Review on three IL-4-related branch points for immune regulation: alternative splicing of IL-4, differential receptor engagement, and differential regulation of macrophage activation by IL-4.
Keywords: alternative splicing, interelukin-4 receptor, interleukin-4 signaling, alternative macrophage activation
Abstract
Studies of IL-4 have revealed a wealth of information on the diverse roles of this cytokine in homeostatic regulation and disease pathogenesis. Recent data suggest that instead of simple linear regulatory pathways, IL-4 drives regulation that is full of alternatives. In addition to the well-known dichotomous regulation of Th cell differentiation by IL-4, this cytokine is engaged in several other alternative pathways. Its own production involves alternative mRNA splicing, yielding at least two functional isoforms: full-length IL-4, encoded by the IL-4 gene exons 1–4, and IL-4δ2, encoded by exons 1, 3, and 4. The functional effects of these two isoforms are in some ways similar but in other ways quite distinct. When binding to the surface of target cells, IL-4 may differentially engage two different types of receptors. By acting on macrophages, a cell type critically involved in inflammation, IL-4 induces the so-called alternative macrophage activation. In this review, recent advances in understanding these three IL-4-related branch points—alternative splicing of IL-4, differential receptor engagement by IL-4, and differential regulation of macrophage activation by IL-4—are summarized in light of their contributions to inflammation.
Introduction
IL-4, a multifunctional pleiotropic cytokine discovered in the mid-1980s, remains a focus of attention and continues to spur vigorous research efforts. This mediator is produced mainly by activated T cells but also by mast cells, basophils, and eosinophils [1]. A typical cytokine structurally, with molecular weight varying between 12 and 20 kDa as a result of variable natural glycosylation, IL-4 shares sequence homology, cell surface receptors, intracellular signaling, and partial functional effects on cells with IL-13 [2–7]. Functionally, IL-4 is best known for defining the so-called Th2 phenotype of lymphocytes and for regulating cell proliferation, apoptosis, and expression of numerous genes in various cell types, including lymphocytes, macrophages, and fibroblasts, as well as epithelial and endothelial cells [1–7].
Considering the already staggering and still rapidly growing volume of information available about IL-4, it is hardly possible to provide, in a journal article, a systematic review of this pivotal regulator alone, even without considering important relevant aspects, for example, recent advances concerning functionally related Th2 mediators, such as thymic stromal lymphopoietin [8–10], IL-33 [11], IL-21 [12], or IL-25 [13]. As the field of IL-4 research has expanded, it has become obvious that the initial broad-strokes picture of IL-4 function created in the 1980s and 1990s needs to be adjusted to reflect the numerous intricacies related to IL-4 generation, receptor use, and the effects on cells. Specifically, it has emerged that instead of linear scenarios, the field of IL-4 biology is full of alternative pathways. In addition to the well-appreciated—now described at the textbook level—role of IL-4 in determining the alternative fates of Th lymphocytes [14], particularly in promoting Th2 and inhibiting Th1 [15] and Th17 [16] differentiation, new knowledge about other IL-4-related, alternative branchings has emerged.
In this review, we focus on specific alternatives related to IL-4 production, receptor use, activation of a cell type (macrophages), and the relationships of these alternative branchings to the process of inflammation. These three areas have gained much attention in the past decade. Recent studies have shown that IL-4 is produced in several mRNA and protein variants arising as a result of alternative splicing. IL-4 can bind to alternative cell-surface receptors that each induces functionally different intracellular signaling. In addition to defining the alternative fate of lymphocytes, IL-4 drives the so-called “alternative macrophage activation”. The discussion of these IL-4-related alternative branch points below focuses on their contribution to the regulation of immune homeostasis and inflammation.
SPLICE VARIANTS OF IL-4
An important recent realization has been that a previously unappreciated level of complexity exists in the IL-4/IL-13 system, defined by the natural occurrence and functional activity of the splice variants of IL-4. The gene for IL-4 is composed of four exons [17, 18], and exon 2 is the shortest (48 bp, encoding 16 aa); exon 3 the longest. The full-length IL-4 protein, which is encoded by all four exons, is the variant that is commonly referred to as simply IL-4, and it is by far the most studied isoform, structurally and functionally. The first definitive demonstration of alternative exclusion of exon 2 from IL-4 mRNA in humans—by direct sequencing of the transcript—dates back to 1996 [19]. The resulting transcript and the corresponding protein isoform were dubbed IL-4δ2 [19–22]. Minimal information is available about other splice variants of IL-4 [23, 24], the discussion of which goes beyond the scope of this review article.
The IL-4δ2 mRNA occurs naturally in humans and also in chimpanzees, cynomolgus macaques, rabbits, and woodchucks [25] and in cattle [26]. In mice, this splice variant is present in minute amounts, detectable only in the spleen and bone marrow [27]; nevertheless, the IL-4δ2 protein is fully functional in mice [28, 29]. That IL-4δ2 is conserved across numerous species suggests an important yet still poorly understood functional role. The IL-4δ2 mRNA is expressed by all cell types that express full-length IL-4, and both mRNA variants are abundant in various tissues [19, 20, 30–35]. The steady-state levels of IL-4δ2 mRNA are usually but not always below those of full-length IL-4 mRNA [36–38]. How the relative levels of these two alternatively spliced mRNAs are regulated is unknown, but the correlation between their steady-state levels is remarkably strong, as found in several independent studies [36–38], suggesting tightly controlled regulation.
One of the main challenges has been to show definitively the existence of not only the IL-4δ2 mRNA but also the corresponding IL-4δ2 protein. Overall, the IL-4δ2 isoform is difficult to assess, as it is not detected or not distinguished from full-length IL-4 by currently available commercial ELISA assays or even by the probes included in Affymetrix or Illumina microarray kits (some of which target IL-4δ2 rather than full-length IL-4), leading to grossly misleading data. The natural production of IL-4δ2 protein has been particularly difficult to assess as a result of the initial unavailability of an antibody that would recognize IL-4δ2 without cross-reacting with full-length IL-4. These two splice isoforms are 100% homologous and differ only in the 16 aa that are absent in the IL-4δ2 isoform. Therefore, commercial anti-IL-4 antibodies react with both isoforms indiscriminately or recognize only full-length IL-4. Only recently has an antibody been developed that reacts selectively with IL-4δ2 protein without cross-reactivity to full-length IL-4. Combined with a commercial antibody that reacts selectively with full-length IL-4 without cross-reactivity to IL-4δ2, this has become a valuable tool, allowing for a clear demonstration of natural IL-4δ2 protein production [38]. In contrast to mRNA expression, IL-4δ2 protein is not produced by a wide variety of cells, and at the moment, the complete set of cell types responsible for IL-4δ2 protein production remains to be identified. It is clear that activated T cells produce IL-4δ2 protein, but neither Th1 nor Th17 cells do so, whereas Th2 cells produce only minute amounts [38].
Association of IL-4δ2 with disease
The levels of IL-4δ2 mRNA have been measured relative to full-length IL-4 mRNA in a variety of diseases, such as Fasciola hepatica infection [26], Helicobacter pylori infection [31, 32], and sepsis [39], with differences noted between patients and healthy controls. However, the majority of the studies associating IL-4δ2 with disease has been on scleroderma, asthma, and TB, as discussed below. To date, the expression of IL-4δ2 protein has been assessed in comparison with the full-length IL-4 isoform only in asthma [38].
Scleroderma.
The first association between IL-4δ2 mRNA and human pathology was established in patients with systemic sclerosis, or scleroderma, particularly those with pulmonary involvement, which is the most serious complication of this disease [40–42]. This finding allowed a controversy about the role of Th2 cytokines in scleroderma to be resolved. On the one hand, Th2 cytokines, which are directly profibrotic, have been hypothesized to propel immune inflammation combined with tissue fibrosis—the hallmark of scleroderma. On the other hand, unlike patients with asthma, whose disease is clearly Th2-driven, patients with scleroderma do not routinely exhibit overt eosinophilia or goblet cell hyperplasia, which are key features of IL-4-induced immune inflammation. We hypothesized that the short isoform (IL-4δ2) may not be a typical Th2 cytokine. Indeed, the in vivo studies discussed below have demonstrated that full-length IL-4 and IL-4δ2 induce immune inflammation, with the notable difference that full-length IL-4 changes the cytokine milieu and cellular composition toward the Th2 pattern, whereas IL-4δ2 does not have such effects [28]. Combined, these data suggest that IL-4δ2, whose levels are increased in scleroderma and which promotes immune inflammation without Th2 skewing, may be an important contributor to the disease pathogenesis.
Asthma.
Several studies compared the expression levels of full-length IL-4 and IL-4δ2 mRNAs in patients with asthma and healthy controls, with somewhat contradictory results [35–38]. A recent report [38] has added clarity to this issue. Various levels of IL-4δ2 and full-length IL-4 mRNAs were observed in PBMCs and in purified peripheral blood T cells from asthma patients and healthy controls, but there was no association with disease or its severity, therapies, levels of IgE, or clinical status at the time of blood draw. The possibility was then considered that the differences may be elucidated by stimulation of T cells, but there was again significant variability among and no difference between patients with asthma and healthy controls in the expression of IL-4δ2 and full-length IL-4 mRNAs in response to stimulation. These observations prompted further investigation of the possibility that a contribution from post-transcriptional regulation could make the levels of full-length IL-4 and IL-4δ2 proteins more reliable indicators of asthma than the corresponding mRNAs. This was the first study in which the existence of IL-4δ2 protein was demonstrated definitively in relation to a disease. This has become possible as a result of a newly developed antibody that reacts very selectively with IL-4δ2 and not full-length IL-4. Following activation with PMA/ionomycin, T lymphocytes from patients with asthma but not from healthy controls secreted IL-4δ2 into the culture supernatant in a time-dependent fashion. Of note, levels of full-length IL-4 tended to peak after 12–24 h of cell stimulation, declined by 48 h, and declined further at 72 h. In contrast, levels of IL-4δ2 became detectable only at 12–24 h, increased at 48 h, and increased further at 72 and 96 h. This study thus demonstrated that IL-4δ2 is naturally produced as a protein, particularly in T cells from patients with asthma but not in T cells from healthy individuals; that the kinetics of IL-4δ2 production are different from that of full-length IL-4; and that measuring both proteins may provide more valuable information than measuring their corresponding mRNAs [38].
Tuberculosis.
IL-4δ2 mRNA expression was elevated in blood and in BAL cells from TB patients compared with controls [43–46], although in TB patients coinfected with HIV, an elevated IL-4δ2 mRNA level was found exclusively in lavage, where IL-4δ2 was the dominant variant [45]. Interestingly, the increased IL-4δ2 mRNA level was seen in T cells and non-T cells [46] but more strikingly in the latter, echoing the recent finding that T cells might not be the major source of IL-4δ2 [38]. During treatment, the levels of IL-4δ2 rose significantly, whereas the levels of full-length IL-4 tended to fall [46]. This observation was confirmed emphatically when cells from TB patients were tested before treatment and after 1 week of standard anti-TB chemotherapy. Only two cytokines showed changed levels of expression during this period: mRNA encoding full-length IL-4 showed a fourfold decrease, whereas in sharp contrast, IL-4δ2 mRNA showed a striking 32-fold increase [47]. There were no significant changes in the expression of TGF-β, TGF-βRII, FoxP3, IFN-γ, or GATA-3 over the same 1-week period. An important point here is that when the same authors used a RT-PCR protocol that measured both IL-4 cytokines together, the changes canceled one another out, and no significant changes in expression were noted [47]. This situation also applies to most of the TB literature, which needs to be revised and reinterpreted with appropriate methods that distinguish between full-length IL-4 and IL-4δ2.
It remains unclear whether the observed changes reflect the rapidly changing antigen loads or represent irrelevant bystander effects. However, several studies of individuals with latent TB suggest that IL-4δ2 might be important. Unstimulated peripheral blood cells from healthy Ethiopian individuals who had contact with TB, with long-established latent infection that had not progressed to clinical disease, had increased levels of mRNA encoding IL-4δ2 compared with TB patients and with individuals without latent infection [48]. Similar observations were reported from Gambia [49] and Mexico [50]. These findings suggest that high IL-4δ2 mRNA levels could be a correlate of immune protection. This view was supported by a further study of very recent contacts, some of who developed active disease. The risk that such contacts will develop progressive TB is greatest if their in vitro responses to the TB antigen ESAT-6 are strong. Interestingly, a strong response to ESAT-6 correlated with elevated expression of full-length IL-4 and reduced expression of IL-4δ2, suggesting the possibility that in these at-risk individuals, immunity is skewed away from a protective Th1 response and that the IL-4δ2/full-length IL-4 ratio is a potential biomarker of this effect [51].
Functional effects of IL-4δ2
To assess the possible mechanistic contribution of IL-4δ2 protein to regulation of inflammation, direct studies with recombinant protein in cell culture [19–21, 38, 40] and with IL-4δ2 gene delivery in vivo [28, 29] have been performed. In cell culture, rIL-4δ2 had no independent effects on T cell proliferation [20] or expression of IFN-γ, IL-1, IL-6, IL-8, MCP-1, CD14, CD23, CD16, or TLR2 in primary human monocytes. However, IL-4δ2 inhibited full-length IL-4-induced T cell proliferation [20], attenuated production of full-length IL-4 by polarized Th2 cells [38], blocked inhibitory action of full-length IL-4 on LPS-induced COX-2 expression and subsequent PGE2 secretion in monocytes, and antagonized the full-length IL-4-induced synthesis of IgE and expression of CD23 in B cells [21]. Furthermore, secretion of IL-4δ2 from activated T cells occurs later when the levels of full-length IL-4 decline [38]. It is therefore possible that the switch from full-length IL-4 to IL-4δ2 production represents the early stages of a regulatory mechanism in which Th2 activity is inhibited by IL-4δ2 at the end of a physiological Th2 immune reaction. The purpose of such regulation would be to prevent an unnecessarily prolonged or exaggerated Th2 response, thus making IL-4δ2 functionally a Th1 cytokine [38].
Additional studies revealed that there is more to IL-4δ2 function than mere neutralization of full-length IL-4 production and function. In cultured primary human T lymphocytes, IL-4δ2 activated production of IFN-γ, IL-6, IL-10, MCP-1, and TNF-α [38]. The stimulating effect on the production of IFN-γ was the most consistent, in agreement with the notion already mentioned that IL-4δ2 is a Th1 and not a Th2 cytokine. Moreover, studies in vivo in a full-length IL-4 and IL-4δ2 gene delivery model [28, 29] have shown that IL-4δ2 is independently active in causing immune inflammation; its effects are different from those of full-length IL-4, and it is not an IL-4 antagonist, whereas the combined effect of these two isoforms is additive of their independent effects. Expression of IL-4δ2 in vivo caused accumulation of T and B lymphocytes and a tendency to collagen accumulation similar to the effects of full-length IL-4, but these effects of delivered IL-4δ2 were independent of endogenous full-length IL-4 [28, 29]. Unlike full-length IL-4, IL-4δ2 did not induce pulmonary eosinophilia or goblet cell hyperplasia [28]. Also, delivery of IL-4δ2 altered the gene-expression pattern and the cytokine milieu differentially from that of full-length IL-4, with a notable IL-4δ2-stimulated increase in IFN-γ, further strengthening the notion that IL-4δ2 is a Th1 rather than a Th2 cytokine [28]. The mechanistic explanations for these partial similarities and differences in functional effects between full-length IL-4 and IL-4δ2 in vivo are yet to be elucidated, but some evidence for receptor sharing between these two isoforms is discussed below. Thus, IL-4δ2 is an independent regulator of lymphocytic infiltration; its effects are different from and independent of the effects of full-length IL-4; and instead of viewing IL-4δ2 as a potential anti-full-length IL-4 therapeutic agent, differential therapeutic targeting of the two isoforms should be considered in the treatment of various diseases.
IL-4R STRUCTURE AND SIGNALING PATHWAYS
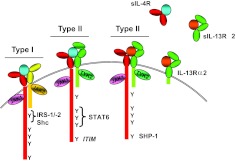
There are two different types of IL-4R and IL-13R complexes, termed type I and II receptors (Fig. 1). IL-4 first binds to the IL-4Rα chain with picomolar affinity, leading to the recruitment of the IL-2Rγ γc chain to form the type I receptor complex or alternatively, the IL-13Rα1 to form the type II receptor complex. Thus, IL-4 uses both types of receptors. On the other hand, IL-13 first binds to the IL-13Rα1 chain with nanomolar affinity, leading to the recruitment of the IL-4Rα chain. Thus, IL-13 is not able to engage the type I receptor, and its affinity for the type II receptor complex is limited. Once the cytokines bind to their respective receptors, they induce a cascade of signaling events, as described below. Another chain, termed IL-13Rα2, binds IL-13 with high (picomolar) affinity and is thought to act predominantly as a decoy receptor.
Figure 1. Alternative use of receptor complexes by IL-4 and IL-13.
The IL-4R is composed of two transmembrane proteins. IL-4Rα chain (red) binds IL-4 (blue) with high affinity, leading to dimerization with γc (yellow) or IL-13Rα1 (green), forming the type I or II receptor complex, respectively. IL-13 (orange) binds to IL-13Rα1 with lower affinity, followed by heterodimerization with IL-4Rα to form a high-affinity complex. IL-13 also binds to IL-13Rα2 at the cell surface or in soluble form, but this interaction fails to activate the JAK/STAT pathway, and generally, it is thought to be inhibitory. Soluble (s)IL-4Rα, sIL-13Rα1, and IL-13Rα2 exist and can also bind ligand. Following ligand binding and heterodimerization, JAKs are activated. Tyrosine residues in the cytoplasmic tail of the IL-4Rα become phosphorylated and act as docking sites for signaling molecules. The first cytoplasmic tyrosine residue (Y1) is in a sequence motif called the I4R motif, which interacts with the PTB domains of IRS1 or IRS2. Tyrosine residues 2–4 in the IL-4Rα interact with the SH2 domain of STAT6. The fifth cytoplasmic Y in the IL-4Rα lies in a consensus motif, termed an ITIM that can recruit SHP-1 to the activated receptor complex.
Expression pattern and structure of the IL-4R and IL-13R chains
High-affinity IL-4-binding sites were first characterized on cells of the hematopoietic lineage [52, 53]. These receptors were expressed at varying levels in primary cells and cell lines of B cell, T cell, mast cell, myeloid, and monocytic lineages. Whereas resting primary B and T cells and thymocytes expressed very few IL-4Rs (20–300/cell), IL-4 was able to exert a potent biologic effect with only a few molecules bound to the cell surface. Lymphocyte activation with LPS or Con A increased the number of IL-4-binding sites by five- to tenfold [52, 53]. Furthermore, cell lines derived from monocyte/macrophage and mast cell lineages expressed between 1000 and 4000 receptors/cell. Subsequently, Lowenthal and colleagues [54] showed that IL-4Rs were also expressed by several nonhematopoietic cells, including epithelial, endothelial, and fibroblast cells.
Most cells express the type I or II receptor complex, whereas some express both. The two subunits of the type I IL-4R complex, IL-4Rα and γc, were identified first [55, 56]. Although IL-4Rα was widely expressed, it was found that γc had a more restricted expression pattern. Most cells of the hematopoietic lineage express the γc chain [57, 58], whereas only very low levels of γc mRNA were found in the heart, skeletal muscle, and kidney [58]. The γc chain recognizes IL-4 complexed to the IL-4Rα chain and forms a heterodimeric receptor complex [59]. Association of γc with IL-4Rα leads to only a modest increase (2.5- to threefold) in the binding affinity for IL-4 but is important for activation of downstream signaling [55]. As a result of the limited tissue distribution of the γc subunit, expression of the type I receptor is limited to hematopoietic cells (T and B cells, macrophages, NK cells, eosinophils, basophils, and mast cells) and can bind only IL-4. However, it is now known that cellular stress, viral infection, and proinflammatory cytokines, such as IFN-γ and TNF-α, can induce expression of γc mRNA and protein expression [60]. In addition, some human bronchial epithelial cell lines express this receptor chain [61, 62].
The ability of IL-4 to act on cells lacking γc suggested that other receptors can bind this cytokine. Obiri et al. [63] demonstrated that IL-4 and IL-13 bind to a second receptor complex, which did not use γc. They also found that T cells contained negligible levels of IL-13R, and monocytes and human B cells expressed low levels of this receptor (100–300 receptors/cell). However, renal carcinoma cell lines expressed high levels of this receptor (5000–15,000 receptors/cell) [63]. It is now known that in addition to T cells, NK cells, basophils, mast cells, and most mouse B cells express little or no IL-13Rα1. Hilton et al. [64] then showed that IL-13 was able to bind to the IL-13Rα1 chain with low affinity, whereas IL-13Rα1 could associate with IL-4Rα to form a second receptor complex in mouse and in human cells [64, 65]. Thus, the type II receptor is expressed on nonhematopoietic cells, such as epithelial and smooth muscle cells, and on monocytes, macrophages, and human B cells. The type II receptor, unlike the type I receptor, is responsive to IL-4 and IL-13.
IL-13 has also been reported to interact with high affinity with another cell surface receptor, IL-13Rα2, which contains a very short intracellular domain [66, 67]. IL-13Rα2 mRNA expression was initially found in murine brain and spleen tissues, as well as human brain, liver, lung, and thymus [66]. A secreted form of this receptor also exists, which can be detected in the serum [68]. The functional significance of this receptor chain is controversial. Several studies suggest that it acts as a decoy receptor for IL-13 and reduces IL-13-induced STAT6 activation [67, 69]. Another group demonstrated IL-13 signaling, although the IL-13Rα2 chain induced TGF-β production in macrophages in the presence of TNF-α [70]. Furthermore, blockade of IL-13Rα2 in vivo led to reduced TGF-β production and collagen deposition in bleomycin-induced lung fibrosis.
Signal transduction through IL-4R and IL-13R
The IL-4R complexes lack intrinsic kinase activity, but their cytoplasmic tails are each associated with a member of the JAK family (JAK1, JAK2, JAK3, and TYK2) [71], as well as Fes [72] and Syk [73] (Fig. 2). Ligand-induced dimerization of the receptor complex activates the JAKs, resulting in auto [74–76]- and transphosphorylation [77–79] of the JAKs, as well as phosphorylation of target tyrosine residues in the cytoplasmic domains of both receptor chains [80–83]. The cytoplasmic domains of all three receptor subunits contain tyrosine residues, which when phosphorylated, act as docking sites for downstream adaptor and signaling molecules. The IL-4Rα cytoplasmic domain contains five key tyrosine residues, Y1–Y5, which have been well-defined in terms of binding to downstream signaling molecules and outcomes on cellular functions [84–88]. Y1 is at the core of an insulin-IL-4R motif (PLxxxxNPxYxSxSD) bound by the protein PTB domains of IRS proteins, Dok-1 or Dok-2 and Shc. Y2–Y4 are bound by SH2 domain-containing proteins, such as STAT6 [88]. Y5 is part of an inhibitory tyrosine motif, bound by SH2 domains of SHP-1, SHP-2, and SHIP-1, depending on the cell type.
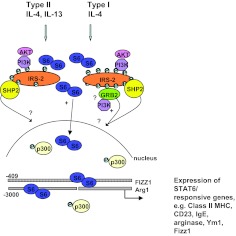
Figure 2. Intracellular signaling pathways activated by IL-4 and IL-13.
IL-4 and IL-13 induce the tyrosine phosphorylation of STAT6. Activation of the type I receptor by IL-4 and the type II by IL-4 or IL-13 will induce the tyrosine phosphorylation of IRS2 with different efficiencies. The Grb2 site is restricted to the type I receptor. Activation of pathways downstream of IRS2, especially the PI3K pathway, have been shown to be critical for the STAT6-dependent transcription of arginase-1 and FIZZ1. Activation of STAT6-dependent genes is dependent on the recruitment of CREB-binding protein/p300 to the transcriptional complex. The mechanism by which the PI3K pathway modulates transcription of these genes is unclear.
One of the major phosphoprotein species activated by IL-4 stimulation is IRS-2 (generally hematopoietically expressed) or IRS-1 (generally nonhematopoietically expressed), depending on the cell type and cellular expression level of each protein. IRS-2 is a large (∼180 kDa) cytoplasmic adapter protein, comprising pleckstrin homology, PTB, Shc, and NPXY binding domains at the N-terminus, with multiple potential tyrosine (≥20; ref. [89]) and serine and threonine (≥70; ref. [90]) phosphorylation sites at the C-terminus. Following docking of the IRS-2 PTB domain with the I4R motif of IL-4Rα, IRS-2 becomes heavily tyrosine-phosphorylated, presumably by virtue of its proximity to the IL-4R-associated JAKs. Our data indicated that γc (i.e., a functional type I receptor) is required for robust tyrosine phosphorylation of IRS-2, and the type II receptor does not activate the IRS-2 pathway efficiently [91]. The pY residues of IRS-2 can serve as initiators of a variety of signaling pathways, such as Sos/Ras/GAP, PI3K/Akt, PKB/mammalian target of rapamycin/p70S6K, or PKC/p70S6K, by recruiting SH2 domain-containing molecules, including p85α, Grb2, and SHP-2. The ability to recruit a variety of signaling molecules allows the IRS proteins to stimulate mitogenesis, promote cell survival [92], and regulate gene expression, reproduction [93], and energy homeostasis/insulin responses [94]. In general, IL-4/IL-13 signaling does not activate Ras/MAPK pathways (with some exceptions), despite the interaction of Grb2 with IRS-2 [91, 95].
IL-4R engagement predominantly activates tyrosine phosphorylation of STAT6, but other members (STAT3, STAT5, and STAT1) can also be activated to a lesser degree. STAT6 forms homodimers through pY641–SH2 interactions [96, 97] and translocates to the nucleus to activate transcription by binding a dyad-symmetric recognition element, TTC-GAA, separated by four nucleotides (N4; ref. [98]). STAT6 often forms cooperative interactions with other transcription factors and coactivators, such as NF-κB or p300/CEBP. Other post-translational modifications of STAT6 that impact its transcriptional function include acetylation [99], methylation [100], and serine phosphorylation [101–104]. The STAT6 pathway plays a central role in gene regulation and allergic responses regulated by IL-4 or IL-13, including T cell proliferation and survival and Th2 differentiation [105].
The SH2 domain of the protein tyrosine phosphatases SHP-1 and -2 bind to the putative ITIM sequence [I/V/L]xY(p)xx[I/V/L] surrounding the fifth tyrosine, Y713, in activated receptors [106]. SHIP can also be recruited by Y713 [107]. SHP-2 is widely expressed, whereas SHP-1 is hematopoietically restricted. Tyrosine phosphorylation of SHP-1 increases its phosphatase activity [108, 109], whereas phosphorylation of SHP-2 creates binding sites for SH2 domain-containing proteins (IRS-1, p85, and Grb2, [110]). Our group demonstrated that SHP-1 downregulates STAT6 signaling initiated by the Type I receptor complex [111]. SHIP-1 hydrolyzes phosphatidylinositol (3,4,5)-trisphosphate, the product of PI-3K activity, to down-regulate signaling.
Down-regulation of IL-4/IL-13-induced signaling is also accomplished by the rapid induction of SOCS proteins [112]. In particular, SOCS-1 and -3 inhibit IL-4 signaling [113, 114]. SOCS proteins associate with their targets through SH2 domain–pY interactions. They can inhibit JAK activity directly via their kinase inhibitory region [115], bind to IL-4Rα and interfere with the binding of downstream signaling proteins to the receptor [116], and mediate degradation of the JAKs and signaling proteins [117, 118].
Receptor use by and cross-species activities of IL-4δ2
The exact receptor identity for and the details of receptor binding by IL-4δ2 are unknown. IL-4δ2 was found to bind specifically to human PBMCs and to tumor lines that are known to express IL-4R and IL-13R [20]. Unlabeled full-length IL-4 inhibited cellular binding of labeled IL-4δ2, suggesting that IL-4δ2 may bind to the same receptors as full-length IL-4[20]. In full-length IL-4, the second exon encodes the main part of the long loop connecting α-helices A and B, as determined by three-dimensional structural superposition [119]. Molecular modeling suggested that in the absence of the second exon-encoded part of the protein (as in IL-4δ2), the left-handed, four-helix bundle structure of full-length IL-4 with the up-up-down-down structural pattern is transformed into a down-up-down-down structural pattern [22]. Such changes would likely affect binding of IL-4δ2 to the cell surface receptors. Indeed, the earlier results from binding studies suggested that IL-4δ2 has lower affinity than full-length IL-4 for their common receptor(s) [20]. Significantly lower levels of full-length IL-4 than IL-4δ2 were required to inhibit cellular binding of radiolabeled IL-4δ2. Nonetheless, the affinity of IL-4δ2 for its receptors was adequate to inhibit the full-length IL-4-induced effects in lymphocytes and monocytes [20, 21]. These findings were later confirmed in a separate study, which showed that both splice variants bound to the cell surface, partially in an IL-4Rα-dependent manner, although the affinity of full-length IL-4 for the IL-4Rα chain appeared to be higher than that of IL-4δ2, based on their differential competition with an M1 antibody for binding to the receptor chain [28]. The M1 antibody is specific for IL-4Rα and is known to compete with full-length IL-4 for binding to this receptor chain. Preincubation with full-length IL-4 but not with IL-4δ2 attenuates binding of the antibody to the cell surface [28]. The in vivo experiments in IL-4Rα-deficient mice, in comparison with WT animals, have confirmed that the functional effects of IL-4δ2 strongly but not completely depend on this receptor chain [28]. Further studies are needed to fully elucidate the spectrum of cell surface receptors for IL-4δ2 and the molecular details of receptor binding by this isoform.
Very little is known about intracellular signaling induced by IL-4δ2, which is mainly a result of the fact that in cell culture, IL-4δ2 is functionally passive in comparison with full-length IL-4 and only exerts mild effects on cytokine production [38]. Therefore, it is difficult to associate mechanistic observations on intracellular signaling with IL-4δ2 function in cell culture. There are greater opportunities for studying IL-4δ2-induced signaling in vivo, where this isoform is fully functional [28]. The in vivo studies in STAT6-deficient mice, in comparison with WT animals, have shown that the effects of IL-4δ2 are strongly but not completely STAT6-dependent [28]. Major further effort is needed to explain IL-4δ2-driven inflammation in vivo mechanistically [28].
Interesting insight into receptor engagement by full-length IL-4 and IL-4δ2 can be gained from studies of the cross-species activities of these isoforms. In cell culture, the functional activity of full-length IL-4 appears to be species-specific [120], although structural analysis suggests that the two key residues of human full-length IL-4 that define its binding to the IL-4Rα, namely Glu-9 and Arg-88, as well as the minor determinants, Arg-53 and Arg-85, are all present in mouse full-length IL-4 [121]. Direct experiments in the BIAcore system have revealed that normally glycosylated mouse full-length IL-4 directly binds to human IL-4Rα [121]. Moreover, several previous reports have shown that human full-length IL-4 is active in mouse or rat in vivo in a fashion similar to that of rodent full-length IL-4 [122, 123]. We found that human full-length IL-4 is active in mice in vivo [29], but this activity is only partially similar to that of mouse full-length IL-4 [28]. Specifically, human or mouse full-length IL-4 induced pulmonary lymphocytosis, whereas only mouse full-length IL-4 induced eosinophilia and goblet cell hyperplasia [28, 29]. The similarities in the effects of human and mouse IL-4δ2 were greater, with each separately inducing pulmonary lymphocytosis and neither inducing eosinophilia or goblet cell hyperplasia [28, 29]. The effects of IL-4δ2 from both species on the pulmonary cytokine milieu in mouse lungs was also similar, with notable induction of TNF-α and IFN-γ [28, 29]. It appears that IL-4δ2 from both species acts on mouse lungs in vivo in a similar way as the effects of human full-length IL-4 and more distinctly from the effects of mouse full-length IL-4, but the molecular reasons for such similarities and differences remain to be understood. The exact reasons for the discrepancy between, on the one hand, a lack of cross-species, full-length IL-4 activity in cell culture and on the other hand, significant cross-species activity in vivo also remain unclear. However, it is reasonable to expect that further comparative investigation of within- and cross-species activities of full-length IL-4 and IL-4δ2 will shed light on specific molecular details of receptor engagement by these two splice variants.
REGULATION OF ALTERNATIVE MACROPHAGE ACTIVATION
The seminal observation that full-length IL-4 induces the expression of the mannose receptor (CD206) on macrophages [124] gave way to the concept of M1 and M2 macrophage activation states coinciding with the polarized Th1 and Th2 cytokine profiles. The M1 and M2 designations now coexist with these phenotypes described as classically or alternatively activated and targeting bacterial or helminthic pathogens, respectively. IL-4 and IL-13 are the archetypal inducers of M2, but it remains completely unknown what effects IL-4δ2 may have on alternative macrophage activation or macrophages that have already been alternatively activated in cell culture or in vivo.
M1 macrophages are a source of proinflammatory cytokines, such as TNF-α, IL-1β, and IL-12. There is less information about M2 production of cytokines, but evidence suggests that macrophages can produce full-length IL-4 and IL-4δ2 [30, 33], and specifically, M2 can generate full-length IL-4 and IL-13 in response to viruses in vitro [125–128]. A subset of M2 produces a chemokine, CCL18, also referred to as pulmonary and activation-regulated chemokine (PARC), dendritic cell-derived chemokine-1 (DC-CK1), or alternative activated macrophage associated CC-Chemokine 1 (AMAC1), explicitly indicating the source cell for this factor [129]. This chemokine attracts mostly T lymphocytes but not monocytes or granulocytes [130–133], and the resulting T lymphocytic infiltration has been associated with a variety of fibrotic inflammatory diseases, especially of the lungs, such as scleroderma [40, 131–139], hypersensitivity pneumonitis and idiopathic pulmonary fibrosis [140, 141], asthma [129, 142], and lung sarcoidosis [141, 143]. The example of CCL18 alone shows the multidimensional nature of the IL-4 effects on inflammation and its severe complication, fibrosis. IL-4 can cause inflammation and fibrosis independently or in combination with other processes that potentiate its effects, for example, CD40 ligation, by acting simultaneously on inflammatory and stromal cells [144]. IL-4 also drives M2 activation, and CCL18 production by M2 induces lymphocytic inflammation, leading to tissue fibrosis through direct CCL18 effects on fibroblasts [134, 139] or through indirect T cell-dependent mechanisms [131–133]. Thus, T cells are not only the main source of IL-4 that drives M2 activation but also responders to IL-4 and an M2 product, CCL18, making this a complex web of interplaying cells, cytokines and chemokines, and cell surface molecules driving inflammation and fibrosis [28, 131–133, 136, 137, 144, 145].
M2 macrophages are reportedly more heterogeneous than M1 and can be divided further into M2a, M2b, M2c, and M2d subsets based on the mechanism of induction (reviewed in ref. [146]). M2a is activated by IL-4/IL-13 and drives classic Th2 immune responses; M2b is activated by immune complexes and by TLR/IL-1R; M2c is activated by IL-10, has immunosuppressive functions, and is important in tissue remodeling; M2d macrophages accumulate in the tumor microenvironment. The M2 macrophages, therefore, are a group of highly specialized cells with a signature gene expression (reviewed in ref. [147]), an inventory of immune mediators, and functions that include tissue repair, clearance of parasites, and regulation of inflammation.
Infections that polarize cytokine profiles have provided a wealth of information on the function of macrophage phenotypes. M2 macrophages play a key role in the type 2 immunity needed for clearance of helminths that preferentially colonize the small intestine [148, 149] but may not have the same importance for helminths that infect the colon [150]. The induction of M2 in schistosomiasis may serve to prevent the development of severe inflammation in the acute phase of infection at the expense of M2-driven fibrotic liver diseases in the chronic phase of infection [151]. Pathogens may circumvent host defenses by the inappropriate induction of M2 to promote survival. M1 macrophages are critical to host defense against intracellular bacteria, yet Francisella tularensis infection induces M2, allowing bacterial replication but resulting in death of the host [127]. Both Mycobacterium tuberculosis and Toxoplasma gondii up-regulate arginase-1 in macrophages to induce the M2 macrophages [152] through a STAT6-independent, TLR-activated mechanism to circumnavigate the M1-protective immune response mediated by NO production by iNOS-2. Recent studies also show that T. gondii virulence may be linked to parasite-derived kinase Toxoplasma rhoptry protein 16-mediated activation of STAT3, the transcription factor for the immunoregulatory cytokine IL-10, to promote development of M2 macrophages [153, 154].
M2 is distinguished from M1 by the presence of surface markers, such as the mannose receptor (CD206) and up-regulation of macrophage expression of arginase-1 by a STAT6-dependent mechanism. This enzyme is believed to promote tissue repair as a result of its role in the synthesis of proline and polyamines. Other markers, such as Ym1 and FIZZ1, are also characteristic of M2 but are expressed by other cells, and their up-regulation on M2 is not STAT6-dependent in some systems such as the gastrointestinal tract [149]. Several new M2 markers have emerged that may further distinguish the M2 subtypes, such as E-cadherin, a junctional protein important in a number of epithelial and endothelial cell functions [155]. The relevance of M2 expression of junctional proteins is unclear, but M2 macrophages have increased expression of claudins 1, 2, and 11, all members of the claudin family of proteins that are critical for epithelial tight junction formation and function. Only claudin 11, however, was linked exclusively to IL-4-driven M2a in highly polarized Th2 environments, whereas claudin 2 was associated with M2d in tumors [156]. Of interest is that loss of claudin -11 increased epithelial cell invasiveness in gastric cancer [157].
Macrophage activation is influenced heavily by the cytokine environment, but the range of factors that modulate M2 is expanding. For example, progesterone, a female hormone elevated in pregnancy, impaired IL-4-induced arginase-1 and CD206, but not Ym1, in M2 [158]. The plasticity of macrophages has prompted considerable interest in the role of M2 in human disease, particularly in those pathologies that feature a mixed immune environment. Highly differentiated M2 can also change their phenotype and function in response to different challenges. IL-4-primed macrophages showed reduced phagocytosis of bacteria, but when these M2 macrophages were challenged subsequently with bacteria, there was a potentiation of proinflammatory cytokine production [159], a finding relevant to human disease. IBD, which includes Crohn's disease and ulcerative colitis, is a chronic, progressive disorder that results from dysregulated immune responses in susceptible individuals. There is an imbalance in the pro- and anti-inflammatory mediators that likely impacts macrophage phenotype, suggesting that M2 macrophages have protective effects. In murine models of colitis, adoptive transfer of macrophages treated with IL-4/IL-13 to induce the M2 phenotype attenuated the inflammation [160–162].
The anti-inflammatory benefits of helminth-induced type 2 immune responses, however, do not extend to inflammation as a consequence of microbial pathogens. The complications associated with concurrent infections have global implications as a result of the overlapping distribution of endemic bacterial and helminthic infections in many developing nations. Failure to induce the appropriate polarized cytokine profile diminishes type 1 immune responses against bacteria and is linked to the promotion of the M2 phenotype, which is associated with impaired killing of internalized bacteria, despite increased production of TNF-α [163]. These effects were not observed in STAT6 knockout mice, which can mount an effective response against microbial pathogens in the absence of IL-4/Il-13 signaling [164]. Concomitant infections also impact therapeutic outcomes. Vaccination is one of the most effective treatments for contagious infections, and vaccine efficacy is reduced in areas where helminth infection is high [165]. Whereas helminth infection in industrialized areas is generally low, the type 2 responses associated with the increasing incidence of asthma and allergy in these regions may have a similar impact on vaccine effectiveness [165].
There are a number of pathologies, including IBD and chronic obstructive pulmonary disease, in which chronic inflammation is linked to inflammation-related cancer [166, 167]. The inflammatory milieu supports the M1 phenotype, which persists during the early tumor stages in which macrophages emerge as the dominant immune cell. Tumor progression features a change from M1 to tumor-associated macrophages of the M2d phenotype expressing arginase-1, IL-10, and TGF-β [168]. The nature of the mechanisms involved in this transition are not elucidated fully, but there is growing appreciation of the number of different molecules and pathways implicated in this process, including COX-2, changes in TLR signaling, and hypoxia-induced changes in the microenvironment leading to up-regulation of hypoxia-inducible factor 1α, as well as Notch (reviewed in ref. [168]). Recent studies also indicate a role for changes in the macrophage expression of MHC class IIhi to MHC class IIlow [169]. The contribution of IL-4 to tumor pathology is evident in that mice deficient in STAT6 have improved immunity to tumors, an effect that may be linked to a reduced resistance to apoptosis [170]. The observations that tumor-associated M2d can revert to the M1 phenotype when exposed to IFN-γ, that depletion of macrophages limits tumor development, and that elevated levels of macrophages are correlated with poor prognosis [169] have further fueled interest in developing therapeutic agents targeting M2d in cancer.
Acknowledgment of the contribution of inflammation to the pathogenesis of obesity and insulin resistance led to investigations of the complex interactions between adipocytes and immune cells in adipose tissue. Macrophages are implicated in the development of the chronic inflammatory status of fat, and the appearance of increased numbers of M1-type macrophages observed in overweight individuals is critical to the development of insulin resistance, a hallmark feature of diabetes, hypertension, and other obesity-linked disorders. The importance of M1 is supported by studies showing that depletion of macrophages or inhibition of their recruitment attenuates insulin resistance [171]. There is a distinct difference in the macrophage phenotype of obese and lean animals, and the latter has M2 macrophages that are fewer in number and evenly dispersed throughout the adipose tissue [172]. In addition, M2 macrophages are an important prophylactic against the complication of obesity, as activation of the PPAR-γ results in recruitment of M2 [173], whereas mice lacking PPAR-γ fail to develop M2 and are predisposed to insulin resistance [174]. Of interest is that removal of adipocytes through apoptosis results in recruitment of M2 macrophages [175]. Factors in adipose tissue that control or promote the transition from M1 to M2 macrophages, such as PPAR-γ or exercise training [176], as well as cells that maintain insulin sensitivity and glucose tolerance, such as the Gr-1+CD11b+ myeloid-derived suppressor cells [177], are potential therapeutic targets.
CONCLUSION
The advances in understanding IL-4 biology reviewed above, specifically, the natural alternatives in the cytokine's production, receptor binding, and effect on macrophages, suggest that simplistic approaches to IL-4 targeting in the clinic will not necessarily lead to the intended effect. For example, inhibition of full-length IL-4 without targeting IL-4δ2 may alleviate eosinophilia and mucus production but not necessarily lymphocytosis and skewing of the cytokine milieu; targeting both of these IL-4 splice isoforms would likely be necessary for a more robust therapeutic effect. On the other hand, there is a possibility that IL-4δ2 acts, in part, as an anti-full-length IL-4 agent, and its inhibition has the potential to slow down the natural mechanisms of Th2 suppression. In either case, IL-4δ2 should be taken into consideration in designing IL-4 targeting therapies. Similar considerations apply to the targeting of IL-4Rs. The existence of two IL-4R types, combined with poorly understood receptor engagement by IL-4δ2, makes the development of therapies interfering with IL-4Rs challenging but clearly possible, as demonstrated by successful clinical trials of an inhibitory IL-4 variant that binds to IL-4Rα complexes and inhibits binding of IL-4 and IL-13 [178]. Finally, it is not clear whether alternative activation of macrophages should be targeted universally, promoted, or not interfered with in disease processes, as these cells may act in an anti-inflammatory fashion and promote normal tissue repair. Th2 diseases are, in major part, driven by IL-4, and continue to pose significant biomedical challenges worldwide. Further studies in the specific aspects of IL-4-driven regulation of inflammation are necessary to form the basis for development of better therapies for successful therapeutic modulation of and perhaps, even cures for these diseases.
ACKNOWLEDGMENTS
Support for this work was provided by VA Merit Award 5I01CX000101 (I.G.L.), U.S. National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases, 1R03AR054946 (I.G.L.), U.S. National Institutes of Health, NIAID, RO1AI038985 (A.D.K.), U.S. National Institutes of Health, NIAID, 2RO1AI049316 (T.S-D.), VA Merit Award 5I01CX000107 (S.P.A.), and U.S. National Institutes of Health, National Heart, Lung, and Blood Institute, 1R21HL106196 (S.P.A.). We thank Dr. Paul Todd for his expert editorial help.
Footnotes
- γc chain
- common γ chain
- BAL
- bronchoalveolar lavage
- Dok-1/2
- downstream of kinase 1/2
- ESAT-6
- 6 kDa early secretory antigenic target
- FIZZ1
- found in inflammatory zone 1
- Grb2
- growth factor receptor-bound protein 2
- IBD
- inflammatory bowel disease
- IRS
- insulin receptor substrate
- NIAID
- National Institute of Allergy and Infectious Diseases
- PPAR-γ
- peroxisome proliferator-activated receptor-γ
- PTB
- protein tyrosine binding
- SH2
- Src homology 2
- SHIP
- Src homology 2 domain-containing inositol-5-phosphatase
- SHP
- Src homology 2 domain-containing phosphatase
- SOCS
- suppressors of cytokine signaling
- TB
- tuberculosis
- TYK2
- tyrosine kinase 2
AUTHORSHIP
All authors contributed equally to this article, including reviewing research literature, summarizing literature observations, and writing individual sections of the text. The overall integration of the article was performed by S.P.A., with help from all coauthors.
REFERENCES
- 1. Nelms K., Keegan A. D., Zamorano J., Ryan J. J., Paul W. E. (1999) The IL-4 receptor: signaling mechanisms and biologic functions. Annu. Rev. Immunol. 17, 701–738 [DOI] [PubMed] [Google Scholar]
- 2. Kelly-Welch A., Hanson E. M., Keegan A. D. (2005) Interleukin-13 (IL-13) pathway. Sci. STKE 2005, cm8. [DOI] [PubMed] [Google Scholar]
- 3. Mueller T. D., Zhang J. L., Sebald W., Duschl A. (2002) Structure, binding, and antagonists in the IL-4/IL-13 receptor system. Biochim. Biophys. Acta 1592, 237–250 [DOI] [PubMed] [Google Scholar]
- 4. Chomarat P., Banchereau J. (1998) Interleukin-4 and interleukin-13: their similarities and discrepancies. Int. Rev. Immunol. 17, 1–52 [DOI] [PubMed] [Google Scholar]
- 5. Murata T., Obiri N. I., Puri R. K. (1998) Structure of and signal transduction through interleukin-4 and interleukin-13 receptors (review). Int. J. Mol. Med. 1, 551–557 [DOI] [PubMed] [Google Scholar]
- 6. Kelly-Welch A. E., Hanson E. M., Boothby M. R., Keegan A. D. (2003) Interleukin-4 and interleukin-13 signaling connections maps. Science 300, 1527–1528 [DOI] [PubMed] [Google Scholar]
- 7. LaPorte S. L., Juo Z. S., Vaclavikova J., Colf L. A., Qi X., Heller N. M., Keegan A. D., Garcia K. C. (2008) Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell 132, 259–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iseki M., Omori-Miyake M., Xu W., Sun X., Takaki S., Rawlings D. J., Ziegler S. F. (2012) Thymic stromal lymphopoietin (TSLP)-induced polyclonal B-cell activation and autoimmunity are mediated by CD4+ T cells and IL-4. Int. Immunol. 24, 183–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okayama Y., Okumura S., Sagara H., Yuki K., Sasaki T., Watanabe N., Fueki M., Sugiyama K., Takeda K., Fukuda T., Saito H., Ra C. (2009) FcϵRI-mediated thymic stromal lymphopoietin production by interleukin-4-primed human mast cells. Eur. Respir. J. 34, 425–435 [DOI] [PubMed] [Google Scholar]
- 10. Omori M., Ziegler S. (2007) Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J. Immunol. 178, 1396–1404 [DOI] [PubMed] [Google Scholar]
- 11. Schmitz J., Owyang A., Oldham E., Song Y., Murphy E., McClanahan T. K., Zurawski G., Moshrefi M., Qin J., Li X., Gorman D. M., Bazan J. F., Kastelein R. A. (2005) IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23, 479–490 [DOI] [PubMed] [Google Scholar]
- 12. Frohlich A., Marsland B. J., Sonderegger I., Kurrer M., Hodge M. R., Harris N. L., Kopf M. (2007) IL-21 receptor signaling is integral to the development of Th2 effector responses in vivo. Blood 109, 2023–2031 [DOI] [PubMed] [Google Scholar]
- 13. Angkasekwinai P., Park H., Wang Y. H., Chang S. H., Corry D. B., Liu Y. J., Zhu Z., Dong C. (2007) Interleukin 25 promotes the initiation of proallergic type 2 responses. J. Exp. Med. 204, 1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu J., Paul W. E. (2008) CD4 T cells: fates, functions, and faults. Blood 112, 1557–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Constant S. L., Bottomly K. (1997) Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu. Rev. Immunol. 15, 297–322 [DOI] [PubMed] [Google Scholar]
- 16. Bi Y., Liu G., Yang R. (2011) Reciprocal modulation between TH17 and other helper T cell lineages. J. Cell. Physiol. 226, 8–13 [DOI] [PubMed] [Google Scholar]
- 17. Otsuka T., Villaret D., Yokota T., Takebe Y., Lee F., Arai N., Arai K. (1987) Structural analysis of the mouse chromosomal gene encoding interleukin 4 which expresses B cell, T cell and mast cell stimulating activities. Nucleic Acids Res. 15, 333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arai N., Nomura D., Villaret D., DeWaal Malefijt R., Seiki M., Yoshida M., Minoshima S., Fukuyama R., Maekawa M., Kudoh J.., et al. (1989) Complete nucleotide sequence of the chromosomal gene for human IL-4 and its expression. J. Immunol. 142, 274–282 [PubMed] [Google Scholar]
- 19. Alms W. J., Atamas S. P., Yurovsky V. V., White B. (1996) Generation of a variant of human interleukin-4 by alternative splicing. Mol. Immunol. 33, 361–370 [DOI] [PubMed] [Google Scholar]
- 20. Atamas S. P., Choi J., Yurovsky V. V., White B. (1996) An alternative splice variant of human IL-4, IL-4 δ 2, inhibits IL-4-stimulated T cell proliferation. J. Immunol. 156, 435–441 [PubMed] [Google Scholar]
- 21. Arinobu Y., Atamas S. P., Otsuka T., Niiro H., Yamaoka K., Mitsuyasu H., Niho Y., Hamasaki N., White B., Izuhara K. (1999) Antagonistic effects of an alternative splice variant of human IL-4, IL-4δ2, on IL-4 activities in human monocytes and B cells. Cell. Immunol. 191, 161–167 [DOI] [PubMed] [Google Scholar]
- 22. Zav'yalov V. P., Denesyuk A. I., White B., Yurovsky V. V., Atamas S. P., Korpela T. (1997) Molecular model of an alternative splice variant of human IL-4, IL-4 δ 2, a naturally occurring inhibitor of IL-4-stimulated T cell proliferation. Immunol. Lett. 58, 149–152 [DOI] [PubMed] [Google Scholar]
- 23. Rhodes S. G., Sawyer J., Whelan A. O., Dean G. S., Coad M., Ewer K. J., Waldvogel A. S., Zakher A., Clifford D. J., Hewinson R. G., Vordermeier H. M. (2007) Is interleukin-4δ3 splice variant expression in bovine tuberculosis a marker of protective immunity? Infect. Immun. 75, 3006–3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yuan Z., Wang R., Lee Y., Chen C. Y., Yu X., Wu Z., Huang D., Shen L., Chen Z. W. (2009) Tuberculosis-induced variant IL-4 mRNA encodes a cytokine functioning as growth factor for (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate-specific Vγ2Vδ2 T cells. J. Immunol. 182, 811–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gautherot I., Burdin N., Seguin D., Aujame L., Sodoyer R. (2002) Cloning of interleukin-4 δ2 splice variant (IL-4δ2) in chimpanzee and cynomolgus macaque: phylogenetic analysis of δ2 splice variant appearance, and implications for the study of IL-4-driven immune processes. Immunogenetics 54, 635–644 [DOI] [PubMed] [Google Scholar]
- 26. Waldvogel A. S., Lepage M. F., Zakher A., Reichel M. P., Eicher R., Heussler V. T. (2004) Expression of interleukin 4, interleukin 4 splice variants and interferon γ mRNA in calves experimentally infected with Fasciola hepatica. Vet. Immunol. Immunopathol. 97, 53–63 [DOI] [PubMed] [Google Scholar]
- 27. Yatsenko O. P., Filipenko M. L., Khrapov E. A., Voronina E. N., Kozlov V. A., Sennikov S. V. (2004) Alternative splicing of mRNA of mouse interleukin-4 and interleukin-6. Cytokine 28, 190–196 [DOI] [PubMed] [Google Scholar]
- 28. Luzina I. G., Lockatell V., Todd N. W., Highsmith K., Keegan A. D., Hasday J. D., Atamas S. P. (2011) Alternatively spliced variants of interleukin-4 promote inflammation differentially. J. Leukoc. Biol. 89, 763–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luzina I. G., Lockatell V., Todd N. W., Keegan A. D., Hasday J. D., Atamas S. P. (2011) Splice isoforms of human interleukin-4 are functionally active in mice in vivo. Immunology 132, 385–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. De Moraes-Pinto M. I., Vince G. S., Flanagan B. F., Hart C. A., Johnson P. M. (1997) Localization of IL-4 and IL-4 receptors in the human term placenta, decidua and amniochorionic membranes. Immunology 90, 87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Orsini B., Vivas J. R., Ottanelli B., Amedei A., Surrenti E., Galli A., Milani S., Pinzani P., Del Prete G., Surrenti C., Baldari C. T., Touati E., D'Elios M. M. (2007) Human gastric epithelium produces IL-4 and IL-4δ2 isoform only upon Helicobacter pylori infection. Int. J. Immunopathol. Pharmacol. 20, 809–818 [DOI] [PubMed] [Google Scholar]
- 32. Orsini B., Ottanelli B., Amedei A., Surrenti E., Capanni M., Del Prete G., Amorosi A., Milani S., D'Elios M. M., Surrenti C. (2003) Helicobacter pylori cag pathogenicity island is associated with reduced expression of interleukin-4 (IL-4) mRNA and modulation of the IL-4δ2 mRNA isoform in human gastric mucosa. Infect. Immun. 71, 6664–6667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pouliot P., Turmel V., Gelinas E., Laviolette M., Bissonnette E. Y. (2005) Interleukin-4 production by human alveolar macrophages. Clin. Exp. Allergy 35, 804–810 [DOI] [PubMed] [Google Scholar]
- 34. Plante S., Semlali A., Joubert P., Bissonnette E., Laviolette M., Hamid Q., Chakir J. (2006) Mast cells regulate procollagen I (α 1) production by bronchial fibroblasts derived from subjects with asthma through IL-4/IL-4 δ 2 ratio. J. Allergy Clin. Immunol. 117, 1321–1327 [DOI] [PubMed] [Google Scholar]
- 35. Glare E. M., Divjak M., Rolland J. M., Walters E. H. (1999) Asthmatic airway biopsy specimens are more likely to express the IL-4 alternative splice variant IL-4δ2. J. Allergy Clin. Immunol. 104, 978–982 [DOI] [PubMed] [Google Scholar]
- 36. Seah G. T., Gao P. S., Hopkin J. M., Rook G. A. (2001) Interleukin-4 and its alternatively spliced variant (IL-4δ2) in patients with atopic asthma. Am. J. Respir. Crit. Care Med. 164, 1016–1018 [DOI] [PubMed] [Google Scholar]
- 37. Glare E. M., Divjak M., Bailey M. J., Walters E. H. (2001) The usefulness of competitive PCR: airway gene expression of IL-5, IL-4, IL-4δ2, IL-2, and IFNγ in asthma. Thorax 56, 541–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Luzina I. G., Lockatell V., Lavania S., Pickering E. M., Kang P. H., Bashkatova Y. N., Andreev S. M., Atamas S. P. (2012) Natural production and functional effects of alternatively spliced interleukin-4 protein in asthma. Cytokine 58, 20–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu H. P., Wu C. L., Chen C. K., Chung K., Tseng J. C., Liu Y. C., Chuang D. Y. (2008) The interleukin-4 expression in patients with severe sepsis. J. Crit. Care 23, 519–524 [DOI] [PubMed] [Google Scholar]
- 40. Atamas S. P., Yurovsky V. V., Wise R., Wigley F. M., Goter Robinson C. J., Henry P., Alms W. J., White B. (1999) Production of type 2 cytokines by CD8+ lung cells is associated with greater decline in pulmonary function in patients with systemic sclerosis. Arthritis Rheum. 42, 1168–1178 [DOI] [PubMed] [Google Scholar]
- 41. Sakkas L. I., Tourtellotte C., Berney S., Myers A. R., Platsoucas C. D. (1999) Increased levels of alternatively spliced interleukin 4 (IL-4δ2) transcripts in peripheral blood mononuclear cells from patients with systemic sclerosis. Clin. Diagn. Lab. Immunol. 6, 660–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Atamas S. P., White B. (1999) Interleukin 4 in systemic sclerosis: not just an increase. Clin. Diagn. Lab. Immunol. 6, 658–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Seah G. T., Rook G. A. (1999) A sensitive, non-radioactive quantitative method for measuring IL-4 and IL-4δ2 mRNA in unstimulated cells from multiple clinical samples, using nested RT-PCR. J. Immunol. Methods 228, 139–149 [DOI] [PubMed] [Google Scholar]
- 44. Seah G. T., Scott G. M., Rook G. A. (2000) Type 2 cytokine gene activation and its relationship to extent of disease in patients with tuberculosis. J. Infect. Dis. 181, 385–389 [DOI] [PubMed] [Google Scholar]
- 45. Dheda K., Chang J. S., Breen R. A., Haddock J. A., Lipman M. C., Kim L. U., Huggett J. F., Johnson M. A., Rook G. A., Zumla A. (2005) Expression of a novel cytokine, IL-4δ2, in HIV and HIV-tuberculosis co-infection. AIDS 19, 1601–1606 [DOI] [PubMed] [Google Scholar]
- 46. Dheda K., Chang J. S., Breen R. A., Kim L. U., Haddock J. A., Huggett J. F., Johnson M. A., Rook G. A., Zumla A. (2005) In vivo and in vitro studies of a novel cytokine, interleukin 4δ2, in pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 172, 501–508 [DOI] [PubMed] [Google Scholar]
- 47. Djoba Siawaya J. F., Bapela N. B., Ronacher K., Beyers N., van Helden P., Walzl G. (2008) Differential expression of IL-4 and IL-4 δ2, but not transforming growth factor β (TGF-β), TGF-β RII, Foxp3, γ interferon, T-bet, or GATA-3 mRNA, in patients with fast and slow responders to antituberculosis treatment. Clin. Vaccine Immunol. 15, 1165–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Demissie A., Abebe M., Aseffa A., Rook G., Fletcher H., Zumla A., Weldingh K., Brock I., Andersen P., Doherty T. M., VACSEL Study Group (2004) Healthy individuals that control a latent infection with M. tuberculosis express high levels of Th-1 cytokines and the IL-4 antagonist IL-4δ2. J. Immunol. 172, 6938–6943 [DOI] [PubMed] [Google Scholar]
- 49. Fletcher H. A., Owiafe P., Jeffries D., Hill P., Rook G. A. W., Zumla A., Doherty T. M., Brookes R., VACSEL Study Group (2004) Increased expression of mRNA encoding IL-4 and its splice variant IL-4δ2 in cells from contacts of Mycobacterium tuberculosis in the absence of in vitro stimulation. Immunology 112, 669–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mendez A., Hernandez-Pando R., Contreras S., Aguilar D., Rook G. A. (2011) CCL2, CCL18 and sIL-4R in renal, meningeal and pulmonary TB; a 2 year study of patients and contacts. Tuberculosis (Edinb) 91, 140–145 [DOI] [PubMed] [Google Scholar]
- 51. Demissie A., Leyten E. M., Abebe M., Wassie L., Aseffa A., Abate G., Fletcher H., Owiafe P., Hill P. C., Brookes R., Rook G., Zumla A., Arend S. M., Klein M., Ottenhoff T. H., Andersen P., Doherty T. M., VACSEL Study Group (2006) Recognition of stage-specific mycobacterial antigens differentiates between acute and latent infections with Mycobacterium tuberculosis. Clin. Vaccine Immunol. 13, 179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ohara J., Coligan J. E., Zoon K., Maloy W. L., Paul W. E. (1987) High-efficiency purification and chemical characterization of B cell stimulatory factor-1/interleukin 4. J. Immunol. 139, 1127–1134 [PubMed] [Google Scholar]
- 53. Park L. S., Friend D., Grabstein K., Urdal D. L. (1987) Characterization of the high-affinity cell-surface receptor for murine B-cell-stimulating factor 1. Proc. Natl. Acad. Sci. USA 84, 1669–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lowenthal J. W., Castle B. E., Christiansen J., Schreurs J., Rennick D., Arai N., Hoy P., Takebe Y., Howard M. (1988) Expression of high affinity receptors for murine interleukin 4 (BSF-1) on hemopoietic and nonhemopoietic cells. J. Immunol. 140, 456–464 [PubMed] [Google Scholar]
- 55. Russell S. M., Keegan A. D., Harada N., Nakamura Y., Noguchi M., Leland P., Friedmann M. C., Miyajima A., Puri R. K., Paul W. E.., et al. (1993) Interleukin-2 receptor γ chain: a functional component of the interleukin-4 receptor. Science 262, 1880–1883 [DOI] [PubMed] [Google Scholar]
- 56. Kondo M., Takeshita T., Ishii N., Nakamura M., Watanabe S., Arai K., Sugamura K. (1993) Sharing of the interleukin-2 (IL-2) receptor γ chain between receptors for IL-2 and IL-4. Science 262, 1874–1877 [DOI] [PubMed] [Google Scholar]
- 57. Takeshita T., Asao H., Ohtani K., Ishii N., Kumaki S., Tanaka N., Munakata H., Nakamura M., Sugamura K. (1992) Cloning of the γ chain of the human IL-2 receptor. Science 257, 379–382 [DOI] [PubMed] [Google Scholar]
- 58. Cao X., Kozak C. A., Liu Y. J., Noguchi M., O'Connell E., Leonard W. J. (1993) Characterization of cDNAs encoding the murine interleukin 2 receptor (IL-2R) γ chain: chromosomal mapping and tissue specificity of IL-2R γ chain expression. Proc. Natl. Acad. Sci. USA 90, 8464–8468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Letzelter F., Wang Y., Sebald W. (1998) The interleukin-4 site-2 epitope determining binding of the common receptor γ chain. Eur. J. Biochem. 257, 11–20 [DOI] [PubMed] [Google Scholar]
- 60. Lesur O., Arsalane K., Berard J., Mukuna J. P., de Brum-Fernandes A. J., Lane D., Rola-Pleszczynski M. (1997) Functional IL-2 receptors are expressed by rat lung type II epithelial cells. Am. J. Physiol. 273, L495–L503 [DOI] [PubMed] [Google Scholar]
- 61. Van der Velden V. H., Naber B. A., Wierenga-Wolf A. F., Debets R., Savelkoul H. F., Overbeek S. E., Hoogsteden H. C., Versnel M. A. (1998) Interleukin 4 receptors on human bronchial epithelial cells. An in vivo and in vitro analysis of expression and function. Cytokine 10, 803–813 [DOI] [PubMed] [Google Scholar]
- 62. Yamamoto S., Kobayashi I., Tsuji K., Nishi N., Muro E., Miyazaki M., Zaitsu M., Inada S., Ichimaru T., Hamasaki Y. (2004) Upregulation of interleukin-4 receptor by interferon-γ: enhanced interleukin-4-induced eotaxin-3 production in airway epithelium. Am. J. Respir. Cell Mol. Biol. 31, 456–462 [DOI] [PubMed] [Google Scholar]
- 63. Obiri N. I., Debinski W., Leonard W. J., Puri R. K. (1995) Receptor for interleukin 13. Interaction with interleukin 4 by a mechanism that does not involve the common γ chain shared by receptors for interleukins 2, 4, 7, 9, and 15. J. Biol. Chem. 270, 8797–8804 [DOI] [PubMed] [Google Scholar]
- 64. Hilton D. J., Zhang J. G., Metcalf D., Alexander W. S., Nicola N. A., Willson T. A. (1996) Cloning and characterization of a binding subunit of the interleukin 13 receptor that is also a component of the interleukin 4 receptor. Proc. Natl. Acad. Sci. USA 93, 497–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Miloux B., Laurent P., Bonnin O., Lupker J., Caput D., Vita N., Ferrara P. (1997) Cloning of the human IL-13R α1 chain and reconstitution with the IL4R α of a functional IL-4/IL-13 receptor complex. FEBS Lett. 401, 163–166 [DOI] [PubMed] [Google Scholar]
- 66. Donaldson D. D., Whitters M. J., Fitz L. J., Neben T. Y., Finnerty H., Henderson S. L., O'Hara R. M., Jr., Beier D. R., Turner K. J., Wood C. R.., et al. (1998) The murine IL-13 receptor α 2: molecular cloning, characterization, and comparison with murine IL-13 receptor α 1. J. Immunol. 161, 2317–2324 [PubMed] [Google Scholar]
- 67. Kawakami K., Taguchi J., Murata T., Puri R. K. (2001) The interleukin-13 receptor α2 chain: an essential component for binding and internalization but not for interleukin-13-induced signal transduction through the STAT6 pathway. Blood 97, 2673–2679 [DOI] [PubMed] [Google Scholar]
- 68. Zhang J. G., Hilton D. J., Willson T. A., McFarlane C., Roberts B. A., Moritz R. L., Simpson R. J., Alexander W. S., Metcalf D., Nicola N. A. (1997) Identification, purification, and characterization of a soluble interleukin (IL)-13-binding protein. Evidence that it is distinct from the cloned Il-13 receptor and Il-4 receptor α-chains. J. Biol. Chem. 272, 9474–9480 [DOI] [PubMed] [Google Scholar]
- 69. Wood N., Whitters M. J., Jacobson B. A., Witek J., Sypek J. P., Kasaian M., Eppihimer M. J., Unger M., Tanaka T., Goldman S. J.., et al. (2003) Enhanced interleukin (IL)-13 responses in mice lacking IL-13 receptor α 2. J. Exp. Med. 197, 703–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fichtner-Feigl S., Strober W., Kawakami K., Puri R. K., Kitani A. (2006) IL-13 signaling through the IL-13α2 receptor is involved in induction of TGF-β1 production and fibrosis. Nat. Med. 12, 99–106 [DOI] [PubMed] [Google Scholar]
- 71. Schindler C., Levy D. E., Decker T. (2007) JAK-STAT signaling: from interferons to cytokines. J. Biol. Chem. 282, 20059–20063 [DOI] [PubMed] [Google Scholar]
- 72. Izuhara K., Feldman R. A., Greer P., Harada N. (1994) Interaction of the c-fes proto-oncogene product with the interleukin-4 receptor. J. Biol. Chem. 269, 18623–18629 [PubMed] [Google Scholar]
- 73. Ennaciri J., Girard D. (2009) IL-4R(α), a new member that associates with Syk kinase: implication in IL-4-induced human neutrophil functions. J. Immunol. 183, 5261–5269 [DOI] [PubMed] [Google Scholar]
- 74. Kurzer J. H., Argetsinger L. S., Zhou Y. J., Kouadio J. L., O'Shea J. J., Carter-Su C. (2004) Tyrosine 813 is a site of JAK2 autophosphorylation critical for activation of JAK2 by SH2-B β. Mol. Cell. Biol. 24, 4557–4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Argetsinger L. S., Kouadio J. L., Steen H., Stensballe A., Jensen O. N., Carter-Su C. (2004) Autophosphorylation of JAK2 on tyrosines 221 and 570 regulates its activity. Mol. Cell. Biol. 24, 4955–4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhou Y. J., Hanson E. P., Chen Y. Q., Magnuson K., Chen M., Swann P. G., Wange R. L., Changelian P. S., O'Shea J. J. (1997) Distinct tyrosine phosphorylation sites in JAK3 kinase domain positively and negatively regulate its enzymatic activity. Proc. Natl. Acad. Sci. USA 94, 13850–13855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Liu K. D., Gaffen S. L., Goldsmith M. A., Greene W. C. (1997) Janus kinases in interleukin-2-mediated signaling: JAK1 and JAK3 are differentially regulated by tyrosine phosphorylation. Curr. Biol. 7, 817–826 [DOI] [PubMed] [Google Scholar]
- 78. Wang R., Griffin P. R., Small E. C., Thompson J. E. (2003) Mechanism of Janus kinase 3-catalyzed phosphorylation of a Janus kinase 1 activation loop peptide. Arch. Biochem. Biophys. 410, 7–15 [DOI] [PubMed] [Google Scholar]
- 79. Fujii H. (2008) Receptor expression is essential for proliferation induced by dimerized Jak kinases. Biochem. Biophys. Res. Commun. 370, 557–560 [DOI] [PubMed] [Google Scholar]
- 80. Wang L. M., Keegan A. D., Paul W. E., Heidaran M. A., Gutkind J. S., Pierce J. H. (1992) IL-4 activates a distinct signal transduction cascade from IL-3 in factor-dependent myeloid cells. EMBO J. 11, 4899–4908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Smerz-Bertling C., Duschl A. (1995) Both interleukin 4 and interleukin 13 induce tyrosine phosphorylation of the 140-kDa subunit of the interleukin 4 receptor. J. Biol. Chem. 270, 966–970 [DOI] [PubMed] [Google Scholar]
- 82. Orchansky P. L., Kwan R., Lee F., Schrader J. W. (1999) Characterization of the cytoplasmic domain of interleukin-13 receptor-α. J. Biol. Chem. 274, 20818–20825 [DOI] [PubMed] [Google Scholar]
- 83. Umeshita-Suyama R., Sugimoto R., Akaiwa M., Arima K., Yu B., Wada M., Kuwano M., Nakajima K., Hamasaki N., Izuhara K. (2000) Characterization of IL-4 and IL-13 signals dependent on the human IL-13 receptor α chain 1: redundancy of requirement of tyrosine residue for STAT3 activation. Int. Immunol. 12, 1499–1509 [DOI] [PubMed] [Google Scholar]
- 84. Koettnitz K., Kalthoff F. S. (1993) Human interleukin-4 receptor signaling requires sequences contained within two cytoplasmic regions. Eur. J. Immunol. 23, 988–991 [DOI] [PubMed] [Google Scholar]
- 85. Seldin D. C., Leder P. (1994) Mutational analysis of a critical signaling domain of the human interleukin 4 receptor. Proc. Natl. Acad. Sci. USA 91, 2140–2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Keegan A. D., Nelms K., White M., Wang L. M., Pierce J. H., Paul W. E. (1994) An IL-4 receptor region containing an insulin receptor motif is important for IL-4-mediated IRS-1 phosphorylation and cell growth. Cell 76, 811–820 [DOI] [PubMed] [Google Scholar]
- 87. Deutsch H. H., Koettnitz K., Chung J., Kalthoff F. S. (1995) Distinct sequence motifs within the cytoplasmic domain of the human IL-4 receptor differentially regulate apoptosis inhibition and cell growth. J. Immunol. 154, 3696–3703 [PubMed] [Google Scholar]
- 88. Wang H. Y., Paul W. E., Keegan A. D. (1996) IL-4 function can be transferred to the IL-2 receptor by tyrosine containing sequences found in the IL-4 receptor α chain. Immunity 4, 113–121 [DOI] [PubMed] [Google Scholar]
- 89. Sun X. J., Wang L. M., Zhang Y., Yenush L., Myers M. G., Jr., Glasheen E., Lane W. S., Pierce J. H., White M. F. (1995) Role of IRS-2 in insulin and cytokine signalling. Nature 377, 173–177 [DOI] [PubMed] [Google Scholar]
- 90. Zick Y. (2005) Ser/Thr phosphorylation of IRS proteins: a molecular basis for insulin resistance. Sci. STKE 2005, pe4. [DOI] [PubMed] [Google Scholar]
- 91. Heller N. M., Qi X., Junttila I. S., Shirey K. A., Vogel S. N., Paul W. E., Keegan A. D. (2008) Type I IL-4Rs selectively activate IRS-2 to induce target gene expression in macrophages. Sci. Signal. 1, ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zamorano J., Wang H. Y., Wang L. M., Pierce J. H., Keegan A. D. (1996) IL-4 protects cells from apoptosis via the insulin receptor substrate pathway and a second independent signaling pathway. J. Immunol. 157, 4926–4934 [PubMed] [Google Scholar]
- 93. Burks D. J., Font de Mora J., Schubert M., Withers D. J., Myers M. G., Towery H. H., Altamuro S. L., Flint C. L., White M. F. (2000) IRS-2 pathways integrate female reproduction and energy homeostasis. Nature 407, 377–382 [DOI] [PubMed] [Google Scholar]
- 94. Withers D. J., Gutierrez J. S., Towery H., Burks D. J., Ren J. M., Previs S., Zhang Y., Bernal D., Pons S., Shulman G. I.., et al. (1998) Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391, 900–904 [DOI] [PubMed] [Google Scholar]
- 95. Lee H. M., Jin H. S., Park J. W., Park S. M., Jeon H. K., Lee T. H. (2003) IL-4 augments anisomycin-induced p38 activation via Akt pathway in a follicular dendritic cell (FDC)-like line. FEBS Lett. 549, 110–114 [DOI] [PubMed] [Google Scholar]
- 96. Hou J., Schindler U., Henzel W. J., Ho T. C., Brasseur M., McKnight S. L. (1994) An interleukin-4-induced transcription factor: IL-4 Stat. Science 265, 1701–1706 [DOI] [PubMed] [Google Scholar]
- 97. Mikita T., Campbell D., Wu P., Williamson K., Schindler U. (1996) Requirements for interleukin-4-induced gene expression and functional characterization of Stat6. Mol. Cell. Biol. 16, 5811–5820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Schindler U., Wu P., Rothe M., Brasseur M., McKnight S. L. (1995) Components of a Stat recognition code: evidence for two layers of molecular selectivity. Immunity 2, 689–697 [DOI] [PubMed] [Google Scholar]
- 99. Shankaranarayanan P., Chaitidis P., Kuhn H., Nigam S. (2001) Acetylation by histone acetyltransferase CREB-binding protein/p300 of STAT6 is required for transcriptional activation of the 15-lipoxygenase-1 gene. J. Biol. Chem. 276, 42753–42760 [DOI] [PubMed] [Google Scholar]
- 100. Chen W., Daines M. O., Hershey G. K. (2004) Methylation of STAT6 modulates STAT6 phosphorylation, nuclear translocation, and DNA-binding activity. J. Immunol. 172, 6744–6750 [DOI] [PubMed] [Google Scholar]
- 101. Pesu M., Takaluoma K., Aittomaki S., Lagerstedt A., Saksela K., Kovanen P. E., Silvennoinen O. (2000) Interleukin-4-induced transcriptional activation by stat6 involves multiple serine/threonine kinase pathways and serine phosphorylation of Stat6. Blood 95, 494–502 [PubMed] [Google Scholar]
- 102. Wick K. R., Berton M. T. (2000) IL-4 induces serine phosphorylation of the STAT6 transactivation domain in B lymphocytes. Mol. Immunol. 37, 641–652 [DOI] [PubMed] [Google Scholar]
- 103. Wang Y., Malabarba M. G., Nagy Z. S., Kirken R. A. (2004) Interleukin 4 regulates phosphorylation of serine 756 in the transactivation domain of Stat6. Roles for multiple phosphorylation sites and Stat6 function. J. Biol. Chem. 279, 25196–25203 [DOI] [PubMed] [Google Scholar]
- 104. Shirakawa T., Kawazoe Y., Tsujikawa T., Jung D., Sato S., Uesugi M. (2011) Deactivation of STAT6 through serine 707 phosphorylation by JNK. J. Biol. Chem. 286, 4003–4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Pernis A. B., Rothman P. B. (2002) JAK-STAT signaling in asthma. J. Clin. Invest. 109, 1279–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ravetch J. V., Lanier L. L. (2000) Immune inhibitory receptors. Science 290, 84–89 [DOI] [PubMed] [Google Scholar]
- 107. Zamorano J., Keegan A. D. (1998) Regulation of apoptosis by tyrosine-containing domains of IL-4R α: Y497 and Y713, but not the STAT6-docking tyrosines, signal protection from apoptosis. J. Immunol. 161, 859–867 [PubMed] [Google Scholar]
- 108. Imani F., Rager K. J., Catipovic B., Marsh D. G. (1997) Interleukin-4 (IL-4) induces phosphatidylinositol 3-kinase (p85) dephosphorylation. Implications for the role of SHP-1 in the IL-4-induced signals in human B cells. J. Biol. Chem. 272, 7927–7931 [DOI] [PubMed] [Google Scholar]
- 109. Haque S. J., Harbor P., Tabrizi M., Yi T., Williams B. R. (1998) Protein-tyrosine phosphatase Shp-1 is a negative regulator of IL-4- and IL-13-dependent signal transduction. J. Biol. Chem. 273, 33893–33896 [DOI] [PubMed] [Google Scholar]
- 110. Lorenz U. (2009) SHP-1 and SHP-2 in T cells: two phosphatases functioning at many levels. Immunol. Rev. 228, 342–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hanson E. M., Dickensheets H., Qu C. K., Donnelly R. P., Keegan A. D. (2003) Regulation of the dephosphorylation of Stat6. Participation of Tyr-713 in the interleukin-4 receptor α, the tyrosine phosphatase SHP-1, and the proteasome. J. Biol. Chem. 278, 3903–3911 [DOI] [PubMed] [Google Scholar]
- 112. Yoshimura A., Mori H., Ohishi M., Aki D., Hanada T. (2003) Negative regulation of cytokine signaling influences inflammation. Curr. Opin. Immunol. 15, 704–708 [DOI] [PubMed] [Google Scholar]
- 113. Losman J. A., Chen X. P., Hilton D., Rothman P. (1999) Cutting edge: SOCS-1 is a potent inhibitor of IL-4 signal transduction. J. Immunol. 162, 3770–3774 [PMC free article] [PubMed] [Google Scholar]
- 114. Haque S. J., Harbor P. C., Williams B. R. (2000) Identification of critical residues required for suppressor of cytokine signaling-specific regulation of interleukin-4 signaling. J. Biol. Chem. 275, 26500–26506 [DOI] [PubMed] [Google Scholar]
- 115. Yasukawa H., Misawa H., Sakamoto H., Masuhara M., Sasaki A., Wakioka T., Ohtsuka S., Imaizumi T., Matsuda T., Ihle J. N.., et al. (1999) The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. EMBO J. 18, 1309–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ram P. A., Waxman D. J. (1999) SOCS/CIS protein inhibition of growth hormone-stimulated STAT5 signaling by multiple mechanisms. J. Biol. Chem. 274, 35553–35561 [DOI] [PubMed] [Google Scholar]
- 117. Monni R., Santos S. C., Mauchauffe M., Berger R., Ghysdael J., Gouilleux F., Gisselbrecht S., Bernard O., Penard-Lacronique V. (2001) The TEL-Jak2 oncoprotein induces Socs1 expression and altered cytokine response in Ba/F3 cells. Oncogene 20, 849–858 [DOI] [PubMed] [Google Scholar]
- 118. Frantsve J., Schwaller J., Sternberg D. W., Kutok J., Gilliland D. G. (2001) Socs-1 inhibits TEL-JAK2-mediated transformation of hematopoietic cells through inhibition of JAK2 kinase activity and induction of proteasome-mediated degradation. Mol. Cell. Biol. 21, 3547–3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wlodawer A., Pavlovsky A., Gustchina A. (1992) Crystal structure of human recombinant interleukin-4 at 2.25 A resolution. FEBS Lett. 309, 59–64 [DOI] [PubMed] [Google Scholar]
- 120. Mosmann T. R., Yokota T., Kastelein R., Zurawski S. M., Arai N., Takebe Y. (1987) Species-specificity of T cell stimulating activities of IL 2 and BSF-1 (IL 4): comparison of normal and recombinant, mouse and human IL 2 and BSF-1 (IL 4). J. Immunol. 138, 1813–1816 [PubMed] [Google Scholar]
- 121. Wang Y., Shen B. J., Sebald W. (1997) A mixed-charge pair in human interleukin 4 dominates high-affinity interaction with the receptor α chain. Proc. Natl. Acad. Sci. USA 94, 1657–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Baumhofer J. M., Beinhauer B. G., Wang J. E., Brandmeier H., Geissler K., Losert U., Philip R., Aversa G., Rogy M. A. (1998) Gene transfer with IL-4 and IL-13 improves survival in lethal endotoxemia in the mouse and ameliorates peritoneal macrophages immune competence. Eur. J. Immunol. 28, 610–615 [DOI] [PubMed] [Google Scholar]
- 123. Volpert O. V., Fong T., Koch A. E., Peterson J. D., Waltenbaugh C., Tepper R. I., Bouck N. P. (1998) Inhibition of angiogenesis by interleukin 4. J. Exp. Med. 188, 1039–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Stein M., Keshav S., Harris N., Gordon S. (1992) Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J. Exp. Med. 176, 287–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Chang Y-J, Kim H. Y., Albacker L. A., Baumgarth N., McKenzie A. N. J., Smith D. E., DeKruyff R. H., Umetsu D. T. (2011) Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat. Immunol. 12, 631–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kim E. Y., Battaile J. T., Patel A. C., You Y., Agapov E., Grayson M. H., Benoit L. A., Byers D. E., Alevy Y., Tucker J.., et al. (2008) Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat. Med. 14, 633–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Shirey K. A., Cole L. E., Keegan A. D., Vogel S. N. (2008) Francisella tularensis live vaccine strain induces macrophage alternative activation as a survival mechanism. J. Immunol. 181, 4159–4167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Shirey K. A., Pletneva L. M., Puche A. C., Keegan A. D., Prince G. A., Blanco J. C. G., Vogel S. N. (2010) Control of RSV-induced lung injury by alternatively activated macrophages is IL-4Rα-, TLR4-, and IFN-β-dependent. Mucosal Immunol. 3, 291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kodelja V., Muller C., Politz O., Hakij N., Orfanos C. E., Goerdt S. (1998) Alternative macrophage activation-associated CC-chemokine-1, a novel structural homologue of macrophage inflammatory protein-1 α with a Th2-associated expression pattern. J. Immunol. 160, 1411–1418 [PubMed] [Google Scholar]
- 130. Adema G. J., Hartgers F., Verstraten R., de Vries E., Marland G., Menon S., Foster J., Xu Y., Nooyen P., McClanahan T.., et al. (1997) A dendritic-cell-derived C-C chemokine that preferentially attracts naive T cells. Nature 387, 713–717 [DOI] [PubMed] [Google Scholar]
- 131. Luzina I. G., Papadimitriou J. C., Anderson R., Pochetuhen K., Atamas S. P. (2006) Induction of prolonged infiltration of T lymphocytes and transient T lymphocyte-dependent collagen deposition in mouse lungs following adenoviral gene transfer of CCL18. Arthritis. Rheum. 54, 2643–2655 [DOI] [PubMed] [Google Scholar]
- 132. Pochetuhen K., Luzina I. G., Lockatell V., Choi J., Todd N. W., Atamas S. P. (2007) Complex regulation of pulmonary inflammation and fibrosis by CCL18. Am. J. Pathol. 171, 428–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Luzina I. G., Todd N. W., Nacu N., Lockatell V., Choi J., Hummers L. K., Atamas S. P. (2009) Regulation of pulmonary inflammation and fibrosis through expression of integrins αVβ3 and αVβ5 on pulmonary T lymphocytes. Arthritis Rheum. 60, 1530–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Atamas S. P., Luzina I. G., Choi J., Tsymbalyuk N., Carbonetti N. H., Singh I. S., Trojanowska M., Jimenez S. A., White B. (2003) Pulmonary and activation-regulated chemokine stimulates collagen production in lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 29, 743–749 [DOI] [PubMed] [Google Scholar]
- 135. Atamas S. P., Luzina I. G., Ingels J., Choi J., Wong W. K., Furst D. E., Clements P. J., Postlethwaite A. E. (2010) Stimulation with type I collagen induces changes in gene expression in peripheral blood mononuclear cells from patients with diffuse cutaneous systemic sclerosis (scleroderma). Clin. Exp. Immunol. 161, 426–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Atamas S. P., White B. (2003) Cytokine regulation of pulmonary fibrosis in scleroderma. Cytokine Growth Factor Rev. 14, 537–550 [DOI] [PubMed] [Google Scholar]
- 137. Luzina I. G., Atamas S. P., Wise R., Wigley F. M., Choi J., Xiao H. Q., White B. (2003) Occurrence of an activated, profibrotic pattern of gene expression in lung CD8+ T cells from scleroderma patients. Arthritis Rheum. 48, 2262–2274 [DOI] [PubMed] [Google Scholar]
- 138. Luzina I. G., Atamas S. P., Wise R., Wigley F. M., Xiao H. Q., White B. (2002) Gene expression in bronchoalveolar lavage cells from scleroderma patients. Am. J. Respir. Cell Mol. Biol. 26, 549–557 [DOI] [PubMed] [Google Scholar]
- 139. Luzina I. G., Tsymbalyuk N., Choi J., Hasday J. D., Atamas S. P. (2006) CCL18-stimulated upregulation of collagen production in lung fibroblasts requires Sp1 signaling and basal Smad3 activity. J. Cell. Physiol. 206, 221–228 [DOI] [PubMed] [Google Scholar]
- 140. Pardo A., Smith K. M., Abrams J., Coffman R., Bustos M., McClanahan T. K., Grein J., Murphy E. E., Zlotnik A., Selman M. (2001) CCL18/DC-CK-1/PARC up-regulation in hypersensitivity pneumonitis. J. Leukoc. Biol. 70, 610–616 [PubMed] [Google Scholar]
- 141. Prasse A., Pechkovsky D. V., Toews G. B., Jungraithmayr W., Kollert F., Goldmann T., Vollmer E., Muller-Quernheim J., Zissel G. (2006) A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18. Am. J. Respir. Crit. Care Med. 173, 781–792 [DOI] [PubMed] [Google Scholar]
- 142. de Nadai P., Charbonnier A. S., Chenivesse C., Senechal S., Fournier C., Gilet J., Vorng H., Chang Y., Gosset P., Wallaert B.., et al. (2006) Involvement of CCL18 in allergic asthma. J. Immunol. 176, 6286–6293 [DOI] [PubMed] [Google Scholar]
- 143. Mrazek F., Sekerova V., Drabek J., Kolek V., du Bois R. M., Petrek M. (2002) Expression of the chemokine PARC mRNA in bronchoalveolar cells of patients with sarcoidosis. Immunol. Lett. 84, 17–22 [DOI] [PubMed] [Google Scholar]
- 144. Atamas S. P., Luzina I. G., Dai H., Wilt S. G., White B. (2002) Synergy between CD40 ligation and IL-4 on fibroblast proliferation involves IL-4 receptor signaling. J. Immunol. 168, 1139–1145 [DOI] [PubMed] [Google Scholar]
- 145. Luzina I. G., Todd N. W., Iacono A. T., Atamas S. P. (2008) Roles of T lymphocytes in pulmonary fibrosis. J. Leukoc. Biol. 83, 237–244 [DOI] [PubMed] [Google Scholar]
- 146. Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686 [DOI] [PubMed] [Google Scholar]
- 147. Martinez F. O., Gordon S., Locati M., Mantovani A. (2006) Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 177, 7303–7311 [DOI] [PubMed] [Google Scholar]
- 148. Anthony R. M., Urban J. F., Alem F., Hamed H. A., Rozo C. T., Boucher J-L., Van Rooijen N., Gause W. C. (2006) Memory TH2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat. Med. 12, 955–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Zhao A., Urban J. F., Jr., Anthony R. M., Sun R., Stiltz J., van Rooijen N., Wynn T. A., Gause W. C., Shea-Donohue T. (2008) Th2 cytokine-induced alterations in intestinal smooth muscle function depend on alternatively activated macrophages. Gastroenterology 135, 217–225.e211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Bowcutt R., Bell L. V., Little M., Wilson J., Booth C., Murray P. J., Else K. J., Cruickshank S. M. (2011) Arginase-1-expressing macrophages are dispensable for resistance to infection with the gastrointestinal helminth Trichuris muris. Parasite Immunol. 33, 411–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Herbert D. B. R., Orekov T., Roloson A., Ilies M., Perkins C., O'Brien W., Cederbaum S., Christianson D. W., Zimmermann N., Rothenberg M. E.., et al. (2010) Arginase I suppresses IL-12/IL-23p40–driven intestinal inflammation during acute schistosomiasis. J. Immunol. 184, 6438–6446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. El Kasmi K. C., Qualls J. E., Pesce J. T., Smith A. M., Thompson R. W., Henao-Tamayo M., Basaraba R. J., Konig T., Schleicher U., Koo M-S.., et al. (2008) Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat. Immunol. 9, 1399–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Butcher B. A., Fox B. A., Rommereim L. M., Kim S. G., Maurer K. J., Yarovinsky F., Herbert D. B. R., Bzik D. J., Denkers E. Y. (2011) Toxoplasma gondii rhoptry kinase ROP16 activates STAT3 and STAT6 resulting in cytokine inhibition and arginase-1-dependent growth control. PLoS Pathog. 7, e1002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Yamamoto M., Standley D. M., Takashima S., Saiga H., Okuyama M., Kayama H., Kubo E., Ito H., Takaura M., Matsuda T.., et al. (2009) A single polymorphic amino acid on Toxoplasma gondii kinase ROP16 determines the direct and strain-specific activation of Stat3. J. Exp. Med. 206, 2747–2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Van den Bossche J., Malissen B., Mantovani A., De Baetselier P., Van Ginderachter J. A. (2012) Regulation and function of the E-cadherin/catenin complex in cells of the monocyte-macrophage lineage and DCs. Blood 119, 1623–1633 [DOI] [PubMed] [Google Scholar]
- 156. Van den Bossche J., Laoui D., Morias Y., Movahedi K., Raes G., De Baetselier P., Van Ginderachter J. A. (2012) Claudin-1, claudin-2 and claudin-11 genes differentially associate with distinct types of anti-inflammatory macrophages in vitro and with parasite- and tumor-elicited macrophages in vivo. Scand. J. Immunol. 75, 588–598 [DOI] [PubMed] [Google Scholar]
- 157. Agarwal R., Mori Y., Cheng Y., Jin Z., Olaru A. V., Hamilton J. P., David S., Selaru F. M., Yang J., Abraham J. M.., et al. (2009) Silencing of claudin-11 is associated with increased invasiveness of gastric cancer cells. PLoS ONE 4, e8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Menzies F. M., Henriquez F. L., Alexander J., Roberts C. W. (2011) Selective inhibition and augmentation of alternative macrophage activation by progesterone. Immunology 134, 281–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Varin A., Mukhopadhyay S., Herbein G., Gordon S. (2010) Alternative activation of macrophages by IL-4 impairs phagocytosis of pathogens but potentiates microbial-induced signalling and cytokine secretion. Blood 115, 353–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Hunter M. M., Wang A., Parhar K. S., Johnston M. J. G., Van Rooijen N., Beck P. L., McKay D. M. (2010) In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology 138, 1395–1405 [DOI] [PubMed] [Google Scholar]
- 161. Rizzo A., Monteleone I., Fina D., Stolfi C., Caruso R., Fantini M. C., Franzè E, Schwendener R., Pallone F., Monteleone G. (2012) Inhibition of colitis by IL-25 associates with induction of alternatively activated macrophages. Inflamm. Bowel Dis. 18, 449–459 [DOI] [PubMed] [Google Scholar]
- 162. Weisser S. B., Brugger H. K., Voglmaier N. S., McLarren K. W., van Rooijen N., Sly L. M. (2011) SHIP-deficient, alternatively activated macrophages protect mice during DSS-induced colitis. J. Leukoc. Biol. 90, 483–492 [DOI] [PubMed] [Google Scholar]
- 163. Weng M., Huntley D., Huang I-F, Foye-Jackson O., Wang L., Sarkissian A., Zhou Q., Walker W. A., Cherayil B. J., Shi H. N. (2007) Alternatively activated macrophages in intestinal helminth infection: effects on concurrent bacterial colitis. J. Immunol. 179, 4721–4731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Chen C-C., Louie S., McCormick B., Walker W. A., Shi H. N. (2005) Concurrent infection with an intestinal helminth parasite impairs host resistance to enteric Citrobacter rodentium and enhances Citrobacter-induced colitis in mice. Infect. Immun. 73, 5468–5481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Urban J. F., Jr., Steenhard N. R., Solano-Aguilar G. I., Dawson H. D., Iweala O. I., Nagler C. R., Noland G. S., Kumar N., Anthony R. M., Shea-Donohue T.., et al. (2007) Infection with parasitic nematodes confounds vaccination efficacy. Vet. Parasitol. 148, 14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Schroedl C., Kalhan R. (2012) Incidence, treatment options, and outcomes of lung cancer in patients with chronic obstructive pulmonary disease. Curr. Opin. Pulm. Med. 18, 131–137 [DOI] [PubMed] [Google Scholar]
- 167. Terzić J., Grivennikov S., Karin E., Karin M. (2010) Inflammation and colon cancer. Gastroenterology 138, 2101–2114 e2105 [DOI] [PubMed] [Google Scholar]
- 168. Schmieder A., Michel J., Schönhaar K., Goerdt S., Schledzewski K. (2012) Differentiation and gene expression profile of tumor-associated macrophages. Semin. Cancer Biol. 22, 289–297 [DOI] [PubMed] [Google Scholar]
- 169. Wang B., Li Q., Qin L., Zhao S., Wang J., Chen X. (2011) Transition of tumor-associated macrophages from MHC class II(hi) to MHC class II(low) mediates tumor progression in mice. BMC Immunol. 12, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Li B. H., Xu S. B., Li F., Zou X. G., Saimaiti A., Simayi D., Wang Y. H., Zhang Y., Yuan J., Zhang W. J. (2012) Stat6 activity-related Th2 cytokine profile and tumor growth advantage of human colorectal cancer cells in vitro and in vivo. Cell. Signal. 24, 718–725 [DOI] [PubMed] [Google Scholar]
- 171. Chawla A., Nguyen K. D., Goh Y. P. S. (2011) Macrophage-mediated inflammation in metabolic disease. Nat. Rev. Immunol. 11, 738–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Olefsky J. M., Glass C. K. (2010) Macrophages, inflammation, and insulin resistance. Ann. Rev. Physiol. 72, 219–246 [DOI] [PubMed] [Google Scholar]
- 173. Stienstra R., Duval C., Keshtkar S., van der Laak J., Kersten S., Müller M. (2008) Peroxisome proliferator-activated receptor γ activation promotes infiltration of alternatively activated macrophages into adipose tissue. J. Biol. Chem. 283, 22620–22627 [DOI] [PubMed] [Google Scholar]
- 174. Odegaard J. I., Ricardo-Gonzalez R. R., Goforth M. H., Morel C. R., Subramanian V., Mukundan L., Eagle A. R., Vats D., Brombacher F., Ferrante A. W.., et al. (2007) Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature 447, 1116–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Fischer-Posovszky P., Wang Q. A., Asterholm I. W., Rutkowski J. M., Scherer P. E. (2011) Targeted deletion of adipocytes by apoptosis leads to adipose tissue recruitment of alternatively activated M2 macrophages. Endocrinology 152, 3074–3081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Kawanishi N., Yano H., Yokogawa Y., Suzuki K. (2010) Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc. Immunol. Rev. 16, 105–118 [PubMed] [Google Scholar]
- 177. Xia S., Sha H., Yang L., Ji Y., Ostrand-Rosenberg S., Qi L. (2011) Gr-1+ CD11b+ myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity. J. Biol. Chem. 286, 23591–23599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Wenzel S., Wilbraham D., Fuller R., Getz E. B., Longphre M. (2007) Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet 370, 1422–1431 [DOI] [PubMed] [Google Scholar]