Abstract
Objectives:
A single-centre, prospective trial was performed aiming to assess the impact of narrow-band imaging (NBI) cystoscopy in cases of non-muscle-invasive bladder cancer (NMIBC) in comparison to standard white light cystoscopy (WLC).
Materials and methods:
A total of 95 NMIBC-suspected consecutive cases were enrolled. The inclusion criteria were hematuria, positive urinary cytology or ultrasound suspicion of bladder tumors. All patients underwent WLC and NBI cystoscopy. Standard resection was performed for all lesions visible in white light and NBI transurethral resection of bladder tumors for only NBI-observed tumors.
Results:
The overall detection rates of NMIBC and carcinoma in situ (CIS) were significantly improved for NBI (96.2% versus 87.2% and 100% versus 66.7% respectively). Also, NBI cystoscopy showed significantly superior detection for CIS, pTa and overall tumors (95.2% versus 61.9%, 93.9% versus 85.2% and 94.8% versus 83.9% respectively). Additional tumors were diagnosed by NBI in a significant proportion of patients with CIS, pTa, pT1 and NMIBC (55.5% versus 11.1%, 26.5% versus 10.2%, 30% versus 10% and 30.8% versus 10.3%). Postoperative treatment was significantly improved due to NBI results (16.7% versus 5.1%).
Conclusions:
NBI cystoscopy represents a valuable diagnostic alternative in patients with NMIBC, with significant improvement in tumor visual accuracy as well as detection. This approach provides a substantial improvement to bladder cancer therapeutic management.
Keywords: narrow-band imaging cystoscopy, non-muscle-invasive bladder cancer, transurethral resection of bladder tumors, white light cystoscopy
Introduction
Non-muscle-invasive bladder cancer (NMIBC) includes stages pTa, pT1 and carcinoma in situ (CIS) and accounts for 75–85% of all newly diagnosed bladder tumors [Grasso, 2008]. This pathology is often present in the form of a multifocal disease with an exceptionally high recurrence rate within the first 5 years after the initial diagnosis [Sylvester et al. 2002].
During the last decades, many methods of improving bladder cancer diagnosis and treatment were evaluated. Quite often, small papillary tumors and flat CIS lesions are not properly visualized during white light cystoscopy (WLC). Consequently, an incomplete resection of the primary tumors is performed, leading to a substantial increase in the short- and medium-term recurrence rates [Filbeck et al. 2002; Oliva Encina et al. 2010]. Moreover, undetected tumors are expected to appear later as recurrences and some might become invasive, highlighting the need to develop alternative endoscopic methods to detect bladder lesions [Naselli et al. 2010a].
Narrow-band imaging (NBI) cystoscopy was introduced as an endoscopic alternative and was presumed to be able to improve the diagnostic accuracy in early malignant lesions [Gono et al. 2004; Mannath et al. 2010; Rastogi et al. 2009]. This relatively new method is based on the light output bandwidth ranging between tight wavelengths and subsequently enhancing the color contrast of urothelial lesions [Bryan et al. 2008].
Reports suggested that NBI cystoscopy is more effective than the standard endoscopic evaluation as far as the detection of malignant lesions is concerned [Gono et al. 2004; Hamamoto et al. 2004]. Moreover, the use of NBI during follow up proved to be effective in discovering recurrent bladder tumors and provided longer recurrence-free intervals in comparison with standard cystoscopy [Herr and Donat, 2008].
On this basis, we performed a prospective trial comparing the accuracy of bladder tumor diagnosis using NBI versus WLC in NMIBC cases.
Materials and methods
A single-centre, prospective trial was performed to assess the impact of NBI cystoscopy in cases of non-muscle-invasive bladder tumors in comparison to the standard approach. The primary endpoint of the study was to determine significant differences in detection rates between the two methods and the resulting postoperative treatment changes.
The study was approved by the local ethics and research committee. A total of 95 NMIBC-suspected consecutive cases (71 men and 24 women, mean age 68.4 years, range 32–81 years) were enrolled in the trial (after properly explaining the aims, methods, anticipated benefits, potential hazards and any other aspects of the study relevant to the patient’s decision to participate and written informed consent was obtained).
All patients underwent a standard investigation protocol which included general clinical examination, blood tests, urinalysis, urinary cytology, abdominal ultrasonography and intravenous pyelography or computed tomography scan. The inclusion criteria were hematuria, positive urinary cytology or ultrasound suspicion of bladder tumors.
The patients underwent WLC followed by NBI cystoscopy, resulting in separate bladder maps of all WLC and NBI diagnosed lesions. White light transurethral resection of bladder tumors (TURBT) was performed for all lesions visible at standard cystoscopy, while NBI resection was applied for lesions observed during NBI alone.
At the end of the procedure, NBI cystoscopic control was used to assess the margins of the resection areas and to identify eventual residual lesions. All resected specimens were subjected to central pathology review. According to the pathological results, all patients without any detected tumor and those with muscle-invasive bladder cancer were excluded from the trial. A specific bladder map of all pathologically confirmed lesions was obtained and compared with previous ones. Subsequently, the overall NMIBC, CIS, pTa and pT1 cases and tumor detection rates were determined for both types of cystoscopy.
Statistical analysis was performed using the SPSS 15.0 software (Statistical Package for Social Sciences 15.0; SPSS Inc., Chicago, IL, USA). The binomial test was applied to assess the statistical significance of differences between the two diagnostic methods at a confidence level of at least 95%.
Results
All procedures were successfully performed. Six cases (6.3%) presented no bladder cancer lesions while 89 patients (93.6%) were diagnosed with this pathology. The 11 muscle-invasive bladder cancer cases (12.6%) were excluded from the study and scheduled for radical cystectomy. A total of 78 patients with NMIBC (87.6%) were pathologically confirmed.
The overall detection rates for patients with NMIBC and CIS were significantly improved for NBI compared with WLC (96.2% versus 87.2% and 100% versus 66.7%, respectively) (Figure 1). NBI also showed improved detection rates for pTa cases; however the results failed to reach statistical significance (93.9% versus 87.8%) (Table 1).
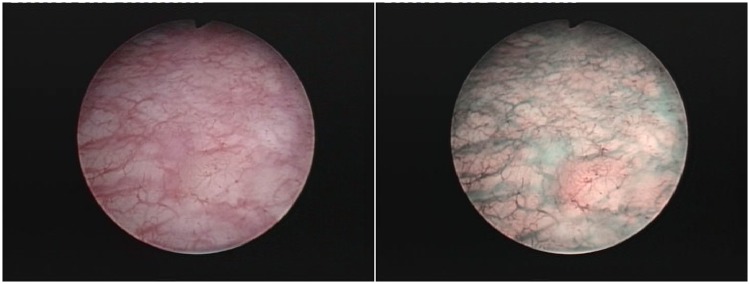
Figure 1.
Carcinoma in situ lesion missed during standard cystoscopy and only visible in narrow-band imaging.
Table 1.
Patient detection rates.
| NBI | WLC | p | Patients (n) | |
|---|---|---|---|---|
| Global | 75 (96.2%) | 68 (87.2%) | 0.007 | 78 |
| CIS | 9 (100%) | 6 (66.7%) | 0.026 | 9 |
| pTa | 46 (93.9%) | 43 (87.8%) | 0.136 | 49 |
| pT1 | 20 (100%) | 19 (95%) | 0.358 | 20 |
CIS, carcinoma in situ; NBI, narrow-band imaging; WLC, white light cystoscopy.
For lesion detection rates, NBI cystoscopy showed significantly superior diagnostic accuracy compared with WLC for CIS, pTa and overall non-muscle-invasive bladder tumors (95.2% versus 61.9%, 93.9% versus 85.2% and 94.8% versus 83.9% respectively) (Figure 2). In addition, more pT1 tumors were found using NBI versus WLC (97.4% versus 92.1%); however the results failed to reach statistical significance (Table 2 and Figure 3).
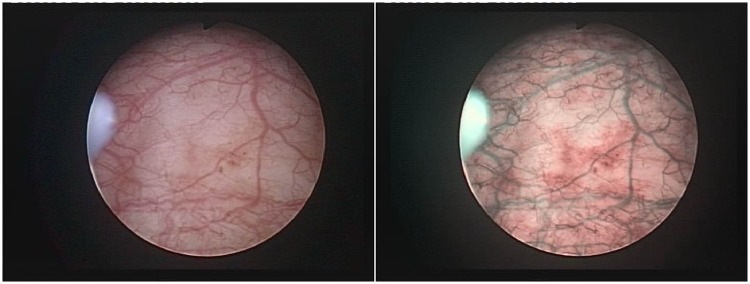
Figure 2.
pTa tumor diagnosed on narrow-band imaging cystoscopy alone.
Table 2.
Tumor detection rates.
| NBI | WLC | p | Tumors (n) | |
|---|---|---|---|---|
| Global | 165 (94.8%) | 146 (83.9%) | <0.0001 | 174 |
| CIS | 20 (95.2% | 13 (61.9%) | 0.001 | 21 |
| pTa | 108 (93.9%) | 98 (85.2%) | 0.003 | 115 |
| pT1 | 37 (97.4%) | 35 (92.1%) | 0.187 | 38 |
CIS, carcinoma in situ; NBI, narrow-band imaging; WLC, white light cystoscopy.
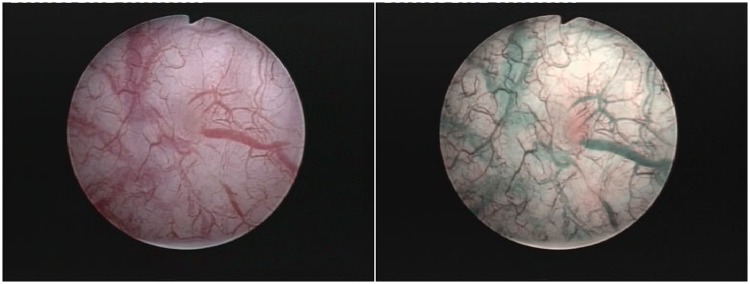
Figure 3.
Narrow-band imaging found pT1 tumor, left behind during white light cystoscopy.
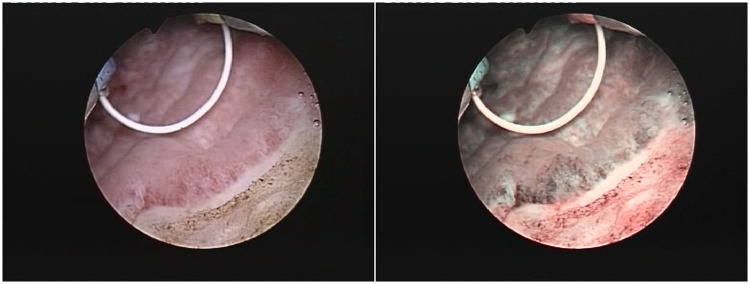
The rate of false-positive results was slightly higher for NBI compared with standard cystoscopy but the results were not statistically significant (13.6% versus 11.5%; p = 0.208, binomial test). Moreover, pathologically confirmed positive tumoral margins secondary to white light TURBT were found during NBI cystoscopic control in 10.3% of cases (two CIS, two pTa and four pT1) (Figure 4).
Figure 4.
Positive tumoral margins missed during standard transurethral resection of bladder tumors and discovered at the narrow-band imaging control.
Additional tumors were diagnosed by NBI cystoscopy in a significantly higher proportion of patients with CIS, pTa, pT1 and NMIBC in comparison to WLC (55.5% versus 11.1%, 26.5% versus 10.2%, 30% versus 10% and 30.8% versus 10.3% respectively) (Table 3).
Table 3.
Additional tumor cases.
| NBI | WLC | p | Patients (n) | |
|---|---|---|---|---|
| Global | 24 (30.8%) | 8 (10.3%) | <0.0001 | 78 |
| CIS | 5 (55.5%) | 1 (11.1%) | 0.001 | 9 |
| pTa | 13 (26.5%) | 5 (10.2%) | 0.005 | 49 |
| pT1 | 6 (30%) | 2 (10%) | 0.035 | 20 |
CIS, carcinoma in situ; NBI, narrow-band imaging; WLC, white light cystoscopy.
As a result of these supplementary diagnostic findings, postoperative instillation treatment was improved due to NBI cystoscopy in a significantly larger number of NMIBC cases (16.7% versus 5.1%; p < 0.0001, test binomial).
Discussion
According to the literature, 50–70% of patients with NMIBC display recurrence after the initial treatment [Sylvester et al. 2002]. Numerous cases of early recurrences are determined by incomplete tumor removal during TURBT [Tatsugami et al. 2010]. Consequently, many methods of improving bladder cancer diagnosis and treatment were evaluated.
Photodynamic diagnosis was proved to be particularly useful in CIS cases as it provided the conditions for a better resection. Blue light cystoscopy emphasized the ability to identify white light ‘overlooked’ flat or small papillary tumors and to target biopsies in cases of positive urinary cytology. The advantages of this technique consisted of achieving a more complete resection, reduced recurrence rates [Denzinger et al. 2007] and a more adequate postoperative instillation treatment [Jocham et al. 2005]. However, this diagnostic approach requires a preoperative intravesical instillation substantially increasing the costs of the procedure, which also becomes more time consuming [Katz and Steinberg, 2008].
NBI cystoscopy is a high-resolution diagnostic alternative that aims to increase the detection of urothelial lesions in comparison to standard white light endoscopic imaging without the use of dyes [Naselli et al. 2010b]. Basically, the optical image enhancement technology increases the visibility of capillaries and other tissue surface structures by emphasizing the contrast between them [Traxer et al. 2011]. The basis of this optical technique is represented by white light being filtered into two discrete center wavelengths for blue (415 nm) and green (540 nm). These light bands are strongly absorbed by hemoglobin and only penetrate the tissue surface [Cauberg et al. 2010]. Therefore, the vascular structures appear dark brown (capillary vessels) and green (veins), thus contrasting with the pink or white background of normal mucosa [Naselli et al. 2010b; Traxer et al. 2011; Cauberg et al. 2010].
Subjectively speaking, both in the literature and in our experience, NBI is described to accurately underline the specific vascular architecture of urothelial lesions, thus creating the impression of a three-dimensional visualization of tumors and clearly defining their limits [Traxer et al. 2011].
One of the main advantages of NBI is that there are no contraindications for the patient and it requires no instillation or disposable items. Moreover, NBI can be used an unlimited number of times and there is no maximum duration for each procedure as costs are minimal (only related to the actual device) because it consists of a software modification to the endoscope’s optical structures [Naselli et al. 2010a]. NBI use was also investigated for several gastrointestinal diseases and showed significant diagnostic benefits [Lambert et al. 2007].
In urological practice, NBI was applied as a viable modality of improving urothelial tumor detection because of the advantages of no additional running costs or morbidity [Naselli et al. 2010b]. In addition, there seems to be no particular learning curve in diagnosing bladder tumors when using this relatively new technique [Herr et al. 2010].
A significantly higher overall tumor detection rate was described for NBI cystoscopy versus WLC both in the literature (94.7% versus 79.2%) [Cauberg et al. 2010] and in the present trial (94.8% versus 83.9%). From the tumor stage point of view, improved CIS (89.7% versus 50%) [Tatsugami et al. 2010] and pTa (98.7% versus 82.9%) [Cauberg et al. 2010) detection rates were described for NBI cystoscopy in comparison to the standard approach according to the literature and these results were confirmed by our findings (100% versus 66.7% and 93.9% versus 87.8% respectively). Moreover, in light of the published data, a significant proportion of patients with NMIBC were diagnosed using NBI cystoscopy alone (12.6–26.9%) [Herr and Donat, 2008; Tatsugami et al. 2010], as also demonstrated by the present trial (12.8%).
For the overall detection rate of patients with NMIBC, a recent study reported the substantial advantage of NBI over the standard method (97.9% versus 88.8%) [Chen et al. 2011]. Our results confirmed the improved accuracy of the NBI mode in comparison to white light (96.2% versus 87.2%).
From another perspective, NBI was shown to offer a significantly more precise delineation of the tumor margins due to the superior visualization of the surface layer [Tatsugami et al. 2010]. In our series, NBI cystoscopy emphasized additional extended positive tumoral margins in the normal-appearing mucosa surrounding the tumors, confirmed by pathology in 10.3% of NMIBC cases.
Improved detection led to a significant proportion of additional tumors being found using NBI, both according to the available studies (35.9–38% [Bryan et al. 2008, 2010; Cauberg et al. 2010] and our experience (30.8%). In addition, in another paper, 34% of patients with NMIBC were found to have residual/recurrent cancer when using an extensive protocol of second transurethral resection followed by NBI biopsies [Naselli et al. 2010a]. Moreover, in a recent article, NBI flexible cystoscopy was reported to significantly improve the detection of unconfirmed positive voided urine cytology over WLC. According to this study, NMIBC was diagnosed on first NBI cystoscopy in 42% of cases [Zhu et al. 2012].
NBI cystoscopy appeared to be associated with higher rates of false-positive results compared with white light (31.6–36% versus 24.5–33%) [Herr and Donat, 2008; Cauberg et al. 2010]. From this perspective, our analysis described reasonably reduced and quite similar results for NBI and standard cystoscopy, despite the more frequent false-positive results found in NBI (13.6% versus 11.5%; no statistically significant difference).
The improved cystoscopic examination using NBI resulting in more tumors being found and more NMIBC cases being diagnosed had a significant impact in terms of improved postoperative treatment (16.7% of the cases benefited from better adjuvant intravesical therapy). This finding may be considered promising, especially since practically similar results (13-17%) were obtained concerning the photodynamic diagnostic, which otherwise implies additional expenses related to the required preoperative instillation of a contrast medium [Fradet et al. 2007; Jocham et al. 2005; Abascal Junquera et al. 2008].
Of course, future studies will need to establish the long-term advantages and general viability of this endoscopic alternative, particularly from the perspective of tumor recurrence.
Conclusions
NBI cystoscopy showed significantly improved detection rates of CIS, overall NMIBC and lesions in comparison to WLC. Moreover, significantly more pTa tumors were found using NBI, while pathologically confirmed positive tumoral margins secondary to standard TURBT were found using NBI cystoscopic control in about 10% of cases. However, the rate of false-positive results was similar for the two methods.
In addition, supplementary tumors were diagnosed by NBI cystoscopy in a significantly higher proportion of patients with CIS, pTa, pT1 and overall NMIBC in comparison to WLC. Consequently, postoperative treatment was improved due to NBI results in a significantly larger number of cases.
In conclusion, NBI cystoscopy seems to represent a valuable diagnostic alternative in patients with NMIBC, showing significant improvement in tumor visual accuracy and detection. This approach also provides a substantial improvement in postoperative therapeutic management of bladder cancer.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Bogdan Geavlete received honorariums from Olympus when he presented lectures at company sponsored symposiums.
Contributor Information
Bogdan Geavlete, ‘Saint John’ Emergency Clinical Hospital, Department of Urology, Bucharest, Romania.
Marian Jecu, ‘Saint John’ Emergency Clinical Hospital, Department of Urology, Bucharest, Romania.
Razvan Multescu, ‘Saint John’ Emergency Clinical Hospital, Department of Urology, Bucharest, Romania.
Petrisor Geavlete, ‘Saint John’ Emergency Clinical Hospital, Department of Urology, Vitan Barzesti Street No. 13, Sector 4, 042122 Bucharest, Romania.
References
- Abascal Junquera J., Hevia Suárez M., Abascal García J., Estébanez C., Astudillo A., Abascal R. (2008) Initial experience in the diagnosis and treatment of superficial bladder tumors with Hexvix. Arch Esp Urol 61: 475–482 [DOI] [PubMed] [Google Scholar]
- Bryan R., Billingham L., Wallace D. (2008) Narrow-band imaging flexible cystoscopy in the detection of recurrent urothelial cancer of the bladder. BJU Int 101: 702–705 [DOI] [PubMed] [Google Scholar]
- Bryan R., Shah Z., Collins S., Wallace D. (2010) Narrow-band imaging flexible cystoscopy: a new user’s experience. J Endourol 24: 1339–1343 [DOI] [PubMed] [Google Scholar]
- Cauberg E., Kloen S., Visser M., de la Rosette J., Babjuk M., Soukup V., et al. (2010) Narrow band imaging cystoscopy improves the detection of non-muscle-invasive bladder cancer. Urology 76: 658–663 [DOI] [PubMed] [Google Scholar]
- Chen G., Wang B., Li H., Ma X., Shi T., Zhang X. (2011) Applying narrow-band imaging in complement with white-light imaging cystoscopy in the detection of urothelial carcinoma of the bladder. Urol Oncol 11 November (epub ahead of print). [DOI] [PubMed] [Google Scholar]
- Denzinger S., Burger M., Walter B., Kneuchel R., Roessler W., Wieland W., et al. (2007) Clinically relevant reduction in risk of recurrence of superficial bladder cancer using 5-aminolevulinic acid induced fluorescence diagnosis: 8 year results of prospective randomized study. Urology 69: 675–679 [DOI] [PubMed] [Google Scholar]
- Filbeck T., Pichlmeier U., Knuechel R., Wieland W., Roessler W. (2002) Clinically relevant improvement of recurrence-free survival with 5-aminolevulinic acid induced fluorescence diagnosis in patients with superficial bladder tumors. J Urol 168: 67–71 [PubMed] [Google Scholar]
- Fradet Y., Grossman H., Gomella L., Lerner S., Cookson M., Albala D., et al. (2007) PC B302/01 Study Group. A comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of carcinoma in situ in patients with bladder cancer: a phase III, multicenter study. J Urol 178: 68–73 [DOI] [PubMed] [Google Scholar]
- Gono K., Obi T., Yamaguchi M., Ohyama N., Machida H., Sano Y., et al. (2004) Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt 9: 568–577 [DOI] [PubMed] [Google Scholar]
- Grasso M. (2008) Bladder cancer: a major public health issue. Eur Urol 7(Suppl. 7): 510–515 [Google Scholar]
- Hamamoto Y., Endo T., Nosho K., Arimura Y., Sato M., Imai K. (2004) Usefulness of narrowband imaging endoscopy for diagnosis of Barrett’s esophagus. J Gastroenterol 39: 14–20 [DOI] [PubMed] [Google Scholar]
- Herr H., Donat S. (2008) A comparison of white-light cystoscopy and narrow-band imaging cystoscopy to detect bladder tumour recurrences. BJU Int 102: 1111–1114 [DOI] [PubMed] [Google Scholar]
- Herr H., Donat M., Dalbagni G., Taylor J. (2010) Narrow-band imaging cystoscopy to evaluate bladder tumours – individual surgeon variability. BJU Int 106: 53–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jocham D., Witjes F., Wagner S., Zeylemaker B., van Moorselaar J., Grimm M., et al. (2005) Improved detection and treatment of bladder cancer using hexaminolevulinate imaging: a prospective, phase III multicenter study. J Urol 174: 862–866 [DOI] [PubMed] [Google Scholar]
- Katz M., Steinberg G. (2008) Should fluorescence cystoscopy be used for transurethral resection in patients with high-grade T1 bladder cancer? Nat Clin Pract Urol 5: 472–473 [DOI] [PubMed] [Google Scholar]
- Lambert R., Kuznetsov K., Rey J. (2007) Narrow-band imaging in digestive endoscopy. ScientificWorldJournal 30: 449–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannath J., Subramanian V., Hawkey C., Ragunath K. (2010) Narrow band imaging for characterization of high grade dysplasia and specialized intestinal metaplasia in Barrett’s esophagus: a meta-analysis. Endoscopy 42: 351–359 [DOI] [PubMed] [Google Scholar]
- Naselli A., Introini C., Bertolotto F., Spina B., Puppo P. (2010a) Narrow band imaging for detecting residual/recurrent cancerous tissue during second transurethral resection of newly diagnosed non-muscle-invasive high-grade bladder cancer. BJU Int 105: 208–211 [DOI] [PubMed] [Google Scholar]
- Naselli A., Introini C., Bertolotto F., Spina B., Puppo P. (2010b) Feasibility of transurethral resection of bladder lesion performed entirely by means of narrow-band imaging. J Endourol 24: 1131–1134 [DOI] [PubMed] [Google Scholar]
- Oliva Encina J., Marco Valdenebro A., Pelegrí Gabarró J., Rioja Sanz C. (2010) Beyond the photodynamic diagnosis: searching for excellence in the diagnosis of non-muscle-invasive bladder cancer. Actas Urol Esp 34: 657–668 [PubMed] [Google Scholar]
- Rastogi A., Keighley J., Singh V., Callahan P., Bansal A., Wani S., et al. (2009) High accuracy of narrow band imaging without magnification for the real-time characterization of polyp histology and its comparison with high-definition white light colonoscopy: a prospective study. Am J Gastroenterol 104: 2422–2430 [DOI] [PubMed] [Google Scholar]
- Sylvester R., van der Meijden A., Lamm D. (2002) Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol 168: 1964–1970 [DOI] [PubMed] [Google Scholar]
- Tatsugami K., Kuroiwa K., Kamoto T., Nishiyama H., Watanabe J., Ishikawa S., et al. (2010) Evaluation of narrow-band imaging as a complementary method for the detection of bladder cancer. J Endourol 24: 1807–1811 [DOI] [PubMed] [Google Scholar]
- Traxer O., Geavlete B., de Medina S., Sibony M., Al-Qahtani S. (2011) Narrow-band imaging digital flexible ureteroscopy in detection of upper urinary tract transitional-cell carcinoma: initial experience. J Endourol 25: 19–23 [DOI] [PubMed] [Google Scholar]
- Zhu Y., Shen Y., Ye D., Wang C., Yao X., Zhang S., et al. (2012) Narrow-band imaging flexible cystoscopy in the detection of clinically unconfirmed positive urine cytology. Urol Int 88: 84–87 [DOI] [PubMed] [Google Scholar]