Abstract
Background: Other than the in vitro erythrocyte hemolysis test, no valid biomarkers of vitamin E status currently exist.
Objective: We hypothesized that the urinary vitamin E metabolite α-carboxyethyl hydroxychroman (α-CEHC) could serve as a biomarker.
Design: The relations between urinary α-CEHC, plasma α-tocopherol, and vitamin E intakes were assessed by using a previously validated multipass, Web-based, 24-h self-administered dietary recall, and we concurrently collected plasma and 24-h urine samples from 233 participants of both sexes.
Results: Median vitamin E intakes were 9.7 mg α-tocopherol/d. Intakes were correlated with plasma α-tocopherol (R = 0.40, P < 0.001) and urinary α-CEHC (R = 0.42, P < 0.001); these correlations were essentially unchanged after multivariate adjustments. On the basis of multiple regression analysis, urinary α-CEHC excretion increased by ∼0.086 μmol/g creatinine (95% CI: 0.047, 0.125) for every 1-mg (2.3-μmol) increase in dietary α-tocopherol. Urinary α-CEHC excretion remained at a plateau (median: 1.39 μmol/g creatinine) until dietary intakes of α-tocopherol exceeded 9 mg α-tocopherol/d. The inflection point at which vitamin E metabolism increased was estimated to be at an intake of 12.8 mg α-tocopherol/d. Daily excretion of >1.39 μmol α-CEHC/g creatinine is associated with a greater than adequate α-tocopherol status, as evidenced by increased vitamin E metabolism and excretion.
Conclusion: Thus, urinary α-CEHC is a valid biomarker of α-tocopherol status that can be used to set a value for the Estimated Adequate Requirement of vitamin E.
INTRODUCTION
Estimation of vitamin E intakes is problematic because vitamin E is fat soluble, and dietary fat is often underreported (1). Moreover, different sources of fat have different amounts and kinds of vitamin E (1). Plants synthesize 8 different molecular forms (each with a chromanol head group) that have antioxidant activities similar to those of α-tocopherol, as discussed previously (2). These forms vary with respect to the number of methyl groups on the chromanol ring as follows: α- (3 methyl groups), β- or γ- (2 methyl groups), δ- (2 methyl groups) tocopherol, or tocotrienol. The tocopherols have saturated side chains, and the tocotrienols have unsaturated side chains. Importantly, these various forms are not interconvertable by humans (1). Given that the biological function of α-tocopherol is to act as an antioxidant, scavenging lipid peroxyl radicals (3), it is important to note that the biological activity of vitamin E depends on regulatory mechanisms that involve the preferential transfer of α-tocopherol from the liver into the plasma, facilitated by the hepatic α-tocopherol transfer protein, along with increased hepatic metabolism and excretion of non-α-tocopherol forms as carboxyethyl hydroxychromanols (4).
The biological activity of α-tocopherol also depends on its stereochemistry with the natural RRR-α-tocopherol [2-methyl-2-(4′,8′,12′-trimethyltridecyl)-chroman-6-ol], which has twice the activity of the synthetic all-rac mixture of RRR, RSR, RRS, RSS, SSS, SRS, SRR, and SSR, which is frequently used in vitamin supplements and food fortificants (1). All 2R-α-tocopherol stereoisomers (RRR, RSR, RRS, and RSS) are included in the vitamin E dietary requirements (1).
In 2000, when the Dietary Reference Intake for vitamin E was established, the Dietary Reference Intakes were based on a test of hemolysis that required in vitro addition of peroxide to erythrocytes from vitamin E–deficient subjects (1). Lipid peroxidation biomarkers were not specific to only vitamin E status, plasma α-tocopherol did not correlate well with dietary intake (5–7), and few data are available concerning the mechanisms regulating urinary excretion of α-tocopherol metabolites (Figure 1): α-carboxyethyl hydroxychroman (α-CEHC)4 and carboxymethylbutyl hydroxychroman (α-CMBHC). We hypothesized that the urinary metabolites α-CEHC and α-CMBHC could serve as reliable biomarkers of dietary vitamin E intakes and as surrogates for vitamin E status. Herein, we report a self-administered, Web-based, 24-h dietary recall to assess dietary vitamin E intake and to assess the validity of these metabolites as biomarkers of vitamin E intakes relative to measures of plasma α-tocopherol.
FIGURE 1.
Structures of α-tocopherol and its urinary metabolites α-CEHC and α-CMBHC. α-CEHC, α-carboxyethyl hydroxychroman; α-CMBHC, carboxymethylbutyl hydroxychroman.
SUBJECTS AND METHODS
Study population
The University of California, Los Angeles (UCLA) Energetics Study was conducted between July 2006 and June 2009. The study population, data collection, and dietary assessment were extensively described elsewhere (8–11). In brief, the study subjects were participants in the Energetics Study—an NIH-supported study that was designed to evaluate dietary assessment. The UCLA Institutional Review Board approved the study protocol, and all participants provided written informed consent.
DietDay is a fully automated, self-administered, Web-based, computer-assisted self-interview, 24-h dietary recall, viewable at www.24hrrecall.com. DietDay applies multipasses similar to the USDA-designed multipass approach (12): a first overview report of all types of food consumed by meal, a comprehensive reporting of details on those foods down to 7 levels of information, a reminder about possibly forgotten snack foods, and a last review of the foods reported to allow additions and changes. DietDay also assesses supplement use and provides feedback in the form of extensive individual dietary evaluations based on National Academy of Science recommendations (13).
DietDay contains 9349 foods and >7000 food images in 61 modules. Portion sizes are quantified by household measures by using images of different amounts of foods on a standard plate, glass, or bowl, as illustrated elsewhere (11). Food-preparation methods, condiments, and other additions were also assessed. DietDay asks about usual consumption by time of day. DietDay applies automatic branching, complex skip routines, range checks, edit checks, and prompts during the questionnaire (14). Nutrient values in the program are based on USDA food composition and expanded to include mixed dishes and product-labeling information.
In total, 333 subjects consented to participate in the study, 268 were scheduled into the study, and 261 completed all clinic visits. A total of 233 subjects completed the study, and the demographic profile is summarized in Table 1. Approximately half of the subjects were African American, half were white, and a greater percentage of females than males completed the study. Plasma concentrations of α- and γ-tocopherol were measured, and 2 separate 24-h urine samples were collected from 233 participants. Plasma samples were analyzed by Craft Technologies, and the urine samples were analyzed at Oregon State University. Both sets were coded for statistical evaluation to the UCLA group.
TABLE 1.
Sample demographics of the University of California, Los Angeles Energetics Study
| Total (n = 233) | |
| Race [n (%)] | |
| White | 118 (50.6) |
| African American | 115 (49.4) |
| Sex [n (%)] | |
| Female | 158 (67.8) |
| Male | 75 (32.2) |
| Age (y) | 33.3 ± 12.51 |
| Age [n (%)] | |
| 21–30 y | 95 (40.8) |
| >30–50 y | 87 (37.3) |
| >50–69 y | 51 (21.9) |
| BMI (kg/m2) | 25.0 ± 6.1 |
| BMI [n (%)] | |
| <25 kg/m2 | 112 (48.1) |
| 25–30 kg/m2 | 68 (29.2) |
| >30 kg/m2 | 53 (22.8) |
| Education [n (%)] | |
| Less than high school | 1 (0.4) |
| High school graduate | 6 (2.6) |
| Some college | 86 (36.9) |
| College graduate | 105 (45.1) |
| Postgraduate | 35 (15.0) |
| Serum lipids (mg/dL) | |
| Cholesterol | 169 ± 40.5 |
| Triglycerides | 77 ± 62.4 |
| Supplement users (%) | |
| Single vitamin E | 2 |
| Multivitamins | 14 |
Median ± SD (all such values).
Over a 2-wk period, subjects visited the UCLA General Clinical Research Center (GCRC) twice, and the diet questionnaires were self-administered 8 times throughout the study. The subjects were asked to complete a total of 8 questionnaires: 3 at the GCRG study visits and 5 on their own. The approximate timing of the 8 self-administered 24-h recalls was as follows: during the first week and then during the 3rd, 5th, 8th, 10th, 13th, 30th, and 60th wks. The data from the first 6 dietary recalls were used for the analyses in the current study.
Laboratory methods
Blood samples were collected in the morning from subjects after a fast of ≥10 h on days coinciding with one 24-h dietary recall. Samples were protected from light and processed within 1 h of collection at the UCLA GCRC core laboratory by centrifugation at 2500 rpm for 15 to 20 min. Plasma aliquots (0.5 mL) were stored at −80°C until the time of analysis. Samples were analyzed in batch. Plasma vitamin E was measured according to the method of Nomura et al (15).
The urinary vitamin E metabolites (Figure 1) α-CEHC, γ-CEHC, and α-CMBHC were extracted and measured by using a modified version of the liquid chromatography–mass spectrometry method of Leonard et al (16). Briefly, an aliquot of urine (100 μL) was added to a 10-mL screw-cap tube containing Milli-Q water and 100 μL 1% ascorbic acid. The sample was incubated for 1 h at 37°C after the addition of 100 μL β-glucuronidase (1 mg in 100 μL of 10 mmol acetate buffer/L, pH 6.8, type H-1; contains a minimum of 300,000 U/g β-glucuronidase activity and a minimum of 10,000 U/g sulfatase activity; Sigma-Aldrich). Metabolites were extracted twice with 3 mL diethyl ether, and an aliquot of the ether fraction was collected and dried under nitrogen. The sample was resuspended in 1:1 (vol:vol) water:methanol containing trolox (Sigma; internal standard) for injection into the liquid chromatography–mass spectrometer. With the use of single-ion recording m/z, data were obtained for α-CEHC (277.6), γ-CEHC (263.6), α-CMBHC (319.6), and trolox (249.6). Typical retention times were 14.93 (trolox), 15.03 (γ-CEHC), 15.90 (α-CEHC), and 20.39 (α-CMBHC) min. Analyte concentrations were calculated from the peak area of the ion corresponding to that of the trolox peak and compared with peak areas of authentic compounds. Urinary creatinine was measured by using kit 55A from Sigma-Aldrich, which is based on the Jaffe reaction (17).
Statistical analysis
Ford et al (18) reported “the arithmetic mean (±SEM) of serum concentrations of the US adult population of α-tocopherol to be 30.09 ± 0.45 μmol/L, the median; 25.94 μmol/L.” On the basis of these data, we separated out subjects with high plasma α-tocopherol (>33 μmol/L, hereafter referred to as “high”) from subjects with typical plasma α-tocopherol (≤33 μmol/L, hereafter referred to as “typical”) for analysis. There were 200 persons in the typical group, none of whom took multivitamins. The high group was separated out to avoid those subjects consuming multivitamins or foods with extraordinarily high levels of vitamin E. The percentage contribution of foods to dietary vitamin E intake was computed by aggregating all reported food items that contained vitamin E for subjects in the high and typical groups and all subjects independently. Foods were then grouped into broader food categories, such as “fruit and vegetables,” and the amount of vitamin E (mg) contributed by each food item (eg, mg vitamin E contributed by avocados) was summed to obtain the total amount of vitamin E (mg) contributed by the specific food category (eg, mg vitamin E contributed by fruit). Each food category was then expressed as a percentage of total vitamin E.
All data were log transformed for analysis to normalize the variances. Standard regression and Pearson correlation analysis were used to compare from each individual the mean dietary α-tocopherol obtained from the first 6 dietary 24-h recalls with mean α-CEHC or mean α-CMBHC concentrations averaged from two 24-h urine collections and plasma α-tocopherol. Differences between the high and typical groups were determined by using Wilcoxon's tests. Results were considered statistically significant at P < 0.05. The statistical software packages used to analyze the data were SAS software (version 9.3; SAS Institute) and Stata software (Stata/SE 12.0 for Windows; StataCorp LP).
For the evaluation of the urinary excretion of α-CEHC relative to dietary α-tocopherol, the median α-CEHC was plotted for each incremental milligram of dietary α-tocopherol; the Spearman rank correlation was then used to assess significance. Shown is a spline curve to assess where the increase in α-CEHC excretion relative to dietary α-tocopherol occurred. The plateau in α-CEHC excretion was calculated as the median value from subjects with dietary vitamin E <9 mg. With the use of multiple regression analysis, the relation between dietary α-tocopherol >9 mg and urinary α-CEHC excretion was calculated and used to estimate the intersection of the plateau with the increase in α-CEHC excretion.
RESULTS
Dietary alpha-tocopherol, plasma alpha-tocopherol, and urinary vitamin E metabolites
Mean dietary α-tocopherol intakes were greater in this study (Table 2) than those previously reported for Americans by others (19, 20). The average α-tocopherol intake of subjects with typical plasma α-tocopherol (≤33 μmol/L) concentrations was 13.3 mg α-tocopherol/d. In contrast, subjects with high plasma α-tocopherol (>33 μmol/L) concentrations consumed on average 23.3 mg α-tocopherol/d. The median dietary α-tocopherol intakes are also reported because the data were not normally distributed (Table 2). For all subjects, the median intake was 9.7 mg α-tocopherol—less than the Estimated Average Requirement for vitamin E of 12 mg (1).
TABLE 2.
Self-reported dietary intakes of α- and γ-tocopherol, plasma α- and γ-tocopherol concentrations, and urinary vitamin E metabolite concentrations1
| All subjects(n = 233) | High group2(n = 33) | Typical group2(n = 200) | P value3 | |
| Dietary α-tocopherol (mg/d)4 | 14.7 ± 12.9 (9.7) | 23.3 ± 17.4 (17.8) | 13.3 ± 11.5 (8.6) | 0.003 |
| Dietary γ-tocopherol (mg/d)4 | 4.5 ± 5.3 (3.2) | 4.8 ± 7.5 (2.5) | 4.4 ± 4.8 (3.3) | 0.46 |
| Plasma α-tocopherol (μmol/L) | 25.4 ± 7.8 (24.3) | 39.9 ± 6.3 (39.7) | 23.0 ± 4.9 (23.3) | 0.001 |
| Plasma α-tocopherol (mmol/mol)5 | 6.4 ± 1.6 (6.1) | 8.6 ± 1.8 (8.5) | 5.9 ± 1.2 (5.8) | 0.001 |
| Plasma γ-tocopherol (mmol/mol)5 | 0.96 ± 0.48 (0.87) | 0.79 ± 0.39 (0.72) | 0.98 ± 0.49 (0.90) | 0.02 |
| Urinary α-CEHC (μmol/g)6 | 2.9 ± 3.2 (1.8) | 5.5 ± 4.7 (4.1) | 2.6 ± 2.7 (1.6) | 0.001 |
| Urinary α-CMBHC (μmol/g)6 | 0.44 ± 0.63 (0.23) | 0.7 ± 0.8 (0.42) | 0.4 ± 0.6 (0.22) | 0.001 |
| Urinary γ-CEHC (μmol/g)6 | 5.1 ± 3.4 (4.3) | 5.6 ± 3.1 (5.3) | 4.9 ± 3.5 (4.2) | 0.12 |
All values are means ± SDs; medians in parentheses. CEHC, carboxyethyl hydroxychroman; CMBHC, carboxymethylbutyl hydroxychroman.
Subjects were divided into high or typical grouping based on a plasma α-tocopherol value >33 μmol/L. Those subjects with plasma values >33 μmol/L were placed in the “High” group, and those subjects with plasma values ≤33 μmol/L were placed in the “Typical” group.
P values for high compared with low comparisons were estimated by using Wilcoxon's tests.
Dietary (http://24hrrecall.com); mean of first 6 dietary recalls.
Plasma concentrations were normalized for total cholesterol.
Mean of two 24-h urine collections; normalized for creatinine.
The average plasma α-tocopherol concentration in the high group was 1.7 times that in the typical group (Table 2). Not unexpectedly, concentrations of the urinary metabolites α-CEHC and α-CMBHC in the high group were double those in the typical group (Table 2). Although the reported dietary γ-tocopherol intakes did not differ between the high and typical groups, plasma γ-tocopherol concentrations were 20% greater in the typical group than in the high group, and urinary γ-CEHC concentrations did not differ between groups (Table 2).
Foods contributing to alpha-tocopherol intakes
The foods that contributed to the intake of dietary vitamin E are shown in Table 3. In the high plasma α-tocopherol subjects, multivitamins were the largest contributors to dietary vitamin E intakes, whereas the food groups that provided the largest contributions were snacks and nuts. In the typical subjects, mixed meals followed by cereals and grains and oils were the largest contributors to vitamin E intakes.
TABLE 3.
Major contributors to dietary vitamin E intake1
| Food | All subjects(n = 233) | High group2(n = 33) | Typical group2(n = 200) |
| Vitamin supplements3 | 17.4 ± 0.9 | 56.0 ± 5.9 | 0.0 ± 0.0 |
| Meals4 | 12.2 ± 3.6 | 4.4 ± 0.2 | 13.7 ± 0.1 |
| Snacks5 | 9.8 ± 0.6 | 10.3 ± 1.7 | 10.1 ± 0.1 |
| Nuts6 | 9.7 ± 1.1 | 9.0 ± 1.1 | 10.2 ± 0.3 |
| Cereals and grains7 | 8.9 ± 1.8 | 6.0 ± 0.9 | 11.3 ± 0.2 |
| Oils and butter8 | 8.3 ± 0.9 | 2.6 ± 0.6 | 11.0 ± 0.1 |
| Fruit | 6.8 ± 2.6 | 4.3 ± 0.3 | 8.1 ± 0.3 |
| Vegetables | 6.7 ± 1.4 | 2.5 ± 0.5 | 6.8 ± 0.05 |
| Seeds | 5.1 ± 7.9 | 0.0 ± 0.0 | 7.6 ± 1.0 |
| Meats | 4.2 ± 0.3 | 1.7 ± 0.1 | 6.1 ± 0.05 |
| Desserts9 | 4.1 ± 0.7 | 1.1 ± 0.1 | 5.8 ± 0.1 |
| Eggs10 | 3.8 ± 0.8 | 1.0 ± 0.1 | 5.3 ± 0.1 |
| Breads | 1.2 ± 0.1 | 0.8 ± 0.1 | 1.5 ± 0.02 |
| Dairy11 | 0.9 ± 0.1 | 0.3 ± 0.1 | 1.2 ± 0.01 |
| Drinks12 | 0.9 ± 0.2 | 0.04 ± 0.01 | 1.2 ± 0.02 |
All values are means ± SDs.
Subjects were divided into high or typical grouping based on a plasma α-tocopherol value >33 μmol/L. Those subjects with plasma values >33 μmol/L were placed in the “High” group, and those subjects with plasma values ≤33 μmol/L were placed in the “Typical” group.
Multivitamins and vitamin water.
Soups, Caesar and meat-containing salads, pasta dishes with mixed ingredients (eg, lo mein and chow mein), calzones, sandwiches (eg, fried chicken or Italian sub), meatloaf, etc.
Pizzas, potato chips, fries, popcorn, crackers, and pretzels.
Nuts and peanut butter.
Cold and hot cereals, rice, and wheat germ.
Oils, salad dressings, margarine, and butter.
Cookies, pies, ice cream, brownies, taffy, etc.
Any style of eggs and egg-salad sandwich.
Milk, cheese, and yogurt.
Diet drinks, soy milk, lemonade, cider, coffee, and espresso.
Correlations between dietary vitamin E intakes and plasma tocopherols
For all subjects, dietary α-tocopherol intakes were correlated with plasma α-tocopherol (R = 0.43; Table 4), both before and after adjustment for various factors (plasma lipids, BMI, age, sex, race, and total energy intake) and inversely with plasma γ-tocopherol concentrations (R = −0.30). This was true of the typical group but not in subjects with high plasma α-tocopherol concentrations, for whom no associations with either plasma α- or γ-tocopherol were observed with either dietary α- or γ-tocopherol (Table 4).
TABLE 4.
Pearson correlations between reported dietary intakes of α- or γ-tocopherol and corresponding plasma α- or γ-tocopherol concentrations1
| Plasma α-tocopherol (mmol/mol)2 |
Plasma γ-tocopherol (mmol/mol)2 |
|||||||||||
| All subjects(n = 233) |
High group3(n = 33) |
Typical group3(n = 200) |
All subjects(n = 233) |
High group3(n = 33) |
Typical group3(n = 200) |
|||||||
| R | P | R | P | R | P | R | P | R | P | R | P | |
| Dietary α-tocopherol (mg/d)4 | 0.40 | 0.001 | 0.32 | NS | 0.33 | 0.001 | −0.29 | 0.001 | −0.07 | NS | −0.30 | 0.001 |
| Dietary γ-tocopherol (mg/d)4 | −0.04 | NS | −0.14 | NS | −0.02 | NS | −0.04 | NS | −0.05 | NS | −0.04 | NS |
| Dietary α-tocopherol (mg/d)5 | 0.43 | 0.001 | −0.30 | 0.001 | ||||||||
| Dietary γ-tocopherol (mg/d)5 | −0.02 | NS | −0.06 | NS | ||||||||
Correlations were performed on log-transformed values.
Plasma concentrations were normalized for total cholesterol.
Subjects were divided into high or typical grouping based on a plasma α-tocopherol value >33 μmol/L. Those subjects with plasma values >33 μmol/L were placed in the “High” group, and those subjects with plasma values ≤33 μmol/L were placed in the “Typical” group.
Dietary (http://24hrrecall.com); mean of first 6 dietary recalls.
Model adjusted for total plasma cholesterol, plasma triglycerides, BMI, age, sex, race, and energy intake.
Correlations between plasma tocopherols and urinary metabolites
For all subjects, urinary α-CEHC was even more strongly correlated with plasma α- and γ-tocopherols (R = 0.54 and −0.45, respectively; Table 5) than were the above reported dietary intakes. Urinary α-CMBHC concentrations were also correlated with plasma α- and γ-tocopherol for all subjects (R = 0.35 and −0.27, respectively; Table 5). Urinary γ-CEHC concentrations were not correlated with either plasma α- or γ-tocopherol concentrations. When the models were adjusted for total plasma cholesterol, plasma triglycerides, BMI, age, sex, race, and energy intake, the correlations were virtually unchanged from the unadjusted models (Table 5).
TABLE 5.
Pearson correlations between reported dietary intake of α-tocopherol or plasma α- and γ-tocopherol concentrations and corresponding tocopherol urinary metabolite concentrations1
| α-CEHC (μmol/g)2 |
α-CMBHC (μmol/g)2 |
γ-CEHC (μmol/g)2 |
||||||||||||||||
| All subjects(n = 233) |
High group3(n = 33) |
Typical group3(n = 200) |
All subjects(n = 233) |
High group3(n = 33) |
Typical group3(n = 200) |
All subjects(n = 233) |
High group3(n = 33) |
Typical group3 (n = 200) |
||||||||||
| R | P | R | P | R | P | R | P | R | P | R | P | R | P | R | P | R | P | |
| Dietary α-tocopherol (mg/d)4 | 0.42 | 0.001 | 0.42 | 0.01 | 0.37 | 0.001 | 0.38 | 0.001 | 0.29 | NS | 0.35 | 0.001 | 0.10 | NS | 0.31 | NS | 0.04 | NS |
| Plasma α-tocopherol (mmol/mol)5 | 0.54 | 0.001 | 0.48 | 0.001 | 0.44 | 0.01 | 0.35 | 0.001 | 0.17 | NS | 0.28 | 0.001 | 0.09 | NS | −0.01 | NS | 0.08 | NS |
| Plasma γ-tocopherol (mmol/mol)5 | −0.45 | 0.001 | −0.39 | 0.02 | −0.44 | 0.001 | −0.27 | 0.001 | −0.20 | NS | −0.28 | 0.001 | 0.05 | NS | −0.10 | NS | 0.09 | NS |
| Dietary α-tocopherol (mg/d)6 | 0.39 | 0.001 | 0.37 | 0.001 | 0.06 | NS | ||||||||||||
| Plasma α-tocopherol (mmol/mol)6 | 0.55 | 0.001 | 0.35 | 0.001 | 0.04 | NS | ||||||||||||
| Plasma γ-tocopherol (mmol/mol)6 | −0.44 | 0.001 | −0.27 | 0.001 | 0.10 | NS | ||||||||||||
Correlations were performed on log-transformed values. CEHC, carboxyethyl hydroxychroman; CMBHC, carboxymethylbutyl hydroxychroman.
Mean of two 24-h urine collections; normalized for creatinine.
Subjects were divided into high or typical grouping based on a plasma α-tocopherol value >33 μmol/L. Those subjects with plasma values >33 μmol/L were placed in the “High” group, and those subjects with plasma values ≤33 μmol/L were placed in the “Typical” group.
Dietary (http://24hrrecall.com); mean of first 6 dietary recalls.
Plasma concentrations were normalized for total cholesterol.
Model adjusted for total plasma cholesterol, plasma triglycerides, BMI, age, sex, race, and energy intake.
Correlations between dietary intake and urinary tocopherol metabolites
The reported dietary intakes of α-tocopherol were significantly correlated with urinary concentrations of α-CEHC (R = 0.42) and α-CMBHC (R = 0.38) (Table 5). When the data were adjusted for all subjects for various factors (plasma lipids, BMI, age, sex, race, and total energy intake), dietary α-tocopherol remained correlated with urinary α-CEHC (R = 0.39) and α-CMBHC (R = 0.37) concentrations (Table 5).
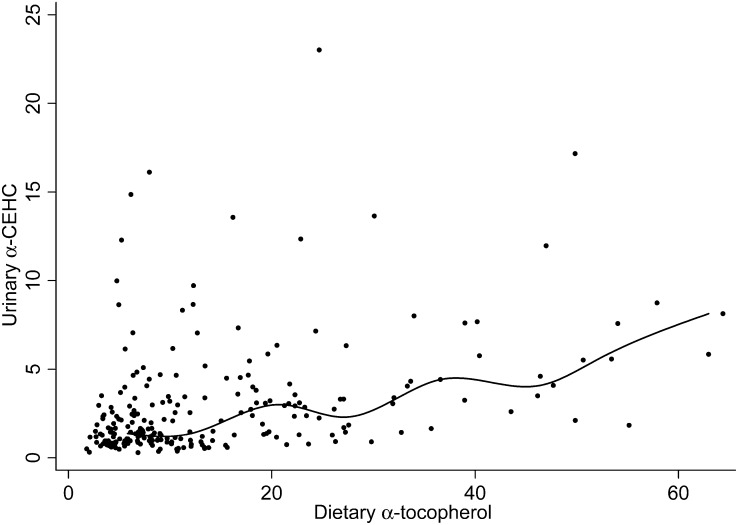
After adjustment through multiple regression for confounders, for all levels of vitamin E intake, a positive correlation was observed such that for every 1-mg (2.3-μmol) increase in dietary α-tocopherol, the urinary α-CEHC excretion increased by 0.086 μmol/g creatinine (Table 6). Other predictors of urinary α-CEHC concentrations included plasma α-tocopherol, total cholesterol, and triglycerides. For evaluation of the urinary excretion of α-CEHC relative to low levels of dietary α-tocopherol, the median α-CEHC excreted daily was plotted for each incremental 1 mg dietary α-tocopherol; a spline curve was used to assess where the increase in α-CEHC excretion relative to dietary α-tocopherol occurred, and a Spearman rank correlation was used to assess significance (P < 0.001). Urinary α-CEHC excretion remained at a plateau until the subjects consumed ∼9 mg α-tocopherol/d (assessed by visual inspection), then the spline curve sharply increased (Figure 2). For subjects consuming 9 mg α-tocopherol/d, the median urinary α-CEHC excretion was 1.39 μmol/g creatinine. When the Spearman rank correlation was calculated by using data only from subjects consuming >9 mg α-tocopherol/d, we observed that urinary α-CEHC excretion increased by 0.091 μmol/g creatinine with every 1-mg (2.3-μmol) increase in dietary α-tocopherol (Table 7). These data suggest that urinary α-CEHC reflects the degree of excess α-tocopherol intake. Specifically, 12.8 mg α-tocopherol/d was the dietary intake calculated by using the latter regression equation to estimate the amount necessary for the daily α-CEHC excretion to exceed 1.39 μmol/g creatinine.
TABLE 6.
Multiple regression analysis between plasma α-tocopherol (normalized for total cholesterol) or urinary metabolites and covariates in all subjects (n = 233)1
| α-CEHC (μmol/g) |
α-CMBHC (μmol/g) |
Plasma α-tocopherol (mmol/mol) |
||||
| Regression coefficient | 95% CI | Regression coefficient | 95% CI | Regression coefficient | 95% CI | |
| Dietary α-tocopherol (mg/d) | 0.0862 | 0.047, 0.125 | 0.018 | 0.010, 0.027 | 0.0842 | 0.059, 0.109 |
| Plasma α-tocopherol (mmol/mol) | 0.8202 | 0.646, 0.994 | 0.0732 | 0.034, 0.112 | ||
| Plasma lipids (mg/dL) | ||||||
| Total cholesterol | −0.0332 | −0.047, −0.0199 | −0.0042 | −0.007, −0.001 | 0.0392 | 0.031, 0.048 |
| Triglycerides | −0.0142 | −0.022, −0.006 | −0.001 | −0.003, 0.001 | 0.0192 | 0.013, 0.024 |
| BMI | ||||||
| Overweight, 25–30 kg/m2 | −0.718 | −1.736, 0.300 | 0.034 | −0.198, 0.266 | 0.045 | −0.717, 0.807 |
| Obese, >30 kg/m2 | −0.711 | −1.882, 0.461 | −0.122 | −0.386, 0.141 | −0.485 | −1.358, 0.388 |
| Age | ||||||
| 30–50 y | 0.323 | −0.661, 1.307 | 0.145 | −0.077, 0.367 | 0.354 | −0.383, 1.091 |
| >50 y | 0.522 | −0.714, 1.758 | 0.252 | −0.029, 0.534 | 0.928 | 0.008, 1.849 |
| Female | 0.359 | −0.565, 1.284 | 0.044 | −0.168, 0.256 | 0.215 | −0.476, 0.905 |
| African American | 0.070 | −0.865, 1.004 | −0.104 | −0.317, 0.109 | −0.329 | −1.031, 0.374 |
| Energy intake (kcal) | 0.230 | −0.215, 0.675 | 0.050 | −0.053, 0.154 | −0.198 | −0.552, 0.156 |
CEHC, carboxyethyl hydroxychroman; CMBHC, carboxymethylbutyl hydroxychroman.
Significant regression coefficients (P < 0.05).
FIGURE 2.
Relation between dietary α-tocopherol and α-CEHC. To evaluate the urinary excretion of α-CEHC relative to dietary α-tocopherol, the median α-CEHC concentration was plotted for each incremental milligram of dietary α-tocopherol, and then the Spearman rank correlation was used to assess significance. Shown is a spline curve to assess where the increase in α-CEHC excretion relative to dietary α-tocopherol occurred. Spearman's ρ = 0.402, P < 0.001. α-CEHC, α-carboxyethyl hydroxychroman.
TABLE 7.
Multiple regression analysis between urinary α-CEHC and covariates1
| α-CEHC (μmol/g) if dietary α-tocopherol >9 mg/d |
||
| Regression coefficient | 95% CI | |
| Dietary α-tocopherol (mg/d) | 0.0912 | 0.034, 0.148 |
| Plasma α-tocopherol (mmol/mol) | 0.8912 | 0.649, 1.132 |
| Plasma lipids (mg/dL) | ||
| Total cholesterol | −0.0382 | −0.058, −0.019 |
| Triglycerides | −0.0142 | −0.024, −0.003 |
| BMI | ||
| Overweight, 25–30 kg/m2 | −0.627 | −2.144, 0.890 |
| Obese, >30 kg/m2 | −0.721 | −2.522, 1.081 |
| Age | ||
| 30–50 y | 0.599 | −0.887, 2.085 |
| >50 y | 0.342 | −1.495, 2.178 |
| Female sex | 0.280 | −1.086, 1.646 |
| African American | 0.554 | −0.848, 1.956 |
| Energy intake (kcal) | 0.233 | −0.337, 0.803 |
CEHC, carboxyethyl hydroxychroman.
Significant regression coefficient (P < 0.05).
DISCUSSION
The goal of this study was to investigate the association between various biomarkers of vitamin E status and dietary α-tocopherol using an exemplary evaluation of dietary intakes. The Energetics Study protocol enrolled Americans living within 50 miles (80 km) of UCLA, who participated in a study to validate a Web-based, automated, self-administered 24-h recall (8, 11). The subjects completed 3 dietary recalls at the study visits and another 5 on their own. The last 2 dietary recalls were completed 1 and 2 mo after the final clinic visit. The data from the first 6 dietary recalls were used for the analyses in the current study. The benefits of assessing vitamin E intakes were that the subjects were well trained for reporting food intakes and became proficient at estimating their own consumption. In addition, doubly labeled water–based validation of reported intakes within this population showed high reporting validity for both African American and white subjects (8). It is therefore not surprising that the α-tocopherol intakes of the typical subjects shown in Table 2 were more generous than those previously reported for Americans by others (19, 20). Given that these subjects were highly motivated and interested in their own diets and lived in an area with excellent year-round access to high-quality fruit and vegetables, they may not be representative of the general population in the United States. Nonetheless, these high-quality measures of food intake allow for the determination of acceptable biomarkers of vitamin E status.
We divided our subjects based on plasma α-tocopherol concentrations into those with typical (≤33 μmol/L) and high (>33 μmol/L) concentrations with the assumption that high plasma α-tocopherol resulted from intake of dietary vitamin E supplements, as has been observed in vitamin E supplementation studies (21, 22). This assumption was borne out by the dietary intake assessments in our subjects, which showed that 56% of the high group and none of the typical group took vitamin supplements (Table 3). It should also be noted that the subjects in our study were normolipidemic; thus, no corrections for circulating lipid concentrations were likely to be necessary (13), and none of the corrections for cholesterol or triglyceride concentrations altered the outcomes of our findings.
In the typical group and in the entire cohort of subjects studied, dietary α-tocopherol intakes correlated with plasma α-tocopherol concentrations and inversely correlated with plasma γ-tocopherol concentrations (Table 4). When the model was adjusted for total plasma cholesterol, plasma triglycerides, BMI, age, sex, race, and energy intake, the correlation was further strengthened. It is likely that the relatively small sample size of subjects with high plasma α-tocopherol concentrations accounted for the lack of correlation between dietary α-tocopherol and plasma α- or γ-tocopherol concentrations for these subjects. A recent study using food-frequency questionnaires (FFQs) to assess plasma α-tocopherol concentrations as a biochemical indicator of dietary intake found a negative correlation between these variables for all subjects; only women had a significantly increased plasma α-tocopherol concentration with increasing FFQ-estimated intakes (adjusted correlation coefficients: 0.26, P < 0.05), which suggests that the accuracy of the dietary measure was problematic, despite the strong correlations for other dietary antioxidants, such as carotenoids (23). McNaughton et al (24) also reported that they could not use “the method of triads,” which uses triangulation techniques to calculate the validity coefficient of an FFQ for vitamin E because 1 of the 3 underlying correlations was negative. Attempts to assess vitamin E intakes and α-tocopherol status in adolescents have also not been very successful. Neuhouser et al (25), using a sample of 285 healthy participants aged 12–17 y from 3 US cities, reported a Pearson correlation for α-tocopherol of 0.16 between diet measured with an FFQ and serum nutrient concentrations (adjusted for total serum cholesterol, age, sex, race, and BMI) among these adolescents (25). Taken together, these findings emphasize that the difficulty in assessing dietary intakes of vitamin E is likely the cause of the inability to assess relations between vitamin E status on the basis of circulating α-tocopherol concentrations and intakes.
More than a decade ago, the vitamin E metabolite α-CEHC was suggested to be an indicator of an adequate vitamin E supply (26). Unfortunately, the methodology at that time was not sufficiently sensitive to detect low urinary concentrations of α-CEHC excreted during times of vitamin E intakes from diet only, although plasma α-CEHC increases were reported with supplemental vitamin E intakes (27). Refinements in extraction methods (28) and the use of deuterium-labeled tocopherols (29) have led to the observations that low concentrations of α-CEHC are continuously excreted and do increase with higher α-tocopherol intakes (30). The mean reported urinary α-CEHC concentrations reported herein are similar to those reported by Morinobu et al (31) in 14 healthy subjects before supplementation (3.6 ± 2.3 μmol/L; 2.4 ± 1.5 μmol/g creatinine). Imai et al (32) reported that 10 Japanese men excreted low urinary α-CEHC concentrations, which increased linearly with vitamin E supplementation. Moreover, a significant correlation was found between dietary vitamin E intake and urinary α-CEHC excretion in a cross-sectional study of 76 free-living individuals (32). These findings further support the utility of urinary α-CEHC as a biomarker of vitamin E status.
The initial step in the conversion of tocopherols to CEHCs is the omega hydroxylation by cytochrome P450 4F2 (CYP4F2) (33, 34). Genome-wide association studies have documented that genetic variants of CYP4F2 are associated with serum α-tocopherol concentrations in both unsupplemented (35) and vitamin E–supplemented (36) subjects, but these genetic variants account for only a few percentage differences in circulating α-tocopherol concentrations, which suggests that the variants are not likely to affect the excretion of α-CEHC discussed here as a biomarker of vitamin E status. CYP4F2 is also involved in the metabolism of vitamin K (37) and in the production of 20-hydroxyeicosatetraenoic acid from arachidonic acid (38). Plant lignans have been used to inhibit CYP4F2, but no changes in serum α-tocopherol were noted despite increases in serum γ-tocopherol and decreases in 20-hydroxyeicosatetraenoic acid. Because γ-tocopherol is more actively metabolized in vivo than is α-tocopherol (29), as was documented by using CYP4F2 in vitro (33), it is likely that the differences in circulating α- and γ-tocopherols observed in vivo are highly dependent on CYP4F2 activity to metabolize non-α-tocopherols, thereby leading to the preference for α-tocopherol. Thus, the genetic variations in CYP4F2 that have not led to differences in circulating α-tocopherol concentrations might not be expected to cause abnormal increases in urinary α-CEHC, but such speculation needs to be formally tested.
Cigarette smokers with elevated concentrations of F2-isoprostanes, a marker of lipid peroxidation and thus higher levels of oxidative stress, were found to have decreased urinary α-CEHC excretion (30), which suggests that the α-CEHC metabolite might be a useful biomarker of vitamin E adequacy. The difficulty, however, was that 3–5 times as much α-CEHC was produced from all-rac-α-tocopherol than from RRR-α-tocopherol (30). Thus, we previously suggested that urinary α-CEHC should be used as a biomarker of vitamin E adequacy only in subjects who are not taking supplements and are not consuming fortified foods (32). In the current study, we separated out subjects who took supplements or had extraordinarily high plasma α-tocopherol concentrations but still found in our typical subjects that urinary α-CEHC was strongly correlated with plasma α-tocopherol and negatively correlated with plasma γ-tocopherol (Table 5), which suggests that increased urinary α-CEHC is associated with increased vitamin E status.
Importantly, in the current study we observed that urinary α-CEHC excretion increased by 0.086 μmol/g creatinine with every 1-mg (2.3-μmol) increase in dietary α-tocopherol (Table 6). However, because urinary α-CEHC excretion remained at a plateau of 1.39 μmol/g creatinine, this relation underestimated the excretion at higher vitamin E intakes. We observed that a sharper increase in vitamin E metabolite excretion occurred at an intake of ∼9 mg α-tocopherol/d (Figure 2), which suggests that vitamin E intake had exceeded α-tocopherol needs and that more α-tocopherol was being metabolized and excreted, as has been observed in a rodent model given excess vitamin E (16, 39). These findings suggest that urinary α-CEHC can be used as a biomarker to estimate when α-tocopherol intakes exceed daily needs. We found that 12.8 mg is the dietary amount that exceeds a person's adequate α-tocopherol intake. The value agrees closely with the Estimated Average Requirement of 12 mg/d (1).
Acknowledgments
We acknowledge the excellent discussions with Gerd Bobe, Linus Pauling Institute, Oregon State University, during the preparation of the manuscript.
The authors’ responsibilities were as follows—LA: designed the research; LA, KML, and MGT: conducted the research; AA: performed the statistical analysis; and KML, MGT, and LA: wrote the manuscript with the assistance of AA. All authors read and approved the final manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: CYP4F2, cytochrome P450 4F2; FFQ, food-frequency questionnaire; GCRC, General Clinical Research Center; UCLA, University of California, Los Angeles; α-CEHC, α-carboxyethyl hydroxychroman; α-CMBHC, carboxymethylbutyl hydroxychroman; γ-CEHC, γ-carboxyethyl hydroxychroman.
REFERENCES
- 1.Food and Nutrition Board, Institute of Medicine Dietary Reference Intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, DC: National Academies Press, 2000 [PubMed] [Google Scholar]
- 2.Eitenmiller R, Lee J. Vitamin E: food chemistry, composition, and analysis. New York, NY: Marcel Dekker, Inc, 2004 [Google Scholar]
- 3.Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med 2007;43:4–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Traber MG. Vitamin E regulatory mechanisms. Annu Rev Nutr 2007;27:347–62 [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Sowell A. Serum alpha-tocopherol status in the United States population: findings from the Third National Health and Nutrition Examination Survey. Am J Epidemiol 1999;150:290–300 [DOI] [PubMed] [Google Scholar]
- 6.Kardinaal AF, van 't Veer P, Brants HA, van den Berg H, van Schoonhoven J, Hermus RJ. Relations between antioxidant vitamins in adipose tissue, plasma, and diet. Am J Epidemiol 1995;141:440–50 [DOI] [PubMed] [Google Scholar]
- 7.Ascherio A, Stampfer MJ, Colditz GA, Rimm EB, Litin L, Willett WC. Correlations of vitamin A and E intakes with the plasma concentrations of carotenoids and tocopherols among American men and women. J Nutr 1992;122:1792–801 [DOI] [PubMed] [Google Scholar]
- 8.Arab L, Tseng CH, Ang A, Jardack P. Validity of a multipass, web-based, 24-hour self-administered recall for assessment of total energy intake in blacks and whites. Am J Epidemiol 2011;174:1256–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arab L, Cambou MC, Craft N, Wesseling-Perry K, Jardack P, Ang A. Racial differences in correlations between reported dietary intakes of carotenoids and their concentration biomarkers. Am J Clin Nutr 2011;93:1102–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arab L, Hahn H, Henry J, Chacko S, Winter A, Cambou MC. Using the web for recruitment, screen, tracking, data management, and quality control in a dietary assessment clinical validation trial. Contemp Clin Trials 2010;31:138–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arab L, Wesseling-Perry K, Jardack P, Henry J, Winter A. Eight self-administered 24-hour dietary recalls using the Internet are feasible in African Americans and Whites: the energetics study. J Am Diet Assoc 2010;110:857–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am J Clin Nutr 2003;77:1171–8 [DOI] [PubMed] [Google Scholar]
- 13.Traber MG, Jialal I. Measurement of lipid-soluble vitamins—further adjustment needed? Lancet 2000;355:2013–4 [DOI] [PubMed] [Google Scholar]
- 14.Bemelmans-Spork M, Sikkel D. Data collection with handheld computers. Proceedings of the 45th session. Den Hague, the Netherlands: International Statistical Institute, 1985 [topic 18(3)]
- 15.Nomura AM, Stemmermann GN, Lee J, Craft NE. Serum micronutrients and prostate cancer in Japanese Americans in Hawaii. Cancer Epidemiol Biomarkers Prev 1997;6:487–91 [PubMed] [Google Scholar]
- 16.Leonard SW, Gumpricht E, Devereaux MW, Sokol RJ, Traber MG. Quantitation of rat liver vitamin E metabolites by LC-MS during high-dose vitamin E administration. J Lipid Res 2005;46:1068–75 [DOI] [PubMed] [Google Scholar]
- 17.Hervey GR. Determination of creatinine by the Jaffe reaction. Nature 1953;171:1125. [DOI] [PubMed] [Google Scholar]
- 18.Ford ES, Schleicher RL, Mokdad AH, Ajani UA, Liu S. Distribution of serum concentrations of alpha-tocopherol and gamma-tocopherol in the US population. Am J Clin Nutr 2006;84:375–83 [DOI] [PubMed] [Google Scholar]
- 19.Gao X, Wilde PE, Lichtenstein AH, Bermudez OI, Tucker KL. The maximal amount of dietary alpha-tocopherol intake in U.S. adults (NHANES 2001-2002). J Nutr 2006;136:1021–6 [DOI] [PubMed] [Google Scholar]
- 20.Maras JE, Bermudez OI, Qiao N, Bakun PJ, Boody-Alter EL, Tucker KL. Intake of alpha-tocopherol is limited among US adults. J Am Diet Assoc 2004;104:567–75 [DOI] [PubMed] [Google Scholar]
- 21.Devaraj S, Adams-Huet B, Fuller CJ, Jialal I. Dose-response comparison of RRR-alpha-tocopherol and all-racemic alpha-tocopherol on LDL oxidation. Arterioscler Thromb Vasc Biol 1997;17:2273–9 [DOI] [PubMed] [Google Scholar]
- 22.Dimitrov NV, Meyer C, Gilliland D, Ruppenthal M, Chenoweth W, Malone W. Plasma tocopherol concentrations in response to supplemental vitamin E. Am J Clin Nutr 1991;53:723–9 [DOI] [PubMed] [Google Scholar]
- 23.Signorello LB, Buchowski MS, Cai Q, Munro HM, Hargreaves MK, Blot WJ. Biochemical validation of food frequency questionnaire-estimated carotenoid, alpha-tocopherol, and folate intakes among African Americans and non-Hispanic Whites in the Southern Community Cohort Study. Am J Epidemiol 2010;171:488–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNaughton SA, Marks GC, Gaffney P, Williams G, Green A. Validation of a food-frequency questionnaire assessment of carotenoid and vitamin E intake using weighed food records and plasma biomarkers: the method of triads model. Eur J Clin Nutr 2005;59:211–8 [DOI] [PubMed] [Google Scholar]
- 25.Neuhouser ML, Rock CL, Eldridge AL, Kristal AR, Patterson RE, Cooper DA, Neumark-Sztainer D, Cheskin LJ, Thornquist MD. Serum concentrations of retinol, alpha-tocopherol and the carotenoids are influenced by diet, race and obesity in a sample of healthy adolescents. J Nutr 2001;131:2184–91 [DOI] [PubMed] [Google Scholar]
- 26.Schultz M, Leist M, Petrzika M, Gassmann B, Brigelius-Flohé R. Novel urinary metabolite of alpha-tocopherol, 2,5,7,8-tetramethyl-2(2'-carboxyethyl)-6-hydroxychroman, as an indicator of an adequate vitamin E supply? Am J Clin Nutr 1995;62(suppl):1527S–34S [DOI] [PubMed] [Google Scholar]
- 27.Radosavac D, Graf P, Polidori MC, Sies H, Stahl W. Tocopherol metabolites 2, 5, 7, 8-tetramethyl-2-(2'-carboxyethyl)-6-hydroxychroman (alpha-CEHC) and 2, 7, 8-trimethyl-2-(2'-carboxyethyl)-6-hydroxychroman (gamma-CEHC) in human serum after a single dose of natural vitamin E. Eur J Nutr 2002;41:119–24 [DOI] [PubMed] [Google Scholar]
- 28.Lodge JK, Ridlington J, Vaule H, Leonard SW, Traber MG. α- and γ-Tocotrienols are metabolized to carboxyethyl-hydroxychroman (CEHC) derivatives and excreted in human urine. Lipids 2001;36:43–8 [DOI] [PubMed] [Google Scholar]
- 29.Leonard SW, Paterson E, Atkinson JK, Ramakrishnan R, Cross CE, Traber MG. Studies in humans using deuterium-labeled α- and γ-tocopherol demonstrate faster plasma γ-tocopherol disappearance and greater γ-metabolite production. Free Radic Biol Med 2005;38:857–66 [DOI] [PubMed] [Google Scholar]
- 30.Bruno RS, Leonard SW, Li J, Bray TM, Traber MG. Lower plasma α-carboxyethyl-hydroxychroman after deuterium labeled α-tocopherol supplementation suggests decreased vitamin E metabolism in smokers. Am J Clin Nutr 2005;81:1052–9 [DOI] [PubMed] [Google Scholar]
- 31.Morinobu T, Yoshikawa S, Hamamura K, Tamai H. Measurement of vitamin E metabolites by high-performance liquid chromatography during high-dose administration of alpha-tocopherol. Eur J Clin Nutr 2003;57:410–4 [DOI] [PubMed] [Google Scholar]
- 32.Imai E, Tsuji T, Sano M, Fukuwatari T, Shibata K. Association between 24 hour urinary alpha-tocopherol catabolite, 2,5,7,8-tetramethyl-2(2'-carboxyethyl)-6-hydroxychroman (alpha-CEHC) and alpha-tocopherol intake in intervention and cross-sectional studies. Asia Pac J Clin Nutr 2011;20:507–13 [PubMed] [Google Scholar]
- 33.Sontag TJ, Parker RS. Influence of major structural features of tocopherols and tocotrienols on their omega-oxidation by tocopherol-omega-hydroxylase. J Lipid Res 2007;48:1090–8 [DOI] [PubMed] [Google Scholar]
- 34.Sontag TJ, Parker RS. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism: novel mechanism of regulation of vitamin E status. J Biol Chem 2002;277:25290–6 [DOI] [PubMed] [Google Scholar]
- 35.Major JM, Yu K, Wheeler W, Zhang H, Cornelis MC, Wright ME, Yeager M, Snyder K, Weinstein SJ, Mondul A, et al. Genome-wide association study identifies common variants associated with circulating vitamin E levels. Hum Mol Genet 2011;20:3876–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Major JM, Yu K, Chung CC, Weinstein SJ, Yeager M, Wheeler W, Snyder K, Wright ME, Virtamo J, Chanock S, et al. Genome-wide association study identifies three common variants associated with serologic response to vitamin E supplementation in men. J Nutr 2012;142:866–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDonald MG, Rieder MJ, Nakano M, Hsia CK, Rettie AE. CYP4F2 Is a vitamin K1 oxidase: an explanation for altered warfarin dose in carriers of the V433M variant. Mol Pharmacol 2009;75:1337–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stec DE, Roman RJ, Flasch A, Rieder MJ. Functional polymorphism in human CYP4F2 decreases 20-HETE production. Physiol Genomics 2007;30:74–81 [DOI] [PubMed] [Google Scholar]
- 39.Mustacich DJ, Vo AT, Elias VD, Payne K, Sullivan L, Leonard SW, Traber MG. Regulatory mechanisms to control tissue alpha-tocopherol. Free Radic Biol Med 2007;43:610–8 [DOI] [PMC free article] [PubMed] [Google Scholar]