Abstract
Background: Improved complementary feeding is cited as a critical factor for reducing stunting. Consumption of meats has been advocated, but its efficacy in low-resource settings has not been tested.
Objective: The objective was to test the hypothesis that daily intake of 30 to 45 g meat from 6 to 18 mo of age would result in greater linear growth velocity and improved micronutrient status in comparison with an equicaloric multimicronutrient-fortified cereal.
Design: This was a cluster randomized efficacy trial conducted in the Democratic Republic of Congo, Zambia, Guatemala, and Pakistan. Individual daily portions of study foods and education messages to enhance complementary feeding were delivered to participants. Blood tests were obtained at trial completion.
Results: A total of 532 (86.1%) and 530 (85.8%) participants from the meat and cereal arms, respectively, completed the study. Linear growth velocity did not differ between treatment groups: 1.00 (95% CI: 0.99, 1.02) and 1.02 (95% CI: 1.00, 1.04) cm/mo for the meat and cereal groups, respectively (P = 0.39). From baseline to 18 mo, stunting [length-for-age z score (LAZ) <−2.0] rates increased from ∼33% to nearly 50%. Years of maternal education and maternal height were positively associated with linear growth velocity (P = 0.0006 and 0.003, respectively); LAZ at 6 mo was negatively associated (P < 0.0001). Anemia rates did not differ by group; iron deficiency was significantly lower in the cereal group.
Conclusion: The high rate of stunting at baseline and the lack of effect of either the meat or multiple micronutrient-fortified cereal intervention to reverse its progression argue for multifaceted interventions beginning in the pre- and early postnatal periods. This trial was registered at clinicaltrials.gov as NCT01084109.
INTRODUCTION
Impairment of linear growth during childhood is associated with adverse health and developmental outcomes in low-resource settings (1). Postnatal linear growth faltering is recognized to begin early in the first year of life and progresses through the first 24 mo (2–4). This pattern of early-onset growth faltering highlights the importance of early determinants, which are likely to be multidimensional. Postnatal nutrition is one important and potentially modifiable factor.
Exclusive breastfeeding for approximately the first 6 mo of life provides important nutritional and survival benefits to young infants and continues to provide energy and important nutritional and nonnutritional benefits in later infancy. As the infant approaches 6 mo of age, complementary foods must meet the nutrient gaps that develop because of the longitudinal changes in milk composition and the older infant's nutritional requirements (5–7). Promotion of optimal complementary feeding, especially in conjunction with infection control (1), has been identified as an effective intervention to reduce stunting and its associated adverse outcomes (8). In its guidelines for complementary feeding, the WHO recommends, if possible, the daily consumption of meat, poultry, fish, or eggs (9). Animal-source foods such as these, which have a high energy density, high-quality protein, and highly bioavailable micronutrients, including iron and zinc, are often particularly lacking in the diets of older infants and young children in low-resource settings (10, 11). In the absence of regular consumption of animal-source foods, fortified foods or micronutrient supplements are recommended (9). These products are typically less expensive but have the challenge of not being routinely available, especially in rural, resource-limited settings, where access to and use of fortified commercial products is often limited (10). Many education interventions to promote dietary diversification with locally available foods have been undertaken and have shown promising results on growth (12–14). To our knowledge, however, no efficacy trials of the effect of routine consumption of meats as a complementary food have been reported.
The broad goal of this study was to improve the growth, health, and development of infants and young children in diverse low-resource settings by improving the quality of complementary feeding. We hypothesized that the daily provision of meat compared with an equicaloric portion of a micronutrient-fortified cereal as an early complementary food would result in greater linear growth velocity from 6 through 18 mo of age. Secondary outcomes included dietary diversity, micronutrient status, neurocognitive development, and infectious disease morbidity.
SUBJECTS AND METHODS
Study design and setting
The details of the protocol for this trial were published previously (15). Briefly, we applied a cluster randomized design to compare the effect of consumption of daily meat with that of a micronutrient-fortified cereal, starting at 6 mo of age and continuing through 18 mo. The study was undertaken through the Eunice Kennedy Shriver National Institute of Child Health and Human Development–supported Global Network for Women's and Children's Health Research (GN)4. Participating GN sites were rural communities in the Democratic Republic of Congo and Zambia, semirural communities in the Western Highlands of Guatemala, and urban communities in Karachi, Pakistan. A total of 40 clusters, 5 in each study arm per site, were included. Cluster inclusion criteria included an estimated prevalence of stunting of ≥20%, based on pilot data obtained in the same sites (10).
Subjects
Inclusion criteria for individual subjects included infants aged 3–4 mo who were primarily or exclusively breastfed and whose mothers intended to continue breastfeeding through at least the first year of life. Individual exclusion criteria included use of free or subsidized fortified complementary foods or infant formula, multiple births, known congenital anomaly, and neurologic deficit. Computer-generated lists of births within each cluster were used to recruit and screen potential participants. The subjects were assigned to the cluster in which they resided. The protocol was approved by ethics boards located in the countries where the studies were located, the partnering US institutions, and RTI International, and the study was conducted pursuant to these guidelines.
Sample size calculations were based on an effect size of 0.3 SD units in linear growth, and statistical power was set at 80% to detect a difference of 0.3 SD units in linear growth velocity from 6 to 18 mo of age between the 2 feeding groups by using a 2-tailed test and a 5% type 1 error rate. An assumed intracluster correlation coefficient of 0.05, adjustment for 20% attrition, and an allowance for cluster loss resulted in a requirement of 20 clusters and 600 subjects per treatment group (15).
Randomization was performed at the community level, stratifying by site and stunting rate. Strata were created by combining communities with similar stunting rates, as measured in a pilot study (10), within each site, and a computer-generated randomization algorithm assigned clusters at a 1:1 ratio within each stratum. For practical implementation reasons, neither the study participants nor the study staff was masked to cluster treatment assignment. Geographic distance between clusters minimized the risk of contamination of the intervention or communication among study subjects in different clusters.
Intervention
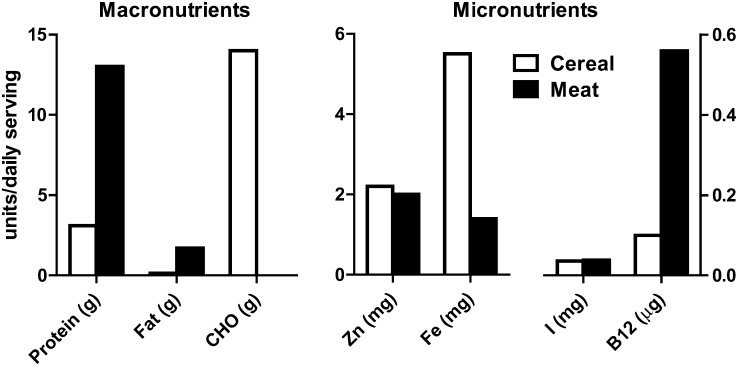
Subjects in the intervention clusters received a daily provision of lyophilized beef (Mountain House Inc), equivalent to 30 g cooked meat/d from 6 through 11 mo of age and 45 g/d from 12 through 18 mo of age. Subjects residing in clusters randomly assigned to the comparison group received a micronutrient-fortified rice-soy cereal product (Nutrica), which was equicaloric to the daily meat portions: 70 and 105 kcal/d for the first and second 6-mo periods, respectively. The levels of micronutrient fortification were based on recommendations from the literature (16). The specific comparison of the micronutrient content of the 2 intervention foods was published previously (15); a graphic comparison of selected aspects of the nutrient composition is presented in Figure 1. In addition, both groups received educational messages about complementary feeding: encourage thickened feeds, offer complementary foods ≥3 times per day, and optimize variety of complementary foods. Messages regarding exclusive breastfeeding through 6 mo, hand washing, and use of boiled water for food preparation were also emphasized. Compliance was monitored at weekly visits by counting empty food packets and by mothers’ reports. Compliance for each subject was calculated by dividing the number of days the subject ate the study food by the total number of days the subject was followed.
FIGURE 1.
Nutrient composition of the intervention foods per daily portion for 6–12-mo-olds. B12, vitamin B-12; CHO, carbohydrate.
Outcome assessments
Study assessments were conducted by trained staff different from the intervention teams; standardized assessments were conducted when participating infants were 6, 9, 12, and 18 mo of age. Assessments included anthropometric measurements, the Infant and Child Feeding Index and its components (17, 18), Bayley Scales of Infant Development at 18 mo, and biochemical indexes of micronutrient status at 18 mo, which were determined in a convenience subsample of ∼300 subjects per group (60% of total subjects), with approximately equal numbers of subjects sampled from each site (15). Hemoglobin concentrations, obtained via finger stick, were obtained from 63–77% of subjects at 3 of the 4 sites. Because of a protocol deviation, measurements of hemoglobin were not obtained from subjects at the Pakistani site.
Statistical methods
A comprehensive analysis of potential confounders at baseline was performed to assess the extent, if any, of baseline treatment group imbalances for sex and socioeconomic status (SES), exclusive breastfeeding at 6 mo, birth weight via maternal report or medical records, length-for-age z score (LAZ) at 6 mo, maternal stature, maternal age, maternal education, maternal health disease history, parity, and site. The SES score is a composite SES variable calculated by using economic indicators (including dwelling, roof, wall, floor, toilet type, and water source) to score the family's relative wealth (an average SES score would be 0, below average <0, and above average >0). Mean z scores at 6, 9, 12, and 18 mo for LAZ, weight-for-age z score, and weight-for-length z score (WLZ) were determined from WHO growth standards (19), as were the prevalence of stunting and wasting (defined as LAZ <−2 and WLZ <−2, respectively). Model-based approaches were used to evaluate treatment-group differences in baseline variables, anthropometric measurements, and primary and secondary outcomes by using generalized estimating equation extensions of logistic models (for binary measures) and linear models (for continuous measures) to account for the correlation of subjects induced by the cluster randomization with control for GN site. Analogous generalized estimating equation models compared outcomes between the treatment arms, adjusting for potential confounders; covariates included both subject level and community level (as above). An additional post hoc analysis was performed to examine the effect of meat consumption on linear growth velocity over time by modeling longitudinal growth data from 6 to 18 mo by using linear mixed models. Data were analyzed by using SAS statistical software (SAS Inc) and are presented as means ± SDs and 95% CIs unless otherwise indicated.
RESULTS
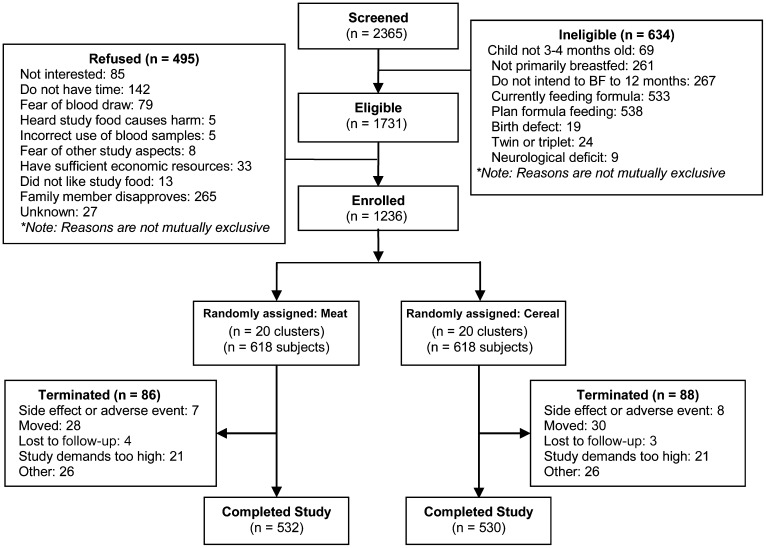
The number of subjects enrolled at each site ranged from 300 to 329. The percentage of potential subjects who were screened and found to be ineligible overall was 26.8%; this value ranged from 4.4% to 43.4% among sites. More subjects were ineligible in the urban and semirural sites, primarily because of current or planned formula feeding. Of those who were eligible but refused, 28.6% overall, the primary reasons were spouse or other family member not wanting the infant to participate (53.5%) and/or not having enough time to participate (28.7%). The final number of participants was 618 in each arm of the study; 532 (86.1%) and 530 (85.8%) completed the study for the meat and cereal arms, respectively (Figure 2). The major reasons for termination from the study included moving (33.3%), voluntary discontinuation because of demands of the study (24.1%), adverse events (8.6%), and other (29.9%). In all cases, these reasons were similar between the 2 study arms. Delivery of the study food was >94% for both groups, and overall compliance was 99% for both groups.
FIGURE 2.
Consort diagram. BF, breastfeed.
The baseline demographic, socioeconomic, and maternal and infant health data for each group are presented in Table 1. The only factor that differed significantly between the 2 arms was the highest level of paternal education; the cereal arm had a higher level. Other factors with limited evidence (P < 0.1) of difference between treatment arms (both favoring the cereal group) included socioeconomic composite score and highest level of maternal education. Positive history of maternal HIV was slightly more frequent in the meat group (P = 0.06), but the absolute number was small.
TABLE 1.
Baseline demographic, socioeconomic, and maternal and infant health data by group1
| Treatment group |
|||
| Meat(n = 614) | Cereal(n = 617) | P value2 | |
| SES | |||
| SES composite score | −0.21 ± 2.843 | 0.20 ± 2.97 | 0.070 |
| Maternal education level (y) | 4.6 ± 3.9 | 5.6 ± 4.0 | 0.072 |
| Paternal education level (y) | 6.4 ± 3.8 | 7.3 ± 3.9 | 0.047 |
| Mother employed [n (%)] | 44 (7) | 55 (9) | 0.460 |
| Father employed [n (%)] | 241 (39) | 239 (39) | 0.992 |
| Maternal demographics | |||
| No. of pregnancies | 3.6 ± 2.4 | 3.3 ± 2.3 | 0.157 |
| No. of children | 3.1 ± 1.9 | 2.8 ± 1.9 | 0.205 |
| Breastfed last child [n (% yes)] | 77 (3) | 101 (16) | 0.355 |
| Has mother ever had | |||
| Malaria [n (%)] | 273 (45) | 266 (43) | 0.739 |
| Tuberculosis [n (%)] | 4 (<1) | 4 (<1) | 1.0004 |
| HIV [n (%)] | 4 (<1) | 0 | 0.0624 |
| Diabetes [n (%)] | 0 | 1 (<1) | 1.0004 |
| Anemia [n (%)] | 77 (13) | 62 (10) | 0.626 |
| Parasites [n (%)] | 165 (27) | 148 (24) | 0.239 |
| Maternal height (cm) | 154.5 ± 8.5 | 153.9 ± 7.6 | 0.353 |
| Maternal weight (kg) | 54.6 ± 9.4 | 55.9 ± 10.5 | 0.106 |
| Maternal BMI (kg/m2) | 23.0 ± 4.19 | 23.6 ± 4.2 | 0.077 |
| Infant demographics | |||
| Child born prematurely [n (% yes)] | 32 (5) | 34 (6) | 0.894 |
| Exclusive BF at 6 mo [n (% yes)]5 | 301 (51) | 286 (49) | 0.341 |
| Sex [n (% male)] | 287 (47) | 312 (51) | 0.122 |
| Birth weight (g) | 3209 ± 595 | 3117 ± 502 | 0.081 |
| Study site [n (%)] | 0.9999 | ||
| DRC | 150 (24) | 150 (24) | |
| Guatemala | 150 (24) | 150 (24) | |
| Pakistan | 165 (27) | 164 (27) | |
| Zambia | 153 (25) | 154 (25) | |
BF, breastfeeding; DRC, Democratic Republic of Congo; SES, socioeconomic status.
Type 3 generalized estimating equation analysis.
Mean ± SD (all such values).
Fisher exact test used because of small sample sizes.
Sample sizes for 6 mo: meat (n = 589) and cereal (n = 587).
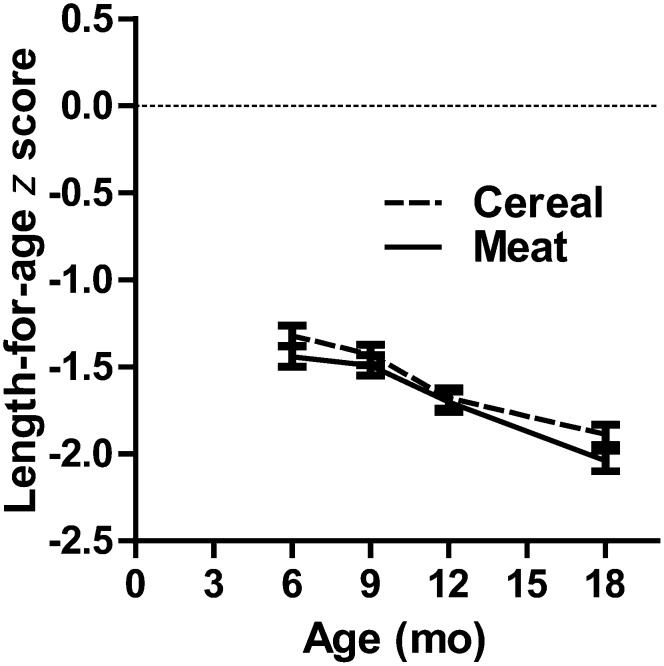
The primary outcome, linear growth velocity, did not differ between the 2 treatment groups: 1.00 (95% CI: 0.99, 1.02) and 1.02 (95% CI: 1.00, 1.04) cm/mo for the meat and cereal groups, respectively (P = 0.39). Post hoc longitudinal analysis of the change in LAZ provided little evidence of an overall treatment effect (P = 0.11), with control for time period and site (Figure 3). Approximately one-third of the infants were stunted at 6 mo of age, and stunting rates increased for both groups by 18 mo, also with limited evidence of a difference between the groups at that time (50% compared with 45% for the meat and cereal groups, respectively; P = 0.08). After control for baseline covariates, the analyses provided no evidence that linear growth was affected by treatment (P = 0.52). Adjustment with a reduced model, which included only the covariates that were significant at α = 0.10 level in the full model, indicated that the highest year of maternal education and maternal height were significant in a positive direction on linear growth, whereas male sex and LAZ at 6 mo of age were significant in a negative direction. Additionally, linear growth differed by site (Table 2).
FIGURE 3.
Longitudinal mean (±SD) length-for-age z score from 6 to 18 mo for meat compared with cereal. Longitudinal modeling yielded a treatment-by-age interaction P value of 0.114 on length-for-age z score when age and site were controlled for. Age was also determined to be the repeated measure, whereas data were clustered on subject identification nested within community. Proc Mixed (SAS Institute Inc) was used for longitudinal analysis.
TABLE 2.
Main effects with a reduced model, which included only the significant covariates from the full model
| Covariate | Main effect estimate (95% CI) | P value1 |
| Treatment, meat | −0.013 (−0.042, 0.015) | 0.360 |
| Maternal education (y) | 0.009 (0.005, 0.013) | <0.001 |
| Has mother ever had malaria? | −0.066 (−0.134, 0.003) | 0.081 |
| Maternal height (cm) | 0.003 (0.001, 0.005) | 0.003 |
| Sex (male vs female) | −0.061 (−0.094, −0.028) | 0.002 |
| Study site | ||
| DRC2 | −0.006 (−0.080, 0.068) | 0.867 |
| Guatemala | −0.103 (−0.147, −0.058) | <0.0001 |
| Pakistan | — | — |
| Zambia | 0.050 (−0.008, 0.108) | 0.094 |
| Length-for-age z score (6 mo) | −0.054 (−0.070, −0.038) | <0.0001 |
Type 3 generalized estimating equation analysis.
DRC, Democratic Republic of Congo.
Additional details of linear growth and of secondary outcomes related to anthropometric measures—including weight-for-age z score, WLZ, head circumference z score, and wasting rates—are presented in Table 3. Mean WLZ did not differ at any point between groups. Mean wasting rates were much lower than stunting rates, both at baseline and at 18 mo, and did not differ by group. Head circumference z score, which was not different at baseline, was significantly lower in the meat group at 18 mo.
TABLE 3.
Anthropometric data by group1
| Treatment group |
|||||
| Meat (n = 532) |
Cereal (n = 530) |
||||
| Variable | Value | (95% CI) | Value | (95% CI) | P value2 |
| Length | |||||
| LAZ | |||||
| 6 mo | −1.44 ± 1.333 | (−1.55, −1.32) | −1.32 ± 1.39 | (−1.44, −1.21) | 0.233 |
| 9 mo | −1.49 ± 1.39 | (−1.61, −1.37) | −1.43 ± 1.43 | (−1.55, −1.31) | 0.542 |
| 12 mo | −1.70 ± 1.39 | (−1.82, −1.58) | −1.68 ± 1.31 | (−1.80, −1.57) | 0.745 |
| 18 mo | −2.04 ± 1.33 | (−2.16, −1.93) | −1.89 ± 1.42 | (−2.01, −1.77) | 0.126 |
| Stunting rate (LAZ <2) [n (%)] | |||||
| 6 mo | 181 (34) | (30, 38) | 177 (33) | (29, 37) | 0.713 |
| 9 mo | 184 (35) | (31, 39) | 179 (34) | (30, 38) | 0.676 |
| 12 mo | 222 (42) | (38, 46) | 212 (40) | (36, 44) | 0.510 |
| 18 mo | 273 (51) | (47, 56) | 239 (45) | (41, 49) | 0.080 |
| Weight | |||||
| Weight-for-age z score | |||||
| 6 mo | −1.01 ± 1.26 | (−1.12, −0.90) | −0.91 ± 1.21 | (−1.02, −0.81) | 0.400 |
| 9 mo | −1.09 ± 1.20 | (−1.19, −0.99) | −1.07 ± 1.17 | (−1.17, −0.97) | 0.830 |
| 12 mo | −1.22 ± 1.21 | (−1.33, −1.12) | −1.16 ± 1.08 | (−1.25, −1.07) | 0.486 |
| 18 mo | −1.28 ± 1.01 | (−1.37, −1.19) | −1.15 ± 1.06 | (−1.24, −1.05) | 0.099 |
| WLZ | |||||
| 6 mo | −0.02 ± 1.47 | (−0.15, 0.10) | 0.02 ± 1.45 | (−0.10, 0.15) | 0.922 |
| 9 mo | −0.27 ± 1.24 | (−0.37, −0.16) | −0.28 ± 1.32 | (−0.40, −0.17) | 0.673 |
| 12 mo | −0.46 ± 1.23 | (−0.57, −0.36) | −0.40 ± 1.17 | (−0.50, −0.30) | 0.638 |
| 18 mo | −0.38 ± 1.04 | (−0.47, −0.29) | −0.29 ± 1.30 | (−0.40, −0.18) | 0.447 |
| Wasting rate (WLZ <2) [n (%)] | |||||
| 6 mo | 46 (9) | (6, 11) | 41 (8) | (5, 10) | 0.859 |
| 9 mo | 38 (7) | (5, 9) | 54 (10) | (8, 13) | 0.132 |
| 12 mo | 44 (8) | (6, 11) | 61 (12) | (9, 14) | 0.177 |
| 18 mo | 28 (5) | (3, 7) | 39 (7) | (5, 10) | 0.241 |
| Head circumference | |||||
| Head circumference–for-age z score | |||||
| 6 mo | −0.41 ± 1.19 | (−0.52, −0.31) | −0.39 ± 1.18 | (−0.49, −0.28) | 0.715 |
| 9 mo | −0.48 ± 1.06 | (−0.57, −0.39) | −0.41 ± 1.11 | (−0.51, −0.32) | 0.404 |
| 12 mo | −0.61 ± 1.09 | (−0.70, −0.52) | −0.58 ± 1.13 | (−0.68, −0.49) | 0.718 |
| 18 mo | −0.69 ± 0.99 | (−0.77, −0.60) | −0.50 ± 1.43 | (−0.62, −0.38) | 0.016 |
LAZ, length-for-age z score; WLZ, weight-for-length z score.
Type 3 generalized estimating equation analysis analysis.
Mean ± SD (all such values).
With respect to dietary and feeding practices, differences were not observed between groups for percentage breastfeeding, which remained >90% at all assessment points. Likewise, bottle feeding did not differ, averaging <10% at 9 mo and <15% at 18 mo. The cereal group reported significantly more “main meals” per day at the 9-, 12-, and 18-mo assessment points, but they reported significantly fewer additional meals (P < 0.05). The meat group reported consuming significantly more food groups at 9 and 12 mo but not at 18 mo. Reported micronutrient supplement use (multivitamin, iron, and zinc) was infrequent, being highest for iron in 4–6% of participants, but not different by group. The highest supplement use was in the “other” category, which was primarily vitamin A, and was reported in ∼25–30% of the 12- and 18-mo-olds.
Overall morbidity and specific diagnoses (diarrhea, respiratory illness, pneumonia, severe pneumonia, and malaria) did not differ between groups. Neither the psychomotor developmental index nor the mental developmental index of the Bayley Scales of Infant Development II differed by group: 99.1 (95% CI: 97.9, 100.3) and 99.7 (95% CI: 98.8, 100.7) (P = 0.54) for meat and cereal, respectively (psychomotor developmental index) and 95.2 (95% CI: 94.2, 96.2) and 95.3 (95% CI: 94.5, 96.2) (P = 0.82) for meat and cereal, respectively (mental developmental index). Mean hemoglobin concentrations, zinc, and vitamin B-12 concentrations did not differ by group; ferritin and transferrin receptor concentrations were significantly more favorable (higher and lower, respectively) in the cereal group (Table 4). With respect to frequency of anemia, 23.7% and 15.7% of subjects had hemoglobin <11 g/dL in the meat and cereal groups, respectively (P < 0.01). On the basis of serum ferritin <12 ng/mL, 27% and 16% of subjects in the meat and cereal groups, respectively, were iron deficient (P = 0.001). Correction for elevated CRP concentrations had no detectable effect on either of the means for ferritin or zinc concentrations. Adverse events and protocol violations did not differ between groups.
TABLE 4.
Biomarkers of micronutrient status at 18 mo of age by group
| Meat |
Cereal |
||||
| Variable | n | Mean ± SD (95% CI) | n | Mean ± SD (95% CI) | P value1 |
| Hemoglobin (g/dL)2 | 287 | 11.5 ± 1.5 (11.3, 11.7) | 267 | 11.7 ± 1.3 (11.5, 11.8) | 0.189 |
| Ferritin (ng/mL) | 290 | 27.6 ± 27.6 (24.4, 30.8) | 261 | 38.2 ± 44.2 (32.8, 43.6) | 0.002 |
| Tranferrin receptor (mg/L) | 335 | 13.7 ± 6.9 (12.9, 14.4) | 301 | 12.1 ± 4.7 (11.5, 12.6) | 0.001 |
| Zinc (μg/dL) | 291 | 63.2 ± 12.3 (61.9, 64.5) | 262 | 62.2 ± 12.4 (60.8, 63.6) | 0.429 |
| Vitamin B-12 (pg/mL) | 312 | 221 ± 103 (210, 233) | 285 | 222 ± 123 (207, 236) | 0.762 |
Type 3 generalized estimating equation analysis.
Measurements not obtained at the Pakistani site.
DISCUSSION
The major observations resulting from this study were the virtually indistinguishable linear growth velocity between the 2 groups and the progressive linear growth faltering for both groups. Assuming that both of the study foods provided an improvement to the subjects’ diets, these findings underscore the likely multifactorial basis of stunting in young children.
Improved micronutrient intakes from complementary foods have been emphasized over energy and macronutrient intakes to improve the growth of young children (5, 8). Consistent with the WHO guidelines for complementary feeding, which endorse routine consumption of either meats or micronutrient-fortified foods (9), the addition of 30 to 45 g meat provided several key micronutrients, including the so-called problem nutrients zinc and iron, in quantities similar to or less than those provided by the fortified cereal (5, 6). Animal-source proteins, both from meats (20) and from milk sources (11), have received renewed attention for their benefits to lean tissue accrual and linear growth, but education interventions demonstrating positive growth effects were in settings where the prevalence and severity of stunting were generally less than in the current study (12–14). Data from the pilot study in the same communities as in the current study indicated that <25% of the infants’ diets included meats, whereas >60% of the toddlers were reported to consume meats; the use of fortified products was notably uncommon, and the use of micronutrient supplements (vitamin A and iron) was highly variable (10). Thus, both of the study foods provided in the current intervention trial were likely to have improved the micronutrient intake of the participating infants and toddlers, but this improvement did not alter the overall trend of progressive growth faltering.
The prevalence (∼33%) and severity of stunting present at the initial assessment at 6 mo of age strongly suggests a potent effect of early determinants of growth faltering. The downward progression of LAZ scores through 18 mo and the failure of either intervention to alter that progression emphasize the difficulty of reversing stunting of such severity and early onset. Although the observed slope of the LAZ decline between 6 and 18 mo was similar to that reported for low-resource countries, the mean LAZ at 6 mo in our study was nearly a full SD below the mean at 6 mo for 54 countries reported by Victora et al (2). Without length measurements before 6 mo of age, we are unable to characterize the onset of linear growth faltering. Recent data from this area in Guatemala, where stunting at 6 mo is well documented (21, 22), indicate sharp growth deceleration between birth and 3 mo and a slower decline thereafter (23). The effect of early determinants is further substantiated by the significantly positive association between maternal height and linear growth over the interval of the current study. The observation that the LAZ at 6 mo was significantly negatively associated with overall linear growth velocity suggests potential responsiveness to improved dietary quality during the transition from exclusive or predominant breastfeeding to complementary feedings. Although the trend (P = 0.11) for greater linear growth velocity in the meat group between 6 and 12 mo is consistent with this possibility, the absence of a significant and sustained effect indicates that other factors prevailed.
The decline in WLZ over the course of the study, although relatively small, raises the possibility of energy insufficiency, especially to support catch-up growth. The food supplements were intentionally quite modest in their caloric contributions because the aim of the study was to specifically evaluate the potential benefit of a daily portion of meat to improve dietary quality, and the pilot data indicated very low rates of wasting in these communities (10). The energy provided by the food supplements was at the low end of the range of increased caloric intake associated with improved growth in studies of complementary feeding in the developing world (24).
Of the nonnutritional factors that were significantly associated with linear growth velocity, irrespective of treatment group, maternal education was strongly significant (P = 0.0006). This finding may represent a surrogate for better SES and/or other favorable practices, including hygiene practices and medical care, although SES was not a significant covariate in the reduced model. A significantly positive association between caregiver education and growth has been reported in several interventions to improve complementary feeding outcomes (8, 13, 14, 25). The association of male sex with slower linear growth velocity was also observed in the pilot study (10) and has been reported in other populations (26). This may represent the males’ greater vulnerability related to slightly more rapid growth (19) and/or to susceptibility to infectious disease morbidity. Significant differences in response to the intervention were observed among the sites; the study was not powered to evaluate outcomes by site. This finding further emphasizes the complexity and probable different origins of stunting in diverse settings, as was the situation in this study.
Although iron status was significantly more favorable for the cereal group, both anemia and iron-deficiency rates for both groups at 18 mo were notably lower than worldwide prevalence estimates and suggest a benefit of both food interventions on iron status. In particular, the anemia rate of <25% for both of the groups, with approximately two-thirds of the hemoglobin measurements from the African sites (171 and 178 for Democratic Republic of Congo and Zambia, respectively), compares very favorably with WHO anemia prevalence estimates of 67.6% for Africa (27). Data for iron deficiency are much more limited, but the prevalence is typically much higher than for iron deficiency anemia. The rates of low ferritin observed for both groups in this study are much lower than prevalence estimates for such settings (28) and are comparable with observed outcomes after home-fortification interventions with micronutrient powders (29, 30). Although the heme iron in meat would be expected to be highly bioavailable, the absorption would have needed to be ∼50% from this source alone to meet the estimated physiologic requirements of 0.69 mg/d for infancy and ∼30% to meet the requirement for toddlers (31). In contrast, absorption efficiency for the fortificant iron (ferrous sulfate) would have needed to be only <15% in both periods to meet the physiologic requirements (31). Thus, the greater effect observed for the cereal is plausible. Overall, however, the results suggest that both the daily meat and the cereal were relatively effective at maintaining or building iron stores in these very vulnerable populations.
The strengths of this study include its size and randomized controlled design and its fidelity in implementation according to the original study design. To our knowledge, this was the first study of the effect of a single animal-source food compared with that of a multiple micronutrient-fortified food on growth. The major limitation of the study was the lack of a true control group (ie, a group that received neither meat nor a micronutrient-fortified product), which precludes comparison of the growth pattern in the study infants to a contemporaneous group in the same communities. The progression of stunting rates from one-third at 6 mo to approximately half of the subjects at 18 mo is consistent with pilot data obtained from 12- to 24-mo-olds in these same communities, which showed overall stunting rates of 53%, with age positively associated with stunting (10, 32). Although reported compliance was very high, and days of missed food delivery were very low, we cannot guarantee that the foods were eaten every day by the index child. Anecdotal reports through the community health workers indicated, however, that mothers in both groups felt that these participating infants were growing and developing better than their older siblings, which suggested the families’ commitment to providing the study foods to the participants.
In conclusion, the findings of this study highlight the complexity of growth faltering in the first 2 y of life. The poor linear growth already evident at 6 mo and the lack of effect of either intervention to reverse this trend strongly argue for attention to earlier stages of both pre- and postnatal growth and for multifaceted interventions to prevent further linear growth failure in older infants and toddlers living in impoverished environments. Both food interventions seem likely to have improved iron status, with the fortified cereal having a more potent beneficial effect. However, meat from foraging and scavenging animals in rural communities and individual households may be more readily available and affordable than micronutrient-fortified products (10). With no indication that the latter are superior in reversing linear growth failure in older infants and toddlers, and with evidence suggesting that both foods improved iron status, regular consumption of meat at this critical stage of development should be encouraged over unfortified staples, consistent with the recommendations of the WHO.
Acknowledgments
The authors’ responsibilities were as follows—NFK, KMH, and MM: conceived of and designed the study, obtained funding, and designed the intervention and evaluation procedures; NFK: supervised the implementation of the study; NG, JW, and KMH: implemented the study; LLW, MK-T, AT, CLB, OP, RLG, EC, and WAC: participated in the design and implementation of the study, the intervention, and the evaluation procedures; and MK, NG, TDH, and EMM: participated in the study design, data management, and statistical analysis. The remaining authors participated in the design and implementation of the study. All authors read and approved the manuscript. None of the authors had any conflicts of interest.
Footnotes
Abbreviations used: GN, Global Network for Women's and Children's Health Research; LAZ, length-for-age z score; SES, socioeconomic status; WLZ, weight-for-length z score.
REFERENCES
- 1.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371(9608):243–60 [DOI] [PubMed] [Google Scholar]
- 2.Victora CG, de Onis M, Hallal PC, Blossner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics 2010;125(3):e473–80 [DOI] [PubMed] [Google Scholar]
- 3.Fenn B, Penny ME. Using the new World Health Organisation growth standards: differences from 3 countries. J Pediatr Gastroenterol Nutr 2008;46():316–21 [DOI] [PubMed] [Google Scholar]
- 4.Umeta M, West CE, Verhoef H, Haidar J, Hautvast JG. Factors associated with stunting in infants aged 5-11 months in the Dodota-Sire District, rural Ethiopia. J Nutr 2003;133:1064–9 [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization Complementary feeding of young children in developing countries: A review of current scientific knowledge. Geneva, Switzerland: World Health Organization, 1998 [Google Scholar]
- 6.Dewey KG, Brown KH. Update on technical issues concerning complementary feeding of young children in developing countries and implications for intervention programs. Food Nutr Bull 2003;24:5–28 [DOI] [PubMed] [Google Scholar]
- 7.Krebs NF, Hambidge KM. Complementary feeding: clinically relevant factors affecting timing and composition. Am J Clin Nutr 2007;85(2):639S–45S [DOI] [PubMed] [Google Scholar]
- 8.Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, Haider BA, Kirkwood B, Morris SS, Sachdev HP, et al. What works? Interventions for maternal and child undernutrition and survival. Lancet 2008;371(9610):417–40 [DOI] [PubMed] [Google Scholar]
- 9.PAHO/WHO. Guiding principles for complementary feeding of the breastfed child. Washington, DC: PAHO, WHO, 2003.
- 10.Krebs NF, Mazariegos M, Tshefu A, Bose C, Sami N, Chomba E, Carlo W, Goco N, Kindem M, Wright LL, et al. Meat consumption is associated with less stunting among toddlers in four diverse low-income settings. Food Nutr Bull 2011;32:185–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dror DK, Allen LH. The importance of milk and other animal-source foods for children in low-income countries. Food Nutr Bull 2011;32:227–43 [DOI] [PubMed] [Google Scholar]
- 12.Penny ME, Creed-Kanashiro HM, Robert RC, Narro MR, Caulfield LE, Black RE. Effectiveness of an educational intervention delivered through the health services to improve nutrition in young children: a cluster-randomised controlled trial. Lancet 2005;365(9474):1863–72 [DOI] [PubMed] [Google Scholar]
- 13.Guldan GS, Fan HC, Ma X, Ni ZZ, Xiang X, Tang MZ. Culturally appropriate nutrition education improves infant feeding and growth in rural Sichuan, China. J Nutr 2000;130:1204–11 [DOI] [PubMed] [Google Scholar]
- 14.Shi L, Zhang J, Wang Y, Caulfield LE, Guyer B. Effectiveness of an educational intervention on complementary feeding practices and growth in rural China: a cluster randomised controlled trial. Public Health Nutr 2010;13(4):556–65 [DOI] [PubMed] [Google Scholar]
- 15.Krebs NF, Hambidge KM, Mazariegos M, Westcott J, Goco N, Wright LL, Koso-Thomas M, Tshefu A, Bose C, Pasha O, et al. Complementary feeding: a Global Network cluster randomized controlled trial. BMC Pediatr 2011;11:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutter CK, Dewey KG. Proposed nutrient composition for fortified complementary foods. J Nutr 2003;133:3011S–20S [DOI] [PubMed] [Google Scholar]
- 17.Sawadogo PS, Martin-Prevel Y, Savy M, Kameli Y, Traissac P, Traore AS, Delpeuch F. An infant and child feeding index is associated with the nutritional status of 6- to 23-month-old children in rural Burkina Faso. J Nutr 2006;136(3):656–63 [DOI] [PubMed] [Google Scholar]
- 18.Ruel MT, Menon P. Child feeding practices are associated with child nutritional status in Latin America: innovative uses of the demographic and health surveys. J Nutr 2002;132:1180–7 [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization The WHO child growth standards. Geneva, Switzerland: World Health Organization, 2006 [Google Scholar]
- 20.Murphy SP, Allen LH. Nutritional importance of animal source foods. J Nutr 2003;133(suppl 2):3932S–5S [DOI] [PubMed] [Google Scholar]
- 21.Mazariegos M, Hambidge KM, Westcott JE, Solomons NW, Raboy V, Das A, Goco N, Kindem M, Wright LL, Krebs NF. Neither a zinc supplement nor phytate-reduced maize nor their combination enhance growth of 6- to 12-month-old Guatemalan infants. J Nutr 2010;140(5):1041–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rivera J, Ruel MT. Growth retardation starts in the first three months of life among rural Guatemalan children. Eur J Clin Nutr 1997;51:92–6 [DOI] [PubMed] [Google Scholar]
- 23.Hambidge KM, Bishop J, Berngard C, Garces A, Mazariegos M, Westcott J, Miller L, Kindem M, Wright L, Krebs NF. Covariates of early linear growth failure in indigenous Guatemalan infants. Boston, MA: PAS, 2012 [Google Scholar]
- 24.Caulfield L, Huffman SL, Piwoz EG. Interventions to improve intake of complementary foods by infants 6 to 12 months of age in developing countries: impact on growth and on the prevalence of malnutrition and potential contribution to child survival. Food Nutr Bull 1999;20:183–200 [Google Scholar]
- 25.Bhandari N, Bahl R, Nayyar B, Khokhar P, Rohde JE, Bhan MK. Food supplementation with encouragement to feed it to infants from 4 to 12 months of age has a small impact on weight gain. J Nutr 2001;131:1946–51 [DOI] [PubMed] [Google Scholar]
- 26.Saha KK, Frongillo EA, Alam DS, Arifeen SE, Persson LA, Rasmussen KM. Use of the new World Health Organization child growth standards to describe longitudinal growth of breastfed rural Bangladeshi infants and young children. Food Nutr Bull 2009;30:137–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr 2009;12:444–54 [DOI] [PubMed] [Google Scholar]
- 28.Cameron BM, Neufeld LM. Estimating the prevalence of iron deficiency in the first two years of life: technical and measurement issues. Nutr Rev 2011;69(suppl 1):S49–56 [DOI] [PubMed] [Google Scholar]
- 29.Zlotkin S, Arthur P, Schauer C, Antwi KY, Yeung G, Piekarz A. Home-fortification with iron and zinc sprinkles or iron sprinkles alone successfully treats anemia in infants and young children. J Nutr 2003;133:1075–80 [DOI] [PubMed] [Google Scholar]
- 30.Suchdev PS, Ruth LJ, Woodruff BA, Mbakaya C, Mandava U, Flores-Ayala R, Jefferds ME, Quick R. Selling Sprinkles micronutrient powder reduces anemia, iron deficiency, and vitamin A deficiency in young children in Western Kenya: a cluster-randomized controlled trial. Am J Clin Nutr 2012;95:1223–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Food and Nutrition Board, Institute of Medicine Dietary Reference Intakes for vitamin A, vitamin K, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. Washington, DC: National Academy Press, 2001 [PubMed] [Google Scholar]
- 32.Phuka JC, Maleta K, Thakwalakwa C, Cheung YB, Briend A, Manary MJ, Ashorn P. Postintervention growth of Malawian children who received 12-mo dietary complementation with a lipid-based nutrient supplement or maize-soy flour. Am J Clin Nutr 2009;89(1):382–90 [DOI] [PubMed] [Google Scholar]