TLR activation by multiple pathways leads to triglyceride accumulation in macrophages that could contribute to the accelerated atherosclerosis seen in chronic infections and inflammatory diseases.
Keywords: LPS, zymosan, toll-like receptors, glucose, fatty acid, oxidation
Abstract
LPS treatment of macrophages induces TG accumulation, which is accentuated by TG-rich lipoproteins or FFA. We defined pathways altered during macrophage activation that contribute to TG accumulation. Glucose uptake increased with activation, accompanied by increased GLUT1. Oxidation of glucose markedly decreased, whereas incorporation of glucose-derived carbon into FA and sterols increased. Macrophage activation also increased uptake of FFA, associated with an increase in CD36. Oxidation of FA was markedly reduced, whereas the incorporation of FA into TGs increased, associated with increased GPAT3 and DGAT2. Additionally, macrophage activation decreased TG lipolysis; however, expression of ATGL or HSL was not altered. Macrophage activation altered gene expression similarly when incubated with exogenous FA or AcLDL. Whereas activation with ligands of TLR2 (zymosan), TLR3 (poly I:C), or TLR4 (LPS) induced alterations in macrophage gene expression, leading to TG accumulation, treatment of macrophages with cytokines had minimal effects. Thus, activation of TLRs leads to accumulation of TG in macrophages by multiple pathways that may have beneficial effects in host defense but could contribute to the accelerated atherosclerosis in chronic infections and inflammatory diseases.
Introduction
Macrophages have a central role in the link of inflammation and infection with atherosclerosis. Macrophages recognize inflammatory stimuli, such as bacterial LPS, by means of a family of TLRs [1–3]. These receptors, named for their homology to the Drosophila Toll protein, are specific for PAMPs and are expressed abundantly on macrophages [1–3]. For example, LPS is recognized by TLR4, whereas the dsRNA viral analog poly I:C is recognized by TLR3, and the fungal product zymosan is recognized by TLR2 [1–3]. In addition to exogenous microbial-derived activators, TLRs may be activated by endogenously produced compounds, such as FA, oxidized LDLs, and heat shock proteins [4, 5].
Interestingly, polymorphisms in TLR4 that attenuate their signaling ability have been associated with a reduced risk of atherosclerosis in humans [6, 7]. Similarly, in murine models of atherosclerosis, a deficiency of TLR2, TLR4, or MyD88, a key adapter protein in TLR signaling, also results in a decrease in lesion formation [8–10]. Conversely, concurrent infections increase the risk of atherosclerosis, and studies in animal models have shown that the administration of LPS accelerates the development of atherosclerosis in apolipoprotein E-deficient mice and cholesterol fed rabbits [11–14]. By means of the specific receptor–ligand interactions, these TLRs activate signaling cascades that induce a number of significant changes in cellular gene expression in macrophages.
A characteristic finding in atherosclerosis is the accumulation of cholesterol esters and TG in macrophages, leading to the formation of foam cells. Our laboratory and others [15–17] have shown previously that LPS-stimulated macrophages store more TG and cholesterol ester. When macrophages were incubated with cholesterol ester-rich lipoproteins (β VLDL or LDL), LPS treatment increased cholesterol ester accumulation by approximately threefold [15]. In contrast, when macrophages were activated by LPS in the absence of cholesterol ester-enriched lipoproteins, there was no increase in cholesterol ester storage [15]. However, there was an increase in the accumulation of TGs [15]. Moreover, in the presence of TG-enriched lipoproteins or FFA, the increase in TG storage in macrophages was markedly enhanced by LPS treatment [15, 18, 19]. Of note, foam cells have been found not only in atherosclerotic lesions but also in other tissues during chronic inflammatory conditions, such as parasitic infections and leprosy [20–22]. Additionally, incubation of bacteria with macrophages results in the accumulation of lipids, which is dependent on TLR signaling [20–22].
The mechanisms by which TLR activation leads to lipid accumulation are not well-defined. Recent studies from our laboratory [18, 19] have shown that TLR activators stimulate the expression of aP2 and Mal1, FA-binding proteins in macrophages. The uptake of FA is enhanced by these FA-binding proteins, and the combined deficiency of aP2 and Mal1 results in a decrease in atherosclerosis, indicating that these FA-binding proteins play an important role in neutral lipid accumulation in macrophages [23]. Additionally, the expression of ADRP/ADFP in macrophages is also increased by TLR agonists [18]. ADRP/ADFP is a member of the perilipin–adipophilin–tail-interacting protein of the 47-kDa family that is involved in the movement and storage of neutral lipids in a variety of cell types [24]. Studies have shown that an increase in ADRP/ADFP allows for the increased storage of cholesterol esters in macrophages, whereas the decreased expression of ADRP/ADFP reduces cholesterol ester accumulation [25]. The purpose of the present study was to determine the pathways in glucose and lipid metabolism that are altered during macrophage activation, which could contribute to this TG accumulation.
MATERIALS AND METHODS
Materials
LPS from Escherichia coli strain O55:B5 was purchased from Difco (Detroit, MI, USA). DMEM was purchased from Fisher Scientific (Pittsburgh, PA, USA). FBS was purchased from Hyclone (Logan, UT, USA), and HSA was obtained from Bayer (Elkhart, IN, USA). TRI Reagent and OA were purchased from Sigma-Aldrich (St. Louis, MO, USA). AcLDL was purchased from Intracel (Frederick, MD, USA). poly I:C and zymosan were purchased from InvivoGen (San Diego, CA, USA). The mouse cytokines TNF-α and IL-1β were purchased from R&D Systems (Minneapolis, MN, USA). C1-BODIPY 500/510 C12 was obtained from Invitrogen (Carlsbad, CA, USA). [1-14C]-OA (51 mCi/mmol), d-[14C(U)]-glucose (3.2 mCi/mmol), 2-[1,2-3H (N)]-DOG (26.5 Ci/mmol), and [α-32P]-dCTP (3000 Ci/mmol) were purchased from PerkinElmer Life Sciences (Boston, MA, USA).
Cell culture
RAW 264.7, a murine macrophage cell line, was obtained from American Type Culture Collection (Manassas, VA, USA). Cells were grown in 75 cm2 flasks in DMEM supplemented with 10% FBS and incubated at 37°C in 5% CO2. Confluent flasks were used to seed six-well plates for experiments. When confluent, cells were washed with serum-free medium once and then treated in medium with 2.5% HSA in the presence or absence of TLR activators. For lipid-loading experiments, AcLDL at 100 μg/ml or 0.3 mM OA was coincubated with LPS at 100 ng/ml for 24 h. For inhibitor studies, cells were preincubated with 500 μg/ml thalidomide for 1 h prior to treatment.
Mouse peritoneal macrophage culture
Peritoneal macrophages were harvested from C57BL/6 mice 3 days after the i.p. injection of 40 μg Con A in 0.5 ml PBS and then cultured as described previously by Tang et al. [26]. Cells were plated in 12-well plates in DMEM containing 10% FBS and 20% L cell culture medium and allowed to adhere to wells for 1 h. Cells were washed with serum-free medium and then treated in DMEM supplemented with 2.5% HSA with LPS (100 ng/ml) for 16 h.
RNA isolation and Northern blot analysis
Total RNA was isolated from 100 mm dishes using TRI Reagent. RAW cell RNA (30 μg) was denatured and electrophoresed on 1% agarose-formaldehyde gels. The uniformity of sample loading was verified by UV visualization of the ethidium-bromide-stained gel prior to electrotransfer to Nytran membrane. 32P-Labeled cDNA was prepared using the random priming method (Amersham Biosciences, Piscataway, NJ, USA). CD36 cDNA was kindly provided by Dr. N. A. Abumrad of Washington University (St. Louis, MO, USA). CPT1α cDNA probe was generated by RT-PCR with the following primers: TCGGTACTCTCTGAAGATGGC and GAGCAGAGTGGAATCGTGGGA. mRNA levels were quantified by means of the Personal FX phosphorimager (Bio-Rad, Hercules, CA, USA).
qRT-PCR
First-strand cDNA was synthesized from 1 μg total RNA with the iScript cDNA synthesis kit (Bio-Rad). The real-time PCR reaction contained 20 ng reverse-transcribed total RNA, 450 nM forward and reverse primers, and 10 μl 2× LightCycler 480 SYBR Green I Master (Roche Applied Science, Indianapolis, IN, USA) in a final volume of 20 μl in 96-well plates using the MyiQ real-time PCR system (Bio-Rad). Products were electrophoresed to confirm the specificity of the reaction. Quantification was performed by the comparative threshold cycle (Ct) method with 36B4 used for normalization. The primers used in these studies are shown in Table 1.
Table 1. Sequences of Primers Used for Real-Time qPCR.
| Gene | Forward | Reverse | Accession # |
|---|---|---|---|
| 36B4 | GCGACCTGGAAGTCCAACTAC | X15267 | |
| GLUT1 | GGATCTCTCTGGAGCACAGG | TCCTCCTGGACTTCACTGCT | NM_011400 |
| GLUT2 | CCCTGGGTACTCTTCACCAA | GCCAAGTAGGATGTGCCAAT | NM_031197 |
| GLUT3 | CGCCAATGGCTTTTAAGTGT | CCCCTTCCCTCCCAATATAA | NM_011401 |
| GLUT4 | ACTCTTGCCACACAGGCTCT | CCTTGCCCTGTCAGGTATGT | NM_009204 |
| GLUT5 | GATAAACGGCTGAAGGGTGA | GCCAAAACCTGAGCAAAGTC | NM_019741 |
| CD36 | GATGTGGAACCCATAACTGGAT | CCCAGTCTCATTTAGCCACAGTA | NM_007643 |
| FATP1 | TGCTTTGGTTTCTGGGACTT | GCTCTAGCCGAACACGAATC | NM_011977 |
| FATP2 | ATGCCGTGTCCGTCTTTTAC | CTTCAGACCTCCACGACTCC | NM_011978 |
| FATP3 | TTGCTTCTGGCATCTGTGAC | GGCTGTAAAATCGCTGCTTC | NM_028817 |
| FATP4 | CATGAGGAGAGTGTGGCTCA | GCTAAGGGCTTATCCCAAGG | NM_011989 |
| FATP5 | TCATTGCTGACCCCCTCAC | TGGCTAGGTGGTCAGAGCTT | NM_009512 |
| FATP6 | GCAACACTTCTGCTGGAACA | TAGCCTGCATGGACTTGATG | NM_144823 |
| FACS1 | CAGGCAAAGCATGTCTTCAA | ATCTGCTGCATCTGCTTGG | NM_007981.3 |
| CPT1a | GCACTGCAGCTCGCACATTACAA | CTCAGACAGTACCTCCTTCAGGAAA | NM_013495.2 |
| CPT1b | CCCATGTGCTCCTACCAGAT | CCTTGAAGAAGCGACCTTTG | NM_009948 |
| Ndufs8 | TGGCGGCAACGTACAAGTAT | CCTCGGATGAGTTCTGTCCA | NM_144870 |
| AtP5g1 | AGTTGGTGTGGCTGGATCA | GCTGCTTGAGAGATGGGTTC | NM_007506.6 |
| Idh3a | CCTCCTGCTTAGTGCTGTGA | CGTTGCCTCCCAGATCTTT | NM_029573 |
| Cox5a | GGGTCACACGAGACAGATGA | GGAACCAGATCATAGCCAACA | NM_007747 |
| GPAT1 | CAACACCATCCCCGACATC | GTGACCTTCGATTATGCGATCA | NM_008149 |
| GPAT3 | GGAGGATGAAGTGACCCAGA | CCAGTTTTTGAGGCTGCTGT | NM_172715.3 |
| GPAT4 | TGTCTGGTTTGAGCGTTCTG | TTCTGGGAAGATGAGGATGG | NM_018743.4 |
| Lipin1 | CGCCAAAGAATAACCTGGAA | TGAAGACTCGCTGTGAATGG | NM_172950.3 |
| Lipin2 | CCTGCTTATCTTGCCACCTC | GCCTGCTCCTCCTTCCTATT | NM_001164885.1 |
| DGAT1 | TCGTGGTATCCTGAATTGGTG | AGGTTCTCTAAAAATAACCTTGCATT | NM_010046.2 |
| DGAT2 | AGTGGCAATGCTATCATCACGT | AAGGAATAAGTGGGAACCAGATCA | NM_026384 |
| ATGL | GCCACAGCGCTGGTCACT | CCTCCTTGGACACCTCAATAATG | NM_025802.3 |
| HSL | GGCTTACTGGGCACAGATACCT | CTGAAGGCTCTGAGTTGCTCAA | NM_010719 |
| PPAR[ | CCTGAACATCGAGTGTCGAATAT | GTTCTTCTTCTGAATCTTGCAGCT | NM_011144 |
| PPARδ | CTCGAGTATGAGAAGTGCGA | CATCCGTCCAAAGCGGATAG | NM_011145 |
| PPARγ | CCACCAACTTCGGAATCA | TTTGTGGATCCGGCAGTTA | NM_00503 |
| TNF-α | TGTCTCAGCCTCTTCTCATT | GCCATTTGGGAACTTCTCAT | NM_013693.2 |
FA and glucose oxidation assays
RAW cells in six-well plates were treated with LPS or control media for 16 h. Cells were scraped into 2 ml KRP buffer and then incubated with 0.4 mM [1-14C]-OA or 0.3 mM d-[14C(U)]-glucose for an additional 1 or 2 h, respectively, at 37°C, according to the protocol described by Kaikaus et al [27]. The scintillation counts were normalized to protein concentration for each sample.
TG synthesis assays
RAW cells in six-well plates were treated with LPS or control media for 16 h and then incubated with 0.4 mM [1-14C]-OA for an additional 6 h in the presence or absence of LPS. Cells in each sample were scraped into 1 ml KRP buffer and dissolved in chloroform:methanol (2:1). Two sets of extractions were performed collecting the lower chloroform phase. Polar and nonpolar lipids were separated by TLC on Partisil high-performance silica gel 60 plates with two different solvent systems, chloroform:methanol:acetic acid:formic acid:water (35:15:6:2:1) for polar and hexane:diethyl ether:water (70:34:1) for nonpolar lipids. TG and FFA spots were identified by comparison with known standards after visualization with iodine vapor. FA incorporation was assessed by exposure of the TLC plate to a Personal FX (Bio-Rad) phosphorimager screen.
2-DOG assay
RAW cells in six-well plates were treated with LPS or control media for 16 h, and 2-DOG uptake was measured as described previously [28]. The uptake was normalized to protein concentration for each sample.
Immunocytochemistry
RAW cells were seeded on chamber glass slides and grown overnight prior to treatment with 100 ng/ml LPS for 16 h. After fixation with 2% formaldehyde, cells were quenched with 100 mM glycine and then permeabilized in 0.5% saponin in PBS. Cells were then blocked for 1 h with 10% goat serum and 0.1% saponin in PBS. Slides were then incubated for 1 h with rabbit anti-GLUT1 at 1:500 (Abcam, Cambridge, MA, UA), followed by incubation for 1 h with Alexa 488 goat anti-rabbit IgG at 1:1000 (Invitrogen). All staining was observed with a Zeiss LSM510 Meta confocal microscope.
BODIPY FA uptake assay
RAW cells were plated in 96-well plates and treated with LPS or control media for 16 h. The cells were subsequently washed twice with PBS and then incubated with 1 μg/ml C1-BODIPY 500/510 C12 for 2 min at 37°C. After an additional four washes, the fluorescence/well was read with the Victor-2 multilabel counter (PerkinElmer Life Sciences).
Saponifiable and nonsaponifiable lipid synthesis assays
RAW cells in six-well plates were treated with LPS or control media for 16 h and then incubated with 0.3 mM d-[14C(U)]-glucose for an additional 2 h at 37°C in the presence or absence of LPS. Cells in each sample were dissolved in 1 ml 0.1 N NaOH and saponified with 3 ml ETOH and 0.5 ml 50% KOH for 1 h at 37°C. Cholesterol and FA standards were added, and petroleum ether extractions were performed to separate saponifiable and nonsaponifiable lipids, according to the method described by Brown and co-workers [29]. TLC on Silica Gel G plates with hexane:ethyl acetate (5:1) as a solvent system for nonsaponifiable lipids or heptane:diethyl ether:acetic acid (90:30:1) as a solvent system for saponifiable lipids was used to isolate the cholesterol and FA spots, respectively. After visualization with iodine vapor, the individual spots were scraped, counted, and normalized to protein concentration.
TG hydrolase activity assay
RAW cells in six-well plates were treated with LPS or control media for 16 h, washed two times with PBS, and then sonicated in 100 mM potassium phosphate lysis buffer (250 mM sucrose, 1 mM EDTA, 1 mM DTT, protease inhibitor cocktail). TG hydrolase activity was measured according to the method described by Chandak et. al [30]. Briefly, 100 μg protein from cell lysates was incubated at 37°C for 1 h with100 μl substrate. TG substrate contained 25 nm triolein/assay and 40,000 cpm/nmol [9,10-3H] triolein (PerkinElmer Life Sciences) as a tracer. The reaction mixture was extracted, radioactivity in 1 ml lipid phase was determined by liquid scintillation counting, and the release of FFA was calculated.
TG accumulation measurement
Following 24 h treatment, cells were washed twice with PBS and scraped into 200 μl PBS. The cell suspensions were sonicated, and TG levels were assayed using a commercially available enzymatic assay (Sigma-Aldrich). The TG accumulation was normalized to protein concentration for each sample.
Lactate assays
RAW cells in six-well plates were treated with LPS or control media for 16 h, and the extracellular media was collected. The pH was measured by means of a pH meter, and the lactate concentration was measured by means of YSI 2300 STAT Plus glucose and lactate analyzer (YSI, Yellow Springs, OH, USA).
Statistics
Data are presented as mean ± sem. Statistical significance between two groups was determined by using the Student's t test. When multiple samples were compared, one-way ANOVA analysis was done to determine statistical significance. A P value <0.05 was considered significant.
RESULTS
Effect of LPS on glucose metabolism in macrophages
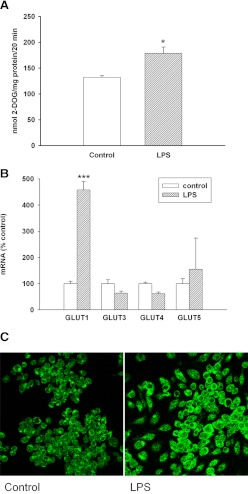
Glucose is a major energy source for macrophages [31]. Therefore, we first examined the effect of LPS treatment on the uptake of glucose by macrophages. As shown in Fig. 1A, the uptake of 2-deoxyglucose is increased by LPS treatment, confirming observations by other investigators [32, 33]. This increase in glucose uptake can be explained by a marked increase in GLUT 1 mRNA (Fig. 1B) and protein levels (Fig. 1C). Of note is an increase in GLUT 1 protein seen on the plasma membrane (Fig. 1C). In contrast, LPS treatment caused little change in the expression of GLUT3, -4, and -5. GLUT2 mRNA was not detected in untreated or LPS-treated macrophages.
Figure 1. (A) The effect of LPS on glucose uptake into macrophages.
RAW cells were treated with LPS at 100 ng/ml in serum-free medium for 16 h and then incubated with 2 mM 2-DOG for 20 min. Data are presented as nmol uptake/mg protein over the 20-min incubation period (mean±sem). (B) Effect of LPS on the expression of GLUTs in macrophages. RAW cells were treated with LPS (100 ng/ml), RNA was isolated, and GLUT expression levels were measured by RT-PCR. Data are presented as percent change of control (mean±sem). *P < 0.05; ***P < 0.001. (C) Immunofluorescence analysis for GLUT1 in RAW cells. Cells were treated with LPS at 100 ng/ml in serum-free medium for 16 h. Immunostaining was performed, as described in Materials and Methods. Fluorescent GLUT1 staining was visualized by confocal microscopy with a 40× oil immersion objective lens. All images were acquired with identical settings.
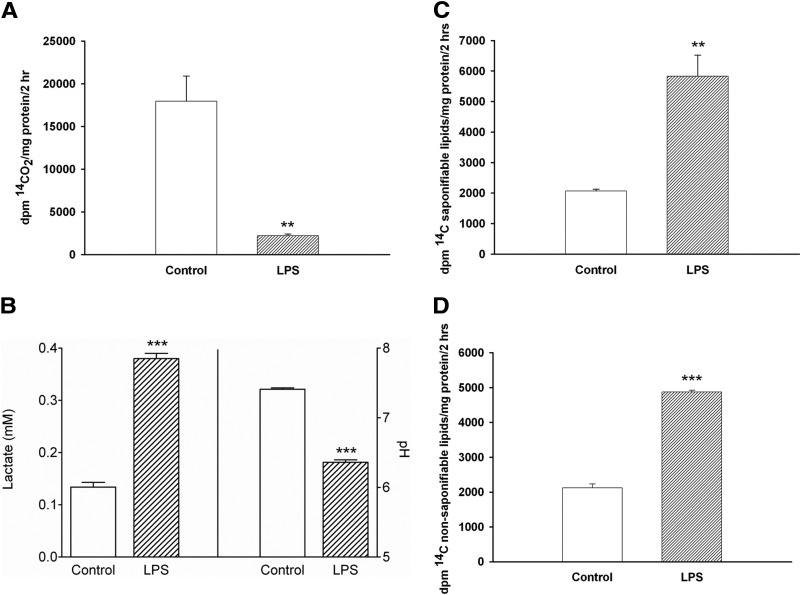
We next examined the metabolism of glucose in LPS-treated compared with control macrophages. As shown in Fig. 2A, the oxidation of glucose to CO2 is strikingly suppressed in LPS-treated macrophages. In contrast, in LPS-treated macrophages, the conversion of glucose to lactate is increased greatly, resulting in a decrease in the pH of the media in the LPS-treated macrophages (Fig. 2B). As shown in Figs. 2C and D, the incorporation of glucose-derived carbon into saponifiable (FA) and nonsaponifiable (sterols) lipids is increased in LPS-treated macrophages.
Figure 2. (A) The effect of LPS on glucose oxidation in macrophages.
RAW cells were treated with LPS at 100 ng/ml in serum-free medium for 16 h and then incubated with 0.3 mM d-[14C(U)]-glucose for 2 h. 14CO2 production was determined, as described in Materials and Methods. Data are presented as dpm 14CO2/mg protein/2 h (mean±sem). (B) Effect of LPS on extracellular lactate and pH levels in macrophages. RAW cells were treated with LPS for 16 h, medium was obtained, and lactate and pH were determined, as described in Materials and Methods. Data are presented as mean lactate or pH ± sem. (C and D) The effect of LPS on the incorporation of glucose into saponifiable (C) and nonsaponifiable lipids (D). RAW cells were treated with LPS at 100 ng/ml in serum-free medium for 16 h and then incubated with 0.3 mM d-[14C(U)]-glucose for an additional 2 h in the presence or absence of LPS. Lipids were isolated by petroleum ether extraction and separated by TLC, as described in Materials and Methods. Data are presented as dpm 14C saponifiable lipids (C) or nonsaponifiable lipids (D)/mg protein/2 h (mean±sem). **P < 0.01; ***P < 0.001.
Thus, the metabolism of glucose is markedly altered in LPS-activated macrophages, resulting in the increased uptake of glucose with a decrease in glucose oxidation to CO2 and an increase in the formation of lactate and an increase in the incorporation of glucose into lipid. This increased conversion of glucose to lipid in LPS-treated macrophages could contribute to the increase in macrophage lipid storage that occurs in LPS-activated macrophages.
Effect of LPS on FA metabolism in macrophages
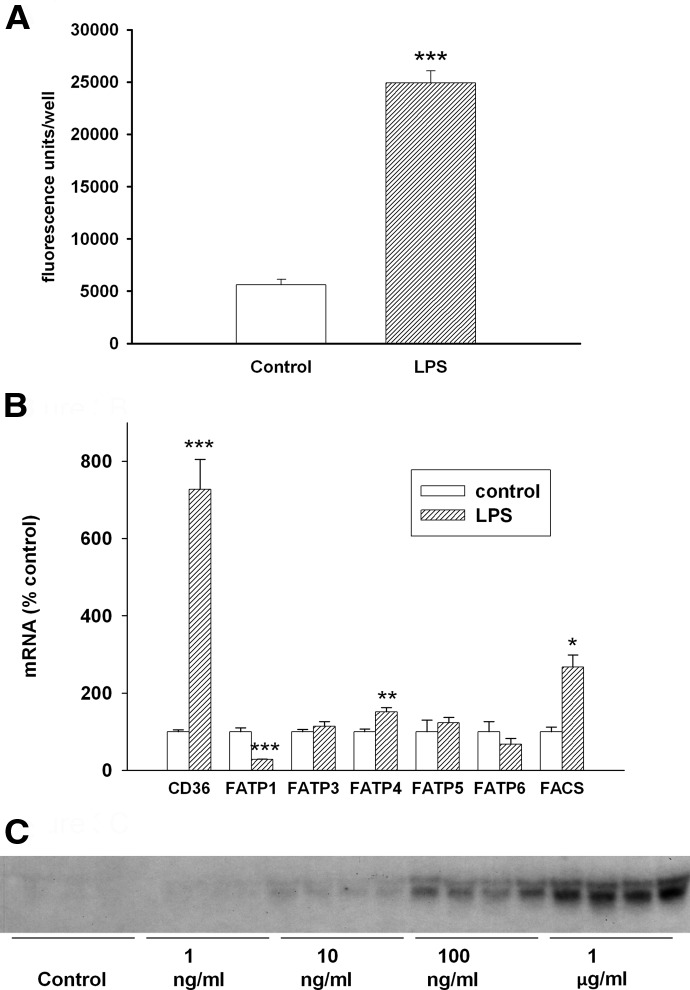
As discussed in Introduction, incubating macrophages with TG-enriched lipoproteins or FFA markedly accentuates the increase in TG accumulation induced by LPS treatment. We therefore next examined the effect of LPS treatment on the uptake of FA. As shown in Fig. 3A, LPS treatment increases FA uptake in macrophages. This increase in FA uptake is associated with a marked increase in CD36 expression (Fig. 3B). The effect of various doses of LPS on CD36 expression is shown in Fig. 3C and indicates that LPS can markedly increase CD36 expression but that relatively high doses are required. The expression of FATP4 is also increased slightly by LPS treatment, whereas FATP1 is decreased greatly (Fig. 3B). FATP3, -5, and -6 expression is not altered by LPS treatment, and FATP2 mRNA is not detected in LPS-treated or control macrophages. In addition, LPS treatment increased levels of fatty acyl-CoA synthase 1 (also named ACSL1), which converts FA to FA-CoA for metabolism (Fig. 3B).
Figure 3. (A) Effect of LPS on FA uptake in macrophages.
RAW cells were treated with LPS at 100 ng/ml in serum-free medium for 16 h and then incubated with C1-BODIPY 500/510 C12 for 2 min. Data are presented as fluorescence units/well (mean±sem). (B) Effect of LPS on the expression of FA transporters in macrophages. RAW cells were treated with LPS at 100 ng/ml in serum-free medium for 16 h, and FA transporter mRNA levels were measured by RT-PCR, as described in Materials and Methods. Data are presented as percent change of control (mean±sem). (C) Effect of different doses of LPS on CD36 expression. Northern blot of CD36 expression as a function of LPS concentration in RAW cells was carried out, as described in Materials and Methods. Cells were treated with LPS at the indicated concentrations (n=4 for each concentration) in serum-free medium for 16 h. *P < 0.05; **P < 0.01; ***P < 0.001.
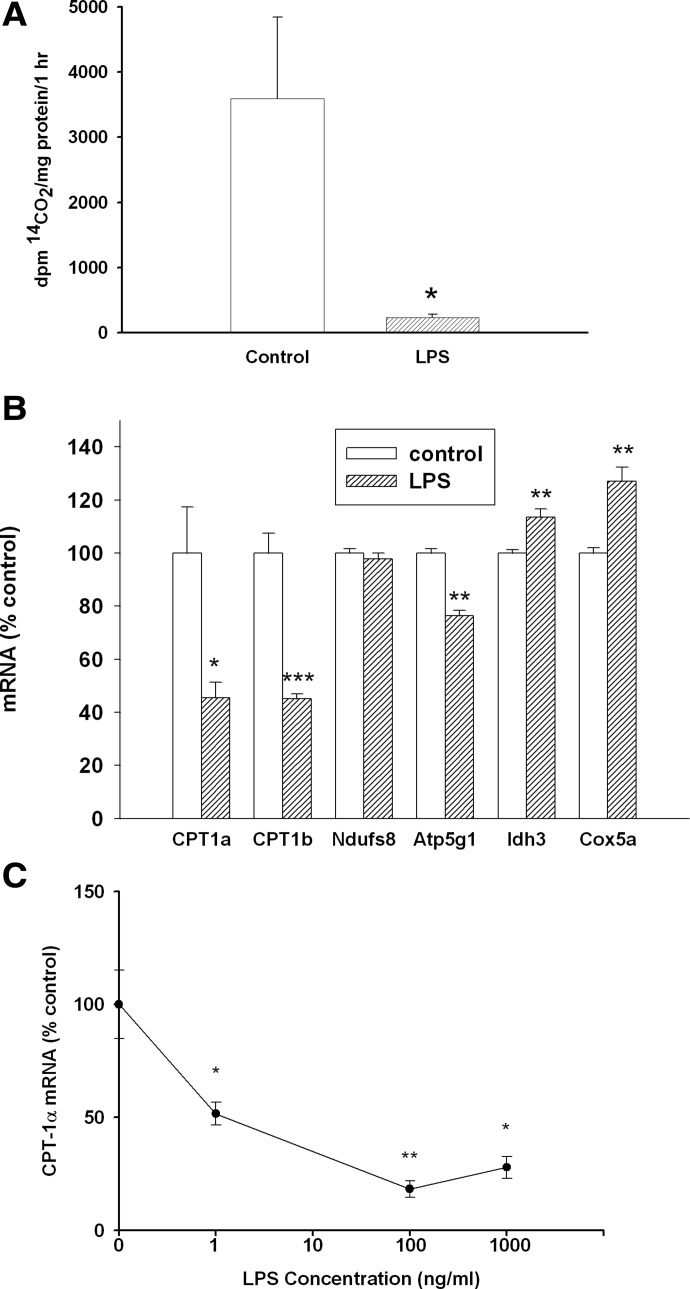
We next examined the effect of LPS treatment on the metabolism of FA in macrophages. LPS treatment results in a striking decrease in the oxidation of FA by macrophages (Fig. 4A). As shown in Fig. 4B, this decrease in FA oxidation is associated with a marked suppression of the expression of CPT1α and -β, proteins that are required for the transport of FA into mitochondria for oxidation. CPT1α is more robustly expressed in macrophages (Ct CPT1α-25; Ct CPT1β-29), and the effect of various doses of LPS on CPT1α expression is shown in Fig. 4C. In contrast to CD36, low doses of LPS markedly inhibit CPT1α expression (one-half maximum inhibition is seen at 1 ng/ml). As glucose and FA oxidation were greatly decreased by LPS treatment, we next determined the effect of LPS treatment on the expression of several mitochondrial proteins. As shown in Fig. 4B, LPS did not markedly affect the expression of a variety of other mitochondrial proteins.
Figure 4. (A) Effect of LPS on FA oxidation in macrophages.
RAW cells were treated with LPS at 100 ng/ml in serum-free medium for 16 h and then incubated with 0.4 mM [1-14C]-OA for an additional 1 h. 14CO2 production was quantified, as described in Materials and Methods. Data are presented as dpm 14CO2/mg protein/h (mean±sem). (B) Effect of LPS on the expression of genes involved in FA oxidation in macrophages. RAW cells were treated with LPS at 100 ng/ml in serum-free medium for 16 h, and oxidation gene mRNA levels were measured by RT-PCR, as described in Materials and Methods. Data are presented as percent change of control (mean±sem). (C) Effect of different doses of LPS on CPT1α expression. Cells were treated with LPS at the indicated concentrations (n=4 for each concentration) in serum-free medium for 16 h. A Northern blot of CPT1α expression as a function of LPS concentration in RAW cells was carried out, as described in Materials and Methods. Data are presented as percent change of control (mean±sem). *P < 0.05; **P < 0.01; ***P < 0.001.
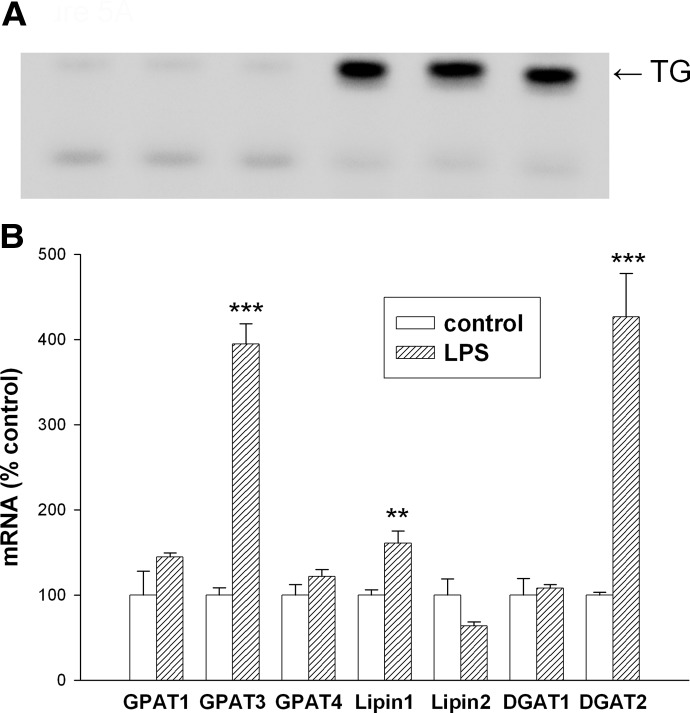
We next examined the incorporation of radiolabeled FA into TGs. As shown in Fig. 5A, the incorporation of FA into TGs is increased in LPS-treated macrophages. In association with this increase in incorporation, the expression of several of the key enzymes in glycerol lipid synthesis is increased greatly (Fig. 5B). Specifically, mRNA levels of GPAT3, Lipin 1, and DGAT2 are increased. In contrast, the expression of GPAT1, GPAT4, lipin 2, and DGAT1 is not increased by LPS treatment. Thus, FA uptake and the incorporation of FA into TGs are increased by LPS treatment and could contribute to the increase in TG storage that occurs in LPS-activated macrophages.
Figure 5. (A) Effect of LPS on FA incorporation into TG in macrophages.
RAW cells were treated with LPS at 100 ng/ml in serum-free medium for 16 h and then incubated with 0.4 mM [1-14C]-OA for an additional 6 h (n=3 for each condition). Lipids were isolated by chloroform extraction and separated by TLC, as described in Materials and Methods. TG spots were identified by comparison with known standards after visualization with iodine vapor, and FA incorporation was assessed by exposure of the TLC plate to a phosphorimager screen. (B) Effect of LPS on the expression of enzymes that synthesize TGs. RAW cells were treated with LPS at 100 ng/ml in serum-free medium for 16 h, and mRNA levels of enzymes that synthesize TGs were measured by RT-PCR, as described in Materials and Methods. Data are presented as percent change of control (mean±sem). **P < 0.01; ***P < 0.001.
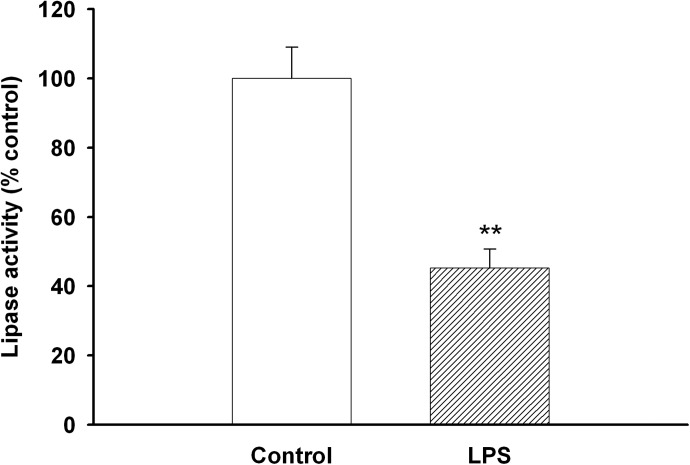
In addition to increasing TG formation, LPS treatment decreased TG lipolysis (Fig. 6). However, the expression of the enzymes that break down intracellular TGs, ATGL and HSL, was not altered by LPS treatment (data not shown). Thus, increased TG synthesis and decreased TG degradation may account for the increase in TG storage in LPS-treated macrophages.
Figure 6. Effect of LPS on TG lipolysis.
RAW cells were treated with LPS at 100 ng/ml in serum-free medium for 16 h, and TG hydrolase activity was measured, as described in Materials and Methods. Data are presented as percent change of control (mean±sem). **P < 0.01.
The expression of many of the genes involved in FA metabolism is regulated by PPARs. PPARα was not detected in RAW cells, whereas PPARδ was expressed abundantly (Ct 25-26) but did not change with LPS treatment. In contrast, PPARγ, which is expressed relatively abundantly (Ct 29-30), decreased by ∼70% with LPS treatment, an observation similar to that reported previously by other investigators [34].
Role of the MyD88 pathway
LPS activates TLR4, which has several intracellular pathways that regulate gene expression [35, 36]. The major pathway is via MyD88, which increases the activity of NF-κB and AP-1. Inhibiting the MyD88 pathway by thalidomide treatment blocked the LPS-induced increase in TNF mRNA levels by ∼90% (positive control). Thalidomide treatment also decreased the ability of LPS to increase GLUT1 expression by 81%. However, the increase in expression of GPAT3, DGAT2, and CD36 was affected only minimally by MyD88 inhibition (20–30% decrease in mRNA levels). This indicates that the postreceptor signaling pathways, by which LPS alters expression, differ among GLUT1 and GPAT3, DGAT2, and CD36 and that the pathway for the LPS-induced increase in GPAT3, DGAT2, and CD36 expression is not dependent on the activation of the MyD88 pathway.
Effect of LPS on gene expression in mouse peritoneal macrophages
To determine whether these LPS effects on lipid metabolism also occurred in primary macrophages, we next examined the effect of LPS treatment in mouse peritoneal macrophages. Similar to our observations in RAW cells, LPS treatment stimulated the expression of GLUT 1 [sevenfold increase, GPAT3 (35-fold increase) and DGAT2 (753-fold increase)]. In contrast to our observations in RAW cells, the expression of CD36 was not increased by LPS treatment in mouse peritoneal macrophages.
Effect of other macrophage activators on gene expression
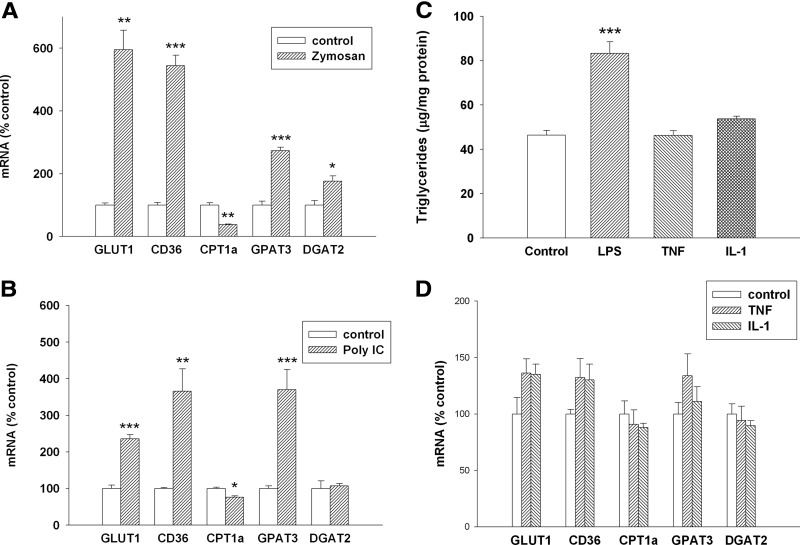
As noted above, the expression of several key genes in macrophages involved in the metabolism of glucose and FAs is altered by treatment with LPS, which binds to TLR4 and activates macrophages. We next examined the effect of zymosan, a TLR2 activator, in macrophages. Similar to LPS, treatment of RAW cells with zymosan increased TG accumulation (control=100%±6.2; zymosan=197%±20.5; P<0.01). As shown in Fig. 7A, similar to what was observed with LPS, zymosan treatment also results in an increase in GLUT1, CD36, GPAT3, and DGAT2, whereas decreasing the expression of CPT1α. Poly I:C, an activator of TLR3, also increases TG accumulation in RAW cells (control=100%±6.7; poly I:C=130%±10.4; P<0.03) and has a similar effect on gene expression with the exception that DGAT2 expression is not increased (Fig. 7B). In contrast, TNF and IL-1 have no effect on TG accumulation in macrophages (Fig. 7C) or the expression of GLUT1, CD36, GPAT3, and DGAT2 (Fig. 7D). Thus, activators of the TLRs have marked effects on several key genes involved in glucose and FA metabolism, whereas cytokines have minimal effects.
Figure 7. Effect of TLR activators and cytokines on gene expression and TG accumulation in macrophages.
RAW cells were treated for 16 h, and mRNA levels were measured by RT-PCR, as described in Materials and Methods. (A) Zymosan treatment at 500 μg/ml. (B) poly I:C treatment at 50 μg/ml. (C) RAW cells were treated with LPS at 100 ng/ml and TNF-α and IL-1β at 10 ng/ml for 24 h. TG levels were measured, as described in Materials and Methods. Data are presented as μg TG/mg protein. (D) RAW cells were treated with TNF-α or IL-1β at 10 ng/ml for 16 h, and mRNA levels were measured by RT-PCR, as described in Materials and Methods. Data are presented as percent change of control (mean±sem). (C and D) One-way ANOVA analysis was done to compare all treatments with controls using Tukey-Kramer as the post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001.
Effect of LPS on gene expression in lipid-loaded macrophages
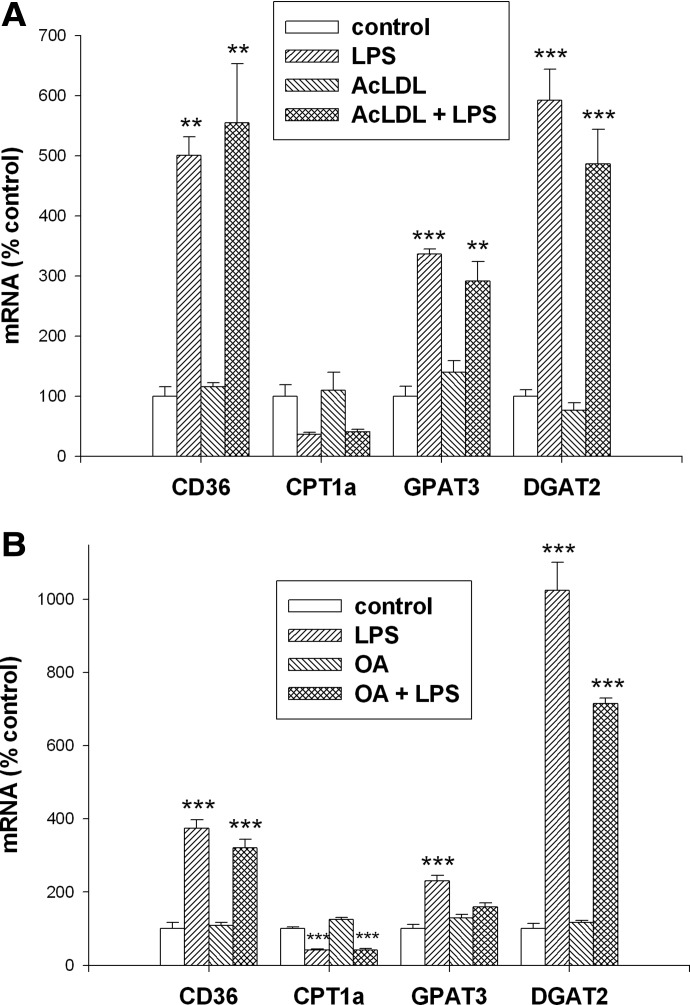
We next determined the effect of LPS treatment in macrophages loaded with cholesterol by incubating with AcLDL. As shown in Fig. 8A, loading with cholesterol does not affect the expression of CD36, CPT1α, GPAT3, or DGAT2. LPS treatment of macrophages loaded with cholesterol results in virtually identical increases in the expression of CD36, GPAT3, and DGAT2 with a decrease in CPT1α expression (Fig. 8A).
Figure 8. Effect of lipid loading on gene expression in macrophages.
Cells were treated for 16 h, and mRNA levels were measured by RT-PCR, as described in Materials and Methods. (A) Cells were coincubated with AcLDL at 100 μg/ml and LPS at 100 ng/ml. (B) Cells were coincubated with OA at 0.3 mM and LPS at 100 ng/ml. Data are presented as percent change of control (mean±sem). One-way ANOVA analysis was done to compare all treatments with controls using Tukey-Kramer as the post hoc test. **P < 0.01; ***P < 0.001 compared with the corresponding controls.
In cells loaded with TG by coincubation with OA, no change in the basal expression of CD36, CPT1α, GPAT3, or DGAT2 is observed (Fig. 8B). The LPS-induced changes in gene expression are similar to control macrophages, with the exception that GPAT3 is not increased significantly by LPS treatment in the TG-loaded cells. Thus, altering the state of macrophages by loading with cholesterol or FAs does not greatly alter the macrophage response to LPS treatment.
DISCUSSION
Previous studies have shown that ligands that activate TLRs lead to the accumulation of TGs in macrophages [15, 18–22]. The primary purpose of the present study was to elucidate and characterize the metabolic pathways that account for this TG accumulation. We identified multiple pathways that could all contribute to the increase in TG accumulation in activated macrophages.
First, in agreement with the results of other investigators, we found that there is an increase in glucose uptake by activated macrophages that is mediated by an increase in the expression of GLUT1 [32, 33]. This increase in glucose uptake may be used in PET scans to allow for the determination of the number of activated macrophages in atherosclerotic lesions [37]. Moreover, we observed that there is a marked decrease in glucose oxidation to CO2 leading to the increased accumulation of lactic acid, resulting in a decrease in the pH of the media. Others have also observed that the activation of macrophages leads to a reduction in media pH, and the area surrounding sites of infection or injury in vivo is typically a more acidic environment [31, 38, 39]. Furthermore, it is of interest that areas of rupture-prone plaque are also acidic [40]. The formation of an acid milieu may be beneficial in the killing of microorganisms but could also have adverse side-effects. For example, it has been proposed that an acidic environment accelerates the oxidation and aggregation of lipoproteins, which would lead to the increased uptake of lipoproteins by macrophages [41]. The decrease in glucose oxidation to CO2 also increases the glucose available for the synthesis of lipids, and we observed that LPS treatment of macrophages resulted in the increased incorporation of glucose-derived carbon into FAs and sterols. Other investigators have reported that LPS activation of macrophages leads to the increased incorporation of acetate into FAs [42]. Very recently, Im and colleagues [43] have shown that LPS treatment of macrophages increases sterol regulatory element-binding protein-1a expression and activation, which could account for the increased expression of the enzymes required for the de novo synthesis of FAs. In addition, a decrease in the oxidation of glucose to CO2 could provide an increase in substrate available for the synthesis of FAs. The increased incorporation of glucose into FAs could contribute to the increased accumulation of TG in activated macrophages.
Second, we found that macrophage activation stimulated FA uptake into the cell, and this increased transport was accompanied by a marked increase in CD36 expression, a protein well-recognized to transport FAs. Of note is that the increase in CD36 required relatively high concentrations of LPS and was not observed in mouse peritoneal macrophages treated with LPS. This suggests that changes in CD36 may occur only with severe insults. Inflammation is characterized by the stimulation of lipolysis in adipose tissue, resulting in the increased release of FAs and an elevation in serum-FFA levels [44]. Additionally, the levels of TG-rich lipoproteins are also increased during inflammation [44]. However, the uptake of FAs from TG-rich lipoproteins may not be increased markedly in macrophages, as studies by other investigators have shown that in LPS-treated macrophages, there is a decrease in the activity of lipoprotein lipase, which is required for the breakdown of the TG in lipoproteins [45, 46]. However, given that FFA levels are increased, it is likely that the quantities of FAs available to macrophages and other cells would be increased during an inflammatory state; thus, the increased ability of activated macrophages to transport these FAs into the cell would result in the increased use of FAs during the inflammatory state.
Similar to the metabolism of glucose, the ability of activated macrophages to oxidize FAs to CO2 was reduced greatly. Expression of CPT1α and -β, proteins that facilitate the transport of FAs into the mitochondria for oxidation, was decreased, which could contribute to the decrease in FA oxidation. However, we also observed that the oxidation of acetate to CO2 was decreased in LPS-treated macrophages (data not shown), indicating other defects in mitochondria function. Studies have shown that LPS treatment disrupts the structure of mitochondria and can lead to mitochondrial dysfunction, which could account for the ability of LPS treatment to decrease the oxidation of acetate, glucose, and FAs [47–50].
Whereas the oxidation of FAs was reduced, the incorporation of FAs into TGs was markedly enhanced. A similar increase in TG synthesis was observed by Posokhova et al. [42]. Associated with this increase in TG synthesis was an increase in the expression of GPAT3 and DGAT2. GPAT3 catalyzes the initial committed step in the synthesis of TGs, the acylation of glycerol-3-phosphate to form lysophosphatidic acid, whereas DGAT catalyzes the final step, the conversion of diacylglycerol to TG. The combination of an increase in substrate uptake, decrease in oxidation, and an increase in the expression of TG synthesis enzymes was accompanied by increased synthesis of TGs, which could contribute to the accumulation of TG in macrophages.
Finally, we found that macrophage activation decreases TG lipolysis. However, the expression of ATGL—which catalyzes the initial step in lipolysis by removing the first FA from TG, thereby generating diacylglycerols—and HSL—which catalyzes the removal of FAs from diacylglycerol, leading to the formation of monoacylglycerols—was not altered by LPS treatment. It is well recognized in adipocytes that the rate of lipolysis is not regulated by alterations in the expression of ATGL and HSL. Instead, the rate of lipolysis is regulated by the phosphorylation state of HSL and the location of other proteins, such as perilipin that interact with lipid droplets and facilitate or inhibit lipolysis [51–53]. A reduction in the breakdown of TGs could also contribute to the net TG accumulation in activated macrophages. Thus, following the activation of macrophages, there are multiple mechanisms that could contribute to the accumulation of TG.
The ability of LPS to alter the expression of genes important in TG accumulation occurred under a variety of different conditions. Specifically, in macrophages incubated with high concentrations of FAs, which results in an increase in TG storage, or AcLDL, which results in an increase in the storage of cholesterol, LPS treatment still resulted in similar alterations in the expression of genes that contribute to TG accumulation. The fact that TLR activation can alter the expression of genes even in cells that have already accumulated cholesterol could explain why infections and inflammation accelerate atherosclerosis. Of note is that the accumulation of TG or cholesterol in macrophages that were not exposed to LPS did not alter the expression of genes important in TG metabolism; these data indicate that the accumulation of lipid per se is not the cause of the changes in gene expression induced by LPS.
LPS (TLR4 ligand and a model of gram-negative infections), zymosan (TLR2 ligand and a model of fungal infections), and poly I:C (TLR3 ligand and a model of viral infections) all increased TG storage and induced changes in the expression of key genes that could contribute to TG accumulation. However, TNF and IL-1 had little or no effect on TG storage and these same genes in macrophages. This pattern is similar to our previous observations where LPS, zymosan, and poly I:C increased the expression of ADRP/ADFP and Mal1, whereas cytokines had little effect [18]. These results suggest that the activation of TLRs has effects on macrophages that are not mimicked by cytokine activation of macrophages. One can postulate that the activation of TLRs is more closely linked to the ability of the host to resist infections than activation of cytokine receptors (see below).
Whereas the alterations of gene expression induced by TLR activation may play a role in atherosclerosis, it is likely that these changes evolved, as they play an important role in host defense and the ability to resist infections. Recent studies have shown that the lipolysis of TGs plays a key role in macrophage phagocytosis [30]. Thus, the accumulation of TG through TLR stimulation may be preparing the macrophage to ingest and kill microorganisms. Alternatively, the increase in fat droplets in macrophages may play a role in sequestering intracellular microorganisms and preventing their replication. It is also possible that the large quantities of TGs or perhaps metabolic products of TG (for example, FFA) may be toxic to microorganisms and play a role in killing bacteria, viruses, or fungi [54]. Additionally, the enzymes required for eicosanoid synthesis are localized to lipid bodies, and eicosanoids are produced in these lipid bodies [55]. The increase in lipid storage in activated macrophages may provide a substrate for the enhanced synthesis of eicosanoids. Lastly, cytokines are also stored in these lipid bodies [55]. Thus, the increased formation of lipid bodies during macrophage activation may be to facilitate the production and storage of bioactive compounds that play a role in host defense. Future studies will need to determine the role that the increased TG accumulation plays in macrophage-defensive functions.
In conclusion, the present study demonstrates that the activation of TLRs in macrophages leads to the accumulation of TG by multiple pathways, including increasing glucose uptake and incorporation into lipid, increased FA uptake, increased synthesis of TGs, and a decrease in TG lipolysis. The beneficial effects of lipid accumulation during macrophage activation remain to be elucidated, but this increased accumulation of lipid could contribute to the acceleration of atherosclerosis observed with chronic infections and inflammatory diseases.
ACKNOWLEDGMENTS
This work was supported by grants from the Research Service of the Department of Veterans Affairs, U.S. National Institutes of Health grants 5 RO1 AR049932 and 2 RO1 HD29706, and the Albert L. and Janet A. Shultz Supporting Foundation, which were administered by the Northern California Institute for Research and Education and with resources of the Veterans Affairs Medical Center (San Francisco, CA, USA).
Footnotes
- 2-DOG
- 2 deoxy-d-glucose
- AcLDL
- acetylated LDL
- ADRP (ADFP)
- adipocyte differentiation-related protein
- ATGL
- adipose triglyceride lipase
- AtP5g1
- ATP synthase, H+ transporting mitochondrial Fo complex subunit c
- Cox5a
- cytochrome c oxidase subunit 5a
- CPT1
- carnitine palmitoyltransferase 1
- DGAT
- diacylglycerol acyltransferase
- FA
- fatty acid
- FATP
- fatty acid transport protein
- FFA
- free fatty acids
- GLUT
- glucose transporter
- GPAT
- glycerol 3-phosphate acyltransferase
- HSL
- hormone-sensitive lipase
- Idh3a
- isocitrate dehyrogenase 3 (NAD+) α
- KRP
- Krebs-Ringer phosphate
- Ndufs8
- NADH dehydrogenase (ubiquinone) Fe-S protein 8
- OA
- oleic acid
- poly I:C
- polyinosinic:polycytidylic acid
- PPAR
- peroxisome proliferator-activated receptor
- qRT-PCR
- quantitative RT-PCR
- TG
- triglyceride
AUTHORSHIP
K.R.F. designed the research and wrote the paper. J.K.S. performed experiments, analyzed the data, and wrote the paper. M.R.K., C.M.M., S.M.P., A.S.C., and A.M. performed experiments and analyzed the data. C.G. designed and supervised the research.
REFERENCES
- 1. Aderem A., Ulevitch R. J. (2000) Toll-like receptors in the induction of the innate immune response. Nature 406, 782–787 [DOI] [PubMed] [Google Scholar]
- 2. Beutler B. (2003) Innate immune responses to microbial poisons: discovery and function of the Toll-like receptors. Annu. Rev. Pharmacol. Toxicol. 43, 609–628 [DOI] [PubMed] [Google Scholar]
- 3. Kaisho T., Akira S. (2000) Critical roles of Toll-like receptors in host defense. Crit. Rev. Immunol. 20, 393–405 [PubMed] [Google Scholar]
- 4. Beg A. A. (2002) Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 23, 509–512 [DOI] [PubMed] [Google Scholar]
- 5. Ionita M. G., Arslan F., de Kleijn D. P., Pasterkamp G. (2010) Endogenous inflammatory molecules engage Toll-like receptors in cardiovascular disease. J Innate Immun. 2, 307–315 [DOI] [PubMed] [Google Scholar]
- 6. Ameziane N., Beillat T., Verpillat P., Chollet-Martin S., Aumont M. C., Seknadji P., Lamotte M., Lebret D., Ollivier V., de Prost D. (2003) Association of the Toll-like receptor 4 gene Asp299Gly polymorphism with acute coronary events. Arterioscler. Thromb. Vasc. Biol. 23, e61–e64 [DOI] [PubMed] [Google Scholar]
- 7. Kiechl S., Lorenz E., Reindl M., Wiedermann C. J., Oberhollenzer F., Bonora E., Willeit J., Schwartz D. A. (2002) Toll-like receptor 4 polymorphisms and atherogenesis. N. Engl. J. Med. 347, 185–192 [DOI] [PubMed] [Google Scholar]
- 8. Bjorkbacka H. (2006) Multiple roles of Toll-like receptor signaling in atherosclerosis. Curr. Opin. Lipidol. 17, 527–533 [DOI] [PubMed] [Google Scholar]
- 9. Michelsen K. S., Wong M. H., Shah P. K., Zhang W., Yano J., Doherty T. M., Akira S., Rajavashisth T. B., Arditi M. (2004) Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc. Natl. Acad. Sci. USA 101, 10679–10684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mullick A. E., Tobias P. S., Curtiss L. K. (2005) Modulation of atherosclerosis in mice by Toll-like receptor 2. J. Clin. Invest. 115, 3149–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Epstein S. E., Zhu J., Burnett M. S., Zhou Y. F., Vercellotti G., Hajjar D. (2000) Infection and atherosclerosis: potential roles of pathogen burden and molecular mimicry. Arterioscler. Thromb. Vasc. Biol. 20, 1417–1420 [DOI] [PubMed] [Google Scholar]
- 12. Lehr H. A., Sagban T. A., Ihling C., Zahringer U., Hungerer K. D., Blumrich M., Reifenberg K., Bhakdi S. (2001) Immunopathogenesis of atherosclerosis: endotoxin accelerates atherosclerosis in rabbits on hypercholesterolemic diet. Circulation 104, 914–920 [DOI] [PubMed] [Google Scholar]
- 13. Ostos M. A., Recalde D., Zakin M. M., Scott-Algara D. (2002) Implication of natural killer T cells in atherosclerosis development during a LPS-induced chronic inflammation. FEBS Lett. 519, 23–29 [DOI] [PubMed] [Google Scholar]
- 14. Pussinen P. J., Mattila K. (2004) Periodontal infections and atherosclerosis: mere associations? Curr. Opin. Lipidol. 15, 583–588 [DOI] [PubMed] [Google Scholar]
- 15. Funk J. L., Feingold K. R., Moser A. H., Grunfeld C. (1993) Lipopolysaccharide stimulation of RAW 264.7 macrophages induces lipid accumulation and foam cell formation. Atherosclerosis 98, 67–82 [DOI] [PubMed] [Google Scholar]
- 16. Lopes-Virella M. F., Klein R. L., Stevenson H. C. (1987) Low density lipoprotein metabolism in human macrophages stimulated with microbial or microbial-related products. Arteriosclerosis 7, 176–184 [DOI] [PubMed] [Google Scholar]
- 17. Oiknine J., Aviram M. (1992) Increased susceptibility to activation and increased uptake of low density lipoprotein by cholesterol-loaded macrophages. Arterioscler. Thromb. 12, 745–753 [DOI] [PubMed] [Google Scholar]
- 18. Feingold K. R., Kazemi M. R., Magra A. L., McDonald C. M., Chui L. G., Shigenaga J. K., Patzek S. M., Chan Z. W., Londos C., Grunfeld C. (2010) ADRP/ADFP and Mal1 expression are increased in macrophages treated with TLR agonists. Atherosclerosis 209, 81–88 [DOI] [PubMed] [Google Scholar]
- 19. Kazemi M. R., McDonald C. M., Shigenaga J. K., Grunfeld C., Feingold K. R. (2005) Adipocyte fatty acid-binding protein expression and lipid accumulation are increased during activation of murine macrophages by Toll-like receptor agonists. Arterioscler. Thromb. Vasc. Biol. 25, 1220–1224 [DOI] [PubMed] [Google Scholar]
- 20. D'Avila H., Maya-Monteiro C. M., Bozza P. T. (2008) Lipid bodies in innate immune response to bacterial and parasite infections. Int. Immunopharmacol. 8, 1308–1315 [DOI] [PubMed] [Google Scholar]
- 21. Mattos K. A., D'Avila H., Rodrigues L. S., Oliveira V. G., Sarno E. N., Atella G. C., Pereira G. M., Bozza P. T., Pessolani M. C. (2010) Lipid droplet formation in leprosy: Toll-like receptor-regulated organelles involved in eicosanoid formation and Mycobacterium leprae pathogenesis. J. Leukoc. Biol. 87, 371–384 [DOI] [PubMed] [Google Scholar]
- 22. Nicolaou G., Erridge C. (2010) Toll-like receptor-dependent lipid body formation in macrophage foam cell formation. Curr. Opin. Lipidol. 21, 427–433 [DOI] [PubMed] [Google Scholar]
- 23. Boord J. B., Maeda K., Makowski L., Babaev V. R., Fazio S., Linton M. F., Hotamisligil G. S. (2004) Combined adipocyte-macrophage fatty acid-binding protein deficiency improves metabolism, atherosclerosis, and survival in apolipoprotein E-deficient mice. Circulation 110, 1492–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bickel P. E., Tansey J. T., Welte M. A. (2009) PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim. Biophys. Acta 1791, 419–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Larigauderie G., Furman C., Jaye M., Lasselin C., Copin C., Fruchart J. C., Castro G., Rouis M. (2004) Adipophilin enhances lipid accumulation and prevents lipid efflux from THP-1 macrophages: potential role in atherogenesis. Arterioscler. Thromb. Vasc. Biol. 24, 504–510 [DOI] [PubMed] [Google Scholar]
- 26. Tang W., Walsh A., Tabas I. (1999) Macrophage-targeted CTP: phosphocholine cytidylyltransferase (1-314) transgenic mice. Biochim. Biophys. Acta 1437, 301–316 [DOI] [PubMed] [Google Scholar]
- 27. Kaikaus R. M., Sui Z., Lysenko N., Wu N. Y., Ortiz de Montellano P. R., Ockner R. K., Bass N.M. (1993) Regulation of pathways of extramitochondrial fatty acid oxidation and liver fatty acid-binding protein by long-chain monocarboxylic fatty acids in hepatocytes. Effect of inhibition of carnitine palmitoyltransferase I. J. Biol. Chem. 268, 26866–26871 [PubMed] [Google Scholar]
- 28. Grunfeld C., Shigenaga J. K. (1984) Nicotinamide and other inhibitors of ADP-ribosylation block deoxyglucose uptake in cultured cells. Biochem. Biophys. Res. Commun. 123, 785–791 [DOI] [PubMed] [Google Scholar]
- 29. Balasubramaniam S., Goldstein J. L., Faust J. R., Brown M. S. (1976) Evidence for regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and cholesterol synthesis in nonhepatic tissues of rat. Proc. Natl. Acad. Sci. USA 73, 2564–2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chandak P. G., Radovic B., Aflaki E., Kolb D., Buchebner M., Frohlich E., Magnes C., Sinner F., Haemmerle G., Zechner R., Tabas I., Levak-Frank S., Kratky D. (2010) Efficient phagocytosis requires triacylglycerol hydrolysis by adipose triglyceride lipase. J. Biol. Chem. 285, 20192–20201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Calder P. C., Dimitriadis G., Newsholme P. (2007) Glucose metabolism in lymphoid and inflammatory cells and tissues. Curr. Opin. Clin. Nutr. Metab. Care 10, 531–540 [DOI] [PubMed] [Google Scholar]
- 32. Fukuzumi M., Shinomiya H., Shimizu Y., Ohishi K., Utsumi S. (1996) Endotoxin-induced enhancement of glucose influx into murine peritoneal macrophages via GLUT1. Infect. Immun. 64, 108–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Spitzer J. J. (1995) Bacterial endotoxin effects on carbohydrate utilization and transport. Biochem. Soc. Trans. 23, 998–1002 [DOI] [PubMed] [Google Scholar]
- 34. Barish G. D., Downes M., Alaynick W. A., Yu R. T., Ocampo C. B., Bookout A. L., Mangelsdorf D. J., Evans R. M. (2005) A nuclear receptor atlas: macrophage activation. Mol. Endocrinol. 19, 2466–2477 [DOI] [PubMed] [Google Scholar]
- 35. Kawai T., Akira S. (2006) TLR signaling. Cell Death Differ. 13, 816–825 [DOI] [PubMed] [Google Scholar]
- 36. Sheedy F. J., O'Neill L. A. (2007) The troll in Toll: Mal and Tram as bridges for TLR2 and TLR4 signaling. J. Leukoc. Biol. 82, 196–203 [DOI] [PubMed] [Google Scholar]
- 37. Rudd J. H., Narula J., Strauss H. W., Virmani R., Machac J., Klimas M., Tahara N., Fuster V., Warburton E. A., Fayad Z. A., Tawakol A. A. (2010) Imaging atherosclerotic plaque inflammation by fluorodeoxyglucose with positron emission tomography: ready for prime time? J. Am. Coll. Cardiol. 55, 2527–2535 [DOI] [PubMed] [Google Scholar]
- 38. Sawyer R. G., Spengler M. D., Adams R. B., Pruett T. L. (1991) The peritoneal environment during infection. The effect of monomicrobial and polymicrobial bacteria on pO2 and pH. Ann. Surg. 213, 253–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Swallow C. J., Grinstein S., Sudsbury R. A., Rotstein O. D. (1991) Cytoplasmic pH regulation in monocytes and macrophages: mechanisms and functional implications. Clin. Invest. Med. 14, 367–378 [PubMed] [Google Scholar]
- 40. Naghavi M., John R., Naguib S., Siadaty M. S., Grasu R., Kurian K. C., van Winkle W. B., Soller B., Litovsky S., Madjid M., Willerson J. T., Casscells W. (2002) pH Heterogeneity of human and rabbit atherosclerotic plaques; a new insight into detection of vulnerable plaque. Atherosclerosis 164, 27–35 [DOI] [PubMed] [Google Scholar]
- 41. Leake D. S. (1997) Does an acidic pH explain why low density lipoprotein is oxidised in atherosclerotic lesions? Atherosclerosis 129, 149–157 [DOI] [PubMed] [Google Scholar]
- 42. Posokhova E. N., Khoshchenko O. M., Chasovskikh M. I., Pivovarova E. N., Dushkin M. I. (2008) Lipid synthesis in macrophages during inflammation in vivo: effect of agonists of peroxisome proliferator activated receptors α and γ and of retinoid X receptors. Biochemistry (Mosc) 73, 296–304 [DOI] [PubMed] [Google Scholar]
- 43. Im S. S., Yousef L., Blaschitz C., Liu J. Z., Edwards R. A., Young S. G., Raffatellu M., Osborne T. F. (2011) Linking lipid metabolism to the innate immune response in macrophages through sterol regulatory element binding protein-1a. Cell Metab. 13, 540–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Khovidhunkit W., Kim M. S., Memon R. A., Shigenaga J. K., Moser A. H., Feingold K. R., Grunfeld C. (2004) Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J. Lipid Res. 45, 1169–1196 [DOI] [PubMed] [Google Scholar]
- 45. Sopher O., Goldman R. (1987) Bacterial lipopolysaccharide suppresses the expression of lipoprotein lipase in murine macrophages: a process independent of tumor necrosis factor or interleukin 1. Immunol. Lett. 15, 261–265 [DOI] [PubMed] [Google Scholar]
- 46. White J. R., Chait A., Klebanoff S. J., Deeb S., Brunzell J. D. (1988) Bacterial lipopolysaccharide reduces macrophage lipoprotein lipase levels: an effect that is independent of tumor necrosis factor. J. Lipid Res. 29, 1379–1385 [PubMed] [Google Scholar]
- 47. Garedew A., Moncada S. (2008) Mitochondrial dysfunction and HIF1α stabilization in inflammation. J. Cell Sci. 121, 3468–3475 [DOI] [PubMed] [Google Scholar]
- 48. Kuwabara T., Imajoh-Ohmi S. (2004) LPS-induced apoptosis is dependent upon mitochondrial dysfunction. Apoptosis 9, 467–474 [DOI] [PubMed] [Google Scholar]
- 49. Lopez A., Albassam M., Yong S., Sharma A., Lillie L. E., Prior M. G. (1987) Profiles of type-II pneumocytes in rats inoculated intratracheally with bacterial lipopolysaccharide. Am. J. Vet. Res. 48, 1534–1539 [PubMed] [Google Scholar]
- 50. Van Bossuyt H., Wisse E. (1988) Structural changes produced in Kupffer cells in the rat liver by injection of lipopolysaccharide. Cell Tissue Res. 251, 205–214 [DOI] [PubMed] [Google Scholar]
- 51. Brasaemle D. L. (2007) Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J. Lipid Res. 48, 2547–2559 [DOI] [PubMed] [Google Scholar]
- 52. Kraemer F. B., Shen W. J. (2002) Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J. Lipid Res. 43, 1585–1594 [DOI] [PubMed] [Google Scholar]
- 53. Zechner R., Kienesberger P. C., Haemmerle G., Zimmermann R., Lass A. (2009) Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J. Lipid Res. 50, 3–21 [DOI] [PubMed] [Google Scholar]
- 54. Drake D. R., Brogden K. A., Dawson D. V., Wertz P. W. (2008) Thematic review series: skin lipids. Antimicrobial lipids at the skin surface. J. Lipid Res. 49, 4–11 [DOI] [PubMed] [Google Scholar]
- 55. Bozza P. T., Magalhaes K. G., Weller P. F. (2009) Leukocyte lipid bodies—biogenesis and functions in inflammation. Biochim. Biophys. Acta 1791, 540–551 [DOI] [PMC free article] [PubMed] [Google Scholar]