Abstract
Background: The inefficiency of HIV breast-milk transmission may be caused by the presence of immunologically active factors, including human milk oligosaccharides (HMOs).
Objective: We investigated whether HMO concentrations are associated with a reduced risk of postnatal HIV transmission.
Design: A nested case-control study was conducted within a larger cohort study of HIV-infected women and their infants followed from birth to 24 mo in Lusaka, Zambia. Breast-milk samples collected at 1 mo from 81 HIV-infected women who transmitted via breastfeeding, a random sample of 86 HIV-infected women who did not transmit despite breastfeeding, and 36 uninfected breastfeeding women were selected. Total and specific HMO concentrations were measured by HPLC and compared between groups with adjustment for confounders by using logistic regression.
Results: HIV-infected women with total HMOs above the median (1.87 g/L) were less likely to transmit via breastfeeding (OR: 0.45; 95% CI: 0.21, 0.97; P = 0.04) after adjustment for CD4 count and breast-milk HIV RNA concentrations; a trend toward higher concentrations of lacto-N-neotetraose being associated with reduced transmission (OR: 0.49; 95% CI: 0.23, 1.04; P = 0.06) was also observed. The proportion of 3′-sialyllactose (3′-SL) per total HMOs was higher among transmitting than among nontransmitting women (P = 0.003) and correlated with higher plasma and breast-milk HIV RNA and lower CD4 counts. Neither Secretor nor Lewis status distinguished between transmitting and nontransmitting women.
Conclusions: Higher concentrations of non-3′-SL HMOs were associated with protection against postnatal HIV transmission independent of other known risk factors. Further study of these novel, potentially anti-HIV components of breast milk is warranted. This trial was registered at clinicaltrials.gov as NCT00310726.
INTRODUCTION
HIV-1 can be transmitted from mother to child throughout the period of breastfeeding. However, the vast majority of breastfed infants do not acquire HIV-1 despite continuous exposure to the virus within their mothers’ milk over many months. In the absence of antiretroviral drugs, ∼10–15% of infants will acquire infection from their HIV-infected mother via breastfeeding, depending on the duration of exposure (1). The plethora of immunologically active milk components (2) may minimize the risk of transmission despite this intensive viral exposure.
We investigated the potential role of human milk oligosaccharides (HMOs)4 in reducing the risk of postnatal HIV transmission via breastfeeding. These complex oligosaccharides are the third most abundant component of breast milk yet are not digestible and reach the mucosal surfaces of the infant's gastrointestinal tract at high concentrations (3–5). HMOs act as prebiotics, which serve as metabolic substrate to promote the growth of desired bacterial communities in the infant's intestine (6, 7). In addition, HMOs structurally resemble the glycans on epithelial cell surfaces and can serve as soluble decoy receptors that inhibit pathogen adhesion (3–5, 8). HMOs can also directly affect intestinal epithelial cell maturation and integrity (9, 10). Furthermore, HMOs have antiinflammatory activity in vitro and in animal models and have been shown to modulate immune cell responses through interactions with a wide variety of immune receptors, including Toll-like receptors, selectins, or dendritic cell–specific C-type lectins (11–18).
Many mechanisms by which HMOs may interfere with HIV infection have been postulated, but the strongest evidence comes from in vitro data, which suggest that HMOs interfere with the binding of HIV to dendritic cell–specific intercellular adhesion molecule 3–grabbing non-integrin (DC-SIGN)—a C-type lectin on human dendritic cells (DCs) (19–21). HIV binds to DC-SIGN, which carries the virus across the mucosal barrier (22–24). DC-SIGN, however, not only binds HIV but has strong affinity to Lewis blood group antigens (25), which are part of HMOs in the milk of some, but not all, women (3, 26, 27).
More than 100 structurally distinct HMOs have been identified (3, 28), but not every woman synthesizes and secretes each and every HMO. HMO composition depends highly on the woman's Secretor and Lewis blood group status (26, 29, 30). We hypothesized that these interindividual differences in HMO amount and composition are associated with postnatal HIV transmission via breastfeeding. We tested this hypothesis by analyzing HMO amount and composition in breast-milk samples collected as part of a larger study of HIV-infected women and their infants followed from birth to 24 mo in Lusaka, Zambia (31). Most of the cohort was recruited and followed before antiretroviral therapy (ART) became available and only single-dose nevirapine was used for the prevention of mother-to-child HIV transmission. Because it has been clearly established that maternal ART or extended infant prophylaxis substantially reduces postnatal transmission (32, 33), our samples provide a unique opportunity to study associations between milk components and transmission in vivo undisturbed by ART and with a large number of infections to yield statistically informative comparisons.
SUBJECTS AND METHODS
Study design
A nested case-control study was conducted within the context of a randomized trial to test the safety and efficacy of abrupt cessation of breastfeeding at 4 mo on postnatal HIV transmission and child mortality. The trial was described in detail elsewhere (31). In brief, 958 HIV-infected women, recruited during pregnancy at 2 antenatal care clinics in Lusaka, Zambia, between May 2001 and September 2004, were followed to delivery and through 24 mo postpartum with their infants. Women were given single-dose nevirapine as prophylaxis to prevent transmission to the child. ART became available in the public sector only toward the end of the enrollment period. All women provided written informed consent for their participation, and all the investigators’ Institutional Review Boards approved the study.
Only women who intended to breastfeed were enrolled, and all were counseled to breastfeed to ≥4 mo. Half of the women were randomly assigned to stop all breastfeeding at 4 mo and the other half to continue breastfeeding for a duration of their own choice. Heel-stick blood samples were collected from all infants at birth, monthly to 6 mo, and every 3 mo to 24 mo of age. DNA was extracted from each infant's blood sample and tested for HIV by real-time polymerase chain reaction. All positive test results were confirmed by testing ≥2 samples. All negative test results were confirmed to be adequate by the amplification of betaglobin. Maternal blood was collected at enrollment during pregnancy and tested for CD4 and CD8 cell counts (FACSCount system, BD Immunocytometry Systems), hemoglobin (Hemocue system), and plasma viral load (Roche Amplicor 1.5). Social and clinical data were collected at enrollment during pregnancy, at the time of delivery, and at each follow-up visit over the postnatal period.
Selection of cases and controls
Cases were defined as HIV-infected women who transmitted HIV to their infants via breastfeeding. HIV infection was inferred to have occurred during breastfeeding if infants had a first positive polymerase chain reaction test after 42 d of age with an earlier negative result. Ninety-three women met the case definition, giving a postnatal transmission rate (by Kaplan-Meier methods) of 12.7% by 24 mo. Of these, 87 attended the 1-mo visit and 81 had breast-milk samples available for testing. As nontransmitting controls, we randomly selected from among HIV-infected mothers who did not transmit despite breastfeeding, a sample of 96 women who breastfed >42 d using incidence-density sampling (31). Of these, 92 attended the 1-mo visit, and 86 had breast-milk samples available for testing. In addition, we selected all 36 HIV-uninfected women who had been recruited and followed as part of the same protocol at the same site but counseled to breastfeed for a duration of their own choice. We considered it important to select a single time point to test as declines in immunologically active components of milk, including HMOs, were described previously (27, 34–36). One-month samples were selected for testing because these samples would be before postnatal HIV acquisition for all infants and are from a period when lactation is fully established for most infants.
Breast-milk processing and viral load measurement
Breast milk was manually expressed from each breast separately into clean sterile collection cups and transported to a central laboratory in Lusaka, where samples were stored at 4°C and processed within 4 h. Breast-milk samples were centrifuged, and the supernatant fluid was separated from the cell pellets. Cell pellets were removed and stored separately. Lipid and aqueous fractions of milk were mixed, portioned into aliquots, and then stored at −70°C.
Samples were shipped on dry ice to the United States for further testing. Copies of HIV-1 RNA per milliliter of breast milk (viral load) were measured with the Roche Amplicor Ultrasensitive HIV-1 1.5 kit (Roche Molecular Systems Inc) with a lower detection limit of 50 copies/mL. The assay has been validated for quantitation of HIV-1 RNA in breast milk (37). Because RNA quantity was nonnormally distributed, log-transformed data were used for analyses. For samples below the detection limit of the assay, a value of 49 copies/mL was imputed.
HMO measurement
HMOs were analyzed as previously described (38, 39). Briefly, raffinose was added to 40 μL milk to serve as an internal standard through sample processing and analysis. Lipids and proteins were removed from the samples by centrifugation and chloroform-methanol extraction. Lactose was removed by overnight incubation on lactase-immobilized beads (Invitrogen) at 37°C. Residual peptides and salt were removed over Sep-Pak C18 cartridges followed by porous graphitized carbon cartridges. The reducing end of the dried oligosaccharides was labeled with the fluorescent tag 2-aminobenzamide (2AB) for 2 h at 65°C (19). Free 2AB label was separated from the 2AB-labeled oligosaccharides by using silica gel cartridges. The 2AB-labeled oligosaccharides were analyzed by HPLC on an amide-80 column (4.6 mm inner diameter × 25 cm, 5 μm; Tosoh Bioscience) with a 50-mmol/L ammonium formate–acetonitrile buffer system. Separation was performed at 25°C and monitored with a fluorescence detector at 360 nm excitation and 425 nm emission. Peak annotation was based on standard retention times and mass spectrometric analysis on a Thermo LCQ Duo Ion trap mass spectrometer equipped with a Nano-ESI-source.
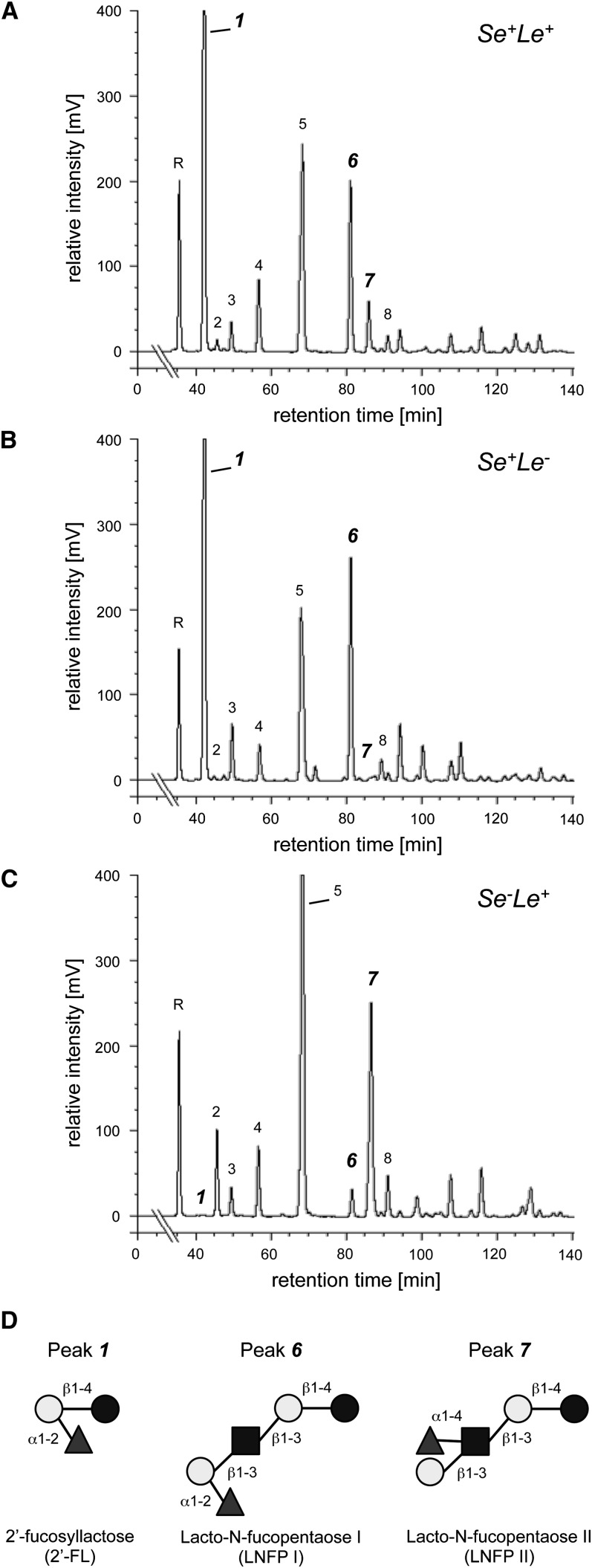
The total concentration of HMOs was calculated as the sum of the most common oligosaccharides, including 2′-fucosyllactose (2′-FL), 3 -fucosyllactose (3-FL), 3′-sialyllactose (3′-SL), lacto-N-tetraose (LNT), lacto-N-neotetraose (LNnT), lacto-N-fucopentaose I (LNFP I), lacto-N-fucopentaose II (LNFP II), and lacto-N-fucopentaose III (LNFP III). The proportion of each HMO per total HMO concentration was calculated. Secretor samples were identified by the presence of 2′-FL and LNFP I. Lewis positive samples were identified by the presence of LNFP II (Figure 1).
FIGURE 1.
HMO HPLC spectra of representative samples from a Lewis-positive Secretor woman (A), a Lewis-negative Secretor woman (B), and a Lewis-positive non-Secretor woman (C). Peak annotation (R: raffinose; 1: 2′-fucosyllactose; 2: 3 -fucosyllactose; 3: lacto-N-neotreaose; 4: 3′-sialyllactose; 5: lacto-N-tetraose; 6: lacto-N-fucopentaose I; 7: lacto-N-fucopentaose II; 8: lacto-N-fucopentaose III). D: Secretor women express the α1–2-fucosyltransferase FUT2, which participates in the biosynthesis of characteristic 2′-FL (peak 1) or LNFP I (peak 6). Women with active Lewis gene express the α1–3/4-fucosyltransferase FUT3, which participates in the biosynthesis of characteristic α1–4-LNFP II (peak 7). Dark circles: glucose; light circles: galactose; squares: N-acetylglucosamine; triangles: fucose. HMO, human milk oligosaccharides; Le+, Lewis positive; Le–, Lewis negative; LNFP, lacto-N-fucopentaose; Se+, Secretor positive; Se–, Secretor negative; 2′-FL, 2′-fucosyllactose.
Statistical methods
The distributions of total HMOs and specific HMOs and proportion of total HMO attributable to each specific type were compared between the groups on a continuous scale and were also categorized as either above or below the median concentration of the HMOs in the nontransmitting group. Student's t and nonparametric Wilcoxon's tests were used for continuous variables, and Pearson's chi-square statistics and Fisher's exact test were used for categorical variables. A Bonferroni correction was applied to adjust for multiple comparisons. Logistic regression was conducted to examine the relations between each HMO and transmission. Unadjusted and adjusted ORs were reported with 95% CIs. Maternal health indicators, including CD4 cell count, hemoglobin concentration, plasma viral load, breast-milk RNA viral load at 1 mo, and clinical stage were investigated as potential confounders. Women were defined as symptomatic if they experienced weight loss, >30 d of diarrhea, or fever or cough in the previous 6 mo or had a history of thrush or tuberculosis. Associations between HMOs and maternal CD4 cell count, plasma viral load, and breast-milk RNA viral load at 1 mo were described by using Spearman's correlation coefficients. Kaplan-Meier methods were used to calculate the duration of breastfeeding. All statistical analyses were performed by using SAS (version 9.2).
RESULTS
Cohort characteristics
Baseline characteristics of the 81 HIV-transmitting, 86 nontransmitting, and 36 uninfected control mother-child pairs are shown in Table 1. Women who transmitted via breastfeeding had significantly lower CD4 cell counts and a higher plasma viral load than did the nontransmitting mothers. More HIV-uninfected mothers were aged <20 y and had a higher hemoglobin concentration than did nontransmitting mothers.
TABLE 1.
Baseline infant and maternal characteristics of TR, NTR, and uninfected control mother-child pairs1
| TR (n = 81) | NTR (n = 86) | Control (n = 36) | P value2(TR vs NTR) | P value2(NTR vs control) | |
| Infant factors | |||||
| Sex [n (%)] | |||||
| Male | 42 (51.9) | 39 (45.4) | 23 (63.9) | 0.40 | 0.06 |
| Female | 39 (48.1) | 47 (54.7) | 13 (36.1) | ||
| Birth weight [n (%)] | |||||
| <2500 g | 13 (16.5) | 8 (9.4) | 4 (11.1) | 0.18 | 0.75 |
| ≥2500 g | 66 (83.5) | 77 (90.6) | 32 (88.9) | ||
| Age (y) | 26.9 ± 4.73 | 26.2 ± 5.2 | 25.8 ± 6.9 | 0.39 | 0.73 |
| <20 y [n (%)] | 4 (4.9) | 5 (5.8) | 8 (22.2) | 0.69 | 0.01 |
| 20–30 y [n (%)] | 58 (71.6) | 66 (76.7) | 19 (52.8) | ||
| >30 y [n (%)] | 19 (23.5) | 15 (17.4) | 9 (25.0) | ||
| Maternal factors (cells/mm3) | |||||
| CD4 count | |||||
| Median (25th, 75th percentiles) | 219.0 (163.0, 300.0) | 367.5 (220.0, 524.0) | 797.0 (676.5, 938.0) | <0.0001 | <0.0001 |
| <350 cells/mm3 [n (%)] | 68 (84.0) | 40 (46.5) | 2 (5.6) | <0.0001 | <0.0001 |
| ≥350 cells/mm3 [n (%)] | 13 (16.1) | 46 (53.5) | 34 (94.4) | ||
| Plasma viral load (copies/mL) | |||||
| Median (25th, 75th percentiles) | 126,047 (41,958, 207,691) | 27,679 (10,217, 82,408) | NA | <0.0001 | |
| ≥50,000 copies/mL [n (%)] | 57 (71.3) | 30 (34.9) | <0.0001 | ||
| <50,000 copies/mL [n (%)] | 23 (28.7) | 56 (65.1) | |||
| Hemoglobin [n (%)] | |||||
| <10 g/dL | 33 (41.8) | 27 (31.4) | 4 (11.1) | 0.17 | 0.02 |
| ≥10 g/dL | 46 (58.2) | 59 (68.6) | 32 (88.9) | ||
| Clinical stage [n (%)] | |||||
| Symptomatic | 34 (42.0) | 37 (43.0) | 13 (36.1) | 0.89 | 0.48 |
| Asymptomatic | 47 (58.0) | 49 (57.0) | 23 (63.9) | ||
| Duration of breastfeeding (mo)4 | |||||
| Median (25th, 75th percentiles) | 334 (126, 547) | 365 (182, 426) | 639 (517, 714) | 1.00 | <0.0001 |
| BMI [n (%)] | |||||
| <18.5 kg/m2 | 14 (17.3) | 11 (12.8) | 6 (16.7) | 0.42 | 0.57 |
| ≥18.5 kg/m2 | 67 (82.7) | 75 (87.2) | 30 (83.3) |
NA, not available; NTR, HIV-nontransmitting; TR, HIV-transmitting.
Student's t and nonparametric Wilcoxon's tests were used to compare continuous variables between groups, and Pearson's chi-square statistics and Fisher's exact test were used for categorical variables.
Mean ± SD (all such values).
Calculated by Kaplan-Meier methods and compared between the groups by using the log-rank test.
Quantitation of human milk oligosaccharides
Median concentrations of total HMO in breast milk were 1.60, 1.87, and 1.64 g/L among transmitting, nontransmitting, and uninfected control mothers, respectively (Table 2). On a continuous scale, the distributions did not differ significantly between the groups. LNT, 2′-FL, and LNFP II+III were the most abundant HMOs, whereas 3-FL had the lowest concentration of the 3 groups. HIV-infected mothers had higher concentrations of 3′-SL (P < 0.01) and LNnT (P = 0.06) than did uninfected controls (Table 2). Concentrations of the other oligosaccharides did not differ significantly between the groups. If only the non-3′-SL HMO concentration was considered, the median concentrations were 1.48 and 1.76 among transmitting and nontransmitting mothers, respectively (P = 0.04).
TABLE 2.
Concentrations of total HMOs and each HMO in rank order and proportion of each HMO per total HMO among TR and NTR mothers and HIV-uninfected controls1
| HIV-infected |
HIV-uninfected |
P value |
|||
| TR (n = 81) | NTR (n = 86) | Control (n = 36) | TR vs NTR | HIV-infected vs control | |
| Total HMO (g/L) | 2.34 ± 2.312 | 2.64 ± 2.49 | 1.97 ± 1.69 | ||
| Median (25th, 75th percentiles) | 1.60 (1.19, 2.54) | 1.87 (1.37, 2.87) | 1.64 (1.16, 2.02) | 0.06 | 0.11 |
| Median concentration (mg/L) | |||||
| LNT | 538.4 | 620.7 | 531.9 | 0.14 | 0.62 |
| 2′-FL | 370.3 | 484.5 | 398.5 | 0.27 | 0.71 |
| LNFP II+III | 178.1 | 168.6 | 121.2 | 0.69 | 0.25 |
| LNnT | 132.0 | 158.3 | 98.9 | 0.32 | 0.06 |
| 3′-SL | 143.3 | 140.3 | 114.1 | 0.71 | 0.006a |
| LNFP I | 106.0 | 120.9 | 138.6 | 0.25 | 0.83 |
| 3-FL | 39.0 | 36.6 | 32.9 | 0.54 | 0.78 |
| Percentage per total HMO | |||||
| LNT | 33.3 | 31.3 | 35.3 | 0.95 | 0.32 |
| 2′-FL | 22.7 | 26.4 | 26.9 | 0.29 | 0.80 |
| LNFP II+III | 10.7 | 8.3 | 7.4 | 0.20 | 0.81 |
| LNnT | 8.0 | 7.2 | 6.8 | 0.41 | 0.28 |
| 3′-SL | 8.6 | 6.6 | 7.0 | 0.003b | 0.37 |
| LNFP I | 5.9 | 6.3 | 7.3 | 0.28 | 0.55 |
| 3-FL | 2.3 | 1.5 | 2.3 | 0.21 | 0.67 |
Wilcoxon's tests were used to compare concentrations between the groups (Bonferroni adjustment): aP = 0.04, bP = 0.02. HMO, human milk oligosaccharide; LNFP, lacto-N-fucopentaose; LNnT, lacto-N-neotetraose; LNT, lacto-N-tetraose; NTR, HIV-nontransmitting; TR, HIV-transmitting; 2′-FL, 2′-fucosyllactose; 3-FL, 3-fucosyllactose; 3′-SL, 3′-sialyllactose.
Mean ± SD (all such values).
The proportion of 3′-SL was significantly higher in HIV-transmitting mothers than in nontransmitting mothers (8.6% compared with 6.6%; P = 0.003), but the proportions of all other oligosaccharides were similar between the groups (Table 2). The association between 3′-SL and transmission remained significant after Bonferroni adjustment (P = 0.02).
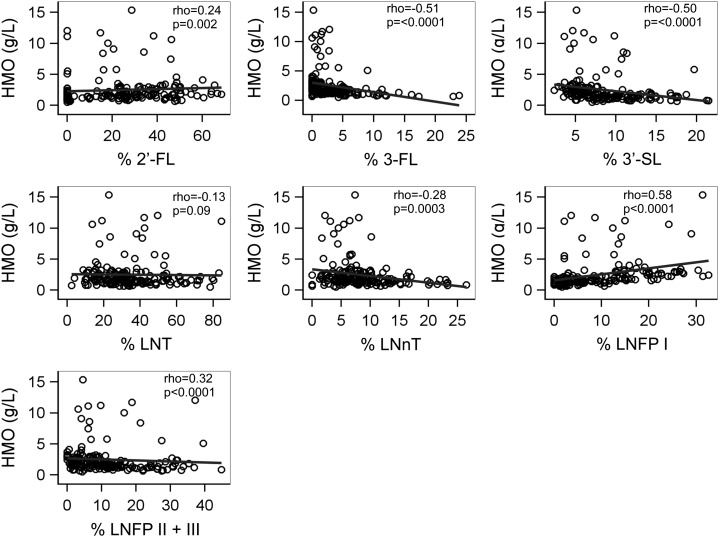
We also examined associations between the total HMO concentration and the proportion of each oligosaccharide per total HMO (Figure 2). The proportion of each oligosaccharide per total HMO was significantly correlated with the total HMO concentration, except for LNT. The lower concentration of total HMO was strongly associated with a higher percentage of 3′-SL (Spearman's correlation coefficient = −0.50, P < 0.001). Similarly, a lower concentration of total HMO was significantly associated with a higher proportion of 3-FL and LNnT but with lower proportions of 2′-FL and LNFP I.
FIGURE 2.
Scatter plots of total HMO (g/L) by proportion of each oligosaccharide per total HMO among 167 HIV-infected mothers. Spearman's coefficients (ρ) and P values are shown. HMO, human milk oligosaccharide; LNFP, lacto-N-fucopentaose; LNnT, lacto-N-neotetraose; LNT, lacto-N-tetraose; 2′-FL, 2′-fucosyllactose; 3-FL, 3-fucosyllactose; 3′-SL, 3′-sialyllactose.
Maternal health and oligosaccharides
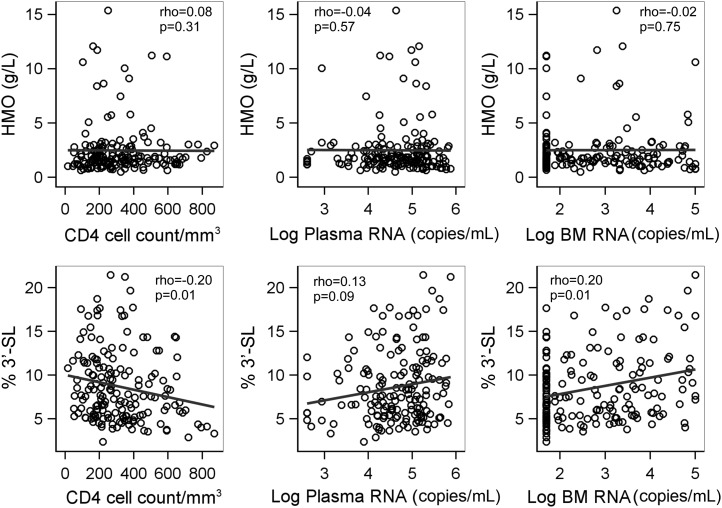
Associations between maternal health factors and oligosaccharides were investigated in HIV-infected women considering oligosaccharides as either absolute concentration or proportion per total HMO (Figure 3). Higher proportions of 3′-SL were significantly associated with a lower CD4 cell count (Spearman's r = −0.20, P = 0.01), a higher plasma viral load (Spearman's r = 0.13, P = 0.09), and a higher breast-milk HIV RNA viral load (Spearman's r = 0.20, P = 0.01). The total HMO concentration and all other oligosaccharides were not associated with any of these indicators of more advanced maternal disease.
FIGURE 3.
Scatter plots of total HMO and percentage of 3′-SL per total HMO by CD4 cell count plasma viral load and breast-milk HIV RNA viral load at 1 mo among 167 HIV-infected mothers. Spearman's coefficients (ρ) and P values are shown. BM, breast milk; HMO, human milk oligosaccharide; 3′-SL, 3′-sialyllactose.
Postpartum HIV transmission and oligosaccharides
On a continuous scale, the total HMO concentration was not significantly associated with a reduced risk of postpartum HIV transmission, although a trend was apparent (adjusted OR for each log10 increase in total HMO concentration: 0.31; 95% CI: 0.08, 1.21) (Table 3). When stratified by below and above the median, a higher total HMO concentration (≥1.87 g/L) was significantly associated with a reduced risk of postpartum transmission after adjustment for the maternal CD4 cell count and breast-milk HIV RNA viral load at 1 mo (log scale) (adjusted OR: 0.45; 95% CI: 0.21, 0.97). If only non-3′-SL HMO was considered, the concentration above the median (1.76 g/L) was more strongly associated with a reduced transmission (adjusted OR: 0.38; 95% CI: 0.17, 0.82) than with the total HMO.
TABLE 3.
Unadjusted and adjusted associations between concentrations of specific HMO and postnatal HIV transmission via breastfeeding1
| TR | NTR | Unadjusted OR(95% CI) | Adjusted OR2 (95% CI) | |
| n (%) | n (%) | |||
| Log10 total HMO | 0.43 (0.14, 1.32) | 0.31 (0.08, 1.21) | ||
| Median HMO (g/L) | ||||
| ≥1.87 | 31 (41.9) | 43 (58.1) | 0.62 (0.34, 1.15) | 0.45 (0.21, 0.97) |
| <1.87 | 50 (53.8) | 43 (46.2) | ||
| Secretor | ||||
| Secretor | 64 (46.7) | 73 (53.3) | 0.64 (0.28, 1.50) | 0.70 (0.25, 1.92) |
| Non-Secretor | 15 (57.7) | 11 (42.3) | ||
| Lewis | ||||
| Lewis-antigen | 67 (48.6) | 71 (51.4) | 1.03 (0.43, 2.49) | 0.81 (0.28, 2.40) |
| Non-Lewis | 11 (47.8) | 12 (52.2) | ||
| Actual concentration (mg/L) | ||||
| 2′-FL | ||||
| ≥485 | 34 (44.2) | 43 (55.8) | 0.72 (0.39, 1.33) | 0.74 (0.35, 1.53) |
| <485 | 47 (52.2) | 43 (47.8) | ||
| 3-FL | ||||
| ≥37 | 42 (49.4) | 43 (50.6) | 1.08 (0.59, 1.98) | 1.03 (0.50, 2.13) |
| <37 | 39 (47.6) | 43 (52.4) | ||
| 3′-SL | ||||
| ≥140 | 41 (48.2) | 44 (51.8) | 0.98 (0.53, 1.80) | 0.70 (0.34, 1.48) |
| <140 | 40 (48.8) | 42 (51.2) | ||
| LNT | ||||
| ≥621 | 30 (41.1) | 43 (58.9) | 0.59 (0.32, 1.09) | 0.55 (0.26, 1.16) |
| <621 | 51 (54.3) | 43 (45.7) | ||
| LNnT | ||||
| ≥158 | 31 (41.9) | 43 (58.1) | 0.62 (0.34, 1.15) | 0.49 (0.23, 1.04) |
| <158 | 50 (53.8) | 43 (46.2) | ||
| LNFP I | ||||
| ≥121 | 34 (44.2) | 43 (55.8) | 0.72 (0.39, 1.33) | 0.89 (0.43, 1.85) |
| <121 | 47 (52.2) | 43 (47.8) | ||
| LNFP II+III | ||||
| ≥169 | 42 (49.4) | 43 (50.6) | 1.08 (0.59, 1.98) | 0.96 (0.46, 2.00) |
| <169 | 39 (47.6) | 43 (52.4) | ||
| As percentage of total HMO | ||||
| 2′-FL | ||||
| ≥26.4 | 31 (41.9) | 43 (58.1) | 0.62 (0.34, 1.15) | 0.67 (0.32, 1.41) |
| <26.4 | 50 (53.8) | 43 (46.2) | ||
| 3-FL | ||||
| ≥1.5 | 48 (53.3) | 42 (46.7) | 1.52 (0.83, 2.81) | 1.28 (0.62, 2.67) |
| <1.5 | 33 (42.9) | 44 (57.1) | ||
| 3′-SL | ||||
| ≥6.6 | 58 (57.4) | 43 (42.6) | 2.52 (1.33, 4.79) | 2.21 (1.04, 4.73) |
| <6.6 | 23 (34.8) | 43 (65.2) | ||
| LNT | ||||
| ≥31.3 | 46 (51.7) | 43 (48.3) | 1.31 (0.71, 2.42) | 1.56 (0.74, 3.28) |
| <31.3 | 35 (44.9) | 43 (55.1) | ||
| LNnT | ||||
| ≥7.2 | 44 (51.2) | 42 (48.8) | 1.25 (0.68, 2.29) | 0.86 (0.41, 1.80) |
| <7.2 | 37 (45.7) | 44 (54.3) | ||
| LNFP I | ||||
| ≥6.3 | 38 (46.9) | 43 (53.1) | 0.88 (0.48, 1.62) | 0.95 (0.46, 1.98) |
| <6.3 | 43 (50.0) | 43 (50.0) | ||
| LNFP II+III | ||||
| ≥8.3 | 49 (53.8) | 42 (46.2) | 1.60 (0.87, 2.97) | 1.32 (0.63, 2.76) |
| <8.3 | 32 (42.1) | 44 (57.9) |
HMO, human milk oligosaccharides; LNFP, lacto-N-fucopentaose; LNnT, lacto-N-neotetraose; LNT, lacto-N-tetraose; NTR, HIV-nontransmitting; TR, HIV-transmitting; 2′-FL, 2′-fucosyllactose; 3-FL, 3-fucosyllactose; 3′-SL, 3′-sialyllactose.
Logistic regression was conducted to examine the relations between each HMO and transmission. Unadjusted and adjusted ORs adjusted for maternal CD4 cell count and log10 breast-milk HIV RNA viral load at 1 mo are reported with 95% CIs.
The association between concentrations of each oligosaccharide and postpartum HIV transmission is described in Table 3. Having Lewis-antigen or Secretor status was not associated with the risk of postpartum transmission. A nonsignificant trend toward higher concentrations of LNnT (mg/L) and a reduced risk of HIV was observed (adjusted OR: 0.49; 95% CI: 0.23, 1.04). All other oligosaccharides were not associated with postpartum transmission. When the proportion of each oligosaccharide in total HMO was considered, a higher proportion of 3′-SL was associated with a 2-fold increased risk of transmission (OR: 2.52; 95% CI: 1.33, 4.79). This remained significant after adjustment for the CD4 cell count and breast-milk HIV RNA viral load (adjusted OR: 2.21; 95% CI: 1.04, 4.73).
DISCUSSION
In the current study, we examined milk from >200 African women to investigate the role of HMO in HIV transmission. Epidemiologic and in vitro studies have shown that HMOs protect infants from a wide variety of pathogens (39–44). Our study indicates that these versatile substances may also play a role in protecting infants from HIV infection. Higher concentrations of total HMO, particularly non-3′-SL HMOs, including LNnT, were associated with protection against postnatal HIV transmission independent of other known risk factors such as CD4 count and breast-milk HIV RNA viral load. We also observed that the proportion of HMO due to 3′-SL was associated with increased transmission and markers of more advanced disease in the mother. These results indicate that it is the specific profile of HMOs that is important at determining whether or not HMOs play a role in mitigating HIV transmission via breastfeeding.
The type and amount of HMOs that infants receive from their mothers are variable and depend on maternal Secretor and Lewis blood group status (26, 29, 30). Lewis X components in human milk bind DC-SIGN, compete with the HIV-1 envelope protein gp120 for binding to DC-SIGN, and inhibit HIV-1 transfer to CD4+ T lymphocytes (19). Lewis X–containing bile salt–stimulated lipase was later identified as one of the inhibitory glycoproteins in human milk that bind to DC-SIGN and inhibit HIV-1 transfer to CD4+ T lymphocytes (20). Whereas bile salt–stimulated lipase concentrations in human milk range from 0.1 to 0.2 g/L (45), HMOs are much more abundant, with concentrations of 5 to 10 g/L. HMOs also carry Lewis blood group antigens and successfully compete with HIV-1-gp120 for binding to DC-SIGN in ELISA- and cell-based assays (21). These data led us to hypothesize that Lewis- and Secretor-related interindividual differences in HMO amount and composition are associated with HIV transmission via breastfeeding. In the current study, however, the concentration of HMOs with Secretor- (2′-FL or LNFP I) or Lewis-active epitopes (LNFP II) did not correlate with HIV transmission risk. These results suggest that other HMOs contribute to the protection against postnatal HIV transmission and are independent of the mother's Secretor or Lewis blood group status. Our data suggest that protection may be mediated in part by concentrations of LNnT. This oligosaccharide is capable of shifting the composition of microbial communities in the intestine in vivo (46), has antiinflammatory effects in vitro, and is produced by helminthes, where it has been found to be a potent suppressor of T helper 1 and inflammatory responses (12). Other more complex HMOs that were not included in our analysis might also contribute to the protective effects.
There are >100 structurally different HMOs, each of which probably has a distinct role as a receptor analog for pathogens and/or as immune modulators. Most intriguing are the unexpected results concerning 3′-SL. The percentage of 3′-SL was higher among transmitting than among nontransmitting mothers. Also, women with a higher percentage of 3′-SL were more likely to have higher plasma and breast-milk HIV RNA concentrations and lower CD4 counts. In contrast with the other HMOs in this analysis, 3′-SL is sialylated and appears to have proinflammatory properties, including T cell stimulation (15) and promotion of intestinal inflammation (47). The correlation between a high percentage of 3′-SL in the mother's milk and high viral load in the mother's plasma and milk raises questions about causalities. Altered antibody glycosylation is well described in inflammatory states such as autoimmune diseases, pregnancy, and aging, and the respective cytokine milieu may play a role (48). Therefore, it is plausible that the inflammatory signals associated with a high viral load may induce the synthesis of 3′-SL in the mammary gland. Indeed, altered cellular glycosylation has been described in HIV-infected cells (49). Whether increased 3′-SL concentrations represent a host response to counteract HIV, or whether they directly or indirectly promote HIV infection, requires further investigation.
Whereas the current study focused on a selected subset of some of the most abundant HMOs, milk bioactive compounds other than HMOs may also decrease the risk of HIV transmission through breastfeeding. In fact, data from the same cohort showed various levels of protection associated with the concentration of breast milk defensins (50), various cytokines, and chemokines as well as several other factors (51, 52). We did not, however, find associations between transmission risk and concentrations of breast-milk IgA HIV-specific antibodies or CCL28 (53, 54). In addition, in vitro data suggest that sulfated glycolipids and glycosaminoglycans in breast milk may also contribute to an inhibition of HIV transmission (55). It is possible that HMOs and other bioactive compounds in breast milk synergize to decrease HIV transmission.
Breastfed infants provide a unique human challenge model in which to investigate factors that modulate the risk of mucosal HIV transmission (56). Whereas maternal ART or extended infant prophylaxis are known to prevent postnatal transmission (32, 33), the samples analyzed in the current study were collected before ART became available (31). Thus, this large and well-defined cohort provided a unique and powerful opportunity to study associations between HMO and HIV transmission in vivo. In the future, a better understanding of how individual HMOs facilitate or obstruct HIV transmission may guide the development of interventions to complement antiretroviral strategies and more effectively prevent transmission.
Acknowledgments
The authors’ responsibilities were as follows—LB, LK, and GMA: designed the research and wrote the manuscript; H-YK, LK, LH, CN, and LB: conducted the research; MS, CK, MM, and DMT: provided the milk samples; LB, LK, H-YK, and GMA: analyzed data; and LB: had primary responsibility for the final content. All authors read and approved the final manuscript. All authors declared that they had no conflict of interest.
Footnotes
Abbreviations used: ART, antiretroviral therapy; DC, dendritic cell; DC-SIGN, dendritic cell–specific intercellular adhesion molecule 3–grabbing non-integrin; HMO, human milk oligosaccharide; LNnT, lacto-N-neotetraose; LNT, lacto-N-tetraose; LNFP, lacto-N-fucopentaose; 2AB, 2-aminobenzamide; 2′-FL, 2′-fucosyllactose; 3-FL, 3 -fucosyllactose; 3′-SL, 3′-sialyllactose.
REFERENCES
- 1.Coutsoudis A, Dabis F, Fawzi W, Gaillard P, Haverkamp G, Harris DR, Jackson JB, Leroy V, Meda N, Msellati P, et al. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis 2004;189:2154–66 [DOI] [PubMed] [Google Scholar]
- 2.Labbok MH, Clark D, Goldman AS. Breastfeeding: maintaining an irreplaceable immunological resource. Nat Rev Immunol 2004;4:565–72 [DOI] [PubMed] [Google Scholar]
- 3.Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr 2000;20:699–722 [DOI] [PubMed] [Google Scholar]
- 4.Bode L. Recent advances on structure, metabolism, and function of human milk oligosaccharides. J Nutr 2006;136:2127–30 [DOI] [PubMed] [Google Scholar]
- 5.Bode L. Human milk oligosaccharides: prebiotics and beyond. Nutr Rev 2009;67(suppl 2):S183–91 [DOI] [PubMed] [Google Scholar]
- 6.Sela DA, Mills DA. Nursing our microbiota: molecular linkages between bifidobacteria and milk oligosaccharides. Trends Microbiol 2010;18:298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chichlowski M, German JB, Lebrilla CB, Mills DA. The influence of milk oligosaccharides on microbiota of infants: opportunities for formulas. Annu Rev Food Sci Technol 2011;2:331–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newburg DS, Ruiz-Palacios GM, Morrow AL. Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr 2005;25:37–58. [DOI] [PubMed] [Google Scholar]
- 9.Kuntz S, Rudloff S, Kunz C. Oligosaccharides from human milk influence growth-related characteristics of intestinally transformed and non-transformed intestinal cells. Br J Nutr 2008;99:462–71. [DOI] [PubMed] [Google Scholar]
- 10.Kuntz S, Kunz C, Rudloff S. Oligosaccharides from human milk induce growth arrest via G2/M by influencing growth-related cell cycle genes in intestinal epithelial cells. Br J Nutr 2009;101:1306–15. [DOI] [PubMed] [Google Scholar]
- 11.Atochina O, Daly-Engel T, Piskorska D, McGuire E, Harn DA. A schistosome-expressed immunomodulatory glycoconjugate expands peritoneal Gr1(+) macrophages that suppress naive CD4(+) T cell proliferation via an IFN-gamma and nitric oxide-dependent mechanism. J Immunol 2001;167:4293–302 [DOI] [PubMed] [Google Scholar]
- 12.Terrazas LI, Walsh KL, Piskorska D, McGuire E, Harn DA., Jr The schistosome oligosaccharide lacto-N-neotetraose expands Gr1(+) cells that secrete anti-inflammatory cytokines and inhibit proliferation of naive CD4(+) cells: a potential mechanism for immune polarization in helminth infections. J Immunol 2001;167:5294–303 [DOI] [PubMed] [Google Scholar]
- 13.Bode L, Kunz C, Muhly-Reinholz M, Mayer K, Seeger W, Rudloff S. Inhibition of monocyte, lymphocyte, and neutrophil adhesion to endothelial cells by human milk oligosaccharides. Thromb Haemost 2004;92:1402–10 [DOI] [PubMed] [Google Scholar]
- 14.Bode L, Rudloff S, Kunz C, Strobel S, Klein N. Human milk oligosaccharides reduce platelet-neutrophil complex formation leading to a decrease in neutrophil beta 2 integrin expression. J Leukoc Biol 2004;76:820–6 [DOI] [PubMed] [Google Scholar]
- 15.Eiwegger T, Stahl B, Schmitt J, Boehm G, Gerstmayr M, Pichler J, Dehlink E, Loibichler C, Urbanek R, Szepfalusi Z. Human milk–derived oligosaccharides and plant-derived oligosaccharides stimulate cytokine production of cord blood T-cells in vitro. Pediatr Res 2004;56:536–40 [DOI] [PubMed] [Google Scholar]
- 16.Atochina O, Harn D. LNFPIII/LeX-stimulated macrophages activate natural killer cells via CD40-CD40L interaction. Clin Diagn Lab Immunol 2005;12:1041–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eiwegger T, Stahl B, Haidl P, Schmitt J, Boehm G, Dehlink E, Urbanek R, Szepfalusi Z. Prebiotic oligosaccharides: in vitro evidence for gastrointestinal epithelial transfer and immunomodulatory properties. Pediatr Allergy Immunol 2010;21:1179–88 [DOI] [PubMed] [Google Scholar]
- 18.Zhu B, Trikudanathan S, Zozulya AL, Sandoval-Garcia C, Kennedy JK, Atochina O, Norberg T, Castagner B, Seeberger P, Fabry Z, et al. Immune modulation by Lacto-N-fucopentaose III in experimental autoimmune encephalomyelitis. Clin Immunol 2012;142:351–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naarding MA, Ludwig IS, Groot F, Berkhout B, Geijtenbeek TB, Pollakis G, Paxton WA. Lewis X component in human milk binds DC-SIGN and inhibits HIV-1 transfer to CD4+ T lymphocytes. J Clin Invest 2005;115:3256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naarding MA, Dirac AM, Ludwig IS, Speijer D, Lindquist S, Vestman EL, Stax MJ, Geijtenbeek TB, Pollakis G, Hernell O, et al. Bile salt-stimulated lipase from human milk binds DC-SIGN and inhibits human immunodeficiency virus type 1 transfer to CD4+ T cells. Antimicrob Agents Chemother 2006;50:3367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong P, Ninonuevo MR, Lee B, Lebrilla C, Bode L. Human milk oligosaccharides reduce HIV-1-gp120 binding to dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN). Br J Nutr 2009;101:482–6 [DOI] [PubMed] [Google Scholar]
- 22.Su SV, Gurney KB, Lee B. Sugar and spice: viral envelope-DC-SIGN interactions in HIV pathogenesis. Curr HIV Res 2003;1:87–99 [DOI] [PubMed] [Google Scholar]
- 23.van Kooyk Y, Geijtenbeek TB. DC-SIGN: escape mechanism for pathogens. Nat Rev Immunol 2003;3:697–709 [DOI] [PubMed] [Google Scholar]
- 24.Wu L. KewalRamani VN. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol 2006;6:859–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Liempt E, Bank CM, Mehta P, Garcia-Vallejo JJ, Kawar ZS, Geyer R, Alvarez RA, Cummings RD, Kooyk Y, van Die I. Specificity of DC-SIGN for mannose- and fucose-containing glycans. FEBS Lett 2006;580:6123–31 [DOI] [PubMed] [Google Scholar]
- 26.Stahl B, Thurl S, Henker J, Siegel M, Finke B, Sawatzki G. Detection of four human milk groups with respect to Lewis-blood-group-dependent oligosaccharides by serologic and chromatographic analysis. Adv Exp Med Biol 2001;501:299–306 [DOI] [PubMed] [Google Scholar]
- 27.Gabrielli O, Zampini L, Galeazzi T, Padella L, Santoro L, Peila C, Giuliani F, Bertino E, Fabris C, Coppa GV. Preterm milk oligosaccharides during the first month of lactation. Pediatrics 2011;128:e1520–31 [DOI] [PubMed] [Google Scholar]
- 28.Kobata A. Structures and application of oligosaccharides in human milk. Proc Jpn Acad, Ser B, Phys Biol Sci 2010;86:731–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thurl S, Henker J, Siegel M, Tovar K, Sawatzki G. Detection of four human milk groups with respect to Lewis blood group dependent oligosaccharides. Glycoconj J 1997;14:795–9 [DOI] [PubMed] [Google Scholar]
- 30.Coppa GV, Gabrielli O, Zampini L, Galeazzi T, Ficcadenti A, Padella L, Santoro L, Soldi S, Carlucci A, Bertino E, et al. Oligosaccharides in 4 different milk groups, Bifidobacteria, and Ruminococcus obeum. J Pediatr Gastroenterol Nutr 2011;53:80–7 [DOI] [PubMed] [Google Scholar]
- 31.Kuhn L, Aldrovandi GM, Sinkala M, Kankasa C, Semrau K, Mwiya M, Kasonde P, Scott N, Vwalika C, Walter J, et al. Effects of early, abrupt weaning on HIV-free survival of children in Zambia. N Engl J Med 2008;359:130–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taha TE, Kumwenda J, Cole SR, Hoover DR, Kafulafula G, Fowler MG, Thigpen MC, Li Q, Kumwenda NI, Mofenson L. Postnatal HIV-1 transmission after cessation of infant extended antiretroviral prophylaxis and effect of maternal highly active antiretroviral therapy. J Infect Dis 2009;200:1490–7. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro RL, Hughes MD, Ogwu A, Kitch D, Lockman S, Moffat C, Makhema J, Moyo S, Thior I, McIntosh K, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med 2010;362:2282–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaturvedi P, Warren CD, Altaye M, Morrow AL, Ruiz-Palacios G, Pickering LK, Newburg DS. Fucosylated human milk oligosaccharides vary between individuals and over the course of lactation. Glycobiology 2001;11:365–72 [DOI] [PubMed] [Google Scholar]
- 35.Davidson B, Meinzen-Derr JK, Wagner CL, Newburg DS, Morrow AL. Fucosylated oligosaccharides in human milk in relation to gestational age and stage of lactation. Adv Exp Med Biol 2004;554:427–30 [DOI] [PubMed] [Google Scholar]
- 36.Thurl S, Munzert M, Henker J, Boehm G, Muller-Werner B, Jelinek J, Stahl B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br J Nutr 2010;104:1261–71 [DOI] [PubMed] [Google Scholar]
- 37.Ghosh MK, Kuhn L, West J, Semrau K, Decker D, Thea DM, Aldrovandi GM. Quantitation of human immunodeficiency virus type 1 in breast milk. J Clin Microbiol 2003;41:2465–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jantscher-Krenn E, Zherebtsov M, Nissan C, Goth K, Guner YS, Naidu N, Choudhury B, Grishin AV, Ford HR, Bode L. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut (Epub ahead of print 11 December 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jantscher-Krenn E, Lauwaet T, Bliss LA, Reed SL, Gillin FD, Bode L. Human milk oligosaccharides reduce Entamoeba histolytica attachment and cytotoxicity in vitro. Br J Nutr 2012;23:1–8 [DOI] [PubMed] [Google Scholar]
- 40.Andersson B, Porras O, Hanson LA, Lagergard T, Svanborg-Eden C. Inhibition of attachment of Streptococcus pneumoniae and Haemophilus influenzae by human milk and receptor oligosaccharides. J Infect Dis 1986;153:232–7 [DOI] [PubMed] [Google Scholar]
- 41.Crane JK, Azar SS, Stam A, Newburg DS. Oligosaccharides from human milk block binding and activity of the Escherichia coli heat-stable enterotoxin (STa) in T84 intestinal cells. J Nutr 1994;124:2358–64 [DOI] [PubMed] [Google Scholar]
- 42.Lesman-Movshovich E, Lerrer B, Gilboa-Garber N. Blocking of Pseudomonas aeruginosa lectins by human milk glycans. Can J Microbiol 2003;49:230–5 [DOI] [PubMed] [Google Scholar]
- 43.Ruiz-Palacios GM, Cervantes LE, Ramos P, Chavez-Munguia B, Newburg DS. Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha 1, 2Gal beta 1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J Biol Chem 2003;278:14112–20 [DOI] [PubMed] [Google Scholar]
- 44.Morrow AL, Ruiz-Palacios GM, Altaye M, Jiang X, Guerrero ML, Meinzen-Derr JK, Farkas T, Chaturvedi P, Pickering LK, Newburg DS. Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J Pediatr 2004;145:297–303 [DOI] [PubMed] [Google Scholar]
- 45.Bläckberg L, Angquist KA, Hernell O. Bile-salt-stimulated lipase in human milk: evidence for its synthesis in the lactating mammary gland. FEBS Lett 1987;217:37–41 [DOI] [PubMed] [Google Scholar]
- 46.Marcobal A, Barboza M, Sonnenburg ED, Pudlo N, Martens EC, Desai P, Lebrilla CB, Weimer BC, Mills DA, German JB, et al. Bacteroides in the infant gut consume milk oligosaccharides via mucus-utilization pathways. Cell Host Microbe 2011;10:507–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fuhrer A, Sprenger N, Kurakevich E, Borsig L, Chassard C, Hennet T. Milk sialyllactose influences colitis in mice through selective intestinal bacterial colonization. J Exp Med 2010;207:2843–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J, Balog CI, Stavenhagen K, Koeleman CA, Scherer HU, Selman MH, Deelder AM, Huizinga TW, Toes RE, Wuhrer M. Fc-glycosylation of IgG1 is modulated by B-cell stimuli. Mol Cell Proteomics. 2011;10:M110 004655. [DOI] [PMC free article] [PubMed]
- 49.Giordanengo V, Ollier L, Lanteri M, Lesimple J, March D, Thyss S, Lefebvre JC. Epigenetic reprogramming of UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase (GNE) in HIV-1-infected CEM T cells. FASEB J 2004;18:1961–3 [DOI] [PubMed] [Google Scholar]
- 50.Kuhn L, Trabattoni D, Kankasa C, Semrau K, Kasonde P, Lissoni F, Sinkala M, Ghosh M, Vwalika C, Aldrovandi GM, et al. Alpha-defensins in the prevention of HIV transmission among breastfed infants. J Acquir Immune Defic Syndr 2005;39:138–42 [PMC free article] [PubMed] [Google Scholar]
- 51.Walter J, Kuhn L, Ghosh MK, Kankasa C, Semrau K, Sinkala M, Mwiya M, Thea DM, Aldrovandi GM. Low and undetectable breast milk interleukin-7 concentrations are associated with reduced risk of postnatal HIV transmission. J Acquir Immune Defic Syndr 2007;46:200–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walter J, Ghosh MK, Kuhn L, Semrau K, Sinkala M, Kankasa C, Thea DM, Aldrovandi GM. High concentrations of interleukin 15 in breast milk are associated with protection against postnatal HIV transmission. J Infect Dis 2009;200:1498–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuhn L, Trabattoni D, Kankasa C, Sinkala M, Lissoni F, Ghosh M, Aldrovandi G, Thea D, Clerici M. Hiv-specific secretory IgA in breast milk of HIV-positive mothers is not associated with protection against HIV transmission among breast-fed infants. J Pediatr 2006;149:611–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castelletti E, Lo Caputo S, Kuhn L, Borelli M, Gajardo J, Sinkala M, Trabattoni D, Kankasa C, Lauri E, Clivio A, et al. The mucosae-associated epithelial chemokine (MEC/CCL28) modulates immunity in HIV infection. PLoS ONE 2007;2:e969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Viveros-Rogel M, Soto-Ramirez L, Chaturvedi P, Newburg DS, Ruiz-Palacios GM. Inhibition of HIV-1 infection in vitro by human milk sulfated glycolipids and glycosaminoglycans. Adv Exp Med Biol 2004;554:481–7 [DOI] [PubMed] [Google Scholar]
- 56.Aldrovandi GM, Kuhn L. What infants and breasts can teach us about natural protection from HIV infection. J Infect Dis 2010;202(suppl 3):S366–70 [DOI] [PMC free article] [PubMed] [Google Scholar]