Abstract
Objective.
Among adults, slower and more variable reaction times are associated with worse cognitive function and increased mortality risk. Therefore, it is important to elucidate risk factors for reaction time change over the life course.
Method.
Data from the Health and Lifestyle Survey (HALS) were used to examine predictors of 7-year decline in reaction time (N = 4,260). Regression-derived factor scores were used to summarize general change across 4 reaction time variables: simple mean, 4-choice mean, simple variability, and 4-choice variability (53.52% of variance).
Results.
Age (B = .02, p < .001) and HALS1 baseline reaction time (B = −.10, p = .001) were significant risk factors for males (N = 1,899). In addition to these variables, in females (N = 2,361), neuroticism was significant and interacted synergistically with baseline reaction time (B = .06, p = .04). Adjustment for physiological variables explained the interaction with neuroticism, suggesting that candidate mechanisms had been identified.
Discussion.
A priority for future research is to replicate interactions between personality and reaction time in other samples and find specific mechanisms. Stratification of population data on cognitive health by personality and reaction time could improve strategies for identifying those at greater risk of cognitive decline.
Keywords: Cognition, Life course and developmental change, Neuroticism, Personality
Identifying the determinants of cognitive decline is important because cognitive decline and dementia have high morbidity, treatment, and care costs (Department of Health, 2002). As life expectancy continues to increase, a greater number of people are experiencing cognitive decline in old age (Brayne, 2007). Loss of cognitive function is the most feared aspect of aging by many adults and impairs quality of life in old age (Deary et al., 2007; Deary, Corley, & Gow, 2009). This fear is justified because cognitive decline is a strong risk factor for dementia and can reflect preclinical disease (Bennett et al., 2002). Cognitive decline is also a risk factor for all-cause (Shipley, Der, Taylor, & Deary, 2006) and cause-specific mortality, as is low cognitive ability (B. A. Roberts, Der, Deary, & Batty, 2009). A large and growing body of research has identified candidate risk factors for cognitive change (Deary, Allerhand, & Der, 2009). These include older age, prior cognitive ability, lower socioeconomic status (SES) in adulthood, and health status including medication usage (Deary, MacLennan, & Starr, 1998; Deary, Corley et al., 2009). Other risk factors may include personality traits such as neuroticism, unhealthy behaviors (Dahl et al., 2010; Sabia et al., 2009), genetic factors such as APOE e4 status (K. Anstey & Christensen, 2000), and gene–environment interactions (Deary, Corley et al., 2009; Whalley et al., 2008).
Personality traits may be associated with cognitive decline, particularly neuroticism, which describes stable tendencies to experience negative emotionality and proneness to distress. For example, associations between neuroticism and impaired cognitive function have been reported cross-sectionally (Boyle et al., 2010; Gale et al., 2010; Reid & MacLullich, 2006; Schaie, Willis, & Caskie, 2004). One hypothesis is that neuroticism impairs stress reactivity and recovery (Hutchinson & Ruiz, 2011). Chronic exposure to psychosocial stressors can create allostatic load, the cumulative burden of “wear and tear” across multiple bodily systems (McEwen, 2000). Over time, dysregulation of the Hypothalamic–Pituitary–Adrenal (HPA) axis could result in cognitive decline because excessive levels of corticosteroids are neurotoxic, having been implicated in hippocampal atrophy and in dementia risk (Huang et al., 2009). Cognitive ability and personality traits both have strong test–retest stability (Deary, Whalley, Lemmon, Crawford, & Starr, 2000; B. W. Roberts & DelVecchio, 2000). Compared with more transient states such as mood, personality traits are therefore useful as risk factors in predictive models.
An association between neuroticism and cognitive decline has not been consistently replicated across studies, which may reflect differences in sampling and methods. In one birth cohort, neuroticism at age 13 was associated with worse cognitive function at age 53 (Gale et al., 2010). The association was not significant after controlling for prior cognitive ability and level of educational attainment. Longitudinal studies have been particularly informative because they provide a measure of baseline cognitive function and change over time. In the Georgia Centenarian Study, lower neuroticism and higher extraversion were associated with even worse Mini Mental State Examination (MMSE) scores for “disengaged” cohort members (Martin, Baenziger, MacDonald, Siegler, & Poon, 2009). Among a large sample of elderly participants in the Chicago Healthy Aging Project, 5-year cognitive decline was associated with higher distress proneness (Wilson et al., 2005). This was no longer significant, however, after adjustment for depression. Neuroticism was associated with increased risk of cognitive impairment after 25 years in a case-control study of 4,039 twins but not in a co-twin control design. Average levels of extraversion reduced the risk compared with high or low extraversion (Crowe, Andel, Pedersen, Fratiglioni, & Gatz, 2006). In two longitudinal studies, neuroticism was unrelated to baseline cognitive performance or cognitive decline in a comprehensive battery of tests (Jelicic et al., 2003; Wetherell, Reynolds, Gatz, & Pedersen, 2002), except for three tests: block design, symbol digit substitution, and a “names and faces” task (Wetherell et al., 2002). The follow-up period in both studies, however, was only three years. Commentators have emphasized the need for longer follow-up periods and measures of cognitive function that are more sensitive than the MMSE, only intended as a brief screening tool for dementia (Starr, Deary, Inch, Cross, & MacLennan, 1997).
Reaction time scores provide an index of the speed of information processing and decision making or basic neuropsychological function that have moderately strong correlations with higher level cognitive abilities (Deary, Der, & Ford, 2001). Mean reaction times and variability in reaction time are positively correlated (K. J. Anstey, Dear, Christensen, & Jorm, 2005), producing a general factor that can be used as a marker of general cognitive speed (Deary, Allerhand et al., 2009; Penke et al., 2010). In a representative sample of adults aged 56 followed for 14 years, the association between low cognitive ability and mortality risk was removed after adjusting for reaction time, suggesting that reaction time explains the association or is a mechanism (Deary & Der, 2005).
Reaction time scores become markedly slower with age (Salthouse, 1996), leading some researchers to suggest that they are good markers of cognitive aging (Deary, Johnson, & Starr, 2010), although slowing of processing speed does not fully explain cognitive decline (Zimprich, 2002). Nonetheless, reaction time could provide an important source of information about more general age-related changes in multiple bodily systems. Researchers have been encouraged to consider the possibility that reaction time and physiological variables share common variance in age-related decline (Salthouse, 1996), particularly sensory variables (e.g., vision and hearing) but also biomarkers, such as lung function (K. J. Anstey et al., 2005). In addition to becoming slower, reaction times become more variable with age, suggesting that increased variability is an important component of cognitive decline (Bielak, Hultsch, Strauss, MacDonald, & Hunter, 2010b; Der & Deary, 2006; Salthouse, 1996; Tse, Balota, Yap, Duchet, & McCabe, 2010). More variable performance on reaction time tasks has been shown to predict later cognitive decline (MacDonald, Hultsch, & Dixon, 2003) and cognitive impairment (Bielak, Hultsch, Strauss, MacDonald, & Hunter, 2010a). Reaction time is an indicator of ability to perform many different kinds of processing operations (Salthouse, 1996). For all these reasons, there is increasing interest in adding reaction time tasks to large epidemiological studies (K. J. Anstey et al., 2005; Deary & Der, 2005; Shipley, Der, Taylor, & Deary, 2007).
One unresolved issue is the extent to which personality and reaction time interact to influence cognitive change. In a recent cross-sectional study of early stage dementia patients, neuroticism was associated with the positive tail of the reaction time distribution (Tse et al., 2010), which the authors interpreted as evidence that neuroticism can influence attentional control systems and impair cognitive performance. If the combined risk from slow baseline reaction time and personality is also greater than the sum of the two separate effects (an interaction), then this has important implications for identifying people most at risk. A necessary step, then, is to identify the existence of effect modification (interaction) and evaluate whether such modification is attenuated following adjustment for known confounding factors (e.g., health behaviors that might influence baseline reaction time) and possible mechanisms (e.g., biomarkers or physiological mediators).
Adjusting associations between psychological risk factors and cognitive change for physiological variables provides important clues about whether physiological variables might explain an association. Physiological variables are not confounding factors because they are not thought to influence personality traits. They are better conceptualized as possible mechanisms or mediating variables (Babyak, 2009). Lung function was the only variable to be associated with all four reaction time measures considered in a recent cross-sectional population study (K. J. Anstey et al., 2005). The study demonstrated that lung function accounted for a large proportion of age-related variance in reaction time measures. Additional biomarkers, such as Body Mass Index (BMI) and blood pressure, were not considered in that study. Both BMI (MacDonald, Dixon, Cohen, & Hazlitt, 2004) and blood pressure (Singh-Manoux & Marmot, 2005) are associated with cognitive function and may lie on the causal pathway between personality, cognitive ability, and cognitive change (Shipley et al., 2007).
The purpose of the present study was to identify predictors of 7-year decline in reaction time, including baseline reaction time, personality traits, their possible interaction, and adjusting for a wide range of possible confounding factors and mechanisms. Variables such as SES and health behaviors are conceptualized as confounders in our study, whereas physiological variables are considered as candidate mechanisms/mediators. This means that adjusting for physiological variables is expected to lead to a reduction/removal of the any association between the risk factors (personality and baseline reaction time) and the outcome (cognitive decline) because physiological variables lie on the causal chain between the risk factor and the outcome. Physiological variables are treated as explanatory rather than confounding factors (Babyak, 2009). This addresses a clear need in the literature to examine both the independent effects of personality, prior processing speed, and their possible interaction. The U.K. Health and Lifestyle Survey (HALS) is unique in that mean and intraindividual variability data are available on simple and four-choice reaction times for a representative sample of U.K. adults across the entire adult age range, many of whom were retested 7 years later. Additionally, the personality traits of neuroticism and extraversion were measured, providing an opportunity to test whether personality traits modify the risk associated with poorer baseline reaction times, on 7-year decline in reaction time. Previous studies have found important sex differences, such as females scoring higher on neuroticism (Costa, Terracciano, & McCrae, 2001), having more variable reaction time (Der & Deary, 2006), and being more at risk of cognitive decline (Deary, Whiteman, Starr, Whalley, & Fox, 2004; cf. Meinz & Salthouse, 1998). Therefore, we also aimed to describe any gender differences in the role of personality, reaction time, and their possible interaction, as predictors of 7-year cognitive decline.
METHOD
Sample
The Health and Lifestyle Survey 1.—
HALS is a prospective study of a representative sample of U.K. adults (age range 18–97), conducted in 1984 and 1985 (hereafter, HALS1). The sample (N = 9,003) contained slightly more females, fewer single people, and fewer older women than the general population (Cox, 1988). HALS1 involved two home visits, in response to an invitation letter. The first visit comprised a baseline interview and self-completed questionnaire, including personality trait measures. The second involved a visit from a nurse who collected information on physiological data: height, weight, blood pressure, pulse rate, respiratory function, exhaled carbon monoxide, and reaction time.
The Health and Lifestyle Survey 2.—
A second wave of testing included reaction time tests and was performed on the same sample 7 years later, in 1991 and 1992 (hereafter, HALS2). Attrition due to mortality, migration, withdrawal, and loss-to-follow-up resulted in 5,352 participants participating in both HALS1 and HALS2. Compared with the population, HALS2 (N = 5,352) contained fewer older adults, more middle-aged adults, and the age range became 25–99 (Cox, 1995).
Missing data.—
Participants with data on reaction time change had reaction time data at both time points and were slightly younger (OR = 0.991, 95% CI = 0.988–0.993), had higher occupational social class (OR = 1.05, 95% CI = 1.01–1.09) and educational attainment (OR = 1.06, 95% CI = 1.03–1.09). Potential bias associated with casewise deletion of missing data on covariates was evaluated using multiple imputation (see below).
Measures
Simple reaction time.—
The stand-alone reaction time box and its “simple” and “four-choice” reaction time tasks have been described previously (Shipley et al., 2007). The device was specifically designed for the study. The simple reaction time test involved the participant pressing a key (marked “0”), as quickly as possible after being presented with a stimulus “0” on the small LCD screen. Participants were all given eight practice trials and 20 test trials. Both the mean and standard deviation of the 20 reaction times were recorded in milliseconds for each participant. Standard deviation of the reaction times provides data on intra-individual variability in processing speed.
Choice reaction time.—
The four-choice reaction time task involved four stimuli (digits 1–4) presented on the screen. Participants rested two fingers of each hand on buttons marked “1”–“4” and were requested to press the correct button to match the stimulus number. Eight practice trials and 40 test trials involved each digit being presented 10 times at interstimulus intervals of between 1 and 3 s. In the analyses presented below, choice reaction time data are used for correct trials only.
Neuroticism and extraversion.—
Personality traits were measured at HALS1 using the Eysenck Personality Inventory (EPI), a self-report measure of neuroticism and extraversion (Eysenck & Eysenck, 1964). An example item for neuroticism is “Does your mood often go up and down.” An example item for extraversion is “Would you be very unhappy if you could not see lots of people most of the time.” There are 57 items, each having response options coded 1 (yes) and 0 (no). Negatively worded items are reversed keyed so that higher scores indicate higher neuroticism and extraversion (range 0–25). The internal consistencies (Cronbach alpha) of neuroticism and extraversion were .84 and .76 in HALS1. One-year test–retest reliabilities were established in a previous study (.84 and .88 respectively; Eysenck & Eysenck, 1964).
Socioeconomic and demographic variables.—
All socioeconomic information was taken from HALS1. Age was recorded in years. Registrar General’s Occupational Social Classes comprise six categories (Office of Population Censuses and Surveys, 1980): professional, managerial, skilled nonmanual, skilled manual, semiskilled manual, and unskilled. These were treated as continuous and recoded, so that higher scores indicated higher social class, ranging from 1 (lowest) to 6 (highest). Married women were classified by their husband’s occupation, widowed women by their ex-husband’s occupation, single and divorced women by their own occupation. Educational attainment was coded on a 6-point scale ranging from 1 (no educational qualifications) to 6 (degree level qualification). The correlation between occupational social class and education was R = .49 (p < .001) in males and R = .36 (p < .001) in females.
Health behaviors.—
Alcohol intake was the number of units of alcohol consumed in the previous week, derived from alcohol drinking diaries. Cigarette smoking was the number of cigarettes consumed daily (“How many cigarettes do you generally smoke in a day?”). Saturated fat was calculated in grams consumed weekly, by calibrating a food frequency questionnaire against a weighed national dietary survey; a method described previously (Tefft & Boniface, 2000). Physical activity was recorded in minutes, using a checklist of 17 moderate or vigorous sporting activities (e.g., keep fit, cycling, jogging). All four health behaviors were treated as continuous.
Physiological variables.—
BMI (kg/m2) was derived from height and weight measurements. Height was measured using a portable stadiometer and weight was measured using portable scales provided by the nurse. Systolic and diastolic blood pressure recordings were taken as the lowest of four serial recordings made at 1-min intervals, using an automatic blood pressure monitor. Forced expiratory volume in litres in 1 s (FEV1, adjusted for individual differences in body size, age, and sex) provided a measure of lung function.
Residualized reaction time change scores.—
Residualized change scores were created by regressing HALS2 on HALS1 reaction time scores as we have done previously (Shipley et al., 2007). The standardized residuals from these models describe the size and direction of change over seven years but are not associated with baseline scores. This reduces the influence of regression to the mean (Menard, 1991). For each reaction time variable (simple, four-choice, simple variability, four-choice variability), the HALS2 score was treated as the dependent variable and the HALS1 measure was the independent variable. Standardized residuals were calculated from each model, representing cognitive change between HALS1 and HALS2.
Statistical Analysis
Residual change scores for participants with reaction time data were subjected to principal components analysis. A single component accounted for 53.52% of the variance in residual cognitive change. Regression-derived factor scores were calculated, which represent general change in reaction time and reaction time variability across 7 years. Component loadings for reaction time errors were not statistically significant in preliminary analyses (not shown) and were therefore excluded. Sex differences in the component loadings were evaluated by performing a multiple group analysis. A model in which the factor loadings were free to vary did not provide a significantly better fit to the data than a model in which the factor loadings were equal across both sexes (χ2 (5) = 3.98, p = .26). Therefore, the component loadings were treated as equivalent for males and females: .84 (reaction time mean), .86 (choice reaction time mean), .76 (reaction time variability), and .72 (choice reaction time variability).
RESULTS
The analytic sample comprised a total of 4,260 individuals with complete data on reaction time measures at HALS1 and HALS2 (age range 18–96 in males, 18–87 in females). Table 1 shows the means, standard deviations, and proportion of available data for each variable. Table 2 shows the bivariate correlations between the baseline risk factors and 7-year cognitive change. In the models described below, age was centered at mean age; cognitive ability and personality traits were centered by converting them to z scores. The z scores have the additional advantage of producing estimates that refer to 1 SD increase in each trait, per standard deviation increase in residual cognitive change. Because slower reaction times are indicative of worse cognitive functioning, higher scores on the general reaction time factor indicate worse (worsening) function.
Table 1.
Descriptive Statistics
| Total (N = 4,260) | Males (n = 1,899) | Females (n = 2,361) | ||||
| M (SD) | N | M (SD) | N | M (SD) | N | |
| Age | 44.19 (15.36) | 4,260 | 44.73 (15.74) | 1,899 | 43.76 (15.04) | 2,361 |
| Baseline reaction time (HALS1, SD) | −0.12 (0.84) | 4,260 | −0.09 (0.89) | 1,899 | −0.15 (0.79) | 2,361 |
| Neuroticism | 9.46 (5.02) | 3,910 | 8.18 (4.87) | 1,729 | 10.47 (4.91) | 2,181 |
| Extraversion | 12.52 (4.26) | 3,910 | 12.31 (4.32) | 1,729 | 12.68 (4.20) | 2,181 |
| Social class | 3.55 (1.30) | 4,212 | 3.53 (1.29) | 1,874 | 3.56 (1.31) | 2,338 |
| Educational attainment | 1.90 (2.01) | 4,241 | 2.06 (2.05) | 1,891 | 1.78 (1.98) | 2,350 |
| Alcohol intake (units weekly) | 8.58 (14.50) | 4,260 | 14.64 (18.97) | 1,899 | 3.71 (6.05) | 2,361 |
| Cigarettes smoked (daily) | 5.03 (8.97) | 4,260 | 5.65 (9.81) | 1,899 | 4.53 (8.21) | 2,361 |
| Saturated fat intake (g weekly) | 272.56 (125.82) | 4,084 | 324.51 (136.41) | 1,840 | 229.96 (97.63) | 2,244 |
| Physical activity (min daily) | 1.66 (23.61) | 4,260 | 13.11 (29.07) | 1,899 | 8.70 (17.82) | 2,361 |
| Systolic blood pressure (mmHg) | 124.65 (17.28) | 4,251 | 128.58 (16.07) | 1,896 | 121.48 (17.57) | 2,355 |
| Diastolic blood pressure (mmHg) | 75.46 (11.51) | 4,251 | 77.91 (11.05) | 1,896 | 73.50 (11.49) | 2,355 |
| Body mass index (kg/m2) | 24.59 (3.98) | 4,197 | 24.93 (3.60) | 1,897 | 24.30 (4.24) | 2,300 |
| Lung function (FEV1, litres) | 2.76 (0.87) | 3,907 | 3.21 (0.90) | 1,747 | 2.40 (0.65) | 2,160 |
Note. FEV1 = Forced expiratory volume in 1s; HALS = Health and Lifestyle Survey.
Table 2.
Bivariate Correlations Between Baseline Variables and 7-Year Reaction Time Change
| 1 | 2 | 3 | 4 | 5 | |
| 1. Male | 1 | ||||
| 2. Age | .03* | 1 | |||
| 3. Neuroticism | −.23** | −.07** | 1 | ||
| 4. Extraversion | −.04** | .26** | .06** | 1 | |
| 5. Baseline reaction time score | .04* | .41** | .04* | .07** | 1 |
| 6. 7-year reaction time change | .00 | .36** | .02 | .06** | .16** |
Notes. Correlations calculated using available data for each pair of variables. Higher values on reaction time measures indicate slower and more variable reaction time, indicating worse performance.
*p < .05. **p < .01.
Principal risk factors (age, baseline reaction time, neuroticism, extraversion, neuroticism–baseline reaction time, extraversion–baseline reaction time, and interactions between baseline reaction time, personality traits, and age) were entered into the model first. In preliminary models in which we screened for sex differences (not shown), significant interaction terms were observed for neuroticism–reaction time–sex (p = .04) and neuroticism–reaction time–sex–age (p = .02). There were also trends suggesting possible interactions for neuroticism–sex (p = .09) and reaction time–sex (p = .09). This demonstrated that the neuroticism–reaction time interaction was different for males and females, leading us to test models separately. Socioeconomic variables were grouped into a second block (education and social class) and health behavior variables into a third block. Physiological variables (systolic blood pressure, diastolic blood pressure, FEV, BMI) comprised a fourth block. This order was chosen to allow more proximal determinants (i.e., physiological variables) to enter the model last, after adjustment for confounding factors. Results did not differ substantively when cases with missing data were excluded or when missing data were replaced using multiple imputation with 10 replications. Results in Tables 3 and 4 are therefore from the multiple imputation analysis, having a larger sample size. All analyses were performed in Mplus version 6.1.
Table 3.
Summary of Hierarchical Multiple Regression Analysis Showing Predictors of Residual Reaction Time Change in Males
| N = 1,899 | Model 1: baseline risk factors | Model 2: additional adjustment for socioeconomic variables | Model 3: additional adjustment for health behaviors | Model 4: additional adjustment for physiological variables | ||||||||
| B (SE) | β | p Value | B (SE) | β | p Value | B (SE) | β | p Value | B (SE) | β | p Value | |
| General baseline reaction time (g) | −.095 (0.030) | −.09 | .001 | −.152 (0.030) | −.14 | <.001 | −.155 (0.030) | −.14 | <.001 | −.166 (0.031) | −.15 | <.001 |
| Neuroticism | .041 (0.023) | .04 | .07 | .034 (0.022) | .03 | .13 | .034 (0.022) | .03 | .13 | .033 (0.022) | .03 | .14 |
| Extraversion | −.025 (0.023) | −.03 | .28 | −.001 (0.023) | −.00 | .96 | −.003 (0.024) | −.00 | .90 | −.002 (0.024) | −.00 | .93 |
| Age | .023 (0.002) | .38 | <.001 | .024 (0.002) | .38 | <.001 | .024 (0.002) | .38 | <.001 | .020 (0.002) | .32 | <.001 |
| Age × Age | .001 (0.0001) | .15 | <.001 | .001 (0.0001) | .13 | <.001 | .001 (0.0001) | .13 | <.001 | .001 (0.0001) | .13 | <.001 |
| Reaction time × Age | .006 (0.002) | .10 | <.001 | .007 (0.002) | .12 | <.001 | .007 (0.002) | .12 | <.001 | .008 (0.002) | .13 | <.001 |
| Neuroticism × Age | .002 (0.002) | .03 | .23 | .002 (0.002) | .03 | .23 | .002 (0.002) | .03 | .23 | .002 (0.002) | .03 | .30 |
| Extraversion × Age | −.002 (0.002) | −.04 | .18 | −.002 (0.002) | −.03 | .21 | −.002 (0.002) | −.03 | .24 | −.002 (0.002) | −.03 | .23 |
| Neuroticism × g | .012 (0.028) | .01 | .67 | .007 (0.027) | .01 | .81 | .007 (0.027) | .01 | .80 | .010 (0.027) | .01 | .72 |
| Extraversion × g | −.006 (0.030) | −.01 | .83 | −.020 (0.030) | −.02 | .50 | −.020 (0.030) | −.02 | .50 | −.018 (0.030) | −.02 | .54 |
| R 2 (%) | 16.6 | 18.8 | 21.4 | 21.9 | ||||||||
Note. p Value refers to the unstandardized coefficient (B). β = standardized coefficient.
Table 4.
Summary of Hierarchical Multiple Regression Analysis Showing Predictors of Residual Reaction Time Change in Females
| N = 2,361 | Model 1: baseline risk factors | Model 2: additional adjustment for socioeconomic variables | Model 3: additional adjustment for health behaviors | Model 4: additional adjustment for physiological variables | ||||||||
| B (SE) | β | p Value | B (SE) | β | p Value | B (SE) | β | p Value | B (SE) | β | p Value | |
| General baseline reaction time (g) | −.035 (0.028) | −.03 | .22 | −.069 (0.029) | −.06 | .02 | −.073 (0.029) | −.06 | .01 | −.101 (0.030) | −.08 | <.001 |
| Neuroticism | .074 (0.021) | .07 | <.001 | .064 (0.021) | .06 | .00 | .061 (0.021) | .06 | <.001 | .056 (0.021) | .06 | .01 |
| Extraversion | −.009 (0.021) | −.01 | .67 | .0001 (0.021) | −.00 | .98 | −.003 (0.022) | −.00 | .88 | −.0004 (0.021) | −.00 | .99 |
| Age | .020 (0.001) | .31 | <.001 | .020 (0.001) | .31 | <.001 | .020 (0.002) | .31 | <.001 | .017 (0.002) | .26 | <.001 |
| Age × Age | .001 (0.0001) | .18 | <.001 | .001 (0.0001) | .17 | <.001 | .001 (0.0001) | .18 | <.001 | .001 (0.0001) | .16 | <.001 |
| Reaction time × Age | .003 (0.002) | .04 | .11 | .003 (0.002) | .04 | .07 | .003 (0.002) | .05 | .05 | .003 (0.002) | .05 | .03 |
| Neuroticism × Age | .002 (0.002) | .03 | .20 | .002 (0.002) | .03 | .22 | .002 (0.002) | .03 | .20 | .002 (0.002) | .03 | .25 |
| Extraversion × Age | .001 (0.002) | .01 | .57 | .001 (0.002) | .01 | .52 | .001 (0.002) | .01 | .53 | .001 (0.001) | .01 | .58 |
| Neuroticism × g | .058 (0.029) | .05 | .04 | .059 (0.029) | .05 | .04 | .057 (0.029) | .05 | .05 | .051 (0.029) | .04 | .07 |
| Extraversion × g | .014 (0.034) | .01 | .69 | .009 (0.034) | .01 | .79 | .008 (0.034) | .01 | .82 | .011 (0.034) | .01 | .76 |
| R 2 (%) | 16.8 | 17.6 | 17.8 | 18.9 | ||||||||
Note. p Value refers to the unstandardized coefficient (B). β = standardized coefficient.
Males
In males, adjusting for baseline risk factors, greater cognitive decline was associated with higher age (B = .023, p < .001) and faster baseline reaction time (B = −.095, p = .001), perhaps indicating some regression to the mean. The nonlinear effect of age was significant (B = .001, p < .001), suggesting slightly higher rates of change at higher years of age. The influence of baseline reaction time became stronger at older ages (B = .006, p < .001). Personality traits did not interact with age nor with baseline reaction time. Adjustment for socioeconomic variables (education and social class) slightly increased the risk associated with baseline reaction time (B = −.152, p < .001) and the interaction between reaction time and age (B = .007, p < .001). Adjustment for multiple health behaviors did little to alter these associations. In the final model (Model 4) adjusting for blood pressure, BMI and lung function, age (B = .020, p < .001), baseline reaction time (B = −.166, p < .001), age over time (B = .001, p < .001), and the interaction between baseline reaction time and age (B = .008, p < .001) were significant. The interaction between age and baseline reaction time implies that the risk conferred by slower reaction time is stronger at older ages. In summary, the risk factors for greater 7-year cognitive decline in males were higher age, slower baseline reaction time, and particularly slower baseline reaction time for older males. Neuroticism did not interact with baseline reaction time. As a set, the variables in the fully adjusted model accounted for 21.9% of the variance in reaction time change.
Females
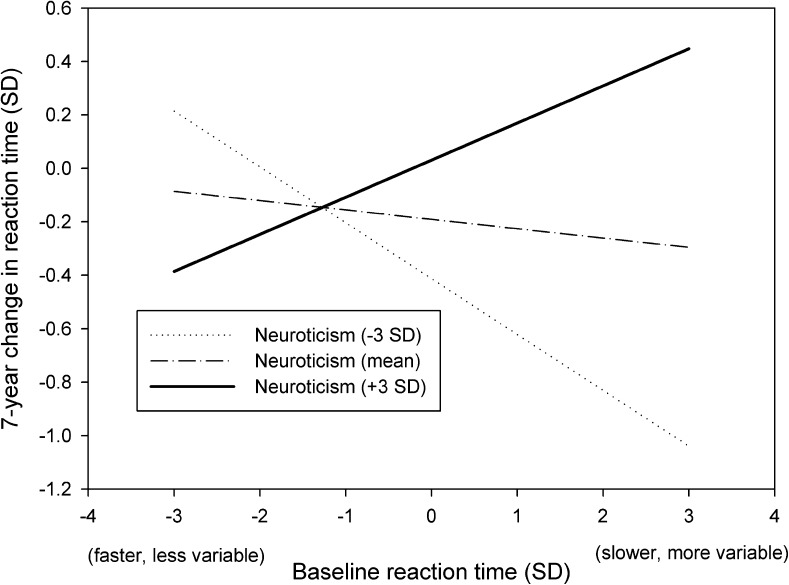
In females, greater reaction time slowing was associated with higher age (B = .020, p < .001), higher neuroticism (B = .074, p < .001), and the nonlinear effect of age suggesting slightly greater risk of slowing reaction times at older ages (B = .001, p < .001). A significant interaction was observed between neuroticism and baseline reaction time (B = .058, p = .04), illustrating synergism between baseline reaction time and neuroticism (Figure 1). Those individuals that were higher in neuroticism (dashed line) and had slower baseline reaction times (higher values on the x axis) experienced greater cognitive decline over the 7-year period (higher values on the y axis), at average levels of age. The effect size is small but is relevant from a population perspective and may be clinically significant. Females tend to have higher mean scores on neuroticism (Costa et al., 2001), there are a greater number of older females then males in the population (Der & Deary, 2006), and there is more cognitive decline at higher levels of age (Deary, Corley, & Gow et al., 2009). There was little attenuation of these associations following adjustment for education, social class, and multiple health behaviors. Following adjustment for physiological variables, age, baseline cognitive speed, and neuroticism remained significant, as did interactions between g-speed and age and age over time. The interaction between baseline reaction time and neuroticism was not significant after adjustment for physiological variables (Table 4). Physiological variables, however, are not confounding factors because they are not known to influence personality traits. Rather than “explaining away” the interaction, the removal of the significant interaction term at this stage suggests that candidate mechanisms may have been identified. The variables in the final model accounted for 18.9% of the variance in cognitive change. Taken together, the results reveal an interaction that is specific to females. Baseline reaction time combined with neuroticism contributed to cognitive decline over seven years.
Figure 1.
Interaction plot showing estimated slower and more variable reaction time scores as predictors of 7-year change, at three different levels of neuroticism in females.
A supplementary analysis was performed in order to identify which of the four physiological variables might have the greatest attenuating effect (explanatory role) on the interaction term. The percentage attenuation was calculated using the coefficient from Model 3 (adjusted for education, social class, and health behaviours) as the comparison using the formula 100 × [(Model 3 estimate−new estimate)/(Model 3 estimate)]. Systolic blood pressure attenuated the interaction term by 1.75%, diastolic by 3.51%, BMI increased it by 1.75%, and lung function attenuated it by 7.02%. This illustrated that although physiological variables, considered collectively, attenuated the association beyond statistical significance at the traditional p < .05 level, none individually explained the interaction substantially. Lung function had the largest effect, followed by diastolic then systolic blood pressure, with BMI having no attenuating effect.
DISCUSSION
Among females, neuroticism interacted with baseline reaction time to accelerate cognitive decline 7 years later. Adjustment for physiological variables attenuated the interaction, suggesting that physiological variables are candidate mechanisms. In both sexes, higher age, slower, and more variable reaction times were associated with 7-year decline in fully adjusted models. The results illustrate that baseline performance, sex, and neuroticism are risk factors for cognitive decline, beyond normal age-related slowing in processing speed (Salthouse, 1996). In females, baseline performance and neuroticism are synergistic risk factors.
The strengths of the study extend from several unique features of the data. HALS1 was broadly representative of the U.K. population, covered the entire adult age range, and reaction time data were available for many participants 7 years later. Very few large epidemiological studies contain data on both personality traits and cognitive speed, along with a wide range of relevant covariates. The data allowed us to demonstrate that the psychological risk factors identified were not explained by SES or by multiple health behaviors. The principal limitation of our study is that some participants were not present for the HALS1 nurse visit, the HALS2 study, or did not provide data for other reasons. This may have led to restriction of range on some variables and underestimation of effect sizes. Additionally, it would be useful to evaluate cognitive decline across a more comprehensive battery of psychometric tests. Reaction time is an important component of cognitive ability, but there are other important components (Deary, Johnson, et al., 2010; Tse et al., 2010). Reaction time tasks, however, are well validated and are available to researchers and clinicians at no charge (Deary, Liewald, & Nissan, 2011). Similarly, brief measures of the big five personality traits are now available which have been validated (e.g., Gosling, Rentfrow, & Swann, 2003), and these may be suitable for clinical settings. Another limitation is that we described but were not able to explain why interaction observed was specific to females. Identifying reasons for this specificity, if replicated, is a priority for future research. Finally, the effect sizes are small (shown in Figure 1 at 3 SDs above and below the mean) but refer to change over a relatively short time period (7 years). They are relevant when considered across the entire life course and also in terms of the excess risk of cognitive decline associated with each risk factor and their interaction at the population level.
Our results also add to a growing body of evidence that neuroticism may increase the risk of cognitive decline (Crowe et al. 2006; Gale et al., 2010; Martin et al., 2009; Wilson et al., 2005), with apparent sex differences and the novel finding that neuroticism interacts with reaction time in females. It is not clear at this stage why these sex differences exist. Physiological variables may be involved in the interaction between neuroticism and reaction time among females, such as blood pressure, diastolic blood pressure, FEV, BMI, or other cardiovascular disease risk factors. It is important not to confuse confounding factors with possible mediators (Babyak, 2009) nor to confuse statistical and biological interaction (Rothman, Greenland, & Walker, 1980). We found no association between extraversion and cognitive decline, which could reflect methodological or sampling differences when compared with other studies (cf. Crowe et al., 2006; Martin et al., 2009).
It is possible that the combination of high neuroticism and slow baseline speed may exacerbate the disruption in attentional control mechanisms seen in the early stages of dementia (Tse et al., 2010), by influencing physiological variables that facilitate attention and response time. Several reports have shown that neuroticism and cognitive ability are associated cross-sectionally with physiological variables and cardiovascular disease risk factors in particular. In a recent report from the Vietnam Experience Study, low cognitive ability and neuroticism were correlated (R = .18, p < .001) and both associated with the metabolic syndrome. Neuroticism was specifically associated with obesity, triglycerides, hypertension, and diabetes (Phillips et al., 2010). Impaired or delayed cardiovascular reactivity and recovery among females exposed to psychosocial stressors may underlie the association between neuroticism and development of cardiovascular disease risk factors (Hutchinson & Ruiz, 2011).
If the interaction we report here is replicated in other studies, there are at least four applied implications (Rothman, et al., 1980). First, there are statistical advantages to including interaction terms in models, most notably that the model will fit the data better. Had the interaction been ignored, the degree of cognitive change observed for females with slower/faster baseline reaction time would have been under-/overestimated at higher/lower levels of neuroticism (see Figure 1). Second, interactions can sometimes point to candidate mechanisms or where to focus future research efforts. Our findings suggest that research into lung function, blood pressure, and BMI among women in relation to their neuroticism and slow reaction time factor scores could be highly informative. Dysregulation of the HPA axis could be involved, particularly if high neuroticism and slow reaction time influence bodily systems in a way that has a cumulative and neurotoxic effect on hippocampal neurons (Huang et al., 2009). Third, understanding interactions between cognitive ability and personality traits can inform clinicians’ decisions about prioritizing treatment, taking into account a patient’s gender, reaction time, and personality traits (Deary, Weiss, & Batty, 2010). Finally, strategies designed to monitor or protect the cognitive health of the population would benefit from knowledge about how risk factors might interact (Rothman et al., 1980). Other personality traits in the Five Factor Model may predict cognitive aging or interact with reaction time, such as openness to experience (Booth, Schinka, Brown, Mortimer, & Borenstein, 2006; cf. Sharp, Reynolds, Pedersen, & Gatz, 2010), agreeableness, and conscientiousness (Tse et al., 2010; Wilson, Schneider, Arnold, Bienias, & Bennett, 2007).
In summary, this study illustrates that slower reaction time and more variable reaction time was a risk factor for 7-year reaction time deterioration, particularly among older adults. Furthermore, neuroticism and baseline reaction time scores interacted among females. The findings illustrate the need to increase precision when estimating the impact of low baseline performance on cognitive decline, by controlling for personality and including interaction terms with personality traits. The need to consider patient’s personality traits when evaluating cognitive function and subjective memory complaints has been raised elsewhere (Reid & MacLullich, 2006). Whether cognitive function and reaction time should be adjusted for personality traits, remains uncertain. Future research will need to replicate the methods and results reported here and ultimately elucidate mechanisms that explain the interaction between traits and reaction time, if it is replicated, and account for why sex differences may exist. There are also emerging and innovative methods in applied health research that take personality and individual differences into account (see Lahey, 2009). Interventions may be more successful and cost effective if they are informed by knowledge about how health and disease are influenced by cognitive ability, personality traits, interactions, and physiological variables that might explain those interactions. Future studies of cognitive decline should consider age, sex, personality traits, and cognitive function at baseline, and test for the possible existence of effect modification. It remains to be seen whether other personality traits interact with baseline performance and sex and how replicable these interactions are.
FUNDING
G. E. Hagger-Johnson is supported by a grant from the National Institute on Aging, NIH (R01AG034454, Principal Investigators: Singh-Manoux & Kivimaki). The University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology is part of the cross council Lifelong Health and Wellbeing Initiative (G0700704/84698). Funding from the Biotechnology and Biological Sciences Research Council (BBSRC), Engineering and Physical Sciences Research Council (EPSRC), Economic and Social Research Council (ESRC), and the Medical Research Council (MRC) is gratefully acknowledged.
Acknowledgments
We are grateful to David Boniface for providing the saturated fat data and to the people who participated in the Health and Lifestyle Surveys of 1984 and 1991.
References
- Anstey K, Christensen H. Education, activity, health, blood pressure and Apolipoprotein E as predictors of cognitive change in old age: A review. Gerontology. 2000;46:163–177. doi: 10.1159/000022153. doi:10.1159%2F000022153. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Dear K, Christensen H, Jorm AF. Biomarkers, health, lifestyle, and demographic variables as correlates of reaction time performance in early, middle, and late adulthood. Quarterly Journal of Experimental Psychology Section A: Human Experimental Psychology. 2005;58:5–21. doi: 10.1080/02724980443000232. doi:10.1080%2F02724980443000232. [DOI] [PubMed] [Google Scholar]
- Babyak MA. Understanding confounding and mediation. Evidence Based Mental Health. 2009;12:68–71. doi: 10.1136/ebmh.12.3.68. doi:10.1136%2Febmh.12.3.68. [DOI] [PubMed] [Google Scholar]
- Bielak AAM, Hultsch DF, Strauss E, Macdonald SW, Hunter MA. Intraindividual variability in reaction time predicts cognitive outcomes 5 years later. Neuropsychology. 2010a;24:731–741. doi: 10.1037/a0019802. doi:10.1037%2Fa0019802. [DOI] [PubMed] [Google Scholar]
- Bielak AAM, Hultsch DF, Strauss E, MacDonald SWS, Hunter MA. Intraindividual variability is related to cognitive change in older adults: Evidence for within-person coupling. Psychology and Aging. 2010b;25:575–586. doi: 10.1037/a0019503. doi:10.1037%2Fa0019503. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- Booth JE, Schinka JA, Brown LM, Mortimer JA, Borenstein AR. Five-factor personality dimensions, mood states, and cognitive performance in older adults. Journal of Clinical and Experimental Neuropsychology. 2006;28:676–683. doi: 10.1080/13803390590954209. doi:10.1080%2F13803390590954209. [DOI] [PubMed] [Google Scholar]
- Boyle LL, Lyness JM, Duberstein PR, Karuza J, King DA, Messing S, Tu X. Trait neuroticism, depression, and cognitive function in older primary care patients. American Journal of Geriatric Psychiatry. 2010;18:305–312. doi: 10.1097/JGP.0b013e3181c2941b. doi:10.1097%2FJGP.0b013e3181c2941b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayne C. The elephant in the room? Healthy brains in later life, epidemiology and public health. Nature Reviews Neuroscience. 2007;8:233–239. doi: 10.1038/nrn2091. doi:10.1038%2Fnrn2091. [DOI] [PubMed] [Google Scholar]
- Costa PT, Terracciano A, McCrae RR. Gender differences in personality traits across cultures: robust and surprising findings. Journal of Personality and Social Psychology. 2001;81:322–331. doi: 10.1037/0022-3514.81.2.322. doi:10.1037%2F%2F0022-3514.81.2.322. [DOI] [PubMed] [Google Scholar]
- Cox BD. Health and Lifestyle Survey, 1984–1985 (HALS1) user manual. Colchester, UK: Economic and Social Data Service; 1988. [Google Scholar]
- Cox BD. Health and Lifestyle Survey: Seven year follow-up, 1991–1992 (HALS2) user manual. Colchester, UK: Economic and Social Data Service; 1995. [Google Scholar]
- Crowe M, Andel R, Pedersen NL, Fratiglioni L, Gatz M. Personality and risk of cognitive impairment 25 years later. Psychology and Aging. 2006;21:573–580. doi: 10.1037/0882-7974.21.3.573. doi:10.1037/0882-7974.21.3.573. [DOI] [PubMed] [Google Scholar]
- Dahl A, Hassing LB, Fransson E, Berg S, Gatz M, Reynolds CA, Pedersen NL. Being overweight in midlife is associated with lower cognitive ability and steeper cognitive decline in late life. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2010;65:57–62. doi: 10.1093/gerona/glp035. doi:10.1093%2Fgerona%2Fglp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Allerhand M, Der G. Smarter in middle age, faster in old age: A cross-lagged panel analysis of reaction time and cognitive ability over 13 years in the West of Scotland Twenty-07 Study. Psychology and Aging. 2009;24:40–47. doi: 10.1037/a0014442. doi:10.1037%2Fa0014442. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Corley J, Gow AJ, Harris SE, Houlihan LM, Marioni RE, et al. Age-associated cognitive decline. British Medical Bulletin. 2009;92:135–152. doi: 10.1093/bmb/ldp033. doi:10.1093%2Fbmb%2Fldp033. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Der G. Reaction time explains IQ’s association with death. Psychological Science. 2005;16:64–69. doi: 10.1111/j.0956-7976.2005.00781.x. doi:10.1111%2Fj.0956-7976.2005.00781.x. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Der G, Ford G. Reaction times and intelligence differences: A population-based cohort study. Intelligence. 2001;29:389–399. doi:10.1016%2FS0160-2896%2801%2900062-9. [Google Scholar]
- Deary IJ, Gow A, Taylor M, Corley J, Brett C, Wilson V, et al. The Lothian Birth Cohort 1936: A study to examine influences on cognitive ageing from age 11 to age 70 and beyond. BMC Geriatrics. 2007;7:28. doi: 10.1186/1471-2318-7-28. doi:10.1186%2F1471-2318-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Johnson W, Starr JM. Are processing speed tasks biomarkers of cognitive aging? Psychology and Aging. 2010;25:219–228. doi: 10.1037/a0017750. doi:10.1037%2Fa0017750. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Liewald D, Nissan J. A free, easy-to-use, computer-based simple and four-choice reaction time programme: The Deary-Liewlad reaction time task. Behavior Research Methods. 2011;43:258–268. doi: 10.3758/s13428-010-0024-1. doi:10.3758%2Fs13428-010-0024-1. [DOI] [PubMed] [Google Scholar]
- Deary IJ, MacLennan WJ, Starr JM. Is age kinder to the initially more able? Differential ageing of a verbal ability in the Healthy Old People in Edinburgh study. Intelligence. 1998;26:357–375. doi:10.1016%2FS0160-2896%2899%2900005-7. [Google Scholar]
- Deary IJ, Weiss A, Batty GD. Intelligence and personality as predictors of illness and death. Psychological Science in the Public Interest. 2010;11:53–79. doi: 10.1177/1529100610387081. doi:10.1177%2F1529100610387081. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Whalley LJ, Lemmon H, Crawford JR, Starr JM. The stability of individual differences in mental ability from childhood to old age: Follow-up of the 1932 Scottish Mental Survey. Intelligence. 2000;28:49–55. doi:10.1016%2FS0160-2896%2899%2900031-8. [Google Scholar]
- Deary IJ, Whiteman MC, Starr JM, Whalley LJ, Fox HC. The impact of childhood intelligence on later life: Following up the Scottish mental surveys of 1932 and 1947. Journal of Personality and Social Psychology. 2004;86:130–147. doi: 10.1037/0022-3514.86.1.130. doi:10.1037%2F0022-3514.86.1.130. [DOI] [PubMed] [Google Scholar]
- Department of Health. National Dementia Strategy. London: HMSO; 2002. [Google Scholar]
- Der G, Deary IJ. Age and sex differences in reaction time in adulthood: Results from the United Kingdom Health and Lifestyle Survey. Psychology and Aging. 2006;21:62–73. doi: 10.1037/0882-7974.21.1.62. doi:10.1037%2Fa0015515. [DOI] [PubMed] [Google Scholar]
- Eysenck SB, Eysenck HJ. An improved short questionnaire for the measurement of extraversion and neuroticism. Life Sciences. 1964;3:1103–1109. doi: 10.1016/0024-3205(64)90125-0. doi:10.1016%2F0024-3205%2864%2990125-0. [DOI] [PubMed] [Google Scholar]
- Gale CR, Deary IJ, Kuh D, Huppert F, Richards M HALCyon Study Team. Neuroticism in adolescence and cognitive function in midlife in the British 1946 birth cohort: The HALCyon program. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2010;65:50–56. doi: 10.1093/geronb/gbp082. doi:10.1093%2Fgeronb%2Fgbp082. [DOI] [PubMed] [Google Scholar]
- Gosling S. D.,, Rentfrow, P. J.,, Swann, W. B. Jr., A very brief measure of the Big Five personality domains. Journal of Research in Personality. 2003;37:504–528. doi:10.1016/S0092-6566(03)00046-1. [Google Scholar]
- Huang CW, Lui CC, Chang WN, Lu CH, Wang YL, Chang CC. Elevated basal cortisol level predicts lower hippocampal volume and cognitive decline in Alzheimer's disease. Journal of Clinical Neuroscience. 2009;16:1283–1286. doi: 10.1016/j.jocn.2008.12.026. doi:10.1016%2Fj.jocn.2008.12.026. [DOI] [PubMed] [Google Scholar]
- Hutchinson JG. Ruiz JM. Neuroticism and cardiovascular response in women: Evidence of effects on blood pressure recovery. Journal of Personality. 2011;79:277–302. doi: 10.1111/j.1467-6494.2010.00679.x. doi:10.1111/j.1467-6494.2010.00679.x. [DOI] [PubMed] [Google Scholar]
- Jelicic M, Bosma H, Ponds RW, Van Boxtel MP, Houx PJ, Jolles J. Neuroticism does not affect cognitive functioning in later life. Experimental Aging Research. 2003;29:73–78. doi: 10.1080/03610730303704. doi:10.1080%2F03610730303704. [DOI] [PubMed] [Google Scholar]
- Lahey BB. Public health significance of neuroticism. American Psychologist. 2009;64:241–256. doi: 10.1037/a0015309. doi:10.1037%2Fa0015309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald SWS, Dixon RA, Cohen AL, Hazlitt JE. Biological age and 12-Year cognitive change in older adults: Findings from the Victoria longitudinal study. Gerontology. 2004;50:64–81. doi: 10.1159/000075557. doi:10.1159%2F000075557. [DOI] [PubMed] [Google Scholar]
- MacDonald SWS, Hultsch DF, Dixon RA. Performance variability is related to change in cognition: Evidence from the Victoria Longitudinal Study. Psychology and Aging. 2003;18:510–523. doi: 10.1037/0882-7974.18.3.510. doi:10.1037%2F0882-7974.18.3.510. [DOI] [PubMed] [Google Scholar]
- Martin P, Baenziger J, MacDonald M, Siegler I, Poon L. Engaged lifestyle, personality, and mental status among centenarians. Journal of Adult Development. 2009;16:199–208. doi: 10.1007/s10804-009-9066-y. doi:10.1007%2Fs10804-009-9066-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. doi:10.1016%2FS0893-133X%2899%2900129-3. [DOI] [PubMed] [Google Scholar]
- Meinz EJ, Salthouse TA. Is age kinder to females than to males? Psychonomic Bulletin and Review. 1998;5:56–70. doi:10.3758%2FBF03209457. [Google Scholar]
- Menard S. Longitudinal research. Newbury Park, CA: SAGE; 1991. [Google Scholar]
- Office of Population Censuses and Surveys. Classification of occupations 1980. London: Her Majesty’s Stationery Office; 1980. [Google Scholar]
- Penke L, Maniega SM, Murray C, Gow AJ, Valdes Hernandez MC, Clayden JD, et al. A general factor of brain white matter integrity predicts information processing speed in healthy older people. Journal of Neuroscience. 2010;30:7569–7574. doi: 10.1523/JNEUROSCI.1553-10.2010. doi:10.1523%2FJNEUROSCI.1553-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AC, Batty GD, Weiss A, Deary I, Gale CR, Thomas GN, Carroll D. Neuroticism, cognitive ability, and the metabolic syndrome: The Vietnam Experience Study. Journal of Psychosomatic Research. 2010;69:193–201. doi: 10.1016/j.jpsychores.2010.01.016. doi:10.1016/j.jpsychores.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Reid LM, MacLullich AMJ. Subjective memory complaints and cognitive impairment in older people. Dementia and Geriatric Cognitive Disorders. 2006;22:471–485. doi: 10.1159/000096295. doi:10.1159%2F000096295. [DOI] [PubMed] [Google Scholar]
- Roberts BA, Der G, Deary IJ, Batty GD. Reaction time and established risk factors for total and cardiovascular disease mortality: Comparison of effect estimates in the follow-up of a large, UK-wide, general-population based survey. Intelligence. 2009;37:561–566. doi:10.1016%2Fj.intell.2009.02.001. [Google Scholar]
- Roberts BW, DelVecchio WF. The rank-order consistency of personality from childhood to old age: A quantitative review of longitudinal studies. Psychological Bulletin. 2000;126:3–25. doi: 10.1037/0033-2909.126.1.3. doi:10.1037%2F0033-2909.126.1.3. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, Walker AM. Concepts of interaction. American Journal of Epidemiology. 1980;112:467–470. doi: 10.1093/oxfordjournals.aje.a113015. [DOI] [PubMed] [Google Scholar]
- Sabia S, Nabi H, Kivimaki M, Shipley MJ, Marmot MG, Singh-Manoux A. Health behaviors from early to late midlife as predictors of cognitive function: The Whitehall II study. American Journal of Epidemiology. 2009;170:428–437. doi: 10.1093/aje/kwp161. doi:10.1093%2Faje%2Fkwp161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. doi:10.1037%2F0033-295X.103.3.403. [DOI] [PubMed] [Google Scholar]
- Schaie KW, Willis SL, Caskie GI. The Seattle longitudinal study: Relationship between personality and cognition. Aging, Neuropsychology and Cognition. 2004;11:304–324. doi: 10.1080/13825580490511134. doi:10.1080%2F13825580490511134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp ES, Reynolds CA, Pedersen NL, Gatz M. Cognitive engagement and cognitive aging: Is openness protective? Psychology and Aging. 2010;25:60–73. doi: 10.1037/a0018748. doi:10.1037%2Fa0018748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley BA, Der G, Taylor MD, Deary IJ. Cognition and all-cause mortality across the entire adult age range: Health and Lifestyle Survey. Psychosomatic Medicine. 2006;68:17–24. doi: 10.1097/01.psy.0000195867.66643.0f. doi:10.1097%2F01.psy.0000195867.66643.0f. [DOI] [PubMed] [Google Scholar]
- Shipley BA, Der G, Taylor MD, Deary IJ. Association between mortality and cognitive change over 7 years in a large representative sample of UK residents. Psychosomatic Medicine. 2007;69:640–650. doi: 10.1097/PSY.0b013e31814c3e7c. doi:10.1097%2FPSY.0b013e31814c3e7c. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Marmot M. High blood pressure was associated with cognitive function in middle-age in the Whitehall II study. Journal of Clinical Epidemiology. 2005;58:1308–1315. doi: 10.1016/j.jclinepi.2005.03.016. doi:10.1016/j.jclinepi.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Starr, J. M., , Deary, I. J., , Inch, S., , Cross, S., , MacLennan, W. J. Age-associated cognitive decline in healthy old people. Age and Ageing, 1997;26:295–300. doi: 10.1093/ageing/26.4.295. Retrieved from http://dx.doi.org/10.1093/ageing/26.4.295. [DOI] [PubMed] [Google Scholar]
- Tefft MEE, Boniface DRR. Estimating food and nutrient intake from food frequency questionnaire data by reference to a standard weighed diet survey. Journal of Human Nutrition and Dietetics. 2000;13:219–224. doi: 10.1046/j.1365-277x.2000.00231.x. doi:10.1046%2Fj.1365-277x.2000.00231.x. [DOI] [PubMed] [Google Scholar]
- Tse C, Balota DA, Yap MJ, Duchet JM, McCabe DP. Effects of healthy aging and early stage dementia of the Alzheimer’s type on components of response time distributions in three attention tasks. Neuropsychology. 2010;24:300–315. doi: 10.1037/a0018274. doi:10.1037%2Fa0018274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherell JLL, Reynolds CA, Gatz M, Pedersen NL. Anxiety, cognitive performance, and cognitive decline in normal aging. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2002;57:246–255. doi: 10.1093/geronb/57.3.p246. doi:10.1093%2Fgeronb%2F57.3.P246. [DOI] [PubMed] [Google Scholar]
- Whalley LJ, Deary IJ, Starr JM, Wahle KW, Rance KA, Bourne VJ, Fox HC. n-3 Fatty acid erythrocyte membrane content, APOE epsilon-4, and cognitive variation: An observational follow-up study in late adulthood. American Journal of Clinical Nutrition. 2008;87:449–454. doi: 10.1093/ajcn/87.2.449. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Bennett D, Mendesdeleon C, Bienias J, Morris M, Evans D. Distress proneness and cognitive decline in a population of older persons. Psychoneuroendocrinology. 2005;30:11–17. doi: 10.1016/j.psyneuen.2004.04.005. doi:10.1016%2Fj.psyneuen.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Arnold SE, Bienias JL, Bennett DA. Conscientiousness and the incidence of Alzheimer disease and mild cognitive impairment. Archives of General Psychiatry. 2007;64:1204–1212. doi: 10.1001/archpsyc.64.10.1204. doi:10.1001%2Farchpsyc.64.10.1204. [DOI] [PubMed] [Google Scholar]
- Zimprich D. Cross-sectionally and longitudinally balanced effects of processing speed on intellectual abilities. Experimental Aging Research. 2002;28:231–251. doi: 10.1080/03610730290080290. doi:10.1080%2F03610730290080290. [DOI] [PubMed] [Google Scholar]