Abstract
Objectives.
We take a biopsychosocial perspective on age-related diseases by examining psychological correlates of having multiple chronic conditions and determining whether positive psychological functioning predicts advantageous profiles of biological risk factors.
Method.
Respondents to the national survey of Midlife in the United States who participated in clinical assessments of health and biological processes (n = 998) provided information on chronic medical conditions and multiple domains of psychological functioning. Serum concentrations of interleukin-6 (IL-6) and C-reactive protein (CRP) were determined from fasting blood samples.
Results.
Life satisfaction declined with increasing comorbidity while negative affect increased. In contrast, positive affect, purpose in life, and positive relations with others were unrelated to comorbidity status. Significant interactions showed that although IL-6 and CRP increased with increasing number of chronic conditions, respondents with higher levels of purpose in life, positive relations with others, and (in the case of CRP) positive affect had lower levels of inflammation compared with those with lower well-being scores.
Discussion.
The results suggest that many older adults with medical comorbidities maintain high levels of positive psychological functioning that are in turn linked to better profiles of biological disease risk.
Keywords: Chronic conditions, Inflammation, Successful aging, Well-being,
Life expectancy in the United States has increased dramatically over the course of the past century (Arias, Rostron, & Tejada-Vera, 2010), and with it has come increased burden of medical comorbidity related to aging. According to a recent report from the 2004 Medical Expenditures Panel Survey, 49% of all adults in the United States have at least one chronic medical condition and 26% have two or more. Among those aged 65 years or older, 72% have two or more conditions (Anderson, 2007). Living with medical comorbidity has thus become the norm for older adults, and the extent to which one can live well with multiple chronic conditions is a pressing challenge for an aging society. We address this challenge in several ways. First, we examine the associations of medical comorbidity with multiple domains of psychological functioning in a national sample of middle-aged and older adults, placing particular emphasis on positive functioning. Second, and most importantly, we determine whether maintaining relatively high levels of well-being in the face of medical comorbidity will afford biological advantages in the form of lower levels of systemic inflammation. Finally, we sharpen the focus on positive functioning by assessing its independence from negative affect and refine the analyses further by examining the potential role of health behaviors as mediators of links between positive psychological functioning and inflammation in the context of medical comorbidity.
Declines in health present a primary threat to subjective well-being in later life. Although older adults tend to report higher levels of subjective well-being (across multiple domains of well-being) than younger adults (George, 2010), health problems are linked to significantly lower levels of positive affect and life satisfaction and higher levels of depression and depressive symptoms in both cross-sectional and longitudinal analyses (Gerstorf et al., 2010; Kunzmann, Little, & Smith, 2000; Mroczek & Spiro, 2005; Strawbridge, Wallhagen, & Cohen, 2002). Health-related declines in well-being are particularly steep for those nearing the end of life (Gerstorf et al., 2010; Mroczek & Spiro, 2005) Nevertheless, mean levels of subjective well-being remain relatively high among adults with poor health. Indeed, many older adults consider themselves to be aging successfully, despite having multiple chronic conditions (Strawbridge et al., 2002; Weir, Meisner, & Baker, 2010). These latter observations suggest that high levels of subjective well-being may be present in contexts of medical comorbidities, which we investigate in the current study.
We bring two distinct domains of psychological well-being —hedonic and eudaimonic (Ryan & Deci, 2001; Waterman, 1993)—to the inquiry. Hedonic well-being is associated with pleasure, contentment, and the avoidance of physical and psychic discomfort. It is typically assessed using the frequency and intensity of positive and negative mood and ratings of life satisfaction (Diener, 2000; Ryan & Deci, 2001). Studies of health-related changes in well-being typically involve hedonic measures, such as life satisfaction (Mroczek & Spiro, 2005), positive and negative affect (Kunzmann et al., 2000), or a combination of these (Gerstorf et al., 2010). In contrast, eudaimonic well-being stems from the Aristotelian ideal of the pursuit of personal excellence (Ryan & Deci, 2001; Waterman, 1993) and has been operationalized with Ryff’s Psychological Well-Being (PWB) scales (Ryff & Keyes, 1995). Here, we focus on two specific dimensions of psychological well-being—purpose in life and positive relations with others—that predicted inflammation in our earlier work (Friedman, Hayney, Love, Singer, & Ryff, 2007). Purpose in life has also been recently linked to reduced mortality (Boyle, Barnes, Buchman, & Bennett, 2009). To our knowledge, links between eudaimonic well-being and medical comorbidity have not yet been studied.
A central aim of this study was to determine whether higher levels of positive psychological functioning in adults with medical comorbidities will afford biological advantages in the form of lower circulating levels of inflammatory proteins. Some of the chronic conditions most common in aging individuals—cardiovascular disease, hypertension, and diabetes—are associated with higher circulating levels of inflammatory proteins (Abraham et al., 2007; Blake & Ridker, 2003; Cesari et al., 2003; Chae, Lee, Rifai, & Ridker, 2001; Fernandez-Real & Ricart, 2003) such as the proinflammatory cytokine interleukin-6 (IL-6) and the acute-phase protein C-reactive protein (CRP). Importantly, IL-6 and CRP have also been linked prospectively with adverse health outcomes, including mortality, in individuals with a range of clinical diagnoses (Aggarwal & Gehlot, 2009; Blake & Ridker, 2003; Leitch et al., 2007). This connection between inflammation and adverse health outcomes is particularly strong in elderly adults (Stork et al., 2006) who also likely suffer from multiple medical comorbidities. Inflammation thus provides a useful index of risk for future mortality and morbidity among those with comorbid clinical illnesses, especially older adults.
Importantly, inflammation is also associated with both positive and negative aspects of psychosocial functioning. A recent meta-analysis of 136 studies published between 1967 and 2008 found that inflammatory proteins, predominantly IL-6 and CRP, are positively associated with depression (Howren, Lamkin, & Suls, 2009). This association was dose dependent and equally evident in clinical and community-based samples (Howren et al., 2009). Positive psychosocial functioning, in contrast, has been linked to lower levels of inflammation. Circulating levels of IL-6 were lower in two independent samples of older women who reported higher levels of psychological well-being (Friedman et al., 2007; Friedman, Love, Singer, & Ryff, 2008), and greater positive affect predicted lower levels of IL-6 and CRP in female, but not in male, participants from the Whitehall II study (Steptoe, O’Donnell, Badrick, Kumari, & Marmot, 2008). The degree to which psychological functioning may moderate the link between chronic illness and inflammation is unknown, although the possibility is supported by earlier research showing that high-quality social relationships predict lower levels of IL-6 in women with advanced ovarian cancer (Costanzo et al., 2005). We test this possibility in the present study.
We also examine whether any links between positive psychological functioning and inflammation in adults with medical comorbidities are independent of negative affect. Broadly, well-being and ill-being are considered to be constructs that are related but distinct, and although the two are typically inversely correlated, the correlation is rarely perfect or even strong (Diener, 1984). Importantly, there is both a theoretical foundation for and an empirical evidence of the possibility of experiencing positive and negative emotions at the same time over the same event (Cacioppo & Berntson, 1994; Larsen, McGraw, & Cacioppo, 2001; Norris, Gollan, Berntson, & Cacioppo, 2010), and neuroscience research has shown that positive and negative emotions involve different regions of the brain (Cacioppo & Berntson, 1999). Of particular relevance for this study are two recent literature reviews showing that higher levels of well-being predict better health and reduced mortality, and studies that have accounted for ill-being typically find residual associations between well-being and multiple indices of health (Chida & Steptoe, 2008; Pressman & Cohen, 2005).
Finally, we assess the potential role of three health behaviors—cigarette smoking, alcohol consumption, and physical activity—in mediating links between positive psychological functioning and inflammation in the context of medical comorbidities. Smoking is linked to higher circulating levels of inflammatory proteins (Helmersson, Larsson, Vessby, & Basu, 2005), whereas light alcohol intake (Volpato et al., 2004) and regular physical activity (Colbert et al., 2004) are associated with lower levels. Individuals with higher levels of well-being are generally more likely to engage in good health behaviors, and health behaviors are thought to contribute to the relationship between positive psychological functioning and better health outcomes (Steptoe, Dockray, & Wardle, 2009). Using a well-established approach to meditational analyses (Baron & Kenny, 1986), we examine the extent to which health behaviors explain the association of positive psychological functioning and inflammation in adults with medical comorbidities.
We test the following hypotheses:
Positive psychological functioning will decline and negative functioning will increase with increasing number of chronic conditions, but consistent with research on older adult well-being (George, 2010), overall levels of subjective well-being will remain relatively high.
At similar levels of comorbidity, higher levels of subjective well-being predict lower levels of IL-6 and CRP.
The links between positive psychological functioning and inflammation, as well as the interactive association with chronic conditions, will be independent of negative affect.
Health behaviors will mediate, at least in part, the association of psychological functioning measures (and their interactions with chronic conditions) and inflammation.
METHOD
Participants
Data for the current study are from the longitudinal survey of Midlife in the United States (MIDUS), a national study of the physical and mental health of middle-aged and older adults. MIDUS comprises a national probability sample of noninstitutionalized English-speaking adults (N = 3,487) living in the coterminus United States and recruited by random digit dialing (RDD). A sample of monozygotic and dizygotic twin pairs (N = 1,914) was also recruited from a national twin registry. The first wave of MIDUS data collection (MIDUS 1) was completed in 1995–1996, and a follow-up study (MIDUS 2) was completed in 2004–2006. Mortality-adjusted retention from the original study was 71% for the RDD sample and 82% for the twins. All respondents completed telephone interviews and self-administered questionnaires. Data collection for MIDUS 2 included an oversampling of African Americans living in Milwaukee County, but given marked differences in sociodemographic and biological variables compared with the RDD and twin samples, the Milwaukee oversample is excluded from these analyses.
A subsample of MIDUS 2 RDD and twin respondents (N = 1,028) participated in a detailed clinic-based assessment of health, disease-related biomarkers, and physiological function (“biomarker sample”). Recruitment was by letter and a follow-up telephone call, and data collection was completed in 2004–2009. Participation in the biomarker sample was open to all MIDUS 2 respondents who had completed the telephone interview and self-administered questionnaires and were willing to travel to a general clinical research center (GCRC) for an overnight stay. Recruitment was by letter and a follow-up telephone call. Compared with the full MIDUS 2 sample, participants in the biomarker sample were more likely to have graduated from a 4-year college, were more likely to be Caucasian, and had higher scores on purpose in life, but were otherwise no different. Although the inclusion of respondents from the twins sample has not influenced the results of previous analyses involving MIDUS data (Friedman, Williams, Singer, & Ryff, 2009), we accounted for possible effects of genetic and familial relatedness among the twins here by including a variable indicating twin status in all analyses. CRP values in excess of 10 mg/L are thought to indicate acute infectious illness, and exclusion of cases with high CRP values is recommended. We identified 30 cases with high CRP values, yielding a final analytical sample of 998 respondents.
Three regional GCRCs participated in the MIDUS biomarker study—one on the West coast, one in the Midwest, and one on the East coast—and participants were invited to stay at whichever GCRC imposed the least travel burden. Upon arrival at the GCRC, each respondent provided a detailed medical history interview with a GCRC clinician. Participants were also asked to bring all current medications to the GCRC, and these were inventoried by the project staff. Fasting blood samples were obtained the next morning between 08:00 and 10:00 a.m. Serum was isolated from all samples, aliquoted, frozen at −80 °C, shipped on dry ice to the appropriate laboratory, and stored at −80 °C for analyses. The time between completion of questionnaires and collection of blood samples varied from simultaneously to several years. To ensure that this variability did not confound the analyses, a variable for time between data collection points was included in all analyses.
Collection of data for MIDUS 2 and analysis of those data for the current study were both approved by the Health Sciences Institutional Review Board at the University of Wisconsin-Madison.
Measures
Chronic conditions.—
Participants indicated whether they had received a physician diagnosis for any of 12 chronic conditions. These included autoimmune disorders, cardiovascular and cerebrovascular diseases, hypertension, arthritis, asthma, diabetes, gastrointestinal diseases, liver disease, and cancer. From these responses, an index of total chronic disease conditions was constructed with possible responses ranging from 0 to 12.
Inflammatory proteins.—
Serum IL-6 from fasting blood samples was measured using a high-sensitivity enzyme-linked immunosorbent assay according to the manufacturer’s guidelines (R&D Systems, Minneapolis, MN) in the laboratory of Dr. Christopher Coe at the University of Wisconsin-Madison. CRP was measured using a particle-enhanced immunonephelometric assay (BNII nephelometer; Dade Behring, Inc., Deerfield, IL) in the laboratory of Dr. Russell Tracy at the University of Vermont. The laboratory intra-assay and interassay coefficients of variance for both proteins were in acceptable ranges (<10%). As is typically seen, distributions for both IL-6 and CRP were positively skewed, and data were log transformed for statistical analyses. Due to cases where blood samples were missing or had inadequate volume for assays, analyses for IL-6 involved 988 participants, whereas those for CRP involved 984 participants. Circulating levels of IL-6 typically increase with age and range from less than 1 pg/ml in young adults to more than 2.5 pg/ml in older adults (Ferrucci et al., 2005).
Eudaimonic well-being.—
Assessments of purpose in life and positive relations with others were based on responses to 2 seven-item subscales from Ryff’s PWB inventory (Ryff & Keyes, 1995). Examples of items from the purpose-in-life scale include “I enjoy making plans for the future and working to make them a reality” and “I sometimes feel as if I’ve done all there is to do in life” (reverse scored). Examples of items from the positive-relations-with-others scale include “I know that I can trust my friends, and they know they can trust me” and “Maintaining close relationships has been difficult and frustrating for me” (reverse scored). Internal validity alphas for purpose in life and positive relations with others for the biomarker sample were .69 and .78, respectively. These are almost identical to the internal validity statistics for the full MIDUS 2 sample (.70 and .78, respectively).
Hedonic well-being.—
Positive and negative affect were measured using adjectives from the Positive and Negative Affect Scale (PANAS; Mroczek & Kolarz, 1998). For positive affect, respondents were asked to indicate how often in the past 30 days they felt “enthusiastic,” “attentive,” “proud,” or “active” and to respond using a 5-point scale (1 = all of the time; 5 = none of the time). Negative adjectives were “afraid,” “jittery,” “irritable,” “ashamed,” and “upset.” Internal validity (determined from the biomarker sample) was .85 for the positive adjectives and .80 for the negative ones. These are comparable with internal validity assessments from the full MIDUS 2 sample (alphas = .90 and .86 for positive and negative affect, respectively).
Life satisfaction was assessed using a single item: “At present, how satisfied are you with your life?” Responses ranged from 1 = a lot to 4 = not at all.
Covariates
In addition to age, sex, marital status, educational attainment, and race, analyses controlled for the potential confounding influence of risk factors for poor health, most notably obesity and use of medications that may affect the inflammatory proteins, and health behaviors.
Education.—
Participants were asked about their highest level of educational attainment. Responses were grouped into 12 categories ranging from “no school/some grade school” to “PhD, MD, JD, or other professional degrees.” Responses were then grouped into three categories—high school degree or Graduate Equivalency Degree, some college, and college degree or more—and this variable was used in all analyses.
Obesity.—
Height and weight were measured by the GCRC staff and used to calculate body mass index (BMI; weight in kilograms divided by the square of height in meters). A continuous measure of BMI was included in all analyses. Waist and hip circumferences were measured directly on skin or over a single layer of clothing (e.g., camisole or undershirt) using a tape measure. A continuous measure of waist-to-hip ratio was used in all analyses.
Medications.—
Antihypertensive (Fliser, Buchholz, & Haller, 2004; Tatli & Kurum, 2005), cholesterol-lowering (Jain & Ridker, 2005), and antidepressant (Kenis & Maes, 2002) medications have all been shown to have anti-inflammatory properties, and steroid medications, particularly as part of a hormone replacement regimen, have been shown to increase CRP levels (Silvestri et al., 2003). Dichotomous variables indicating the use of any of these medications were included in all analyses.
Health behavior.—
Health behavior indicators are based on self-reported information from questionnaires completed at the GCRC. Participants were asked about history of cigarette smoking, and the responses were coded as never smoked, former smoker, or current smoker. Participants also indicated the number of drinks consumed during an average day, and the responses were categorized (none, 1–2, 3 or more). Finally, respondents were asked whether they engage in regular exercise or physical activity for 20 min or more at least 3 times per week, and the responses were categorized as yes or no.
Statistical Analyses
Bivariate associations between key predictor and outcome variables were initially assessed. Hierarchical multivariate linear regression models were then used to estimate the independent and interactive associations of chronic conditions and well-being measures with inflammatory proteins—separate models were estimated for IL-6 and CRP. Initial models examined direct associations between each inflammatory protein and chronic conditions and well-being, and the Well-being × Chronic Conditions interaction net of the effects of age, sex, race, educational attainment, and marital status. To examine the independence of well-being measures and negative affect, the models for positive affect, purpose in life, and positive relations with others then added the variable for negative affect from the PANAS (Model 2). Finally, to determine whether the aspects of health behavior accounted for associations of hedonic or eudaimonic well-being measures and inflammatory markers, Model 3 added BMI, waist–hip ratio, medications, smoking status, alcohol consumption, and exercise. Collinearity diagnostics indicated that associations among these measures were not sufficiently great to compromise the models. The threshold for statistically significant associations was α = .05.
RESULTS
Descriptive statistics for all variables are shown in Table 1.
Table 1.
Descriptive Statistics for Analytical Sample (N = 1,028)
| Variable | Mean (±SEM) or % | Range |
| Age, years | 58.0 (0.4) | 35–86 |
| Sex (% female) | 55.0 | |
| Race (% non-White) | 7.0 | |
| Education (% with high school degree or GED) | 24.4 | |
| Marital status (% married) | 70.0 | |
| Chronic conditions | 2.6 (0.6) | 0–12 |
| Eudaimonic well-being | ||
| Purpose in life | 39.6 (0.2) | 10–49 |
| Positive relations with others | 41.1 (0.2) | 14–49 |
| Hedonic well-being | ||
| PANAS positive affect | 3.6 (0.7) | 1–5 |
| PANAS negative affect | 1.5 (0.5) | 1–4.6 |
| Life satisfaction | 7.9 (1.2) | 0–10 |
| Serum IL-6 (pg/ml) | 2.0 (1.0) | 0.2–9.8 |
| Serum CRP (mg/L) | 1.3 (1.0) | 0.1–10.0 |
| BMI (% >30) | 38.2 | |
| Waist-to-hip ratio | 0.9 (0.0) | 0.6–1.6 |
| Medications (% yes) | ||
| Antihypertensive | 34.8 | |
| Cholesterol lowering | 29.3 | |
| Steroids | 12.3 | |
| Antidepressants | 15.1 | |
| Exercise (20 min, 3 times per week; % yes) | 79.0 | |
| Alcohol consumption per day (% none) | 35.1 | |
| Smoking status (% current smokers) | 11.5 |
Note. BMI = body mass index; CRP = C-reactive protein; GED = Graduate Equivalency Degree; IL-6 = interleukin-6; PANAS = Positive and Negative Affect Scale; SEM = Standard Error of the Mean.
Bivariate Associations
Table 2 displays bivariate associations among the key predictor and outcomes variables as well as the covariates. IL-6 (r = .29, p < .01) and CRP (r = .17, p < .01) were both significantly positively correlated with the number of chronic conditions. Number of chronic conditions was significantly positively correlated with negative affect (r = .07, p < .05) and negatively correlated with life satisfaction (r = −.08, p < .05) but not associated with other measures of psychological functioning. IL-6 was inversely associated with purpose in life (r = −.07, p < .05), positive affect (r =−.09, p < .01), and life satisfaction (r = −.06, p < .05), and CRP was negatively correlated with life satisfaction (r = −.07, p < .05) and positively correlated with positive relations with others (r = .08, p < .05). Finally, measures of hedonic and eudaimonic well-being were all moderately correlated with one another.
Table 2.
Bivariate Associations Among Key Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | ||
| 1 | Age | |||||||||||||
| 2 | Chronic conditions | .43** | ||||||||||||
| 3 | IL-6 | .23** | .29** | |||||||||||
| 4 | CRP | .03 | .17** | .46** | ||||||||||
| 5 | BMI | −.05 | .18** | .34** | .42** | |||||||||
| 6 | Waist–hip ratio | .16** | .13** | .21** | .11** | .34** | ||||||||
| 7 | Smoking status (0 = never smoked) | .01 | .07* | .09** | .06 | −.01 | .12** | |||||||
| 8 | Drinks per day | −.11** | −.09** | −.05 | .01 | −.04 | .09** | .14** | ||||||
| 9 | Exercise (1 = yes) | .05 | .12** | .17** | .16** | .15** | .11** | .08* | −.04 | |||||
| 10 | Purpose in life | .06 | −.06 | −.07* | −.01 | −.06 | −.11** | −.13** | −.03 | −.07* | ||||
| 11 | Positive relations with others | .19** | .02 | .03 | .08* | −.04 | .09** | −.08* | −.04 | −.03 | .57** | |||
| 12 | Positive affect (PANAS) | .21** | −.05 | −.09** | −.03 | −.14** | −.04 | −.12** | .01 | −.06 | .53** | .46** | ||
| 13 | Negative affect (PANAS) | −.26** | .07* | −.01 | −.01 | .08* | −.02 | .15** | .01 | .06 | −.37** | −.42** | −.50** | |
| 14 | Life satisfaction | .26** | −.08** | −.06* | −.07* | −.15** | −.11** | −.12** | −.02 | −.06 | .43** | .47** | .52** | −.47** |
Notes. BMI = body mass index; CRP = C-reactive protein; IL-6 = interleukin-6; PANAS = Positive and Negative Affect Scale.
†p < .10. *p < .05. **p < .01. ***p < .001.
Regression Analyses
Interleukin-6.—
The results of the regression analyses for IL-6 are shown in Table 3.
Table 3.
IL-6 Regressed on Chronic Conditions, Indices of Psychological Functioning, and Their Interactions
| Predictors | Model 1 | Model 2 | Model 3 |
| Number of chronic conditions | 0.21** | — | 0.09* |
| Purpose in life | 0.05 | 0.04 | 0.05 |
| Purpose in Life × Number of Chronic Conditions | −0.43* | −0.43* | −0.36* |
| Positive relations with others | 0.10† | 0.10† | 0.08 |
| Positive Relations With Others × Number of Chronic Conditions | −0.38* | −0.38* | −0.32† |
| PANAS positive affect | −0.10† | −0.12* | −0.10† |
| PANAS Positive Affect × Number of Chronic Conditions | −0.03 | −0.03 | 0.08 |
| Life satisfaction | 0.02 | 0.02 | 0.02 |
| Life Satisfaction × Number of Chronic Conditions | 0.01 | 0.01 | −0.01 |
| PANAS negative affect | −0.01 | — | 0.02 |
| PANAS Negative Affect × Number of Chronic Conditions | 0.03 | — | −0.08† |
Notes. IL-6 = interleukin-6; PANAS = Positive and Negative Affect Scale. Standardized regression coefficients are shown. Separate models were estimated for each psychological measure. Model 1: adjusted for age, sex, race, educational attainment, marital status, sample type, and time between data collections; Model 2: adjusted for Model 1 variables plus PANAS negative affect; and Model 3: adjusted for Model 2 variables plus body mass index, waist–hip ratio, medications, smoking status, alcohol consumption, and physical activity.
†p < .10. *p < .05. **p < .01. ***p < .001.
After adjustment for age, sex, race, educational attainment, marital status, sample type (RDD vs. twin), and time elapsed between data collections (base model), chronic conditions were positively associated with IL-6 (β = .21, p < .01), and this association remained significant albeit diminished after the addition of other covariates (β = .09, p < .05).
Purpose in life was not associated with IL-6 in the base model or after adjustments for health and health behavior covariates. In contrast, the interaction between chronic conditions and purpose in life was significantly associated with IL-6 in the base model (Model 1: β = −.43, p < .05), and this association was maintained after controlling for negative affect (Model 2: β = −.43, p < .05) and other covariates (Model 3: β = −.36, p < .05).
Positive relations with others was marginally significantly associated with IL-6 in both the base model (Model 1: β = .10, p = .05) and after adjustments for negative affect (Model 2: β = .10, p = .05), but this association was reduced to statistical nonsignificance after inclusion of health and health behavior covariates (Model 3: β = .08, p > .05). The interaction of positive relations with others and chronic conditions was significant in the base model (Model 1: β = −.37, p = .05), and after controlling for negative affect (Model 2: β = −.38, p < .05), but was reduced to marginal significance after adjustment for health and health behavior covariates (Model 3: β = −.32, p = .08).
Positive affect was marginally associated with IL-6 in the base (Model 1: β = −.10, p = .06) and full models (Model 3: β = −10, p = .06), and significantly associated with IL-6 in the model after adjusting for negative affect (Model 2: β = −.12, p < .05). The interaction between positive affect and chronic conditions was not significantly associated with IL-6 in any of the models (see Table 3). Neither life satisfaction nor the interaction between life satisfaction and chronic conditions was significantly associated with IL-6 in any of the models (see Table 3). Similarly, negative affect and the interaction of negative affect and chronic conditions did not significantly predict IL-6 in any of the models (see Table 3).
C-reactive protein.—
The results of the regression analyses for CRP are shown in Table 4.
Table 4.
CRP Regressed on Chronic Conditions, Indices of Psychological Functioning, and Their Interactions
| Predictors | Model 1 | Model 2 | Model 3 |
| Number of chronic conditions | 0.17*** | — | 0.07† |
| Purpose in life | 0.11* | 0.09† | 0.10* |
| Purpose in Life × Number of Chronic Conditions | −0.41* | −0.42* | −0.36* |
| Positive relations with others | 0.16** | 0.15** | 0.12* |
| Positive Relations With Others × Number of Chronic Conditions | −0.40* | −0.41* | −0.33† |
| PANAS positive affect | 0.09† | 0.06 | 0.09† |
| PANAS Positive Affect × Number of Chronic Conditions | −0.36* | −0.36* | −0.19 |
| Life satisfaction | −0.01 | 0.01 | 0.02 |
| Life Satisfaction × Number of Chronic Conditions | 0.07 | 0.08 | 0.05 |
| PANAS negative affect | −0.07 | — | −0.02 |
| PANAS Negative Affect × Number of Chronic Conditions | 0.04 | — | −0.11 |
Notes. CRP = C-reactive protein; PANAS = Positive and Negative Affect Scale. Standardized regression coefficients are shown. Separate models were estimated for each psychological measure. Model 1: adjusted for age, sex, race, educational attainment, marital status, sample type, and time between data collections; Model 2: adjusted for Model 1 variables plus PANAS negative affect; and Model 3: adjusted for Model 2 variables plus body mass index, waist–hip ratio, medications, smoking status, alcohol consumption, and physical activity.
†p < .10. *p < .05. **p < .01. ***p < .001.
Chronic conditions were significantly associated with CRP in the base model (β = .17, p < .001), but the association was reduced to marginal significance in the full model (β = .07, p = .05).
Purpose in life was significantly associated with CRP in the base model (Model 1: β = .11, p < .05; Table 4), but this association became marginally significant after adjusting for negative affect (Model 2: β = .09, p = .09). Interestingly, purpose in life predicted CRP in the full model (Model 3: β = .10, p < .05), suggesting modest suppression effects associated with the health and health behavior covariates. The interaction between chronic conditions and purpose in life was consistently significantly related to CRP in all the three models (see Table 4).
Positive relations with others was significantly associated with CRP in all models (see Table 4). The interaction of positive relations with others and chronic conditions was significant in the base model and after controlling for negative affect but was reduced to marginal significance in the full model (Model 3: β = −.33, p = .06).
Positive affect was marginally associated with CRP in the base model (Model 1: β = .09, p = .09), but this association was reduced to nonsignificance after adjustment for negative affect and other covariates (see Table 4). The interaction between positive affect and chronic conditions was significant in the base model (β = −.36, p < .05) and after adjustment for negative affect (Model 2: β = −.36, p < .05), but nonsignificant after adjustment for health and health behavior covariates (β = −.19, p > .05). Life satisfaction, negative affect, and their respective interactions with chronic conditions were not significantly associated with CRP in any of the models (see Table 4).
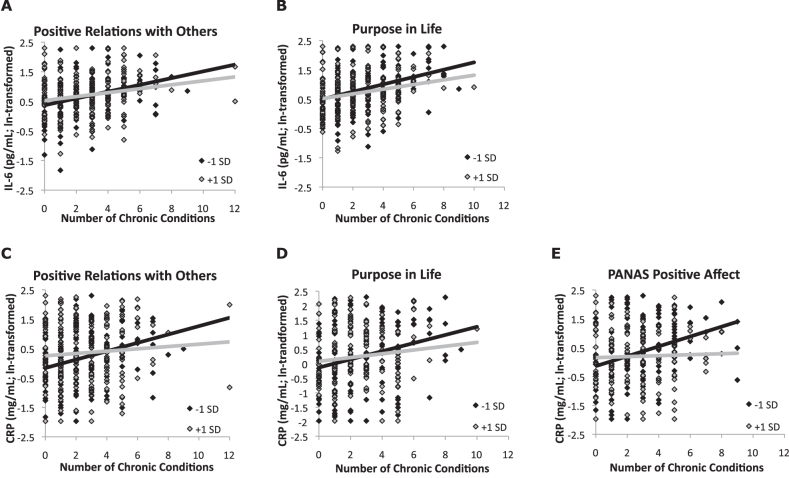
The interactions of chronic conditions with measures of hedonic and eudaimonic well-being are shown in Figure 1.
Figure 1.
Scatter plots of statistically significant interactions (raw values). Scores on hedonic and eudaimonic well-being measures ±1 SD from their respective means are shown. (A) Interaction of positive relations with others and number of chronic conditions predicting interleukin-6 (IL-6; p < .05). (B) Interaction of purpose in life and number of chronic conditions predicting IL-6 (p < .05). (C) Interaction of positive relations with others and number of chronic conditions predicting C-reactive protein (CRP; p < .05). (D) Interaction of purpose in life and number of chronic conditions predicting CRP (p < .05). (E) Interaction of PANAS positive affect and number of chronic conditions predicting CRP (p < .05). PANAS = Positive and Negative Affect Scale.
Analytic Refinements
We probed the aforementioned results in three ways to clarify their interpretation. First, the significant bivariate association between purpose in life and positive relations with others (r = .58, p < .01) raises the question of whether the associations of each domain of eudaimonic well-being with IL-6 and CRP were independent of each other. To address this question, positive relations with others was included in the regression models relating purpose in life to both IL-6 and CRP—and vice versa—and the results showed that neither the associations with the well-being variable nor the association of the relevant interaction term was affected (data not shown). As noted previously, collinearity diagnostics indicated that inclusion of both variables in the same model did not compromise the model.
Second, hedonic and eudaimonic aspects of well-being are considered as related but distinct constructs (Keyes, Shmotkin, & Ryff, 2002). To determine whether these different aspects of well-being shared their associations with inflammatory proteins or were independent, we included positive affect in the regression models for purpose in life and positive relations with others. In every instance, inclusion of positive affect left the coefficients for eudaimonic well-being measures virtually identical.
Finally, IL-6 is a potent stimulator of the synthesis and release of CRP from the liver (Mortensen, 2001; Tennent et al., 2007), and we recently reported that the association of income and CRP was mediated by IL-6 (Friedman & Herd, 2010). To examine a possible mediating role for IL-6 in these analyses, IL-6 was added to the full CRP models. With the exception of the interaction between chronic conditions and positive relations with others, which was reduced to marginal statistical significance (p = .08), associations were unchanged (data not shown).
DISCUSSION
This study examined the psychological correlates of multiple disease comorbidity in a national sample of middle-aged and older adults in the United States. Consistent with prior literature on subjective well-being (Fortin, Dubois, Hudon, Soubhi, & Almirall, 2007; Kunzmann et al., 2000; Mroczek & Spiro, 2005; Strawbridge et al., 2002), we found that life satisfaction declined with increasing number of chronic conditions while negative affect increased. These results are also consistent with a broader literature focused on changes in well-being related to advancing age. These studies show that disease is a significant predictor of age-related declines in well-being (Mroczek & Spiro, 2005; Scheibe & Carstensen, 2010; Strawbridge et al., 2002). In contrast, positive affect was not significantly related to number of chronic conditions, although the association was in the expected direction. This observation differs from earlier reports of declines in positive affect associated with greater disease burden (Kunzmann et al., 2000). Finally, neither purpose in life nor positive relations with others was significantly associated with the number of chronic conditions. To the best of our knowledge, this is a novel finding, and it suggests that eudaimonic well-being, which reflects investment in life goals and personal relationships, may be relatively insensitive to the number of chronic conditions in older adults, at least as assessed in this study. It is worth noting that the effect sizes for the interaction terms were small—the interaction of purpose in life and chronic conditions explained 1% of the variance in IL-6 and 2% of the variance in CRP (effect sizes were smaller for positive relations with others and positive affect)—but with one exception: the increments in the variance explained were statistically significant. By comparison, even chronic conditions explained only 4% of the variance in IL-6 and 2% of the variance in CRP, a reflection of the fact that inflammation is determined by myriad factors, any one of which is likely to have a relatively small effect.
Despite declines in some aspects of subjective well-being with increasing number of chronic conditions, mean levels of hedonic and eudaimonic well-being were high. This result is consistent with research suggesting that aging adults become particularly adept at using cognitive and behavioral strategies to manage positive psychological functioning (Scheibe & Carstensen, 2010). Indeed, scores on some measures of subjective well-being—positive relations with others, positive affect, and life satisfaction—were positively associated with age, whereas depressive symptom scores were associated negatively; scores on the purpose-in-life scale were quadratically associated with age, rising into later life before falling among the oldest participants (those in their late 70s and early 80s). The present results thus suggest that although physical health is an important determinant of some aspects of well-being in later life (Kunzmann et al., 2000; Mroczek & Spiro, 2005; Strawbridge et al., 2002), multiple medical comorbidities are not obstacles to positive psychological functioning.
The final aim of this study was to determine whether these high levels of subjective well-being would be associated with favorable profiles of disease risk in the form of lower circulating levels of inflammatory proteins. The results showed that at similar numbers of chronic conditions, those with higher levels of positive affect, purpose in life, and positive relations with others had lower levels of inflammation. These associations were largely preserved after adjustments for multiple potential confounds. Further analytic refinements suggested that hedonic and eudaimonic well-being make unique contributions to lower levels of inflammation in older adults with multiple chronic conditions.
The findings extend our earlier research showing that higher ratings of purpose in life and positive relations with others predicted lower levels of inflammatory proteins in a community sample of older women (Friedman et al., 2007) and that multiple aspects of well-being moderate the association of educational attainment and IL-6 (Morozink, Friedman, Ryff, & Coe, 2010). Here, we document that aspects of well-being also moderate the linkage between later-life chronic conditions and inflammatory markers. Collectively, such findings underscore the role of positive psychological functioning in offsetting the extent to which various life challenges (social inequalities, unfolding health problems) contribute to biological processes (inflammation) implicated in future profiles of morbidity and mortality. That is, higher levels of inflammation are linked to adverse health outcomes in a range of age-related chronic disease conditions. CRP in particular has been positively linked to poorer prognosis in adults with cardiovascular disease (Blake & Ridker, 2003) and some forms of cancer (Aggarwal & Gehlot, 2009). Other studies have shown that higher levels of inflammation predict increased risk for morbidity and mortality in healthy individuals, particularly in elderly adults (Harris et al., 1999; Stork et al., 2006). Factors that moderate levels of inflammation in aging individuals thus emerge as important influences on health and longevity. Higher levels of purpose in life (Boyle et al., 2009) and positive affect (Chida & Steptoe, 2008; Pressman & Cohen, 2005; Steptoe et al., 2009) have been linked to better health and reduced mortality across the life span, but the mechanisms underlying such associations are unclear. The current results suggest that reduced inflammation may contribute to these general associations between well-being and health.
Our results contribute to the broader discussion of what constitutes successful aging. One of the most influential definitions of successful aging (Rowe & Kahn, 1998) consists of three elements: absence of physical illness or disability, high levels of cognitive function and physical functioning, and active engagement with life. However, only a small percentage of aging individuals in the United States would be considered successful agers by these criteria (McLaughlin, Connell, Heeringa, Li, & Roberts, 2010; Strawbridge et al., 2002), in part because having multiple medical conditions is increasingly the norm for older adults (Anderson, 2007). Moreover, many older adults who do not meet Rowe and Kahn standards consider themselves to be aging successfully. We believe that a compelling alternative formulation of successful aging is the ability to remain actively engaged even in the face of age-related disease (Young, Frick, & Phelan, 2009). As the current results show, even high levels of medical comorbidities do not preclude high levels of subjective well-being. More importantly, maintaining high levels of well-being into later life, particularly a sense of life purpose and strong social relationships, predicts lower levels of inflammatory proteins that themselves predict better health outcomes in the context of chronic illness.
Interpretation of these results should be tempered by several limitations of the study, the most important being that the analyses were cross-sectional, and thus, causal relationships among the variables of interest are unclear. For example, the ability of inflammatory proteins such as IL-6 to influence mood, particularly depression, is well documented (Raison, Capuron, & Miller, 2006). The results are also limited to a single measurement of inflammatory proteins, which, given variability in their levels within individuals, might produce inaccurate estimates of each person’s circulating levels. Nevertheless, such variability would be expected to reduce the likelihood of detecting associations, meaning that the strength of the relationships observed may be underestimated. Finally, given the small effect sizes, it is important not to overstate the significance of these results. The effect sizes for the interactions of chronic conditions and well-being measures were, however, comparable with those for chronic conditions and inflammation alone, suggesting that this interaction is not a trivial one.
In sum, these results add to a growing literature on the potential salutary effects of positive psychological functioning, and they suggest that among those most at risk for age-related morbidity and mortality, experiences of positive affect, purposeful life engagement, and robust social ties may contribute to better health outcomes.
FUNDING
This work was supported by grant K01-AG029381 (to E. M. F.) from the National Institute on Aging (NIA), and the longitudinal follow-up of the MIDUS investigation was supported in part by grant P01-AG020166 from the NIA. The original MIDUS study was supported in part by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development.
Acknowledgments
The authors gratefully acknowledge Barry Radler and Gayle Love for their expert preparation of data files and for assistance with the creation of variables for the current analyses.
References
- Abraham J, Campbell CY, Cheema A, Gluckman TJ, Blumenthal RS, Danyi P. C-reactive protein in cardiovascular risk assessment: A review of the evidence. Journal of Cardiometabolic Syndrome. 2007;2:119–123. doi: 10.1111/j.1559-4564.2007.05950.x. doi:10.1111/j.1559-4564.2007.05950.x. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Gehlot P. Inflammation and cancer: How friendly is the relationship for cancer patients? Current Opinion in Pharmacology. 2009;9:351–369. doi: 10.1016/j.coph.2009.06.020. doi:S1471-4892(09)00090-3[pii]10.1016/j.coph.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G. Chronic conditions: Making the case for ongoing care. 2007. Retrieved from Johns Hopkins University website: http://fightchronicdisease.com/pdfs/ChronicCareChartbook_FINAL.pdf. [Google Scholar]
- Arias E, Rostron BL, Tejada-Vera B. United States life tables, 2005. National Vital Statistics Reports. 2010;58(10):1–132. [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. doi:10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Blake GJ, Ridker PM. C-reactive protein and other inflammatory risk markers in acute coronary syndromes. Journal of the American College of Cardiology. 2003;41(4 Suppl. S):37S–42S. doi: 10.1016/s0735-1097(02)02953-4. doi:S0735109702029534[pii] [DOI] [PubMed] [Google Scholar]
- Boyle PA, Barnes LL, Buchman AS, Bennett DA. Purpose in life is associated with mortality among community-dwelling older persons. Psychosomatic Medicine. 2009;71:574–579. doi: 10.1097/PSY.0b013e3181a5a7c0. doi:PSY.0b013e3181a5a7c0[pii]10.1097/PSY.0b013e3181a5a7c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG. Relationship between attitudes and evaluative space: A critical review with emphasis on the separability of positive and negative substrates. Psychological Bulletin. 1994;115:401–423. doi:10.1037/0033-2909.115.3.401. [Google Scholar]
- Cacioppo JT, Berntson GG. The affect system: Architecture and operating characteristics. Current Directions in Psychological Science. 1999;8:133–137. doi:10.1111/1467-8721.00031. [Google Scholar]
- Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Harris T. Inflammatory markers and cardiovascular disease (The Health, Aging and Body Composition [Health ABC] Study) American Journal of Cardiology. 2003;92:522–528. doi: 10.1016/s0002-9149(03)00718-5. doi:10.1016/S0002-9149(03)00718-5. [DOI] [PubMed] [Google Scholar]
- Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38:399–403. doi: 10.1161/01.hyp.38.3.399. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Positive psychological well-being and mortality: A quantitative review of prospective observational studies. Psychosomatic Medicine. 2008;70:741–756. doi: 10.1097/PSY.0b013e31818105ba. doi:PSY.0b013e31818105ba[pii]10.1097/PSY.0b013e31818105ba. [DOI] [PubMed] [Google Scholar]
- Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB, Harris TB. Physical activity, exercise, and inflammatory markers in older adults: Findings from the Health, Aging and Body Composition Study. Journal of the American Geriatrics Society. 2004;52:1098–1104. doi: 10.1111/j.1532-5415.2004.52307.x. doi:10.1111/j.1532-5415.2004.52307.x[doi]JGS52307[pii] [DOI] [PubMed] [Google Scholar]
- Costanzo ES, Lutgendorf SK, Sood AK, Anderson B, Sorosky J, Lubaroff DM. Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer. 2005;104:305–313. doi: 10.1002/cncr.21147. doi:10.1002/cncr.21147. [DOI] [PubMed] [Google Scholar]
- Diener E. Subjective well-being. Psychological Bulletin. 1984;95:542–575. doi:10.1037/0033-2909.95.3.542. [PubMed] [Google Scholar]
- Diener E. Subjective well-being. The science of happiness and a proposal for a national index. American Psychologist. 2000;55:34–43. doi:10.1037/0003-066X.55.1.34. [PubMed] [Google Scholar]
- Fernandez-Real JM, Ricart W. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocrine Reviews. 2003;24:278–301. doi: 10.1210/er.2002-0010. doi:10.1210/er.2002-0010. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Longo DL. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliser D, Buchholz K, Haller H. Antiinflammatory effects of angiotensin II subtype 1 receptor blockade in hypertensive patients with microinflammation. Circulation. 2004;110:1103–1107. doi: 10.1161/01.CIR.0000140265.21608.8E. doi:10.1161/01.CIR.0000140265.21608.8E. [DOI] [PubMed] [Google Scholar]
- Fortin M, Dubois MF, Hudon C, Soubhi H, Almirall J. Multimorbidity and quality of life: A closer look. Health and Quality of Life Outcomes. 2007;5:52. doi: 10.1186/1477-7525-5-52. doi:1477-7525-5-52[pii]10.1186/1477-7525-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM, Hayney M, Love GD, Singer BH, Ryff CD. Plasma interleukin-6 and soluble IL-6 receptors are associated with psychological well-being in aging women. Health Psychology. 2007;26:305–313. doi: 10.1037/0278-6133.26.3.305. doi:10.1037/0278-6133.26.3.305. [DOI] [PubMed] [Google Scholar]
- Friedman EM, Herd P. Income, education, and inflammation: Differential associations in a national probability sample (The MIDUS study) Psychosomatic Medicine. 2010;72:290–300. doi: 10.1097/PSY.0b013e3181cfe4c2. doi:PSY.0b013e3181cfe4c2[pii]10.1097/PSY.0b013e3181cfe4c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM, Love GD, Singer BH, Ryff CD. Social well-being predicts reduced inflammation in aging women. The Gerontologist. 2008;48(Special Issue III):291. [Google Scholar]
- Friedman EM, Williams DR, Singer BH, Ryff CD. Chronic discrimination predicts higher circulating levels of E-selectin in a national sample: The MIDUS study. Brain Behavior and Immunity. 2009;23:684–692. doi: 10.1016/j.bbi.2009.01.002. doi:S0889-1591(09)00004-X[pii]10.1016/j.bbi.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George LK. Still happy after all these years: Research frontiers on subjective well-being in later life. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2010;65:331–339. doi: 10.1093/geronb/gbq006. doi:gbq006 [pii] 10.1093/geronb/gbq006. [DOI] [PubMed] [Google Scholar]
- Gerstorf D, Ram N, Mayraz G, Hidajat M, Lindenberger U, Wagner GG, Schupp J. Late-life decline in well-being across adulthood in Germany, the United Kingdom, and the United States: Something is seriously wrong at the end of life. Psychology and Aging. 2010;25:477–485. doi: 10.1037/a0017543. doi:2010-11857-022[pii]10.1037/a0017543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr., Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. American Journal of Medicine. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. doi:10.1016/S0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Helmersson J, Larsson A, Vessby B, Basu S. Active smoking and a history of smoking are associated with enhanced prostaglandin F(2alpha), interleukin-6 and F2-isoprostane formation in elderly men. Atherosclerosis. 2005;181:201–207. doi: 10.1016/j.atherosclerosis.2004.11.026. doi:10.1016/S0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosomatic Medicine. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. doi:PSY.0b013e3181907c1b[pii]10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Jain MK, Ridker PM. Anti-inflammatory effects of statins: Clinical evidence and basic mechanisms. Nature Reviews Drug Discovery. 2005;4:977–987. doi: 10.1038/nrd1901. doi:10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- Kenis G, Maes M. Effects of antidepressants on the production of cytokines. International Journal of Neuropsychopharmacology. 2002;5:401–412. doi: 10.1017/S1461145702003164. doi:10.1017/S1461145702003164. [DOI] [PubMed] [Google Scholar]
- Keyes CL, Shmotkin D, Ryff CD. Optimizing well-being: The empirical encounter of two traditions. Journal of Personality and Social Psychology. 2002;82:1007–1022. doi:10.1037/0022-3514.82.6.1007. [PubMed] [Google Scholar]
- Kunzmann U, Little TD, Smith J. Is age-related stability of subjective well-being a paradox? Cross-sectional and longitudinal evidence from the Berlin Aging Study. Psychology and Aging. 2000;15:511–526. doi: 10.1037//0882-7974.15.3.511. [DOI] [PubMed] [Google Scholar]
- Larsen JT, McGraw AP, Cacioppo JT. Can people feel happy and sad at the same time? Journal of Personality and Social Psychology. 2001;81:684–696. doi:10.1037/0022-3514.81.4.684. [PubMed] [Google Scholar]
- Leitch EF, Chakrabarti M, Crozier JE, McKee RF, Anderson JH, Horgan PG, McMillan DC. Comparison of the prognostic value of selected markers of the systemic inflammatory response in patients with colorectal cancer. British Journal of Cancer. 2007;97:1266–1270. doi: 10.1038/sj.bjc.6604027. doi:6604027[pii]10.1038/sj.bjc.6604027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin SJ, Connell CM, Heeringa SG, Li LW, Roberts JS. Successful aging in the United States: Prevalence estimates from a national sample of older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2010;65:216–226. doi: 10.1093/geronb/gbp101. doi:gbp101[pii]10.1093/geronb/gbp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozink J, Friedman EM, Ryff CD, Coe CL. Socioeconomic and psychosocial predictors of interleukin-6 in the MIDUS national sample. Health Psychology. 2010;29(6):626–635. doi: 10.1037/a0021360. doi:10.1037/a0021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen RF. C-reactive protein, inflammation, and innate immunity. Immunologic Research. 2001;24:163–176. doi: 10.1385/IR:24:2:163. doi:IR:24:2:163[pii]10.1385/IR:24:2:163. [DOI] [PubMed] [Google Scholar]
- Mroczek DK, Kolarz CM. The effect of age on positive and negative affect: A developmental perspective on happiness. Journal of Personality and Social Psychology. 1998;75:1333–1349. doi: 10.1037//0022-3514.75.5.1333. doi:10.1037/0022-3514.75.5.1333. [DOI] [PubMed] [Google Scholar]
- Mroczek DK, Spiro A., III. Change in life satisfaction during adulthood: Findings from the veterans affairs normative aging study. Journal of Personality and Social Psychology. 2005;88:189–202. doi: 10.1037/0022-3514.88.1.189. doi:2004-22407-014[pii]10.1037/0022-3514.88.1.189. [DOI] [PubMed] [Google Scholar]
- Norris CJ, Gollan J, Berntson GG, Cacioppo JT. The current status of research on the structure of evaluative space. Biological Psychology. 2010;84:422–436. doi: 10.1016/j.biopsycho.2010.03.011. doi:S0301-0511(10)00083-9[pii]10.1016/j.biopsycho.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman SD, Cohen S. Does positive affect influence health? Psychological Bulletin. 2005;131:925–971. doi: 10.1037/0033-2909.131.6.925. doi:10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. doi:S1471-4906(05)00288-7[pii]10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JW, Kahn RL. Successful aging. New York: Pantheon/Random House; 1998. [Google Scholar]
- Ryan RM, Deci EL. On happiness and human potentials: A review of research on hedonic and eudaimonic well-being. Annual Review of Psychology. 2001;52:141–166. doi: 10.1146/annurev.psych.52.1.141. doi:10.1146/annurev.psych.52.1.141. [DOI] [PubMed] [Google Scholar]
- Ryff CD, Keyes CL. The structure of psychological well-being revisited. Journal of Personality and Social Psychology. 1995;69:719–727. doi: 10.1037//0022-3514.69.4.719. doi:10.1037/0022-3514.69.4.719. [DOI] [PubMed] [Google Scholar]
- Scheibe S, Carstensen LL. Emotional aging: Recent findings and future trends. The Journals of Gerontology, Series B: Psychological and Social Sciences. 2010;65:135–144. doi: 10.1093/geronb/gbp132. doi:gbp132[pii]10.1093/geronb/gbp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri A, Gebara O, Vitale C, Wajngarten M, Leonardo F, Ramires JA, Rosano GM. Increased levels of C-reactive protein after oral hormone replacement therapy may not be related to an increased inflammatory response. Circulation. 2003;107:3165–3169. doi: 10.1161/01.CIR.0000074208.02226.5E. doi:10.1161/01.CIR.0000074208.02226.5E. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Dockray S, Wardle J. Positive affect and psychobiological processes relevant to health. Journal of Personality. 2009;77:1747–1776. doi: 10.1111/j.1467-6494.2009.00599.x. doi:JOPY599[pii]10.1111/j.1467-6494.2009.00599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, O’Donnell K, Badrick E, Kumari M, Marmot M. Neuroendocrine and inflammatory factors associated with positive affect in healthy men and women: The Whitehall II study. American Journal of Epidemiology. 2008;167:96–102. doi: 10.1093/aje/kwm252. doi:kwm252[pii]10.1093/aje/kwm252. [DOI] [PubMed] [Google Scholar]
- Stork S, Feelders RA, van den Beld AW, Steyerberg EW, Savelkoul HF, Lamberts SW, Bots ML. Prediction of mortality risk in the elderly. American Journal of Medicine. 2006;119:519–525. doi: 10.1016/j.amjmed.2005.10.062. doi:10.1016/j.amjmed.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Strawbridge WJ, Wallhagen MI, Cohen RD. Successful aging and well-being: Self-rated compared with Rowe and Kahn. The Gerontologist. 2002;42:727–733. doi: 10.1093/geront/42.6.727. [DOI] [PubMed] [Google Scholar]
- Tatli E, Kurum T. A controlled study of the effects of carvedilol on clinical events, left ventricular function and proinflammatory cytokines levels in patients with dilated cardiomyopathy. Canadian Journal of Cardiology. 2005;21:344–348. [PubMed] [Google Scholar]
- Tennent GA, Brennan SO, Stangou AJ, O’Grady J, Hawkins PN, Pepys MB. Human plasma fibrinogen is synthesized in the liver. Blood. 2007;109:1971–1974. doi: 10.1182/blood-2006-08-040956. doi:blood-2006-08-040956[pii]10.1182/blood-2006-08-040956. [DOI] [PubMed] [Google Scholar]
- Volpato S, Pahor M, Ferrucci L, Simonsick EM, Guralnik JM, Kritchevsky SB, Harris TB. Relationship of alcohol intake with inflammatory markers and plasminogen activator inhibitor-1 in well-functioning older adults: The Health, Aging, and Body Composition study. Circulation. 2004;109:607–612. doi: 10.1161/01.CIR.0000109503.13955.00. doi:10.1161/01.CIR.0000109503.13955.00. [DOI] [PubMed] [Google Scholar]
- Waterman AS. Two conceptions of happiness: Contrasts of personal expressiveness (eudaimonia) and hedonic enjoyment. Journal of Personality and Social Psychology. 1993;64:678–691. doi:10.1037/0022-3514.64.4.678. [Google Scholar]
- Weir PL, Meisner BA, Baker J. Successful aging across the years: Does one model fit everyone? Journal of Health Psychology. 2010;15:680–687. doi: 10.1177/1359105309353648. doi:15/5/680[pii]10.1177/1359105309353648. [DOI] [PubMed] [Google Scholar]
- Young Y, Frick KD, Phelan EA. Can successful aging and chronic illness coexist in the same individual? A multidimensional concept of successful aging. Journal of the American Medical Directors Association. 2009;10:87–92. doi: 10.1016/j.jamda.2008.11.003. doi:S1525-8610(08)00422-2[pii]10.1016/j.jamda.2008.11.003. [DOI] [PubMed] [Google Scholar]