Abstract
Objective
Ocular motility abnormalities may be a marker of neuro-degeneration beyond motor neurons in amyotrophic lateral sclerosis (ALS). We formally compared clinical neuro-ophthalmic abnormalities in ALS patients and a control population.
Methods
Patients attending a multidisciplinary ALS clinic (n = 63, age 60.8 +/− 16.4 years) and their caregivers serving as controls (n = 37, ages 55.0 +/− 12.7 years) participated in this cross-sectional study. Visual acuity was assessed. Video recordings of a standardized ocular motility exam including gaze fixation, voluntary saccades, reflex saccades, smooth pursuit, eyelid opening and Bell's phenomenon were rated by two senior neuro-ophthalmologists who were masked to subject group.
Results
Visual acuity was lower in ALS patients versus control subjects (OR 0.81 (0.71–0.93), p = 0.003, logistic regression). Inter- and intra-rater reliability for ocular motility examination ratings were good (Cohen's Kappa > 0.6). Findings observed only in ALS subjects included gaze impersistence (14%, p = 0.01), moderately or severely restricted voluntary upgaze (13%, p = 0.01), and moderate or severe eyelid opening apraxia (27%, p = 0.0002). Accounting for age, moderately or severely saccadic horizontal smooth pursuits distinguished ALS from control subjects (OR 3.6 (1.2– 10.9), p = 0.02, logistic regression).
Conclusions
Clinical findings of decreased visual acuity, gaze impersistence, voluntary upgaze restriction, eyelid opening apraxia, and saccadic horizontal smooth pursuits are more frequent in patients with ALS than in similar-aged controls. These findings are potential clinical markers of neurodegeneration beyond upper and lower motor neuron disease in ALS. Further study is warranted regarding their application to disease categorization and outcomes assessment.
Keywords: Prevalence study, Eye movements, Vision, Amyotrophic lateral sclerosis
1. Introduction
Amyotrophic lateral sclerosis (ALS) is classically defined by degeneration of upper and lower motor neurons causing corresponding symptoms and clinical signs. Post-mortem studies demonstrate pathological spread beyond the motor system in some ALS patients [1]. The presence of symptoms and signs that do not localize to motor neurons characterizes ALS-Plus syndromes, which may be the clinical correlate of pathological spread. These syndromes might represent distinct disease phenotypes with implications for diagnosis and therapy [2].
Abnormal eye movements are attractive as a potential marker of ALS-plus syndromes [3] because the ocular lower motor neurons (i.e. cranial nerves 3, 4 and 6) and the actions of the muscles they innervate are typically spared until late in the disease [4–6]. Therefore the neuro-ophthalmic examination remains relatively accessible when motor weakness obscures other portions of the neurological exam [7]. Gaze fixation instability [8,9], saccadic abnormalities [10–14], supra-nuclear gaze palsy [15], anti-saccade errors [9] smooth pursuit impairment [12,16–18], reduced Bell's phenomenon [19], eyelid apraxia [10], poor suppression of vestibular ocular reflex [11] and nystagmoid movements [20–22] have been described in ALS subjects. Correlations between some of these findings, duration of ALS symptoms [16] and severity of ALS disease [17,23] suggest that extra-motor neuron involvement is the consequence of advanced disease. However, associations with bulbar symptoms [11,14], parkinsonism [24], cognitive deficits [8,9], and onset pattern [8] in individuals without advanced disease suggest that they may be markers for a distinct disease phenotype. We examined the ocular motility in an ALS population to determine the prevalence of clinically apparent findings. Visual acuity was measured to screen for concurrent afferent visual impairment.
2. Methods
2.1. Study participants
This is a cross-sectional study of a population of individuals with possible, probable or definite ALS according to the revised El Escorial criteria [25] attending a multidisciplinary ALS clinic serving the greater Philadelphia region. These clinical criteria were used due to their good sensitivity for prediction of pathological findings diagnostic of ALS [26]. Control subjects were recruited from healthy individuals accompanying those receiving care in the ALS multidisciplinary clinic. Controls were included as a comparison group to account for cofounding influences on clinical ocular motility characterization. Participants were excluded if they had a history of neurological disease other than migraine. Recruitment and data collection occurred during a two and a half month period from October to December, 2009. The number of subjects enrolled during the study period determined the sample size.
This study was carried out in compliance with the guidelines set forth by and with the approval of the University of Pennsylvania Office for Regulatory Affairs. Informed consent was obtained from all participants.
Impairment in ALS subjects was assessed using the revised ALS functional rating scale including respiration (FRS-R), a validated measure of symptom severity and functional limitation (range 0–48, 48 is normal) [27] and forced vital capacity (FVC), a measure of pulmonary strength that is normalized to predicted values for age and gender. Both were assessed on the date of study participation. Rate of functional decline was calculated as the quotient of FRS-R and duration of disease symptoms. Bulbar impairment in ALS subjects was characterized using the FRS-R bulbar sub-score (range 0–12, 12 is normal). The presence of pseudobulbar affect associated with bulbar upper motor neuron dysfunction was assessed using the Center for Neurologic Study Lability Scale (CNS-LS), a validated measure of affective lability (range 7–35, normal less than 14) [28]. Executive language function was assessed with phonemic fluency (number of words starting with ‘F’ generated in 60 s, normal greater than 12) [29]. These are collected at regular intervals as part of on-going clinical care. The worst recorded score for each patient was obtained from the medical record.
2.2. Neuro-ophthalmic examination
Binocular high and low (2.5%, 1.25%) contrast visual acuity at 2 m was measured by Sloan charts using the subject's own corrective lenses in the examination room. A single examiner assessed pupillary constriction to light, eyelid symmetry, corneal reflex and blink reflex. Horizontal and vertical ocular alignment was measured using Maddox rod and hand held prisms with a light source at 24 in.
A standardized ocular motility examination of primary and eccentric gaze fixation, smooth pursuit, visually guided saccades, anti-saccades, eyelid opening and closing, ocular deviation during attempted eyelid closure (i.e. Bell's phenomenon) and vestibular ocular reflex (Table 2) was recorded using a video camera positioned directly in front of the subject at 1 m. Subjects' gaze was directed by the examiner's fingers, which were presented at measured positions on a plane 1 m in front of the subject. The subject was instructed to hold his/her head position constant during each task. Videos were limited to subjects' eyes and cropped to prevent identification of subject and group.
Table 2.
Standardized clinical ocular motility examination.
| Instruction given to subject | Target presented | Abnormal findings assessed by reviewers |
|---|---|---|
| Look at camera | Camera (3 s) | Square wave jerks |
| Nystagmus | ||
| Look and keep looking right, left, up, down (3 s each) | None | Nystagmus |
| Gaze limitation | ||
| Gaze impersistence | ||
| Visually follow moving target | Target moving at | Saccadic intrusions into smooth pursuit |
| 30°/s horizontally | ||
| 15°/s horizontally | ||
| 30°/s vertically | ||
| 15°/s vertically | ||
| (3 cycles of each) | ||
| If you see a target in the periphery, look at it | Stationary target at 25° from center on horizontal (random left and right), 25° from center on vertical (random left and right) (3 of each) | Lack of visually guided saccades |
| Dysmetria | ||
| Anticipatory movements (i.e. eye Movement generated when no target provided) | ||
| If you see a target in the periphery, look in the opposite direction | Stationary target at 25° from center on horizontal (random left and right) 25° from center on vertical (random up and down) (3 of each) | Anti-saccade error (i.e. eye movement toward target) or no movement when target provided |
| Anticipatory movements (i.e. eye movement when no target provided) | ||
| Close your eyes, open your eyes | None | Eyelid opening apraxia |
| Eyelid closure apraxia | ||
| Close your eyes while one eye is held open | None | Impaired Bell's phenomenon (i.e. limited ocular deviation during attempted eye closure) |
| Look at the camera while your head is moved | Camera | Limited vestibular ocular reflex |
Two senior neuro-ophthalmologists who were masked to subject group independently reviewed each video once and ten randomly selected videos a second time. They rated all findings except anticipatory saccades, saccade errors and saccade absence according to their own clinical judgment using ordered categories (normal, mildly abnormal, moderately abnormal, severely abnormal). These were dichotomized to normal/mildly abnormal and moderately/severely abnormal. Intra-rater agreement was assessed for each rater based on the ten videos reviewed on two occasions. Inter-rater reliability was assessed between raters for each finding across all subjects using Cohen's Kappa (K), a chance corrected reliability measure. K greater than 0.6 was used as the standard for good reliability based on reliability studies of clinical rating scales in the movement disorders field [30]. Findings were classified as moderately/severely abnormal for analysis only if both raters classified them as such. A single reviewer classified anticipatory movements and saccadic errors during reflex and anti-saccade tasks.
2.3. Statistical analysis
Analysis was performed using Stata 11 (Statacorp LP, College Station, TX). Low- and high-contrast acuity scores were compared between ALS and control groups using Wilcoxon rank-sum and t-tests. Acuity was compared between ALS subgroups using linear regression, accounting for age. Ocular motility tasks were compared between ALS and control groups using Fisher's exact test. Significant differences were further evaluated with logistic regression of subject groups, accounting for age. Subgroup analysis was performed using logistic regression. Alpha of 0.05 was used for between group comparisons and 0.01 was used for subgroup comparisons.
For independent variable data points (i.e. those characterizing ALS) not available for the precise examination date, one from another date was used if clinical status was similar. Subjects without an appropriate substitute data point were excluded from portions of the analysis involving that independent variable. Subjects with missing dependent variable data points were excluded from analyses requiring that data point.
3. Results
Sixty-three consecutive ALS subjects (mean age 60.8, range 28–90 years, 52% female) were enrolled (Table 1). An additional 15 agreed to enroll, but were excluded for neurological co-morbidities (n = 5) and not meeting the ALS definition (n = 10). 66 subjects declined to participate due to fatigue, emotional stress and not having time. For a small number of patients, FRS-R (n = 6) and FVC (n = 3) were obtained on a different day than the neuro-ophthalmic examination. For ventilated subjects (n = 2), in whom FVC measurements were not obtained, a value of 20% predicted was used. CNS-LS data and phonemic fluency were not available for three and nine subjects respectively.
Table 1.
ALS subject characteristics (n=63 unless otherwise noted).
| Central tendency | Range | |
|---|---|---|
| Age (years) | 60.8+/−16.4 (mean+/−SD) | 18–90 |
| Gender (female) | 52% (n=33) | |
| El Escorial diagnostic classification | ||
| Possible ALS | 11% (n=7) | |
| Probable ALS | 32% (n=20) | |
| Definite ALS | 57% (n=36) | |
| Duration of symptoms (years) | 3.6+/−3.1 (mean+/−SD) | 0.6–17 |
| Bulbar onset | 28.6% (n=18) | |
| Family history of ALS | 15.9% (n=10) | |
| Forced vital capacity (% predicted) | 58+/−27 (mean+/−SD) | 8–111 |
| ALS revised functional rating score, current | 29 (median) | 6–46 |
| Worst bulbar function (FRS-R bulbar) | 9 (median) | |
| Rate of functional decline (points/yr) | 6.5 (median) | 0.3–43.5 |
| Phonemic fluency (F-words), lowest ever (n=54) | 10 (median) | 4–23 |
| Affective lability (CNS-LS), highest ever (n=60) | 10 (median) | 7–28 |
Thirty-seven control subjects were recruited (mean age 54.9, range 20–82 years, 62% female). An additional 2 agreed to enroll, but were excluded due to neurological co-morbidities. Subject groups did not differ by age (p = 0.09 by Mann Whitney) or gender (p = 0.34 by chi square).
High and low contrast visual acuity, accounting for age, were lower in ALS (n = 54) than control (n = 35) subjects (Table 3). Subjects with co-morbid conditions including glaucoma (n = 6), macular degeneration (n = 3), no knowledge of Roman alphabet (n = 1) and wearing sunglasses (n = 1) were excluded from this analysis. Regression analysis did not identify differences in ALS subject visual acuity based on familial disease pattern, forced vital capacity, level of functional disability, rate of functional decline, duration of symptoms, bulbar symptoms, phonemic fluency or affect lability.
Table 3.
Visual acuity in ALS vs. control subjects.
| Acuity measure | ALS | Control | OR* (5%–95% CI) | p |
|---|---|---|---|---|
| High contrast | 53.1+/−7.4 | 57.6+/−3.1 | 0.81 (0.71–0.93) | 0.003 |
| 2.5% low contrast | 29.4+/−12.2 | 38.3+/−8.6 | 0.89 (0.83–0.95) | 0.001 |
| 1.25% low contrast | 24.1+/−13.1 | 32.6+/−9.3 | 0.91 (0.86–0.97) | 0.002 |
Acuity recorded in letters (mean +/− SD) seen on 2 m Sloan chart,
Logistic regression, accounting for age.
All subjects had intact corneal reflex, blink reflex and pupillary response to light. There was no significant difference between ALS and control groups in terms of ocular alignment.
Cervical rigidity prevented effective vestibular ocular reflex assessment in many ALS subjects and therefore this finding was not analyzed. Intra-rater agreement exceeded 80% for all remaining tasks except vertical smooth pursuit. Inter-rater reliability was poor for square wave jerks, nystagmus, down gaze limitation, horizontal gaze limitations, and saccadic dysmetria. All except the latter had a prevalence of less than 5% for both reviewers. Inter-rater reliability for gaze impersistence (K = 0.68), upgaze limitation (K = 0.66), saccadic intrusion into horizontal smooth pursuit (K = 0.64), delayed eyelid opening (K = 0.65) and limited ocular deviation during attempted eye closure (K = 0.74) was good. These findings were included in subsequent analysis. Missing data for ocular motility findings was ≤5% and reflects inability to interpret it from video or poor subject comprehension of the task. Smooth pursuit at 15 degrees/s data was not available for 33/100 subjects because it was incorporated into the protocol after these subjects had been recruited.
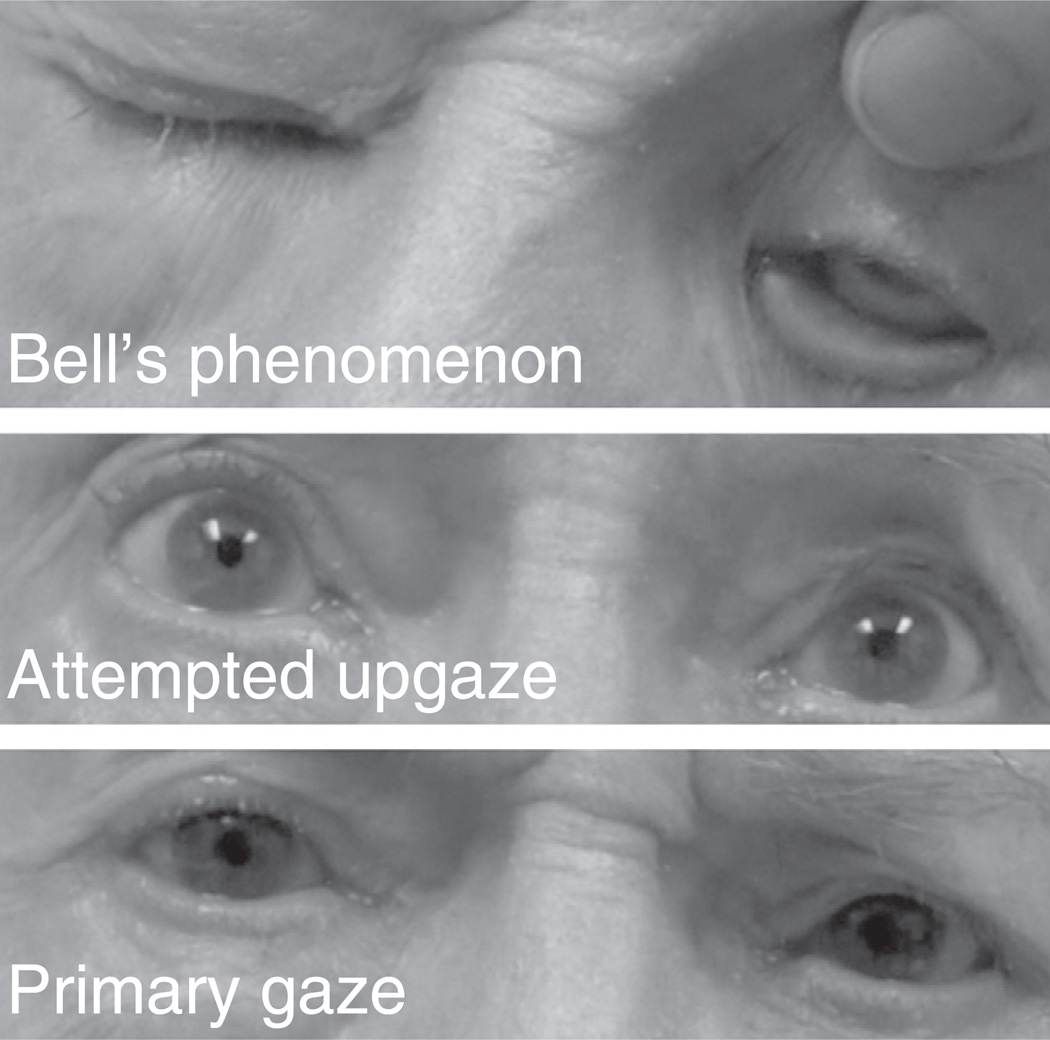
Gaze impersistence (Video 1 in the Supplementary material), upgaze limitation, saccadic intrusion into high and medium speed horizontal smooth pursuits (Video 2 in the Supplementary material) and eyelid opening apraxia were more common in the ALS population, accounting for age (Table 4). The difference persisted after accounting for visual acuity. For subjects with upgaze limitation, more complete upgaze was demonstrated in other parts of the examination, suggesting that the disorder was supranuclear in origin (Fig. 1). Absence of visually guided saccades (i.e. no response to peripherally presented visual stimuli) was observed only in ALS subjects (Video 3 in the Supplementary material). This abnormality did not reach statistical significance. Each of these subjects demonstrated conjugate gaze deviation during other tasks, suggesting that the visually guided saccade limitation was a gaze apraxia.
Table 4.
Ocular motility abnormalities in ALS vs. control subjects.
| ALS | Control | p* | |
|---|---|---|---|
| Gaze impersistence | 14.3% (9/63) | 0% (0/37) | 0.01 |
| Upgaze limitation | 12.7% (8/63) | 0% (0/37) | 0.02 |
| Absent visually guided saccades | |||
| horizontal | 3.4% (2/59) | 0% (0/36) | 0.38 |
| vertical | 5% (3/59) | 0% (0/36) | 0.23 |
| Anticipatory visually guided saccades | |||
| horizontal | 25.4% (16/63) | 24.3% (9/37) | 0.24 |
| vertical | 20% (12/60) | 13.9% (5/36) | 0.14 |
| Anti-saccade errors (>33%) | |||
| Horizontal | 16.7% (10/60) | 5.4% (2/37) | 0.054 |
| Vertical | 25.9% (15/58) | 19.4% (7/36) | 0.19 |
| Reduced Bell's phenomenon | 25.9% (15/58) | 8.1% (3/37) | 0.08 |
| Eyelid opening apraxia | 26.7% (16/60) | 0% (0/37) | 0.0002 |
| ALS | Control | OR** (5–95% CI) | |
| Saccadic intrusion into horizontal smooth pursuit | |||
| High speed (30°/s) | 39.7% (25/63) | 13.5% (5/37) | 3.6 (1.2–10.9) |
| Low speed (15°/s) | 42.9% (18/42) | 3.8% (1/26) | 15.5 (1.9–128.8) |
Fisher's exact test,
logistic regression, accounting for age.
Fig. 1.
Supranuclear upgaze limitation in an ALS patient. During voluntary upgaze ocular supraduction is limited (middle panel). During attempted eye closure, reflexive ocular supraduction is full (upper panel).
In ALS subjects presence of eyelid opening apraxia correlated inversely with forced vital capacity (OR 15, 95% CI 2.7–82.5, p = 0.002 logistic regression accounting for age). Further analysis of ALS subpopulations using logistic regression accounting for age did not identify correlations between ocular motility abnormalities and visual acuity, family history of ALS, duration of ALS symptoms, functional disability, rate of functional decline, bulbar symptoms, phonemic fluency or affect lability.
4. Discussion
This comprehensive clinical assessment of ocular motor function in ALS subjects has identified specific clinical supranuclear ocular motility findings that are more common in ALS than control subjects. The results provide evidence of pathology outside of upper and lower motor neurons and strengthen the candidacy of these clinical ocular motor findings as biomarkers of disease spread in ALS.
Limitations of the current study are primarily related to the qualitative clinical methodology employed. While extensive efforts were made to mask the reviewers to subject group, video clues may have made this incomplete. The lack of quantitative characterization likely introduced variability into the data, in turn decreasing statistical power. Our methodology did not capture saccade latency, saccade speed or square wave jerks that quantitative studies have found to be prolonged in the ALS population. Internal validity was implied using a gold standard of clinical expertise. While this may be reasonable in this study, in which the reviewers were constant across all subjects, external validity would need to be addressed for any future study in which not all reviewers rate all of the subjects.
Reduced visual acuity in ALS subjects was a surprising result. Though afferent visual pathway pathology is well described in other neurological diseases including multiple sclerosis [31], Alzheimer's disease [32] and Parkinson's disease [33] there is no published literature regarding such a finding in ALS. It is feasible that pathological spread beyond motor neurons could negatively impact afferent visual function at various anatomical locations. Monocular acuity measurements using best refractive correction and controlled lighting are necessary to confirm this finding. The functional importance also remains to be determined as the mean high contrast difference between ALS and control subjects was less than 5 letters (i.e. one line), which is not typically clinically significant.
We found saccadic abnormalities that have not been previously described in ALS patients. Subjects with gaze impersistence could not maintain eccentric gaze despite good attention to the task, with the gaze returning to midline without corrective saccade back to eccentric gaze. Serial examinations would be required to determine if this is an isolated finding or a precursor to the gaze apraxia seen in three ALS subjects in this study and described in the literature [15]. We confirm eye lid apraxia, previously reported in a case series [10], to bemore common in ALS subjects. We found Bell's phenomenon abnormalities, also previously reported in a case series [19], to not have increased prevalence in the ALS group.
Incidence of ocular motility abnormalities did not correlate with markers of disease severity or duration and therefore our results do not suggest that ocular motor abnormalities are simply population markers of advanced disease [23]. Serial examinations of ocular motility in individuals would be necessary to determine if they are markers of disease progression in individuals as has been reported by Palmowski et al. [7].
These observations provide clinical support for the histopathologic observation that TDP-43 pathology, the predominant pathologic finding to accompany upper and lower motor neuron cell loss in sporadic ALS, extends beyond upper and lower motor neurons [1] and provide support for a multi-system disorder in TDP-43 proteinopathy. Structure–function correlation with imaging and pathological studies has the potential to investigate the relationship between molecular pathology, neuro-anatomical involvement and ocular motility abnormalities as it relates to phenotypic variation in ALS.
There is overlap between ocular motility findings seen in our ALS subjects with those described in other neurodegenerative diseases with distinct pathological substrates. Supranuclear upgaze limitation of the kind seen in our subjects has previously been described as a distinguishing feature of progressive supranuclear palsy [34] and has been proposed as a marker of tau pathology. Saccadic intrusion into smooth pursuits is well described in parkinsonism as the ocular equivalent to cogwheel rigidity. In contrast with Gizzi et al., who found eye movement abnormalities exclusively in ALS patients with extrapyramidal signs [24], none of our subjects had extrapyramidal signs. This supports the notion of a continuum of clinical overlap between pathological disease entities [35].
We identified the clinical findings of gaze impersistence, supranuclear upgaze limitation, eyelid opening apraxia, and saccadic intrusion into horizontal smooth pursuit to be more common in ALS subjects. These findings have potentially important applications to clinical descriptions of ALS phenotypes and will help to generate hypotheses regarding underlying pathology and disease mechanisms. The clinical nature of the abnormalities makes them candidates for use in bedside categorization of ALS phenotypes.
Supplementary Material
Acknowledgments
Financial disclosures
Dr. Grossman has received support from NIH (AG32953, AG17586, NS44266, AG15116 and NS53488). He is a consultant for Forest Labs, Allon Pharmaceuticals, and Pfizer Pharmaceuticals. Dr. Balcer has received speaking and consulting honoraria from Biogen Idec, Novartis, and Bayer. Dr. Galetta has received speaking honoraria from Biogen Idec, Teva and Novaritis and has received royalty payments for his part in writing the book, “Neuro-Ophthalmology: Diagnosis and Management,” 2nd edition, Elsevier (2010). Dr. Liu has received personal compensation for speaking and consulting for Lundbeck and Ipsen and has received royalty payments for his part in writing the book, “Neuro-Ophthalmology: Diagnosis and Management,” 2nd edition, Elsevier (2010).
Funding/support
This study was supported in part by NIHAG32593 and NIH AG17586 to Dr. Grossman for study of Amyotrophic Lateral Sclerosis and Frontal Temporal Dementia.
Footnotes
Appendix A. Supplementary data
Supplementary materials related to this article can be found online at doi:10.1016/j.jns.2011.10.016.
References
- 1.Geser F, Brandmeir N, Kwong L, Martinez-Lage M, Elman L, McCluskey L, et al. Evidence of multisystem disorder in whole-brain map of pathological TDP-43 in amyotrophic lateral sclerosis. Arch Neurol. 2008;65(5):636–641. doi: 10.1001/archneur.65.5.636. [DOI] [PubMed] [Google Scholar]
- 2.Takeda S, Yamada M, Kawasaki K, Oyanagi K, Ikuta F, Arai M, et al. Motor neuron disease with multi-system involvement presenting as tetraparesis, ophthalmoplegia and sensori-autonomic dysfunction. Acta Neuropathol. 1994;88(3):193–200. doi: 10.1007/BF00293393. [DOI] [PubMed] [Google Scholar]
- 3.Donaghy C, Thurtell MJ, Pioro EP, Gibson JM, Leigh RJ. Eye movements in amyotrophic lateral sclerosis and its mimics: a review with illustrative cases. J Neurol Neurosurg Psychiatry. 2011;82(1):110. doi: 10.1136/jnnp.2010.212407. [DOI] [PubMed] [Google Scholar]
- 4.Okamoto K, Hirai S, Amari M, Iizuka T, Watanabe M, Murakami N, et al. Oculomotor nuclear pathology in amyotrophic lateral sclerosis. Acta Neuropathol. 1993;85(5):458–462. doi: 10.1007/BF00230482. [DOI] [PubMed] [Google Scholar]
- 5.Ahmadi M, Liu JX, Brannstrom T, Andersen PM, Stal P, Pedrosa-Domellof F. Human extraocular muscles in ALS. Invest Ophthalmol Vis Sci. 2010 Jul;51(7):3494–3501. doi: 10.1167/iovs.09-5030. [DOI] [PubMed] [Google Scholar]
- 6.Whitehouse PJ, Wamsley JK, Zarbin MA, Price DL, Kuhar MJ. Neurotransmitter receptors in amyotrophic lateral sclerosis: possible relationship to sparing of eye movements. Ann Neurol. 1985 May;17(5):518. doi: 10.1002/ana.410170518. [DOI] [PubMed] [Google Scholar]
- 7.Palmowski A, Jost WH, Prudlo J, Osterhage J, Kasmann B, Schimrigk K, et al. Eye movement in amyotrophic lateral sclerosis: a longitudinal study. Ger J Ophthalmol. 1995 Nov;4(6):355–362. [PubMed] [Google Scholar]
- 8.Donaghy C, Pinnock R, Abrahams S, Cardwell C, Hardiman O, Patterson V, et al. Ocular fixation instabilities inmotor neurone disease. A marker of frontal lobe dysfunction? J Neurol. 2009 Mar;256(3):420–426. doi: 10.1007/s00415-009-0109-x. [DOI] [PubMed] [Google Scholar]
- 9.Shaunak S, Orrell RW, O'Sullivan E, Hawken MB, Lane RJ, Henderson L, et al. Oculomotor function in amyotrophic lateral sclerosis: evidence for frontal impairment. Ann Neurol. 1995 Jul;38(1):38–44. doi: 10.1002/ana.410380109. [DOI] [PubMed] [Google Scholar]
- 10.Averbuch-Heller L, Helmchen C, Horn AK, Leigh RJ, Buttner-Ennerver JA. Slow vertical saccades in motor neuron disease: correlation of structure and function. Ann Neurol. 1998 Oct;44(4):641–648. doi: 10.1002/ana.410440410. [DOI] [PubMed] [Google Scholar]
- 11.Ohki M, Kanayama R, Nakamura T, Okuyama T, Kimura Y, Koike Y. Ocular abnormalities in amyotrophic lateral sclerosis. Acta Otolaryngol Suppl. 1994;114(S511):138–142. doi: 10.3109/00016489409128318. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs L, Bozian D, Heffner RR, Jr, Barron SA. An eye movement disorder in amyotrophic lateral sclerosis. Neurology. 1981 Oct;31(10):1282–1287. doi: 10.1212/wnl.31.10.1282. [DOI] [PubMed] [Google Scholar]
- 13.Leveille A, Kiernan J, Goodwin JA, Antel J. Eye movements in amyotrophic lateral sclerosis. Arch Neurol. 1982 Nov;39(11):684–686. doi: 10.1001/archneur.1982.00510230010003. [DOI] [PubMed] [Google Scholar]
- 14.Donaghy C, Pinnock R, Abrahams S, Cardwell C, Hardiman O, Patterson V, et al. Slow saccades in bulbar-onset motor neurone disease. J Neurol. 2010:1–7. doi: 10.1007/s00415-010-5478-7. [DOI] [PubMed] [Google Scholar]
- 15.Ushio M, Iwasaki S, Sugasawa K, Murofushi T. Atypical motor neuron disease with supranuclear vertical gaze palsy and slow saccades. Auris Nasus Larynx. 2009 Feb;36(1):85–87. doi: 10.1016/j.anl.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Marti-Fabregas J, Roig C. Oculomotor abnormalities in motor neuron disease. J Neurol. 1993 Sep;240(8):475–478. doi: 10.1007/BF00874116. [DOI] [PubMed] [Google Scholar]
- 17.Abel L, Williams I, Gibson K, Levi L. Effects of stimulus velocity and acceleration on smooth pursuit in motor neuron disease. J Neurol. 1995;242(7):419–424. doi: 10.1007/BF00873543. [DOI] [PubMed] [Google Scholar]
- 18.Cohen B, Caroscio J. Eye movements in amyotrophic lateral sclerosis. J Neural Transm Suppl. 1983;19:305–315. [PubMed] [Google Scholar]
- 19.Esteban A, De Andres C, Gimenez-Roldan S. Abnormalities of Bell's phenomenon in amyotrophic lateral sclerosis: a clinical and electrophysiological evaluation. J Neurol Neurosurg Psychiatry. 1978 Aug;41(8):690–698. doi: 10.1136/jnnp.41.8.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kushner MJ, Parrish M, Burke A, Behrens M, Hays AP, Frame B, et al. Nystagmus in motor neuron disease: clinicopathological study of two cases. Ann Neurol. 1984 Jul;16(1):71–77. doi: 10.1002/ana.410160114. [DOI] [PubMed] [Google Scholar]
- 21.Thakore NJ, Pioro EP, Rucker JC, Leigh RJ. Motor neuronopathy with dropped hands and downbeat nystagmus: a distinctive disorder? A case report. BMC Neurol. 2006;6(3):1–6. doi: 10.1186/1471-2377-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balaratnam MS, Leschziner GD, Seemungal BM, Bronstein AM, Guiloff RJ. Amyotrophic lateral sclerosis and ocular flutter. Amyotroph Lateral Scler. 2010 May 3;11(3):331–334. doi: 10.3109/17482960902875133. [DOI] [PubMed] [Google Scholar]
- 23.Mizuno M. Neurotological findings in amyotrophic lateral sclerosis. Auris Nasus Larynx Suppl. 1986;13:S139–S146. doi: 10.1016/s0385-8146(86)80067-0. [DOI] [PubMed] [Google Scholar]
- 24.Gizzi M, DiRocco A, Sivak M, Cohen B. Ocular motor function in motor neuron disease. Neurology. 1992 May;42(5):1037–1046. doi: 10.1212/wnl.42.5.1037. [DOI] [PubMed] [Google Scholar]
- 25.Brooks B, Miller R, Swash M, Munsat T. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2000;1(5):293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 26.Chaudhuri K, Crump S, Al-Sarraj S, Anderson V, Cavanagh J, Leigh P. The validation of El Escorial criteria for the diagnosis of amyotrophic lateral sclerosis: a clinico-pathological study. J Neurol Sci. 1995;129:11. doi: 10.1016/0022-510x(95)00050-c. [DOI] [PubMed] [Google Scholar]
- 27.Cedarbaum J, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRSR: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169(1–2):13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 28.Moore S, Gresham L, Bromberg M, Kasarkis E, Smith R. A self report measure of affective lability. J Neurol Neurosurg Psychiatry. 1997;63(1):89–93. doi: 10.1136/jnnp.63.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruff R, Light R, Parker H. Benton controlled oral word association test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11(4):329–338. [PubMed] [Google Scholar]
- 30.Ramaker C, Marinus J, Stiggelbout AM, van Hilten BJ. Systematic evaluation of rating scales for impairment and disability in Parkinson's disease. Mov Disord. 2002;17(5):867–876. doi: 10.1002/mds.10248. [DOI] [PubMed] [Google Scholar]
- 31.Balcer L, Baier M, Cohen J, Kooijmans M, Sandrock A, Nano-Schiavi M, et al. Contrast letter acuity as a visual component for the Multiple Sclerosis Functional Composite. Neurology. 2003;61(10):1367. doi: 10.1212/01.wnl.0000094315.19931.90. [DOI] [PubMed] [Google Scholar]
- 32.Kirby E, Bandelow S, Hogervorst E. Visual impairment in Alzheimer's disease: a critical review. J Alzheimers Dis. 2010;21(1):15–34. doi: 10.3233/JAD-2010-080785. [DOI] [PubMed] [Google Scholar]
- 33.Bodis-Wollner I, Marx M, Mitra S, Bobak P, Mylin L, Yahr M. Visual dysfunction in Parkinson's disease. Loss in spatiotemporal contrast sensitivity. Brain. 1987;110(6):1675–1698. doi: 10.1093/brain/110.6.1675. [DOI] [PubMed] [Google Scholar]
- 34.Litvan I, Agid Y, Jankovic J, Goetz C, Brandel J, Lai E, et al. Accuracy of clinical criteria for the diagnosis of progressive supranuclear palsy (Steele–Richardson–Olszewski syndrome) Neurology. 1996;46(4):922–930. doi: 10.1212/wnl.46.4.922. [DOI] [PubMed] [Google Scholar]
- 35.Garbutt S, Matlin A, Hellmuth J, Schenk A, Johnson J, Rosen H, et al. Oculomotor function in frontotemporal lobar degeneration, related disorders and Alzheimer's disease. Brain. 2008;131(5):1268. doi: 10.1093/brain/awn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.