Abstract
BACKGROUND
Corticosteroid therapy has been the time-honoured treatment for autoimmune hepatitis; however, the emergence of new immunosuppressive agents has afforded opportunities to improve or replace the standard regimens.
OBJECTIVE:
To describe technological advances and feasible treatment interventions that promise to supplant the current generation of corticosteroids.
METHODS:
A review of the MEDLINE database for published experiences from 1984 to 2011 was conducted.
RESULTS:
Cyclosporine and tacrolimus have been uniformly successful as salvage therapies for steroid-refractory autoimmune hepatitis. Ten reports of cyclosporine therapy involving 133 patients had positive outcomes in 93%, whereas therapy with tacrolimus in three reports involving 41 patients had positive outcomes in 98%. Salvage therapy with mycophenolate mofetil had a favourable outcome in 47%, especially in patients with azathioprine intolerance. Front-line therapy with mycophenolate mofetil normalized liver parameters in 88% and allowed corticosteroid tapering in 58%. Front-line therapy with budesonide combined with azathioprine for six months normalized liver parameters more frequently (47% versus 18%) and with fewer side effects (28% versus 53%) than prednisone combined with azathioprine. Monoclonal antibodies to CD3 and recombinant cytotoxic T lymphocyte antigen 4 fused with immunoglobulin represent feasible molecular interventions for study in autoimmune hepatitis.
DISCUSSION:
Nonstandard drug therapies must be used in highly selected clinical situations including steroid failure (calcineurin inhibitors), azathioprine intolerance (mycophenolate mofetil), and mild disease or fragile patients (budesonide combined with azathioprine). Molecular interventions for autoimmune hepatitis are feasible for study because of their use in other immune-mediated diseases.
CONCLUSION:
Opportunities to improve or replace standard corticosteroid regimens have emerged.
Keywords: Autoimmune, Front-line, Hepatitis, Molecular, Salvage, Therapy
Abstract
HISTORIQUE :
La corticothérapie est le traitement immuable de l’hépatite auto-immune. Cependant, l’émergence de nouveaux immuno-suppresseurs donne l’occasion d’améliorer ou de remplacer les médicaments habituels.
OBJECTIF :
Décrire les progrès technologiques et les interventions thérapeutiques possibles qui promettent de supplanter la génération actuelle de corticoïdes.
MÉTHODOLOGIE :
Les chercheurs ont procédé à une analyse de la base de données MEDLINE afin d’extraire les expériences publiées entre 1984 et 2011.
RÉSULTATS :
La cyclosporine et le tacrolimus ont toujours été efficaces comme thérapies de rattrapage de l’hépatite auto-immune réfractaire aux stéroïdes. Sept rapports de traitements à la cyclosporine auprès de 33 patients ont fait foi d’une issue positive dans 82 % des cas, tandis que trois rapports au sujet de 41 patients subissant un traitement au tacrolimus ont souligné une issue positive dans 98 % des cas. La thérapie de rattrapage au mofétil mycophénolate a eu une issue positive dans 47 % des cas, notamment chez des patients intolérants à l’azathioprine. La thérapie de première ligne au mofétil mycophénolate normalise les paramètres hépatiques dans 88 % des cas et permet de réduire graduellement la corticothérapie dans 58 % des cas. La thérapie de première ligne au budésonide associée à l’azathioprine administrée pendant six mois a normalisé les paramètres hépatiques plus fréquemment (47 % par rapport à 18 %) et causé moins d’effets secondaires (28 % par rapport à 53 %) que la prednisone associée à l’azathioprine. Les anticorps monoclonaux anti-CD3 et l’antigène 4 du lymphocyte T cytotoxique recombinant fusionnés avec l’immunoglobuline représentent des interventions moléculaires possibles en vue d’études sur l’hépatite autoimmune.
EXPOSÉ :
Il faut utiliser les médicothérapies non standard dans des situations cliniques extrêmement bien sélectionnées, y compris l’échec des stéroïdes (inhibiteurs de la calcineurine), l’intolérance à l’azathioprine (mofétilmycophénolate) et une maladie bénigne ou des patients fragiles (budésonide associée à l’azathioprine). Il est possible d’étudier des interventions moléculaires pour le traitement de l’hépatite auto-immune en raison de leur utilisation dans d’autres maladies à médiation immunitaire.
CONCLUSION :
Des possibilités d’améliorer ou de remplacer la corticothérapie standard ont vu le jour.
Prednisone (or prednisolone), alone or in a lower dose combined with azathioprine, is the standard treatment for autoimmune hepatitis (1); this therapy has been a benchmark for decades (2–4). At least 70% of treated patients experience clinical, laboratory and histological improvement within 24 months (3,5,6). Ten- and 20-year life expectancies are normal (5,7), and hepatic fibrosis is prevented or reduced in 79% (8,9). These achievements, however, have been outweighed by deficiencies in the current regimens including treatment-ending side effects in 13% (10), refractory disease (treatment failure) in 7% (11), incomplete response in 13% (12) and relapse after drug withdrawal in 50% to 86% (13,14).
Only recently has the emergence of powerful immunosuppressive agents, mainly from liver transplantation, challenged the supremacy of the corticosteroid regimens (15–17). Drugs outside of the standard repertoire now promise greater immune suppression than conventional medications, offer site-specific actions and satisfactory patient tolerance (15,17). Site-specific molecular interventions are also feasible because of improved understanding of the critical pathogenic disease pathways and technological advances that now enable modulation of these pathways (16,17). Furthermore, successes in animal models and humans with other immune-mediated diseases have primed these molecular interventions for study in autoimmune hepatitis (16–18).
The aim of the present review is to describe contemporary advances and feasible treatment strategies that promise to supplant the current generation of corticosteroids. The new nonstandard drugs have emerged as front-line and salvage therapies, and include calcineurin inhibitors (cyclosporine and tacrolimus), a next-generation purine antagonist (mycophenolate mofetil) and an alternative glucocorticoid agent (budesonide) (17). These agents have been assessed mainly in small, single-centre studies for off-label indications. The potential molecular interventions include monoclonal antibodies that interfere with the immune response and recombinant molecules that favourably modulate the cytokine pathways responsible for lymphocyte differentiation and proliferation (15–17). The challenges are to incorporate these new, nonstandard, off-label medications into safe and effective management strategies, and to facilitate the rigorous study of these emerging molecular interventions.
CALCINEURIN INHIBITORS
Calcineurin activates nuclear factor-κB via a pathway dependent on phosphatase activity (Figure 1) (19). The activated nuclear factor binds to promoter regions of the interleukin (IL)-2 gene and increases transcription of IL-2. In turn, IL-2 stimulates the cell cycle by binding to the IL-2 receptor, and lymphocytes proliferate along a type 1 cytokine pathway. Cyclosporine and tacrolimus are calcineurin inhibitors that impair phosphatase activity, interfere with the proliferation of lymphocytes and blunt cell-mediated immune responses. Cyclosporine and tacrolimus have each been used in autoimmune hepatitis patients, primarily as salvage therapies for steroid-refractory disease (15,17).
Figure 1).
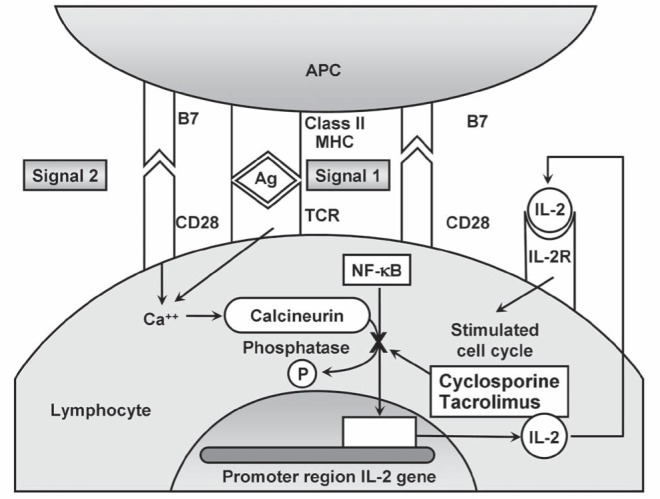
Mechanisms of action of calcineurin inhibitors within lymphocytes. Lymphocyte activation requires recognition of the antigen (Ag) presented by the class II molecule of the major histocompatibility complex (MHC) on the surface of the antigen presenting cell (APC) by the T cell antigen receptor (TCR) of the lymphocyte (Signal 1). Binding of the B7 ligands on the surface of the APC to CD28 molecules on the surface of the lymphocyte completes the second costimulatory signal (signal 2) and activates the lymphocyte. Lymphocyte activation releases calcium (Ca2+), which activates calcineurin and stimulates lymphocyte proliferation. Cyclosporine and tacrolimus each block calcineurin-mediated activation of nuclear factor-kappa B (NF-κB) via a phosphatase (P) pathway within lymphocytes and thereby impair production of interleukin (IL)-2. Activation of the IL-2 receptor (IL-2R) is impaired; cell cycles are stalled; and lymphocyte proliferation is blunted
Cyclosporine
Cyclosporine has been used as a salvage therapy for autoimmune hepatitis since 1985 (20). Ten reports involving 133 patients over the past 26 years (20–26) indicated that a positive response of any degree was achieved in 93%, and a negative response, defined as no response or drug intolerance, was reported in 7% (Table 1). In the most recent experience involving 19 patients treated for 26 weeks with cyclosporine (Neoral, Novartis Pharma, Switzerland) 2 mg/kg to 5 mg/kg daily (26), serum aminotransferase levels decreased significantly, histological activity indexes improved and the medication was well tolerated.
TABLE 1.
Alternative drugs to prednisone for autoimmune hepatitis
| Drug, dose (reference[s]) | Mechanism of action (reference[s]) | Clinical indication (reference[s]) | Outcomes (reference[s]) |
|---|---|---|---|
| Cyclosporine, 2 mg/kg/day to 5 mg/kg/day (26) | Calcineurin inhibitor (19) Impairs NF-κB (19) Reduces IL-2 (19) Impairs lymphocyte proliferation (19) |
Steroid-failure (20–26) | Composite results (20–26):
|
| Tacrolimus, 0.5 mg/day to 3 mg twice/day (27–29) | Calcineurin inhibitor (19) Impairs NF-κB (19) Reduces IL-2 (19) Impairs lymphocyte proliferation (19) |
Steroid-failure (27–29) | Composite results (27–29):
|
| Mycophenolate mofetil, 0.5 g/day to 3 g/day (41) | Purine antagonist (30) Inhibits inosine monophosphate dehydrogenase (30) Limits purine nucleotides (30) Impairs lymphocyte proliferation (30) |
Azathioprine intolerance (main) (37,41) Steroid-failure (less effective) (37,41) Front-line therapy (uncertain preference) (42) |
Salvage outcomes (31–41):
|
Front-line outcomes (42):
|
|||
| Budesonide, 3 mg three times/day combined with azathioprine (47) | Anti-inflammatory (46) High hepatic clearance (48) Inactive metabolites (48) |
Front-line therapy (47) No cirrhosis (53) Mild disease (49) Prednisone risk (49) |
After 6 months of therapy (47):
|
IL-2 Interleukin-2; NF-κB Nuclear factor kappa-B
Tacrolimus
Tacrolimus has been used as a salvage therapy for autoimmune hepatitis since 1995 (27). The starting dose of tacrolimus in the various studies has been as low as 0.5 mg/day (28,29) to as high as 3 mg twice/day (27). The combined experience with this drug in autoimmune hepatitis consists of three reports involving 41 patients over the past 16 years (27–29) (Table 1). A positive response of any degree was reported in 98%, and a negative response, defined as no response or treatment-ending drug intolerance, was reported in 2%.
The success of the calcineurin inhibitors as a salvage therapy for autoimmune hepatitis has been impressive, but the overall reported clinical experience with these agents has been lacking. Calcineurin inhibitors still lack a uniform dosing schedule, an acceptable safety profile and an established monitoring protocol for autoimmune hepatitis despite their longstanding empirical use in this disease. The paucity of reports may reflect the gradual unendorsed assimilation of these drugs into current clinical practice or the emergence of alternative, more promising treatment options. Efforts to launch large, multi-centre, clinical trials have been frustrated by low patient recruitment. Calcineurin inhibitors remain empirical, off-label treatments reserved for steroid-refractory disease.
MYCOPHENOLATE MOFETIL
Mycophenolate mofetil is a next-generation purine antagonist that has been used as a front-line drug and as a salvage agent for autoimmune hepatitis (15,17). It is a prodrug that is hydrolyzed by liver esterases to mycophenolic acid, which in turn acts as a reversible noncompetitive inhibitor of inosine monophosphate dehydrogenase (30) (Figure 2). Mycophenolate mofetil can thereby selectively impair the synthesis of purine-based nucleotides, inhibit the creation of new DNA and impair the proliferation of activated lymphocytes. Its activation and elimination are independent of the thiopurine methyltransferase pathway.
Figure 2).
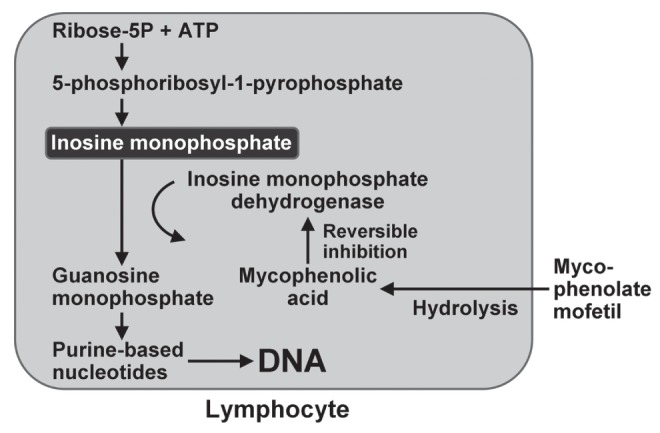
Mechanisms of action of mycophenolate mofetil within lymphocytes. Mycophenolate mofetil is hydrolyzed to mycophenolic acid, which is a noncompetitive reversible inhibitor of inosine monophosphate dehydrogenase. The synthesis of purine-based nucleotides is impaired, the creation of new DNA is reduced and lymphocyte proliferation is inhibited. Inosine monophosphate is produced from ribose-5 phosphate (ribose-5P) and adenosine triphosphate (ATP)
Eleven small single-centre experiences have indicated that mycophenolate mofetil is effective in problematic patients in doses ranging from 0.5 g/day to 3 g/day (31–41). In a compilation of four experiences reported over the past three years (36–38,41), a positive response of any degree was recorded in 47% of treated patients, whereas a negative response, defined as no response or drug intolerance, occurred in 53% (Table 1). Complete corticosteroid withdrawal was possible in 40% of patients included in the 11 studies, and the overall frequency of treatment-ending side effects was 15%. Patients treated with mycophenolate mofetil because of azathioprine intolerance demonstrated improvement more frequently than patients treated for refractory liver disease (58% versus 12%) (37,40,41), whereas children with autoimmune hepatitis and sclerosing cholangitis were nonresponders (39).
Mycophenolate mofetil has also been used as a front-line therapy in treatment-naive patients. Of 59 previously untreated individuals with autoimmune hepatitis who received mycophenolate mofetil for up to 92 months, 88% experienced normalized serum aminotransferase and gamma-globulin levels (usually within three months) and 12% experienced partial response (42). Corticosteroids were withdrawn in 58% (usually within eight months) and serious side effects occurred in 3%. Mycophenolate mofetil can be administered effectively and safely as a front-line treatment, but the reasons for preferring this treatment as a front-line strategy are unclear.
The most common side effects of treatment with mycophenolate mofetil in autoimmune hepatitis patients have been gastrointestinal discomfort (nausea, diarrhea and abdominal pain) (11%), rash (including skin cancers) (7%), fatigue (7%) and leukopenia (1%) (41,42). The frequency of side effects has ranged from 3% to 33% (41,42), and the frequency of treatment-ending complications has been as high as 13% (41). Mycophenolate mofetil has been designated a category D drug in pregnancy by the United States Food and Drug Administration.
The clinical applications of mycophenolate mofetil in autoimmune hepatitis have been based on outcomes in fewer than 300 patients who have been treated with varying doses in different medical centres over the past 11 years (31–42). This limited and disparate experience underscores the need for a highly individualized and carefully monitored management strategy when using mycophenolate mofetil off label. Mycophenolate mofetil is six to seven times more expensive than azathioprine (43,44); treatment ending side effects occur in 3% to 13% (41,42); most patients require continuous corticosteroid therapy; the duration of treatment is indefinite; and the drug is more effective in rescuing patients with azathioprine intolerance than steroid-refractory liver disease (37,40,41). Mycophenolate mofetil has a limited and evolving off-label role in autoimmune hepatitis, and its use as a salvage therapy for azathioprine intolerance is currently its most effective application (45).
BUDESONIDE
Budesonide is a next-generation glucocorticoid that has >90% first-pass clearance in the liver, with metabolites devoid of glucocorticoid activity (46) (Table 1). Its steroidal nature does not disqualify its consideration in the present review because budesonide promises to move current therapies away from reliance on prednisone or prednisolone. Furthermore, budesonide is the first drug in autoimmune hepatitis to be assessed in a large randomized clinical trial in more than 40 years (47). In this regard, it has legitimized its place in the treatment algorithm to a greater degree than the true alternatives to traditional steroids.
Budesonide was first used in the treatment of autoimmune hepatitis in 1994 (48) and, over the past 17 years, six studies involving 162 patients, 100 of whom were enrolled in a randomized clinical trial (47), have helped define its role in autoimmune hepatitis (47–52) (Table 1). Early experiences indicated difficulties in using this drug as a salvage agent in prednisone-dependent patients (49). Attempts to switch from prednisone to budesonide were complicated by severe withdrawal arthralgias and typical corticosteroid-induced side effects (49). Altered hepatic metabolism in patients with cirrhosis was associated with the development of serious drug-related complications (53); immune-mediated diseases concurrent with autoimmune hepatitis could exacerbate (49); and flares of autoimmune hepatitis during therapy were always possible (54). These experiences characterized the ideal target population for the drug and directed its use toward treatment-naive, noncirrhotic and uncomplicated patients.
A large randomized clinical trial involving 203 patients with these attributes subsequently demonstrated the advantages of budesonide over conventional prednisone-based treatment (47). Budesonide (3 mg three times/day) combined with azathioprine (1 mg/kg/day to 2 mg/kg/day) normalized serum aminotransferase levels more often (47% versus 18%) and with fewer side effects (28% versus 53%) than the standard regimen of prednisone (40 mg daily, tapered to 10 mg daily) combined with azathioprine (1 mg/kg/day to 2 mg/kg/day) when administered for six months (47). The frequency of histological resolution and the durability of the response remain unknown, and the low frequency of response (only 18%) and the high frequency of side effects (53%) in the patients treated with the standard regimen remain unexplained.
In combination with azathioprine, budesonide is emerging as an alternative front-line treatment for autoimmune hepatitis, but its off-label use must be cautious and conservative. There is still insufficient experience with this drug in treating the diverse presentations of autoimmune hepatitis to justify its blanket substitution for prednisone or prednisolone. Treatment appears to be best suited for treatment-naive, noncirrhotic and uncomplicated patients, and it has appeal as a first-line therapy in patients with asymptomatic, mild autoimmune hepatitis in whom the benefit-to-risk ratio of conventional prednisone treatment may be low. Patients with pre-existent osteopenia, hypertension, diabetes, obesity and emotional instability may be other candidates for the budesonide-azathioprine combination.
FEASIBLE MOLECULAR INTERVENTIONS
Site-specific molecular interventions are now feasible in autoimmune hepatitis mainly because of successes already achieved in other immune-mediated diseases (16,17) (Table 2). These interventions include the use of monoclonal antibodies and recombinant molecules that alter lymphocyte activation pathways, differentiation and proliferation. They constitute new treatment opportunities that are based on available technology and ongoing experiences in animal models and humans with autoimmune diseases. Other interventions, including synthetic peptides that block autoantigen display, oral tolerization against triggering antigens, T cell vaccination against cytotoxic T cell clones, adoptive transfer of regulatory T cells and silencing of genes that promote the autoimmune reaction, are feasible, but they are either untried, too preliminary, or inconsistently effective in animals or humans (16,17).
TABLE 2.
Future feasible drug alternatives for autoimmune hepatitis (AIH)
| Feasible molecular intervention | Mechanisms of action (reference[s]) | Precedents in human diseases (reference[s]) |
|---|---|---|
| Nonmitogenic monoclonal antibodies to CD3 (anti-CD3) | Targets T cell antigen receptor (17,55) | Type 1 diabetes (17,55) |
| Induces apoptosis of lymphocytes (17) | Few side effects (fever, rash, anemia, EBV infection) (17) | |
| Releases TGF-β (17) | ||
| Induces regulatory T cells (17) | ||
| Suppresses immune response (17) | ||
| Monoclonal antibodies to CD20 (anti-CD20) | Targets CD20 expressed on B cells (17) | Hematological malignancies (17) |
| Depletes B lymphocytes (17) | Rheumatoid arthritis, ITP (17) | |
| Impairs type 2 cytokine pathway (17) | AIH and cryoglobulinemia (17) | |
| Interferes with antibody-dependent cell-mediated cytotoxcities (17) | AIH and B cell lymphoma (17) | |
| Rare serious toxicities (progressive multifocal leukoencephalopathy) (17) | ||
| Monoclonal antibodies to TNF-α (anti-TNF-α) or its receptor (etanercept) | Interferes with type 1 cytokine pathway (15–17) | Crohn disease (16,17) |
| Impairs proliferation of cytotoxic T cells (15–17) | Rheumatoid arthritis (16,17) | |
| Alcoholic hepatitis (ineffective) (16,17) | ||
| NAFLD (ineffective) (16,17) | ||
| Serious toxicities (infection, death, lung disease, SLE, AIH) (16,17) | ||
| Recombinant cytotoxic T lymphocyte antigen 4 fused with immunoglobulin | Resembles CD28 on lymphocyte (15–17) | Rheumatoid arthritis (15–17) |
| Binds B7 ligands on APC (15–17) | Mismatched bone marrow transplantation (15–17) | |
| Interferes with second costimulatory signal of lymphocyte activation (15–17) | Multiple sclerosis (15–17) | |
| Few side effects (infection) (16,17) | ||
| Recombinant interleukin-10 | Counters type 2 cytokine path (15–17) | Chronic hepatitis C (15–17) |
| Reduces TNF-α (15–17) | Inflammatory bowel disease (15–17) | |
| Decreases inflammation (15–17) | Few dose-related side effects (anemia, thrombocytopenia, fever, chills, myalgias, headache) (16,17) | |
| Impairs differentiation and proliferation of T lymphocytes (15–17) |
Numbers in parentheses indicate reference(s). APC Antigen presenting cell; EBV Epstein-Barr virus; ITP Idiopathic thrombocytopenic purpura; NAFLD Nonalcoholic fatty liver disease; SLE Systemic lupus erythematosus; TNF-α Tumour necrosis factor-alpha; TGF-β Transforming growth factor-beta
Monoclonal antibodies
Nonmitogenic monoclonal antibodies to CD3 target the T cell antigen receptor of liver-infiltrating cytotoxic T cells and induce apoptosis (55) (Table 2). This treatment has already been used successfully in animal models and humans with type 1 diabetes and awaits study in autoimmune hepatitis. Antibodies to CD20 can blunt clonal expansion of B cells and dampen antibody-mediated forms of cytotoxicity. Rituximab has already been used successfully in patients with rheumatoid arthritis (17). Monoclonal antibodies to tumour necrosis factor-alpha or its receptor are also feasible, but enthusiasm for this intervention has been dampened by the potential for serious toxicities (16,17). Anti-CD3 is the most promising intervention in this category because of its relevance to the pathogenic mechanisms of autoimmune hepatitis and its success in other immune-mediated human diseases (55).
Recombinant molecules
Recombinant cytotoxic T lymphocyte antigen 4 fused with immuno-globulin blocks the second costimulatory signal for immunocyte activation and can blunt the immune response (16,17,55) (Table 2). Abatacept is already approved in the United States and Europe for rheumatoid arthritis, and awaits study in autoimmune hepatitis. Recombinant IL-10 has anti-inflammatory effects that counterbalance the type 1 cytokine pathway and has been used in humans with chronic hepatitis C or inflammatory bowel disease (16). Recombinant IL-10 has had a satisfactory safety profile when administered in other human diseases and is a another candidate for study in autoimmune hepatitis (16). Cytotoxic T lymphocyte antigen-4 fused with immuno-globulin is the most promising intervention in this category because of its relevance to the pathogenic mechanisms of autoimmune hepatitis and its success in other immune-mediated human diseases (16).
THERAPEUTIC ANIMAL MODELS
The key to the emergence of new pharmacological and molecular therapies for autoimmune hepatitis is a therapeutic animal model of the human disease (18). Two promising mouse models have emerged while others are also being evaluated. One model is based on the immunization of female mice with plasmids of cytomegalovirus containing the antigenic region of the human cytochrome, CYP2D6, and human formiminotransferase cyclodeaminase (56). These are the antigenic targets of type 2 autoimmune hepatitis. The other model is based on the infection of mice with an adenovirus expressing the antigenic region of human CYP2D6 (57). The infection model has the added advantages of recruiting inflammatory and immune cells to the damaged liver, generating a promiscuous immune response against self-antigens by molecular mimicry and developing extensive hepatic fibrosis. The major deficiencies in these current models are their short life-spans and, as a result, the inability to assess the long-term impact of treatment (18).
THERAPEUTIC DILEMMA
The new nonstandard drugs for autoimmune hepatitis create a treatment dilemma (58). Ideally, an international collaborative network of committed clinical investigators will be formed, funding resources will be available and large, rigorously designed clinical trials will be launched that fully define the value and role of these new agents. The quandary is whether to wait for more evidence or to treat with these new agents now. For autoimmune hepatitis and other uncommon diseases, expectations for clinical trials have been difficult to realize, and the promising new drugs warrant prudent, highly individualized and timely treatment decisions that are now applicable to clinical practice.
Budesonide in combination with azathioprine can be considered as a front-line therapy, and it may be especially appropriate in treatment-naive patients with mild, early stage disease or with obesity, osteopenia, diabetes or hypertension that might be worsened by prednisone treatment. A calcineurin inhibitor (cyclosporine or tacrolimus) might be considered for refractory liver disease; mycophenolate mofetil appears to be best suited for patients with azathioprine intolerance.
The treatment dilemma in autoimmune hepatitis requires clinical judgment that is based on the immediacy of the clinical situation, failure of conventional treatment options, knowledge of the new agents and restriction of the new therapies to well-defined clinical situations that can be closely monitored. The societal need for fully evaluated new treatments stresses the need for a continuing effort to introduce new agents to standard clinical practice only after rigorous investigational scrutiny (58).
Footnotes
DISCLOSURES: Presented in part during the Canadian Digestive Diseases Week and annual winter meeting of the Canadian Association for the Study of the Liver, Montreal, Quebec, February 24, 2012. This review did not receive financial support from a funding agency or institution, and Albert J Czaja MD has no conflict of interests to declare.
REFERENCES
- 1.Manns MP, Czaja AJ, Gorham JD, et al. Practice Guidelines of the American Association for the Study of Liver Diseases. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193–213. doi: 10.1002/hep.23584. [DOI] [PubMed] [Google Scholar]
- 2.Cook GC, Mulligan R, Sherlock S. Controlled prospective trial of corticosteroid therapy in active chronic hepatitis. Q J Med. 1971;40:159–85. doi: 10.1093/oxfordjournals.qjmed.a067264. [DOI] [PubMed] [Google Scholar]
- 3.Soloway RD, Summerskill WH, Baggenstoss AH, et al. Clinical, biochemical, and histological remission of severe chronic active liver disease: A controlled study of treatments and early prognosis. Gastroenterology. 1972;63:820–33. [PubMed] [Google Scholar]
- 4.Murray-Lyon IM, Stern RB, Williams R. Controlled trial of prednisone and azathioprine in active chronic hepatitis. Lancet. 1973;1:735–7. doi: 10.1016/s0140-6736(73)92125-9. [DOI] [PubMed] [Google Scholar]
- 5.Kanzler S, Lohr H, Gerken G, Galle PR, Lohse AW. Long-term management and prognosis of autoimmune hepatitis (AIH): A single center experience. Z Gastroenterol. 2001;39:339–41. 44–8. doi: 10.1055/s-2001-13708. [DOI] [PubMed] [Google Scholar]
- 6.Czaja AJ. Rapidity of treatment response and outcome in type 1 autoimmune hepatitis. J Hepatol. 2009;51:161–7. doi: 10.1016/j.jhep.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 7.Roberts SK, Therneau TM, Czaja AJ. Prognosis of histological cirrhosis in type 1 autoimmune hepatitis. Gastroenterology. 1996;110:848–57. doi: 10.1053/gast.1996.v110.pm8608895. [DOI] [PubMed] [Google Scholar]
- 8.Czaja AJ, Carpenter HA. Decreased fibrosis during corticosteroid therapy of autoimmune hepatitis. J Hepatol. 2004;40:646–52. doi: 10.1016/j.jhep.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Mohamadnejad M, Malekzadeh R, Nasseri-Moghaddam S, et al. Impact of immunosuppressive treatment on liver fibrosis in autoimmune hepatitis. Dig Dis Sci. 2005;50:547–51. doi: 10.1007/s10620-005-2472-5. [DOI] [PubMed] [Google Scholar]
- 10.Czaja AJ. Safety issues in the management of autoimmune hepatitis. Expert Opin Drug Saf. 2008;7:319–33. doi: 10.1517/14740338.7.3.319. [DOI] [PubMed] [Google Scholar]
- 11.Montano-Loza AJ, Carpenter HA, Czaja AJ. Features associated with treatment failure in type 1 autoimmune hepatitis and predictive value of the model of end-stage liver disease. Hepatology. 2007;46:1138–45. doi: 10.1002/hep.21787. [DOI] [PubMed] [Google Scholar]
- 12.Czaja AJ, Davis GL, Ludwig J, Taswell HF. Complete resolution of inflammatory activity following corticosteroid treatment of HBsAg-negative chronic active hepatitis. Hepatology. 1984;4:622–7. doi: 10.1002/hep.1840040409. [DOI] [PubMed] [Google Scholar]
- 13.Hegarty JE, Nouri Aria KT, Portmann B, Eddleston AL, Williams R. Relapse following treatment withdrawal in patients with autoimmune chronic active hepatitis. Hepatology. 1983;3:685–9. doi: 10.1002/hep.1840030510. [DOI] [PubMed] [Google Scholar]
- 14.Czaja AJ, Menon KV, Carpenter HA. Sustained remission after corticosteroid therapy for type 1 autoimmune hepatitis: A retrospective analysis. Hepatology. 2002;35:890–7. doi: 10.1053/jhep.2002.32485. [DOI] [PubMed] [Google Scholar]
- 15.Czaja AJ. Current and future treatments of autoimmune hepatitis. Expert Rev Gastroenterol Hepatol. 2009;3:269–91. doi: 10.1586/egh.09.15. [DOI] [PubMed] [Google Scholar]
- 16.Czaja AJ. Emerging opportunities for site-specific molecular and cellular interventions in autoimmune hepatitis. Dig Dis Sci. 2010;55:2712–26. doi: 10.1007/s10620-009-1122-8. [DOI] [PubMed] [Google Scholar]
- 17.Czaja AJ. Promising pharmacological, molecular and cellular treatments of autoimmune hepatitis. Curr Pharm Design. 2011;17:3120–40. doi: 10.2174/138161211798157568. [DOI] [PubMed] [Google Scholar]
- 18.Czaja AJ. Animal models of autoimmune hepatitis. Expert Rev Gastroenterol Hepatol. 2010;4:429–43. doi: 10.1586/egh.10.42. [DOI] [PubMed] [Google Scholar]
- 19.Allison AC. Immunosuppressive drugs: The first 50 years and a glance forward. Immunopharmacology. 2000;47:63–83. doi: 10.1016/s0162-3109(00)00186-7. [DOI] [PubMed] [Google Scholar]
- 20.Mistilis SP, Vickers CR, Darroch MH, McCarthy SW. Cyclosporin, a new treatment for autoimmune chronic active hepatitis. Med J Aust. 1985;143:463–5. doi: 10.5694/j.1326-5377.1985.tb123140.x. [DOI] [PubMed] [Google Scholar]
- 21.Hyams JS, Ballow M, Leichtner AM. Cyclosporine treatment of autoimmune chronic active hepatitis. Gastroenterology. 1987;93:890–3. doi: 10.1016/0016-5085(87)90454-9. [DOI] [PubMed] [Google Scholar]
- 22.Person JL, McHutchison JG, Fong TL, Redeker AG. A case of cyclosporine-sensitive, steroid-resistant, autoimmune chronic active hepatitis. J Clin Gastroenterol. 1993;17:317–20. doi: 10.1097/00004836-199312000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Sherman KE, Narkewicz M, Pinto PC. Cyclosporine in the management of corticosteroid-resistant type I autoimmune chronic active hepatitis. J Hepatol. 1994;21:1040–7. doi: 10.1016/s0168-8278(05)80615-4. [DOI] [PubMed] [Google Scholar]
- 24.Jackson LD, Song E. Cyclosporin in the treatment of corticosteroid resistant autoimmune chronic active hepatitis. Gut. 1995;36:459–61. doi: 10.1136/gut.36.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandes NF, Redeker AG, Vierling JM, Villamil FG, Fong TL. Cyclosporine therapy in patients with steroid resistant autoimmune hepatitis. Am J Gastroenterol. 1999;94:241–8. doi: 10.1111/j.1572-0241.1999.00807.x. [DOI] [PubMed] [Google Scholar]
- 26.Malekzadeh R, Nasseri-Moghaddam S, Kaviani MJ, et al. Cyclosporin A is a promising alternative to corticosteroids in autoimmune hepatitis. Dig Dis Sci. 2001;46:1321–7. doi: 10.1023/a:1010683817344. [DOI] [PubMed] [Google Scholar]
- 27.Van Thiel DH, Wright H, Carroll P, et al. Tacrolimus: A potential new treatment for autoimmune chronic active hepatitis: Results of an open-label preliminary trial. Am J Gastroenterol. 1995;90:771–6. [PMC free article] [PubMed] [Google Scholar]
- 28.Aqel BA, Machicao V, Rosser B, et al. Efficacy of tacrolimus in the treatment of steroid refractory autoimmune hepatitis. J Clin Gastroenterol. 2004;38:805–9. doi: 10.1097/01.mcg.0000139050.67178.be. [DOI] [PubMed] [Google Scholar]
- 29.Larsen FS, Vainer B, Eefsen M, Bjerring PN, Adel Hansen B. Low-dose tacrolimus ameliorates liver inflammation and fibrosis in steroid refractory autoimmune hepatitis. World J Gastroenterol. 2007;13:3232–6. doi: 10.3748/wjg.v13.i23.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47:85–118. doi: 10.1016/s0162-3109(00)00188-0. [DOI] [PubMed] [Google Scholar]
- 31.Richardson PD, James PD, Ryder SD. Mycophenolate mofetil for maintenance of remission in autoimmune hepatitis in patients resistant to or intolerant of azathioprine. J Hepatol. 2000;33:371–5. doi: 10.1016/s0168-8278(00)80271-8. [DOI] [PubMed] [Google Scholar]
- 32.Devlin SM, Swain MG, Urbanski SJ, Burak KW. Mycophenolate mofetil for the treatment of autoimmune hepatitis in patients refractory to standard therapy. Can J Gastroenterol. 2004;18:321–6. doi: 10.1155/2004/504591. [DOI] [PubMed] [Google Scholar]
- 33.Chatur N, Ramji A, Bain VG, et al. Transplant immunosuppressive agents in non-transplant chronic autoimmune hepatitis: The Canadian Association for the Study of the Liver (CASL) experience with mycophenolate mofetil and tacrolimus. Liver Int. 2005;25:723–7. doi: 10.1111/j.1478-3231.2005.01107.x. [DOI] [PubMed] [Google Scholar]
- 34.Czaja AJ, Carpenter HA. Empiric therapy of autoimmune hepatitis with mycophenolate mofetil: Comparison with conventional treatment for refractory disease. J Clin Gastroenterol. 2005;39:819–25. doi: 10.1097/01.mcg.0000177260.72692.e8. [DOI] [PubMed] [Google Scholar]
- 35.Inductivo-Yu I, Adams A, Gish RG, et al. Mycophenolate mofetil in autoimmune hepatitis patients not responsive or intolerant to standard immunosuppressive therapy. Clin Gastroenterol Hepatol. 2007;5:799–802. doi: 10.1016/j.cgh.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 36.Hlivko JT, Shiffman ML, Stravitz RT, et al. A single center review of the use of mycophenolate mofetil in the treatment of autoimmune hepatitis. Clin Gastroenterol Hepatol. 2008;6:1036–40. doi: 10.1016/j.cgh.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Hennes EM, Oo YH, Schramm C, et al. Mycophenolate mofetil as second line therapy in autoimmune hepatitis? Am J Gastroenterol. 2008;103:3063–70. doi: 10.1111/j.1572-0241.2008.02180.x. [DOI] [PubMed] [Google Scholar]
- 38.Wolf DC, Bojito L, Facciuto M, Lebovics E. Mycophenolate mofetil for autoimmune hepatitis: A single practice experience. Dig Dis Sci. 2009;54:2519–22. doi: 10.1007/s10620-008-0632-0. [DOI] [PubMed] [Google Scholar]
- 39.Aw MM, Dhawan A, Samyn M, Bargiota A, Mieli-Vergani G. Mycophenolate mofetil as rescue treatment for autoimmune liver disease in children: A 5-year follow-up. J Hepatol. 2009;51:156–60. doi: 10.1016/j.jhep.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 40.Sharzehi K, Huang MA, Schreibman IR, Brown KA. Mycophenolate mofetil for the treatment of autoimmune hepatitis in patients refractory or intolerant to conventional therapy. Can J Gastroenterol. 2010;24:588–92. doi: 10.1155/2010/891252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baven-Pronk AM, Coenraad MJ, van Buuren HR, et al. The role of mycophenolate mofetil in the management of autoimmune hepatitis and overlap syndromes. Aliment Pharmacol Ther. 2011;34:335–43. doi: 10.1111/j.1365-2036.2011.04727.x. [DOI] [PubMed] [Google Scholar]
- 42.Zachou K, Gatselis N, Papadamou G, Rigopoulou EI, Dalekos GN. Mycophenolate for the treatment of autoimmune hepatitis: Prospective assessment of its efficacy and safety for induction and maintenance of remission in a large cohort of treatment-naive patients. J Hepatol. 2011;55:636–46. doi: 10.1016/j.jhep.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 43.Seikaly MG. Mycophenolate mofetil – is it worth the cost? The in-favor opinion. Pediatr Transplant. 1999;3:79–82. doi: 10.1034/j.1399-3046.1999.00015.x. [DOI] [PubMed] [Google Scholar]
- 44.Heneghan MA, Al-Chalabi T, McFarlane IG. Cost-effectiveness of pharmacotherapy for autoimmune hepatitis. Expert Opin Pharmacother. 2006;7:145–56. doi: 10.1517/14656566.7.2.145. [DOI] [PubMed] [Google Scholar]
- 45.Czaja AJ. Mycophenolate mofetil to the rescue in autoimmune hepatitis: A fresh sprout on the decision tree. J Hepatol. 2009;51:8–10. doi: 10.1016/j.jhep.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 46.Clissold SP, Heel RC. Budesonide. A preliminary review of its pharmacodynamic properties and therapeutic efficacy in asthma and rhinitis. Drugs. 1984;28:485–518. doi: 10.2165/00003495-198428060-00001. [DOI] [PubMed] [Google Scholar]
- 47.Manns MP, Woynarowski M, Kreisel W, et al. Budesonide induces remission more effectively than prednisone in a controlled trial of patients with autoimmune hepatitis. Gastroenterology. 2010;139:1198–206. doi: 10.1053/j.gastro.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 48.Danielsson A, Prytz H. Oral budesonide for treatment of autoimmune chronic active hepatitis. Aliment Pharmacol Ther. 1994;8:585–90. doi: 10.1111/j.1365-2036.1994.tb00334.x. [DOI] [PubMed] [Google Scholar]
- 49.Czaja AJ, Lindor KD. Failure of budesonide in a pilot study of treatment-dependent autoimmune hepatitis. Gastroenterology. 2000;119:1312–6. doi: 10.1053/gast.2000.0010000001. [DOI] [PubMed] [Google Scholar]
- 50.Wiegand J, Schuler A, Kanzler S, et al. Budesonide in previously untreated autoimmune hepatitis. Liver Int. 2005;25:927–34. doi: 10.1111/j.1478-3231.2005.01122.x. [DOI] [PubMed] [Google Scholar]
- 51.Csepregi A, Rocken C, Treiber G, Malfertheiner P. Budesonide induces complete remission in autoimmune hepatitis. World J Gastroenterol. 2006;12:1362–6. doi: 10.3748/wjg.v12.i9.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zandieh I, Krygier D, Wong V, et al. The use of budesonide in the treatment of autoimmune hepatitis in Canada. Can J Gastroenterol. 2008;22:388–92. doi: 10.1155/2008/509459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geier A, Gartung C, Dietrich CG, et al. Side effects of budesonide in liver cirrhosis due to chronic autoimmune hepatitis: Influence of hepatic metabolism versus portosystemic shunts on a patient complicated with HCC. World J Gastroenterol. 2003;9:2681–5. doi: 10.3748/wjg.v9.i12.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lohse AW, Gil H. Reactivation of autoimmune hepatitis during budesonide monotherapy, and response to standard treatment. J Hepatol. 2011;54:837–9. doi: 10.1016/j.jhep.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 55.Czaja AJ, Manns MP. Advances in the diagnosis, pathogenesis and management of autoimmune hepatitis. Gastroenterology. 2010;139:58–72. doi: 10.1053/j.gastro.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 56.Lapierre P, Djilali-Saiah I, Vitozzi S, Alvarez F. A murine model of type 2 autoimmune hepatitis: Xenoimmunization with human antigens. Hepatology. 2004;39:1066–74. doi: 10.1002/hep.20109. [DOI] [PubMed] [Google Scholar]
- 57.Holdener M, Hintermann E, Bayer M, et al. Breaking tolerance to the natural human liver autoantigen cytochrome P450 2D6 by virus infection. J Exp Med. 2008;205:1409–22. doi: 10.1084/jem.20071859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manns MP. Autoimmune hepatitis: The dilemma of rare diseases. Gastroenterology. 2011;140:1874–6. doi: 10.1053/j.gastro.2011.04.026. [DOI] [PubMed] [Google Scholar]


