Natural Killer cells can augment the expression of TLR7 by resting B cells via IFNIII leading to enhanced ability to respond to TLR7 ligand.
Keywords: IL-28, IL-6, microarray analysis, ISG
Abstract
The PRR TLR7 plays a key role in the activation of autoantigen-reactive B cells. This response is increased markedly by IFN-α, produced by accessory cells, as a result of the up-regulation of TLR7. We report herein an alternative pathway by which TLR7 expression can be augmented. This finding was derived from continuation of ongoing studies to uncover interactions between NK and B cells. Here, we have compared gene expression profiles by microarray analysis of B cells before and after their interaction with purified NK cells. The most outstanding alteration of genes transcribed in B cells is a significant increase in the expression of many members of the ISG family, among which is TLR7. Further analysis revealed that the enhancement of TLR7 on B cells is not mediated via type I or type II IFN but by another cytokine, IL-28, a type III IFN, which acts in concert with contact-mediated interactions with NK cells. This increased expression allows B cells to respond more readily upon stimulation by its ligand and may increase in vivo responses to other TLR7 ligands, such as autoantigens, prior to or jointly with stimulation by other cytokines.
Introduction
With the recent explosion in studies of the PRRs, there is much interest in investigating the modulation of the adaptive responses via these elements of innate recognition [1]. Even before the discovery of the importance of these receptors, there is much evidence that NK and B cells, also representing these two arms, can interact productively at different stages of their differentiation (reviewed in refs. [2, 3]). The earliest in vivo record for this interaction comes from the evidence that IFN-γ, secreted by activated NK cells, can enhance the extent of B cell production of IgG2a [4, 5]. In these instances, NK cells need to be activated by IFN-α/β or IL-12, secreted by activated monocyte-macrophages or DCs. Activated NK cells can also initiate processes required for switch recombination in antigen-stimulated B cells by activating germline transcription of downstream exons [6, 7]. This effect is mediated via interaction between CD2 or CD244 on NK cells and CD48 on B cells and does not appear to require the participation of cytokines. To investigate whether NK cells can activate additional B cell processes, we have compared gene expression profiles of follicular B cells before and after they have been allowed to interact with NK cells. Surprisingly, although both cell types used were obtained from IFN-γ0/0 mice, the most distinct cohort of gene sequences up-regulated in B cells belonged to the ISG family. Interestingly, among the augmented B cell transcripts is that for TLR7, suggesting that IFN-α may be mediating the effect [8]. However, TLR7 mRNA expression in B cells, obtained from IFNAR0/0 animals, can also be enhanced. Thus, NK cells can apparently stimulate B cell expression of ISGs via alternate pathways. We show, in this report, that the best candidate for this mediator is IL-28, a member of type III IFNs. Therefore, these findings interject a further role for TLRs into the interaction between NK and B cells. More importantly, given the pivotal role of TLR7 in the induction of autoantibodies [9–11], these results further illustrate the important role that NK cells may play on B cell responses.
MATERIALS AND METHODS
Cell preparations and culture
NK cells were purified from BALB/c, BALB/c IFN-γ0/0 [12], C57BL/6, CD2/2B40/0 [13], or B6.IFNAR0/0 [14] mice, maintained in accord with institution guidelines. B6. TLR70/0 mice were provided by Dr. Edward Wakeland (University of Texas Southwestern Medical Center, Dallas, TX, USA). Spleen cells were first passed over a nylon wool column to remove adherent cells prior to isolation of NK cells by positive selection using PE-anti-CD49b (DX5; BioLegend, San Diego, CA, USA) antibodies, followed by magnetic bead selection (BD Biosciences, San Jose, CA, USA). Purified cells were used directly or propagated in IL-2, as described previously [6]. B cells were purified by negative selection by binding to anti-CD43 and anti-CD11b MicroBeads, followed by automax isolation (Miltenyi Biotec, Bergisch Gladbach, Germany). Recovered cells that were routinely >95%, positive for the CD19 marker, were Percoll-fractionated to obtain resting B cells that contain <2% marginal zone B cells, as defined by high CD21 and low CD23 staining. NK and B cells were cultured together or separately at 0.5–1 × 106/ml in the presence of 200 U/ml IL-2 in 24- or 48-well Falcon tissue-culture plates (BD Biosciences), with or without a membrane insert, at a ratio of 2 B cells:1 NK cell.
Antibodies and reagents
Culture additives include goat anti-IL-28a (Abscam, Cambridge, MA, USA), rIL-28 and rat anti-IL-28 antibody (R&D Systems, Minneapolis, MN, USA), the TLR7 ligand Gardiquimod (InvivoGen, San Diego, CA, USA), or whole anti-IgG or (Fab)′2 fragments of anti-IgM (Jackson ImmunoResearch, West Grove, PA, USA). Poly(I:C) was obtained from Amersham Biosciences (Piscataway, NJ, USA).
FACS analysis and sorting
Stained cells were collected on the FACScan flow cytometer and analyzed on CellQuest (BD Biosciences) or sorted on the MoFlo cytometer. Post sort analysis indicated >95% purity for each preparation.
Semiquantitative RT-PCR analysis
RNA was prepared and analyzed by RT-PCR analysis, as described previously [15]. All primers were ascertained to span intronic regions. Amplified products for each set of primers were authenticated by size. For all primer pairs, titration curves were performed to ascertain that the cycle number used fell within the linear range as cDNA concentrations were increased.
Microarray analysis
Microarray analysis was performed in the Microarray Core Facility of University of Texas Southwestern Medical Center. High-quality total RNA was amplified into cRNA, labeled and hybridized to the Mouse WG-6 v2.0 Expression Beadchip that queries 45,281 transcripts that cover >19,000 unique, curated genes in the National Center for Biotechnology Information RefSeq database (Build 36, Release 22). The entire experiment was performed twice. Processing and analysis of chips are described in the Appendix (see supplementary material). Four samples were analyzed for each run. These included: NK only; NK cells sorted from NKB cocultures; B cells only after culture; and B cells sorted from NKB cocultures.
RESULTS AND DISCUSSION
Microarray analysis of B cell genes altered by interaction with NK cells
Our previous experiments showed that activated NK cells can induce and/or enhance various differentiation markers on B cells [2]. To more broadly survey the influence of NK cells on B cell gene expression, we conducted genome-wide analysis of B cell transcripts, before and after a short period of interaction with NK cells. Follicular B cells were isolated to avoid the more heterogeneous B cell subsets that may have already undergone various stages of differentiation. Both preparations were obtained from IFN-γ0/0 mice to eliminate from the analysis B cell transcripts that may be activated via stimulation by IFN-γ, produced by NK cells. RNA extracted from the sorted cells were subjected to microarray analysis. Of the 45,000 sequences queried, ∼400 sequences exhibited a greater than twofold increase in expression level when comparing NK-stimulated B cells with nonstimulated B cells. Although the sorting after coculture of the two cell types was performed to >95% purity, the hybridization levels revealed some cross-contamination, as indicated by measurable hybridization to some genes that are supposedly not expressed by B cells but expressed at high levels in NK cells. To rule out the effect of contamination, we eliminated from consideration the candidates with hybridization levels at <15% of the signal exhibited by NK cells. As this is a relatively conservative exclusion, some relevant transcripts may have been discounted. In addition, sequences that did not correspond to annotated genes, could not be analyzed and were not considered further. Initial perusal of the remaining 250 genes (shown in Supplemental Table 1), whose expression was up-regulated, indicated that many of the ones shown by others to be augmented in B cells after a 24-h stimulation with anti-IgM, CD40 ligand, and IL-4 [16], such as NF-κB, TRAF1, IκBα, growth arrest and DNA damage 45β, LIF, activating transcription factor 3, or CD44, were not present within this cohort.
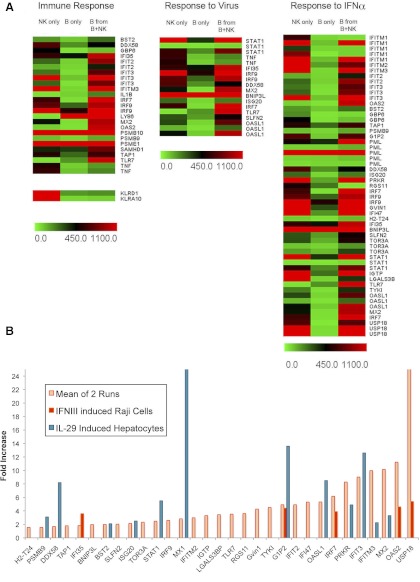
In an attempt to identify gene expression patterns in the 250 candidates that showed up-regulation in B cells after culture with NK cells, differentially expressed genes were clustered in functional categories based on the “biological process” principle of the Gene Ontology Consortium (http://www.ncbi.nlm.nih.gov). The analysis showed that the most significant enrichment of up-regulated genes occurred in two clusters in the category of “immune response” and in “response to virus”. As shown in the heat maps of these two clusters, most of these genes grouped under immune response also appeared under the category of response to virus. Many of the genes up-regulated in this category have been shown to be a result of IFN signaling, which results in the activation of the ISG family (reviewed in ref. [17]). In fact, we found that the expression of all of the genes within this cluster has been identified to be up-regulated upon stimulation of mouse spleen cells by IFN-α [18]. In addition, Fig. 1A shows a number of other genes induced in B cells by NK cells that are not included in the functional Gene Ontology functional categories but have been shown previously to be up-regulated by IFN-α/β in murine splenocytes cells [8] and in LN B cells upon injection of whole animals [22].
Figure 1. Clustering of B cell genes, whose expression was up-regulated by interaction with NK cells.
Follicular B cells were sorted by MoFlo cell sorter (BD Biosciences) on the basis of CD23hi and CD21lo (BioLegend) expression, as described previously [19]. The recovered cells were incubated overnight before coculture with purified NK cells that have been propagated for 7 days in IL-2 and subsequently, sort-purified based on NKp46 staining. Twenty hours after culture, the cells were harvested and NK and B cells separated by FACS sorting (bio-NKp46 and FL CD19). High-quality RNA was extracted and amplified into cRNA and subjected to microarray analysis as described in Materials and Methods. (A) Heat map of up-regulated genes revealed in one of the two microarray runs that fall within the Immune Response and Response to Virus clusters, defined by the Gene Ontology Consortium. The column labeled “Response to IFNα” includes the former two sets, as well as additional ISGs, up-regulated in B cells and extracted from various literature sources. Readings from some genes, where identical oligo probes were slotted several times, are also shown. To illustrate the extent of contamination of the B cell signal by high levels of expression in NK cells, signal from hybridization to sequences from killer cell lectin-like receptor subfamily D, member 1 (KLRD1), and killer cell lectin-like receptor subfamily A, member 10 (KLRA10), genes that are not expressed by B cells, are shown. (B) As the exact levels of increase in expression are not visualized easily in the heat maps, the fold increase in expression of B cell genes as a result of NK stimulation (average of two independent microarray experiments), which has also been shown to be increased by IFN-α or in response to virus infection in various cell types, was plotted. Also shown for comparison is the fold increase induced by IL-28 or IL-29 in Raji cells [20] or human hepatocytes [21], respectively.
To confirm these results, the entire experiment was repeated to obtain another set of microarray data. For the two experiments, the hybridization values for NK cells were almost identical, but the extent of induction of B cell expression was, in general, lower. However, the fold induction for most of the transcripts of interest from the two runs was similar. Fig. 2B shows the average level of enhancement of these ISGs in B cells obtained from the two independent microarray determinations. Although all of the genes presented show an average increase in transcript level of at least 1.8-fold, the ones with the highest expression level are clearly contained within the ISG56/IFIT1 gene family [23].
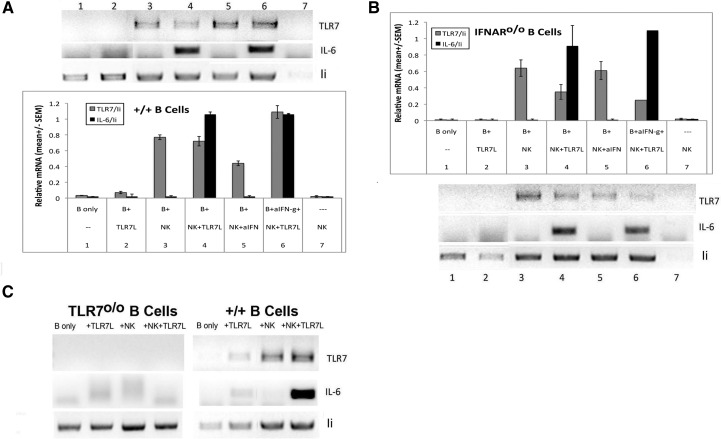
Figure 2. Stimulation of TLR7 mRNA expression on B cells by NK cells.
Resting B cells from B6 (A) or IFNAR0/0 (B) mice were cultured for 24 h in the presence of various reagents as indicated, with or without IL-2-propagated NK cells before TLR7 ligand (5 μg/ml) was added for another 20 h (ratio of NK:B=0.5). RNA prepared from each culture was then processed for semiquantitative RT-PCR analysis. Level of expression of each product was normalized to the level of invariant chain (Ii) of MHC II within each sample. ImageJ, a public domain, Java-based image processing program, developed at the U.S. National Institutes of Health, was used for semiquantitative determination of relative expression from gel bands. Mean (±sem) for each culture condition was derived from eight similar experiments for WT B cells and four for IFNAR0/0 B cells. (C) B cells from TLR70/0 or B6 mice were stimulated with NK cells and assayed as in A and B. Results shown are representative of two independent experiments. Sequence of primers: TLR7, forward 5′TGGCCAGAGAAATTGAGGAGGCCA, reverse 5′CCGCAACTCTCTCAACGGCCA; IL-6, forward 5′CCGGAGAGGAGACTTCACAG, reverse 5′GGAAATTGGGGTAGGAAGGA; invariant chain, forward 5′GAGGCTAGAGCCATGGATGAC, reverse 5′AGATGCTCCAGATTCTCTGGG.
Infection by some viruses stimulates, in addition to genes induced by type I IFN, expression of genes that are activated by type III IFN (IFN-λ; reviewed in ref. [24]). The expression pattern of these genes has not been well documented and not at all in B lymphocytes, but in other cell types, it appears that they represent a subset of the ones induced by type I IFN. For illustrative purposes, the levels of induction of genes induced in human cells by IL-28 or IL-29, which share the same receptor [21], are also shown in Fig. 1B. It is interesting that MxI transcripts, usually considered to be a gold standard for induction of the ISG family, were not induced by type III IFN in human Raji cells [20], a B cell lymphoma, but only in hepatocytes [21] (Fig. 1B). Notably, despite the induction of many ISG family genes, MxI was found to be up-regulated only to a relatively low extent in mouse B cells stimulated by NK cells. Furthermore, other IL-28 responsive genes, including IRF7 and IRF9, which were not induced by IFN-β in B cells from IFNAR0/0 mice [22], were clearly induced by IL-28, as well as by NK cells (Fig. 1B). These correlations, showing difference between type I and type III IFN induction of B cells, indicate a unique role of NK cell-mediated enhancement.
Other than the distinctive clustering of IFN-responsive genes, the remaining B cell genes, that showed large increases in expression levels, such as Phf11 and Syndecan-3 (Supplemental Table 1), were not found to be up-regulated in the repeat microarray analysis; therefore, they were not analyzed further. We have also attempted to perform cluster analysis of the B cell genes that were down-regulated by greater than twofold as a result of the interaction with NK cells (∼200 sequences). Other than the expression of some isolated antiapoptotic genes, the results did not reveal clear-cut, functional categories that merit further consideration.
The microarray analysis also includes assessment of transcript levels in NK cells before and after interaction with B cells. Analysis of the results revealed very few known genes, previously shown to be enhanced in NK cells after this interaction. This result, although disappointing, corresponds to our previous finding that marginal zone, rather than follicular B cells, is a much more effective inducer of NK cells [25].
IFN-α/β-independent induction of TLR7 mRNA by NK cells
The induction of TLR7 expression by 3.6-fold, revealed in the microarray assessment, is of particular interest to us, as increased levels of TLR7 expression have been implicated in the production of autoantibodies by B cells [9, 11, 26] and as we recently showed that NK cells may play a role in the induction of autoantibodies [27]. We therefore selected this gene for confirmation by RT-PCR analysis. Fig. 2A shows that the expression of TLR7 mRNA in high-density, resting B cells is barely detectable, but the levels could be enhanced significantly by coculture with NK cells (Lanes 3–6). Correspondingly, IL-6 mRNA was also close to our minimal detection level. Therefore, the functionality of an increase in TLR7 mRNA expression can be confirmed by assessing the level of IL-6 mRNA induced in the B cells upon the addition of a ligand for TLR7. Clearly IL-6 mRNA was induced to relatively high levels in cultures that contained NK cells (Lanes 4 and 6). It is important to note that IL-6 mRNA was not detected in cultures containing only NK cells, showing that there were insufficient contaminating non-NK cells to respond to the ligand. Furthermore, as shown in Fig. 2C, the induction of IL-6 mRNA by NK cells was completely dependent on the presence of TLR7. It is possible that IFN-γ, secreted by NK cells, may activate contaminating, adherent cells to mediate the enhancement. However, addition of anti-IFN-γ to the cocultures demonstrated minimal effects. Furthermore, we found that addition of rIFN-γ does not affect TLR7 mRNA expression in B cells (data not shown; two independent experiments). The relative levels of TLR7 and IL-6 mRNA were quantified by normalizing each RNA sample with the level of expression of a B cell marker invariant chain of MHC II, and the mean levels of enhancement obtained from four to eight independent experiments for each culture condition are shown.
Assessment of TLR7 mRNA in various B cell subpopulations, separated by Percoll gradient, indicated that the level of expression increases with increasing B cell activation. Supplemental Fig. 1 shows that cells in the low-density fraction, containing an increased proportion of marginal zone B cells, expressed higher levels of TLR7 as well as IL-6 upon addition of ligand. Because of this, we found that the initial level of TLR7 mRNA expression varied between experiments, although high-density B cells were always isolated. The enhancing effect of NK cells was, however, always apparent.
The revelation by microarray analysis of the large repertoire of ISGs induced by NK cells in the absence of IFN-γ was surprising, as NK cells have not been reported to produce IFN-α/β to a significant extent. Nonetheless, to assess the participation of type I IFN, we examined the effect of NK cells on B cells obtained from IFNAR0/0 mice in a parallel experiment using the same preparation of NK cells. Fig. 2B shows that a similar pattern of induction was obtained. As shown by the quantification, further repeat experiments (n=4) confirmed the augmentation. The finding that the effect of NK cells clearly does not require the activation of the IFNAR on B cells provides further assurance that the augmentation of TLR7 mRNA is not a result of indirect effects mediated by type I IFN [8], produced during culture.
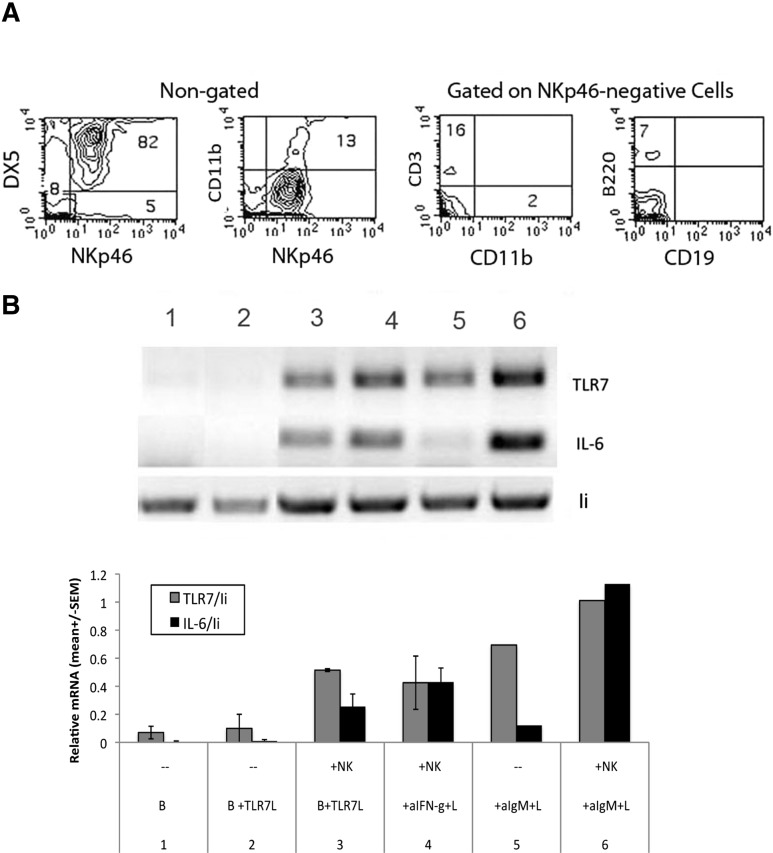
To determine if the enhancement of TLR7 mRNA expression requires prior activation of NK cells, we isolated fresh splenic NK cells based on binding to PE-anti- DX5, followed by SA. An example of the purity of routine preparations is shown in Fig. 3A. Thus, >80% of the DX5-positive cells were NKp46+, and of the 8% NKp46-negative population, <18% were identifiable to be T cells, macrophages, or B cells, equivalent to 1.5% of the total population. The remaining cells may be red blood cells that remain in the preparation. Fig. 3B shows that their ability to induce B cells obtained from IFNAR0/0 mice did not differ significantly from that mediated by IL-2-propagated NK cells.
Figure 3. Induction of TLR7 and IL-6 mRNA in B cells from IFNAR0/0 mice by freshly isolated NK cells.
Freshly isolated NK cells, stained with bio-NKp46 (R&D Systems), PE-Cy7, anti-CD11b, PE-anti-CD3, and FL-anti-CD19 (BioLegend), were analyzed by FACS for the presence of other cell types (A) and subsequently incubated with purified, resting B cells, obtained from IFNAR0/0 mice, for 20 h in the presence of additives, as indicated, followed by semiquantitative RT-PCR analysis (B), as described in Fig. 2. Where shown, mean (±sem) was derived from two independent experiments.
NK induction of CD86 on B cells also does not require IFN-α/β
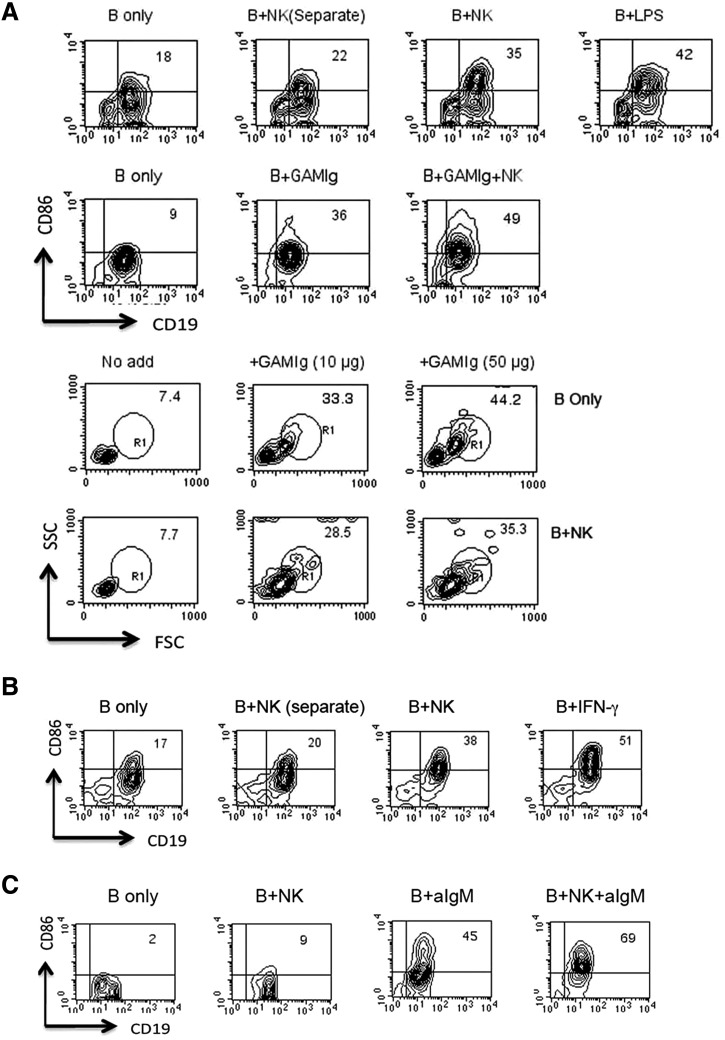
In addition to the induction of transcription of genes involved in switch recombination, NK cells can enhance other aspects of B cells differentiation. In confirmation of our previous findings [2] and as shown in a representative experiment in Fig. 4A, coincubation of IL-2-propagated NK cells with purified, resting B cells augmented the expression of CD86 in an IFN-γ-independent process. In addition, as shown in another representative experiment, NK cells significantly enhanced the level of increase of CD86 if B cells were first activated via cross-linking of the BCR. It is important to note, however, that the effect of NK cells does not entail a global enhancement of all B cell differentiation processes, as they could not initiate blast transformation nor could they enhance this induction rendered by BCR ligation.
Figure 4. Enhancement of B cell differentiation by IL-2-propagated or freshly isolated NK cells.
CD43-negative, Percoll-fractionated resting B cells were cultured for 18 h in the presence of additives, as indicated alone or together with splenic NK cells isolated from IFN-γ0/0 mice, which were propagated for 7 days in IL-2 (A) or not propagated (B). B + NK (Separate) indicates B and NK cells cultured separately but combined before analysis to ensure appropriate gating. B cell activation was assessed by increases in CD86 (BioLegend) expression on CD19-positive cells and by blast transformation indicated by increases in forward (FSC)- and side-scatter (SSC) of NKp46-negative cells. Results shown in A and B are representative of two or three independent experiments. (C) B cells isolated from IFNAR0/0 mice were cultured with freshly isolated NK cells in the presence or absence of goat anti-IgM (GAMIg; aIgM) for 20 h before analysis by FACS (results are representative of two independent experiments).
Fig. 4B shows that freshly isolated NK cells can also enhance B cell expression of CD86. Testing cells from IFNAR knockout mice, we found, and as shown in a representative experiment, that the induction of CD86, as well as the enhancement by NK cells of the induction by anti-IgM, similarly does not require the IFNAR on B cells (Fig. 4C). By comparison of independent experiments, it is apparent that as with the expression of TLR7 mRNA, the level of expression of CD86 varies, despite the isolation of Percoll fractionated high-density B cells. The rapid induction of CD86, requiring only a minimum of 4 h (data not shown), also suggests that the increase does not require transcription and is confirmed by the absence of up-regulation of CD86 mRNA in our microarray analysis.
NK cells enhance TLR7 gene expression via IL-28
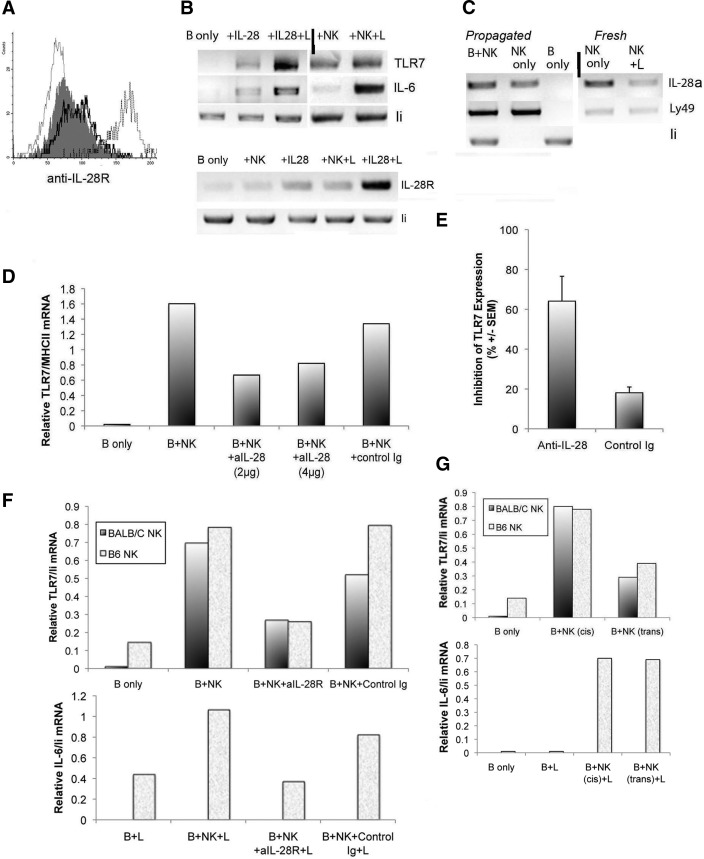
The finding that NK cells can induce a plethora of IFN response genes, including TLR7, which does not require type I or type II IFN, led us to hypothesize that the induction may be mediated via IFN type III. The type III IFNR is composed of a heterodimeric complex consisting of two chains: the IL-28R and IL-10R2 chain [28]. The receptor can respond to IL-28 and IL-29 and is expressed on hematopoietic and nonhematopoietic cells [28, 29] but in a more tissue-restricted manner than that for type I IFN. We had found in one, but not both, of the two microarrays performed that the transcript level of the IL-28R was up-regulated by 2.4-fold in B cells. Fig. 5A shows IL-28R is indeed expressed at low (but variable) levels on B cells and could be enhanced incrementally by NK cells. The sparse expression of the IL-28R on B cells probably explains why responses to IL-28 have been reported to be restricted to plasmacytoid DCs and epithelial cells [29]. Whereas addition of IL-28 to B cells did not increase the receptor expression (data not shown), a minor enhancement of TLR7 mRNA can be detected (Fig. 5B). Interestingly, the level of surface expression as well as mRNA for the receptor (Fig. 5A) and TLR7 mRNA (Fig. 5B) was increased significantly upon addition of the ligand for TLR7. Because of the minimal enhancement of IL-28R by NK cells, we sought to confirm that NK cells do indeed express IL-28. Fig. 5C shows the presence of IL-28 mRNA in IL-2-activated as well as freshly isolated NK cells but not in B cells; however, the level was not increased in cocultures with B cells. To determine whether this apparently intrinsic expression of IL-28 by NK cells is functional, we tested whether anti-IL-28 can inhibit their effects on B cells. As shown in Fig. 5D and in the summary of five experiments (Fig. 5E), the enhancement of B cell TLR7 mRNA was indeed reduced but not completely (60% inhibition; P<0.028 for one-tailed t test). We also tested an antibody against the receptor itself, which resulted in only partial inhibition as well (Fig. 5F). In the presence of the TLR7 ligand, the induction of IL-6 mRNA was correspondingly inhibited to the level induced in B cells cultured alone, which in this case, was somewhat above background, probably as a result of partially activated B cells in this preparation. If at least part of the enhancement of the TLR7 mRNA expression is mediated by a cytokine, the effect should not require direct cell contact. As shown in Fig. 5G, insertion of a membrane between NK and B cells in the cocultures (trans) resulted in a partial, but not complete, transmission of the effect on TLR7 mRNA expression. The level of TLR7 functional expression was, however, not affected by the interposition of the membrane, as registered by the equivalent levels of IL-6 mRNA, induced upon addition of the TLR7 ligand. These results indicate that IL-28, derived from NK cells, can activate B cells without direct contact if B cell TLR7 is enhanced sufficiently. In contrast, the incomplete inhibition by antibodies against the cytokine or against the receptor for the cytokine together with the results from the transwell experiments indicate that additional signals, mediated by direct contact between the two cell types, may be required for the enhancement of TLR7 expression on B cells.
Figure 5. Role of IL-28 in the NK cell-mediated enhancement of B cell TLR7 mRNA.
(A) Histograms from FACS analysis of purified, resting B cells stained with GαIL-28αR (Abcam, Cambridge, MA, USA), followed by FL-donkey anti-goat Ig before (shaded, dark) and after overnight culture with NK cells (dark line) or with IL-28 plus TLR7 ligand (L; dotted line). Light line represents cells stained without primary antibody. (B and C) RT-PCR analysis of RNA prepared from overnight cultures of B cells after stimulation with IL-28 (2 μg/ml) or with NK cells, with or without TLR7 ligand under conditions as indicated and using primers as noted. Rows separated by vertical lines are images taken from a different segment of the same gel. Similar results were obtained from at least two additional experiments. Representative experiment of the relative TLR7 mRNA expressed in cells cultured for 20 h, alone or with IL-2-propagated NK cells in the presence of anti-IL-28 or control antibody (rat Ig). (E) Average inhibition of NK cell-mediated enhancement of TLR7 mRNA by anti-IL-28, calculated from the results of four to five experiments using freshly isolated or IL-2-propagated NK cells. Percent inhibition = (relative mRNA in NKB cocultures−relative mRNA in the presence of inhibitor/relative mRNA without inhibitor)100. P ≤ 0.028 for the possibility that the two sets of data are identical. (F) Relative TLR7 mRNA expression in cocultures containing additives, as indicated from two independent experiments, and relative IL-6 mRNA in the presence of ligand. (G) Relative TLR7 mRNA in cultures of B cells placed in direct contact with NK cells (cis) or separated by a semipermeable membrane (trans) and relative IL-6 mRNA in cultures containing TLR7 ligand. Sequence of primers: IL-28a, forward 5′GCAGCTGCAGGTCCAAGAGCG, reverse 5′ TCCCGGGTGAGCAGGCGAA; IL-28R, forward 5′GAAGGCCCCGTCTAGCCCCA, reverse 5′TCCAGCGACGGCATGCAAGG; Ly49, forward 5′ATCATCCCAAGATGAGTGAGC, reverse 5′TTAGATGGGCCATTGTCAATC.
As we had shown that acting as ligand, CD2 or 2B4 on NK cells can stimulate B cell production of germline transcripts for IgG2a via activation of CD48, albeit after a longer period of incubation [21], we tested NK cells from CD2-2B4 double-knockout mice for their ability to induce TLR7. The results showed clearly that induction by these cells was not compromised (data not shown). In addition, as indicated in Fig. 5F and G, the use of propagated NK cells from two different strains cultured with B cells from BALB/c mice indicated that the interaction does not require histocompatibility between NK and B cells. Therefore, at this point, we have scant information regarding the possible role of any of the other presently known receptors expressed by NK cells [30]. IL-28-independent interaction may account for the enhancement of B cell CD86 expression as well, because we found that this induction was also not inhibited by anti-IL-28 (data not shown).
Requirement for NK cell activation
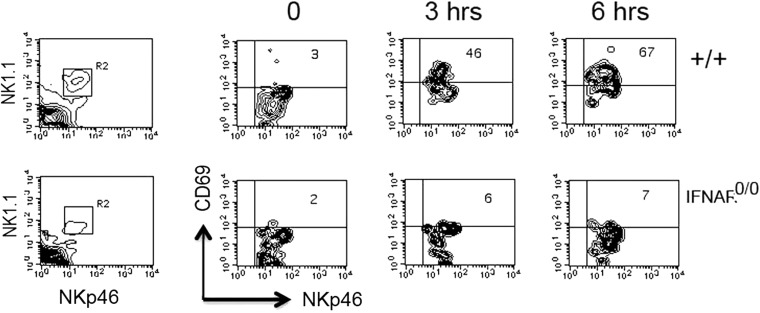
The finding that NK cell expression of IL-28 mRNA is not affected by coincubation with B cells (Fig. 5C) suggests that NK cells may be activated via alternative factors, possibly produced during culture. Poly(I:C), a TLR3 ligand, is a potent activator of many cell types, resulting in, among other effects, the production of IL-12 and TNF-α, which is only partially synergistic with signals from IFN-β [31]. We tested IFNAR0/0 mice for their response to poly(I:C) to determine whether NK cells can be activated via these alternative pathways. As mouse NK cells do not express TLR3 [32], they can only be activated indirectly via IFN-α/β, IL-12, or TNF-α. As we have shown previously [33], in WT mice, poly(I:C) stimulation resulted in extensive activation of NK cells, as measured by the expression of CD69 (Fig. 6). In contrast, NK cells from IFNAR0/0 mice were affected only minimally. These results confirm the status of IFNAR deletion in the mice used in our experiments but more importantly, also provide additional confirmation that the effect of NK cells on B cell TLR7 expression does not require activation of NK cells but is mediated by a combination of pre-existing cell-surface determinants acting in concert with IL-28.
Figure 6. NK cells in IFNAR0/0 mice cannot be activated by poly(I:C).
Splenoctyes were from WT or IFNAR0/0 mice, injected with 100 μg poly(I:C) and harvested at various times, as indicated, and were stained with PE NK1.1 (BioLegend) and NKp46 (R&D Systems) to assess the level of CD69 (BioLegend) expression on NK cells (R2 gate).
In this respect, whereas NK cells have been implicated previously in augmentation of tumor immunity mediated by IL-28 [34, 35], whether the effect requires participation of other cell types has not been validated. Further insight into this effect may be afforded by our finding that purified NK cells can enhance B cell TLR7 expression via IL-28, even without extensive priming.
The results presented herein also add further evidence for the importance of the cytokine network that links cells of the innate response to adaptive responses. The increased expression of TLR7 on B cells induced by NK cells via an IFN-α/β-independent pathway indicates an alternative manner by which B cells can be rendered more susceptible to stimulation by TLR7 ligands. This activation by NK cells may be responsible for the continued development of polyclonal antibodies of the IgG2a subclass, even in IFNAR0/0 New Zealand black mice [36] and antinuclear antibodies in IFNAR0/0129Sv mice induced by 2,6,10,14-tetramethylpentadecane [37]. Interestingly, the persistent production of IgG2a correlates with our recent finding that depletion of NK cells significantly reduces the extent of IgG2a autoantibody expression when they are produced under T-independent conditions [27]. The preferential induction of IgG2a by NK cells is consistent with the increased expression of IRF9 revealed by our microarray analysis, in that this signaling intermediate is believed to act downstream of TLR7 in the induction of IgG2a upon activation of B cells by type I IFN [38].
Even in the presence of intact IFN type I signaling, NK cells could play a role, in that increased expression of TLR7 may provide the required threshold for activation of B cells by autoantigens prior to receiving signals from IFN-α/β produced by other cell types. This activation by the appropriate ligand would in turn result in enhanced production of IFN-α/β, resulting in a feedback loop that could contribute to conditions for augmentation of autoantibody production [8, 39, 40].
Finally, it should be noted that studies with various virus infections have revealed a contribution for type III IFN to some of the responses (reviewed in ref. [24]); however, missing from this assessment is the evaluation of possible participation of NK cells in these infections, which merits some exploration.
Supplementary Material
ACKNOWLEDGMENTS
The study was supported by U.S. National Institutes of Health grant R01 AI69253. We thank Dr. Edward Wakeland (UT Southwestern Medical Center) for providing the B6. TLR70/0 mice and Dr. Zhijian Chen (UT Southwestern Medical Center) for providing breeders for the IFNAR0/0 mice.
SEE CORRESPONDING EDITORIAL ON PAGE 695
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- 0/0
- deficient
- FL
- fluorescinated
- IFIT
- IFN-induced protein with tetratricopeptide
- IFNAR
- type I IFNR
- IL-28R
- IL-28Rα
- IRF
- IFN regulatory factor
- ISG
- IFN-stimulated gene
- poly(I:C)
- polyinosinic:polycytidylic acid
- SA
- streptavidin
AUTHORSHIP
S.T. and Y.G. performed some of the experiments. D.Y. designed the experiments. S.S. and D.Y. interpreted the results and wrote the manuscript.
REFERENCES
- 1. Palm N. W., Medzhitov R. (2009) Pattern recognition receptors and control of adaptive immunity. Immunol. Rev. 227, 221–233 [DOI] [PubMed] [Google Scholar]
- 2. Yuan D. (2004) Interactions between NK cells and B lymphocytes. Adv. Immunol. 84, 1–42 [DOI] [PubMed] [Google Scholar]
- 3. Yuan D., Gao N., Jennings P. (2010) Interactions between B Lymphocytes and NK Cells: an Update. Springer, Berlin, Heidelberg, Germany [Google Scholar]
- 4. Wilder J. A., Koh C. Y., Yuan D. (1996) The role of NK cells during in vivo antigen-specific antibody responses. J. Immunol. 156, 146–152 [PubMed] [Google Scholar]
- 5. Markine-Goriaynoff D., Hulhoven X., Cambiaso C. L., Monteyne P., Briet T., Gonzalez M. D., Coulie P., Coutelier J. P. (2002) Natural killer cell activation after infection with lactate dehydrogenase-elevating virus. J. Gen. Virol. 83, 2709–2716 [DOI] [PubMed] [Google Scholar]
- 6. Gao N., Dang T., Yuan D. (2001) IFN-γ-dependent and -independent initiation of switch recombination by NK cells. J. Immunol. 167, 2011–2018 [DOI] [PubMed] [Google Scholar]
- 7. Gao N., Jennings P., Yuan D. (2008) Requirements for the natural killer cell-mediated induction of IgG1 and IgG2a expression in B lymphocytes. Int. Immunol. 20, 645–657 [DOI] [PubMed] [Google Scholar]
- 8. Green N. M., Laws A., Kiefer K., Busconi L., Kim Y. M., Brinkmann M. M., Trail E. H., Yasuda K., Christensen S. R., Shlomchik M. J., Vogel S., Connor J. H., Ploegh H., Eilat D., Rifkin I. R., van Seventer J. M., Marshak-Rothstein A. (2009) Murine B cell response to TLR7 ligands depends on an IFN-β feedback loop. J. Immunol. 183, 1569–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lau C. M., Broughton C., Tabor A. S., Akira S., Flavell R. A., Mamula M. J., Christensen S. R., Shlomchik M. J., Viglianti G. A., Rifkin I. R., Marshak-Rothstein A. (2005) RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J. Exp. Med. 202, 1171–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Berland R., Fernandez L., Kari E., Han J. H., Lomakin I., Akira S., Wortis H. H., Kearney J. F., Ucci A. A., Imanishi-Kari T. (2006) Toll-like receptor 7-dependent loss of B cell tolerance in pathogenic autoantibody knockin mice. Immunity 25, 429–440 [DOI] [PubMed] [Google Scholar]
- 11. Subramanian S., Tus K., Li Q. Z., Wang A., Tian X. H., Zhou J., Liang C., Bartov G., McDaniel L. D., Zhou X. J., Schultz R. A., Wakeland E. K. (2006) A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc. Natl. Acad. Sci. USA 103, 9970–9975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dalton D. K., Pitts-Meek S., Keshav S., Figari I. S., Bradley A., Stewart T. A. (1993) Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science 259, 1739–1742 [DOI] [PubMed] [Google Scholar]
- 13. Sinha S. K., Gao N., Guo Y., Yuan D. (2010) Mechanism of induction of NK activation by 2B4 (CD244) via its cognate ligand. J. Immunol. 185, 5205–5210 [DOI] [PubMed] [Google Scholar]
- 14. Muller U., Steinhoff U., Reis L. F., Hemmi S., Pavlovic J., Zinkernagel R. M., Aguet M. (1994) Functional role of type I and type II interferons in antiviral defense. Science 264, 1918–1921 [DOI] [PubMed] [Google Scholar]
- 15. Abuodeh R., Wei H., Yuan D. (1998) Effect of upstream RNA processing on selection of μ S versus μ M poly(A) sites. Nucleic Acids Res. 26, 5417–5424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shaffer A. L., Rosenwald A., Hurt E. M., Giltnane J. M., Lam L. T., Pickeral O. K., Staudt L. M. (2001) Signatures of the immune response. Immunity 15, 375–385 [DOI] [PubMed] [Google Scholar]
- 17. Liu S. Y., Sanchez D. J., Cheng G. (2011) New developments in the induction and antiviral effectors of type I interferon. Curr. Opin. Immunol. 23, 57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zimmerer J. M., Lesinski G. B., Radmacher M. D., Ruppert A., Carson W. E., III (2007) STAT1-dependent and STAT1-independent gene expression in murine immune cells following stimulation with interferon-α. Cancer Immunol. Immunother. 56, 1845–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gao N., Schwartzberg P., Wilder J. A., Blazar B. R., Yuan D. (2006) B cell induction of IL-13 expression in NK cells: role of CD244 and SLAM-associated protein. J. Immunol. 176, 2758–2764 [DOI] [PubMed] [Google Scholar]
- 20. Zhou Z., Hamming O. J., Ank N., Paludan S. R., Nielsen A. L., Hartmann R. (2007) Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J. Virol. 81, 7749–7758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Doyle S. E., Schreckhise H., Khuu-Duong K., Henderson K., Rosler R., Storey H., Yao L., Liu H., Barahmand-pour F., Sivakumar P., Chan C., Birks C., Foster D., Clegg C. H., Wietzke-Braun P., Mihm S., Klucher K. M. (2006) Interleukin-29 uses a type 1 interferon-like program to promote antiviral responses in human hepatocytes. Hepatology 44, 896–906 [DOI] [PubMed] [Google Scholar]
- 22. Chang W. L., Coro E. S., Rau F. C., Xiao Y., Erle D. J., Baumgarth N. (2007) Influenza virus infection causes global respiratory tract B cell response modulation via innate immune signals. J. Immunol. 178, 1457–1467 [DOI] [PubMed] [Google Scholar]
- 23. Fensterl V., Sen G. C. (2011) The ISG56/IFIT1 gene family. J. Interferon Cytokine Res. 31, 71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mordstein M., Michiels T., Staeheli P. (2010) What have we learned from the IL28 receptor knockout mouse? J. Interferon Cytokine Res. 30, 579–584 [DOI] [PubMed] [Google Scholar]
- 25. Gao N., Dang T., Dunnick W. A., Collins J. T., Blazar B. R., Yuan D. (2005) Receptors and counterreceptors involved in NK-B cell interactions. J. Immunol. 174, 4113–4119 [DOI] [PubMed] [Google Scholar]
- 26. Marshak-Rothstein A. (2006) Tolling for autoimmunity-prime time for 7. Immunity 25, 397–399 [DOI] [PubMed] [Google Scholar]
- 27. Yuan D., Thet S., Zhou X. J., Wakeland E. K., Dang T. (2011) The role of NK cells in the development of autoantibodies. Autoimmunity 44, 641–651 [DOI] [PubMed] [Google Scholar]
- 28. Lasfar A., Lewis-Antes A., Smirnov S. V., Anantha S., Abushahba W., Tian B., Reuhl K., Dickensheets H., Sheikh F., Donnelly R. P., Raveche E., Kotenko S. V. (2006) Characterization of the mouse IFN-λ ligand-receptor system: IFN-λs exhibit antitumor activity against B16 melanoma. Cancer Res. 66, 4468–4477 [DOI] [PubMed] [Google Scholar]
- 29. Ank N., Iversen M. B., Bartholdy C., Staeheli P., Hartmann R., Jensen U. B., Dagnaes-Hansen F., Thomsen A. R., Chen Z., Haugen H., Klucher K., Paludan S. R. (2008) An important role for type III interferon (IFN-λ/IL-28) in TLR-induced antiviral activity. J. Immunol. 180, 2474–2485 [DOI] [PubMed] [Google Scholar]
- 30. Vivier E., Raulet D. H., Moretta A., Caligiuri M. A., Zitvogel L., Lanier L. L., Yokoyama W. M., Ugolini S. (2011) Innate or adaptive immunity? The example of natural killer cells. Science 331, 44–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Krummen M., Balkow S., Shen L., Heinz S., Loquai C., Probst H. C., Grabbe S. (2010) Release of IL-12 by dendritic cells activated by TLR ligation is dependent on MyD88 signaling, whereas TRIF signaling is indispensable for TLR synergy. J. Leukoc. Biol. 88, 189–199 [DOI] [PubMed] [Google Scholar]
- 32. Lee C. K., Rao D. T., Gertner R., Gimeno R., Frey A. B., Levy D. E. (2000) Distinct requirements for IFNs and STAT1 in NK cell function. J. Immunol. 165, 3571–3577 [DOI] [PubMed] [Google Scholar]
- 33. Gao N., Jennings P., Guo Y., Yuan D. (2011) Regulatory role of natural killer (NK) cells on antibody responses to Brucella abortus. Innate Immun. 17, 152–163 [DOI] [PubMed] [Google Scholar]
- 34. Sato A., Ohtsuki M., Hata M., Kobayashi E., Murakami T. (2006) Antitumor activity of IFN-λ in murine tumor models. J. Immunol. 176, 7686–7694 [DOI] [PubMed] [Google Scholar]
- 35. Numasaki M., Tagawa M., Iwata F., Suzuki T., Nakamura A., Okada M., Iwakura Y., Aiba S., Yamaya M. (2007) IL-28 elicits antitumor responses against murine fibrosarcoma. J. Immunol. 178, 5086–5098 [DOI] [PubMed] [Google Scholar]
- 36. Santiago-Raber M. L., Baccala R., Haraldsson K. M., Choubey D., Stewart T. A., Kono D. H., Theofilopoulos A. N. (2003) Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J. Exp. Med. 197, 777–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nacionales D. C., Kelly-Scumpia K. M., Lee P. Y., Weinstein J. S., Lyons R., Sobel E., Satoh M., Reeves W. H. (2007) Deficiency of the type I interferon receptor protects mice from experimental lupus. Arthritis Rheum. 56, 3770–3783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thibault D. L., Chu A. D., Graham K. L., Balboni I., Lee L. Y., Kohlmoos C., Landrigan A., Higgins J. P., Tibshirani R., Utz P. J. (2008) IRF9 and STAT1 are required for IgG autoantibody production and B cell expression of TLR7 in mice. J. Clin. Invest. 118, 1417–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mathian A., Weinberg A., Gallegos M., Banchereau J., Koutouzov S. (2005) IFN-α induces early lethal lupus in preautoimmune (New Zealand Black × New Zealand White) F1 but not in BALB/c mice. J. Immunol. 174, 2499–2506 [DOI] [PubMed] [Google Scholar]
- 40. Zhuang H., Kosboth M., Lee P., Rice A., Driscoll D. J., Zori R., Narain S., Lyons R., Satoh M., Sobel E., Reeves W. H. (2006) Lupus-like disease and high interferon levels corresponding to trisomy of the type I interferon cluster on chromosome 9p. Arthritis Rheum. 54, 1573–1579 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.