Abstract
PtdIns3P is recognized as an important player in the control of the endocytotic pathway and in autophagy. Recent data also suggest that PtdIns3P contributes to molecular mechanisms taking place at the plasma membrane and at the midbody during cytokinesis. This lipid is present in low amounts in mammalian cells and remains difficult to quantify either by traditional techniques based on radiolabelling followed by HPLC to separate the different phosphatidylinositol monophosphates, or by high-sensitive liquid chromatography coupled to MS, which is still under development. In the present study, we describe a mass assay to quantify this lipid from various biological samples using the recombinant PtdIns3P 5-kinase, PIKfyve. Using this assay, we show an increase in the mass level of PtdIns3P in mouse and human platelets following stimulation, loss of this lipid in Vps34-deficient yeasts and its relative enrichment in early endosomes isolated from BHK cells.
Keywords: class III phosphoinositide 3-kinase, mass assay, PIKfyve, PtdIns3P
Abbreviations: EEA1, early endosome antigen 1; GFP, green fluorescent protein; GST, glutathione transferase; MTM, myotubularin; MTMR, MTM-related; PE, phosphatidylethanolamine; PI3K, phosphoinositide 3-kinase; PtdInsP, phosphatidylinositol monophosphate; PtdInsP2, phosphatidylinositol diphosphate; PX, Phox homology; TRAP, thrombin-receptor-agonist peptide
INTRODUCTION
Phosphoinositides are considered as highly dynamic players in the spatial and temporal organization of signalling networks that regulate many important cellular processes, including cytoskeleton organization, membrane trafficking or cell survival and proliferation [1,2]. Among PtdInsPs (phosphatidylinositol monophosphates), much attention has been paid to PtdIns3P over the last few years. This lipid can be produced by the class III and possibly by class II PI3Ks (phosphoinositide 3-kinases) [3]. The mammalian class III PI3K, hVps34, was identified as a homologue of Vps34, the only PI3K present in yeast [3–5], which plays an important role in the regulation of the endocytic pathway and in autophagy [5]. It has a lipid substrate specificity restricted to PtdIns and is considered as the major producer of the constitutive pool of PtdIns3P. It is still unclear whether class III PI3K has a basal activity or if it is also regulated by extracellular stimuli.
It is generally acknowledged that the amount of PtdIns3P does not exceed 20–25% of the total PtdInsPs in mammalian cells, whereas in yeast it represents nearly 50% of PtdInsPs. Using biochemical approaches and fluorescence microscopy to localize PtdIns3P with a specific probe [GFP (green fluorescent protein)–2×FYVE], it has been demonstrated that this lipid is concentrated in early endosomes [6]. PtdIns3P regulates membrane transport and dynamics through the recruitment of proteins containing FYVE or PX (Phox homology) domains [7]. For instance, EEA1 (early endosome antigen 1), a central regulator of endosome fusion, is recruited to this compartment by Rab5-GTP and PtdIns3P through interaction with its FYVE domain [8]. The implication of PtdIns3P in the control of vesicular trafficking and intracellular protein sorting has been well demonstrated in yeast and in Caenorhabditis elegans, and accumulating data point to a similar role in mammalian cells [4,6,9].
PtdIns3P and class III PI3K, in complex with beclin-1, have also been shown to control the autophagic pathway [10,11]. In neutrophiles, they are important for the recruitment and the assembly of proteins of the NADPH oxydase complex, including p40phox, which binds PtdIns3P by its PX domain on phagosomes [12,13].
However, the role of PtdIns3P is probably not confined to endosomal/phagosomal structures. Evidence is accumulating that this lipid can also act as a dynamic intracellular second messenger [14,15]. Indeed, insulin stimulation induces the formation of a pool of PtdIns3P at the plasma membrane, where it controls the translocation of GLUT4 (glucose transporter 4) [15,16]. In contrast with PtdIns(3,4,5)P3 or PtdIns(3,4)P2, the global level of PtdIns3P does not undergo rapid and dramatic increase following mammalian cell stimulation. However, moderate and transient changes have been reported in insulin-stimulated cells [16] or in activated blood platelets [17], suggesting that besides a pool of PtdIns3P that would act in the basal regulation of trafficking, agonist-dependent pools of PtdIns3P can indeed be generated. In this context, class II PI3Ks have been proposed to be responsible for the synthesis of PtdIns3P upon cellular stimulation by extracellular cues [18].
Finally, recent data suggest unexpected new functions for this lipid in the control of cytokinesis [19] and the entry of pathogen effectors into host cells [20]. Moreover, important changes in the level of PtdIns3P have been reported in mammalian cells infected by pathogens, such as Plasmodium falciparum and Mycobacterium tuberculosis [21].
In addition to a tight control by class III, and probably class II, PI3Ks, the amount of PtdIns3P is also regulated by the PtdIns3P 5-kinase PIKfyve, PtdIns(3,4)P2 4-phosphatases such as inositol polyphosphate 4-phosphatase type I and II, and PtdIns3P 3-phosphatases of the MTM (myotubularin) family [22,23]. Importantly, several MTM genes are mutated in genetic diseases such as X-linked centronuclear myopathy (for MTM1) and Charcot-Marie-Tooth 4B neuropathy [for MTMR (MTM-related) 2 and MTMR13]. Mutations in the gene encoding PIKfyve have been found in patients affected with François-Neetens fleck corneal dystrophy [24,25] and knockout experiments in mice have demonstrated that loss of PIKfyve leads to early embryonic lethality [26]. Moreover, polyphosphate 4-phosphatase type II is involved in cancer aggressiveness [27]. Although a clear implication of PtdIns3P modifications has not been directly demonstrated in the aetiology of these pathologies, an important role of this lipid in cell regulation is highly suspected. Modifications of the level of PtdIns3P may act in concert with scaffolding activities of the enzymes responsible for its metabolism [22].
Quantifying the changes in PtdIns3P elicited by different conditions is still challenging, and monitoring its amounts in biopsies or isolated subcellular compartments remains difficult. To separate and quantify the isomers of PtdInsP (PtdIns3P, PtdIns4P and PtdIns5P), the classical method used requires equilibrium [3H]inositol or [32P]Pi radiolabelling of cells, lipid extraction and deacylation, followed by separation and analysis by ion-exchange HPLC [28]. This method allows analysis of the phosphoinositide content of cultured cell lines and of some primary cells. However, it does not allow quantification of biopsies of solid tissues and is relatively unsensitive to minor phosphoinositides [particularly PtdIns5P, PtdIns3P and PtdIns(3,5)P2]. Moreover, to discriminate between an increase in mass or a change in the turn-over of the phosphate on a phosphoinositide, it is essential to reach the isotopic equilibrium, which is not always possible when primary cells are used. To circumvent these drawbacks, a protocol combining liquid chromatography and MS has been developed, allowing analysis of phosphoinositides from lipid extracts of biological samples [29]. This promising approach is still under development and requires specific equipments and expertise to enable routine profiling of phosphoinositides. It is still particularly difficult to separate and quantify some of the isomers of PtdInsP and PtdInsP2 (phosphatidylinositol diphosphate) by MS. As an alternative, methods using protein domains selectively recognizing a lipid in highly controlled conditions (recently commercially available for different phosphoinositides) or a kinase assay specifically phosphorylating a lipid (e.g. PtdIns5P [30]) can be developed. In the present study, we propose a convenient mass assay to accurately quantify PtdIns3P from various biological samples, including yeast and mammalian cell extracts by using recombinant PIKfyve.
MATERIALS AND METHODS
Materials
GMEM (Glasgow minimal essential medium) and FBS (fetal bovine serum) were from Invitrogen. TRAP (thrombin-receptor-agonist peptide)-7 was from Bachem. Silica gel G60 TLC plates were from Merck. [32P]Pi and [γ-32P]ATP were from PerkinElmer. Brain L-α-phosphatidylethanolamine and brain L-α-PtdIns4P were from Avanti Polar Lipids. C16:0/C16:0-PtdIns3P and C16:0/C16:0-PtdIns5P were from Echelon. The Saccharomyces cerevisiae strains used in the present study were wild-type (BY4741, MATa; his3Δ1; leu2Δ0; met15Δ0; ura3Δ0) and vps34Δ (BY4741 vps34::KanMX4) and were purchased from Euroscarf. All other reagents were purchased from Sigma–Aldrich unless otherwise indicated.
Production of recombinant GST (glutathione transferase)–PIKfyve
The short form of murine PIKfyve was cloned and a plasmid encoding the tagged enzyme at the N-terminus with GST was generated. GST–PIKfyve protein was produced in SF9-infected cells and purified (GTP-Technology). Briefly, after 36 h of infection, SF9 cells were lysed by sonication in a buffer containing 10 mM NaPO4, pH7.4, 150 mM NaCl and a cocktail of inhibitors composed of 4 mM Na3VO4, 5 μM iodoacetamide; 200 μM TLCK (tosyl-lysylchloromethane), 2 μM benzamidine, 100 μM AEBSF [4-(2-aminoethyl)benzenesulfonyl fluoride] and 1 mM PMSF and centrifuged (20 min at 16000 g). The supernatant fraction was incubated for 2 h with glutathione–agarose and the GST–PIKfyve protein was eluted using an excess of reduced glutathione and dialysed against 25 mM Hepes, pH 7.4, containing 150 mM NaCl. Protein purification was assessed by SDS/PAGE and Coomassie Blue staining as well as with Western blotting with an anti-GST antibody. The purified enzyme was aliquoted and kept at −80°C.
Cell preparation and lipid extraction
Yeast culture and lipid extraction were carried out as published previously [31]. Whole blood was drawn from the inferior vena cava of mice anaesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). Ethical approval for animal experiments was received from the French Ministry for Research in accordance with European Union guidelines. Mouse platelets were isolated by successive centrifugations as described previously [32]. Where indicated, platelets (5×108) were pre-incubated at 37°C with wortmannin (100 nM for 15 min). Platelets were then stimulated for 3 min with thrombin (0.5 unit/ml) under aggregating conditions, and stimulation was stopped by the addition of a mixture of CHCl3:CH3OH (v/v). Lipids were immediately extracted by the acidic Bligh and Dyer procedure [28,33]. For metabolic labelling, platelets were incubated with 0.4 mCi/ml [32P]Pi, stimulated for 3 min with thrombin (0.5 unit/ml) under aggregating conditions, and their phosphoinositide content was analysed as described previously [33]. Human platelets were isolated from blood by successive centrifugation steps [33]. Peripheral blood was collected from three healthy donors within the French National Blood Establishment fulfilling the national principles of ethics and the regulatory requirements. Research using human platelets was carried out in accordance with the Declaration of Helsinki (2008) of the World Medical Association. Before stimulation, platelets (109) were preincubated at 37°C for 10 min with fibrinogen (250 μg/ml) and, when indicated, with wortmannin (100 nM for 15 min) before the addition of fibrinogen. Platelets were then stimulated for the indicated times with TRAP (50 μM). The lipid extraction was performed following the same procedure as for mouse platelets.
Endosomal fractions were isolated from BHK cells as described previously [34].
Lipid phosphorylation and analyses
Lipids extracted from the different cells or subcellular fractions were resolved by TLC using a solvent of CHCl3:CH3OH:4.3 M NH4OH (90/70/20, by vol.) on silica-coated glass plates pre-activated for 30 min at 70°C after migration in a solution of 50% (v/v) CH3OH, 1% potassium oxalate and 2 mM EDTA. The migration of authentic standards visualized by iodine vapours allowed the localization of PtdInsPs in the different samples, which were protected from the iodine vapors by a glass plate. PtdInsPs were then scraped off and extracted by the acidic Bligh and Dyer procedure [28,33].
For vesicle formation, PE (phosphatidylethanolamine; 20 nmoles) was added to TLC-purified PtdInsPs or commercial PtdInsPs when indicated and dried under nitrogen. Dried lipids were then resuspended in 30 μl of lipid kinase buffer (25 mM Hepes, 150 mM NaCl, 1.7 mM EDTA, 1.7 mM dithiothreitol and 8 mM 2-glycerophosphate) and sonicated twice for 30 s in a bath sonicator (45 kHz). After the addition of MgCl2 (2.5 mM), ATP (50 μM, final concentration) and 0.2 μg of recombinant GST–PIKfyve, phosphorylation reactions were initiated by the addition of 20 μCi of [γ-32P]ATP. After incubation at 37°C for 15 min, phosphorylated lipids were extracted and analysed by TLC using a solvent of CHCl3:CH3OH:4.3 M NH4OH (90/70/20, by vol.) as described above. The radioactive PtdInsP2 products formed were scraped off, deacylated and futher analysed by HPLC [28,33]. A standard curve with known concentrations of C16:0/C16:0-PtdIns3P was processed under the same conditions.
The amount of phospholipids in yeast was quantified by phosphorus measurement by the Fiske and SubbaRow [35] method.
RESULTS AND DISCUSSION
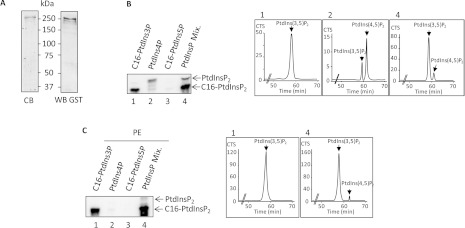
To develop a PtdIns3P mass assay capable of determining accurately the amount of this lipid in cell extracts, we used recombinant PIKfyve and [γ-32P]ATP to produce and quantify radiolabelled PtdIns(3,5)P2 formed from PtdIns3P. Recombinant GST–PIKfyve protein was produced in the baculovirus-SF9 insect cell system and the purified enzyme (Figure 1A) was stable for months at −80°C. Its substrate specificity was first evaluated using pure C16:0/C16:0-PtdIns3P, PtdIns4P purified from brain extracts and C16:0/C16:0-PtdIns5P. PtdIns3P was efficiently phosphorylated into PtdIns(3,5)P2 by recombinant PIKfyve as demonstrated by the HPLC analysis (Figure 1B, panel 1). As expected, PtdIns5P was not phosphorylated by PIKfyve. However, when PtdIns4P purified from brain extract was used as a substrate, we observed the production of radiolabelled PtdIns(3,5)P2 (Figure 1B, panel 2), suggesting that some PtdIns3P was present in the samples, since the procedure used to purify PtdIns4P from the brain does not preclude contamination with other PtdInsPs. Recombinant PIKfyve also phosphorylated small amounts of PtdIns4P into PtdIns(4,5)P2, as shown by the HPLC analysis (Figure 1B, panel 2). When a mixture of PtdInsPs containing 80% PtdIns4P, 10% PtdIns3P and 10% PtdIns5P was used to reproduce the ratio classically found in resting mammalian cells, PtdIns3P appeared as the preferred substrate of recombinant PIKfyve (Figure 1B, panel 4). However, to further decrease the residual phosphorylation of PtdIns4P, which could cause a bias of quantification since it is much more abundant in mammalian cells than PtdIns3P, we optimized the assay by testing different conditions, especially variation of ions concentrations and lipid vesicle composition. Interestingly, in the presence of 0.4 μM PE and 2.5 mM MgCl2, recombinant PIKfyve selectively phosphorylated PtdIns3P (Figure 1C). Under these conditions, even with a large excess of PtdIns4P (80% PtdIns4P, 10% PtdIns3P and 10% PtdIns5P), PIKfyve produced 50-fold more PtdIns(3,5)P2 than PtdIns(4,5)P2 (Figure 1C, panel 4).
Figure 1. Purification and substrate specificity of recombinant PIKfyve.
(A) Recombinant PIKfyve was produced in SF9 insect cells, purified and analysed by Coomassie Blue (CB) staining or Western blotting (WB) using an anti-GST antibody. (B) The substrate specificity of purified recombinant PIKfyve was evaluated using vesicles composed of three isomers of PtdInsP alone (i.e. C16:0/C16:0-PtdIns3P, natural PtdIns4P or C16:0/C16:0-PtdIns5P) or mixed (Mix.) (10% PtdIns3P, 80% PtdIns4P and 10% PtdIns5P, by weight). The products of the reactions were analysed by TLC (left-hand panel) and HPLC (right-hand panels). (C) Lipid vesicles containing PE improved the substrate specificity of recombinant PIKfyve towards PtdIns3P. Vesicles composed of 99.25% natural PE and 0.75% of either C16:0/C16:0-PtdIns3P, natural PtdIns4P or C16:0/C16:0-PtdIns5P were used as a substrate for purified recombinant PIKfyve. Lipid vesicles composed of a mixture (Mix.) of natural PE (99.25%), C16:0/C16:0-PtdIns3P (0.075%), natural PtdIns4P (0.60%) and C16:0/C16:0-PtdIns5P (0.075%) (by weight) were also used as a substrate for recombinant PIKfyve. The products of the reactions were analysed as in (B). Results are representative of three independent experiments. CTS, counts per second.
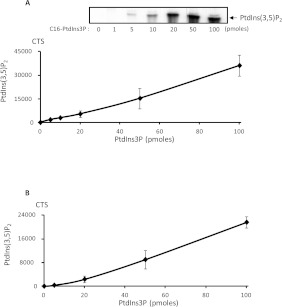
The assay was found to be linear with pure PtdIns3P quantities varying from a few pmol up to 100 pmol (Figure 2A). To assay the linearity in the context of a cellular extract, increasing amounts of pure PtdIns3P were then mixed with HeLa cell lysates (Figure 2B). Again, the assay was linear over a relatively large range of concentrations, indicating that the method displayed good sensitivity. However, it is noteworthy that the yield of lipid extraction is different from one biological sample to another (i.e. cultured cells, yeasts or biopsies such as muscle extracts), mostly because of the difference in mechanical resistance of tissues compared with cultured cells, and must therefore be optimized to accurately quantify PtdIns3P.
Figure 2. Sensitivity and linearity of the PtdIns3P mass assay.
(A) Vesicles composed of 20 nmol of natural PE and increasing amounts of pure C16:0/C16:0-PtdIns3P (from 0 to 100 pmol), as indicated, were submitted to the assay and the formed [32P]PtdIns(3,5)P2 was separated by TLC (upper panel) and quantified by HPLC (lower panel). (B) Increasing amounts of C16:0/C16:0-PtdIns3P (from 0 to 100 pmol) were added to 3×105 HeLa cell lysate. Lipids were then extracted and PtdInsPs were TLC-purified and submitted to the PtdIns3P mass assay to check the sensitivity and linearity of the assay in a biological sample. [32P]PtdIns(3,5)P2 was quantified in each sample by HPLC. The basal level of PtdIns3P detected in HeLa cells (10 pmoles/3×105 HeLa cells) was subtracted to each point of the curve. Results are the means±S.D. from three experiments. CTS, counts per second.
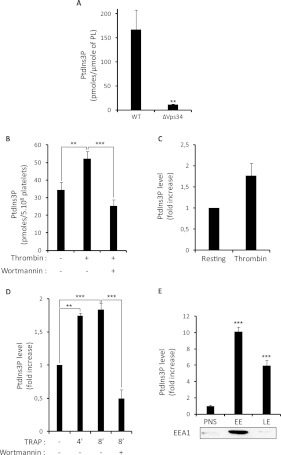
As a proof of principle, we tested our assay by measuring the amount of PtdIns3P in yeast, where the only PI3K present is encoded by the vps34 gene. In this model, a previous study demonstrated that PtdIns3P could hardly be detected by [3H]inositol labelling and HPLC analysis in the vps34-deleted strain [4]. Accordingly, lipid extraction and mass assay showed a dramatic decrease of PtdIns3P in the vps34-deleted strain (Figure 3A), demonstrating that the mass assay is accurate in quantifying PtdIns3P in yeasts. To further validate the assay, we used mouse platelets stimulated by thrombin. As shown in Figure 3(B), the mass of PtdIns3P increased following stimulation, whereas inhibition of PI3Ks by wortmannin impaired this elevation. Importantly, the increase in PtdIns3P measured by the mass assay correlated with the increase in 32P-PtdIns3P detected by HPLC after metabolic labelling in a parallel experiment (Figure 3C). We also observed an increase in the mass of PtdIns3P in human platelets stimulated through the thrombin receptor PAR1 (protease-activated receptor 1) with the agonist peptide (TRAP) in the presence of fibrinogen to fully engage the integrin GpIIbIIIa (Figure 3D). Again, preincubation of platelets with the PI3K inhibitor wortmannin abolished the PtdIns3P production measured after 8 min of TRAP stimulation (Figure 3D). These results are consistent with a previous report showing an increase in 32P-labelled PtdIns3P upon stimulation under similar conditions using HPLC analysis, after metabolic labelling of human platelets [17]. Thus, in this case, the incorporation of the radiolabel did reflect an increase in the mass of this lipid. The role of PtdIns3P in stimulated platelets is still unknown and the development of mouse models with deficiencies in enzymes involved in its metabolism might bring new light on the functions of this lipid in the near future.
Figure 3. Application of the PtdIns3P mass assay to yeast and mammalian cells.
(A) The amount of PtdIns3P was quantified in wild-type (WT) or vps34-deleted S. cerevisae (ΔVps34) using the mass assay. The amount of PtdIns3P found is expressed as pmole/μmole of total phospholipids (PL) determined by lipid phosphorus assay. Results are the means±S.E.M. from three independent experiments. **P<0.01 according to Student's t test. (B) The amount of PtdIns3P was quantified in resting mouse platelets and in platelets stimulated by 0.5 unit/ml thrombin and pre-incubated or not with wortmannin (100 nM for 15 min). Results are expressed as pmole of PtdIns3P in 5×108 platelets and are the means±S.E.M. from six independent experiments. (C) 32P-labelled mouse platelets were stimulated by 0.5 unit/ml thrombin for 3 min and the level of [32P]PtdIns3P was quantified by HPLC. Results are expressed as the fold increase compared to the level measured in resting platelets, and are the means±S.E.M. of two independent experiments. (D) The amount of PtdIns3P was quantified from human platelets stimulated by 50 μM TRAP for the indicated times (4 or 8 min) in the presence of fibrinogen, with or without wortmannin pretreatment (100 nM for 15 min). Results are expressed as fold increase and are means±S.E.M. of three independent experiments (three different donors). (E) PtdIns3P was quantified in the post-nuclear fraction (PNS) of BHK cells and in isolated early (EE) and late endosomal (LE) fractions. Results are expressed as fold increase and are the means±S.E.M. from three independent experiments. As a control of fractionation, a representative Western blot shows the localization of the early endosome protein EEA1. **P<0.01 and ***P<0.001 determined by one-way ANOVA test.
To test whether our assay would be efficient to quantify PtdIns3P in isolated subcellular compartments, we then separated early and late endosomal fractions from BHK cells following a well-established procedure [34]. We observed an enrichment of the relative amount of PtdIns3P in early endosomes (10-fold increase compared with the post-nuclear fraction) and to a lesser extent in the late endosomal fraction when compared with post-nuclear fractions (6-fold increase) (Figure 3E). Subcellular fractionation can induce artifactual changes in lipid levels due to potential transfer of material or to modification of their metabolism during the isolation procedure. However, the results of our mass assay are consistent with the imaging data showing that the GFP–2×FYVE probe used in living cells to visualize PtdIns3P pools preferentially decorates early endosomes [6].
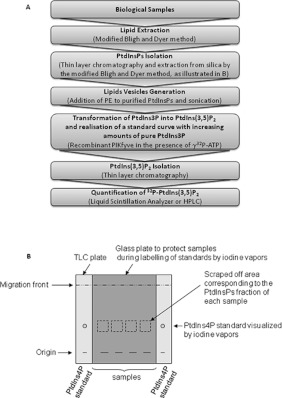
Overall, these results validate the application and the sensitivity of the mass assay we developed in various biological samples (i.e. yeast, primary human cells and isolated subcellular fractions). This assay is relatively simple (Figure 4), robust and should be useful to quantify PtdIns3P in various tissues from mice deficient for PtdIns3P-metabolizing enzymes (i.e. Vps34, PIKfyve, MTMs, inositol polyphosphate 4-phosphatase type I and II) or models exhibiting knockin forms of these enzymes that are under development. Accordingly, we already validated this assay by measuring the amount of PtdIns3P in muscle samples from mice deficient for myotubularin 1 and infected with wild-type or inactive MTM1-expressing adeno-associated virus (L. Amoasii, K. Hnia, G. Chicanne, A. Brech, B. S. Cowling, M. Mueller, Y. Scwab, P. Koebel, A. Ferry, B. Payrastre and J. Laporte, unpublished work).
Figure 4. Schematic representation of the major steps of the mass assay.
(A) The different steps of the mass assay are summarized. (B) Schematic representation of the PtdInsPs isolation procedure.
In conclusion, we propose a new sensitive mass assay for PtdIns3P quantification in various samples with significant advantages compared with the method requiring radiolabelling of cultured cells followed by HPLC analysis.
AUTHOR CONTRIBUTION
Gaëtan Chicanne and Sonia Severin performed the experiments and analysed the data. Cécile Boscheron performed the experiments using yeast. Marie-Pierre Gratacap and Anne-Dominique Terrisse performed experiments with blood platelets. Hélène Tronchère performed subcellular fractionation experiments. Frédérique Gaits-Iacovoni, Hélène Tronchère and Bernard Payrastre controlled experimental approaches and analysed data, Bernard Payrastre wrote the paper. All authors checked the final version of the paper.
ACKNOWLEDGEMENTS
We thank S. Dupuis-Coronas, S. Manenti and J. Laporte for stimulating discussions.
FUNDING
This work was supported by grants from the ANR (Agencie Nationale de la Recherche) programme Blanc [grant number ANR-07-BLAN-0065] and E-Rare, Inserm-Transfert and Région Midi-Pyrénées.
References
- 1.Di Paolo G., De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 2.Payrastre B., Missy K., Giuriato S., Bodin S., Plantavid M., Gratacap M. Phosphoinositides: key players in cell signalling, in time and space. Cell. Signalling. 2001;13:377–387. doi: 10.1016/s0898-6568(01)00158-9. [DOI] [PubMed] [Google Scholar]
- 3.Vanhaesebroeck B., Guillermet-Guibert J., Graupera M., Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell. Biol. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 4.Schu P. V., Takegawa K., Fry M. J., Stack J. H., Waterfield M. D., Emr S. D. Phosphatidylinositol 3-kinase encoded by yeast vps34 gene essential for protein sorting. Science. 1993;260:88–91. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- 5.Backer J. M. The regulation and function of Class III PI3Ks: novel roles for Vps34. Biochem. J. 2008;410:1–17. doi: 10.1042/BJ20071427. [DOI] [PubMed] [Google Scholar]
- 6.Gillooly D. J., Morrow I. C., Lindsay M., Gould R., Bryant N. J., Gaulier J. M., Parton R. G., Stenmark H. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 2000;19:4577–4588. doi: 10.1093/emboj/19.17.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawe D. C., Patki V., Heller-Harrison R., Lambright D., Corvera S. The FYVE domain of early endosome antigen 1 is required for both phosphatidylinositol 3-phosphate and Rab5 binding. Critical role of this dual interaction for endosomal localization. J. Biol. Chem. 2000;275:3699–3705. doi: 10.1074/jbc.275.5.3699. [DOI] [PubMed] [Google Scholar]
- 8.Simonsen A., Lippe R., Christoforidis S., Gaulier J. M., Brech A., Callaghan J., Toh B. H., Murphy C., Zerial M., Stenmark H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- 9.Nicot A. S., Laporte J. Endosomal phosphoinositides and human diseases. Traffic. 2008;8:1240–1249. doi: 10.1111/j.1600-0854.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Axe E. L., Walker S. A., Manifava M., Chandra P., Roderick H. L., Habermann A., Griffiths G., Ktistakis N. T. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J. Cell Biol. 2008;182:685–701. doi: 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vergne I., Deretic V. The role of PI3P phosphatases in the regulation of autophagy. FEBS Lett. 2010;584:1313–1318. doi: 10.1016/j.febslet.2010.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellson C., Davidson K., Anderson K., Stephens L. R., Hawkins P. T. PtdIns3P binding to the PX domain of p40phox is a physiological signal in NADPH oxidase activation. EMBO J. 2006;25:4468–4478. doi: 10.1038/sj.emboj.7601346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson K. E. CD18-dependent activation of the neutrophil NADPH oxidase during phagocytosis of E. coli or S. aureus is regulated by class III but not class I or II PI3K. Blood. 2008;112:5202–5211. doi: 10.1182/blood-2008-04-149450. [DOI] [PubMed] [Google Scholar]
- 14.Falasca M., Maffucci T. Rethinking phosphatidylinositol 3-monophosphate. Biochim. Biophys. Acta. 2009;1793:1795–1803. doi: 10.1016/j.bbamcr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Falasca M., Maffucci T. Emerging roles of phosphatidylinositol 3-monophosphate as a dynamic lipid second messenger. Arch. Physiol. Biochem. 2006;112:274–284. doi: 10.1080/13813450601094664. [DOI] [PubMed] [Google Scholar]
- 16.Maffucci T., Brancaccio A., Piccolo E., Stein R. C., Falasca M. Insulin induces phosphatidylinositol-3-phosphate formation through TC10 activation. EMBO J. 2003;22:4178–4189. doi: 10.1093/emboj/cdg402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J., Banfic H., Straforini F., Tosi L., Volinia S., Rittenhouse S. E. A type II phosphoinositide 3-kinase is stimulated via activated integrin in platelets. A source phosphatidylinositol 3-phosphate. J. Biol. Chem. 1998;273:14081–14084. doi: 10.1074/jbc.273.23.14081. [DOI] [PubMed] [Google Scholar]
- 18.Falasca M., Maffucci T. The role of class II phosphoinositide 3-kinase in cell signalling. Biochem. Soc. Trans. 2007;35:211–214. doi: 10.1042/BST0350211. [DOI] [PubMed] [Google Scholar]
- 19.Sagona A. P., Nezis I. P., Pedersen N. M., Liestøl K., Poulton J., Rusten T. E., Skotheim R. I., Raiborg C., Stenmark H. PtdIns(3)P controls cytokinesis through KIF13A-mediated recruitment of FYVE-CENT to the midbody. Nat. Cell Biol. 2010;12:362–371. doi: 10.1038/ncb2036. [DOI] [PubMed] [Google Scholar]
- 20.Kale S. D., Gu B., Capelluto D. G. S., Dou D., Feldman E., Rumore A., Arredondo F. D., Hanlon R., Fudal I., Rouxel T., et al. External lipid PI3P mediates entry of eukaryotic pathogen effectors into plant and animal host cells. Cell. 2010;142:284–295. doi: 10.1016/j.cell.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Payrastre B., Gaits-Iacovoni F., Sansonetti P., Tronchère H. Phosphoinositides and cellular pathogens. Subcell. Biochem. 2012;59:363–388. doi: 10.1007/978-94-007-3015-1_12. [DOI] [PubMed] [Google Scholar]
- 22.Tronchère H., Bolino A., Laporte J., Payrastre B. Myotubularins and associated neuromuscular diseases. Clin. Lipidol. 2012;7:151–162. [Google Scholar]
- 23.Blero D., Payrastre B., Schurmans S., Erneux C. Phosphoinositide phosphatases in a network of signalling reactions. Pflugers Arch. 2007;455:31–44. doi: 10.1007/s00424-007-0304-5. [DOI] [PubMed] [Google Scholar]
- 24.Pendaries C., Tronchere H., Plantavid M., Payrastre B. Phosphoinositide signaling disorders in human diseases. FEBS Lett. 2003;546:25–31. doi: 10.1016/s0014-5793(03)00437-x. [DOI] [PubMed] [Google Scholar]
- 25.McCrea H. J., De Camilli P. Mutations in phosphoinositide metabolizing enzymes and human disease. Physiology. 2009;24:8–16. doi: 10.1152/physiol.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikonomov O. C., Sbrissa D., Delvecchio K., Xie Y., Jin J.-P., Rappolee D., Shisheva A. The phosphoinositide kinase PIKfyve is vital in early embryonic development. J. Biol. Chem. 2011;286:13404–13413. doi: 10.1074/jbc.M111.222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fedele C. G., Ooms L. M., Ho M., Vieusseux J., O'Toole S. A., Millar E. K., Lopez-Knowles E., Sriratana A., Gurung R., Baglietto L., et al. Inositol polyphosphate 4-phosphatase II regulates PI3K/Akt signalling and is lost in human basal-like breast cancers. Proc. Natl. Acad. Sci. U.S.A. 2010;107:22231–22236. doi: 10.1073/pnas.1015245107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Payrastre B. Phosphoinositides: lipid kinases and phosphatases. Methods Mol. Biol. 2004;273:201–212. doi: 10.1385/1-59259-783-1:201. [DOI] [PubMed] [Google Scholar]
- 29.Pettitt T. R., Dove S. K., Lubben A., Calaminus S. D. J., Wakelam M. J. O. Analysis of intact phosphoinositides in biological samples. J. Lipid Res. 2006;47:1588–1596. doi: 10.1194/jlr.D600004-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Morris J. B., Hinchliffe K. A., Ciruela A., Letcher A. J., Irvine R. F. Thrombin stimulation of platelets causes an increase in phosphatidylinositol 5-phosphate revealed by mass assay. FEBS Lett. 2000;475:57–60. doi: 10.1016/s0014-5793(00)01625-2. [DOI] [PubMed] [Google Scholar]
- 31.Hama H., Takemoto J. Y., DeWald D. B. Analysis of phosphoinositides in protein trafficking. Methods. 2000;20:465–473. doi: 10.1006/meth.2000.0959. [DOI] [PubMed] [Google Scholar]
- 32.Séverin S., Gratacap M. P., Lenain N., Alvarez L., Hollande E., Penninger J. M., Gachet C., Plantavid M., Payrastre B. Deficiency of Src homology 2 domain-containing inositol 5-phosphatase 1 affects platelet responses and thrombus growth. J. Clin. Invest. 2007;117:944–952. doi: 10.1172/JCI29967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gratacap M. P., Payrastre B., Viala C., Mauco G., Plantavid M., Chap H. Phosphatidylinositol 3,4,5-trisphosphate-dependent stimulation of phospholipase C-γ2 is an early key event in FcγRIIA-mediated activation of human platelets. J. Biol. Chem. 1998;273:24314–24321. doi: 10.1074/jbc.273.38.24314. [DOI] [PubMed] [Google Scholar]
- 34.Gorvel J. P., Chavrier P., Zerial M., Gruenberg J. Rab 5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- 35.Fiske C. H., SubbaRow Y. The colorimetric determination of phosphorus. J. Biol. Chem. 1925;66:375–400. [Google Scholar]