogen and stored at −80°C.
FA-composition analysis
FAs were analyzed in total lipid fractions for all pools except plasma, which was further separated into phosphatidylcholine, CEs, NEFAs and triglycerides. Total lipid was extracted into chloroform:methanol (2:1, vol:vol) from plasma, MNCs, RBCs, platelets, BUs, and homogenized AT; butylated hydroxytoluene (50 mg/L) was added as an antioxidant. Plasma lipid fractions were separated and isolated by solid-phase extraction on aminopropylsilica cartridges. CEs and triglycerides were eluted with chloroform. Phosphatidylcholine, which is the major plasma phospholipid, was eluted with chloroform:methanol (60:40, vol:vol). NEFAs were eluted by using chloroform:methanol:glacial acetic acid (100:2:2, vol:vol:vol). A second cartridge was used to separate CEs and triglycerides: CEs were eluted with hexane, and triglycerides were eluted with hexane:chloroform:ethyl acetate (100:5:5, vol:vol:vol). All lipids were dried under nitrogen and redissolved in toluene. Fatty acid methyl esters (FAMEs) were formed by incubation with methanol that contained 2% (vol:vol) H2SO4 at 50°C for 2 h. After allowing the tubes to cool, samples were neutralized with a solution of 0.25 mol KHCO3/L and 0.5 mol K2CO3/L. FAMEs were extracted into hexane, dried down, redissolved in a small volume of hexane, and separated by using gas chromatography. Gas chromatography was performed on a Hewlett-Packard 6890 gas chromatograph fitted with a BPX-70 column (30 m × 0.22 mm × 0.25 μm). The inlet temperature was 300°C. The oven temperature was initially 115°C and was maintained for 2 min after injection. The oven temperature was programmed to increase to 200°C at the rate of 10°C/min to hold at 200°C for 16 min and increase to 240°C at the rate of 60°C/min to hold at 240°C for 2 min. The total run time was just longer than 29 min. Helium was used as the carrier gas. FAMEs were detected by using a flame ionization detector held at a temperature of 300°C. The instrument was controlled by, and data collected, with HPChemStation software (Hewlett-Packard). FAMEs were identified by comparison of retention times with those of authentic standards run previously. Within-run CVs for analysis of EPA and DHA as methyl esters were 3% and 2%, respectively. Between-run CVs for analysis of EPA and DHA as methyl esters were 5% and 2.5%, respectively.
Statistics
All analyses were performed with Stata software (version 11; StataCorp LP). Data were analyzed for participants who completed the 12-mo intervention. Primary comparisons between treatment groups were analyzed by using multiple regression with the factors treatment or portion (with amounts of 4, 2, 1, and zero portions), age (with levels of young, middle, and old), sex (men compared with women, if needed), study center, baseline BMI, and average compliance over the 12 mo (%). A repeated-measures mixed-effects model was used to analyze how FA concentrations changed over time; because the incorporation of EPA and DHA was nonlinear over time, time was included in the model as a categorical factor. A chi-square likelihood-ratio test of the interaction between treatment group and time was used to confirm whether FA concentrations varied between treatment and time. Residuals were plotted across dose to check for the deviation of the variance homogeneity assumption, and residuals were also checked for normality by using a quantile-quantile plot.
The dose response between pools was assessed by comparing slopes from a multivariate linear regression model of the proportion of EPA plus DHA in each pool at 12 mo in response to dose, with linearity assumed for the effect of dose.
To determine whether comparisons may be made between the effect of dose on the relative abundance of EPA and DHA between different pools, relationships between EPA and DHA at 12 mo were assessed in the different pools by using Spearman's rank correlations. Spearman's rank correlation was used rather than Pearson's correlation because we did not wish to assume a straight-line relation between any of the 2 variables. The determination of which pools are well correlated may aid in interpretation of past and future studies conducted in single but different pools.
To determine the rate of incorporation, 95% of the value of the asymptote was used to define the maximum FA concentration for each treatment group. A nonlinear model was used to explain the FA concentration over time, and this functional form is
where a represents the asymptote, b is the change from baseline to the asymptote, and ctime is the rate of increase in the outcome to the asymptote.
This model assumes that the FA concentration increases monotonically over time until it eventually reaches an asymptote. These model variables and their respective variance-covariance matrix were used to estimate the time taken to reach within 5% of the asymptote with its corresponding 95% CI.
RESULTS
Baseline characteristics
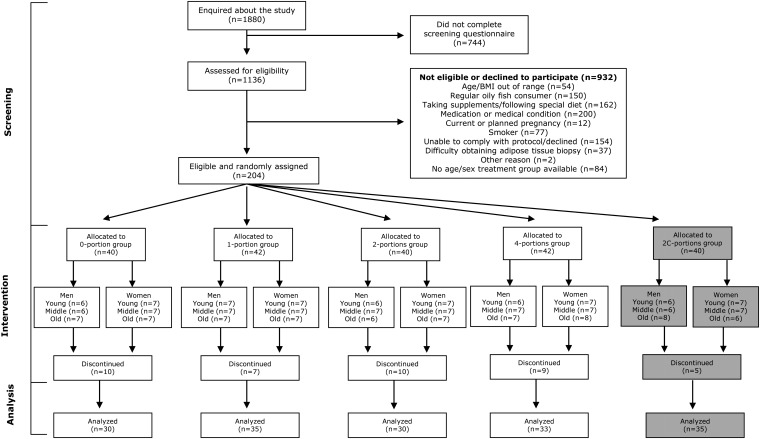
The study design and flow of participants through the study are shown in Figure 1. A total of 204 participants met the inclusion criteria and were recruited into the intervention study. All baseline measurements were completed between September 2006 and March 2008. Baseline characteristics of participants are shown in Table 1. There were no significant differences among groups with respect to participant characteristics or baseline levels of EPA or DHA in any of the pools.
FIGURE 1.
Flow of participant involvement in the study. Randomization group 2C (shaded in gray) is not considered further in this article. The 2C-portions group received the equivalent amount of EPA plus DHA that was provided in the 2-portions group but which was distributed evenly over the 7 d.
A total of 41 participants withdrew from the study between baseline and the final 12-mo time point, which left 163 participants who completed the study with measurable outcomes at all visits. The reasons for withdrawal were as follows: adverse event or concomitant illness (15 subjects), change of medication (5 subjects), poor compliance with the intervention (6 subjects), unwilling to have a repeat adipose biopsy (6 subjects), informed consent withdrawn (6 subjects), or no reason given (3 subjects).
Compliance and dietary intake data
Data from returned capsule counts were used to assess compliance. In study completers, compliance was high (mean: 98.1%; IQR: 2.2). The range of compliance values at 12 mo for individual participants was 87.4–100%. Compliance was similar across intervention groups but tended to be lower at the 9-mo and 12-mo time points.
Data from 4-d unweighed food diaries showed no significant differences between groups or between time points for total reported energy or macronutrient intake. The mean (±SD) intake of total dietary n−3 PUFAs (including α-linolenic acid) was 1.7 ± 0.7 g/d in the total study population at baseline. This intake was lower than that reported for UK national data (2.2 ±1.3 g/d in the National Diet and Nutrition Survey), which reflected the specific recruitment of participants with low or no oily fish intake. Direct questioning on fish consumption at each study time point revealed no consumption of oily fish at any point during the study.
Dose response
Adjusted mean differences between groups in EPA, DHA, and EPA plus DHA for each of the 9 sample types analyzed are shown in Tables 2–4. The mixed-effects model showed a significant dose response for all sample types (all P < 0.05) except for RBC EPA (P = 0.057), which meant the adjusted mean differences of each portion group were different within each sample type.
TABLE 2.
EPA in the zero-portions group and differences in changes in EPA between treatment groups at 12 mo1
| EPA2 |
Change in EPA3 |
|||||
| 0 portions | 1 compared with 0 portions | 2 compared with 0 portions | 4 compared with 0 portions | P | ||
| % of total FAs | % of total FAs | |||||
| Plasma PC | 1.28 ± 0.75 | 0.40 (0.07, 0.73) | 1.10 (0.75, 1.45) | 2.28 (1.94, 2.62) | <0.0001 | |
| Plasma CEs | 1.17 ± 0.73 | 0.30 (−0.01, 0.60) | 1.04 (0.73, 1.36) | 2.30 (2.00, 2.61) | <0.0001 | |
| Plasma NEFAs | 0.27 ± 0.17 | 0.17 (−0.05, 0.38) | 0.28 (0.05, 0.50) | 0.57 (0.35, 0.79) | <0.0001 | |
| Plasma TAGs | 0.51 ± 0.43 | 0.13 (−0.17, 0.43) | 0.57 (0.26, 0.88) | 1.35 (1.05, 1.65) | <0.0001 | |
| MNCs | 0.53 ± 0.25 | 0.31 (0.05, 0.56) | 0.85 (0.58, 1.12) | 1.83 (1.57, 2.09) | <0.0001 | |
| RBCs | 2.83 ± 2.48 | 0.56 (−0.34, 1.46) | 1.17 (0.23, 2.11) | 0.99 (0.07, 1.90) | 0.057 | |
| PLATs | 0.98 ± 0.40 | 0.46 (0.16, 0.76) | 1.13 (0.81, 1.44) | 2.41 (2.10, 2.71) | <0.0001 | |
| BUs4 | — | — | — | — | <0.0001 | |
| AT | 0.18 ± 0.09 | 0.05 (0.01, 0.10) | 0.04 (−0.01, 0.08) | 0.11 (0.07, 0.16) | <0.0001 | |
n = 30 (0-portions group), n = 35 (1-portion group), n = 30 (2-portions group), and n = 33 (4-portions group). Mixed-effects models were adjusted for baseline EPA, age, sex, study center, baseline BMI, and average compliance (%). The P value for the global test is presented for each outcome variable. AT, adipose tissue; BUs, buccal cells; CEs, cholesteryl esters; FAs, fatty acids; MNCs, blood mononuclear cells; NEFAs, nonesterified fatty acids; PC, phosphatidylcholine; PLATs, platelets; RBCs, erythrocytes (red blood cells); TAGs, triglycerides.
All values are means ± SDs.
All values are adjusted mean differences (95% CIs) between groups at 12 mo calculated in the mixed-effects model.
No mean-difference data are presented for BUs because data were nonnormally distributed and analyzed by using a Kruskal-Wallis rank test. The median (IQR) for each portion group is as follows: 0-portions group, 0.66 (0.41, 1.28); 1-portion group, 0.72 (0.57, 0.85); 2-portions group, 1.03 (0.71, 1.81); and 4-portions group: 1.45 (0.94, 1.89).
TABLE 3.
DHA in the zero-portions group and differences in changes in DHA between treatment groups at 12 mo1
| DHA2 |
Change in DHA3 |
|||||
| 0 portions | 1 compared with 0 portions | 2 compared with 0 portions | 4 compared with 0 portions | P | ||
| % of total FAs | % of total FAs | |||||
| Plasma PC | 3.56 ± 1.34 | 0.63 (0.18, 1.08) | 1.59 (1.12, 2.06) | 2.36 (1.90, 2.82) | <0.0001 | |
| Plasma CEs | 0.50 ± 0.22 | 0.13 (−0.02, 0.29) | 0.27 (0.11, 0.43) | 0.49 (0.33, 0.64) | <0.0001 | |
| Plasma NEFAs | 1.19 ± 0.53 | 0.60 (0.05, 1.14) | 0.81 (0.25, 1.38) | 1.60 (1.04, 2.15) | <0.0001 | |
| Plasma TAGs | 0.82 ± 0.70 | 0.24 (−0.16, 0.64) | 1.12 (0.71, 1.54) | 1.97 (1.56, 2.37) | <0.0001 | |
| MNCs | 1.62 ± 0.51 | 0.57 (0.28, 0.86) | 1.02 (0.72, 1.32) | 1.61 (1.31, 1.90) | <0.0001 | |
| RBCs | 4.84 ± 1.10 | 1.23 (0.61, 1.86) | 2.38 (1.73, 3.03) | 3.42 (2.78, 4.06) | <0.0001 | |
| PLATs | 1.87 ± 0.56 | 0.54 (0.30, 0.78) | 1.21 (0.97, 1.46) | 2.09 (1.84, 2.33) | <0.0001 | |
| BUs4 | — | — | — | — | <0.0001 | |
| AT | 0.22 ± 0.13 | 0.05 (−0.01, 0.10) | 0.06 (0.00, 0.11) | 0.13 (0.07, 0.18) | <0.0001 | |
n = 30 (0-portions group), n = 35 (1-portion group), n = 30 (2-portions group), and n = 33 (4-portions group). Mixed-effects models were adjusted for baseline DHA, age, sex, study center, baseline BMI, and average compliance (%). The P value for the global test is presented for each outcome variable. AT, adipose tissue; BUs, buccal cells; CEs, cholesteryl esters; FAs, fatty acids; MNCs, blood mononuclear cells; NEFAs, nonesterified fatty acids; PC, phosphatidylcholine; PLATs, platelets; RBCs, erythrocytes (red blood cells); TAGs, triglycerides.
All values are means ± SDs.
All values are adjusted mean differences (95% CIs) between groups at 12 mo calculated in the mixed-effects model.
No mean-difference data are presented for BUs because data were nonnormally distributed and analyzed by using the Kruskal-Wallis rank test. The median (IQR) for each portion group is as follows: 0-portions group, 0.52 (0, 0.74); 1-portion group, 0.90 (0.61, 1.09); 2-portions group, 1.13 (0.93, 1.36); and 4-portions group: 1.43 (1.07, 1.62).
TABLE 4.
EPA plus DHA in the zero-portions group and the adjusted mean differences in EPA plus DHA between treatment groups at 12 mo1
| EPA plus DHA2 |
Change in EPA plus DHA3 |
|||||
| 0 portions | 1 compared with 0 portions | 2 compared with 0 portions | 4 compared with 0 portions | P | ||
| % of total FAs | % of total FAs | |||||
| Plasma PC | 4.84 ± 2.00 | 1.07 (0.40, 1.73) | 2.72 (2.03, 3.41) | 4.66 (3.99, 5.34) | <0.0001 | |
| Plasma CEs | 1.67 ± 0.93 | 0.41 (0.05, 0.78) | 1.30 (0.92, 1.68) | 2.79 (2.42, 3.16) | <0.0001 | |
| Plasma NEFAs | 1.46 ± 0.65 | 0.76 (0.05, 1.48) | 1.09 (0.34, 1.84) | 2.17 (1.44, 2.90) | <0.0001 | |
| Plasma TAGs | 1.32 ± 1.10 | 0.38 (−0.28, 1.03) | 1.69 (1.01, 2.37) | 3.32 (2.65, 3.98) | <0.0001 | |
| MNCs | 2.15 ± 0.70 | 0.88 (0.43, 1.33) | 1.88 (1.41, 2.34) | 3.44 (2.99, 3.90) | <0.0001 | |
| RBCs | 7.68 ± 2.71 | 1.83 (0.92, 2.74) | 3.63 (2.68, 4.58) | 4.52 (3.59, 5.45) | <0.0001 | |
| PLATs | 2.85 ± 0.87 | 1.00 (0.53, 1.48) | 2.36 (1.87, 2.85) | 4.49 (4.01, 4.97) | <0.0001 | |
| BUs4 | — | — | — | — | <0.0001 | |
| AT | 0.40 ± 0.21 | 0.10 (0.01, 0.18) | 0.10 (0.01, 0.18) | 0.24 (0.15, 0.32) | <0.0001 | |
n = 30 (0-portions group), n = 35 (1-portion group), n = 30 (2-portions group), and n = 33 (4-portions group). Mixed-effects models were adjusted for baseline EPA plus DHA, age, sex, study center, baseline BMI, and average compliance (%). The P value for the global test is presented for each outcome variable. AT, adipose tissue; BUs, buccal cells; CEs, cholesteryl esters; FAs, fatty acids; MNCs, blood mononuclear cells; NEFAs, nonesterified fatty acids; PC, phosphatidylcholine; PLATs, platelets; RBCs, erythrocytes (red blood cells); TAGs, triglycerides.
All values are means ± SDs.
All values are adjusted mean differences (95% CIs) between groups at 12 mo calculated in the mixed-effects model.
No mean-difference data are presented for BUs because data were nonnormally distributed and analyzed by using the Kruskal-Wallis rank test. The median (IQR) for each portion group is as follows: 0-portions group, 1.39 (0.93, 2.96); 1-portion group: 1.63 (1.42, 2.14); 2-portions group: 2.33 (1.89, 3.44); and 4-portions group: 2.97 (2.22, 3.70).
A summary of the dose response of EPA plus DHA at 12 mo [expressed as the relative abundance (ie, percentage of total FAs)] for comparison of the 9 sample types is shown in Figure 2. Mostly consistent with the mixed-effects model, the sample types that showed the strongest response to dose at 12 mo were plasma phosphatidylcholine and platelets. BUs and AT demonstrated particularly poor ability for EPA plus DHA (or EPA or DHA alone; data not shown) to differentiate clearly between groups.
FIGURE 2.
Comparison of mean (±SD) EPA plus DHA amounts (percentage of total fatty acids) between the 9 sample types at 12 mo for the groups who received EPA plus DHA equivalent to 0, 1, 2, and 4 portions of oily fish per week. The dose response in the pools was compared by using a multivariate regression model in which each pool was a component of the outcome, and linearity with dose was assumed. Regression coefficients (slopes) and associated P values for the effect of dose in each pool are shown, and pairwise comparisons between the slopes were made. +Significant difference between adipose tissue and other pools, P < 0.001; *significant difference between plasma phosphatidylcholine and other pools, P < 0.001; #significant difference between platelets and other pools, P < 0.001; £significant difference between nonesterified fatty acids and other pools, P < 0.001.
In the exploration of the relation between EPA, DHA, or EPA plus DHA at 12 mo, significant correlations for each of the 9 sample types were shown. For EPA plus DHA (Table 5), the strongest correlations were seen between plasma phosphatidylcholine, plasma CEs, MNCs, and platelets. This result was consistent for EPA, but for DHA, the strongest correlations were between plasma phosphatidylcholine, MNCs, and platelets but not plasma CEs (data not shown). AT and BUs showed the weakest correlations with each of the other sample types.
TABLE 5.
Spearman's rank correlation coefficients of associations between EPA plus DHA (percentage of total FAs) in different sample types at 12 mo1
| Plasma CEs | Plasma NEFAs | Plasma TAG | MNCs | RBCs | PLATs | BUs | AT | |
| Plasma PC | 0.843 | 0.610 | 0.768 | 0.736 | 0.514 | 0.826 | 0.459 | 0.419 |
| Plasma CEs | 0.607 | 0.707 | 0.753 | 0.475 | 0.819 | 0.491 | 0.354 | |
| Plasma NEFAs | 0.577 | 0.599 | 0.225 | 0.567 | 0.325 | 0.196 | ||
| Plasma TAGs | 0.720 | 0.409 | 0.803 | 0.498 | 0.328 | |||
| MNCs | 0.523 | 0.891 | 0.442 | 0.385 | ||||
| RBCs | 0.596 | 0.286 | 0.365 | |||||
| PLATs | 0.494 | 0.469 | ||||||
| BUs | 0.271 |
n = 30 (0-portions group), n = 35 (1-portion group), n = 30 (2-portions group), and n = 33 (4-portions group). A correlation >0.15 was significant by using Fisher's z′-transformation to test the null hypothesis that there was no correlation; therefore all correlations were significant (P < 0.05). AT, adipose tissue; BUs, buccal cells; CEs, cholesteryl esters; FAs, fatty acids; MNCs, blood mononuclear cells; NEFAs, nonesterified fatty acids; PC, phosphatidylcholine; PLATs, platelets; RBCs, erythrocytes (red blood cells); TAGs, triglycerides.
Rate of incorporation
The estimate of the time to maximum (95% of the asymptote) incorporation of EPA and DHA is shown in Table 6. Values were not included for sample types (BU EPA and DHA and CE DHA) for which the model fitting did not converge because the data did not follow the asymptote model (this effect can occur when there is too much variability in data). In addition, AT data could not be modeled because of the limited number of time points. EPA and DHA reached maximum incorporation most quickly in plasma fractions, particularly phosphatidylcholine and triglycerides, in 1–2 and 2–4 wk, respectively. EPA and DHA reached a maximum in platelets in 3–4 wk and 1–2 mo, respectively, and in MNCs in 6–9 mo.
TABLE 6.
Estimate of the time to peak incorporation of EPA or DHA and the maximum incorporation in each sample type1
| Time |
Maximum incorporation |
||||||
| 1 portion | 2 portions | 4 portions | 1 portion | 2 portions | 4 portions | ||
| d (95% CI) | % of total FAs (95% CI) | ||||||
| EPA | |||||||
| Plasma PC | 5 (0, 18) | 13 (3, 22) | 18 (13, 23) | 1.7 (1.6, 1.8) | 2.1 (2.0, 2.2) | 3.5 (3.4, 3.6) | |
| Plasma CEs | 20 (−4, 43) | 53 (18, 88) | 24 (18, 30) | 1.4 (1.3, 1.5) | 1.9 (1.8, 2.0) | 3.2 (3.1, 3.3) | |
| Plasma NEFAs | 87 (−158, 332) | 81 (−30, 192) | 38 (18, 57) | 0.6 (0.5, 0.7) | 0.7 (0.6, 0.8) | 1.1 (1.1, 1.2) | |
| Plasma TAGs | 6 (0, 30) | 6 (0, 25) | 16 (9, 23) | 0.7 (1.0, 1.3) | 0.9 (2.0, 2.2) | 1.7 (3.4, 3.6) | |
| MNCs | 259 (0, 1453) | 1489 (0, 9452) | 249 (149, 350) | 0.9 (0.5, 1.3) | 2.0 (−2.3, 6.3) | 2.3 (2.1, 2.5) | |
| RBCs | 0 (0, 0) | 130 (1, 259) | 55 (17, 94) | NA2 | 3.1 (2.6, 3.6) | 3.7 (3.4, 4.1) | |
| PLATs | 29 (0, 107) | 20 (8, 33) | 25 (18, 31) | 1.5 (1.4, 1.6) | 1.9 (1.8, 2.0) | 3.1 (3.0, 3.2) | |
| DHA | |||||||
| Plasma PC | 12 (0, 26) | 32 (13, 50) | 23 (16, 29) | 4.1 (4.0, 4.3) | 4.8 (4.6, 4.9) | 5.9 (5.7, 6.0) | |
| Plasma NEFAs | 117 (−77, 312) | 83 (6, 159) | 32 (15, 49) | 2.2 (1.9, 2.5) | 2.6 (2.3, 2.8) | 3.1 (2.9, 3.3) | |
| Plasma TAGs | 9 (0, 30) | 978 (0, 6110) | 20 (11, 28) | 1.1 (1.0, 1.3) | 2.4 (−1.3, 6.1) | 2.5 (2.4, 2.7) | |
| MNCs | 273 (0, 670) | 268 (41, 495) | 207 (118, 297) | 2.3 (1.9, 2.6) | 2.6 (2.3, 2.9) | 3.3 (3.1, 3.5) | |
| RBCs | 63 (0, 206) | 299 (0, 681) | 136 (81, 191) | 5.9 (5.5, 6.2) | 7.2 (6.2, 8.1) | 7.7 (7.4, 8.0) | |
| PLATs | 22 (0, 44) | 56 (25, 88) | 32 (24, 39) | 2.4 (2.3, 2.5) | 2.9 (2.8, 3.0) | 3.6 (3.6, 3.7) | |
n = 30 (0-portions group), n = 35 (1-portion group), n = 30 (2-portions group), and n = 33 (4-portions group). Data were calculated as the time (d) to reach the maximum concentration of EPA or DHA as a percentage of total FAs and the maximum incorporation (percentage of total FA). To determine the time to maximum incorporation, a nonlinear model (FA = a + b × ctime, where a represents the asymptote, b is the change from baseline to the asymptote, and ctime is the rate of increase in the outcome to the asymptote) was used to explain the FA concentration over time with the assumption that the FA concentration increases monotonically over time until it eventually reaches an asymptote. Ninety-five percent of the asymptote was used to define the maximal FA incorporation, and these model variables and their respective variance-covariance matrix were used to estimate the time taken to reach within 5% of the asymptote with its corresponding 95% CI. The rate of incorporation data were not calculated for adipose tissue because of the limited number of sampling time points or for buccal cells or plasma CEs (DHA only) because of the shape of the dose-response curves where it was not possible to fit a hyperbolic curve to estimate the value of the curve variable. CEs, cholesteryl esters; FA, fatty acid; MNCs, blood mononuclear cells; NEFAs, nonesterified fatty acids; PC, phosphatidylcholine; PLATs, platelets; RBCs, erythrocytes (red blood cells); TAGs, triglycerides.
No data are presented because this model did not converge.
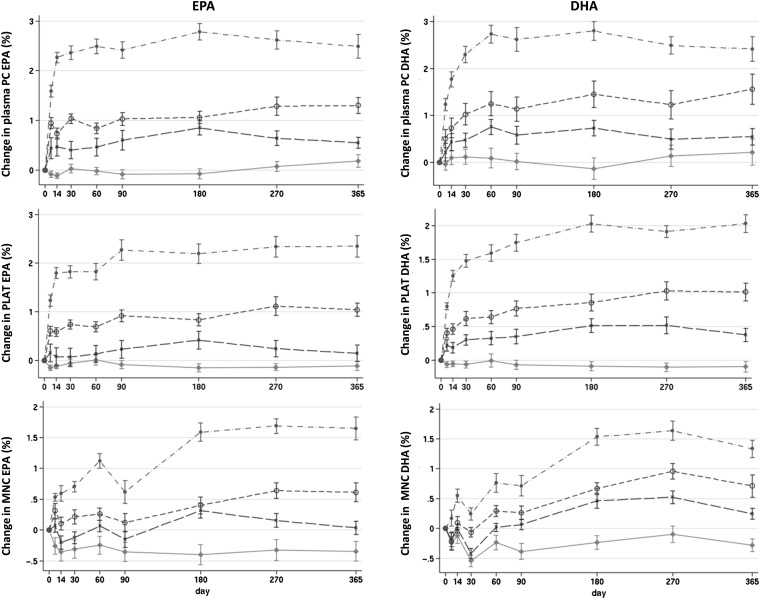
Overall incorporation profiles for the change in EPA and DHA in plasma phosphatidylcholine, MNCs, and platelets, representing a range of incorporation rates, are shown in Figure 3. See Figure 1 under “Supplemental data” in the online issue for incorporation profiles of the remaining pools. The incorporation of EPA tended to be faster than that of DHA for all pools, and there was no obvious consistent difference in the rate of incorporation within a pool among different groups.
FIGURE 3.
Mean (±SE) changes from baseline of EPA and DHA concentration (percentage of total fatty acids) in plasma PC, PLAT, and MNC in response to EPA and DHA equivalent to 0, 1, 2, and 4 portions of oily fish per week for 12 mo. The control group is represented by diamonds, the 1-portion group is represented by Xs, the 2-portions group is represented by open circles, and the 4-portions group is represented by closed circles. The panels provide graphical representation of EPA and DHA responses to doses over the 12-mo intervention. Statistical analyses of responses are shown in Tables 2–4. MNC, mononuclear cells; PC, plasma phosphatidylcholine; PLAT, platelets.
DISCUSSION
This study examined a number of pools in which EPA and DHA are currently routinely measured in intervention or surveillance studies, in addition to some novel pools, to characterize the relative dose and time responses of incorporation and to identify the most suitable pools in which to measure EPA and DHA. The pools studied represent transport, functional, and storage pools. Plasma lipid pools were studied separately to gain a detailed understanding of how these different transport pools respond to increased intake of EPA and DHA and because these pools show different rates of turnover. This study showed plasma phosphatidylcholine and platelets to be the best pools for discriminating between the amounts of EPA and DHA consumed across a range equivalent to 0–4 portions of oily fish per week. In studying the time for incorporation of EPA and DHA into various pools, plasma phosphatidylcholine was shown to reflect rapid changes in EPA and DHA intakes, whereas platelets and, more so, MNCs reflected changes over a longer time and were more appropriate measures of habitual intake. The approach used in this study provided lower amounts of EPA and DHA across a week than in previous intervention studies in which high doses of EPA plus DHA have been administered on a daily basis. Furthermore, the intake of EPA plus DHA was sporadic, which allowed the characterization of EPA and DHA incorporation into various pools by a dietary regimen that more closely reflected oily fish consumption.
There was a significant dose response for EPA, DHA, and EPA plus DHA in all sample types (with the single exception of erythrocyte EPA, for which there was a strong trend for a dose response), and there was a clear linear trend of increasing EPA and DHA with increasing doses at 12 mo. The strongest dose-response relations were obtained for platelets, MNCs, and plasma phosphatidylcholine, triglycerides, and CEs. However CEs had very low concentrations of DHA (which did show a dose-response relation) as shown previously (14, 15), and DHA was difficult to detect in some samples, which led to a high variability. For this reason, it seems that plasma CE is not as robust a sample type in which to assess EPA plus DHA status. Plasma triglyceride EPA, DHA, and EPA plus DHA, although a clear dose-response to VLC n−3 PUFA intake was shown, also showed large SDs at higher levels of intake and, therefore, were not considered as useful to discriminate VLC n−3 PUFA intake as some other sample types.
The AT FA composition has generally been considered representative of a habitual diet because of the slow turnover time in weight-stable individuals and has been considered the gold-standard measurement for dietary FA status (10). However, EPA and DHA are present at very low concentrations in AT. An AT biopsy is also a very invasive procedure and not practical for surveys or field work. For these reasons, the AT FA composition may not be the best biomarker for the measurement of intake of these FAs, particularly when the consumption of VLC n−3 PUFAs is within normal dietary amounts. However, AT EPA and DHA responded to increased intakes.
BUs were investigated as a potential simple noninvasive marker of EPA and DHA intakes. Previous studies have significantly associated FAs in BUs with those in plasma and RBCs (16, 17). Indeed, in the current study, EPA and DHA in BUs correlated moderately well and significantly with EPA and DHA in the other sample types assessed. Although there was an overall dose response in BUs, by 12 mo, there was no linear dose trend for EPA and DHA, which suggested that this pool may not adequately reflect long-term FA intake. BU data in the current study were also shown to be variable. This variability was most likely due to the lower overall concentration of FAs in BUs compared with in other sample types. In a previous 2-y supplementation trial that aimed to use BU VLC n−3 PUFA concentrations to monitor compliance, the variation was also shown to be too high, and values were unreliable, because of the low total yield of FAs (18).
In the current study, RBCs showed a weaker (and nonsignificant) dose-response relation for EPA than several other pools, and data were highly variable, which indicated that they may not discriminate well between VLC n−3 PUFAs at normal dietary levels. EPA and DHA are commonly measured in RBCs in both population surveillance (19) and VLC n−3 PUFA intervention studies (14, 15, 20). Intervention studies have often used much-higher doses of EPA and DHA than used in the current study; eg, in a meta-analysis of the effects on serum markers of cardiovascular risk, the median dose used was 3 g EPA plus DHA/d (21). The results of the current study indicated that other sample types may be preferable to RBCs for short-term (<3-mo) dietary intervention studies and to discriminate between low intakes of VLC n−3 PUFAs, particularly EPA, in dietary surveillance studies.
Fatty acids in different locations may be considered to represent transport (plasma phospholipids, triglycerides, CEs, and NEFAs), functional (cellular phospholipids), or storage (AT triglycerides) roles. This study demonstrated the time to maximal incorporation of FAs increased from days to weeks for transport pools, weeks to months for functional pools, and months to years for storage pools. Correspondingly, the pool in which EPA and DHA should be measured depends on the nature of investigation. To measure acute changes in short-term intervention studies, this study identified transport pools, in particular plasma phosphatidylcholine, as the most suitable biomarker. In contrast, to measure longer-term and habitual intakes, the more-stable functional pools, in particular MNCs or platelets, should be used. In this study, EPA and DHA status in plasma phosphatidylcholine, triglycerides, and CEs, MNCs, and platelets were all strongly related at 12 mo. However, this was assessed in a situation in which the intake was intermittent but regular. Because the amounts of EPA and DHA were shown to change acutely in transport pools, these are unsuitable pools in which to try to assess habitual intake because recent changes in fish intake are likely to have a large impact on EPA and DHA amounts in these pools. AT and BU EPA, DHA, and EPA plus DHA and RBC EPA were not strongly related to status in other sample types at 12 mo. This result may have been due to the higher variability of FA data within these sample types, which was reflected in the relative lack of a strong dose response. EPA and DHA have been measured in a variety of pools in epidemiologic and intervention studies. These studies have included whole blood and total plasma, which were not included in the current analysis. This lack of inclusion represents a limitation of the current study. However, it seems likely that the FA composition of whole blood would be dominated by that of RBCs, whereas the FA composition of whole plasma would represent that of the combination of individual lipid classes reported in the current study. Of these fractions, plasma phosphatidylcholine has the highest DHA content and demonstrated the greatest increase in DHA. Thus, plasma phosphatidylcholine would likely make a major contribution to total plasma DHA. Phosphatidylcholine and CEs would likely make the biggest contributions to total plasma EPA.
The proportion of DHA, as a percentage of total FAs, was higher than that of EPA in all sample types irrespective of VLC n−3 PUFA intake, as has been shown previously in a limited number of pools (14, 15), except for plasma CEs, for which the proportion of DHA was much lower than that of EPA. Higher intakes of VLC n−3 PUFAs resulted in greater proportional increases in EPA than in DHA, especially in plasma phosphatidylcholine, MNCs, and platelets, which was a result that was consistent with previous findings in plasma phospholipids, plasma CEs, MNCs, RBCs, and AT (14, 15, 22, 23). Background levels of EPA and DHA were highest in RBCs, but the increase in the response to VLC n−3 PUFA intake was lowest in this pool. This result may reflect the importance and potential enrichment of VLC n−3 PUFA compared with the diet in this functional pool. Proportions of EPA and DHA as a percentage of total FAs were lowest in AT, and AT showed the lowest proportional increases in response to VLC n−3 PUFA intakes, as described previously in comparison to RBCs and CEs (14). This preferential partitioning of FAs is likely to reflect the importance of EPA and DHA in transport and functional pools, rather than in storage, and also the large mass of AT FAs that had built up over the lifetime.
A combined measure of EPA plus DHA was shown to be a better discriminator of the amount of VLC n−3 PUFAs consumed than the individual measures in all pools. A combined measure of EPA plus DHA in RBCs (the n−3 index) is increasingly being adopted as a risk marker for coronary heart disease (24), mostly in cross-sectional analyses, although it has more recently been used in intervention studies (25). Results from the current study indicated that studies that assess this biomarker in intervention settings should be of sufficient duration (>2 mo, and longer for higher doses) to capture changes in RBC EPA plus DHA.
In conclusion, this study characterized the FA composition of a variety of sample types in response to a sporadic intake of VLC n−3 PUFA as seen with oily fish consumption. Although increased EPA plus DHA intake was reflected in almost all pools assessed, the most suitable biomarkers of oily fish intake within the range likely to be consumed by the UK population (1–4 portions/wk) were identified. These biomarkers were plasma phosphatidylcholine EPA plus DHA for acute changes in intake and platelet and MNC EPA plus DHA for habitual (long-term) intake.
Acknowledgments
We acknowledge the following contributors—the Medical Research Council Human Nutrition Research: Louise Timbers, Clionadh O'Reilly, Mariana Eberhard, Katey Bergstralh, and Sarah Gibbings (research assistants), Darren Cole (database manager), Christine Clewes (FA analysis), Alison Lennox, Birgit Teucher, Anna Gent, and Celia Greenberg (coding and analysis of dietary data), Mario Siervo, Rosemary Hall, and Sue Fisher (clinical support); the University of Southampton: Christiaan Gelauf and Jade Pretorius (FA analysis); and the research nursing team at the Wellcome Trust Clinical Research Facility, University Hospital Southampton National Health Service Foundation Trust.
The authors’ responsibilities were as follows—LMB, SAJ, and PCC: designed the study; LMB, CGW, ALW, JM, JMG, SY, and LW: conducted the research; LMB, CGW, and APM: analyzed data; LMB, CGW, SAJ, and PCC: wrote the manuscript; and all authors: read and approved the final manuscript. PCC serves on Scientific Advisory Boards of the Danone Research Centre in Specialised Nutrition and Aker Biomarine; acts as a consultant to Mead Johnson Nutritionals, Vifor Pharma, and Amarin Corporation; has received speaking honoraria from Solvay Healthcare, Solvay Pharmaceuticals, Pronova Biocare, Fresenius Kabi, B Braun, Abbott Nutrition, Baxter Healthcare, Nestle, Unilever, and DSM; currently receives research funding from Vifor Pharma; serves on the executive board of the International Society for the Study of Fatty Acids and Lipids, which is an organization that is partly supported by corporate membership fees, mainly the food and supplements industries; and is a member of the board of directors of ILSI Europe, the board of directors of the European Nutraceutical Association, and the Council of the British Nutrition Foundation; these organizations are each supported in part by the food and supplements industries. SAJ previously served on Scientific advisory boards for Tanita, PepsiCo, Nestlé, Coca-Cola, California Almond Board, Heinz, and Kelloggs; is currently a member of the Tanita Medical Advisory Board; and receives a fee for nutrition articles in the Rosemary Conley Diet and Fitness Magazine. LMB, CGW, APM, ALW, JM, JMG, SY, and LW had no conflicts of interest.
Footnotes
Abbreviations used: AT, adipose tissue; BU, buccal cell; CE, plasma cholesteryl ester; FA, fatty acid; FAME, fatty acid methyl ester; MNC, blood mononuclear cell; NEFA, nonesterified fatty acid; PBS, phosphate-buffered saline; RBC, erythrocyte (red blood cell); VLC, very-long-chain.
REFERENCES
- 1.Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev 2010;68:280–9 [DOI] [PubMed] [Google Scholar]
- 2.Kromhout D, Bosschieter EB, de Lezenne Coulander C. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med 1985;312:1205–9 [DOI] [PubMed] [Google Scholar]
- 3.Cole GM, Ma Q-L, Frautschy SA. Omega-3 fatty acids and dementia. Prostaglandins Leukot Essent Fatty Acids 2009;81:213–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez E, Chatenoud L, La Vecchia C, Negri E, Franceschi S. Fish consumption and cancer risk. Am J Clin Nutr 1999;70:85–90 [DOI] [PubMed] [Google Scholar]
- 5.Saravanan P, Davidson NC, Schmidt EB, Calder PC. Cardiovascular effects of marine omega-3 fatty acids. Lancet 2010;376:540–50 [DOI] [PubMed] [Google Scholar]
- 6.Calder PC. n−3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr 2006;83:1505S–19S [DOI] [PubMed] [Google Scholar]
- 7.Scientific Advisory Committee on Nutrition (SACN) and Committee on Toxicity (COT) Advice on fish consumption: benefits & risks. London, United Kingdom: Her Majesty's Stationery Office, 2004 [Google Scholar]
- 8.Kris-Etherton PM, Harris WS, Appel LJ. ; American Heart Association. Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002;106:2747–57 (Published erratum appears in Circulation 2003;107:512.) [DOI] [PubMed] [Google Scholar]
- 9.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n–3 fatty acids in humans. Am J Clin Nutr 2006;83:1467S–76S [DOI] [PubMed] [Google Scholar]
- 10.Hodson L, Skeaff C, Fielding B. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008;47:348–80 [DOI] [PubMed] [Google Scholar]
- 11.Gurr MI, Harwood JL, Frayn KN. Lipid biochemistry. 5th ed. Oxford, United Kingdom: Blackwell Science, 2005 [Google Scholar]
- 12.Fielding B. Tracing the fate of dietary fatty acids: metabolic studies of postprandial lipaemia in human subjects. Proc Nutr Soc 2011;70:342–50 [DOI] [PubMed] [Google Scholar]
- 13.Food Standards Agency. McCance and Widdowson's the composition of foods. 6th summary ed. Cambridge, United Kingdom: Royal Society of Chemistry, 2002. [Google Scholar]
- 14.Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res 1997;38:2012–22 [PubMed] [Google Scholar]
- 15.Vidgren H, Ågren J, Schwab U, Rissanen T, Hänninen O, Uusitupa M. Incorporation of n−3 fatty acids into plasma lipid fractions, and erythrocyte membranes and platelets during dietary supplementation with fish, fish oil, and docosahexaenoic acid-rich oil among healthy young men. Lipids 1997;32:697–705 [DOI] [PubMed] [Google Scholar]
- 16.Connor SL, Zhu N, Anderson GJ, Hamill D, Jaffe E, Carlson J, Connor WE. Cheek cell phospholipids in human infants: a marker of docosahexaenoic and arachidonic acids in the diet, plasma, and red blood cells1. Am J Clin Nutr 2000;71:21–7 [DOI] [PubMed] [Google Scholar]
- 17.Harris WS, Sands SA, Windsor SL, Ali HA, Stevens TL, Magalski A, Porter CB, Borkon AM. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients. Circulation 2004;110:1645–9 [DOI] [PubMed] [Google Scholar]
- 18.Dangour AD, Allen E, Elbourne D, Fasey N, Fletcher AE, Hardy P, Holder GE, Knight R, Letley L, Richards M, et al. Effect of 2-y n−3 long-chain polyunsaturated fatty acid supplementation on cognitive function in older people: a randomized, double-blind, controlled trial. Am J Clin Nutr 2010;91:1725–32 [DOI] [PubMed] [Google Scholar]
- 19.Harris WS. The omega-3 index as a risk factor for coronary heart disease. Am J Clin Nutr 2008;87:1997S–2002S [DOI] [PubMed] [Google Scholar]
- 20.Metherel AH, Armstrong JM, Patterson AC, Stark KD. Assessment of blood measures of n−3 polyunsaturated fatty acids with acute fish oil supplementation and washout in men and women. Prostaglandins Leukot Essent Fatty Acids 2009;81:23–9 [DOI] [PubMed] [Google Scholar]
- 21.Hartweg J, Farmer A, Perera R, Holman R, Neil H. Meta-analysis of the effects of n−3 polyunsaturated fatty acids on lipoproteins and other emerging lipid cardiovascular risk markers in patients with type 2 diabetes. Diabetologia 2007;50:1593–602 [DOI] [PubMed] [Google Scholar]
- 22.Yaqoob P, Pala HS, Cortina-Borja M, Newsholme EA, Calder PC. Encapsulated fish oil enriched in α-tocopherol alters plasma phospholipid and mononuclear cell fatty acid compositions but not mononuclear cell functions. Eur J Clin Invest 2000;30:260–74 [DOI] [PubMed] [Google Scholar]
- 23.Thies F, Nebe-von-Caron G, Powell JR, Yaqoob P, Newsholme EA, Calder PC. Dietary supplementation with γ-linolenic acid or fish oil decreases T lymphocyte proliferation in healthy older humans. J Nutr 2001;131:1918–27 [DOI] [PubMed] [Google Scholar]
- 24.Harris WS, Lemke S, Hansen S, Goldstein D, DiRienzo M, Su H, Nemeth M, Taylor M, Ahmed G, George C. Stearidonic acid-enriched soybean oil increased the omega-3 index, an emerging cardiovascular risk marker. Lipids 2008;43:805–11 [DOI] [PubMed] [Google Scholar]
- 25.Lemke SL, Vicini JL, Su H, Goldstein DA, Nemeth MA, Krul ES, Harris WS. Dietary intake of stearidonic acid-enriched soybean oil increases the omega-3 index: randomized, double-blind clinical study of efficacy and safety. Am J Clin Nutr 2010;92:766–75 [DOI] [PubMed] [Google Scholar]