Abstract
The study analyzed outcomes of a consecutive case series of 37 patients with peripheral T-cell non-Hodgkin lymphoma, from related and unrelated donors, using allogeneic hematopoietic cell transplantation (allo-HCT), between the years 2000 and 2007. All patients were pretreated; the majority had either relapsed or progressive disease (n=25, 68%), 13 had cutaneous histologies (CTCL), and all were ineligible for autologous transplant. Fully ablative conditioning regimens were used in 13 patients while 24 patients underwent reduced intensity conditioning (RIC). At five years the overall survival (OS) and progression-free survival (PFS) probabilities were 52.2% and 46.5%, respectively. At the time of analysis, 9 (24.3%) patients had either relapsed (n=6) or progressed (n=3) post allo-HCT. The cumulative incidences of relapse/progression and non-relapse mortality at 5 years were 24.3% and 28.9%. No statistically significant variables for survival or relapse were discovered by univariate Cox-regression analysis of disease and patient characteristics; differences between CTCL and other histologies were not significant. The median follow-up of 64.0 months (range: 16.4–100.4) indicates a mature data-set with probable cure in the survivors. The relapse/progression curves reached and maintained plateaus after 1 year post-transplant, demonstrating that long-term disease control is possible after allo-HCT in PTCL patients with advanced disease.
Keywords: allogeneic transplant, T cell lymphoma, non-Hodgkin lymphoma, NHL
INTRODUCTION
Mature T-cell non-Hodgkin Lymphoma (NHL) and NK-cell neoplasms, collectively called peripheral T cell lymphomas (PTCL), comprise about 12% of all NHL and 15–20% of aggressive lymphomas worldwide [1]. PTCLs exhibit great morphological diversity and histological variation even within individual disease entities [2]; the current 2008 WHO classification of lymphoid neoplasms recognizes over 20 types of PTCLs [3,4]. The most common histologies include: peripheral T-cell lymphomas not otherwise specified (PTCL-nos), anaplastic large-cell lymphomas (ALCL), and angioimmunoblastic T cell lymphomas (AITL). The most aggressive histologies include: hepatosplenic T-cell NHL, gamma/delta T-cell malignancies and NK T-cell lymphomas [5]. Cutaneous T-cell neoplasms (CTCL) are classified separately and are considered a clinically unique entity [6], generally with a more indolent behavior, excepting a few subtypes including Sézary syndrome and transformed mycosis fungoides.
For aggressive lymphomas, a T-cell phenotype confers a worse clinical outcome compared to B-cell lymphomas, with the exception of ALK-positive ALCL [7,8]. Long-term survival at 5 years remains at 10–30% for most histologies [9]. Advanced disease stage, high prognostic index at presentation [10] and inherent chemomoresistance [11] contribute to this dismal outcome [12]. For CTCL patients, Sézary syndrome and advanced mycosis fungoides are often preceded by prolonged disease courses with multiple treatment regimens, ultimately resulting in chemoresistance [13]. Current therapeutic strategies for T-cell NHL remain poorly defined and tend to be extrapolated from treatment paradigms for B-cell NHL. As a result, relapsed and chemo-refractory disease remains a significant clinical dilemma in the care of these patients.
High dose chemotherapy followed by autologous stem cell transplantation (ASCT) is the standard therapeutic option for patients with aggressive B-cell lymphomas failing primary therapy [14,15]. However, many patients with relapsed PTCL are not candidates for ASCT due to chemo-refractory/progressive disease, extensive bone marrow involvement, or failure to adequately mobilize stem cells [16,17]. Furthermore, 25–30% of patients who achieve a complete response to ASCT will relapse [18]. For those PTCL patients who fail, or are ineligible for ASCT, allogeneic (related or unrelated) hematopoietic cell transplantation (allo-HCT) has been offered in an attempt to harness a potential graft-versus-lymphoma (GVL) effect [18,19]. In these studies, the GVL effects are inferred from response to reduced intensity conditioning regimens, donor lymphocyte infusions (DLI) and withdrawal of immunosuppression at the time of relapse (10 responding patients in all 3 studies), and the correlation of GVHD with decreased relapse rate [19–25] The specific factors related to disease biology that impact survival outcomes, disease control, mortality and morbidity in the setting of allo-HCT for PTCL remain poorly defined.
Due to the rarity of these diseases, most single center studies of allo-HCT in T-cell NHL are limited by small numbers of patients, heterogeneity of histologies and treatment regimens and short follow-up. Three multicenter studies [20, 23,26] have reported consolidated data on a relatively large number of patients in an attempt to understand the efficacy of allogeneic stem cell therapy in PTCL and CTCL, in general, and in specific subtypes where the numbers permit. Here we report the results of allo-HCT performed in 37 patients with PTCL at the City of Hope between 2000 and 2007. We demonstrate an overall survival of 52.2% at 5 years post allo-HCT and long-term disease control in a population with advanced disease.
PATIENTS AND METHODS
The City of Hope (COH) prospective longitudinal transplant database identified 37 consecutive patients with PTCL as defined by the 1999 WHO criteria [27], that were treated with allo-HCT, from HLA matched related and unrelated donors, between the years of 2000 and 2007. This use of data for retrospective analysis was approved by the COH Institutional Review Board (IRB) and anonymity of all patient information was maintained. Pathology review of biopsy specimens was conducted by the COH hematopathology department to confirm the diagnosis of PTCL prior to transplant as per institutional policy. Disease status at time of transplant was confirmed by clinical assessment including physical examination and laboratory evaluation, imaging by CT scans and nuclear imaging, bone marrow biopsies as well as other tissue biopsies and photo documentation (per institutional standard operating procedures). Post transplant evaluation of disease status with imaging studies, bone marrow biopsies and engraftment analysis occurred at 30 days, 60 days, 1 year post transplant and yearly thereafter or as clinically indicated. International Working Group criteria (IWG) criteria [28] were used to define disease response post transplant.
Patient Characteristics
There were 37 patients with a diagnosis of PTCL including: PTCL nos (n=8); AILT (n=4); ALCL (n=6: 3 ALK+, 2 ALK−, 1 ALK unknown); rare histologies (n=6) including NK/T cell lymphomas both nasal and extra-nasal, enteropathy-type-T-cell NHL, and hepatosplenic T-cell lymphoma. There were 13 patients with cutaneous T-cell lymphomas including mycosis fungoides (MF) and Sézary syndrome; these patients were analyzed as a separate subgroup called CTCL, with all other patients grouped together as Other PTCL. The analysis excluded patients with a diagnosis of T-cell lymphoblastic leukemias/lymphomas and HTLVI/II associated T-cell lymphoma/leukemia. Patient and transplant characteristics are summarized in Table I for the entire population and also for the CTCL and Other PTCL subgroups. The median age at transplant was 40 years (range: 7–72) and there were 27 males and 10 females. Disease status at the time of transplant was as follows: 17 patients had failed primary chemotherapy and were classified as induction failure, 1 patient had progressed on primary therapy and was considered to have primary progressive disease, 7 patients were in complete remission (CR) following therapy (including 3 patients in CR1 and 4 in CR2), and 12 patients were either in partial remission after relapsing (n=5) or in first relapse (RL1, n=7). The distribution of PIT scores, for the Other PTCL subgroup, as defined by Gallamini et al. [10] is shown in Table I. Patients were treated with a median of three prior regimens for the entire cohort. The median number of prior regimens was 3 (1–8) in patients from the Other PTCL group and 6 (4–9) in the CTCL subset. The median number of months from diagnosis to transplant was 17 (4–112) for the entire cohort, 38 (9–88) for the CTCL group and 11 (4–112) for the Other PTCL group. Only one patient had received a prior ASCT in this cohort. The median KPS at time of transplant was 90 (range: 60–100).
Table I.
Patient, Disease and Transplant Characteristics
| Total Population | Other PTCL | CTCL) | |
|---|---|---|---|
| Characteristic | N (%) or Median (Range) |
N (%) or Median (Range) |
N (%) or Median (Range) |
| Number of patients (N) | 37 | 24 | 13 |
| Patient Gender | |||
| Male | 27 | 18 | 9 |
| Female | 10 | 6 | 4 |
| Age at Transplant (years) | 40 (7–72) | 39.5 (7–72) | 50 (18–61) |
| Donor Type | |||
| Sibling | 26 (70.3) | 19 (79.2) | 7 (53.8) |
| Matched Unrelated Donor | 11 (29.7) | 5 (20.8) | 6 (46.2) |
| Disease Status at Transplant | |||
| 1st Complete Remission | 3 | 2 | 1 |
| 2nd Complete Remission | 4 | 4 | 0 |
| Partial Remission | 5 | 5 | 0 |
| 1st Relapse | 7 | 5 | 2 |
| Primary Progressive | 1 | 0 | 1 |
| Primary Induction Failure | 17 (46.0) | 8 | 9 |
| PIT Groups | |||
| 1 (PIT Score = 0) | NA | 13 (54.2) | NA |
| 2 (PIT Score = 1) | NA | 2 (8.3) | NA |
| 3 (PIT Score = 2) | NA | 6 (25.0) | NA |
| Unknown | NA | 3 (12.5) | NA |
| Prior Regimens | 3 (1–9) | 3 (1–8) | 6 (4–9) |
|
Months from Diagnosis to Transplant |
17 (4–112) | 11 (4–112) | 38 (9–88) |
| Stem Cell Source | |||
| Bone Marrow | 5 (13.5) | 4 (16.7) | 1 (7.7) |
| Peripheral Blood | 31 (83.8) | 19 (79.2) | 12 (92.3) |
| Cord Blood | 1 (2.7) | 1 (4.2) | 0 |
| Conditioning Intensity | |||
| Reduced | 22 (59.4) | 13 (54.2) | 9 (69.2) |
| Non-myeloablative | 2 (5.4) | 1 (4.2) | 1 (7.7) |
| Myeloablative | 13 (35.1) | 10 (41.7) | 3 (23.1) |
| Conditioning Regimen | |||
| Bu | Cy | 1 | 0 | 1 |
| FTBI | Cy | 7 | 5 | 2 |
| FTBI | Cy | ATG | 1 | 1 | 0 |
| FTBI | VP-16 | 4 | 4 | 0 |
| Fludarabine | TBI | 2 | 1 | 1 |
| Fludarabine | Melphalan | 22 (59.5) | 13 | 9 |
| GVHD Prophylaxis | |||
| CSA/MMF Based | 20 (54.1) | 12 (50.0) | 8 (61.5) |
| Tacro/Siro Based | 17 (45.9) | 12 (50.0) | 5 (38.5) |
MF–mycosis fungoides, SS–Sézary Syndrome, CTCL–cutaneous T-cell lymphoma, DX–diagnosis, Bu–busulfan, CTX–cyclophosphamide, FTBI–fractionated total body irradiation, ATG–anti-thymocyte globulin, VP-16–etoposide, CSA–cyclosporine, MMF–mycophenylate mofetil
Eligibility Criteria
Patients with very aggressive histologies such as gamma/delta T-cell NHL were offered allo-HCT early in the disease course (CR1/PR1) due to the known poor prognosis and aggressive nature of these diseases. Patients who relapsed after conventional chemotherapy or had primary chemo-refractory disease were offered allo-HCT after they were found to be ineligible for high-dose therapy and ASCT. Patients with CTCL were not offered ASCT as it provides poor long-term disease control [29].
Donor Selection
Donor selection was based on the availability of an HLA-identical or single-antigen mismatched family donor, or alternatively from an HLA matched unrelated donor based on molecular typing of HLA A, B, C, DR, and DQ loci. A total of 26 (70%) patients were transplanted using a sibling donor, and 11 (30%) patients had a matched unrelated donor. Source of stem cells was as follows: bone marrow (n=5), growth factor mobilized stem cells (n=31), cord blood (n=1).
Conditioning Regimens and GVHD Prophylaxis
A total of 24 patients received reduced-intensity conditioning regimens (RIC) and 13 received fully ablative regimens. Myeloablative conditioning regimens included: 1) fractionated total body irradiation (FTBI) / cyclophosphamide (Cy) – TBI at 1320 cGY given in 11 fractions over 4 days plus IV Cy at 60 mg/kg ideal bodyweight for 2 days, 2) FTBI/etoposide (VP-16) – FTBI at 1320 cGY given in 11 fractions over 4 days plus IV VP-16 at 60 mg/kg adjusted bodyweight for one day, and 3) busulfan (Bu)/Cy – IV Bu adjusted to AUC between 1000 and 1200 plus IV Cy at 60 mg/kg ideal bodyweight for 2 days. Reduced intensity conditioning regimens included: regimen 4) fludarabine (Flu)/ total body irradiation (TBI) – IV Flu at 25 mg/m2 for 5 days plus a single dose of TBI at 200 cGY given on day -1, and regimen 5) Flu/melphalan (Mel) – IV Flu at 25 mg/m2 for 5 days plus IV Mel at 140 mg/m2 for one day. Specific GVHD prophylactic regimens are shown in Table I.
Statistical Methods
Survival estimates were calculated using the Kaplan-Meier product-limit method; 95% confidence intervals were calculated using the logit transformation and the Greenwood variance estimate [30]. Differences between Kaplan-Meier curves were assessed by the log-rank test. Patients who were alive at the time of analysis were censored at the last contact date. Overall survival (OS) was measured from the day of stem cell infusion to death from any cause. Progression-free survival (PFS) was defined as time from stem cell infusion to recurrence, progression or death from any cause, whichever occurred first. The relapse/progression incidence (RP) was defined as time from stem cell infusion to recurrence or progression. Non-relapse mortality (NRM) was measured from stem cell infusion to death from any cause other than disease relapse or disease progression. Non-relapse-related mortality and relapse-related mortality were considered competing risks for mortality. The cumulative incidence of NRM and relapse-related mortality was calculated using the method described by Gooley et al [31]. Differences between cumulative incidence curves in the presence of a competing risk were tested using the Gray method [32]. The significance of demographic, disease, and treatment features was assessed using either univariate Cox regression analysis [33] or its competing-risks analogue [34]. Univariate models were used to model time to event endpoints (e.g., OS, PFS, RP, and NRM), as a function of the prognostic variables. The list of prognostic variables was determined from a literature review that identified factors associated with survival and/or disease relapse/recurrence in patients treated with allo-HCT. These variables were: histopathological subtype, patient age at allo-HCT (<40 years, ≥40 years), disease status at the time of allo-HCT (CR or PR; relapse/PD/IF), donor type (sibling, unrelated), and conditioning regimen (ablative, reduced-intensity). The impact of GVHD (acute, chronic, any GVHD) was analyzed as a time-dependent covariate [35,36]. For the time-dependent covariate analyses, GVHD evaluation began after the infusion of stem cells. The time-dependent covariate took on the value of ‘1’ if the GVHD assessment was positive and ‘0’ otherwise. The value of the time-dependent covariate remained the same until the next GVHD assessment. All calculations were preformed using SAS 9.2 (SAS Institute, Cary, NC) and R (version 2.4.1; http://www.r-project.org). Statistical significance was set at the P <0.05 level; all P values were two-sided. The data were locked for analysis on February 1, 2009 (analytic date).
RESULTS
Outcomes
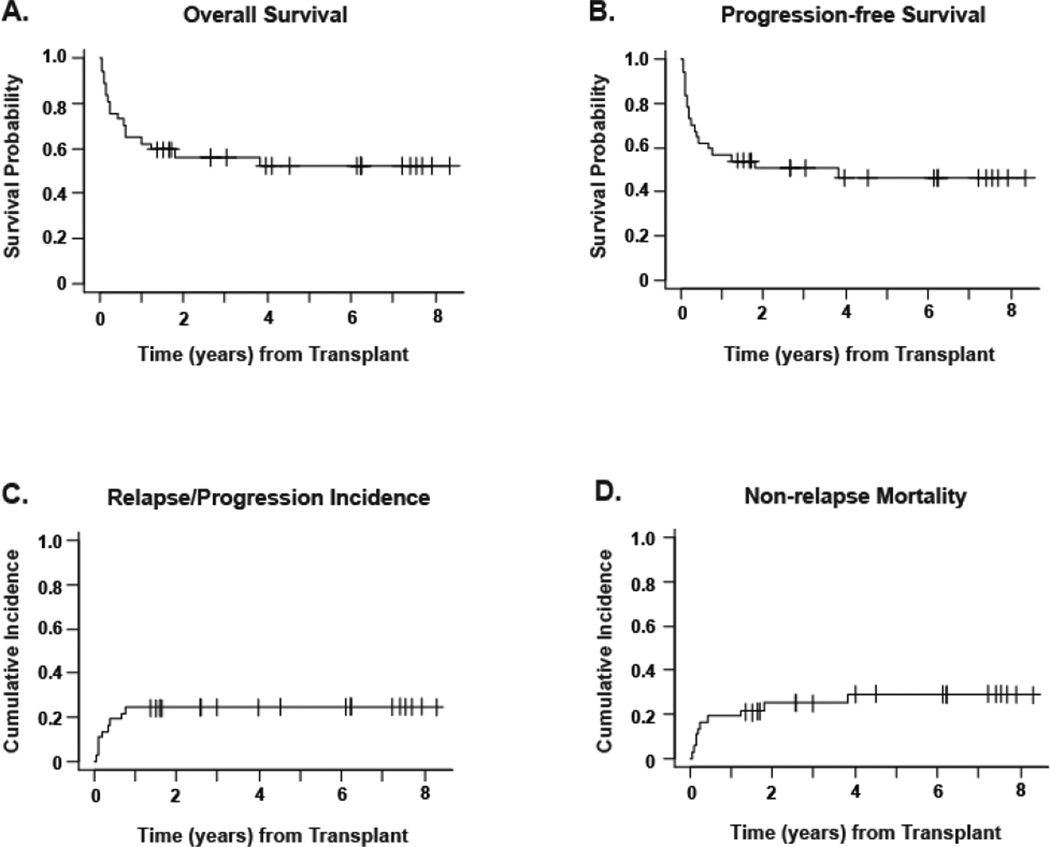
Study outcomes are summarized in Table II, both for the entire population and for the Other PTCL and CTCL subgroups. The median follow up was 20.3 months (range: 0.7–100.4 months) for all patients and 64.0 months (range: 16.4–100.4 months) for surviving patients. The median follow-up was 49.2 months (range: 16.4–100.4) for surviving Other PTCL patients and 86.4 (range: 31.9–95.3) for CTCL patients. At last contact, 20 patients (54.1%) were alive and 17 (45.9%) had expired. The OS probability at 5 years was 52.2% (95%CI: 43.0–60.5) (Figure 1). The primary causes of death included: disease progression (n=6), infection (n=6), acute GVHD (n=1), chronic GVHD (n=1), secondary malignancy (duodenal cancer, n=1), and multi-organ failure (n=2). There were 9 deaths prior to day 100 mainly due to transplant-related causes, 4 in the Other PTCL group and 5 in the CTCL group. The proportion of patients who either relapsed or progressed following transplant was 24.3% including 6 relapses and 3 progression events (defined as disease progression without remission); 7 of these patients died from their disease and 2 relapsed patients remained alive at the analysis date, 1 in CR and 1 with active disease. No relapsed/progressed patients received donor lymphocyte infusions, and all patients received additional treatments, except for 2 progressives who died rapidly. The only relapsed or progressed patient who was alive and disease-free at the analytic date, had relapse only in the skin and achieved remission via topical steroids and retinoids.
Table II.
Summary of Outcomes
| Total | Other PTCL | CTCL | |
|---|---|---|---|
| Outcome | N (%) | N (%) | N (%) |
| Number of patients (N) | 37 | 24 | 13 |
| aGVHD | |||
| Yes | 19 (51.4) | 12 (50.0) | 7 (53.9) |
| Grade I | 5 (13.5) | 4 (16.7) | 1 (7.7) |
| Grade II | 8 (21.6) | 6 (25.0) | 2 (15.4) |
| Grade III | 2 (5.4) | 1 (4.2) | 1 (7.7) |
| Grade IV | 4 (10.8) | 1 (4.2) | 3 (23.1) |
| No | 18 (48.6) | 12 (50.0) | 6 (46.2) |
| cGVHD | |||
| Yes | 23 (62.2) | 16 (66.7) | 7 (53.9) |
| Limited | 3 (8.1) | 3 (12.5) | 0 |
| Extensive | 20 (54.1) | 13 (54.2) | 7 (53.9) |
| No | 5 (13.5) | 4 (16.7) | 1 (7.7) |
| Expired < 100 days post-HCT | 9 (24.3) | 4 (16.7) | 5 (38.5) |
| Any GVHD (aGVHD/cGVHD) | |||
| Yes | 29 (78.4) | 19 (79.2) | 10 (76.9) |
| No | 8 (21.6) | 5 (20.8) | 3 (23.1) |
| Relapse/ Progression post-HCT | |||
| Yes | 9 (24.3) | 6 (25.0) | 3 (23.1) |
| No | 28 (75.7) | 18 (75.0) | 10 (76.9) |
| Vital Status post-HCT | |||
| Alive | 20 (54.1) | 15 (62.5) | 5 (38.5) |
| Dead | 17 (45.9) | 9 (37.5) | 8 (61.5) |
| Cause of Death | |||
| Disease Progression | 6 (16.2) | 4 (44.4) | 2 (25.0) |
| Infection | 6 (16.2) | 3 (33.3) | 3 (37.5) |
| multi-organ failure | 2 (5.4) | 1 (11.1) | 1 (12.5) |
| aGVHD | 1 (2.7) | 0 | 1 (12.5) |
| cGVHD | 1 (2.7) | 0 | 1 (12.5) |
| secondary malignancy (duodenal) | 1 (2.7) | 1 (11.1) | 0 |
aGVHD–acute graft-versus-host disease, NA–not applicable, cGVHD–chronic graft-versus-host disease, HCT–hematopoietic cell transplant
Figure 1. Outcomes for total population.
All curves are for the 37-patient population with a median follow-up of 64 months for survivors. Panel A shows the Kaplan-Meier estimate of survival probability. Panel B shows progression-free survival, defined as time from stem cell infusion to recurrence, progression, or death from any cause, whichever occurred first. Panel C shows cumulative incidence of relapse/progression, defined as time from stem cell infusion to recurrence or progression. Panel D shows non-relapse mortality, measured as time from stem cell infusion to death from any cause other than disease relapse or disease progression. In panels C and D, relapse/progression and non-relapse mortality were treated as competing risks.
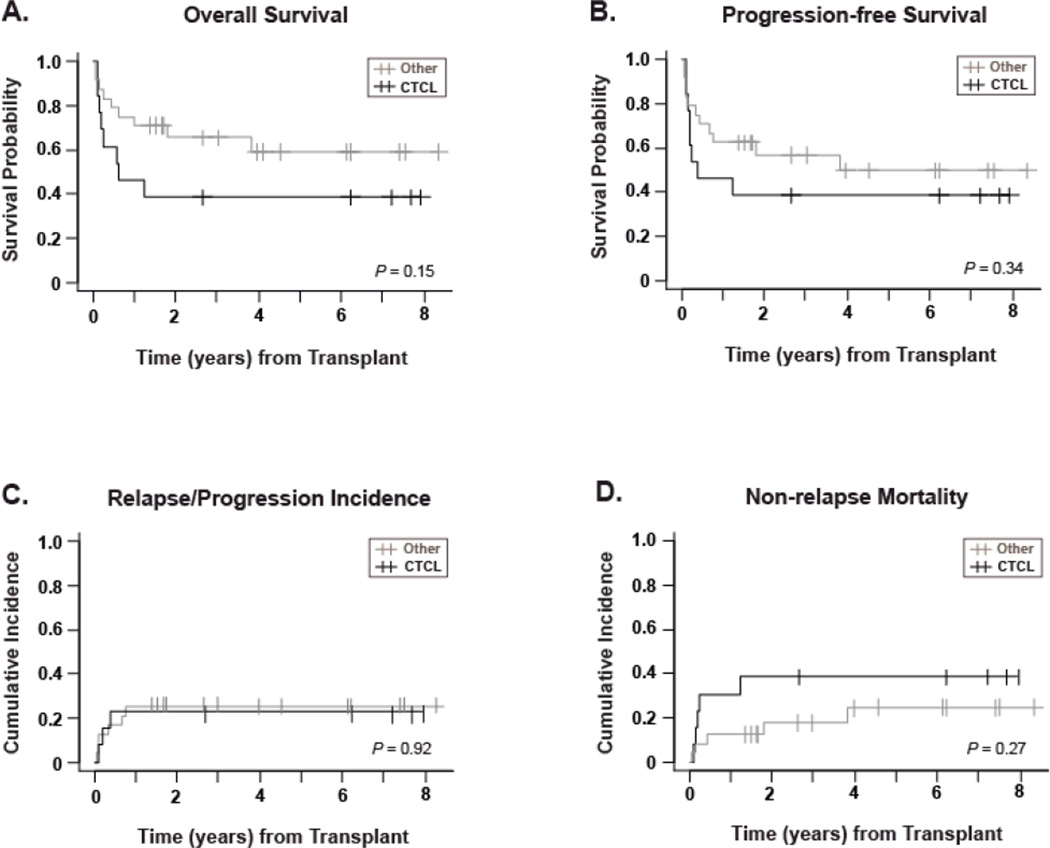
Curves for OS, PFS, relapse incidence and NRM are shown for the entire population in Figure 1, and are stratified based on CTCL or Other PTCL subgroups in Figure 2. The probability of PFS was 46.5% (95%CI: 38.4–54.1) at 5 years for the entire population. For CTCL and Other PTCL, 5-year PFS was 38.5% (95%CI: 28.4–48.5) and 49.7% (95%CI: 38.3–60.1) respectively, with no statistically significant difference (P=0.34). A plateau in the cumulative incidence of relapse/progression at 24.3% was achieved 1 year post allo-HCT. The cumulative incidence of non-relapse mortality at 100 days, 1 year and 5 years was 16.2%, 18.9% and 28.9% respectively for the total population, and was not statistically significantly different for the CTCL versus Other PTCL subgroups. Patients with active disease at the time of transplant showed a trend toward poorer overall survival, with 5-year OS estimates of 43.2%, for patients not in CR/PR, compared to 72.9% for patients in CR/PR (p=0.07) indicating that remission status could impact the outcome of the transplant.
Figure 2. Outcomes stratified by histological subgroup.
All curves are stratified for histology: CTCL (n=13, black lines) versus Other PTCL (n=24, gray lines), with a median follow-up of 86 months for the CTCL surviving patients and 49 months for the Other PTCL group. P-values for log-rank comparison of curves are given in the bottom right hand corner of each graph. Panel A shows the Kaplan-Meier estimate of survival probability. Panel B shows progression-free survival, defined as time from stem cell infusion to recurrence, progression, or death from any cause, whichever occurred first. Panel C shows cumulative incidence of relapse/progression, defined as time from stem cell infusion to recurrence or progression. Panel D shows non-relapse mortality, measured as time from stem cell infusion to death from any cause other than disease relapse or disease progression. For data in panels C and D, relapse/progression and non-relapse mortality were treated as competing risks.
Graft-versus-host disease
Of the 37 evaluable patients, acute GVHD was seen in 19 (51.4%) patients (Table II). Of these, 13 had grade I/II acute GVHD, the remaining 6 experienced grade III/IV acute GVHD. Chronic GVHD developed in 23/28 evaluable patients (82.1%); extensive chronic GVHD in 20 (71.4%). In the final analysis, 29/37 patients (78.4%) experienced some form of GVHD.
Univariate Analyses
The univariate analyses results for OS, PFS, and RP are summarized in Table III. There were no statistically significant differences in OS, PFS, and RP in patients who had CTCL versus Other PTCL. Similarly, there were no statistically significant differences in outcome between patients who received fully ablative conditioning and those conditioned with a reduced intensity regimen., For patients who underwent transplantation with advanced disease, there was a trend toward a 3-fold increased risk of death (HR: 3.1, 95%CI: 0.9–10.7; p=0.08). Prognostic Index for PTCL-unspecified (PIT score) [10] was also analyzed as a potential influence on hazard risk for OS, PFS and RPR in the Other PTCL group only. The analysis was performed on the following separate groupings: PIT group 1/2 vs. 3/4, PIT group 1 vs. 2/3/4, and PIT Level: 1 vs. 2 and 1vs. 3/4. There was no significant difference found by any grouping method. Table III shows the results for PIT group 1/2 versus 3/4.
Table III.
Univariate Analysis of Outcomes
| Overall Survival | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Reference Group |
Total n= |
Events | Censored | Hazard Ratio |
Confidence Interval |
p-Value |
| Age at HCT | Age < 40 | 37 | 17 | 20 | 0.96 | 0.37–2.48 | 0.93 |
| PTCL versus CTCL | PTCL | 37 | 17 | 20 | 2.00 | 0.77–5.20 | 0.16 |
| Disease Status at HCT | 1CR/2CR/PR | 37 | 17 | 20 | 3.06 | 0.87–10.69 | 0.08* |
| PIT Group | 1 / 2 | 21 | 7 | 14 | 1.06 | 0.21–5.50 | 0.94 |
| Donor Type | Sibling | 37 | 17 | 20 | 0.96 | 0.34–2.74 | 0.94 |
| Conditioning Regimen | RIC | 37 | 17 | 20 | 0.69 | 0.25–1.87 | 0.46 |
| aGVHD Max Grade | None-II | 37 | 17 | 20 | 2.17 | 0.49–9.62 | 0.31 |
| cGVHD Max Grade | Limited | 23 | 7 | 16 | 0.68 | 0.16–2.88 | 0.60 |
| Progression-Free Survival | |||||||
| Variable |
Reference Group |
Total n= |
Events | Censored |
Hazard Ratio |
Confidence Interval |
p-Value |
| Age at HCT | Age < 40 | 37 | 19 | 18 | 0.94 | 0.38–2.32 | 0.90 |
| PTCL versus CTCL | PTCL | 37 | 19 | 18 | 1.56 | 0.62–3.89 | 0.34 |
| Disease Status at HCT | 1CR/2CR/PR | 37 | 19 | 18 | 1..80 | 0.65–5.02 | 0.26 |
| PIT Group | 1 / 2 | 21 | 9 | 12 | 0.74 | 0.15–3.55 | 0.70 |
| Donor Type | Sibling | 37 | 19 | 18 | 0.76 | 0.27–2.10 | 0.59 |
| Conditioning Regimen | RIC | 37 | 19 | 18 | 0.58 | 0.22–1.54 | 0.27 |
| aGVHD Max Grade | None-II | 37 | 19 | 18 | 1.70 | 0.39–7.43 | 0.438 |
| cGVHD Max Grade | Limited | 23 | 9 | 14 | 1.46 | 0.37–5.79 | 0.59 |
| Relapse/Progression | |||||||
| Variable |
Reference Group |
Total n= |
Events | Censored |
Hazard Ratio |
Confidence Interval |
p-Value |
| Age at HCT | Age < 40 | 37 | 9 | 18 | 0.67 | 0.18–2.49 | 0.55 |
| PTCL versus CTCL | PTCL | 37 | 9 | 18 | 1.10 | 0.27–4.42 | 0.89 |
| Disease Status at HCT | 1CR/2CR/PR | 37 | 9 | 18 | 1.28 | 0.32–5.15 | 0.73 |
| PIT Group | 1 / 2 | 21 | 5 | 12 | 0.64 | 0.07–5.74 | 0.69 |
| Donor Type | Sibling | 37 | 9 | 18 | 0.27 | 0.03–2.16 | 0.22 |
| Conditioning Regimen | RIC | 37 | 9 | 18 | 0.69 | 0.17–2.75 | 0.59 |
| aGVHD Max Grade | None-II | 37 | 9 | 18 | 1.00 | 0.00-UND | 0.99 |
| cGVHD Max Grade | Limited | 23 | 5 | 14 | 1.98 | 0.28–14.17 | 0.50 |
Univariate Cox proportional hazards analysis:
significance: p ≤0.05;
trend: p ≤ 0.1; Confidence intervals shows 95% confidence ranges. HCT–hematopoietic cell transplant, aGVHD–acute graft-versus-host disease, cGVHD–chronic graft-versus-host disease, PTCL–peripheral T-cell lymphoma, 1CR–first complete remission, 2CR–second complete remission, PR–partial remission, RIC-reduced-intensity conditioning, UND-undetermined.
Time-dependent covariate analysis: In this analysis because the timing of GVHD is considered as part of the risk calculation, the model assesses benefit or harm after GVHD onset for the endpoint of interest. This analysis showed no significant impact on survival, relapse/progression or mortality hazard risk for any of the following variables: 1) time to onset of any GVHD (acute or chronic), 2) time to onset of aGVHD (any grade), 3) time to onset of acute GVHD ≥ grade II, 4) time to onset of aGVHD ≥ grade III, 5) time to onset of chronic GVHD (limited or extensive).
DISCUSSION
T-cell phenotype in aggressive lymphomas confers a poor prognosis as reported by the International T-cell lymphoma project [9], a collaborative epidemiological study involving over 22 centers and 1500 patients worldwide. This study reports 5-year overall survival of the most common types of T-cell lymphoma as follows: PTCL-NOS and AILT (32%), ALCL ALK+ (70%), ALK− (49%), NK/T cell (nasal type 42%, extra nasal type 9%) hepatosplenic and enteropathy type T-cell NHL (7–10%). Relapse and death rates for T-cell lymphomas are unacceptably high with standard therapies.
The suggestion of a graft-versus-lymphoma effect was first introduced in the early 1990s after demonstration that an allogeneic transplant for lymphoma was associated with a lower risk of relapse as compared to autologous transplant [37,38]. These initial studies included both B- and T-cell lymphomas and did not examine graft versus T-cell lymphoma independently. The high transplant-related mortality associated with allo-HCT offset any benefit obtained from the decreased relapse rate thus negating any survival advantage. Over the last decade the introduction of reduced-intensity conditioning regimens and improved supportive care, including prophylaxis and treatment of GVHD and infections, has reduced the upfront morbidity and mortality of allogeneic transplant, making it more accessible to an aging population [39,40]. The RIC therapeutic modality relies primarily on a graft-versus-tumor effect and it is becoming routine to offer RIC transplants for histologies such as low-grade B-cell lymphomas [41,42] where the graft-versus-lymphoma effect has been well defined. These studies have also demonstrated improved disease control in the presence of GVHD and documented treatment of post-transplant relapse by withdrawal of immunosuppression and administration of DLI as evidence of a graft-versus-tumor effect.
The role of allogeneic transplant in patients with T-cell NHL is evolving but systematic prospective studies for this group of patients are lacking. Le Gouill et al [23], in a large multi-center retrospective study of 77 patients with T-cell NHL, report a 5-year OS and event-free survival (EFS) of 57% and 53% respectively with a TRM of 33%. In multivariate analysis, chemo-resistant disease and the occurrence of grade 3/4 acute GVHD are associated with poor outcome. The presence of CR or PR at the time of transplant is associated with a statistically significant improvement in OS at 5 years as compared to stable, progressive or refractory disease: 69% vs. 29% (p=0.04). This study fails to show an association between GVHD and improved outcome; rather, the presence of acute GVHD was a poor prognostic factor for survival. They propose a GVL effect by demonstrating that 2 patients receiving DLI achieve sustained remission. Similarly, Kim et al. [20] report 5-year survivals of 70% with PTCL and 30% with NK/T-cell lymphomas in 54 patients. Their analysis shows that chronic GVHD was associated with a poor outcome, but they also report that 3 patients receiving DLI for relapsed disease achieve a sustained response. Wosserman et al. [43] and Molina et al. [44] focus only on the specific histologies of ALCL and CTCL respectively and report impressive long-term survival in these patients after allogeneic stem cell transplant. Corradini et al. [25] report a positive GVL effect in a prospective phase II trial of 17 PTCL patients using a RIC regimen of thiotepa/Cy/Flu. Overall survival of 80% at 3 years and PFS of 64% at 3 years points to long-term disease control via allogeneic transplant but most notable is their NRM of only 6%. Fifteen of these 17 PTCL patients were in CR or PR at the time of transplant, which likely contributes to the excellent outcomes. Providing further evidence for GVL, this group administered DLI to 2 relapsed patients and also withdrew immunosuppression from one patient, successfully inducing remission in all three. A recently published retrospective study by Kyriakou et al. of allogeneic transplant for AITL [45] shows a decrease in relapse rate associated with development of cGVHD, lending further support to a GVL effect in T-lymphoma. In this study, the 45 patients, 44% of whom received RIC, exhibit a 3-year PFS of 53%, OFS of 64% and relapse rate of 20%. In summary, the literature demonstrates that it is possible to achieve survival rates of 40–60% at 3–5 years with allogeneic transplants for T-cell lymphoma. High rates of NRM (up to 50%) are reported with fully ablative conditioning regimens but the use of RIC is associated with lower toxicity [40] and there is some evidence for a graft-versus-T-cell lymphoma effect.
Since 35% of patients in this study had mycosis fungoides or Sézary syndrome, we investigated possible differences between the CTCL patient subgroup and other subtypes of PTCL. CTCL has a clinical course distinct from other forms of PTCL, with a tendency for multiple treatment regimens over many years, as evidenced by the fact that our CTCL subgroup had double the median number of prior regimens as did the Other PTCL group. Once patients progress to advanced phase disease the median survival is between 1.5 to 4 years.[46,47]. The documentation in the literature of transplant outcomes for CTCL, consists primarily of case studies and series, including a previous City of Hope report [44]. A recent review of 60 CTCL patients from the EBMT registry [26] estimates an OS of 54% at 3 years; however, the extensive use of T-cell depletion (42%) in this study was associated with a reduced PFS.
Here we report the results of a single institution experience using allogeneic stem cell transplant in 37 patients with PTCL followed for over 5 years. We obtained 52% OS and 46.5% PFS at 5 years, with best results seen in patients transplanted in a state of a complete or partial remission compared to patients who had active disease at the time of transplant. Patients were offered allogeneic in lieu of autologous stem cell transplant due to progressive disease, bone marrow involvement or histology. Sixty-five percent of the patients received a reduced intensity conditioning regimen (RIC) with no statistically significant differences in OS or PFS compared to the ablative therapy patients. Histology subtype CTCL versus Other PTCL also made no significant difference in any survival outcome measure. While CTCL tends to have a less aggressive clinical course, the more indolent disease manifestation is offset by the larger number of prior regimens, higher median age, and extended time between diagnosis and transplant (much of it spent on immunosuppressive therapy) in our CTCL group, resulting in higher NRM.
Presence or absence of any form of GVHD, was not found to impact any survival outcomes, based on a time-dependent analysis. As our study population is relatively small, the lack of statistical associations is not surprising. The proportion of patients who relapsed or progressed was 24.3% with relapses occurring within the first 1 year post transplant. Overall mortality was 46% with only 5 deaths attributed to disease relapse while remaining deaths were secondary to infection or complications of GVHD. These data indicate that allogeneic stem cell transplant is capable of conferring long-term disease control even in relapsed patients, with improved overall outcomes attainable through reduction of NRM. The low risk of relapse, and the plateaus in survival and relapse curves, are encouraging and indicate that a cure may be possible in over half the patients, who have now been followed for over 5 years.
Although patients in remission tend to have better overall survival than those with active disease at transplant (73% vs. 43% at 5-years), a 5-year PFS of 43% for patients with active disease is still a chance that many would consider worth taking, given the lack of alternatives. Achieving a remission state prior to transplant typically improves the outcome of allogenic stem cell transplant, emphasizing the importance of more effective T-lymphoma salvage therapies.
In this analysis, only 6/37 patients died of disease progression (5-yr RP cumulative incidence of 24.3%), again emphasizing the excellent disease control that can be achieved by the modality of allogeneic stem cell transplant, even in these heavily pretreated patients. Our 5-yr overall survival of 52% and NRM of 29%, while promising, highlight the need for improved remediation of infections, GVHD and other complications of allo-HCT. The advent of novel agents with activity against T-cell lymphomas including HDAC inhibitors [48–52], antifolates like pralatrexate [53], antibodies such as alemtuzumab (Campath) [54], and anti CD4 (Humax) [55] may change the paradigms in T-cell lymphoma therapy. Several of these agents, although still in clinical trials, show strong indications of activity against T-cell lymphoproliferative disorders, potentially enabling refractory patients to attain remission as a bridge to more effective stem cell transplantation. We have shown that allo-HCT can provide effective long-term disease control for PTCL, even in a population with advanced disease status. A follow-up period of over five years for surviving patients suggests that this long-term disease control is actually cure. The future combination of allogeneic hematopoietic cell transplant with emerging low-toxicity therapies for T-cell lymphoma will further enhance this curative potential.
ACKNOWLEDGEMENTS
This work was supported by the following grants: P01-CA030206, P50-CA107399, P30-CA33572 and the Marcus Foundation. We would like to thank the dedicated nurses and staff of City of Hope, who have made this research possible.
Footnotes
DECLARATION OF INTERESTS
The authors declare no conflicts of interest.
REFERENCES
- 1.Groves FD, Linet MS, Travis LB, Devesa SS. Cancer surveillance series: non-Hodgkin's lymphoma incidence by histologic subtype in the United States from 1978 through 1995. J Natl Cancer Inst. 2000;92:1240–1251. doi: 10.1093/jnci/92.15.1240. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JR, Armitage JO, Weisenburger DD. Epidemiology of the non-Hodgkin's lymphomas: distributions of the major subtypes differ by geographic locations. Non-Hodgkin's Lymphoma Classification Project. Ann Oncol. 1998;9:717–720. doi: 10.1023/a:1008265532487. [DOI] [PubMed] [Google Scholar]
- 3.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. WHO Press; 2008. [Google Scholar]
- 4.Rudiger T, Weisenburger DD, Anderson JR, et al. Peripheral T-cell lymphoma (excluding anaplastic large-cell lymphoma): results from the Non-Hodgkin's Lymphoma Classification Project. Ann Oncol. 2002;13:140–149. doi: 10.1093/annonc/mdf033. [DOI] [PubMed] [Google Scholar]
- 5.Savage KJ, Chhanabhai M, Gascoyne RD, Connors JM. Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann Oncol. 2004;15:1467–1475. doi: 10.1093/annonc/mdh392. [DOI] [PubMed] [Google Scholar]
- 6.Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 7.Coiffier B, Brousse N, Peuchmaur M, et al. Peripheral T-cell lymphomas have a worse prognosis than B-cell lymphomas: a prospective study of 361 immunophenotyped patients treated with the LNH-84 regimen. The GELA (Groupe d'Etude des Lymphomes Agressives) Ann Oncol. 1990;1:45–50. doi: 10.1093/oxfordjournals.annonc.a057673. [DOI] [PubMed] [Google Scholar]
- 8.Gisselbrecht C, Gaulard P, Lepage E, et al. Prognostic significance of T-cell phenotype in aggressive non-Hodgkin's lymphomas. Groupe d'Etudes des Lymphomes de l'Adulte (GELA) Blood. 1998;92:76–82. [PubMed] [Google Scholar]
- 9.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 10.Gallamini A, Stelitano C, Calvi R, et al. Peripheral T-cell lymphoma unspecified (PTCL-U): a new prognostic model from a retrospective multicentric clinical study. Blood. 2004;103:2474–2479. doi: 10.1182/blood-2003-09-3080. [DOI] [PubMed] [Google Scholar]
- 11.Jillella AP, Murren JR, Hamid KK, et al. P-glycoprotein expression and multidrug resistance in cutaneous T-cell lymphoma. Cancer Invest. 2000;18:609–613. doi: 10.3109/07357900009032827. [DOI] [PubMed] [Google Scholar]
- 12.Escalon MP, Liu NS, Yang Y, et al. Prognostic factors and treatment of patients with T-cell non-Hodgkin lymphoma: the M. D. Anderson Cancer Center experience. Cancer. 2005;103:2091–2098. doi: 10.1002/cncr.20999. [DOI] [PubMed] [Google Scholar]
- 13.Gardner JM, Evans KG, Musiek A, Rook AH, Kim EJ. Update on treatment of cutaneous T-cell lymphoma. Curr Opin Oncol. 2009;21:131–137. doi: 10.1097/CCO.0b013e3283253190. [DOI] [PubMed] [Google Scholar]
- 14.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995;333:1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 15.Mounier N, Gisselbrecht C, Briere J, et al. All aggressive lymphoma subtypes do not share similar outcome after front-line autotransplantation: a matched-control analysis by the Groupe d'Etude des Lymphomes de l'Adulte (GELA) Ann Oncol. 2004;15:1790–1797. doi: 10.1093/annonc/mdh471. [DOI] [PubMed] [Google Scholar]
- 16.Jagasia M, Morgan D, Goodman S, et al. Histology impacts the outcome of peripheral T-cell lymphomas after high dose chemotherapy and stem cell transplant. Leuk Lymphoma. 2004;45:2261–2267. doi: 10.1080/10428190412331272749. [DOI] [PubMed] [Google Scholar]
- 17.Zamkoff KW, Matulis MD, Mehta AC, et al. High-dose therapy and autologous stem cell transplant does not result in long-term disease-free survival in patients with recurrent chemotherapy-sensitive ALK-negative anaplastic large-cell lymphoma. Bone Marrow Transplant. 2004;33:635–638. doi: 10.1038/sj.bmt.1704392. [DOI] [PubMed] [Google Scholar]
- 18.Gutierrez A, Caballero MD, Perez-Manga G, Rodriguez J. Hematopoietic SCT for peripheral T-cell lymphoma. Bone Marrow Transplant. 2008;42:773–781. doi: 10.1038/bmt.2008.332. [DOI] [PubMed] [Google Scholar]
- 19.Dhedin N, Giraudier S, Gaulard P, et al. Allogeneic bone marrow transplantation in aggressive non-Hodgkin's lymphoma (excluding Burkitt and lymphoblastic lymphoma): a series of 73 patients from the SFGM database. Societ Francaise de Greffe de Moelle. Br J Haematol. 1999;107:154–161. doi: 10.1046/j.1365-2141.1999.01666.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim SW, Tanimoto TE, Hirabayashi N, et al. Myeloablative allogeneic hematopoietic stem cell transplantation for non-Hodgkin lymphoma: a nationwide survey in Japan. Blood. 2006;108:382–379. doi: 10.1182/blood-2005-02-0596. [DOI] [PubMed] [Google Scholar]
- 21.Maloney DG. Graft-vs.-lymphoma effect in various histologies of non-Hodgkin's lymphoma. Leuk Lymphoma. 2003;44(Suppl 3):S99–S105. doi: 10.1080/10428190310001623694. [DOI] [PubMed] [Google Scholar]
- 22.Armand P, Gannamaneni S, Kim HT, et al. Improved survival in lymphoma patients receiving sirolimus for graft-versus-host disease prophylaxis after allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning. J Clin Oncol. 2008;26:5767–5774. doi: 10.1200/JCO.2008.17.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Gouill S, Milpied N, Buzyn A, et al. Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study by the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. J Clin Oncol. 2008;26:2264–2271. doi: 10.1200/JCO.2007.14.1366. [DOI] [PubMed] [Google Scholar]
- 24.Hamadani M, Awan FT, Elder P, et al. Allogeneic hematopoietic stem cell transplantation for peripheral T cell lymphomas; evidence of graft-versus-T cell lymphoma effect. Biol Blood Marrow Transplant. 2008;14:480–483. doi: 10.1016/j.bbmt.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Corradini P, Dodero A, Zallio F, et al. Graft-versus-lymphoma effect in relapsed peripheral T-cell non-Hodgkin's lymphomas after reduced-intensity conditioning followed by allogeneic transplantation of hematopoietic cells. J Clin Oncol. 2004;22:2172–2176. doi: 10.1200/JCO.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 26.Duarte RF, Canals C, Onida F, et al. Allogeneic hematopoietic cell transplantation for patients with mycosis fungoides and Sezary syndrome: a retrospective analysis of the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2010;28:4492–4499. doi: 10.1200/JCO.2010.29.3241. [DOI] [PubMed] [Google Scholar]
- 27.Harris NL, Jaffe ES, Diebold J, et al. The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. Report of the Clinical Advisory Committee meeting, Airlie House, Virginia, November, 1997. Ann Oncol. 1999;10:1419–1432. doi: 10.1023/a:1008375931236. [DOI] [PubMed] [Google Scholar]
- 28.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 29.Olavarria E, Child F, Woolford A, et al. T-cell depletion and autologous stem cell transplantation in the management of tumour stage mycosis fungoides with peripheral blood involvement. Br J Haematol. 2001;114:624–631. doi: 10.1046/j.1365-2141.2001.02919.x. [DOI] [PubMed] [Google Scholar]
- 30.Breslow NE, Day NE. Statistical methods in cancer research: volume II, the design and analysis of cohort studies. IARC Sci Publ. 1987;82:1–406. [PubMed] [Google Scholar]
- 31.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 32.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics. 1988;16:1140–1154. [Google Scholar]
- 33.Cox DR. Regression models and life tables. Journal of the Royal Statistical Society. 1972;B34:187–220. [Google Scholar]
- 34.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 35.Therneau TM, Grambsch PM, editors. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer; 2000. [Google Scholar]
- 36.Klein JP, Moeschberger ML, editors. Survival Analysis: Techniques for Censored and Truncated Data. 2nd ed. New York, NY: Springer; 2003. [Google Scholar]
- 37.Grigg A, Ritchie D. Graft-versus-lymphoma effects: clinical review, policy proposals, and immunobiology. Biol Blood Marrow Transplant. 2004;10:579–590. doi: 10.1016/j.bbmt.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 38.Ratanatharathorn V, Uberti J, Karanes C, et al. Prospective comparative trial of autologous versus allogeneic bone marrow transplantation in patients with non-Hodgkin's lymphoma. Blood. 1994;84:1050–1055. [PubMed] [Google Scholar]
- 39.Champlin R, Khouri I, Kornblau S, Molldrem J, Giralt S. Reinventing bone marrow transplantation: reducing toxicity using nonmyeloablative, preparative regimens and induction of graft-versus-malignancy. Curr Opin Oncol. 1999;11:87–95. doi: 10.1097/00001622-199903000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez R, Nademanee A, Ruel N, et al. Comparison of reduced-intensity and conventional myeloablative regimens for allogeneic transplantation in non-Hodgkin's lymphoma. Biol Blood Marrow Transplant. 2006;12:1326–1334. doi: 10.1016/j.bbmt.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 41.Khouri IF, McLaughlin P, Saliba RM, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111:5530–5536. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barr PM, Lazarus HM. Follicular non-Hodgkin lymphoma: long-term results of stem-cell transplantation. Curr Opin Oncol. 2008;20:502–508. doi: 10.1097/CCO.0b013e32830b61ac. [DOI] [PubMed] [Google Scholar]
- 43.Woessmann W, Peters C, Lenhard M, et al. Allogeneic haematopoietic stem cell transplantation in relapsed or refractory anaplastic large cell lymphoma of children and adolescents--a Berlin-Frankfurt-Munster group report. Br J Haematol. 2006;133:176–182. doi: 10.1111/j.1365-2141.2006.06004.x. [DOI] [PubMed] [Google Scholar]
- 44.Molina A, Zain J, Arber DA, et al. Durable clinical, cytogenetic, and molecular remissions after allogeneic hematopoietic cell transplantation for refractory Sezary syndrome and mycosis fungoides. J Clin Oncol. 2005;23:6163–6171. doi: 10.1200/JCO.2005.02.774. [DOI] [PubMed] [Google Scholar]
- 45.Kyriakou C, Canals C, Finke J, et al. Allogeneic stem cell transplantation is able to induce long-term remissions in angioimmunoblastic T-cell lymphoma: a retrospective study from the lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol. 2009;27:3951–3958. doi: 10.1200/JCO.2008.20.4628. [DOI] [PubMed] [Google Scholar]
- 46.Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, Hoppe RT. Long-term Outcome of 525 Patients With Mycosis Fungoides and Sezary Syndrome: Clinical Prognostic Factors and Risk for Disease Progression. Arch Dermatol. 2003;139:857–866. doi: 10.1001/archderm.139.7.857. [DOI] [PubMed] [Google Scholar]
- 47.Arulogun SO, Prince HM, Ng J, et al. Long-term outcomes of patients with advanced-stage cutaneous T-cell lymphoma and large cell transformation. Blood. 2008;112:3082–3087. doi: 10.1182/blood-2008-05-154609. [DOI] [PubMed] [Google Scholar]
- 48.Olsen EA, Kim YH, Kuzel TM, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:3109–3115. doi: 10.1200/JCO.2006.10.2434. [DOI] [PubMed] [Google Scholar]
- 49.Ellis L, Pan Y, Smyth GK, et al. Histone deacetylase inhibitor panobinostat induces clinical responses with associated alterations in gene expression profiles in cutaneous T-cell lymphoma. Clin Cancer Res. 2008;14:4500–4510. doi: 10.1158/1078-0432.CCR-07-4262. [DOI] [PubMed] [Google Scholar]
- 50.Gimsing P, Hansen M, Knudsen LM, et al. A phase I clinical trial of the histone deacetylase inhibitor belinostat in patients with advanced hematological neoplasia. Eur J Haematol. 2008;81:170–176. doi: 10.1111/j.1600-0609.2008.01102.x. [DOI] [PubMed] [Google Scholar]
- 51.Piekarz RL, Frye R, Turner M, et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol. 2009;27:5410–5417. doi: 10.1200/JCO.2008.21.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Demierre M, Whittaker S, Kim Y, et al. Pooled analyses of two international, multicenter clinical studies of romidepsin in 167 patients with cutaneous T-cell lymphoma (CTCL) Journal of Clinical Oncology (ASCO Meeting Abstracts) 2009;27:8546. [Google Scholar]
- 53.O'Connor O, Pro B, Pinter-Brown L, et al. PROPEL: A Multi-Center Phase 2 Open-Label Study of Pralatrexate (PDX) with Vitamin B12 and Folic Acid Supplementation in Patients with Relapsed or Refractory Peripheral T-Cell Lymphoma. Blood (ASH Annual Meeting Abstracts_) 2008;112:261. [Google Scholar]
- 54.Lundin J, Hagberg H, Repp R, et al. Phase 2 study of alemtuzumab (anti-CD52 monoclonal antibody) in patients with advanced mycosis fungoides/Sezary syndrome. Blood. 2003;101:4267–4272. doi: 10.1182/blood-2002-09-2802. [DOI] [PubMed] [Google Scholar]
- 55.Kim YH, Duvic M, Obitz E, et al. Clinical efficacy of zanolimumab (HuMax-CD4): two phase 2 studies in refractory cutaneous T-cell lymphoma. Blood. 2007;109:4655–4662. doi: 10.1182/blood-2006-12-062877. [DOI] [PubMed] [Google Scholar]