SUMMARY
Optimization of membrane protein stability under different solution conditions is essential for obtaining crystals that diffract to high resolution. Traditional methods that evaluate protein stability require large amounts of material, and are therefore ill-suited for medium- to high-throughput screening of membrane proteins. Here we present a rapid and efficient fluorescence-detection size-exclusion chromatography-based thermostability assay (FSEC-TS). In this method, the target protein is fused to GFP. Heated protein samples, treated with a panel of additives, are then analyzed by FSEC. FSEC-TS allows one to evaluate the thermostability of nanogram to microgram amounts of the target protein under a variety of conditions without purification. We applied this method to the Danio rerio P2X4 receptor and Caenorhabditis elegans GluCl to screen ligands, ions and lipids, including newly designed cholesterol derivatives. In the case of GluCl, the screening results were used to obtain crystals of the receptor in the presence of lipids.
INTRODUCTION
Growth of membrane protein crystals for the purpose of determining the underlying atomic structure traditionally requires a labor-intensive screening approach to identify conditions that stabilize the protein of interest. Traditionally, this “pre-crystallization” screening is performed with purified protein, which places a requirement for large amounts of pure material and, necessarily, the ability to purify the protein of interest. Membrane proteins, particularly those from eukaryotes, are typically expressed at low levels and are often unstable during and after purification. The challenge of eukaryotic membrane protein crystallography bears itself out in the Protein Data Bank where <1% of the deposited structures are from this elusive category. The degree to which one can identify, in advance of protein purification, conditions and compounds that stabilize a given protein candidate, the higher the likelihood that protein purification will proceed smoothly and well-diffracting crystals will be obtained. Atomic structures of membrane proteins are of tremendous interest from both basic science and drug development perspectives; relatively little structural information exists for this class of proteins, molecular mechanisms of signal transduction across cell membranes are not well understood, and drug design for targets of many currently prescribed therapeutics (Zambrowicz and Sands, 2003) is rendered relatively blind.
One useful technique for pre-crystallization screening of membrane proteins is fluorescence-detection size-exclusion chromatography (FSEC) (Kawate and Gouaux, 2006). In this method, the target protein is expressed as a GFP fusion, and the SEC profile of the resulting fusion protein is monitored by fluorescence spectroscopy. This simple method allows for rapid evaluation of protein expression level and monodispersity of the target protein using nanograms to micrograms of unpurified material from whole-cell extracts. This method has proved to be a powerful tool for screening panels of membrane protein orthologs, detergents and conformationally-sensitive antibodies, and thus has led to the recent determination of a number of membrane protein structures from this laboratory including LeuT, ApcT, ASIC, P2X, GluA2 and GluCl (Hattori and Gouaux, 2012; Hibbs and Gouaux, 2011; Jasti et al., 2007; Kawate et al., 2009; Krishnamurthy and Gouaux, 2012; Shaffer et al., 2009; Sobolevsky et al., 2009; Yamashita et al., 2005).
There are several methods to evaluate protein stability that have already been described, but these techniques often require large amounts of pure protein, and therefore are unsuitable for high-throughput screening of eukaryotic membrane proteins. Fluorescent dye-based thermal stability assays using a thiol-reactive fluorophore N-[4-(7-diethylamino-4-methyl-3-coumarinyl)phenyl]maleimide (CPM) (Alexandrov et al., 2008), have been developed to evaluate the thermostability of membrane proteins in a high-throughput manner. Although the method requires only microgram samples of protein, because of the high sensitivity of the CPM dye, it requires free cysteine residues embedded in the protein core. A thermostability assay that relies upon analytical size-exclusion chromatography was used effectively to screen compounds and conditions for stabilizing membrane proteins for crystallization (Czyzewski and Wang, 2012; Mancusso et al., 2011), but this latter method requires a relatively large amount of purified sample, due to the low sensitivity of UV absorbance.
In the current study, we present a new FSEC-based thermostability assay (FSEC-TS). In this method, nanogram to microgram quantities of purified or unpurified proteins are incubated over a range of temperatures, and then are applied to a SEC column in line with a fluorescence detector to monitor GFP or tryptophan fluorescence. The results from FSEC-TS provide a denaturation or “melting” temperature (Tm), which is used as a reference point to test the degree of protein thermostabilization by a panel of small molecules. We applied this simple and rapid method to the D. rerio P2X4 receptor and the C. elegans GluCl channel to screen ligands, divalent cations and lipids, including new synthetic cholesterol derivatives. We find that thermostabilization of both proteins by different compounds is not qualitatively altered by the presence of GFP, and that in proof-of-principle experiments, Tm by FSEC-TS agrees well with Tm determined by a radioligand binding assay. The results are useful in identifying compounds for stabilizing the proteins during purification and in crystallization, and in the case of GluCl have been used to obtain a new crystal form.
RESULTS
Validation of FSEC-TS
To validate our assay, we used three proteins: EGFP, D. rerio P2X4 (zfP2X4) receptor and C. elegans glutamate-gated chloride channel α (GluCl). Protein samples were incubated over a range of temperatures for 10 min using a thermal cycler (Figure 1), followed by ultracentrifugation to remove precipitated material. The supernatant was then applied to a SEC column attached to a fluorescence detector to monitor GFP or tryptophan fluorescence (Figure 1). In these experiments, we used detergents that we knew from previous experience would stabilize the native oligomeric state of the receptors.
Figure 1. Flow chart of FSEC-TS.
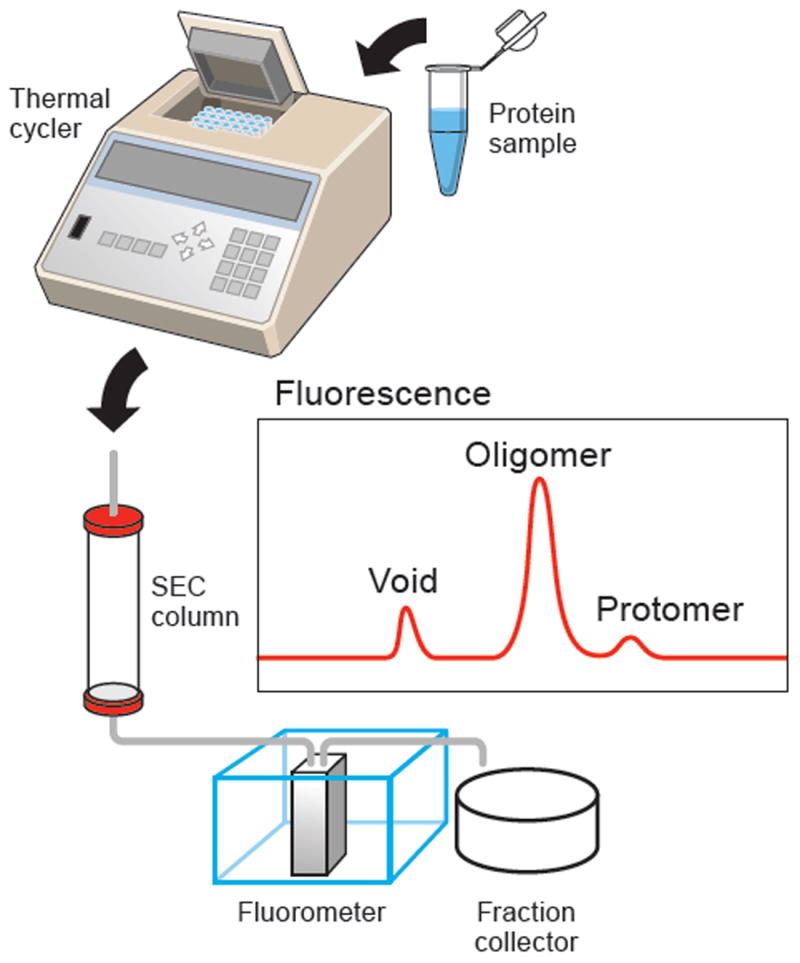
The protein sample, after heat treatment and centrifugation, is loaded onto a SEC column, connected to a fluorescence detector to monitor GFP or tryptophan fluorescence. The panel labeled “Fluorescence” shows a hypothetical elution profile from GFP or tryptophan fluorescence.
As shown in Figure 2A, peak profiles from EGFP samples incubated between 4 °C and 65 °C were sharp and symmetrical, indicating that the protein remained monodisperse. In contrast, a clear thermal shift of the peak profiles was observed from the samples incubated at temperatures near to and above 70 °C (Figure 2A). Aggregation and precipitation after heating resulted in a significant decrease in the peak height, and a melting curve, from the fluorescence signal intensity at the respective temperatures, was observed (Figure 2B). The calculated Tm is 76 °C, which is consistent with the reported melting temperature for GFP (76 °C), the temperature where one half of the intrinsic fluorescence is lost (Bokman and Ward, 1981; Ward, 1981).
Figure 2. Thermostability profiles of zfP2X4 and GluCl.
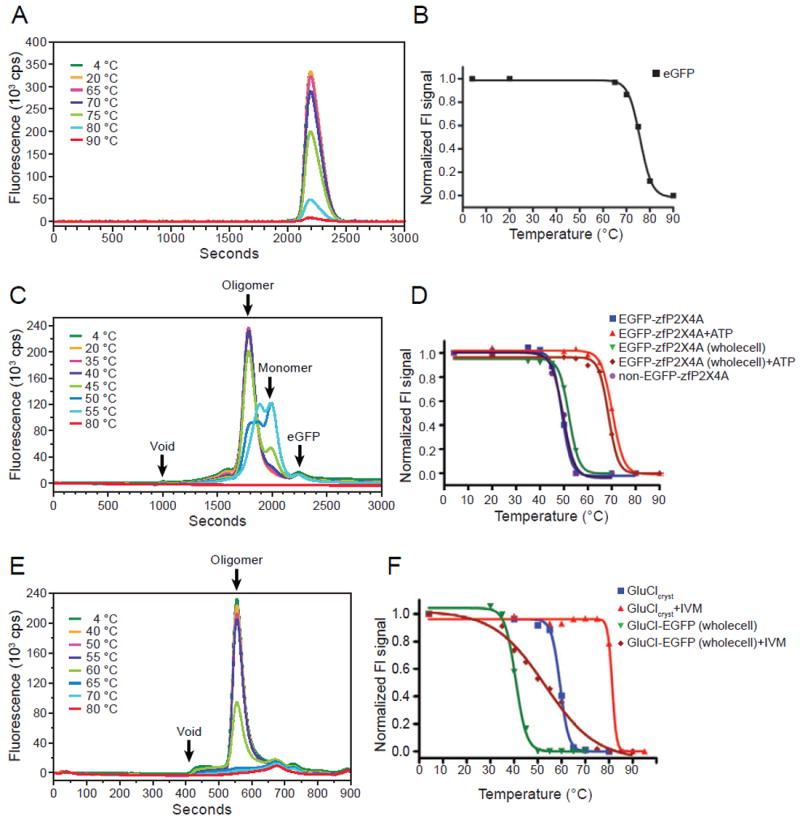
Representative FSEC profiles from EGFP (A), EGFP-ΔP2X4-A (C) and GluClcryst (E), heated at the respective temperatures, detected by GFP fluorescence (A, C) or by Trp fluorescence (E). Representative melting curves of EGFP (B), EGFP-tagged and EGFP-free ΔP2X4-A (D) and GluClcryst and GluCl-EGFP (F). Melting temperatures (Tm) were determined by fitting the curves to a sigmoidal dose-response equation. The fitted curves are shown as a black line. See also Figure S1.
P2X receptors are non-selective cation channels gated by extracellular ATP, and are implicated in diverse physiological processes such as synaptic transmission, inflammation, and taste and pain sensing (Jarvis and Khakh, 2009; North, 2002; Surprenant and North, 2009). Seven P2X receptor subtypes assembled into either homomeric or heteromeric trimers (Jarvis and Khakh, 2009; North, 2002). Recently our group reported the crystal structure of zfP2X4 in an apo, closed-channel conformation (Kawate et al., 2009). Precrystallization screening of P2X orthologs by FSEC had identified zfP2X4 as a promising target for the crystallization trials.
The well-behaved, crystallized construct of a N-terminal EGFP fusion to zebrafish P2X4 (ΔP2X4-A)(Kawate et al., 2009) was expressed with an octa-histidine affinity tag and purified for the assay (Figure S1A). FSEC peak profiles from the EGFP-tagged ΔP2X4-A samples incubated between 4 °C and 40 °C were sharp and symmetrical (Figure 2C); these peaks correspond to the functional P2X receptor trimer. A clear thermal shift of the peak profiles was observed from the samples incubated around 50 °C (Figure 2C). Protein denaturation led to a significant decrease in the trimer peak height and concomitant increase in the putative monomer peak height. A clear melting curve from the trimer peak height at the respective temperatures was observed (Figure 2D). The calculated Tm is 49°C, based on the trimer peak height.
To verify that the result from FSEC-TS is relevant to binding-competent receptor, we measured the ATP binding activity of the heat-treated samples (Figure S1B). The experiment was performed at 100 nM ATP concentration, approximately 5-fold above the Kd value of 19.6±3.8 nM (Figure S1C). The calculated Tm is 47.8±0.3 °C, consistent with FSEC-TS (49 °C). To assess the effect of the GFP-tag on Tm, we also obtained a melting curve from EGFP-free ΔP2X4-A samples by monitoring tryptophan fluorescence from the endogenous Trp residues in ΔP2X4-A (Figure 2D); the estimated Tm is 50 °C, not significantly different from the Tm for EGFP-tagged ΔP2X4-A (49 °C).
GluCl is a pentameric chloride channel gated by glutamate and ivermectin (Cully et al., 1994), and is a member of the Cys-loop receptor superfamily (Thompson et al., 2010). This receptor family also includes the ion channels activated by acetylcholine, serotonin, γ-aminobutyric acid and glycine. GluCl was identified as a promising target for crystallization by FSEC screening of Cys-loop receptor orthologs, which subsequently led to the determination of the structure of a GluCl-Fab complex with the allosteric agonist ivermectin bound and the channel in an open conformation (Hibbs and Gouaux, 2011).
We carried out FSEC-TS with GluCl to test the validity of the assay and to identify compounds for stabilizing the receptor in a resting, closed channel state. The crystallized construct of EGFP-free GluCl (GluClcryst) was purified (Figure S1D) and incubated at 4-80 °C and applied to a SEC column, with the eluted material monitored by tryptophan fluorescence (Figure 2E). We used the TSKgel SuperSW2000 column for GluCl, which requires only 15 minutes per sample, to test many different lipid combinations in a high-throughput manner, as described later. A melting curve based on the peak height of the pentameric species was observed (Figure 2F), with a calculated Tm of a remarkably high 59 °C. Determination of Tm based on a radioligand binding experiment using 3H ivermectin yields a similar melting temperature of 58 °C (Figure S1E).
The FSEC-TS results from EGFP, zfP2X4 and GluCl demonstrate that a combination of heat-treatment and FSEC can be applied to monitor the temperature-induced unfolding of soluble and membrane proteins by detecting GFP fluorescence from GFP-tagged proteins or by measuring tryptophan fluorescence from endogenous Trp residues.
Evaluation of ligands for cocrystallization by FSEC-TS
To test whether FSEC-TS can be used to estimate the effect of ligands on membrane proteins for crystallization, we evaluated the thermostabilizing effects of ATP on zfP2X4 and of ivermectin on GluClcryst. Both ligands bind to their cognate receptors with high affinity and thus we can use them to examine whether or not we can pick up stabilizing effects of tightly bound ligands. In FSEC-TS, both ATP and ivermectin had dramatic stabilizing effects, increasing the calculated Tm by 21 °C for ΔP2X4-A and by 22 °C for GluCl (Figures 2D and 2F, respectively). In accordance with these results, crystallization of zfP2X4 with ATP yields crystals that diffract to 2.8 Å resolution (Hattori and Gouaux, 2012) and crystals of the GluCl-ivermectin complex diffract to 3.3 Å resolution (Hibbs and Gouaux, 2011).
FSEC-TS with unpurified protein
One of the advantages of FSEC using GFP-tagged protein is that unpurified protein can be analyzed, due to the wide spectral separation between GFP fluorescence and the fluorescence intrinsic to most proteins. To test whether unpurified protein can be analyzed using FSEC-TS, EGFP-tagged ΔP2X4-A and GluCl obtained from whole-cell lysates were heat-treated in the presence and absence of ATP and ivermectin, respectively, and applied to an SEC column after centrifugation.
For EGFP-ΔP2X4-A, the calculated Tm values in the absence and presence of ATP are 52 °C and 69 °C respectively (Figure 2D), consistent with the Tm values from the purified EGFP-fusion ΔP2X4-A, which are 49 °C in the absence of ATP and 70 °C in the presence of ATP.
For GluCl-EGFP, the apparent Tm values in the absence and presence of ivermectin are 41 and 53 °C (Figure 2F), respectively. These values are significantly different from the apparent Tm values from the purified GluClcryst (58 °C in the absence of ivermectin and 81 °C in the presence of ivermectin). There are two likely sources of the difference in Tm between GluCl-EGFP and GluClcryst. First, unlike in zfP2X4 wherein EGFP is positioned at the receptor terminus, in GluCl the EGFP is inserted into an internal loop, between the third and fourth transmembrane helices. We suggest that insertion of GFP into this loop destabilizes the protein. Second, GluCl-EGFP contains the full-length, wild-type sequence of the GluCl gene, while GluClcryst is a truncated construct used for crystallization. Regardless of the differences in Tm, however, the qualitative effect of ivermectin on GluCl thermostability remains unchanged. Taken together, we conclude that FSEC-TS can be used to analyze unpurified GFP-tagged protein.
Effect of divalent cations on zfP2X4
The effects of three different divalent cations (Ca2+, Mg2+ and Zn2+) on zfP2X4 thermostability were tested by FSEC-TS (Figure 3A). EGFP-tagged ΔP2X4-A samples in a series of buffers containing the respective additives were heat-treated at 50 °C for 10 minutes, which is close to the calculated Tm of apo protein. As in the case for other P2X receptors, ATP-responses at P2X4 receptors can be modulated by divalent cations such as Zn2+ (Garcia-Guzman et al., 1997; Wildman et al., 1999), and Mg2+ ions (Negulyaev and Markwardt, 2000). The significant divalent cation-induced stabilization of the trimer peak is observed in the presence of all tested divalent cation (Figure 3A). Consistently, best crystals of zfP2X4 in an apo, closed state (Kawate et al., 2009) as well as crystals in the presence of ATP (Hattori and Gouaux, 2012) were obtained in presence of Mg2+.
Figure 3. Effects of additives on the thermostability of zfP2X4.
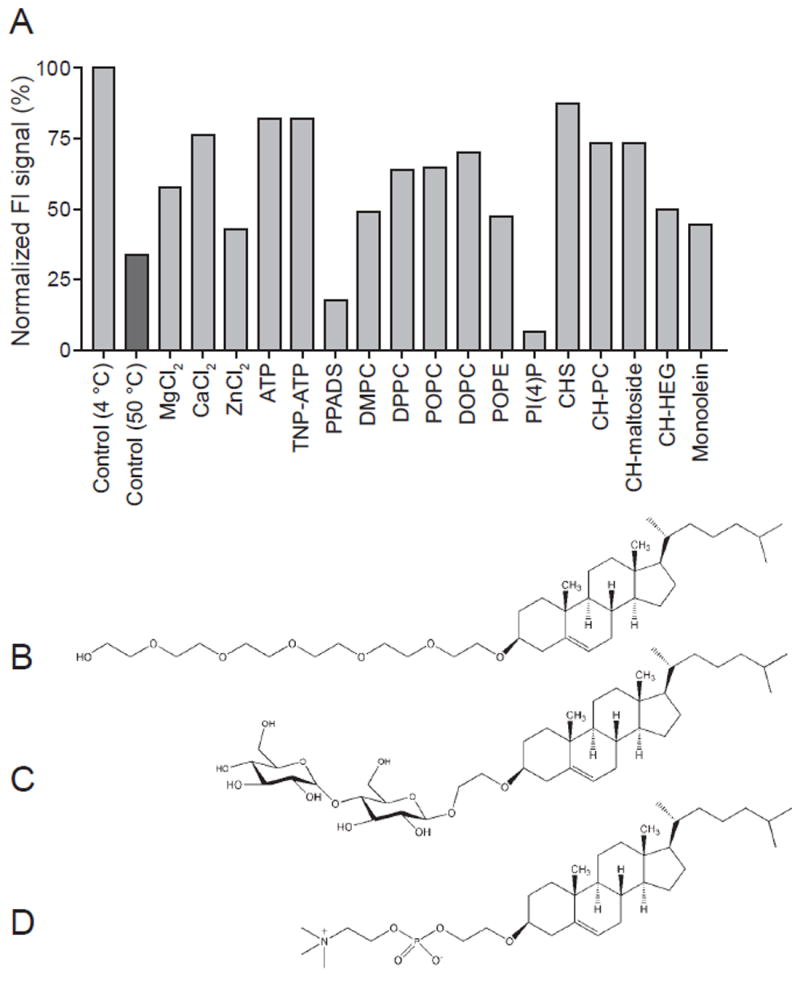
The normalized peak heights from EGFP-fusion ΔP2X4-A (A), heat-treated at 50 °C for 10 minutes, in the presence of the respective additives. The peak heights were normalized to that from the sample at 4 °C without any additive. (C-E) Chemical structures of novel cholesterol derivatives, hexaethyleneglycol hydroxyethyl cholesterol (C) maltoside hydroxyethyl cholesterol (D) and phosphocholine hydroxyethyl cholesterol (E), employed in the FSEC-TS studies. Structures were created using the ChemDraw (Cambridge Scientific Computing). See also Figure S2 and Table S1.
Effect of ligands on zfP2X4
The effects of different P2X ligands, 2′,3′-O-(2,4,6-trinitrophenyl) adenosine 5 ′-triphosphate (TNP-ATP) (antagonist) and pyridoxalphosphate-6-azophenyl-2’,4’-disulphonic acid (PPADS) (antagonist) on the thermostability of GFP-fusion ΔP2X4-A were tested by FSEC-TS (Figure 3A). TNP-ATP stabilized the zfP2X4 trimer to a similarly strong extent as was observed with ATP (Figure 3A). PPADS, by contrast, destabilized the protein (Figure 3A). In correlation to the effects of ligands on thermostability, we have successfully crystallized zfP2X4 in the presence of TNP-ATP but have not been able to produce crystals with PPADS.
Effect of lipids on zfP2X4
Purification of membrane proteins from their native environment requires the use of detergents. However, detergent solubilization of membrane proteins often leads to aggregation, because membrane proteins tend to be less stable in detergent micelles than in the lipid bilayer. To remedy this behavior, addition of lipids during purification or crystallization can be used to improve membrane protein behavior for the purposes of biophysical characterization, crystallization and structure determination (Guan et al., 2006; Hattori et al., 2007; Hibbs and Gouaux, 2011; Krishnamurthy and Gouaux, 2012; Lemieux et al., 2003; Sobolevsky et al., 2009; Zhang et al., 2003).
The effects of ten different lipids on the thermostability of GFP-fusion ΔP2X4-A were examined by FSEC-TS (Figure 3A) and also by ATP binding assay (Figure S2). Among the tested lipids, phosphocholine hydroxyethyl cholesterol (CH-PC), maltoside hydroxyethyl cholesterol (CH-maltoside) and hexaethyleneglycol hydroxyethyl cholesterol (CH-HEG) are newly designed cholesterol derivatives (Figure 3B-D). Hydroxyl groups of cholesterol are connected to phosphocholine (CH-PC), maltose (CH-maltoside), or hexaethyleneglycol (CH-HEG) via hydroxyethyl group. Cholesterol cholesteryl hemisuccinate (CHS) as well as cholesterol has been successfully used for purification and crystallization of GPCRs (Cherezov et al., 2007; Rasmussen et al., 2011; Rosenbaum et al., 2011; Soriano et al., 2009). However, the ester group of CHS can be hydrolysis- sensitive, and cholesterol itself is sparingly soluble. Our cholesterol derivatives are more stable because their head groups are connected to hydroxyl groups of cholesterol via a hydroxyethyl group. Additionally, the new head groups of the derivatives might have stabilizing effects on membrane proteins. Therefore, we tested these cholesterol derivatives as well as other lipids by FSEC-TS.
Surprisingly, most of the lipids except for the phosphoinositide, PI(4)P, significantly stabilized the trimer peak of zfP2X4 in FSEC (Figure 3A), which is largely consistent with thermostabilization observed by the same compounds in a radioligand binding assay (Figure S2). Among phosphocholine-containing lipids, the lipids with longer alkyl chains more effectively stabilized the trimer peak (Figure 3A). We also tested one of the phosphoglycerol-containing lipids, POPE (Figure 3A). Although the chain length of POPE is the same as that of POPC, POPC stabilized the trimer peak of zfP2X4 more effectively than POPE.
Among cholesterol derivatives, CH-HEG was the poorest additive in terms of both FSEC-TS and the radioligand binding assay (Figure 3A and Figure S2). Based on FSEC-TS, CHS was slightly better than CH-PC and CH-maltoside, but based on the binding assay, CH-PC had the strongest stabilizing effect among the tested lipids (Figure 3A and Figure S2).
We also tested monoolein (MO), which has been used for membrane protein crystallization in the lipidic cubic phase (Johansson et al., 2009). MO also stabilized the trimer peak of zfP2X4 by FSEC-TS, but was not better than other “good” lipids such as POPC (Figure 3A).
Among all tested lipids, only PI(4)P totally destabilized the trimer peak of zfP2X4 by FSEC-TS (Figure 3A), and consistently in the presence of this lipid the receptor showed almost no ATP binding activity (Figure S2). Interestingly, phosphoinositides are known to regulate the activity of P2X4 receptors through direct interactions (Bernier et al., 2008).
Effect of lipids on GluCl
We previously found that either POPC or DPPC were required for growth of well-diffracting crystals of the GluCl-Fab-ivermectin complex (Hibbs and Gouaux, 2011). To screen lipids for a more stabilizing effect on GluCl, we tested 24 different lipid combinations using FSEC-TS with pure protein (Figure 4). GluClcryst samples in a series of buffers containing the respective lipids were heat-treated for 10 minutes at 57 °C, which is close to the calculated Tm of apo protein. Most lipids had some significant stabilizing effect on GluCl, while total soy lipid, brain ceramide, and DMPE did not stabilize GluCl (Figure 4). Among tested lipids, sphingomyelin and PO-containing lipids consistently had strong stabilizing effects, and the addition of CHS often further increased thermostability. The lipids identified in this experiment have been fed into crystallization trials of GluClcryst with the goal of obtaining well-diffracting crystals in an ivermectin free condition, and a new lipid-dependent crystal form of GluClcryst has been obtained (Figure S3A). These crystals are from the C2 space group, currently diffract X-rays to between 3 and 4 Å (Figure S3B), and are a promising lead in obtaining the structure of a closed-channel conformation of a eukaryotic Cys-loop receptor.
Figure 4. Effects of lipids on the thermostability of GluCl.
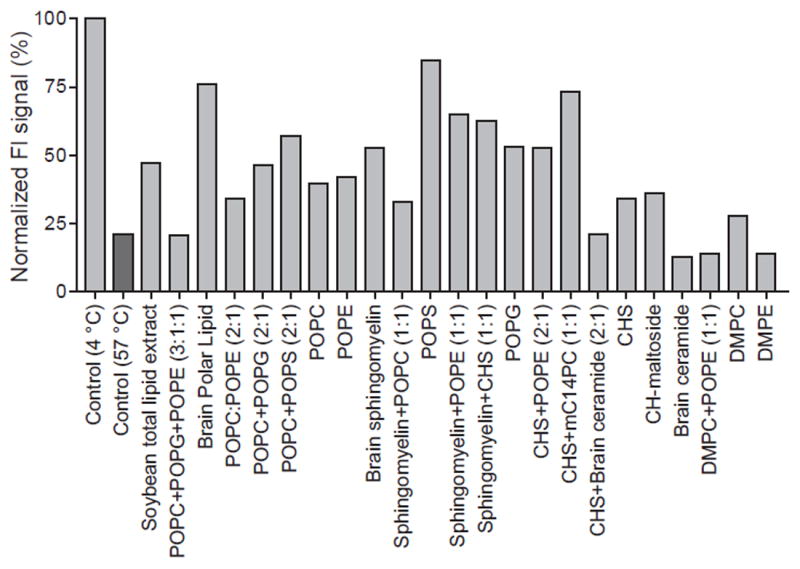
The normalized peak heights from GluClcryst heat-treated at 57 °C for 10 minutes, in the presence of the respective lipids. The peak heights were normalized to that from the sample at 4 °C without any additive. See also Figure S3 and Table S2.
CONCLUSIONS
In this study, we applied FSEC-TS to zfP2X4 and GluCl. This method carries over the advantages of conventional FSEC: it requires only nanogram to microgram quantities of protein, and allows for screening of different stabilizing conditions in an efficient manner with both purified and unpurified protein. Using this method, we screened various conditions such as ligands and lipids to stabilize zfP2X4 and GluCl. Ligand screening demonstrated that ATP, a biological agonist of P2X, and its synthetic analog, TNP-ATP, dramatically stabilized zfP2X4, suggesting that these are suitable ligands for co-crystallization. Lipid screening showed that our newly designed cholesterol derivatives and many other lipids significantly stabilized zfP2X4 or GluCl (Figures 3 and 4). Employing the ligands and lipids identified in this study has led to a new, well-diffracting crystal form of GluCl.
EXPERIMENTAL PROCEDURES
Expression and purification
The crystallized construct of zfP2X4 (ΔP2X4-A) was expressed as a N-terminal EGFP fusion and purified as described previously (Kawate et al., 2009). The EGFP-tagged and EGFP-free purified proteins were concentrated and dialyzed overnight at 4 °C against three changes of buffer I (20mM HEPES-NaOH [pH 7.0], 80mM NaCl, 20mM KCl and 2mM cymal-6), and then stored at -80 °C before use. The crystallized construct of C. elegans GluCl (GluClcryst)) with a C-terminal 8x-His tag was expressed in Sf9 cells and purified as described previously (Hibbs and Gouaux, 2011), omitting addition of Fab before the SEC purification step.
For GluCl experiments in crude cell extracts, a fusion of GluCl with EGFP was made with GFP inserted into the full-length, wild-type C. elegans GluClα receptor between Asn392 and Ser393 in the mature protein sequence. Sf9 cells at a density of 3-4 × 106 cells per mL were infected with baculovirus encoding GluCl-EGFP and harvested 72 hours later by centrifugation; cell pellets were stored at -80 °C.
Radioligand saturation binding assay for zfP2X4
Measurements of total ATP binding was obtained by adding GFP-fusion ΔP2X4-A to a final concentration of 30 nM in 250 μL buffer I containing 0-1000 nM radiolabeled ATP where the hot ATP was diluted with cold ATP in a ratio of 1:4, yielding a final specific activity of 6 Ci/mmol. Reactions were incubated at 4 °C overnight and then terminated by filtering through GSWP 02500 nitrocellulose membranes pre-equilibrated with buffer I containing 100 μM cold ATP. Filters were washed three times with 2 mL of buffer I, placed in 6 mL of Ultima Gold scintillation cocktail, and counted after 1 hour. Estimates of nonspecific binding were obtained by reactions in the presence of 100 μM cold ATP. The experiment was performed in triplicate, and the data were fit to a rectangular hyperbola using the GraphPad Prism4 program.
FSEC-TS with purified proteins
For EGFP, 500 ng of EGFP in 50 μl of buffer II (20mM Tris-HCl [pH 8.0], 150mM NaCl) was incubated at 4-80 and 4-70 °C for 10 minutes, respectively, using a thermal cycler and then centrifuged at 87,000 × g for 20 minutes. A fraction of the supernatant (25 μL) was loaded onto a SEC column (Superose 6 10/300 GL, GE Healthcare) pre-equilibrated with buffer II and run at a flow rate of 0.5 mL/min.
For zfP2X4, 1 μg of GFP-fusion and GFP-free ΔP2X4-A in 50 μL of buffer I was incubated at 4-80 and 4-70 °C for 10 minutes, centrifuged, at 87,000 × g for 20 minutes. 25 μL of the supernatant was loaded onto a SEC column (Superose 6 10/300 GL) pre-equilibrated with buffer III (20mM HEPES-NaOH [pH 7.0], 80mM NaCl, 20mM KCl and 1 mM n-dodecyl β-D-maltoside, C12M) and run at a flow rate of 0.5 mL/min.
For GluCl, 2 μg of GFP-free GluClcryst in 100 μL of buffer IV (20mM Tris-HCl [pH 7.4], 150 mM NaCl and 2 mM C12M) was incubated at 4-80 for 10 minutes, respectively, and was centrifuged as described above. 25 μL of the supernatant was loaded onto a TSKgel SuperSW2000 column (TOSOH Bioscience), pre-equilibrated with buffer IV and run at a flow rate of 0.35 mL/min.
The eluent from the SEC column was passed through a fluorometer (Kawate and Gouaux, 2006) (λexc of 480 nm and λem of 510 nm for GFP fluorescence; λexc of 280 nm and λem of 325 nm for tryptophan fluorescence). The peak heights from EGFP (Figure 2B) or oligomers (Figure 2D and 2F) were normalized to that from samples incubated at 4 °C, and were fit to the Hill equation using the GraphPad Prism 4 program. Melting temperatures (Tm) were determined by fitting the curves to a sigmoidal dose-response equation.
Gaussian fitting was performed using PeakFit software (SeaSolve Software, Inc) as described previously (Kawate and Gouaux, 2006). Initial peak detection and fitting were performed with the AutoFit peaks I Residuals option. The obtained Gaussian functions were further refined and fitted to the original FSEC profile using a least-squares minimization procedure (Figure S1F). The obtained melting curves based on the peak height are consistent with the melting curves based on the peak area of EGFP (Figure S1G), zfP2X4 trimer (Figure S1H) or GluCl pentamer (Figure S1H) estimated by Gaussian fitting.
To test ATP and ivermectin effects, GFP-fusion ΔP2X4-A in the presence of 1 mM ATP and EGFP-free GluClcryst in the presence of 10 μM ivermectin were incubated at 4 °C for 1 hour and 30 minutes before heat-treatment, respectively.
FSEC-TS with whole-cell lysates
The GFP-fusion of ΔP2X4-A was expressed in Sf9 insect cells as described (Kawate et al., 2009), and the harvested cells were stored at -80 °C before use. Sf9 cells expressing GFP-fusion ΔP2X4-A from 7.5 ml culture were re-suspended in 1 mL of buffer V (50mM Tris-HCl [pH 8.0], 150 mM NaCl) containing 40 mM C12M in the presence and absence of 1 mM ATP, rotated at 4 °C for 1 hour, and then centrifuged at 87,000 × g for 40 minutes. 100 μL of the supernatant was dispensed into thin-walled PCR tubes, and incubated at the respective temperatures for 10 min, using a thermal cycler. After centrifuge at 87,000 × g for 20 minutes, 50 μL of the supernatant were applied to a Superose 6 column, pre-equilibrated with buffer V containing 1 mM C12M.
Sf9 cell pellets from 30 mL culture containing GluCl-EGFP were re-suspended in 3 mL of buffer IV containing 40 mM C12M, rotated at 4 °C for 40 minutes then centrifuged at 87,000 × g for 40 minutes. The supernatant was divided into two tubes, and ivermectin (100 μM final concentration from a 10 mM stock in DMSO) was added to one of the tubes, and was rotated at 4 °C for 10 minutes. 100 μL aliquots of the supernatant were dispensed into thin-walled PCR tubes, and incubated at the respective temperatures for 10 minutes using a thermal cycler. After centrifugation at 87,000 × g for 20 minutes, 20 μL of the supernatant were applied to a TSKgel SuperSW2000 column, pre-equilibrated with buffer IV.
The eluent from the SEC column was passed through a fluorometer (Kawate and Gouaux, 2006) (excitation, 480 nm; emission, 510 nm for GFP fluorescence). Data were normalized and fit to the Hill equation, as described above.
FSEC-TS screening of additives
To determine the effect of lipids on the stability of zfP2X4, 5 ×g of GFP-fusion ΔP2X4-A in 250 μl of buffer I was rotated at 4 °C for 1 hour after addition of 2.5 μL of a 100x lipid suspension (20% DMSO, 80% buffer I). The mixture was next incubated at 50°C for 10 min and then centrifuged. 25 μL of the sample was used for FSEC as described above. The rest of the sample was stored at 4°C for use in binding assays. 100x additive solutions were added to GFP-fusion ΔP2X4-A at the respective final concentration shown in Table S1.
For GluCl, 850 ng of GluClcryst in 10 μl of buffer IV was rotated at 4 °C for 40 minutes after addition of 90 μL of lipid stock solution in buffer IV containing 40 mM C12M. The mixture was next incubated at 59°C for 10 min and then centrifuged. A 25 μL aliquot of the sample was used for FSEC as described above. Lipids were added to GluClcryst at the respective final concentrations as shown in Table S2.
Binding assay of heat-treated ΔP2X4-A
These experiments were performed as described above except that the binding was initiated by adding heat-treated GFP-fusion ΔP2X4-A to a final concentration of 30 nM in 250 μL of buffer I containing 100 nM ATP (1:4 3H:1H; 6 Ci/mmol final specific activity). The entire experiment was performed in triplicate.
Binding assay of heat treated GluClcryst
Binding of 3H-ivermectin to GluClcryst post-heat treatment was performed using a scintillation proximity assay (SPA). A solution of pure GluClcryst was made in buffer IV at a subunit concentration of 200 nM, and 70 μL were aliquotted into thin-walled PCR tubes and incubated for 10 minutes at temperatures ranging from 4 °C to 80 °C. A suspension was made containing 2 mg/mL SPA beads (YSi copper his tag, GE Healthcare) and 100 nM ivermectin (1:9 3H:1H; 5 Ci/mmol final specific activity; 3H-ivermectin purchased from American Radiolabeled Chemicals and 1H-ivermectin purchased from Sigma) in buffer IV. The SPA suspension was mixed 1:1 (50 μL to 50 μL) with the protein solution by pipetting in a 96-well microtiter plate, and the cpm data were collected after 18 hr incubation at room temperature.
Supplementary Material
Acknowledgments
We thank A. Penmatsa for providing the lipid screening kit for GluCl, J. Michel for technical help and L. Vaskalis for assistance with figures. This work was supported by the Uehara Memorial Foundation (M.H.), the American Asthma Foundation (E.G.) and the National Institutes of Health (R.H. and E.G.). E.G. is an investigator with the Howard Hughes Medical Institute.
Footnotes
SUPPLEMENTAL DATA
The Supplemental Data including three figures and two tables can be found with this article online at http://www.structure.org/cgi/content/full/xxx
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexandrov AI, Mileni M, Chien EY, Hanson MA, Stevens RC. Microscale fluorescent thermal stability assay for membrane proteins. Structure. 2008;16:351–359. doi: 10.1016/j.str.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Bernier LP, Ase AR, Chevallier S, Blais D, Zhao Q, Boue-Grabot E, Logothetis D, Seguela P. Phosphoinositides regulate P2X4 ATP-gated channels through direct interactions. J Neurosci. 2008;28:12938–12945. doi: 10.1523/JNEUROSCI.3038-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokman SH, Ward WW. Renaturation of Aequorea green-fluorescent protein. Biochem Biophys Res Commun. 1981;101:1372–1380. doi: 10.1016/0006-291x(81)91599-0. [DOI] [PubMed] [Google Scholar]
- Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, et al. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cully DF, Vassilatis DK, Liu KK, Paress PS, Van der Ploeg LH, Schaeffer JM, Arena JP. Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature. 1994;371:707–711. doi: 10.1038/371707a0. [DOI] [PubMed] [Google Scholar]
- Czyzewski BK, Wang DN. Identification and characterization of a bacterial hydrosulphide ion channel. Nature. 2012;483:494–497. doi: 10.1038/nature10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Guzman M, Soto F, Gomez-Hernandez JM, Lund PE, Stuhmer W. Characterization of recombinant human P2X4 receptor reveals pharmacological differences to the rat homologue. Mol Pharmacol. 1997;51:109–118. doi: 10.1124/mol.51.1.109. [DOI] [PubMed] [Google Scholar]
- Guan L, Smirnova IN, Verner G, Nagamori S, Kaback HR. Manipulating phospholipids for crystallization of a membrane transport protein. Proc Natl Acad Sci U S A. 2006;103:1723–1726. doi: 10.1073/pnas.0510922103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M, Gouaux E. Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature. 2012;485:207–212. doi: 10.1038/nature11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M, Tanaka Y, Fukai S, Ishitani R, Nureki O. Crystal structure of the MgtE Mg2+ transporter. Nature. 2007;448:1072–1075. doi: 10.1038/nature06093. [DOI] [PubMed] [Google Scholar]
- Hibbs RE, Gouaux E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature. 2011;474:54–60. doi: 10.1038/nature10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF, Khakh BS. ATP-gated P2X cation-channels. Neuropharmacology. 2009;56:208–215. doi: 10.1016/j.neuropharm.2008.06.067. [DOI] [PubMed] [Google Scholar]
- Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- Johansson LC, Wohri AB, Katona G, Engstrom S, Neutze R. Membrane protein crystallization from lipidic phases. Curr Opin Struct Biol. 2009;19:372–378. doi: 10.1016/j.sbi.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Kawate T, Gouaux E. Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure. 2006;14:673–681. doi: 10.1016/j.str.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Kawate T, Michel JC, Birdsong WT, Gouaux E. Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature. 2009;460:592–598. doi: 10.1038/nature08198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy H, Gouaux E. X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature. 2012;481:469–474. doi: 10.1038/nature10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux MJ, Song J, Kim MJ, Huang Y, Villa A, Auer M, Li XD, Wang DN. Three-dimensional crystallization of the Escherichia coli glycerol-3-phosphate transporter: a member of the major facilitator superfamily. Protein Sci. 2003;12:2748–2756. doi: 10.1110/ps.03276603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancusso R, Karpowich NK, Czyzewski BK, Wang DN. Simple screening method for improving membrane protein thermostability. Methods. 2011;55:324–329. doi: 10.1016/j.ymeth.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negulyaev YA, Markwardt F. Block by extracellular Mg2+ of single human purinergic P2X4 receptor channels expressed in human embryonic kidney cells. Neurosci Lett. 2000;279:165–168. doi: 10.1016/s0304-3940(99)00976-3. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Rasmussen SG, Choi HJ, Fung JJ, Pardon E, Casarosa P, Chae PS, Devree BT, Rosenbaum DM, Thian FS, Kobilka TS, et al. Structure of a nanobody-stabilized active state of the beta(2) adrenoceptor. Nature. 2011;469:175–180. doi: 10.1038/nature09648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DM, Zhang C, Lyons JA, Holl R, Aragao D, Arlow DH, Rasmussen SG, Choi HJ, Devree BT, Sunahara RK, et al. Structure and function of an irreversible agonist-beta(2) adrenoceptor complex. Nature. 2011;469:236–240. doi: 10.1038/nature09665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer PL, Goehring A, Shankaranarayanan A, Gouaux E. Structure and mechanism of a Na+-independent amino acid transporter. Science. 2009;325:1010–1014. doi: 10.1126/science.1176088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevsky AI, Rosconi MP, Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009;462:745–756. doi: 10.1038/nature08624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano A, Ventura R, Molero A, Hoen R, Casado V, Cortes A, Fanelli F, Albericio F, Lluis C, Franco R, et al. Adenosine A2A receptor-antagonist/dopamine D2 receptor-agonist bivalent ligands as pharmacological tools to detect A2A-D2 receptor heteromers. J Med Chem. 2009;52:5590–5602. doi: 10.1021/jm900298c. [DOI] [PubMed] [Google Scholar]
- Surprenant A, North RA. Signaling at purinergic P2X receptors. Annu Rev Physiol. 2009;71:333–359. doi: 10.1146/annurev.physiol.70.113006.100630. [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Lester HA, Lummis SC. The structural basis of function in Cys-loop receptors. Quarterly reviews of biophysics. 2010;43:449–499. doi: 10.1017/S0033583510000168. [DOI] [PubMed] [Google Scholar]
- Ward WW. Properties of the coelenterate green-fluorescent protein. In: DeLuca M, McElroy WD, editors. Bioluminescence and Chemiluminescence: Basic Chemistry and Analytical Applications. New York: Academic Press; 1981. pp. 235–242. [Google Scholar]
- Wildman SS, King BF, Burnstock G. Modulation of ATP-responses at recombinant rP2X4 receptors by extracellular pH and zinc. Br J Pharmacol. 1999;126:762–768. doi: 10.1038/sj.bjp.0702325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl--dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- Zambrowicz BP, Sands AT. Knockouts model the 100 best-selling drugs--will they model the next 100? Nat Rev Drug Discov. 2003;2:38–51. doi: 10.1038/nrd987. [DOI] [PubMed] [Google Scholar]
- Zhang H, Kurisu G, Smith JL, Cramer WA. A defined protein-detergent-lipid complex for crystallization of integral membrane proteins: The cytochrome b6f complex of oxygenic photosynthesis. Proc Natl Acad Sci U S A. 2003;100:5160–5163. doi: 10.1073/pnas.0931431100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


