Abstract
Although Stat1 is required for many IFN-dependent responses, recent work has shown that IFNγ functions independently of Stat1 to affect the growth of tumor cells or immortalized fibroblasts. We now demonstrate that both IFNγ and IFNα/β regulate proliferative responses in cells of the mononuclear phagocyte lineage derived from Stat1-null mice. Using both representational difference analysis and gene arrays, we show that IFNγ exerts its Stat1-independent actions on mononuclear phagocytes by regulating the expression of many genes. This result was confirmed by monitoring changes in expression and function of the corresponding gene products. Regulation of the expression of these genes requires the IFNγ receptor and Jak1. The physiologic relevance of IFN-dependent, Stat1-independent signaling was demonstrated by monitoring antiviral responses in Stat1-null mice. Thus, the IFN receptors engage alternative Stat1-independent signaling pathways that have important physiological consequences.
The interferons (IFNs) are antiviral cytokines that also have profound immunomodulatory activities and exert their pleiotropic effects by interacting with distinct receptors expressed on nearly all cells (1–3). These receptors use both specific and overlapping components of the JAK-STAT signaling pathway (4) to form different transcription factor complexes, all of which contain Stat1 (3, 5). The physiologic importance of Stat1 in mediating IFN-induced responses has been validated in studies of mice that lack an intact Stat1 gene (6, 7). These studies showed that Stat1 is used in a highly restricted manner for signaling by the IFNα/β and IFNγ receptors and plays a critical role in promoting many IFN-induced responses.
Recently, IFNγ and IFNα/β were shown to regulate expression of the c-myc gene in Stat1-deficient tumor cells and immortalized fibroblasts (8), revealing the presence of at least one IFN-induced, Stat1-independent signaling pathway. To better define the importance of the alternative IFN signaling pathways, we explored the functional consequences of engaging these receptors in primary macrophages derived from Stat1-null mice. Herein, we report that both IFNα/β and IFNγ induce physiologically important responses in primary cells of the mononuclear phagocyte lineage derived from Stat1-null mice. Because IFNγ is the major macrophage activating factor (9), we explored the Stat1-independent pathway of IFNγ signaling in more detail. In cells that lack Stat1, IFNγ was found to regulate the expression of a surprisingly large number of genes, including many that encode immunologically important proteins. Whereas expression of some of these genes was regulated by IFNγ in either the presence or absence of Stat1, others responded to IFNγ only when Stat1 was absent. The physiologic relevance of IFN-dependent signaling in the absence of Stat1 was validated by using in vivo models of viral infection. This study thus reveals the existence of alternative signaling pathways used by the IFN receptors in nontransformed, primary cells.
Materials and Methods
Cytokines, Mice, and Bone-Marrow-Derived Macrophages (BMM).
Purified recombinant murine IFNγ was provided by Genentech and human IFNαA/D was obtained from Hoffmann–La Roche. Recombinant murine macrophage colony stimulating factor (M-CSF), IL-3, and granulocyte–macrophage colony-stimulating factor (GM-CSF) were from R & D Systems. Wild-type (WT) mice (strain 129/Sv/Ev) and mice with null mutations in the genes encoding the IFNγ receptor (IFNγR−/−) (10), the IFNα⋅β receptor (IFNαβR−/−), and both receptors (IFNαβγR−/−), were obtained from Michel Aguet (11) and bred at Washington University (St. Louis). Stat1−/− (strain 129/Sv/Ev) and Jak1−/− mice were generated in our laboratory (6, 12). A second line of Stat1−/− mice on the C57BL/6 background (7) was obtained from Joan Durbin (Ohio State University Medical School, Columbus, OH). C57BL/6 mice were purchased from Taconic Farms. PKR−/− mice on the 129/Sv/Ev background (13) were obtained from Bryan Williams (Lerner Research Institute of the Cleveland Clinic, Cleveland) and PKR−/− × STAT1−/− mice were generated by interbreeding at Washington University (St. Louis). Bone marrow cells (BMC) from the femurs of adult mice were cultured with cytokine growth factors as described (12, 14).
Representational Difference Analysis.
This analysis was performed as described (15) by using 30 × 106 BMM derived from Stat1−/− mice stimulated at 37°C for 6 h with or without IFNγ (14 ng/ml).
Affymetrix GeneChip Analysis.
Ninety million BMM, derived from either WT 129/Sv/Ev mice or Stat1−/− mice, were incubated for 1 h at 37°C with or without IFNγ (14 ng/ml) and total RNA was harvested by using RNAzol (Tel-Test, Friendswood, TX). Preparation of cRNA and hybridization to the Mu6400 and Mu11000 GeneChip sets were performed as described by the manufacturer (Affymetrix, San Jose, CA). Stained chips were read and analyzed by using an Affymetrix GeneChip scanner and the accompanying software.
Northern Analysis.
Fifteen micrograms of total RNA was fractionated in 1.2% agarose-formaldehyde gels and subsequently transferred to nylon membranes (Zeta Probe from Bio-Rad). Hybridization and washes were performed as indicated by the manufacturer. All of the experiments were performed at least three times.
The probe for CXCR4 was generated by PCR from a murine spleen cDNA library (CLONTECH) as described (16). A plasmid corresponding to the mouse IL-1β full-length cDNA (SGBS mIL-1β) was kindly provided by David Chaplin (Washington University School of Medicine, St. Louis). Probes for murine MIP-1α and MCP-1 were purchased from Genome Systems (St. Louis).
Analysis of Calcium Mobilization.
The presence of CXCR4 on macrophage surfaces was determined functionally by monitoring the ability of SDF-1, the natural ligand for CXCR4 to induce a calcium ion flux in macrophages after treatment with IFNγ or buffer. One million BMM, adherent to glass cover slips, were incubated with or without IFNγ (14 ng/ml) for 10 or 24 h. The cover slips where then placed in medium containing 5 μM fura-2/acetoxymehtyl ester (Molecular Probes) and 2.5 M probenecid (Sigma) (17). The image acquisition and calibration were performed as described (18).
Viral Infections.
Murine cytomegalovirus, Smith strain (MCMV), was obtained from the American Type Culture Collection (VR-194, Lot 10), and salivary gland MCMV was generated as described (19). Sindbis virus (strain dsTE12Q) was prepared and titered as described (20). Mice were infected i.p. with sgMCMV and s.c. with Sindbis virus.
Results
IFNα/β and IFNγ Regulate the Growth of Stat1−/− BMC.
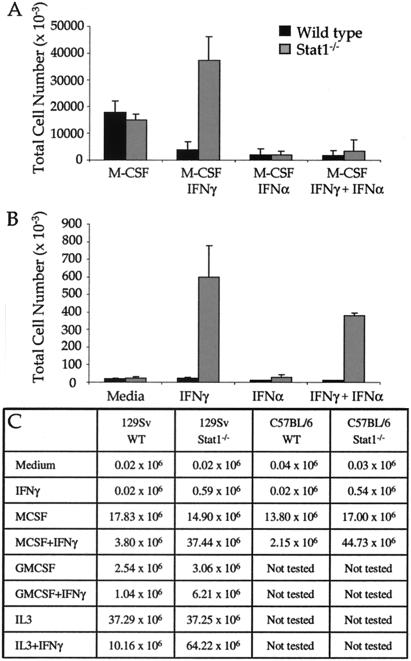
IFNγ has been reported to regulate the growth of transformed/immortalized Stat1-deficient fibroblast cell lines (8); therefore, we asked whether it has similar effects on primary Stat1−/− cells. We chose to study cells of the myeloid lineage because their development and activities are highly influenced by IFNγ, the major, physiologically relevant macrophage activating factor (9). Primary BMC from either WT or Stat1−/− mice, when cultured with the macrophage growth factor M-CSF, proliferated and differentiated into macrophages comparably (Fig. 1 A and C). As expected, IFNα or IFNγ exerted profound antiproliferative effects on M-CSF-dependent growth of WT BMC leading to an approximately 4-fold reduction in cell numbers. IFNα exerted a profound antiproliferative effect on M-CSF-treated BMC from Stat1−/− mice, whereas IFNγ enhanced the M-CSF-induced cell growth of Stat1−/− BMC by almost 3-fold. These results reveal that both IFNα/β and IFNγ regulate BMC growth in a Stat1-independent manner, although with opposite biologic outcomes.
Figure 1.
IFNγ induces survival and/or proliferation of Stat1 deficient cells. (A and B) Ten million BMC obtained from Stat1−/− and WT mice were plated in the presence of medium, rM-CSF (50 ng/ml), IFNγ (14 ng/ml), IFNα (8.5 ng/ml), or different combinations of these cytokines. After 7 days, viable cells were counted. Shown are the total viable cells obtained in the presence (A) or absence (B) of M-CSF. The averages of five independent experiments are shown. (C) Total cells obtained after culturing 1 × 107 BMC for 7 days. The cytokines and growth factors used were: M-CSF (50 ng/ml); IFNγ (14 ng/ml); GM-CSF (5 ng/ml); and IL-3 (10 ng/ml). The averages of three independent experiments are shown.
We next asked whether IFNγ was sufficient to induce the proliferation and survival of Stat1−/− myeloid precursor cells. BMC from either WT or Stat1−/− mice did not proliferate or survive when cultured for 7 days in medium alone (Fig. 1B). In addition, no growth or survival was observed in cultures of WT cells incubated with either IFNα or IFNγ or in cultures of Stat1−/− BMC treated with IFNα. In contrast, IFNγ induced the growth and survival of Stat1−/− BMC leading, after 7 days, to cultures that contained 70% macrophages and 30% polymorphonuclear leukocytes. The validity and specificity of the IFNγ-induced growth of Stat1−/− BMC was confirmed by showing the same effect of IFNγ (i) on BMC from another Stat1−/− mouse line generated by using a different targeting strategy (ref. 7; Fig. 1C) and (ii) on Stat1−/− BMC treated with either IL-3 or GM-CSF instead of M-CSF (Fig. 1C). Thus, IFNγ (but not IFNα/β) functions as a growth and survival factor for immature myeloid cells that lack Stat1.
IFNγ Regulates Gene Expression in Macrophages (BMM) from Stat1-Null Mice.
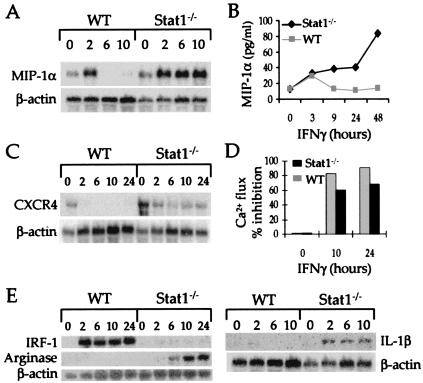
To study whether IFNγ also exerted Stat1-independent effects on mature macrophages, we treated Stat1−/− BMM with IFNγ or buffer and used representational difference analysis (RDA) to monitor changes in gene expression. In total, six genes were identified that were affected by IFNγ in these cells. Four of these genes were regulated in a truly Stat1-independent manner. Specifically, IFNγ induced expression of the MIP-1α (Fig. 2A), MIP-1β, and MCP-1 (data not shown) genes and inhibited expression of the CXCR4 gene (Fig. 2C), regardless of the presence or absence of Stat1. In contrast, the IL-1β and Arginase genes (Fig. 2E) were induced by IFNγ only in cells that lacked Stat1. The induction of the MIP-1α and MCP-1 genes in both WT and Stat1-deficient macrophages was rapid (Fig. 2A). However, gene expression decayed more slowly in cells that lacked Stat1. Similar patterns were also observed for expression of the corresponding protein (Fig. 2B). The repression of CXCR4 gene expression and the disappearance of the CXCR4 protein on cell surfaces (as determined functionally in IFNγ-treated or -untreated cells by monitoring Ca2+ fluxes induced by SDF-1α, the natural ligand for CXCR4) was sustained in both types of macrophages although repression was somewhat less in the Stat1−/− cells after 24 h (Fig. 2 C and D). In contrast, IFNγ induced expression of IRF-1, a classical Stat1-dependent, IFNγ-induced gene (6, 21, 22), only in Stat1-containing macrophages, thereby confirming the specificity of the gene expression analysis (Fig. 2E). Similar results were obtained when macrophages from the second strain of Stat1−/− mice were used (data not shown). These results therefore identify several genes whose expression is regulated by IFNγ in cells that lack Stat1 and also demonstrate that Stat1 is required for terminating the expression of other IFN-induced genes.
Figure 2.
IFNγ regulates gene and protein expression in Stat1−/− macrophages. Fifteen μg of total RNA from macrophages treated with either buffer or IFNγ (14 ng/ml) for different periods of time were subjected to Northern analyses by using probes specific for MIP-1α (A), CXCR4 (C), IRF-1, Arginase, IL-1β (E), and β-actin. The quantitation of mRNA expression was performed by using a Molecular Dynamics PhosphorImager with the corresponding IMAGEQUANT software. (B) Twenty million BMM derived from WT (■) or Stat1−/− (♦) mice were incubated with or without IFNγ (14 ng/ml). After the indicated times the supernatant was harvested, concentrated 7-fold by ultrafiltration and screened by ELISA for MIP-1α (Quantikine M from R&D Systems). The result shown is representative of three experiments. (D) One million BMM were incubated with or without IFNγ (14 ng/ml) for 10 or 24 h. The analysis of calcium mobilization, after the addition of SDF-1α (0.01 mM), was performed as described in Materials and Methods.
IFNγ-Dependent Signaling in the Absence of Stat1 Requires the IFNγ Receptor and Jak1.
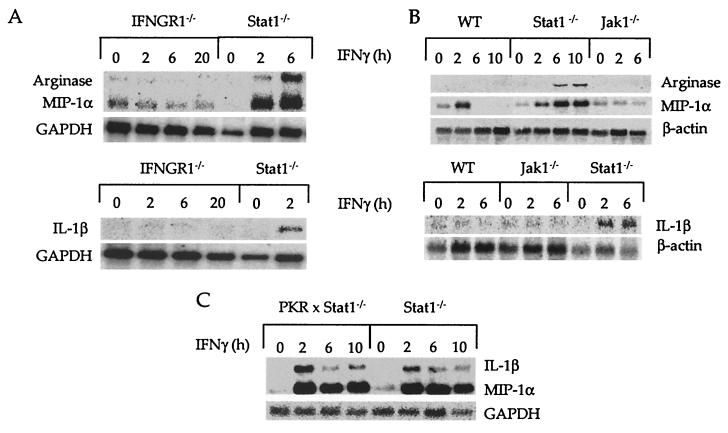
To identify other signaling components that participate in the induction of IFNγ-dependent responses in BMM that lack Stat1, we monitored expression of the MIP-1α, IL-1β, and Arginase genes in either IFNGR1−/− (Fig. 3A) or Jak1−/− BMM (Fig. 3B). No induction was seen in these cells. We also tested the same responses in BMM that lack PKR, a protein serine/threonine kinase that is induced and activated in IFN-treated cells, and that participates in the development of certain IFNγ-dependent cellular responses (24). However, no differences were observed between BMM that lack either Stat1 or the combination of Stat1 and PKR (Fig. 3C). These results show that the alternative IFNγ receptor signaling pathway requires the IFNγ receptor and Jak1 but not Stat1 or PKR. We also considered the possibility that, in the absence of Stat1, the activated IFNγ receptor might be able to recruit other Stat family members. As detected by electrophoretic mobility-shift assays (EMSA) using the M67 oligonucleotide probe, IFNγ induced activation of Stat3 in Stat1−/− BMM, but at a level that was considerably less than that observed in WT IFNγ-treated BMM (data not shown). Thus, there was not a compensatory activation of another Stat in the absence of Stat1.
Figure 3.
The IFNγ receptor and Jak1, but not PKR, are required for IFNγ-dependent regulation of Stat1-independent genes. Thirty million BMM derived from Stat1−/− mice (A–C), IFNγR−/− mice (A), Jak1−/− and WT mice (B), and PKR−/− × Stat1−/− (C) were incubated with or without IFNγ (14 ng/ml) for different periods of time and mRNA levels corresponding to the indicated genes were determined by Northern analyses.
Many Genes Are Regulated by IFNγ in Stat1−/− BMM.
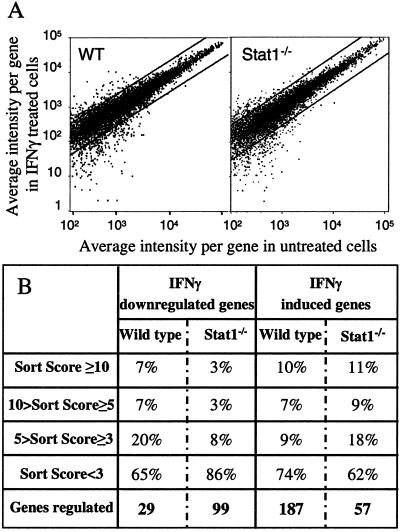
To assess more comprehensively the biological influence of the alternative IFNγ signaling pathway in macrophages, we conducted several gene profiling analyses on WT or Stat1−/− BMM after stimulation for 1 h with IFNγ, using the GeneChip system. When IFNγ-dependent changes in gene expression were compared between WT and Stat1-null BMM, a surprisingly similar pattern of regulated genes was observed (Fig. 4A). The data were analyzed by using stringent criteria that restricted the scored genes to those that showed average difference values equal to or greater than 100 (for the induced genes) and that also showed specific hybridization values (increase or decrease ratios) equal to or greater than 0.5. When using these parameters, IFNγ was found to regulate the expression of 216 genes in WT BMM (187 were increased and 29 decreased) and 150 genes in Stat1−/− BMM (51 were increased and 99 decreased) (Fig. 4B). The changes in gene expression were of similar magnitudes in the two cell types.
Figure 4.
Many genes are regulated by IFNγ in Stat1−/− macrophages. Biotin-labeled cRNA samples were prepared from Stat1−/− and WT BMM, unstimulated or stimulated for 60 min with IFNγ (14 ng/ml). After hybridization to an Affymetrix oligonucleotide array complimentary to more than 11,000 mouse genes and Expressed Sequence Tags (ESTs), and staining and scanning of the arrays, the data were analyzed with the Affymetrix software. This experiment was performed three times [twice with the 6,400 and once with the 11,000 (11k) GeneChips], using freshly prepared RNA for each experiment, with similar results. (A) Representation of the average differences (relative indicator of the level of expression of a transcript) for the untreated- and IFNγ-treated WT and Stat1−/− BMM. The plots contain only those genes that showed an average difference of greater than 100 compared with the untreated sample. Each graph represents the changes observed for ≈8,500 genes. (B) Comparison of IFNγ-dependent gene regulation in WT and Stat1−/− BMM, assessed by using the sort score, a parameter based on a combination of the relative change in mRNA expression levels and the intensity differences shown for a specific gene after IFNγ stimulation.
Table 1 lists a sampling of the known genes whose expression in WT and Stat1-null BMM changed by more than 4-fold in response to IFNγ. For WT cells, at least 13 of the 37 genes listed represent known IFNγ-regulated genes, including IRF-1, ICSBP, IP-10, and SOCS1 (JAB) (2, 24). These genes were not regulated by IFNγ in BMM that lack Stat1, a result that confirms the specificity of the GeneChip analyses. In contrast, genes such as MCP-1, pim-1, fibronectin, and SOCS3 were regulated by IFNγ in both Stat1-deficient and WT macrophages. In addition, many genes were identified that were regulated by IFNγ only in cells lacking Stat1, a result consistent with the representational difference analysis (RDA) experiments discussed above. Thus, IFNγ can regulate the expression of a large number of genes in the absence of Stat1.
Table 1.
Genes regulated by IFNγ in the presence or absence of Stat1
| Mice | Regulation | Accession no. | Description | Average difference change | Fold change | Sort score |
|---|---|---|---|---|---|---|
| Wild-type | Induced | ab000677 | JAB/SOCS1 | 7,811 | ≈123.4 | 82.21 |
| m63961 | IFNγ inducible protein (mag-1) | 1,993 | ≈32.2 | 27.14 | ||
| m35590 | Macrophage inflammatory protein 1-β | 1,957 | ≈31.7 | 26.66 | ||
| m19681 | MCP-1 (JE) | 6,532 | 22 | 41.18 | ||
| y07711 | zyxin | 1,357 | ≈21.9 | 17.8 | ||
| M34815 | Monokine induced by IFNγ (MIG) | 1,214 | ≈20.0 | 15.87 | ||
| m33266 | Interferon inducible protein 10 (IP-10) | 14,853 | 19.5 | 58.24 | ||
| U44731 | Purine nucleotide binding protein | 2,285 | 17.4 | 21.42 | ||
| U88328 | Sup. of cytokine signalling-3 (SOCS-3) | 13,190 | 12.8 | 42.23 | ||
| M21065 | Interferon regulatory factor 1 | 6,096 | 11.1 | 14.51 | ||
| M63630 | GTP binding protein (IRG-47) | 1,700 | 9.8 | 12.34 | ||
| U19119 | G-protein-like LRG-47 | 5,529 | 9.5 | 21.81 | ||
| L27990 | Ro protein | 1,928 | 9.4 | 12.75 | ||
| M31419 | 204 interferon-activatable protein | 763 | 9.4 | 7.98 | ||
| af022371 | Interferon-inducible protein 203 | 3,770 | 9 | 17.21 | ||
| U28404 | MIP-1 alpha receptor | 754 | 8.7 | 7.41 | ||
| U43085 | Glucocorticoid-attenuated response 39 | 423 | ≈7.6 | 4.74 | ||
| x56123 | Talin | 575 | 7.1 | 5.35 | ||
| m31419 | 204 interferon-activatable protein | 3,730 | 7 | 13.46 | ||
| U53219 | GTPase IGTP | 5,600 | 6.7 | 15.87 | ||
| l38444 | T-cell specific protein | 1,282 | 6.5 | 7.36 | ||
| M31418 | 202 interferon-activatable protein | 6,697 | 6.4 | 16.48 | ||
| d38417 | Arylhydrocarbon receptor | 1,295 | 5.6 | 6.21 | ||
| m26071 | Tissue factor (mtf) | 359 | 5.1 | 2.93 | ||
| D13759 | Cot proto-oncogene | 1,277 | 4.7 | 4.91 | ||
| M18194 | Fibronectin | 5,686 | 4.5 | 10.03 | ||
| u59463 | ICH-3 | 1,146 | 4.4 | 4.32 | ||
| M13945 | pim-1 proto-oncogene | 856 | 4.3 | 3.57 | ||
| L20450 | DNA-binding protein | 447 | 4.2 | 2.51 | ||
| Repressed | j04596 | Platelet-derived growth factor-ind. KC | −1,302 | ≈−21.4 | −17.7 | |
| v00727 | c-fos oncogene | −805 | ≈−13.4 | −10.47 | ||
| U29088 | RNA binding protein Mel-N1 | −518 | ≈−9.1 | −5.66 | ||
| m84145 | Fumarylacetoacetate hydrolase | −365 | ≈−6.7 | −3.85 | ||
| X61940 | Growth factor-inducible 3CH134 | −576 | −5.5 | −4.03 | ||
| X53798 | Macrophage inflammatory protein-2 | −284 | ≈−5.4 | −2.76 | ||
| X89749 | mTGIF | −591 | −4.4 | −3.07 | ||
| X99581 | CXCR4 | −2,872 | −4 | −5.93 | ||
| Stat1−/− | Induced | m19681 | MCP-1 (JE) | 910 | ≈19.6 | 13.1 |
| U44088 | TDAG51 | 530 | ≈11.9 | 7.45 | ||
| u67321 | Lice2 cysteine protease | 322 | ≈7.6 | 2.57 | ||
| AF020194 | Retinal taurine transporter | 1,905 | 5.6 | 7.51 | ||
| aa155321 | PRP8 | 848 | 5 | 4.34 | ||
| aa217487 | PIM-1 proto-oncogene | 520 | 5 | 3.43 | ||
| M18194 | Fibronectin (FN) | 2,299 | 4.1 | 5.5 | ||
| U88328 | SOCS-3 | 4,380 | 4 | 7.23 | ||
| Repressed | j04181 | A-X actin | −1,547 | ≈−32.7 | −23.94 | |
| AA033408 | XPE UV-damaged DNA binding factor | −973 | ≈−20.9 | −15.03 | ||
| D50523 | TI-227 | −427 | −9.1 | −5.81 | ||
| AA590859 | Melanoma X actin | −9,743 | −8 | −24.83 | ||
| U17252 | Metabotropic glutamate receptor 8 | −590 | ≈−5.1 | −0.8 | ||
| M32309 | Zfx, zinc finger protein | −323 | −4.5 | −2.37 | ||
| M26391 | Retinoblastoma susceptibility protein | −466 | −4.2 | −2.57 |
Shown are a sampling of IFNγ-regulated genes with fold changes greater than or equal to 4. ≈, The value for this gene at time 0 was below the background and thus the calculation of the fold change is approximate.
Demonstration of an IFN-Dependent, Stat1-Independent Antiviral Effect in Vivo.
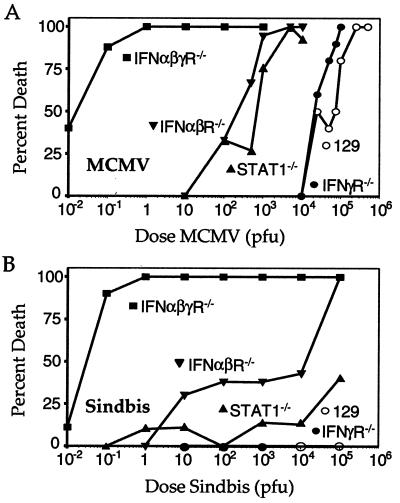
The results presented thus far confirm the presence of IFN-dependent, Stat1-independent signaling pathways that effect biologic responses in primary cells in vitro. To determine whether these signaling pathways have physiologic significance, we asked whether Stat1−/− mice were able to manifest an antiviral response in vivo. We reasoned that if all of the effects of the IFNs require Stat1 in vivo, then the resistance of Stat1−/− mice to viral infection should be similar to that of mice that lack both the IFNα/β receptor and the IFNγ receptor. As described (25), mice lacking only the IFNγ receptor were slightly more susceptible to infection with MCMV (a DNA virus) compared with their WT counterparts, whereas mice lacking the IFNα/β receptor were ≈100-fold more susceptible than WT mice (Fig. 5A). In contrast, mice lacking both receptors were ≈100,000-fold more susceptible to MCMV infection than WT mice. Surprisingly, mice lacking Stat1 displayed a susceptibility that was similar to mice lacking only the IFNα/β receptor and thus were much more resistant to MCMV infection than mice lacking both IFN receptors. This result shows that the IFNs have significant antiviral effects in the absence of Stat1.
Figure 5.
Antiviral responses in Stat1−/− mice. (A) Groups of sex- and age-matched 129/Sv/Ev (n = 58; ○), IFNγR−/− (n = 40; ●), IFNαβR−/− (n = 71; ▾), IFNαβγR−/− (n = 49; ■), or Stat1−/− (n = 79; ▴) mice were infected i.p. with between 0.01 and 5 × 105 plaque-forming units (pfu) of sgMCMV and followed for mortality for 21 days. (B) Groups of sex- and age-matched mice were infected with between 0.01 and 1 × 105 pfu of Sindbis virus s.c. and followed for mortality for 21 days. Data were derived from the following numbers of mice: 129/Sv/Ev (n = 10), IFNγR−/− (n = 38), IFNαβR−/− (n = 57), IFNαβγR−/− (n = 63), and Stat1−/− (n = 89).
To determine the generality of this finding, we performed a similar experiment with Sindbis, another IFN-sensitive virus that is genetically and biologically unrelated to MCMV (26). Similar to the findings with MCMV, mice lacking both IFNα/βR and IFNγR showed uniform lethality when infected with as little as 1 plaque-forming unit (pfu) of Sindbis virus, whereas no mortality was observed in WT mice infected with up to 100,000 pfu of virus. In contrast, mice lacking Stat1 were only slightly more susceptible than WT mice to death from Sindbis infection (Fig. 5B). Taken together, these in vivo findings show that the IFN-dependent, Stat1-independent signaling pathway plays a physiologically relevant role in mediating protective host responses against at least two viral pathogens.
Discussion
In this communication we show that, in primary cells, both the IFNα/β and IFNγ receptors can engage alternative signal transduction pathways in addition to the conventional Stat1-dependent signaling mechanism. Our results demonstrate that, in macrophages derived from Stat1-deficient mice, IFNγ, the major macrophage activating factor, can induce changes in the expression of a large number of genes by a process that requires the presence of the IFNγ receptor and Jak1. This observation was validated by studying altered expression of the proteins encoded by a representative group of the regulated genes. The alternative IFN-receptor signaling pathways have physiologic relevance because they regulate the in vitro proliferation and differentiation of bone marrow derived myeloid cells and contribute to antiviral responses in vivo. Our results also reveal that the alternative signaling pathways can be influenced by the presence or absence of Stat1. Some of the target genes of the alternative pathway are regulated equally by IFNγ in either WT or Stat1-deficient cells, defining the regulation of these genes as truly Stat1-independent. However, we show here that many genes are also regulated by IFNγ only when Stat1 is absent, a result that amplifies the previous observation that IFNγ can induce expression of the c-myc gene only in cells that lack Stat1 (8). It remains unclear whether this action is a result of a direct effect of Stat1 on the gene or through the ability of Stat1 to regulate expression of other transactivating factors.
Much of the data in this study were derived from three separate GeneChip analyses and an independent representational difference analysis (RDA) experiment. All support the same conclusions. Moreover, gene expression patterns were confirmed by using Northern analyses and by monitoring the expression of proteins encoded by a representative number of genes. It is of interest that many of the IFNγ-regulated, Stat1-independent genes encode immunologically important proteins such as chemokines or their receptors, suggesting that the alternative IFNγ receptor signaling pathway may play an important role in directing cellular migration. It is also noteworthy that the alternative pathway also regulates the SOCS3 gene. Thus, IFNγ induces expression of a JAK inhibitor in cells regardless of whether they can manifest Stat1-dependent responses. Because Jak1 is required for the alternative pathway and because SOCS3 inhibits the tyrosine kinase activity of the JAKs (27, 28), the alternative and classical IFNγ receptor signaling pathways may be subject to at least some of the same regulatory mechanisms.
A previous study using growth-dysregulated cells (i.e., mutagenized transformed human tumor cells and immortalized murine embryonic fibroblasts) was the first to suggest the presence of an alternative IFNγ receptor signaling pathway (8). This report showed that IFNγ inhibited c-myc expression in cells containing Stat1, but induced c-myc expression in cells that lacked Stat1. The current study extends the original observations in four distinct directions. First, we have demonstrated the physiologic relevance of this pathway by documenting its importance in mediating host resistance to virus infection in vivo. Second, we have demonstrated the existence of this pathway in nontransformed, primary cells. Third, we have identified a number of genes that are regulated by IFNγ in a manner that is truly Stat1 independent—i.e., that occurs equally in cells that contain or lack Stat1. Fourth, we have demonstrated the wide scope of this pathway by showing that a large number of genes are regulated by IFNγ in cells that lack Stat1.
The demonstration of alternative pathways of IFN receptor signaling raises two important issues for the future. The first is the identity of the transcription factors that mediate the regulation of IFNγ-dependent, Stat1-independent genes. One possibility that we considered was that, in the absence of Stat1, another Stat family member is recruited to and activated at the Stat1 docking site on the activated IFNγ receptor. Although Stat3 was indeed activated following IFNγ treatment of Stat1−/− BMM, the amount of activated Stat3 produced in the Stat1-deficient cells was much less than that produced in WT BMM. This observation indicates that a compensatory activation of another Stat does not occur when Stat1-deficient cells are stimulated with IFNγ. Moreover, the accompanying paper (29) shows that the alternative pathway does not require the Stat1 recruitment site on the IFNγ receptor. Although more work is needed to explore this possibility in greater detail, the results of these two companion studies unequivocally demonstrate that IFNγ does indeed induce Stat1-independent biologic responses in cells. The second is the definition of the physiologic situations where the alternative signaling pathways predominate. Clearly for those genes regulated in a Stat1-independent manner, this pathway operates whenever cells are exposed to IFNγ. The chemokines MIP-1α, MIP-1β, and MCP-1 and the chemokine receptor CXCR4 all fit into this category. The products of these genes promote biologically important functions and are known to be required for productive antiviral responses in vivo (30, 31). However, the physiologic situations in which IFN-dependent gene regulation occurs in the absence of Stat1 remain undefined. This situation will occur if Stat1 is depleted by chronic stimulation or inhibited during viral infection (32, 33). In fact, evidence exists that certain viruses have the capacity to block the activation of Stat1 and thus the presence of Stat1-independent antiviral responses provide the host with distinct survival advantages. Our work now focuses on further elucidating the mechanisms and physiologic consequences of the alternative IFN signaling pathways.
Acknowledgments
We thank Dr. Thomas Steinberg (Washington University School of Medicine, St. Louis) for his help with the calcium mobilization assays. We are grateful to Dr. Lesleyann Hawthorn (Lerner Research Institute, Cleveland) and Dr. Mark Watson (Washington University School of Medicine) for assistance with the GeneChip experiments. This study was supported by National Institutes of Health Grants CA43059 (to R.D.S.), AI39616 (to H.W.V.), CA62220 (to G.R.S.), and AI44157 (to B.L.).
Abbreviations
- BMM
bone-marrow-derived macrophages
- MCMV
murine cytomegalovirus
- WT
wild type
- M-CSF
macrophage colony stimulating factor
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- BMC
bone marrow cells
References
- 1.Farrar M A, Schreiber R D. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 2.Boehm U, Klamp T, Groot M, Howard J C. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 3.Stark G R, Kerr I M, Williams B R, Silverman R H, Schreiber R D. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 4.Darnell J E, Jr, Kerr I M, Stark G R. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 5.Bach E A, Aguet M, Schreiber R D. Annu Rev Immunol. 1997;15:563–591. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]
- 6.Meraz M A, White J M, Sheehan K C, Bach E A, Rodig S J, Dighe A S, Kaplan D H, Riley J K, Greenlund A C, Campbell D, et al. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- 7.Durbin J E, Hackenmiller R, Simon M C, Levy D E. Cell. 1996;84:443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- 8.Ramana C V, Grammatikakis N, Chernov M, Nguyen H, Goh K C, Williams B R, Stark G R. EMBO J. 2000;19:263–272. doi: 10.1093/emboj/19.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schreiber R D, Celada A. Lymphokines. 1985;11:87–118. [Google Scholar]
- 10.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 11.van den Broek M F, Muller U, Huang S, Aguet M, Zinkernagel R M. J Virol. 1995;69:4792–4796. doi: 10.1128/jvi.69.8.4792-4796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodig S J, Meraz M A, White J M, Lampe P A, Riley J K, Arthur C D, King K L, Sheehan K C, Yin L, Pennica D, et al. Cell. 1998;93:373–383. doi: 10.1016/s0092-8674(00)81166-6. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y L, Reis L F, Pavlovic J, Aguzzi A, Schafer R, Kumar A, Williams B R, Aguet M, Weissmann C. EMBO J. 1995;14:6095–6106. doi: 10.1002/j.1460-2075.1995.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Celada A, Gray P W, Rinderknecht E, Schreiber R D. J Exp Med. 1984;160:55–74. doi: 10.1084/jem.160.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouyang W, Jacobson N G, Bhattacharya D, Gorham J D, Fenoglio D, Sha W C, Murphy T L, Murphy K M. Proc Natl Acad Sci USA. 1999;96:3888–3893. doi: 10.1073/pnas.96.7.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tachibana K, Nakajima T, Sato A, Igarashi K, Shida H, Iizasa H, Yoshida N, Yoshie O, Kishimoto T, Nagasawa T. J Exp Med. 1997;185:1865–1870. doi: 10.1084/jem.185.10.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Virgilio F, Steinberg T H, Swanson J A, Silverstein S C. J Immunol. 1988;140:915–920. [PubMed] [Google Scholar]
- 18.Jorgensen N R, Geist S T, Civitelli R, Steinberg T H. J Cell Biol. 1997;139:497–506. doi: 10.1083/jcb.139.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollock J L, Virgin H W t. J Virol. 1995;69:1762–1768. doi: 10.1128/jvi.69.3.1762-1768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joe A K, Ferrari G, Jiang H H, Liang X H, Levine B. J Virol. 1996;70:7744–7751. doi: 10.1128/jvi.70.11.7744-7751.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyamoto M, Fujita T, Kimura Y, Maruyama M, Harada H, Sudo Y, Miyata T, Taniguchi T. Cell. 1988;54:903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- 22.Pine R, Decker T, Kessler D S, Levy D E, Darnell J E. Mol Cell Biol. 1990;10:2448–2457. doi: 10.1128/mcb.10.6.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar A, Yang Y L, Flati V, Der S, Kadereit S, Deb A, Haque J, Reis L, Weissmann C, Williams B R. EMBO J. 1997;16:406–416. doi: 10.1093/emboj/16.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Der S D, Zhou A, Williams B R, Silverman R H. Proc Natl Acad Sci USA. 1998;95:15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Presti R M, Pollock J L, Dal Canto A J, O'Guin A K, Virgin H W t. J Exp Med. 1998;188:577–588. doi: 10.1084/jem.188.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryman K D, Klimstra W B, Nguyen K B, Biron C A, Johnston R E. J Virol. 2000;74:3366–3378. doi: 10.1128/jvi.74.7.3366-3378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yasukawa H, Sasaki A, Yoshimura A. Annu Rev Immunol. 2000;18:143–164. doi: 10.1146/annurev.immunol.18.1.143. [DOI] [PubMed] [Google Scholar]
- 28.Stoiber D, Kovarik P, Cohney S, Johnston J A, Steinlein P, Decker T. J Immunol. 1999;163:2640–2647. [PubMed] [Google Scholar]
- 29.Ramana C V, Gil M P, Han Y, Ransohoff R H, Schreiber R D, Stark G R. Proc Natl Acad Sci USA. 2001;98:6674–6679. doi: 10.1073/pnas.111164198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sallusto F, Lanzavecchia A, Mackay C R. Immunol Today. 1998;19:568–574. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- 31.Kim C H, Broxmeyer H E. J Leukocyte Biol. 1999;65:6–15. doi: 10.1002/jlb.65.1.6. [DOI] [PubMed] [Google Scholar]
- 32.Heise M T, Connick M, Virgin H W. J Exp Med. 1998;187:1037–1046. doi: 10.1084/jem.187.7.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller D M, Rahill B M, Boss J M, Lairmore M D, Durbin J E, Waldman J W, Sedmak D D. J Exp Med. 1998;187:675–683. doi: 10.1084/jem.187.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]