Abstract
Background: The apolipoprotein A5 gene (APOA5) is a major gene that regulates lipid metabolism and is modulated by dietary factors. A novel variant rs964184 in APOA5 was identified to be associated with lipids in genome-wide association studies.
Objective: We examined whether this variant modified changes in lipid concentrations in response to a 2-y weight-loss diet intervention in a randomized trial.
Design: The current analyses were secondary analyses of a data set from the Pounds Lost Trial. We genotyped APOA5 rs964184 in 734 overweight or obese adults who were randomly assigned to one of 4 diets that differed in percentages of energy derived from fat, protein, and carbohydrate for 2 y. We evaluated changes in fasting serum concentrations of total cholesterol (TC), LDL cholesterol, HDL cholesterol, and triglyceride from baseline to 2 y of follow-up.
Results: After a 2-y dietary intervention, we showed significant interactions between the APOA5 rs964184 polymorphism and dietary fat intake (low compared with high) in the determination of changes in TC, LDL cholesterol, and HDL cholesterol (P-interaction = 0.007, 0.017, and 0.006, respectively). In the low-fat intake group (20% of energy derived from fat), carriers of the risk allele (G allele) exhibited greater reductions in TC and LDL cholesterol than did noncarriers (P = 0.036 and 0.039, respectively), whereas in the high-fat diet group (40% of energy derived from fat), participants with the G allele had a greater increase in HDL cholesterol than did participants without this allele (P = 0.038).
Conclusion: Our data showed better improvement in lipid profiles from long-term low-fat diet intake in the APOA5 rs964184 risk allele. The Pounds Lost Trial was registered at clinicaltrials.gov as NCT00072995.
INTRODUCTION
Unfavorable blood lipid concentrations, including high triglyceride, total cholesterol (TC), and LDL cholesterol and low HDL cholesterol, have been associated with increased risk of cardiovascular disease (1–3). Lipid profiles are determined by interactions between genetic and environmental factors, such as diet and lifestyle (4). Genetic variants in the apolipoprotein A5 gene (APOA5), which are located in the APOA1-APOC3-APOA4-APOA5 gene cluster on human chromosome 11q23, are widely studied in relation to lipid profiles in candidate gene studies (5–8). In addition, observational studies and short-term (<3 mo) intervention trials have shown that several common variants in the APOA5 gene interact with dietary factors, especially dietary fat, in the determination of blood lipid concentrations (5, 9–17). However, gene-diet interactions in long-term randomized intervention settings have been rarely explored.
In genome-wide association studies, a novel variant rs964184 in the APOA5 locus was identified to be associated with increased triglyceride, TC, and LDL cholesterol and decreased HDL cholesterol for the risk allele (18–21). Furthermore, this variant showed the strongest associations with serum triglyceride concentrations in a dyslipidemic population (22). rs964184 resides 11 kb upstream of APOA5 and in the 5′ untranslated region of a zinc finger protein, which may be involved in signal transduction and have multiple physiologic functions (22). However, to our knowledge, no study has examined the effect of rs964184 on protein function or availability. In the current study, we aimed to investigate whether the APOA5 rs964184 genotype may interact with weight-loss diets that vary in macronutrients on 2-y changes in lipid concentrations in the randomized intervention study the Pounds Lost Trial (www.clinicaltrials.gov; NCT00072995).
SUBJECTS AND METHODS
Study population
The Pounds Lost Trial was conducted from October 2004 through December 2007 at the following 2 sites: the Harvard School of Public Health and Brigham & Women's Hospital (Boston, MA) and the Pennington Biomedical Research Center of the Louisiana State University System (Baton Rouge, LA). The study design and sample collection have been previously described in detail (23). Briefly, the study population was composed of 811 overweight or obese participants who were aged 30–70 y and had a BMI [in kg/m2 (weight in kilograms divided by the square of the height in meters)] of 25–40. Major criteria for exclusion were the presence of diabetes treated with oral medications or insulin, unstable cardiovascular disease, the use of medications that affect body weight, and insufficient motivation as assessed by an interview and questionnaire. Individuals with type 2 diabetes controlled with diet or with hypertension or hyperlipidemia treated with diet or drugs were eligible to participate. Participants were randomly assigned to one of 4 diets that constituted a 2-by-2 factorial design; target percentages of energy derived from fat, protein, and carbohydrate in the 4 diets were 20%, 15%, and 65%, respectively; 20%, 25%, and 55%, respectively; 40%, 15%, and 45%, respectively; and 40%, 25%, and 35%, respectively. After 2 y, 645 participants (80% of the total population) completed the trial. The study was approved by the human subjects committee at each institution and by a data and safety monitoring board appointed by the National Heart, Lung, and Blood Institute. All participants provided written informed consent.
The current analyses were secondary analyses of a data set obtained from the Pounds Lost Trial, which was not designed for testing the hypothesis in the current analysis. A total of 734 participants with APOA5 rs964184 genotype data available were included in the current study (90.5% of participants in the Pounds Lost Trial). Consistent with the entire Pounds Lost Trial, the mean (±SD) age of participants in the current analysis was 50.9 ± 9.2 y, and the mean BMI (±SD) was 32.7 ± 3.9 kg/m2. There was no significant difference in basic characteristics between participants with and without APOA5 rs964184 genotype data.
Measurements
Body weight and waist circumference were measured in the morning before breakfast on 2 d at baseline, 6 mo, and 2 y. Dietary intake was assessed in a random sample of 50% of participants by a review of the 5-d diet record at baseline and by a 24-h recall during a telephone interview on 3 nonconsecutive days at 6 mo and 2 y. Fasting blood samples, 24-h urine samples, and measurement of the resting metabolic rate were obtained on 1 d, and blood pressure was measured on 2 d at baseline, 6 mo, and 2 y. Analyses of serum lipids, glucose, and urinary nitrogen were performed at the Clinical Laboratory at Pennington Biomedical Research Center of the Louisiana State University System. The respiratory quotient was obtained by using the DeltaTrac II metabolic cart (Datex-Ohmeda) (23). Triglyceride, TC, LDL cholesterol, and HDL cholesterol were measured by using the Beckman Synchron CX7 analyzer (Beckman Coulter). LDL cholesterol was calculated for each participant according to the following formula (24):
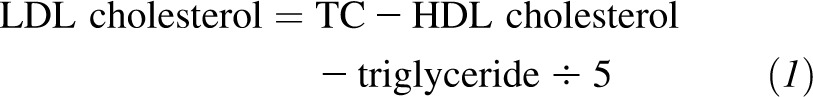 |
except when the triglyceride concentration was >400 mg/dL, in which case LDL cholesterol was measured directly on all samples of the participant.
Genotyping
As indicated in the Introduction, we selected APOA5 single nucleotide polymorphism (SNP) rs964184 because it was identified to be associated with multiple lipid traits (18–21), and previous evidence has shown potential interactions between this locus and dietary intakes, especially of fat in relation to lipids (11, 14, 16, 25). With consideration of genotyping cost, we focused on this specific variant rather than all of the reported lipid-associated SNPs. DNA was extracted from the buffy coat fraction of centrifuged blood by using the QIAmp Blood Kit (Qiagen). APOA5 rs964184 was genotyped successfully in 734 total participants, including 587 white subjects, with DNA samples made available by using the OpenArray SNP Genotyping System (BioTrove). The genotype success rate was 99%. Replicated quality-control samples (10%) were included in every genotyping plate with >99% concordance (26). The genotype distribution was in Hardy-Weinberg equilibrium in all participants or in white subjects (P > 0.05).
Statistical analysis
The primary outcomes were 2-y changes in blood lipids. Because APOA5 is sensitive to dietary fat ingestion (17, 27–30), we compared low-fat (20%) with high-fat (40%) diets in the primary analysis and compared average-protein (15%) with high-protein (25%) diets in the secondary analysis. Triglyceride concentrations were log transformed to normalize the distribution of the data. The Hardy-Weinberg equilibrium and comparison of categorical variables were assessed by using the chi-square test. Differences in continuous variables at baseline by genotypes were tested by using general linear models, with adjustment for age, sex, and ethnicity. The main effects of genotype and diet intervention on 2-y changes of lipids were analyzed by using multivariate linear regression models. Moreover, to analyze potential interactions between genotype and diet intervention, an interaction product term was included in the model. Appropriate adjustments, such as age, sex, ethnicity, the baseline value for the respective outcome, baseline BMI, lipid-lowing medication use, and weight loss, were used in the analysis. With the use of time as a repeated variable, linear mixed models were applied to test genetic associations with the trajectory of changes in outcomes according to diet intervention during the 2 y of follow-up by including genotype-time interaction terms. Additive genetic models were used in the analyses. A partial Pearson's correlation was computed to examine the relation between weight loss and changes in lipid profiles. Because the majority of the study population was white (80%), similar analyses were repeated in white participants. All reported P values were 2-sided, and a P = 0.05 was considered statistically significant. All data were analyzed with SAS version 9.1 software (SAS Institute Inc).
RESULTS
Characteristics of study population
Baseline characteristics of participants according to the APOA5 rs964184 genotype are shown in Table 1. Genotype frequencies were similar in men and women but differed by ethnicity (P < 0.0001). HDL cholesterol and triglyceride were significantly different across the genotype after adjustment for age, sex, and ethnicity, and the G allele of the rs964184 was associated with lower HDL-cholesterol and higher triglyceride concentrations; whereas other variables such as weight, BMI, TC, LDL cholesterol, dietary intake, and biomarkers of adherence (urinary nitrogen and respiratory quotient) were not associated with genotype at baseline. In addition, we did not find any significant difference in weight loss at 6 mo and ∼2 y across the rs964184 genotype (all P > 0.05).
TABLE 1.
Baseline characteristics of the study participants according to APOA5 rs964184 genotypes1
| CC (n = 526) | CG (n = 194) | GG (n = 14) | P | |
| Age (y) | 51.0 ± 9.32 | 50.6 ± 9.1 | 53.4 ± 6.7 | 0.384 |
| Sex [n (%)] | 0.687 | |||
| F | 323 (71.9) | 119 (21.5) | 7 (1.6) | |
| M | 203 (71.2) | 75 (26.3) | 7 (2.5) | |
| Race or ethnicity [n (%)] | <0.0001 | |||
| White | 439 (74.8) | 134 (22.8) | 14 (2.4) | |
| Black | 73 (65.8) | 38 (34.2) | 0 (0.0) | |
| Hispanic or other | 14 (38.9) | 22 (61.1) | 0 (0.0) | |
| Weight (kg) | 93.4 ± 15.6 | 93.4 ± 15.4 | 91.4 ± 15.9 | 0.669 |
| BMI (kg/m2) | 32.7 ± 3.9 | 32.9 ± 3.8 | 31.4 ± 3.9 | 0.941 |
| Waist circumference (cm) | 103.8 ± 13.2 | 103.6 ± 12.8 | 101.3 ± 11.6 | 0.471 |
| Glucose (mg/dL) | 91.8 ± 11.1 | 92.3 ± 13.9 | 90.4 ± 7.4 | 0.977 |
| SBP (mm Hg) | 119.4 ± 13.1 | 119.9 ± 14.5 | 122.0 ± 11.0 | 0.674 |
| DBP (mm Hg) | 75.4 ± 9.3 | 76.2 ± 9.5 | 77.1 ± 9.9 | 0.253 |
| TC (mg/dL) | 201.5 ± 36.7 | 204.9 ± 37.5 | 204.2 ± 39.3 | 0.131 |
| HDL cholesterol (mg/dL) | 49.6 ± 14.3 | 46.9 ± 13.8 | 46.5 ± 11.3 | 0.028 |
| LDL cholesterol (mg/dL) | 125.3 ± 31.1 | 126.7 ± 34.0 | 127.3 ± 39.5 | 0.380 |
| Triglyceride (mg/dL)3 | 136.9 ± 85.5 | 160.4 ± 84.6 | 152.3 ± 78.0 | 0.002 |
| Dietary intake per day | ||||
| Energy (kcal) | 1806 ± 790 | 1742 ± 833 | 2187 ± 965 | 0.949 |
| Carbohydrate (%) | 41 ± 8 | 41 ± 7 | 39 ± 6 | 0.189 |
| Fat (%) | 39 ± 8 | 40 ± 7 | 41 ± 7 | 0.090 |
| Protein (%) | 18 ± 3 | 18 ± 3 | 18 ± 2 | 0.160 |
| Dietary fiber (g) | 12.9 ± 6.3 | 12.1 ± 5.9 | 14.5 ± 6.1 | 0.309 |
| Saturated fat (%) | 13.8 ± 3.2 | 13.8 ± 3.0 | 14.9 ± 3.0 | 0.283 |
| Biomarkers of adherence | ||||
| Urinary nitrogen (g) | 12.1 ± 4.4 | 12.3 ± 4.3 | 13.1 ± 4.6 | 0.448 |
| Respiratory quotient | 0.84 ± 0.04 | 0.84 ± 0.04 | 0.83 ± 0.04 | 0.054 |
| Weight loss at 6 mo (kg) | −6.9 ± 5.8 | −5.8 ± 5.4 | −8.7 ± 5.7 | 0.351 |
| Weight loss at 2 y (kg) | −4.3 ± 7.7 | −3.0 ± 6.9 | −7.6 ± 8.2 | 0.768 |
P values were calculated by using the chi-square test for categorical variables and general linear models for continuous variables after adjustment for age, sex, and ethnicity. DBP, diastolic blood pressure; SBP, systolic blood pressure; TC, total cholesterol.
Mean ± SD (all such values).
Log transformed before analysis.
Interactions between APOA5 rs964184 genotype and dietary fat on 2-y changes in lipids
No significant gene-diet interaction on weight loss was shown during the intervention (data not shown). At 2 y, after adjustment for age, sex, ethnicity, baseline BMI, baseline values for respective outcomes, and lipid-lowing medication use, we showed significant interactions between the APOA5 rs964184 genotype and dietary fat intervention (low compared with high) on changes in TC and LDL cholesterol (P-interaction = 0.007 and 0.017, respectively). In the low-fat diet group, participants with the risk allele (G allele) had greater reductions in TC and LDL-cholesterol concentrations than did noncarriers (β ± SE = −9.3 ± 4.4 for TC and −7.5 ± 3.6 for LDL cholesterol; P = 0.036 and 0.039, respectively) (Figure 1), but the genetic variant was not related to changes in TC and LDL cholesterol in the high-fat diet group. Because changes in TC and LDL cholesterol were highly correlated in study samples (r2 ∼ 0.90; P < 0.0001), we further assessed the independence of the genetic effects on these 2 markers. We showed that the genetic effects on change in TC were attenuated to be nonsignificant after additional adjustment for LDL cholesterol in the model (P > 0.1).
FIGURE 1.
Effects of APOA5 rs964184 genotype and fat intervention on mean (±SE) changes in lipid profile at 2 y. Data included 219 and 176 (CC), 50 and 77 (CG), and 3 and 9 (GG) participants in low- and high-fat groups, respectively, at 2 y (total n = 534). P values were calculated by using general linear models with adjustment for age, sex, ethnicity, baseline BMI, baseline values for respective outcomes, and lipid-lowing medication use. HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; TC, total cholesterol; TG, triglyceride.
In addition, we also observed the gene-diet interaction on the change in HDL cholesterol (P-interaction = 0.006), and carriers of risk allele had a greater increase in HDL cholesterol response to the high-fat diet (β ± SE = 1.7 ± 0.8; P = 0.038). We did not find significant interaction between diet fat and the APOA5 rs964184 genotype in relation to changes in serum concentrations of triglyceride (Figure 1). Additional adjustment for weight loss did not substantially change the results.
We did not find significant interactions between the APOA5 rs964184 genotype and protein intake on changes in lipid concentrations (all P > 0.05). Similar interactions were observed when the analysis was restricted to the white participants.
Trajectory of changes in lipids by the APOA5 rs964184 genotype
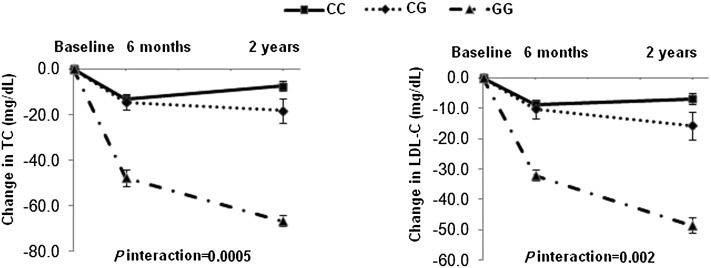
We further examined the dynamic pattern of changes in lipid by APOA5 genotypes through the 2-y intervention period. We observed significant genotype-time interactions on changes in TC and LDL cholesterol in the low-fat diet group (P-interaction = 0.0005 and 0.002, respectively). Differences in reductions of TC and LDL cholesterol by APOA5 genotypes displayed a continued, cumulative pattern throughout the intervention and reached maximum values at 2 y (Figure 2). A similar pattern was shown in the white population.
FIGURE 2.
Changes in TC and LDL-C in the low-fat diet intervention group according to APOA5 rs964184 genotype from baseline to 6 mo and 2 y under intervention. Data included 283, 250, and 219 (CC); 81, 65, and 50 (CG); and 4, 3, and 3 (GG) participants at baseline, 6 mo, and 2 y, respectively. P-interaction was tested by using linear mixed models between genotype and intervention time after adjustment for age, sex, ethnicity, baseline BMI, baseline values for respective outcomes, and lipid-lowing medication use. LDL-C, LDL cholesterol; TC, total cholesterol.
DISCUSSION
In this 2-y randomized, weight-loss intervention trial, we observed a significant interaction between the APOA5 rs964184 polymorphism and dietary fat intake in relation to changes in TC, LDL-cholesterol, and HDL-cholesterol concentrations. Compared with noncarriers, individuals with the G allele exhibited a greater reduction in TC and LDL-cholesterol responses to a low-fat diet but had a greater increase in HDL cholesterol when assigned a high-fat diet. These findings suggest that the genetic variation of APOA5 may modulate the effect of long-term dietary fat intake on blood cholesterol concentrations.
Evidence for an APOA5-diet interaction on lipids in previous studies was focused on the 2 most common tag SNPs T-1131>C (rs662799) and Ser19>Trp (rs3135506) (11, 14, 16, 25), which are related to translation efficiency or gene expression, that contribute to the production and function of apolipoprotein AV (31–33). The Ser19>Trp variant in the APOA5 gene was shown to modulate changes in plasma TC in men with a low-fat and -cholesterol and high-fruit and -vegetable diet for 8 y (11, 25), which is in partial agreement with our results. However, several short-term trials generated inconsistent results regarding APOA5-diet fat interactions on cholesterol (14, 16). In a 3-mo intervention trial with a low-fat diet, reductions of TC and LDL cholesterol concentrations were not associated with the T-1131>C polymorphism in hyperlipemic and overweight individuals (n = 606) (14). Conversely, in another study, APOA5 variants (Ser19>Trp and T-1131>C) had a decrease in plasma LDL cholesterol concentrations in 98 overweight and obese women with 9 wk of lifestyle modification that consisted of a reduction of fat and cholesterol intakes and physical activity changes (16). In the current study, we showed that the genotype difference of TC and LDC cholesterol in response to a low-fat diet showed a continued, cumulative pattern throughout the intervention and reached maximum values at 2 y. These findings suggest that the gene-diet interactions on the changes of cholesterol concentrations are more likely to be detected after a long-term intervention. In addition, it is known that about two-thirds of circulating TC is attributable to LDL cholesterol (34). After additional adjustment for the change in LDL cholesterol, the genetic effect on the change in TC was abolished, suggesting that the effect of the variant on change in TC might be mainly driven by change in LDL cholesterol.
In this study, a significant gene-diet interaction was observed in the change in HDL cholesterol. Different from TC and LDL cholesterol, the changes in HDL cholesterol were more likely to be modulated by the high-fat diet in which individuals carrying the risk allele had a greater increase in HDL cholesterol. Several previous studies have shown that changes in HDL cholesterol concentrations in response to high-fat diets varied across different samples (35–38). Our data suggest that APOA5 genotype may partly account for the interindividual heterogeneity in high-fat-diet induced HDL cholesterol changes, although the underlying mechanisms remain to be determined.
Although a large number of studies have shown strong relation between APOA5 genetic variants and triglyceride concentrations (19, 20, 39–43), the genetic effects on long-term change in triglyceride were NS and not modified by dietary intakes in our study. Of note, a similar result was also shown in an 8-y follow-up study (11, 25) in which changes in plasma triglyceride concentrations in response to dietary composition changes were not affected by APOA5 genetic variation. In contrast, several short-term studies observed that genetic variants at the APOA5 locus could affect the triglyceride concentration response to a dietary intervention. For example, 2 trials in small samples showed that APOA5 −1131 C-allele carriers had higher triglyceride concentrations in response to a 6-d high-carbohydrate and low-fat diet and high-fat experimental meal (12, 27). In contrast, Suchanek et al (16) showed that the reduction of triglyceride concentrations by the 3-mo low-fat intervention was not associated with the APOA5 T-1131>C. The reasons for the differential genetic effects on various lipid components are not clear. rs964184 is independent (22) and is not strongly correlated with T-1131>C and Ser19>Trp (linkage disequilibrium r2 = 0.339 and 0.246, respectively). It is currently unknown whether rs964184 is a functional variant or a marker in linkage disequilibrium with other possible functional variants that causally affect lipid homeostasis. Our results suggest that some serum lipid variables were partly affected by the interactions of the APOA5 genetic variant and fat intake; therefore, we assume that different mechanisms might drive the observed gene-diet interactions for various lipid phenotypes.
In this study, we did not find parallel gene-diet interactions on weight loss and changes in lipids profiles. After additional adjustment for weight loss, the interaction and genetic effect had no substantial change on lipid profiles. The findings suggest that these effects on changes in lipid profiles might be independent of weight loss, and weight loss was not involved in the genotype-lipid interaction. Although weight loss was shown to have long-term beneficial effects on lipids, especially on LDL and TC concentrations (44), in our study samples and different subgroups, weight loss was significantly correlated only with triglyceride and HDL cholesterol and not with TC and LDL cholesterol at the 2-y intervention. Thus, to some extent, it is not surprising that we did not observe a statistically meaningful effect of weight loss on interaction and genetic effects.
This study had several limitations. First, although our study was, to our knowledge, the largest and longest diet intervention trial in which the genetic effects on changes in lipid profiles were tested, the analyses were based on secondary analyses, and the sample size in certain genotype such as GG was relatively small. An original design and larger samples are required to confirm our findings. Second, our analyses were restricted to only the APOA5 rs964184 variant; however, as indicated, the selected rs964184 has been most consistently reported in several genome-wide association studies and is related to all of the lipid traits (18–21). Third, we did not adjust for multiple testing because of a high correlation between outcomes and the repeated measurements. Overadjustment for multiple comparisons may increase the type II error, which reduces the power to detect significant differences. Furthermore, because the participants were all overweight or obese, our results may not be generalized to the general population with a normal range of body weight. Finally, because the majority of our study samples were from white participants, and all of the 14 homozygous variant cases were white, future studies are warranted to verify the gene-diet interactions in other minority groups.
In conclusion, we showed that APOA5 polymorphism might modify dietary fat–induced changes of lipid profiles, especially of LDL cholesterol and TC, in overweight or obese subjects. These findings may provide information to the development of an effective diet intervention in prevention and treatment of obesity-related disorders. However, these findings need to be verified in future intervention studies with large sample sizes.
Acknowledgments
We are particularly grateful to all of the participants in the trial for their dedication and contribution to the research.
The authors’ responsibilities were as follows—XZ, FMS, and LQ: designed the research; XZ, QQ, and LQ: conducted the research; XZ and QQ: analyzed data; XZ and LQ: wrote the manuscript; GAB, FBH, and FMS: contributed to providing materials and critical revision of the manuscript; LQ: had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript. None of the authors had a conflict of interest.
REFERENCES
- 1.Robitaille J, Brouillette C, Lemieux S, Perusse L, Gaudet D, Vohl MC. Plasma concentrations of apolipoprotein B are modulated by a gene–diet interaction effect between the LFABP T94A polymorphism and dietary fat intake in French-Canadian men. Mol Genet Metab 2004;82:296–303 [DOI] [PubMed] [Google Scholar]
- 2.Gotto AM, Jr, Brinton EA. Assessing low levels of high-density lipoprotein cholesterol as a risk factor in coronary heart disease: a working group report and update. J Am Coll Cardiol 2004;43:717–24 [DOI] [PubMed] [Google Scholar]
- 3.Sandhu MS, Waterworth DM, Debenham SL, Wheeler E, Papadakis K, Zhao JH, Song K, Yuan X, Johnson T, Ashford S, et al. LDL-cholesterol concentrations: a genome-wide association study. Lancet 2008;371:483–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ordovas JM. Gene-diet interaction and plasma lipid responses to dietary intervention. Biochem Soc Trans 2002;30:68–73 [DOI] [PubMed] [Google Scholar]
- 5.Lai CQ, Demissie S, Cupples LA, Zhu Y, Adiconis X, Parnell LD, Corella D, Ordovas JM. Influence of the APOA5 locus on plasma triglyceride, lipoprotein subclasses, and CVD risk in the Framingham Heart Study. J Lipid Res 2004;45:2096–105 [DOI] [PubMed] [Google Scholar]
- 6.Lai CQ, Tai ES, Tan CE, Cutter J, Chew SK, Zhu YP, Adiconis X, Ordovas JM. The APOA5 locus is a strong determinant of plasma triglyceride concentrations across ethnic groups in Singapore. J Lipid Res 2003;44:2365–73 [DOI] [PubMed] [Google Scholar]
- 7.Qi L, Liu S, Rifai N, Hunter D, Hu FB. Associations of the apolipoprotein A1/C3/A4/A5 gene cluster with triglyceride and HDL cholesterol levels in women with type 2 diabetes. Atherosclerosis 2007;192:204–10 [DOI] [PubMed] [Google Scholar]
- 8.Austin MA, Talmud PJ, Farin FM, Nickerson DA, Edwards KL, Leonetti D, McNeely MJ, Viernes HM, Humphries SE, Fujimoto WY. Association of apolipoprotein A5 variants with LDL particle size and triglyceride in Japanese Americans. Biochim Biophys Acta 2004;1688:1–9 [DOI] [PubMed] [Google Scholar]
- 9.Dvoráková-Lorenzová A, Suchanek P, Havel PJ, Stavek P, Karasova L, Valenta Z, Tintera J, Poledne R. The decrease in C-reactive protein concentration after diet and physical activity induced weight reduction is associated with changes in plasma lipids, but not interleukin-6 or adiponectin. Metabolism 2006;55:359–65 [DOI] [PubMed] [Google Scholar]
- 10.Evans D, Seedorf U, Beil FU. Polymorphisms in the apolipoprotein A5 (APOA5) gene and type III hyperlipidemia. Clin Genet 2005;68:369–72 [DOI] [PubMed] [Google Scholar]
- 11.Hubacek JA, Bohuslavova R, Skodova Z, Pitha J, Bobkova D, Poledne R. Polymorphisms in the APOA1/C3/A4/A5 gene cluster and cholesterol responsiveness to dietary change. Clin Chem Lab Med 2007;45:316–20 [DOI] [PubMed] [Google Scholar]
- 12.Lin J, Fang DZ, Du J, Shigdar S, Xiao LY, Zhou XD, Duan W. Elevated levels of triglyceride and triglyceride-rich lipoprotein triglyceride induced by a high-carbohydrate diet is associated with polymorphisms of APOA5-1131T>C and APOC3-482C>T in Chinese healthy young adults. Ann Nutr Metab 2011;58:150–7 [DOI] [PubMed] [Google Scholar]
- 13.Havasi V, Szolnoki Z, Talian G, Bene J, Komlosi K, Maasz A, Somogyvari F, Kondacs A, Szabo M, Fodor L, et al. Apolipoprotein A5 gene promoter region T-1131C polymorphism associates with elevated circulating triglyceride levels and confers susceptibility for development of ischemic stroke. J Mol Neurosci 2006;29:177–83 [DOI] [PubMed] [Google Scholar]
- 14.Aberle J, Evans D, Beil FU, Seedorf U. A polymorphism in the apolipoprotein A5 gene is associated with weight loss after short-term diet. Clin Genet 2005;68:152–4 [DOI] [PubMed] [Google Scholar]
- 15.Yu Y, Xue L, Zhao CY. Study on polymorphism in the apolipoprotein A5 gene in patients with premature coronary heart disease Beijing Da Xue Xue Bao 2007;39:576–80 (in Chinese) [PubMed] [Google Scholar]
- 16.Suchanek P, Lorenzova A, Poledne R, Hubacek JA. Changes of plasma lipids during weight reduction in females depends on APOA5 variants. Ann Nutr Metab 2008;53:104–8 [DOI] [PubMed] [Google Scholar]
- 17.Sánchez-Moreno C, Ordovas JM, Smith CE, Baraza JC, Lee YC, Garaulet M. APOA5 gene variation interacts with dietary fat intake to modulate obesity and circulating triglycerides in a Mediterranean population. J Nutr 2011;141:380–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 2010;466:707–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willer CJ, Sanna S, Jackson AU, Scuteri A, Bonnycastle LL, Clarke R, Heath SC, Timpson NJ, Najjar SS, Stringham HM, et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet 2008;40:161–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kathiresan S, Willer CJ, Peloso GM, Demissie S, Musunuru K, Schadt EE, Kaplan L, Bennett D, Li Y, Tanaka T, et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet 2009;41:56–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schunkert H, Konig IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, Preuss M, Stewart AF, Barbalic M, Gieger C, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet 2011;43:333–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Investigation of variants identified in caucasian genome-wide association studies for plasma high-density lipoprotein cholesterol and triglycerides levels in Mexican dyslipidemic study samples. Circ Cardiovasc Genet 2010;3:31–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo N, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 2009;360:859–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502 [PubMed] [Google Scholar]
- 25.Hubacek JA, Skodova Z, Adamkova V, Lanska V, Pitha J. APOA5 variant Ser19Trp influences a decrease of the total cholesterol in a male 8 year cohort. Clin Biochem 2006;39:133–6 [DOI] [PubMed] [Google Scholar]
- 26.Qi Q, Bray GA, Smith SR, Hu FB, Sacks FM, Qi L. Insulin receptor substrate 1 gene variation modifies insulin resistance response to weight-loss diets in a 2-year randomized trial: the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Circulation 2011;124:563–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JY, Kim OY, Koh SJ, Jang Y, Yun SS, Ordovas JM, Lee JH. Comparison of low-fat meal and high-fat meal on postprandial lipemic response in non-obese men according to the -1131T>C polymorphism of the apolipoprotein A5 (APOA5) gene (randomized cross-over design). J Am Coll Nutr 2006;25:340–7 [DOI] [PubMed] [Google Scholar]
- 28.Corella D, Lai CQ, Demissie S, Cupples LA, Manning AK, Tucker KL, Ordovas JM. APOA5 gene variation modulates the effects of dietary fat intake on body mass index and obesity risk in the Framingham Heart Study. J Mol Med (Berl) 2007;85:119–28 [DOI] [PubMed] [Google Scholar]
- 29.Mattei J, Demissie S, Tucker KL, Ordovas JM. Apolipoprotein A5 polymorphisms interact with total dietary fat intake in association with markers of metabolic syndrome in Puerto Rican older adults. J Nutr 2009;139:2301–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai CQ, Corella D, Demissie S, Cupples LA, Adiconis X, Zhu Y, Parnell LD, Tucker KL, Ordovas JM. Dietary intake of n−6 fatty acids modulates effect of apolipoprotein A5 gene on plasma fasting triglycerides, remnant lipoprotein concentrations, and lipoprotein particle size: the Framingham Heart Study. Circulation 2006;113:2062–70 [DOI] [PubMed] [Google Scholar]
- 31.Talmud PJ, Palmen J, Putt W, Lins L, Humphries SE. Determination of the functionality of common APOA5 polymorphisms. J Biol Chem 2005;280:28215–20 [DOI] [PubMed] [Google Scholar]
- 32.Merkel M, Loeffler B, Kluger M, Fabig N, Geppert G, Pennacchio LA, Laatsch A, Heeren J. Apolipoprotein AV accelerates plasma hydrolysis of triglyceride-rich lipoproteins by interaction with proteoglycan-bound lipoprotein lipase. J Biol Chem 2005;280:21553–60 [DOI] [PubMed] [Google Scholar]
- 33.Talmud PJ, Hawe E, Martin S, Olivier M, Miller GJ, Rubin EM, Pennacchio LA, Humphries SE. Relative contribution of variation within the APOC3/A4/A5 gene cluster in determining plasma triglycerides. Hum Mol Genet 2002;11:3039–46 [DOI] [PubMed] [Google Scholar]
- 34.Gilmore LA, Walzem RL, Crouse SF, Smith DR, Adams TH, Vaidyanathan V, Cao X, Smith SB. Consumption of high-oleic acid ground beef increases HDL-cholesterol concentration but both high- and low-oleic acid ground beef decrease HDL particle diameter in normocholesterolemic men. J Nutr 2011;141:1188–94 [DOI] [PubMed] [Google Scholar]
- 35.Jackson RL, Yates MT, McNerney CA, Kashyap ML. Diet and HDL metabolism: high carbohydrate vs. high fat diets. Adv Exp Med Biol 1987;210:165–72 [DOI] [PubMed] [Google Scholar]
- 36.Wolf G. High-fat, high-cholesterol diet raises plasma HDL cholesterol: studies on the mechanism of this effect. Nutr Rev 1996;54:34–5 [DOI] [PubMed] [Google Scholar]
- 37.Wojczynski MK, Glasser SP, Oberman A, Kabagambe EK, Hopkins PN, Tsai MY, Straka RJ, Ordovas JM, Arnett DK. High-fat meal effect on LDL, HDL, and VLDL particle size and number in the Genetics of Lipid-Lowering Drugs and Diet Network (GOLDN): an interventional study. Lipids Health Dis 2011;10:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dallongeville J, Gruson E, Dallinga-Thie G, Pigeyre M, Gomila S, Romon M. Effect of weight loss on the postprandial response to high-fat and high-carbohydrate meals in obese women. Eur J Clin Nutr 2007;61:711–8 [DOI] [PubMed] [Google Scholar]
- 39.Charlton-Menys V, Durrington PN. Apolipoprotein A5 and hypertriglyceridemia. Clin Chem 2005;51:295–7 [DOI] [PubMed] [Google Scholar]
- 40.Hubacek JA. Apolipoprotein A5 and triglyceridemia. Focus on the effects of the common variants. Clin Chem Lab Med 2005;43:897–902 [DOI] [PubMed] [Google Scholar]
- 41.Pennacchio LA, Rubin EM. Apolipoprotein A5, a newly identified gene that affects plasma triglyceride levels in humans and mice. Arterioscler Thromb Vasc Biol 2003;23:529–34 [DOI] [PubMed] [Google Scholar]
- 42.Fruchart-Najib J, Bauge E, Niculescu LS, Pham T, Thomas B, Rommens C, Majd Z, Brewer B, Pennacchio LA, Fruchart JC. Mechanism of triglyceride lowering in mice expressing human apolipoprotein A5. Biochem Biophys Res Commun 2004;319:397–404 [DOI] [PubMed] [Google Scholar]
- 43.Pennacchio LA, Olivier M, Hubacek JA, Cohen JC, Cox DR, Fruchart JC, Krauss RM, Rubin EM. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science 2001;294:169–73 [DOI] [PubMed] [Google Scholar]
- 44.Poobalan A, Aucott L, Smith WC, Avenell A, Jung R, Broom J, Grant AM. Effects of weight loss in overweight/obese individuals and long-term lipid outcomes–a systematic review. Obes Rev 2004;5:43–50 [DOI] [PubMed] [Google Scholar]