Circulating human γδ T cells release ATP upon in vitro stimulation, which signals through P2X4 receptors and governs these cells' function.
Keywords: purinergic, pannexin-1 hemichannels, IPP
Abstract
Purinergic signaling plays a key role in a variety of physiological functions, including regulation of immune responses. Conventional αβ T cells release ATP upon TCR cross-linking; ATP binds to purinergic receptors expressed by these cells and triggers T cell activation in an autocrine and paracrine manner. Here, we studied whether similar purinergic signaling pathways also operate in the “unconventional” γδ T lymphocytes. We observed that γδ T cells purified from peripheral human blood rapidly release ATP upon in vitro stimulation with anti-CD3/CD28-coated beads or IPP. Pretreatment of γδ T cells with 10panx-1, CBX, or Bf A reversed the stimulation-induced increase in extracellular ATP concentration, indicating that panx-1, connexin hemichannels, and vesicular exocytosis contribute to the controlled release of cellular ATP. Blockade of ATP release with 10panx-1 inhibited Ca2+ signaling in response to TCR stimulation. qPCR revealed that γδ T cells predominantly express purinergic receptor subtypes A2a, P2X1, P2X4, P2X7, and P2Y11. We found that pharmacological inhibition of P2X4 receptors with TNP-ATP inhibited transcriptional up-regulation of TNF-α and IFN-γ in γδ T cells stimulated with anti-CD3/CD28-coated beads or IPP. Our data thus indicate that purinergic signaling via P2X4 receptors plays an important role in orchestrating the functional response of circulating human γδ T cells.
Introduction
γδ T cells represent a unique subset of lymphocytes, which exhibit adaptive as well as innate immune cell features. Less than 10% of the circulating lymphocytes in adult human blood bear γδ TCRs, and the majority of these cells are Vγ9Vδ2+ [1–3]. A distinguishing feature of Vγ9Vδ2 T cells is their ability to recognize nonprotein phosphoantigens, such as the intermediate of the isoprenoid synthesis pathway, IPP, which is one of the best-characterized cognate antigens of the Vγ9Vδ2+ subset of γδ T cells. Recognition of IPP by Vγ9Vδ2 has been shown to be independent of antigen processing and presentation by classical and nonclassical MHC molecules, although the exact molecular mechanisms behind this process remain obscure and are under investigation [4–6]. Vγ9Vδ2 T cells have been unambiguously shown to play an important role in antimicrobial and anticancer immunity. Upon encountering certain pathogens, stressed host cells, or transformed cells, Vγ9Vδ2 T cells undergo rapid expansion. They up-regulate and secrete proinflammatory cytokines and chemokines including TNF-α, IFN-γ, MIP-1β, and RANTES, and kill target cells through Fas/Fas ligand- or perforin/granzyme-mediated mechanisms, thus conferring protective immunity on the host [7–9].
In humans, γδ T cells other than the Vγ9Vδ2 subset are broadly denoted as “non-Vδ2” T cells. Among these, Vδ1+ T cells form the most predominant population. Although they are a minor γδ T cell subset in peripheral blood, Vδ1+ T cells constitute >50% of the total lymphocytes in the intestinal epithelium. Compared with Vγ9Vδ2 T cells, the exact nature of antigens recognized by non-Vδ2 γδ T cells and the mode of recognition of these cells are poorly understood. Recognition of stress-induced molecules in the context of MHC class I-like or nonclassical-presenting molecules has been implied [10–12]. Non-Vδ2 T cells have been implicated in a wide spectrum of functional responses ranging from resolution of inflammation, maintenance of homeostasis, and anticancer immunity to induction of autoimmune disorders [13–17]. For more than a decade, strong interest in γδ T cells, especially the Vγ9Vδ2 subset, has been driven by a potential role for these cells in cancer immunotherapy [18–21]. This line of research has provided valuable insight into the signaling pathways that govern the functional responses of these cells.
The current report indicates the involvement of purinergic signaling in the activation of γδ T cells. Purinergic signaling refers to the signaling events mediated mainly by the purine ligand ATP, and its breakdown products, ADP, AMP, and adenosine, and their cognate receptors, the “purinergic receptors”. A total of 19 such purinergic receptors have been cloned and characterized, including four P1 receptors that bind to adenosine, seven P2X receptors, which are ATP-gated ion channels, and eight P2Y receptors that are GPCRs and can bind to ATP, ADP, as well as UTP, UDP, and UDP-glucose [22, 23]. First demonstrated in the context of neurotransmission, the phenomenon of purinergic signaling is now widely recognized and seen to play a substantial role in various physiological functions, such as platelet aggregation, vasodilatation, exocrine and endocrine secretion, and regulation of inflammatory and other immune responses [24]. Autocrine purinergic signaling has been shown to be an important regulatory mechanism of different subsets of innate and adaptive immune cells [25, 26].
In the specific context of T cells, TCR stimulation is known to induce the release of ATP from conventional αβ T cells through panx-1, maxi-anion channels, or vesicular exocytosis. Released ATP binds to P2X1, P2X4, and P2X7 purinergic receptors that mediate Ca2+ influx—a crucial step in T cell activation. The downstream signal transduction pathways triggered by these receptors culminate in up-regulated cytokine production and functional αβ T cell responses. Blockade of ATP release, addition of ATP hydrolyzing enzymes that rapidly clear extracellular ATP, and pharmacological inhibition of purinergic receptors all inhibit αβ T cell activation [27–32].
We thus asked whether similar purinergic signaling mechanisms play a role in the activation of γδ T cells. To address this question, we investigated (1) whether γδ T cells release ATP, (2) what the mechanism of ATP release might be, (3) whether γδ T cells express purinergic receptors, and (4) what the role of these purinergic receptors may be with regard to γδ T cell activation. Our results show that purinergic signaling does indeed participate in the regulation of γδ T cells and that P2X4 receptors have a primary role in this process.
MATERIALS AND METHODS
Reagents
Dynabeads (superparamagnetic polystyrene beads) coated with anti-mouse IgG antibodies (Invitrogen Dynal AS, Oslo, Norway) were incubated with mouse anti-human CD3 and anti-human CD28 antibodies (BD Biosciences, San Jose, CA, USA). These beads were used for in vitro TCR/CD28 costimulation. IPP was purchased from Echelon (Echelon Biosciences, Salt Lake City, UT, USA). 10Panx-1 and the P2 receptor antagonists NF023, TNP-ATP, A438079, NF157, and suramin were from Tocris Bioscience (Ellisville, MO, USA). CBX, Bf A, and DIDS were from Sigma-Aldrich (St. Louis, MO, USA).
Cell isolation and maintenance
Whole blood was drawn from healthy human volunteers. PBMCs were isolated using Ficoll Paque Plus (GE Healthcare Life Sciences, Piscataway, NJ, USA), as per the manufacturer's protocol. γδ T cells were purified from PBMCs by negative selection using a human TCR γδ+ T cell isolation kit (Miltenyi Biotec, Auburn, CA, USA) and cultured in RPMI 1640 (ATCC, Manassas, VA, USA), supplemented with 10% FBS (CellGro, Mediatech, Manassas, VA, USA), and 100 U/ml penicillin + 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA, USA) at 37°C and 5% CO2. Purity of γδ T cells was assessed by double staining with anti-human CD3-PE and anti-human γδ TCR-APC antibodies (BD Biosciences) and was routinely found to be ≥90%. Protocols for isolation and maintenance of CD4+ T cells and culturing Jurkat cells (E6-1 clone, ATCC) were described previously [33]. All human studies were approved by the Institutional Review Board for Human Research of the Beth Israel Deaconess Medical Center (Harvard Medical School, Boston, MA, USA).
Assessment of ATP release
Purified γδ T cells (5×104), suspended in 50 μl RPMI 1640, supplemented with serum and antibiotic as described above, were prewarmed at 37°C in a water bath for 30 min. The cells were then stimulated in situ with anti-CD3/CD28-coated beads (one bead/cell) or with 25 μM IPP for the indicated time periods. Unstimulated samples served as controls to estimate basal ATP release at each of these time-points. ATP concentrations were measured using an ATP Bioluminescence HS II assay kit (Roche, Indianapolis, IN, USA), according to the manufacturer's instructions. The increase in extracellular ATP concentrations compared with basal ATP levels was assessed at each time-point. To determine the mechanisms that contribute to ATP release, purified γδ T cells were treated with release inhibitors, namely 10panx-1, CBX, Bf A, or DIDS for 20 min prior to stimulation with anti-CD3/CD28-coated beads or IPP.
Intracellular Ca2+ measurement
As a result of the limited numbers of purified γδ T cells, we used an unpurified cell population among PBMCs to estimate intracellular Ca2+ signaling in γδ T cells. PBMCs were loaded with 2 μM of the Ca2+-sensitive cell-permeable probe Fluo-4-AM (Invitrogen) and concomitantly stained with anti-human γδ TCR-APC in HEPES-buffered RPMI (ATCC) for 30 min at 37°C, 5% CO2. The cells were then washed twice with, and resuspended in recording buffer consisting of, 140 mM NaCl, 5 M KCl, 1 mM CaCl2, 1 mM MgSO4, 5.5 mM glucose, and 20 mM HEPES (pH=7.4). Ca2+ signaling in response to anti-CD3 stimulation was recorded with a FACSCalibur flow cytometer (BD Biosciences). We used soluble anti-CD3 antibodies instead of anti-CD3/CD28 antibody-coated beads, because of technical difficulties with the latter approach such as skewed scatter plots due to clustering of multiple beads and cells, and excess bead-induced in situ mechanical stimulation. To determine the effect of panx-1 blockade on Ca2+ signaling, we treated the cells with 10panx-1 for 20 min before anti-CD3 stimulation. For each individual donor, the baseline Ca2+ level was estimated with buffer, whereas maximum Ca2+ response was elicited with 0.5 μM ionomycin (Sigma-Aldrich). γδ T cells were gated based on staining with anti-human γδ TCR-APC, and intracellular Ca2+ signaling was measured in the gated population. Flow cytometry data were analyzed using CellQuest Pro software (BD Biosciences). Percent inhibition of TCR-stimulated peak Ca2+ signaling was calculated using the following formula: inhibition % = [1−(Test Fluo4 MFI−Buffer Fluo4 MFI)/(anti-CD3 Fluo4 MFI−Buffer Fluo4 MFI)] × 100.
qPCR
Expression patterns of P1 and P2 receptors in purified γδ T lymphocyte and CD4+ T cell populations (purity >98% by FACS), as well as cultured Jurkat cells, were assessed with qPCR, as described earlier [33, 34]. To assess the effect of P2 receptor inhibition on stimulation-induced up-regulation of CD69, TNF-α, and IFN-γ in γδ T cells, 5 × 104 purified cells in each well of a 96-well plate were pretreated with NF023, TNP-ATP, A438079, suramin, or culture medium (control) for 20 min at 37°C. The cells were then stimulated with anti-CD3/CD28-coated beads (one bead/cell) or 25 μM IPP for 4 h at 37°C. At the end of the incubation period, cells were harvested, and total RNA was isolated using an RNeasy mini kit (Qiagen, Valencia, CA, USA) and subjected to reverse transcription using Superscript III First-Strand Synthesis Supermix (Invitrogen). cDNA synthesized in this fashion was used as template for qPCR using SYBR GreenER qPCR Supermix (Invitrogen) as per the manufacturer's instructions. Primers for human CD69, TNF-α, IFN-γ, and β-actin were purchased from Qiagen. Relative gene expression was normalized to β-actin, and mRNA levels were estimated by the comparative threshold cycle method.
Statistical analysis
Unless otherwise stated, all data are expressed as mean ± sd of ≥3 individual experiments. Statistical analyses were performed with GraphPad Prism 5 software (GraphPad Prism, San Diego, CA, USA). For comparison between groups, two-tailed, unpaired Student's t test was used, and differences were considered significant at P values <0.05.
RESULTS
Purified γδ T cells release ATP upon in vitro stimulation
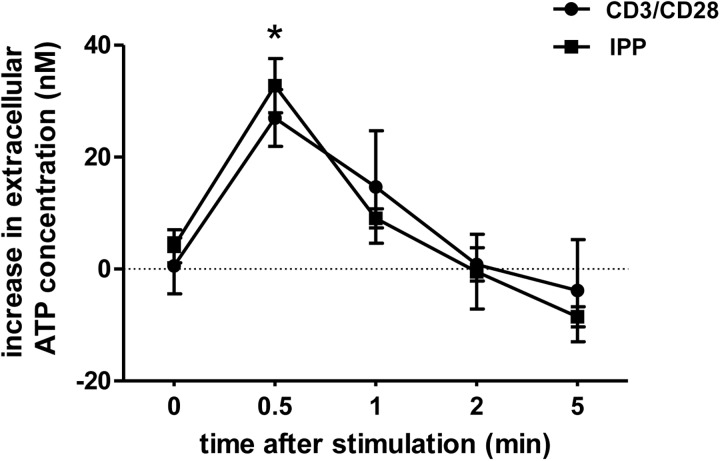
Conventional αβ T cells are known to release ATP in response to TCR cross-linking [27, 29, 30]. To determine whether this phenomenon is also true for γδ T cells, we purified γδ T cells from human peripheral blood using magnetic separation (Supplemental Fig. 1). On in vitro stimulation of purified γδ T cells with anti-CD3/CD28-coated beads or IPP, ATP was rapidly released with the extracellular ATP concentration peaking as early as 30 s after stimulation (Fig. 1). The amount of ATP released with each stimulus was comparable and accounted for ∼50 pmoles/106 cells. The increase in extracellular ATP concentration was highly dynamic in nature, and ATP levels returned to baseline within 5 min after cell stimulation.
Figure 1. γδ T cells release ATP upon in vitro stimulation.
Purified γδ T cells suspended in supplemented RPMI were stimulated with anti-CD3/CD28-coated beads (one bead/cell) or 25 μM IPP for the indicated time periods, and increase in extracellular ATP concentration poststimulation was determined with a luciferin/luciferase ATP bioluminescence assay kit. Data shown are representative of multiple experiments (n=4), and values indicate the increase in ATP concentrations in the culture media after cell stimulation. Basal ATP levels of unstimulated cells were 100 ± 6 nM; data shown are averages ± sd; *P < 0.01 as compared with unstimulated controls.
Gap junction hemichannels and vesicular exocytosis contribute to ATP release from γδ T lymphocytes
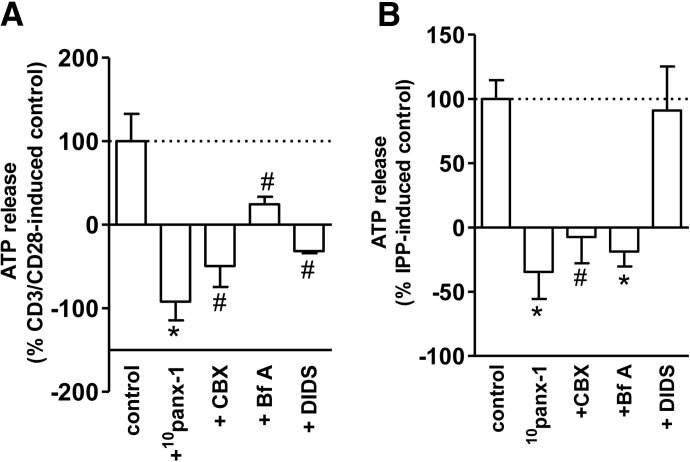
A variety of mechanisms have been proposed to explain the release of ATP from intact mammalian cells [35–38]. These mechanisms include release via panx hemichannels [28, 30, 39], maxianion channels and stretch-activated channels [27], and vesicular transport and exocytosis [31]. All of these mechanisms have been shown to mediate ATP release from conventional αβ T cells. However, no information exists about whether these mechanisms also contribute to the release of ATP from γδ T cells. Therefore, we investigated ATP release in response to γδ T cell activation without or with the pretreatment by the following inhibitors: 10panx-1, CBX, Bf A, and DIDS, which block panx-1 and connexin hemichannels, vesicular exocytosis, and maxianion channels, respectively. At the concentrations used, the viability of the cells pretreated with these inhibitors was comparable with that of the untreated cells, as judged by trypan blue staining. We found that inhibition of panx-1 and connexin hemichannels completely abrogated ATP release in response to cell stimulation with anti-CD3/CD28-coated beads or IPP (Fig. 2). Blockade of vesicular exocytosis with Bf A also significantly reduced ATP release. Interestingly, the suppressive effect of Bf A was more pronounced in IPP-stimulated cells compared with CD3/CD28 stimulation. Although the maxi-anion channel inhibitor DIDS was notably effective in blocking ATP release in response to CD3/CD28 stimulation, it barely altered the release of ATP in response to IPP (Fig. 2A and B). Thus, overall, gap junction hemichannel proteins as well as vesicular exocytosis seem to contribute to the release of ATP from γδ T cells in response to stimulation.
Figure 2. γδ T cells release ATP through panx-1 and/or connexin hemichannels, as well as vesicular exocytosis.
Purified γδ T cells were pretreated for 20 min with 10panx-1 (400 μM), CBX (25 μM), Bf A (50 nM), or DIDS (200 μM) and then stimulated with anti-CD3/CD28-coated beads (one bead/cell; A) or IPP (25 μM; B) for 30 s. The increase in ATP concentration in the culture supernatant was measured with an ATP bioluminescence assay kit as described in Fig. 1. ATP release data are expressed as percentage of the ATP release by control cells stimulated in the absence of inhibitors. Basal ATP concentrations in culture supernatants of unstimulated cells were 87 ± 7 nM. Data shown are averages ± sd; n = 3; #P < 0.05; *P < 0.01 as compared with control.
Ca2+ signaling in γδ T cells requires TCR-induced ATP release
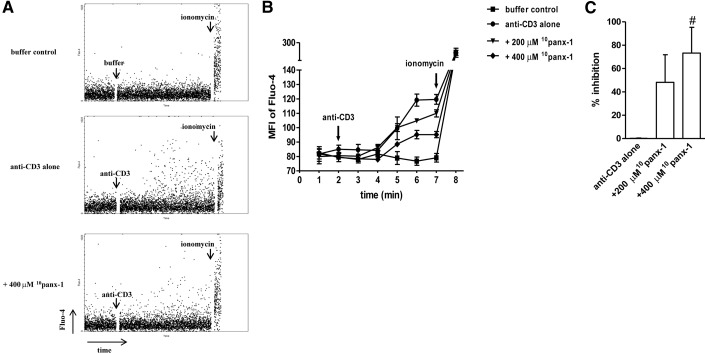
Elevation of cytosolic Ca2+ in response to TCR stimulation is a crucial downstream signaling event in T cell activation. Blocking the release of ATP in response to TCR cross-linking or hastening the clearance of extracellular ATP using ATP hydrolyzing enzymes has been shown to compromise Ca2+ influx and downstream activation signaling events in conventional αβ T cells [27, 28, 31]. As panx-1 seems to have a major role in facilitating ATP release from activated γδ T cells, we studied how inhibition of panx-1 affects stimulation-induced Ca2+ flux in γδ T cells. Inhibition of panx-1 by pretreatment with 10panx-1 dose-dependently blocked Ca2+ signaling in cells stimulated with anti-CD3 antibodies, with a partial but significant decrease observed at a concentration of 400 μM 10panx-1 (Fig. 3). Ca2+ influx in response to ionomycin stimulation was unaffected by 10panx-1 pretreatment. This observation underscores the role for ATP release as a primary step in the purinergic trigger of Ca2+ signaling known to be critical for T cell activation. Hence, to further establish the role of purinergic signaling in γδ T cell activation, we examined the expression profile of purinergic receptors on these cells.
Figure 3. Blockade of panx-1 inhibits anti-CD3-induced Ca2+ signaling in γδ T cells.
Human PBMCs were loaded with Fluo-4 and labeled with anti-γδ TCR-APC antibodies, and Ca2+ signaling, in response to anti-CD3 (1 μg/ml) in the absence or presence of the indicated concentrations of 10panx-1, was recorded with flow cytometry. Baseline and maximum intracellular Ca2+ concentrations were determined using buffer control and ionomycin, respectively. Representative dot plots of Fluo-4 MFI readings over time (total duration: 512 s) are shown in A, and averaged data of a representative experiment are depicted in B. Arrows indicate the time-points at which buffer, anti-CD3, or ionomycin was added to the cells. Inhibition of Ca2+ signaling was calculated by combining data sets from three individual experiments (C). Data shown are averages ± sd; n = 3; #P < 0.05 as compared to stimulation with anti-CD3 alone.
γδ T cells predominantly express A2a, P2X1, P2X4, P2X7, and P2Y11 receptors
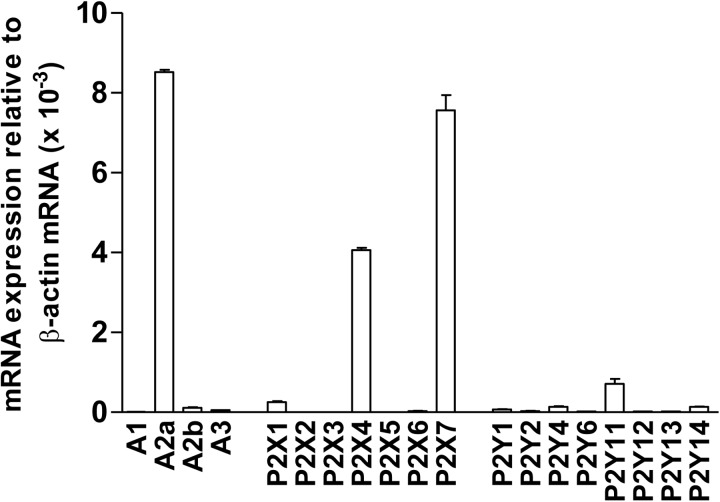
Using qPCR, we found that γδ T cells primarily express the A2a adenosine receptor, the P2X7, P2X4, and P2X1 subtypes of P2X receptors, as well as P2Y11 receptors (Fig. 4). When compared with the purinergic receptor expression profiles of CD4+ T cells and Jurkat cells, both of which belong to the αβ T cell family, we found that γδ T cells express markedly lower levels of A2b, P2X4, P2Y1, P2Y11, and P2Y14 receptors [28]. On the other hand, quite remarkably, the expression levels of P2X7 and A2a receptors on γδ T cells were approximately ten- and four-fold higher compared with CD4+ T cells, respectively (Supplemental Fig. 2).
Figure 4. Peripheral human γδ T cells predominantly express the A2a, P2X1, P2X4, P2X7, and P2Y11 receptor subtypes.
Total RNA isolated from >98% pure γδ T cells was subjected to qPCR using primers specific for the 19 known human purinergic receptor subtypes. Analyses were performed in triplicate, and mRNA expression levels of individual receptors were normalized to β-actin. Data shown are averages ± sd; n = 3.
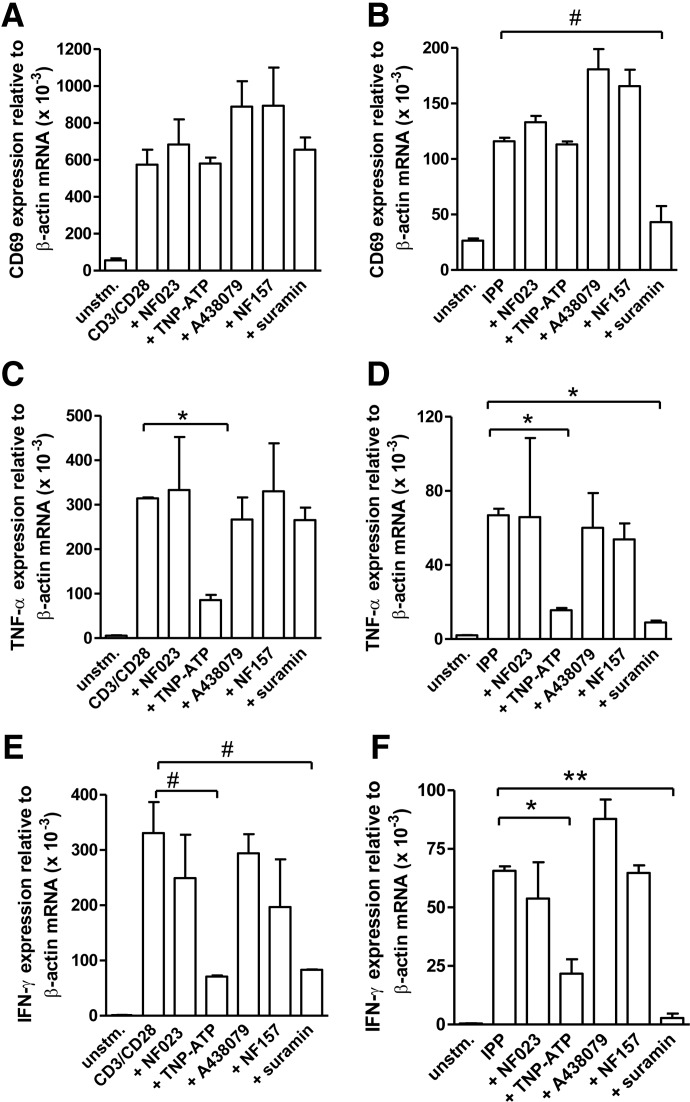
P2X4 receptors regulate γδ T cell activation
To determine the functional significance of the purinergic receptors expressed by γδ T cells, we examined how inhibition of individual P2 receptors influences the expression of CD69, an early T cell activation marker, and of TNF-α and IFN-γ mRNA transcripts. The inhibitors used in these studies were NF023, TNP-ATP, A438079, and NF157, which are antagonists of P2X1, P2X4, P2X7, and P2Y11 receptors, respectively. In addition, we used suramin as a nonspecific pan-P2 receptor antagonist. The viability of the cells in the absence and presence of these inhibitors at the concentrations mentioned was comparable, as assessed by trypan blue staining at the end of the incubation periods. Neither of these inhibitors influenced CD69 expression in response to cell stimulation with anti-CD3/CD28 antibody-coated beads. However, suramin pretreatment significantly inhibited CD69 expression in response to IPP stimulation (Fig. 5A and B). In contrast to NF023, A438079, and NF157, which had no discernible effects on cytokine mRNA expression, we found that TNP-ATP blocked TNF-α and IFN-γ transcription in response to cell stimulation with anti-CD3/CD28-coated beads or IPP. Suramin consistently inhibited IPP-induced TNF-α and IFN-γ transcription and also blocked IFN-γ transcription in response to CD3/CD28 stimulation. However, it had only a modest effect on TNF-α transcription in response to stimulation with anti-CD3/CD28-coated beads (Fig. 5C–F). These results clearly highlighted a special role for P2X4 receptors in the purinergic signaling mechanisms that regulate functional responses of γδ T cells. Our current findings thus suggest that ATP release and autocrine signaling through P2X4 receptors play a key role in the regulation of γδ T cell function.
Figure 5. P2X4 receptors play an important role in the activation of γδ T cells.
Purified γδ T cells were pretreated with NF023 (10 μM; P2X1 antagonist), TNP-ATP (30 μM; P2X4 antagonist), A438079 (10 μM; P2X7 antagonist), NF157 (10 μM; P2Y11 antagonist), or suramin (200 μM; nonselective P2 receptor antagonist) for 20 min. Then, the pretreated cells were stimulated with anti-CD3/CD28-coated beads (one bead/cell; left panels) or IPP (25 μM; right panels) for 4 h, and mRNA expression of CD69 (A and B), TNF-α (C and D), and IFN-γ (E and F) was assessed with qPCR. Individual expression levels were normalized to that of β-actin. Data shown are averages ± sd; n = 4; #P < 0.05; *P < 0.01; **P < 0.001.
DISCUSSION
A growing body of evidence suggests that purinergic signaling events regulate the activation and functional responses of a number of different immune cell types, including neutrophils, dendritic cells, and different T cell subsets [27, 28, 30, 34, 40–57]. However, to our knowledge, this is the first report that provides detailed information about the purinergic signaling system that regulates γδ T cell activation. We demonstrate that the function of circulating human γδ T cells is regulated by purinergic signaling pathways, and that these pathways seem to primarily involve P2X4 receptors.
In human blood, <10% of circulating lymphocytes are γδ TCR+, and of these cells, the Vγ9Vδ2 subset is the predominant γδ T cell subpopulation. The scarcity of these cells is one reason why comparatively little is known about their activation mechanisms. Selective expansion of Vγ9Vδ2 T cells in vitro by treating PBMCs with IPP and IL-2 over a period of 2–3 weeks is a standard protocol to enrich these cells [58, 59]. Although this approach helps to overcome the predicament of working with very limited numbers of purified γδ T cells, it potentially results in the activation-induced phenotype shifts and widespread changes in subsequent cell responses. Thus, in lieu of this protocol, we opted to work with freshly isolated γδ T cells. With the use of magnetic separation by negative rather than positive selection, an additional precaution was taken to preempt the possibility of activating cells by TCR cross-linking. Although this method yielded a relatively low number of purified cells, we believe that results using this approach would better reflect the in vivo properties of γδ T cells compared with approaches based on in vitro γδ T cell propagation.
It should be noted that the γδ T cell population purified in this manner would consist of a majority of cells bearing Vδ2-encoded receptors (>70%), and only a relatively small subset bearing receptors encoded by Vδ1 gene segments [60]. In the present study, we have not attempted to segregate Vδ2+ cells from Vδ2− cells. Thus, our results with CD3/CD28 stimulation reflect the combined responses of all circulating γδ T cell populations, whereas results observed with IPP stimulation reflect the response of Vγ9Vδ2 + cells.
We observed that purified γδ T cells rapidly release ATP upon in vitro stimulation with anti-CD3/CD28-coated beads or IPP. The amount of ATP released was estimated to be ∼50 picomoles/million cells and resulted in an increase of the extracellular ATP concentration by ∼30 nM. However, it should be considered that released ATP can be degraded rapidly by hydrolytic enzymes on the cell surface [61]. Thus, local ATP concentrations near the cell surface of the cells that release ATP can be considerably higher than in the bulk solution that we have used to estimate ATP release. Direct visualization of ATP release using fluorescence microscopy techniques described previously [29] could provide more accurate estimates of the ATP concentrations near the cell surface of stimulated T cells.
Based on previous reports about the mechanism of ATP release from murine and human αβ T cells, we focused our current study on the involvement of gap junction hemichannels, maxi-anion channels, and vesicular exocytosis. We found that panx-1 and connexin hemichannels, as well as vesicular exocytosis, contribute to the ATP release from γδ T cells in response to stimulation with anti-CD3/CD28-coated beads or IPP. Panx-1 seems to play a predominant role in ATP release, as inhibition of panx-1 with the specific inhibitor 10panx-1 was most effective in blocking ATP release. Interestingly, pretreatment with most ATP release blockers not only abolished ATP release in response to cell stimulation but also diminished extracellular ATP concentrations below the levels seen in unstimulated cells (note negative percentages in Fig. 2A and B). This suggests that panx-1 and other mechanisms mentioned above also contribute to basal ATP release from unstimulated cells. The fact that most inhibitors that we used were able to virtually, completely block ATP release in response to cell stimulation suggests that the different ATP release mechanisms might be operating in concert and that the sequential activation of these mechanisms is required for ATP release. Blocking either link in this series of interconnected mechanisms would disable ATP release, regardless of which step is blocked. Nevertheless, this explanation needs to be validated by additional experiments with specialized analytical tools, such as real-time monitoring techniques that we are currently developing to assess the spatiotemporal dynamics of ATP release from living cells.
At the concentrations used, 10panx-1 and CBX were more effective in inhibiting anti-CD3/CD28-induced ATP release compared with IPP-induced ATP release. This suggests that gap junction hemichannels are more critical for ATP release from cells stimulated with anti-CD3/CD28-coated beads compared with cells stimulated with a soluble antigen, i.e., IPP. On the contrary, the involvement of vesicular release seems more important for IPP-induced ATP release compared with anti-CD3/CD28-induced ATP release. A more detailed analysis of the contributions of these ATP release mechanisms to soluble versus immobilized antigens is required to better understand the reasons for these differences.
A central scheme of purinergic signaling is ATP release from an intact cell and binding of released ATP to the purinergic receptors expressed by the same cell or neighboring cells. P2X receptors are ATP-gated ion channels and allow cations, such as Ca2+, to enter the cell. Thus, to study the importance of ATP release in γδ T cell activation, we assessed Ca2+ signaling as a short duration assay of cell activation. Yet, as a result of limitations of cell numbers, we were unable to use purified γδ T cells, but rather used PBMCs and anti-γδ TCR antibody staining to identify γδ T cells within the PBMC pool. Under these circumstances, 10panx-1 pretreatment dose-dependently blocked Ca2+ entry with 400 µM 10panx-1 showing an average inhibition of 70% of the peak response to anti-CD3 stimulation. This percentage reflects the relative contribution of panx-1 to ATP-induced Ca2+ signaling, whereas the remaining 30% could be a result of other mechanisms, such as the recently discovered Orai channels, transient receptor potential channels, and voltage-gated Ca2+ channels. In these experiments, we used soluble anti-CD3 antibodies for cell stimulation. Whereas anti-CD3 stimulation induced a profound Ca2+ response, no Ca2+ signaling was seen in response to IPP stimulation. Morita et al. [62] have demonstrated that IPP induces Ca2+ influx in γδ T cell clones, but Ca2+ signaling in freshly isolated γδ T cells has not been investigated. Moreover, cell-to-cell contact was reported to be an important prerequisite for IPP-induced Ca2+ entry in γδ T cell clones, and the authors used an additional centrifugation step in their experimental protocol to encourage this intercellular contact. Future studies will be required to define the exact modes by which purinergic mechanisms induce Ca2+ signaling in freshly isolated γδ T cells under these different conditions.
Freshly isolated γδ T cells express A2a, P2X1, P2X4, P2X7, and P2Y11 receptors. Of these different P1 and P2 receptor subtypes, P2X7 receptor expression was approximately one order of magnitude higher in γδ T cells than in αβ T cells, suggesting an important role for P2X7 receptors in γδ T cell function. Whereas we did not find evidence for the involvement of P2X7 receptors in cytokine expression in our study, it is likely that P2X7 receptors have a role in other γδ T cell functions not examined in our current study. High expression of P2X7 receptors is a hallmark of regulatory T cells [57], and therefore, P2X7 receptors could possibly be involved in the immunoregulatory functions of non-Vδ2 T cell subsets. In fact, an interesting trend of enhanced functional response upon P2X7 and P2Y11 receptor inhibition (Fig. 5A, B, and F) may be indicative of the involvement of these receptors in γδ T cell regulation.
Our current findings show that P2X4 receptors have a functionally important role in γδ T cell activation. A unique role for P2X4 receptors, notwithstanding the abundant expression of P2X7 receptors, is certainly noteworthy. Selective translocation of P2X4 receptors to the immunological synapse to better avail the released ATP could be one possible reason behind this observation, and this possibility is supported by preliminary data using confocal microscopy. P2X4 receptors are relatively resistant to inhibition by suramin [63, 64], which could explain the relative lack of effect of suramin on CD3/CD28-induced CD69 and TNF-α expression. However, suramin profoundly suppressed all IPP-induced cell responses, as well as expression of IFN-γ in response to CD3/CD28 stimulation. This suggests the involvement of distinct P2 receptor subtypes in addition to P2X4 in the activation of these functional responses to CD3/CD28 and IPP stimulation.
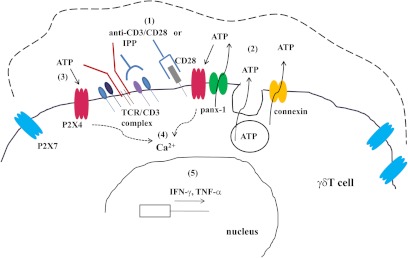
Based on our findings, we propose a model of purinergic signaling in circulating human γδ T cells, as depicted in Fig. 6. Stimulation of γδ T cells with anti-CD3/CD28-coated beads or IPP induces the release of ATP via panx-1 and possibly connexin hemichannels, as well as vesicular exocytosis. P2X4 receptors may selectively translocate to the immunological synapse following TCR stimulation. Thus, released ATP preferentially binds to P2X4 receptors and triggers Ca2+ influx through these receptors. This important, first downstream signaling event promotes subsequent signaling pathways that ultimately lead to the transcription of IFN-γ and TNF-α. In summary, this model highlights a significant role for purinergic signaling in γδ T cell activation. Nevertheless, this model is far from complete. Additional work is needed to provide a comprehensive picture that defines roles for the other P2 and P1 receptor subtypes in γδ TCR signaling, and that delineates how these complex purinergic signaling mechanisms contribute to the various γδ T cell functions pertaining to acute and chronic inflammatory conditions and cancer biology [65].
Figure 6. Proposed purinergic signaling in human γδ T cells.
In response to cell stimulation (1), purified γδ T cells release ATP through panx-1, connexin hemichannels, and vesicular exocytosis (2). P2X4 receptors bind to released ATP at the immunological synapse (3), and open to facilitate the influx of extracellular Ca2+ ions (4). The subsequent downstream signaling cascades culminate in up-regulated transcription and expression of cytokines such as IFN-γ and TNF-α (5). Blockade of ATP release or pharmacological inhibition of P2X4 receptors inhibits purinergic signaling and subsequent functional responses of stimulated γδ T cells.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported in part by a grant from the Congressionally Directed Medical Research Program (PR043034) and by grants from the U. S. National Institutes of Health, GM-51477, GM-60475, AI-072287, and AI-080582.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- 10panx-1
- pannexin-1 mimetic inhibitory peptide (sequence: WRQAAFVDSY)
- A438079
- 3′-{[5-(2, 3-dichlorophenyl)-1H tetrazol-1-yl] methyl} pyridine
- APC
- allophycocyanin
- Bf A
- bafilomycin A
- Ca2+
- calcium ion
- CBX
- carbenoxolone [(3β, 20β)-3-(3-carboxy-1-oxopropoxy)-11-oxoolean-12-en-29-oic acid disodium]
- DIDS
- 4, 4′-diisothiocyano-2, 2′-stilbenedisulfonic acid
- IPP
- isopentenyl pyrophosphate
- NF023
- 8, 8′-[carbonylbis(imino-3,1-phenylenecarbonylimino)]bis-1,3,5-naphthalene trisulfonic acid
- NF157
- 8, 8′-{carbonylbis[imino-3,1-phenylenecarbonylimino(4-fluoro-3,1-phenylene) carbonyl imino]}bis-1,3,5-naphthalenetrisulfonic acid
- panx-1
- pannexin-1 hemichannel
- qPCR
- quantitative real-time PCR
- TNP-ATP
- tri-nitro phenyl ATP
AUTHORSHIP
M.M. and M.I.H. carried out the bulk of these studies. Y.C. and T.W. contributed by assessing ATP levels and performing qPCR and microscope-based experiments. A.A.K. provided M.M. with guidance and advice during this project, which was a part of M.M.'s Ph.D. thesis. M.M. was instrumental in preparing the manuscript. W.G.J. conceived this study, provided overall guidance, and helped finalize the manuscript.
REFERENCES
- 1. Bonneville M., O'Brien R. L., Born W. K. (2010) γδ T cell effector functions: a blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 10, 467–478 [DOI] [PubMed] [Google Scholar]
- 2. Carding S. R., Egan P. J. (2002) γδ T cells: functional plasticity and heterogeneity. Nat. Rev. Immunol. 2, 336–345 [DOI] [PubMed] [Google Scholar]
- 3. Moser B., Eberl M. (2007) γδ T cells: novel initiators of adaptive immunity. Immunol. Rev. 215, 89–102 [DOI] [PubMed] [Google Scholar]
- 4. Morita C. T., Jin C., Sarikonda G., Wang H. (2007) Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vγ2Vδ2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol. Rev. 215, 59–76 [DOI] [PubMed] [Google Scholar]
- 5. Hayday A. C. (2000) γδ cells: a right time and a right place for a conserved third way of protection. Annu. Rev. Immunol. 18, 975–1026 [DOI] [PubMed] [Google Scholar]
- 6. Chien Y., Jores R., Crowley M. P. (1996) Recogntion by γ/δ T cells. Annu. Rev. Immunol. 14, 511–532 [DOI] [PubMed] [Google Scholar]
- 7. Nedellec S., Bonneville M., Scotet E. (2010) Human Vγ9Vδ2 T cells: from signals to functions. Semin. Immunol. 22, 199–206 [DOI] [PubMed] [Google Scholar]
- 8. Hayday A. C. (2009) γδ T cells and the lymphoid stress-surveillance response. Immunity 31, 184–196 [DOI] [PubMed] [Google Scholar]
- 9. Thedrez A., Sabourin C., Gertner J., Devilder M.-C., Allain-Maillet S., Fournié J.-J., Scotet E., Bonneville M. (2007) Self/non-self discrimination by human γδ T cells: simple solutions for a complex issue? Immunol. Rev. 215, 123–135 [DOI] [PubMed] [Google Scholar]
- 10. Groh V., Steinle A., Bauer S., Spies T. (1998) Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science 279, 1737–1740 [DOI] [PubMed] [Google Scholar]
- 11. Spada F. M., Grant E. P., Peters P. J., Sugita M., Melián A., Leslie D. S., Lee H. K., van Donselaar E., Hanson D. A., Krensky A. M., Majdic O., Porcelli S. A., Morita C. T., Brenner M. B. (2000) Self-recognition of CD1 by γ/δ T cells: implications for innate immunity. J. Exp. Med. 191, 937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harly C., Peyrat M-A., Netzer S., Déchanet-Merville J., Bonneville M., Scotet E. (2011) Up-regulation of cytolytic functions of human Vδ2− γδ T lymphocytes through engagement of ILT2 expressed by tumor target cells. Blood 117, 2864–2873 [DOI] [PubMed] [Google Scholar]
- 13. McVay L. D., Li B., Biancaniello R., Creighton M. A., Bachwich D., Lichtenstein G., Rombeau J. L., Carding S. R. (1997) Changes in human mucosal γδ T cell repertoire and function associated with the disease process in inflammatory bowel disease. Mol. Med. 3, 183–203 [PMC free article] [PubMed] [Google Scholar]
- 14. Holtmeier W., Pfänder M., Zollner T. M., Kaufmann R., Caspary W. F. (2002) Distinct TCR δ repertoires are present in the cutaneous lesions and inflamed duodenum of patients with dermatitis herpetiformis. Exp. Dermatol. 11, 527–531 [DOI] [PubMed] [Google Scholar]
- 15. Ebert L. M., Meuter S., Moser B. (2006) Homing and function of human skin γδ T cells and NK cells: relevance for tumor surveillance. J. Immunol. 176, 4331–4336 [DOI] [PubMed] [Google Scholar]
- 16. Ferrarini M., Ferrero E., Dagna L., Poggi A., Zocchi M. R. (2002) Human γδ T cells: a nonredundant system in the immune-surveillance against cancer. Trends Immunol. 23, 14–18 [DOI] [PubMed] [Google Scholar]
- 17. Nanno M., Shiohara T., Yamamoto H., Kawakami K., Ishikawa H. (2007) γδ T cells: firefighters or fire boosters in the front lines of inflammatory responses. Immunol. Rev. 215, 103–113 [DOI] [PubMed] [Google Scholar]
- 18. Lopez R. (2002) Human γδ-T cells in adoptive immunotherapy of malignant and infectious diseases. Immunol. Res. 26, 207–221 [DOI] [PubMed] [Google Scholar]
- 19. Bonneville M., Scotet E. (2006) Human Vγ9Vδ2 T cells: promising new leads for immunotherapy of infections and tumors. Curr. Opin. Immunol. 18, 539–546 [DOI] [PubMed] [Google Scholar]
- 20. Massaia M. (2012) Aminobisphosphonates, statins and the mevalonate pathway: a cross-road to fine-tune the activation of NK and Vγ9Vδ2 T cells. IBMS BoneKEy 9 [Google Scholar]
- 21. Martinet L., Poupot R., Fournié J-J. (2009) Pitfalls on the roadmap to γδ T cell-based cancer immunotherapies. Immunol. Lett. 124, 1–8 [DOI] [PubMed] [Google Scholar]
- 22. Burnstock G., Fredholm B. B., North R. A., Verkhratsky A. (2010) The birth and postnatal development of purinergic signalling. Acta Physiol. 199, 93–147 [DOI] [PubMed] [Google Scholar]
- 23. Burnstock G. (2007) Purine and pyrimidine receptors. Cell. Mol. Life Sci. 64, 1471–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burnstock G. (2006) Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol. Rev. 58, 58–86 [DOI] [PubMed] [Google Scholar]
- 25. Junger W. G. (2011) Immune cell regulation by autocrine purinergic signalling. Nat. Rev. Immunol. 11, 201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Di Virgilio F., Chiozzi P., Ferrari D., Falzoni S., Sanz J. M., Morelli A., Torboli M., Bolognesi G., Baricordi O. R. (2001) Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood 97, 587–600 [DOI] [PubMed] [Google Scholar]
- 27. Yip L., Woehrle T., Corriden R., Hirsh M., Chen Y., Inoue Y., Ferrari V., Insel P. A., Junger W. G. (2009) Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J. 23, 1685–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Woehrle T., Yip L., Elkhal A., Sumi Y., Chen Y., Yao Y., Insel P. A., Junger W. G. (2010) Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood 116, 3475–3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Corriden R., Insel P. A., Junger W. G. (2007) A novel method using fluorescence microscopy for real-time assessment of ATP release from individual cells. Am. J. Physiol. Cell Physiol. 293, C1420–C1425 [DOI] [PubMed] [Google Scholar]
- 30. Schenk U., Westendorf A. M., Radaelli E., Casati A., Ferro M., Fumagalli M., Verderio C., Buer J., Scanziani E., Grassi F. (2008) Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci. Signal. 1, ra6. [DOI] [PubMed] [Google Scholar]
- 31. Tokunaga A., Tsukimoto M., Harada H., Moriyama Y., Kojima S. (2010) Involvement of SLC17A9-dependent vesicular exocytosis in the mechanism of ATP release during T cell activation. J. Biol. Chem. 285, 17406–17416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baricordi O., Ferrari D., Melchiorri L., Chiozzi P., Hanau S., Chiari E., Rubini M., Di Virgilio F. (1996) An ATP-activated channel is involved in mitogenic stimulation of human T lymphocytes. Blood 87, 682–690 [PubMed] [Google Scholar]
- 33. Yip L., Cheung C. W., Corriden R., Chen Y., Insel P. A., Junger W. G. (2007) Hypertonic stress regulates T-cell function by the opposing actions of extracellular adenosine triphosphate and adenosine. Shock 27, 242–250 [DOI] [PubMed] [Google Scholar]
- 34. Chen Y., Corriden R., Inoue Y., Yip L., Hashiguchi N., Zinkernagel A., Nizet V., Insel P. A., Junger W. G. (2006) ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314, 1792–1795 [DOI] [PubMed] [Google Scholar]
- 35. Praetorius H., Leipziger J. (2009) ATP release from non-excitable cells. Purinergic Signal. 5, 433–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yegutkin G. G. (2008) Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim. Biophys. Acta 1783, 673–694 [DOI] [PubMed] [Google Scholar]
- 37. Bodin P., Burnstock G. (2001) Purinergic signalling: ATP release. Neurochem. Res. 26, 959–969 [DOI] [PubMed] [Google Scholar]
- 38. Lazarowski E. R., Boucher R. C., Harden T. K. (2003) Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol. Pharmacol. 64, 785–795 [DOI] [PubMed] [Google Scholar]
- 39. Woehrle T., Yip L., Manohar M., Sumi Y., Yao Y., Chen Y., Junger W. G. (2010) Hypertonic stress regulates T cell function via pannexin-1 hemichannels and P2X receptors. J. Leukoc. Biol. 88, 1181–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen Y., Yao Y., Sumi Y., Li A., To U. K., Elkhal A., Inoue Y., Woehrle T., Zhang Q., Hauser C., Junger W. G. (2010) Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci. Signal. 3, ra45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kobayashi T., Kouzaki H., Kita H. (2010) Human eosinophils recognize endogenous danger signal crystalline uric acid and produce proinflammatory cytokines mediated by autocrine ATP. J. Immunol. 184, 6350–6358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Abbracchio M. P., Paoletti A. M., Luini A., Cattabeni F., De Matteis M. A. (1992) Adenosine receptors in rat basophilic leukaemia cells: transductional mechanisms and effects on 5-hydroxytryptamine release. Br. J. Pharmacol. 105, 405–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ryzhov S., Zaynagetdinov R., Goldstein A. E., Novitskiy S. V., Dikov M. M., Blackburn M. R., Biaggioni I., Feoktistov I. (2008) Effect of A2B adenosine receptor gene ablation on proinflammatory adenosine signaling in mast cells. J. Immunol. 180, 7212–7220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Piccini A., Carta S., Tassi S., Lasiglié D., Fossati G., Rubartelli A. (2008) ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1β and IL-18 secretion in an autocrine way. Proc. Natl. Acad. Sci. USA 105, 8067–8072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ben Yebdri F., Kukulski F., Tremblay A., Sévigny J. (2009) Concomitant activation of P2Y2 and P2Y6 receptors on monocytes is required for TLR1/2-induced neutrophil migration by regulating IL-8 secretion. Eur. J. Immunol. 39, 2885–2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Elliott M. R., Chekeni F. B., Trampont P. C., Lazarowski E. R., Kadl A., Walk S. F., Park D., Woodson R. I., Ostankovich M., Sharma P., Lysiak J. J., Harden T. K., Leitinger N., Ravichandran K. S. (2009) Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461, 282–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kronlage M., Song J., Sorokin L., Isfort K., Schwerdtle T., Leipziger J., Robaye B., Conley P. B., Kim H-C., Sargin S., Schon P., Schwab A., Hanley P. J. (2010) Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci. Signal. 3, ra55. [DOI] [PubMed] [Google Scholar]
- 48. Wilkin F., Duhant X., Bruyns C., Suarez-Huerta N., Boeynaems J-M., Robaye B. (2001) The P2Y11 receptor mediates the ATP-induced maturation of human monocyte-derived dendritic cells. J. Immunol. 166, 7172–7177 [DOI] [PubMed] [Google Scholar]
- 49. La Sala A., Ferrari D., Di Virgilio F., Idzko M., Norgauer J., Girolomoni G. (2003) Alerting and tuning the immune response by extracellular nucleotides. J. Leukoc. Biol. 73, 339–343 [DOI] [PubMed] [Google Scholar]
- 50. Schnurr M., Toy T., Shin A., Wagner M., Cebon J., Maraskovsky E. (2005) Extracellular nucleotide signaling by P2 receptors inhibits IL-12 and enhances IL-23 expression in human dendritic cells: a novel role for the cAMP pathway. Blood 105, 1582–1589 [DOI] [PubMed] [Google Scholar]
- 51. Ben Addi A., Cammarata D., Conley P. B., Boeynaems J-M., Robaye B. (2010) Role of the P2Y12 receptor in the modulation of murine dendritic cell function by ADP. J. Immunol. 185, 5900–5906 [DOI] [PubMed] [Google Scholar]
- 52. Weber F. C., Esser P. R., Müller T., Ganesan J., Pellegatti P., Simon M. M., Zeiser R., Idzko M., Jakob T., Martin S. F. (2010) Lack of the purinergic receptor P2X7 results in resistance to contact hypersensitivity. J. Exp. Med. 207, 2609–2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wilhelm K., Ganesan J., Muller T., Durr C., Grimm M., Beilhack A., Krempl C. D., Sorichter S., Gerlach U. V., Juttner E., Zerweck A., Gartner F., Pellegatti P., Di Virgilio F., Ferrari D., Kambham N., Fisch P., Finke J., Idzko M., Zeiser R. (2010) Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat. Med. 16, 1434–1438 [DOI] [PubMed] [Google Scholar]
- 54. Padeh S., Cohen A., Roifman C. (1991) ATP-induced activation of human B lymphocytes via P2-purinoceptors. J. Immunol. 146, 1626–1632 [PubMed] [Google Scholar]
- 55. Duhant X., Suarez Gonzalez N., Schandené L., Goldman M., Communi D., Boeynaems J-M. (2005) Molecular mechanisms of extracellular adenine nucleotides-mediated inhibition of human CD4+ T lymphocytes activation. Purinergic Signal. 1, 377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gorini S., Callegari G., Romagnoli G., Mammi C., Mavilio D., Rosano G., Fini M., Di Virgilio F., Gulinelli S., Falzoni S., Cavani A., Ferrari D., la Sala A. (2010) ATP secreted by endothelial cells blocks CX3CL1-elicited natural killer cell chemotaxis and cytotoxicity via P2Y11 receptor activation. Blood 116, 4492–4500 [DOI] [PubMed] [Google Scholar]
- 57. Schenk U., Frascoli M., Proietti M., Geffers R., Traggiai E., Buer J., Ricordi C., Westendorf A. M., Grassi F. (2011) ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci. Signal. 4, ra12. [DOI] [PubMed] [Google Scholar]
- 58. Vantourout P., Mookerjee-Basu J., Rolland C., Pont F., Martin H., Davrinche C., Martinez L. O., Perret B., Collet X., Périgaud C., Peyrottes S., Champagne E. (2009) Specific requirements for Vγ9Vδ2 T cell stimulation by a natural adenylated phosphoantigen. J. Immunol. 183, 3848–3857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Maniar A., Zhang X., Lin W., Gastman B. R., Pauza C. D., Strome S. E., Chapoval A. I. (2010) Human γδ T lymphocytes induce robust NK cell-mediated antitumor cytotoxicity through CD137 engagement. Blood 116, 1726–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Parker C. M., Groh V., Band H., Porcelli S. A., Morita C., Fabbi M., Glass D., Strominger J. L., Brenner M. B. (1990) Evidence for extrathymic changes in the T cell receptor γ/δ repertoire. J. Exp. Med. 171, 1597–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zimmermann H. (2000) Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch. Pharmacol. 362, 299–309 [DOI] [PubMed] [Google Scholar]
- 62. Morita C. T., Beckman E. M., Bukowski J. F., Tanaka Y., Band H., Bloom B. R., Golan D. E., Brenner M. B. (1995) Direct presentation of nonpeptide prenyl pyrophosphate antigens to human γδ T cells. Immunity 3, 495–507 [DOI] [PubMed] [Google Scholar]
- 63. Khakh B. S., Burnstock G., Kennedy C., King B. F., North R. A., Séguéla P., Voigt M., Humphrey P. P. A. (2001) International Union of Pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol. Rev. 53, 107–118 [PubMed] [Google Scholar]
- 64. North R. A. (2002) Molecular physiology of P2X receptors. Physiol. Rev. 82, 1013–1067 [DOI] [PubMed] [Google Scholar]
- 65. Stagg J., Smyth M. J. (2010) Extracellular adenosine triphosphate and adenosine in cancer. Oncogene 29, 5346–5358 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.