The pentraxin serum amyloid P, at least in part, uses FcγRI (CD64) and FcγRγ to inhibit fibrocyte differentiation.
Keywords: monocyte, SAP, CD64, CD32, pentraxin
Abstract
Fibrotic diseases, such as cardiac and pulmonary fibrosis, have a poor prognosis with no FDA approved therapies. Monocyte-derived, fibroblast-like cells, called fibrocytes, participate in the formation of fibrotic lesions. The conserved pentraxin protein SAP inhibits fibrocyte differentiation in cell culture, and injections of SAP significantly reduce fibrosis in several animal models. SAP binds to the receptors for the Fc portion of IgG (FcγR) and has been crystallized bound to FcγRIIa (CD32a). The in vivo activity of SAP appears to be dependent on the FcRγ. We find that mutagenesis of the residues critical for SAP binding to FcγRIIa only moderately decreases the ability of SAP to inhibit fibrocyte differentiation. In murine cells, deletion of FcRγ or FcγRI (CD64) significantly reduced sensitivity to SAP. Deletion of the combination of FcγRIIb, FcγRIIIa, and FcγRIV did not significantly affect sensitivity to SAP, whereas deletion of just the inhibitory receptor FcγRIIb (CD32b) increased sensitivity to SAP. In human cells, siRNA-mediated reduction of FcRγ or FcγRI levels significantly decreased sensitivity to SAP, whereas reduction of FcγRIIb levels increased sensitivity to SAP. These observations suggest that SAP, at least in part, uses FcγRI and FcRγ to inhibit fibrocyte differentiation.
Introduction
Fibrosis is an aberrant condition involving tissue injury and inflammation, resulting in scar-like lesions, composed primarily of ECM proteins, such as collagen and fibronectin [1]. This excess buildup of scar tissue leads to tissue dysfunction and eventual organ failure. There are currently no FDA-approved therapies for fibrosis [2, 3]. Increasing evidence suggests that fibroblast-like cells, termed fibrocytes, participate in wound repair and the formation of fibrotic lesions in diseases, such as pulmonary fibrosis, congestive heart failure, cirrhosis of the liver, renal fibrosis, and nephrogenic systemic fibrosis [4–10].
Fibrocytes derive from a subset of CD14+ monocytes, and mature fibrocytes express markers of hematopoietic cells (CD34, CD45, CXCR4, leukocyte-specific protein 1, MHC class II) and stromal cells (collagen I and III and fibronectin) [11–16]. The differentiation of monocytes into fibrocytes in vitro correlates with the presence of tissue fibrocytes in vivo, in terms of time-frame for differentiation, receptor expression, and production of collagen [5, 11–13, 16, 17]. A population of CD34+ CD45+ CXCR4+ cells, which express low levels of collagen I, is also present in peripheral blood, and there are elevated numbers (up to 10% of PBMC) of these cells in patients with inflammatory and fibrotic diseases [5, 17–19]. However, in healthy, young adults (<35 years), the number of CD45+ collagen I+ cells is low compared with older, healthy adults (>60 years) [19]. What proportion of these circulating CD45+ collagen I+ cells is about to enter a tissue and become tissue-resident fibrocytes—or circulating cells, which will never enter a tissue, or mature fibrocytes, which have left a tissue and entered the circulation—is still unclear [17, 20, 21].
Factors that regulate fibrocyte differentiation could potentially be used as a therapeutic for fibrosing diseases. We found that the differentiation of monocytes into fibrocytes in vitro is inhibited by the plasma protein SAP [13], which is a highly conserved glycoprotein secreted by the liver into the blood and is a member of the pentraxin family, including C-reactive protein [22–24]. The pentraxin family is characterized by a planar disc arrangement of five, noncovalently associated monomers [25]. The SAP protomer has a mass of 25.5 kDa [26] and contains a calcium-dependent, ligand binding site on one face of the protomer and a putative receptor binding site on the opposite face [27]. SAP is a pattern recognition protein, which binds components of pathogens, as well as apoptotic and autoimmune material [24, 28]. Injections of SAP significantly reduce bleomycin-induced pulmonary fibrosis in mice and rats [29]. SAP has also been used successfully to treat, in model systems, ischemic cardiomyopathy, Aspergillus fumigatus-induced allergic airway disease, radiation-induced oral mucositis, and renal fibrosis [6, 30, 31]. In these disease models, SAP acted through the reduction of fibrocytes [6, 29] or inhibition of profibrotic inflammatory M2 macrophages [30, 31].
FcγRs are found on a variety of hematopoietic cells and bind the Fc portion of IgG [32]. Once aggregated IgG cross links multiple receptors, a signaling cascade is activated through tyrosine kinases to initiate an immune response [33]. In humans, there are four activating FcγRs: FcγRI (CD64), FcγRIIa (CD32a), FcγRIIIa (CD16a), and FcγRIIIb (CD16b) [34]. In contrast, FcγRIIb (CD32b) triggers an inhibitory signaling pathway to help modulate the immune response [35]. The signaling cascades initiated by FcγRI and FcγRIIIa require an accessory FcRγ, whereas FcγRIIa and FcγRIIb do not need FcRγ for signaling [36]. FcγRs are also defined by their affinity for binding monomeric or aggregated IgG. FcγRI is a high-affinity receptor for monomeric and aggregated IgG, whereas the remaining receptors bind only aggregated IgG with low to medium affinity [37]. We previously found that cross-linked and aggregated IgG inhibit fibrocyte differentiation in vitro, but monomeric IgG did not block SAP bioactivity [38].
In mice, the activating FcγRs are FcγRI, FcγRIII, and FcγRIV, and all three require FcRγ for signaling [39]. The inhibitory receptor is FcγRIIb [37]. Based on sequence similarity of the extracellular domain, mouse FcγRIII is most closely related to human FcγRIIa, whereas mouse FcγRIV is most closely related to human FcγRIIIa [40].
Human (and murine) monocytes express FcγRI, FcγRIIa (FcγRIII in mice), and FcγRIIb [39]. SAP appears to act through FcγR ligation to regulate the differentiation of monocytes into fibrocytes. SAP initiates a signaling cascade consistent with FcγR ligation, and SAP has been crystallized, bound to FcγRIIa [30, 41–46]. In addition, the ability of SAP to inhibit fibrosis in mouse models of pulmonary and kidney fibrosis is dependent on the FcRγ component of activating FcγRs [30, 46].
Whereas the ability of SAP to inhibit fibrosis in vivo relies on FcRγ, little is known about which receptor subtype is responsible for specifying fibrocyte differentiation, nor what domains of SAP are critical for this effect. The current literature is unclear regarding how SAP interacts with FcγRs. One group found that hSAP has a lower affinity for binding to human FcγRs than IgG and that hSAP has the highest affinity for FcγRI among the different receptor subtypes [27]. Another group found that hSAP has a higher affinity for binding to human FcγRs than IgG and that hSAP binds with highest affinity to FcγRIIa and -III [30]. A third group found that hSAP binds with highest affinity to FcγRI and FcγRIIa and a lower affinity to FcγRIIIb [41]. In a crystal structure of hSAP bound to FcγRIIa, a helix region of hSAP (Pro 166 to Gln 174) bound to FcγRIIa [27]. hSAP binds to mouse FcγRs with an affinity of FcγIV > FcγIII ≥ FcγI > FcγII [30]. Finally, we have shown that hSAP is more potent than mSAP at inhibiting human and murine fibrocyte differentiation in vitro [47], and several groups have shown that hSAP is as potent as mSAP at inhibiting fibrosis in mice [29, 30, 48–50].
To elucidate the SAP-FcγR interaction responsible for regulating fibrocyte differentiation, we mutated residues of SAP critical for SAP binding to FcγRIIa, and we examined SAP bioactivity on cells with low levels of specific FcγRs. We found that changing the residues critical for SAP binding to FcγRIIa had little effect on SAP bioactivity. Deletion of FcγRI and FcRγ in murine cells and reduction of FcγRI and FcRγ in human cells reduced sensitivity to SAP. Together, this suggests that SAP, at least in part, uses FcγRI and FcRγ to inhibit fibrocyte differentiation.
MATERIALS AND METHODS
Construction of pcDNA3.1− vector and expression in HEK293 cells
The hSAP coding region (Genbank Accession Number 18375514), including the secretion signal, was amplified via PCR using a commercial human liver cDNA library (Agilent Technologies, Santa Clara, CA, USA). The cDNA was cloned into the pcDNA3.1− plasmid (Invitrogen, Carlsbad, CA, USA) using NotI and AgeI restriction sites, with a RTE-CTE enhancer cloned in 3′ to SAP (PacI to EcoRI, from pNLgagRTE26CTE plasmid) [51]. The vector with hSAP was generously donated by Promedior (Malvern, PA, USA).
The hSAP-pcDNA3.1− vector was transfected into HEK cells (Freestyle 293F cells, Invitrogen) using Lipofectamine 2000 (Invitrogen), following the manufacturer's protocol. Cells were grown at 37°C with 5% CO2 in Freestyle 293 media (Invitrogen), supplemented with 2 mM glutamine and 0.75 mg/ml geneticin to select for the vector. After 5 days, conditioned media were collected for protein purification.
Protein purification from HEK293 cells
Conditioned medium (20 ml) from the hSAP-expressing 293F cells was clarified by centrifugation at 900 g for 10 min at RT. CaCl2 (1 M) was added to a final concentration of 2 mM, and the conditioned medium was then mixed with 1 ml 50% slurry of Sepharose Fast Flow (GE Healthcare BioSciences, Piscataway, NJ, USA) in wash buffer (20 mM Tris, pH 7.4/140 mM NaCl/2 mM CaCl2) for 1 h at RT using an end-over-end mixer (in the presence of Ca++, SAP binds strongly to Sepharose) [52–54]. The Sepharose beads were collected by centrifugation at 900 g for 1 min at RT. The beads were washed five times with 15 ml wash buffer. Bound protein was eluted overnight at 4°C with 400 μl 20 mM Tris, pH 7.4/140 mM NaCl/50 mM EDTA. The eluted protein was dialyzed in a 500-μl, 10-kDa molecular weight cutoff dialysis cassette (Thermo Scientific Pierce, Rockford, IL, USA) against 1.5 L 20 mM NaPO4, pH 7.4/10% glycerol, overnight at 4°C. The purified hSAP was dialyzed further against 20 mM NaPO4, pH 7.4/glycerol (with the glycerol concentration reduced in half each dialysis step and 0% for the final step) for a total of six times with a minimum of 3 h between buffer exchanges. The purified hSAP was filter-sterilized with a 0.2-μm acrodisc syringe filter (Millipore, Billerica, MA, USA), and the hSAP concentration was checked by Western blot, as described previously [13, 29], with the exception that samples were run on 4–15% Tris-glycine gels (Bio-Rad, Hercules, CA, USA), and the detection antibody was a 1:20,000 dilution of rabbit anti-SAP polyclonal antibody (Epitomics, Burlingame, CA, USA). The purity of the hSAP was checked by Coomassie or silver staining of protein gels.
Generation of hSAP mutants
Using the hSAP-pcDNA3.1− vector as a template, the primers in Supplemental Table 1 were used to generate point mutations in the SAP sequence. The PCR reaction and transformation were carried out using a QuikChange II Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA), following the manufacturer's protocol. The resulting plasmids were sequenced to confirm the point mutations and absence of other mutations. Transfection and expression were then carried out as described above.
Gel filtration
Conditioned media from hSAP-expressing 293F cells were filtered with a 0.2-μ acrodisc syringe filter (Millipore). Sample (300 μl) was loaded onto a Superose 12 100/300 GL column (GE Healthcare BioSciences) using an AKTA Purifier UPC-10 with a flow rate of 0.3 ml/min of the gel filtration buffer (20 mM Tris, 140 mM NaCl, 10 mM EDTA, pH 7.4) at 4°C. Fractions (300 μl) were collected and analyzed by Western blots for the presence of SAP. The apparent molecular weight of SAP was estimated by comparing the fractions with the elution profile of gel filtration standards (Bio-Rad), run in the gel filtration buffer.
Fibrocyte differentiation assay
Human peripheral blood was collected into heparin vacutainer tubes (#367874; BD Biosciences, Franklin Lakes, NJ, USA) with written consent from healthy, adult volunteers and with specific approval of the Institutional Review Boards of Rice University (Houston, TX, USA) or Texas A&M University (College Station, TX, USA). PBMCs were isolated by Ficoll-Paque Plus (GE Healthcare Biosciences), as described previously [55]. PBMCs were cultured in SFM, as described previously, using FibroLife basal media (Lifeline Cell Technology, Walkersville, MD, USA) [55]. RPMI medium (5×) was prepared by mixing 2.5 ml 10× RPMI (Sigma-Aldrich, St. Louis, MO, USA) with 250 μl each supplement, described previously [55], 833 μl 6% NaHCO3, and 170 μl H2O. rhSAP was added to flat-bottomed, 96-well tissue-culture plates (BD Biosciences) and diluted as follows: 0.8 μg rhSAP in 20 mM NaPO4, pH 7.4, or an equal volume of buffer was added to the first column of wells, along with a 1/4 vol 5× RPMI medium. The wells were brought to 200 μl with SFM. One hundred microliters was removed from the first column and serially diluted with an equal volume of SFM across the plate to achieve the indicated hSAP concentrations. PBMCs (100 μl) at 5 × 105 cells/ml in SFM were then added to each well. After a 5-day incubation period, the plate was air-dried, fixed with methanol, and stained with Hema 3 stain (Fisher Scientific, Hampton, NH, USA). For each well, fibrocytes were counted in five different, 900 μm-diameter fields, with fibrocytes defined as adherent, elongated, spindle-shaped cells with oval nuclei [13, 15, 38].
Isolation of mSAP
mSAP was purified from murine serum (Gemini Bioproducts, West Sacramento, CA, USA) using calcium-dependent binding to phosphoethanolamine-conjugated agarose, as described previously [6, 29, 47].
Cell fractionation of spleen cells
Four- to 6-week, male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME, USA, or Taconic, Hudson, NY, USA), B6.129P2-Fcgr3tm1Sjv/J mice (The Jackson Laboratory), B6.129S4-Fcgr2btm1TtK N12 mice (Taconic), and B6.129P2-Fcer1gtm1Rav N12 mice (Taconic) were used in this study. Spleen cells from FcγRII/III/IV KO mice in a C57BL6/J background were kindly donated by Dr. Sjef Verbeek at Leiden University Medical Center (The Netherlands) [56, 57]. Spleen cells from FcγRI KO mice [58] were kindly donated by Dr. Bryce Binstadt at the University of Minnesota (Minneapolis, MN, USA). All work was done under Rice University and Texas A&M University Institutional Animal Care and Use Committee-approved protocols. Spleens were harvested (70–100 mg each), and cells were isolated by incubation in a digest cocktail, followed by passage through a cell strainer, and further purified using an ammonium chloride, potassium bicarbonate lysis buffer, as described previously [47].
Flow cytometry
Mouse spleen cells were subjected to flow analysis, as described previously [16, 47], with the following modifications. Cells were stained using 5 μg/ml antibodies against CD3 (clone 17A2, rat IgG2b, BD Biosciences), CD45R/B220 (clone RA3-6B2, rat IgG2a, BD Biosciences), CD11c (clone 223H7, rat IgG2a, MBL International, Woburn, MA, USA), CD16/32 (clone 2.4G2, rat IgG2b, BD Biosciences), CD94 (clone HMa2, rat IgG2a, eBioscience, San Diego, CA, USA), Ly6G (clone 1A8, rat IgG2a, BioLegend, San Diego, CA, USA), GR-1 (clone RB6-8C5, rat IgG2b, BD Biosciences), F4/80 (clone BM8, rat IgG2a, BioLegend), and isotype-matched, irrelevant rat mAb (BioLegend) as controls. The secondary antibody was a FITC-conjugated mouse F(ab′)2 anti-rat IgG (Jackson ImmunoResearch, West Grove, PA, USA), used at 2.5 μg/ml. After blocking with PBS/10% rat serum (Sigma-Aldrich) for 30 min at 4°C, cells were collected by centrifugation at 300 g for 5 min and resuspended in 100 μl PBS/4% BSA containing 2.5 μg/ml PE-conjugated anti-CD11b antibody (clone M1/70, rat IgG2b, eBioscience) and 5 μg/ml biotinylated anti-CD45 antibody (clone 30-F11, rat IgG2b, BD Biosciences). After a 30-min incubation on ice, cells were washed twice with 1.4 ml ice-cold PBS and resuspended in 100 μl ice-cold PBS/BSA containing 2 μg/ml streptavidin-allophycocyanin (BioLegend). After a 30-min incubation on ice, cells were washed twice, resuspended in 100 μl ice-cold PBS/BSA, and analyzed using a C6 flow cytometer (Accuri, Ann Arbor, MI, USA).
Determining the effect of SAP on fibrocyte differentiation for spleen cells
FibroLife (Lifeline Cell Technology) SFM for murine cells was prepared as described previously, including 50 ng/ml murine IL-13 and 25 ng/ml murine M-CSF (PeproTech, Rocky Hill, NJ, USA), for select wells [47]. Spleen cells were cultured in flat-bottomed, 96-well, tissue-culture plates at 3.5 × 105 cells/well in 200 μl with mSAP and hSAP (EMD Calbiochem, Billerica, MA, USA) at the indicated concentrations, as described previously [47]. On Day 3 of the incubation, wells with IL-13 and M-CSF were supplemented further with 5 μl of a cocktail containing 1 μg/ml IL-13 and 0.5 μg/ml M-CSF in FibroLife SFM. After 5 days, plates were air-dried, fixed with methanol, and stained with Hema 3 stain (Fisher Scientific).
siRNA knockdown
Silencer select siRNAs (Applied Biosystems/Ambion, Austin, TX, USA) were reconstituted in H2O to 50 μM. Human PBMCs were isolated and purified as described above. Transfection of human PBMCs was carried out with Lipofectamine 2000 (Invitrogen), following the manufacturer's protocol. siRNA (5.25 pmol) was incubated in 25 μl FibroLife basal media (Lifeline Cell Technology) + HEPES (Sigma-Aldrich) with 0.25 μl Lipofectamine 2000 for 20 min. The mixture was then diluted in 2.1 ml FibroLife SFM, lacking antibiotics, to obtain a concentration of 2.5 nM siRNA. siRNA (200 μl) was added to the top row of a 96-well plate, and 100 μl doubling dilutions were carried out using an equal volume of FibroLife SFM lacking antibiotics to obtain 100 μl of the indicated concentrations of siRNA. PBMCs (100 μl) at 5 × 105 cells/ml in SFM were added to each well, and the plate was incubated for 24 h in a humidified incubator containing 5% CO2 at 37°C. hSAP (EMD Calbiochem) was added at the indicated concentrations, 24 h later, in 3 μl aliquots. The plate was returned to the incubator, and after 4 additional days, the plates were air-dried, fixed with methanol, and stained.
Determination of levels of FcγRs after siRNA knockdown
siRNA plates were set up as described above, except SAP was not added. After 2.5 days, the wells were washed with 100 μl PBS, fixed with 75 μl PBS/4% paraformaldehyde on ice for 15 min, washed twice for 5 min with 100 μl PBS, permeabilized with 75 μl PBS/0.1% Triton X-100 for 10 min at RT, and washed three times for 5 min with 100 μl PBS. The wells were blocked with 100 μl PBS/4% BSA at 4°C overnight. Cells were stained for 1 h with primary antibody or IgG-matched controls (BioLegend and Epitomics, Burlingame, CA, USA) in 50 μl PBS/4% BSA using: 5 μg/ml rabbit anti-human FcϵRI, γ subunit (06-727, Millipore), 5 μg/ml mouse anti-human CD64 (clone 10.1, mouse IgG1, BD Biosciences), 7.5 μg/ml goat anti-human CD32a (AF1875, R&D Systems, Minneapolis, MN, USA), 1:100 rabbit anti-human CD32b (clone EP926Y, Epitomics), or 1:100 rabbit anti-human CD16a (clone EPR4333, Epitomics). The wells were washed four times for 5 min with 100 μl PBS. The primary antibodies were detected with biotinylated F(ab′)2 goat anti-rabbit IgG (Southern Biotech, Birmingham, AL, USA), biotinylated goat F(ab′)2 anti-mouse IgG (Southern Biotech), or biotinylated F(ab′)2 donkey anti-goat IgG (Jackson ImmunoResearch). All secondary antibodies were incubated for 30 min at 2.5 μg/ml in 50 μl PBS/BSA, and the wells were then washed four times for 5 min with 100 μl PBS. After washing, the wells were incubated for 30 min with 2.5 μg/ml streptavidin-conjugated Dylight-488 antibody (Thermo Scientific Pierce). The wells were washed four times for 5 min with PBS, and the wells were coated with 30 μl Vectashield mounting media (Vector Labs, Burlingame, CA, USA), containing 4′,6-diamidino-2-phenylindole. Immunofluorescence images were captured using an Olympus FV1000 confocal microscope (Olympus, Center Valley, PA, USA), and ImageJ (NIH, Bethesda, MD, USA) was used for analysis. Mean fluorescent intensity was measured for each cell, excluding sizes smaller than 156 μm2 to eliminate cell debris and lymphocytes.
Immunohistochemistry
Human PBMCs were cultured in eight-well glass slides (177402; Lab-Tek, Nalge Nunc, Naperville, IL, USA) at 1 × 106 cells/ml and 250 μl/well for 5 days in the presence or absence of siRNA against FcRγ and hSAP. Slides were air-dried overnight, fixed in acetone, and stained for collagen I (rabbit polyclonal; Rockland, Gilbertsville, PA, USA) or negative control rabbit IgG (Jackson ImmunoResearch), as described previously [16].
Mouse spleen cells were cultured in eight-well CC2 glass slides (154941; Nalge Nunc), as described previously [47], with the following modifications. Slides were stained for CD45 (clone Ly-5, rat IgG2b, BD Biosciences) and pro-collagen I (clone Y-18, Santa Cruz Biotechnology, Santa Cruz, CA, USA), along with their respective negative control rat IgG2b (BD Biosciences) and goat IgG (R&D Systems). Primary antibodies were detected using Vectastain Elite ABC kits for rat and goat (Vector Labs), following the manufacturer's protocol and visualized using diaminobenzidine substrate (Vector Labs).
Statistical analysis
Statistical analysis was performed using GraphPad Prism software (GraphPad Software, San Diego, CA, USA). Significance was defined as P < 0.05.
RESULTS
We previously found that hSAP inhibits the differentiation of monocytes to fibrocytes in vitro [13, 15, 38, 47]. Additionally, mSAP significantly reduced the levels of fibrosis in mouse cardiac and lung fibrosis, which correlated with a reduction in the number of fibrocytes [6, 29]. The ability of SAP to inhibit fibrosis in vivo appeared to be dependent on the presence of FcRγ [30, 46], and hSAP has been crystallized, bound to the extracellular domain of FcγRIIa, an activating FcγR [27]. To determine if the inhibition of monocyte to fibrocyte differentiation by SAP is dependent on FcγRs, we cultured human and murine PBMC in SFM. After incubation for 5 days, we observed spindle-shaped cells in the culture. To confirm the identity of the spindle-shaped cells, we stained the cells for a variety of markers, as described previously [13, 15, 16, 38, 47, 55]. The spindle-shaped cells were positive for markers expressed by fibrocytes, including CD11b, CD34, CD44, CD45, and collagen I (Supplemental Fig. 1, and data not shown). In addition, as described previously [6, 13, 16, 29, 31, 38, 47–50, 53, 59, 60], SAP reduced the number of fibrocytes and collagen production (Supplemental Fig. 1A and B, and data not shown).
rhSAP is pentameric and bioactive
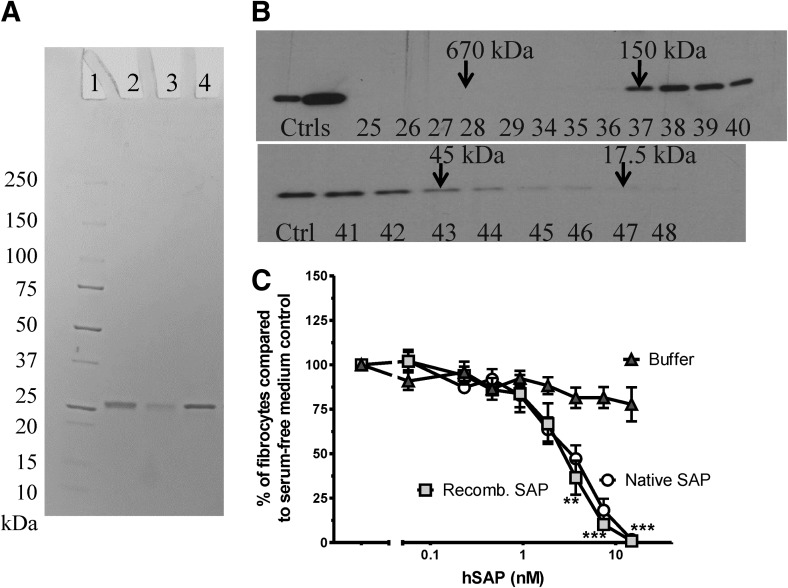
Native SAP is a pentamer in serum [61]. To determine if we could produce rhSAP and use this system to mutagenize SAP, we expressed hSAP in the HEK293 cell culture and measured the molecular mass by size exclusion chromatography. rhSAP was purified from conditioned media with yields between 5 and 50 μg/20 ml media (Fig. 1A). rhSAP had a gel-filtration profile similar to that of native SAP, suggesting that the rhSAP produced by HEK293 cells is pentameric (data not shown and Fig. 1B). To determine if the rhSAP has bioactivity similar to native hSAP, we tested the rhSAP for its ability to inhibit fibrocyte differentiation of human PBMCs in serum-free conditions. The rhSAP significantly reduced the number of fibrocytes, signifying bioactivity (Fig. 1C), and had an IC50, which was statistically indistinguishable from native hSAP and similar to IC50s, which we have observed previously for native hSAP (Fig. 2E) [13, 55]. This suggests that rhSAP is a pentamer and has bioactivity indistinguishable from that of native SAP. Therefore, we could use rhSAP as a platform for measuring changes in bioactivity as a result of site-directed mutagenesis.
Figure 1. rhSAP secreted from HEK293 cells can be readily purified, is predominantly a pentamer, and is bioactive, as determined by its ability to inhibit fibrocyte differentiaiton.
(A) Purified rhSAP was analyzed by PAGE on a 4–20% reducing gel and stained with Coomassie blue. Lane 1, Marker; lane 2, rhSAP; lane 3, rhSAP mutant S171A/Y173A/Q174A; lane 4, native hSAP control. (B) rhSAP was fractionated by size exclusion chromatography on a Superose 12 10/300 GL column. Fractions were analyzed by Western blot using a rabbit anti-SAP primary antibody. The left lanes contain native hSAP controls (Ctrls). The remaining lanes represent 300 μl fractions collected off the column. Arrows indicate the molecular weight of standards previously run on the column. (C) PBMCs were cultured for 5 days in the presence of rhSAP (Recomb. SAP) or an equal volume of buffer in SFM. Results are normalized to the number of fibrocytes in SFM only, and the results are expressed as the mean percentage ± sem (n=3). rhSAP, at and above 4 nM, significantly reduced fibrocyte differentiation compared with buffer-only control, as determined by t test; **P < 0.01; ***P < 0.001. There was no significant difference between the activities of hSAP and rhSAP at any concentration.
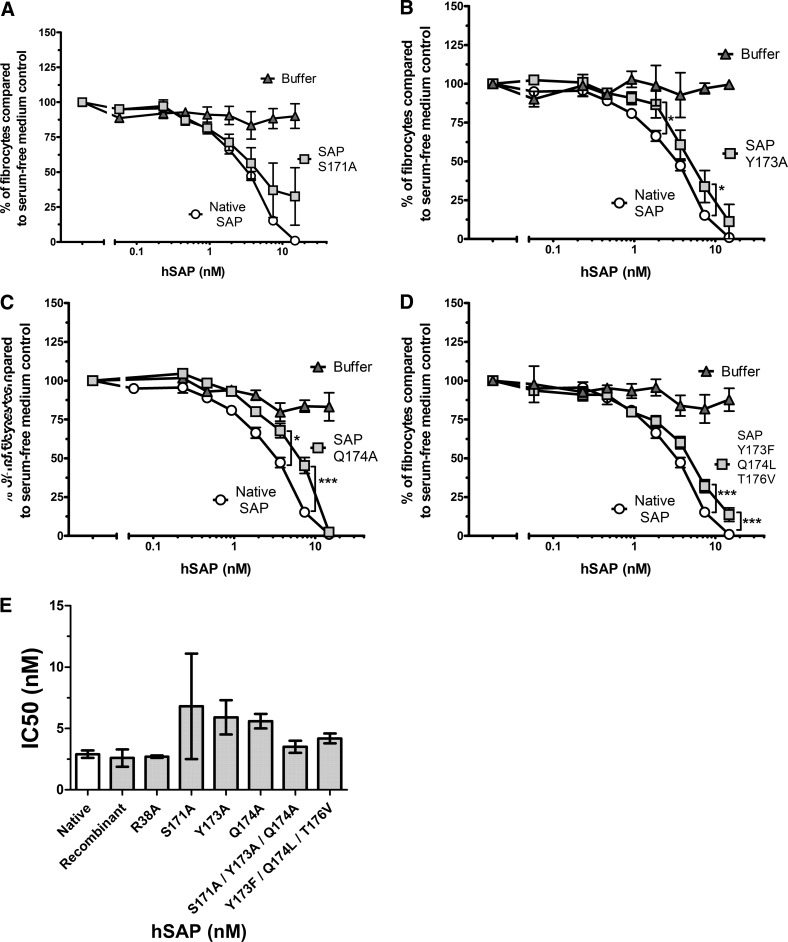
Figure 2. hSAP mutants Y173A, Q174A, and Y173F/Q174L/T176V have reduced bioactivity compared with native hSAP.
PBMCs were cultured for 5 days in the presence of hSAP or an equal volume of SFM/buffer. Results are normalized to the number of fibrocytes in SFM, and the results are expressed as the mean percentage ± sem; n = 12 for native; n = 5 for S171A; n = 4 for Y173A and 173/174/176; and n = 3 for Q174A. (A) hSAP S171A did not have significantly reduced bioactivity compared with native hSAP as a result of the significant variance between assays (two assays showed reduced bioactivity; three assays showed a response similar to that of native SAP). (B–D) hSAP Y173A, Q174A, and Y173F/Q174L/T176V at 7.4 nM have significantly reduced bioactivity compared with native hSAP, as determined by t test; *P < 0.05; ***P < 0.001. (E) IC50 values were obtained by fitting a sigmoidal dose response curve to the data. No significant differences were observed in the IC50s between the mutants and native SAP (one-way ANOVA, Dunnett's test). If mutant S171A is excluded from the ANOVA analysis, mutants Y173A and Q174A have significantly increased IC50 values compared with native hSAP, suggesting reduced hSAP bioactivity (P<0.01; one-way ANOVA, Dunnett's test).
Altering residues at the FcγRIIa binding site modestly reduces SAP bioactivity
The crystal structure of hSAP, bound to the extracellular domain of FcγRIIa, indicated that SAP residues 38, 171–176, and 200–205 are the key contact for SAP binding to FcγRIIa [27]. To determine if these residues are critical for SAP regulating fibrocyte differentiation, a series of single and multiple point mutations (Supplemental Table 1) was tested for their ability to inhibit fibrocyte differentiation in serum-free cell cultures of human PBMCs. As residues 200–204 are near the SAP pentamerization domain, and mutations there could interfere with native structure formation, we focused on region 171–176. To ensure that any changes in bioactivity were not a result of gross misfolding, all hSAP variants tested were assayed by size-exclusion chromatography, and all were found to be pentameric (data not shown). Altering residue 171 did not significantly reduce SAP bioactivity as a result of assay variability (Fig. 2A). Altering residues 173 and 174 and removing the hydrogen-bonding potential from residues 173, 174, and 176 reduced SAP bioactivity (Fig. 2B–D). Comparing the IC50 of native SAP with that of the mutants, no significant differences were observed (Fig. 2E), unless the variable results of mutant S171A were excluded from the analysis. Excluding the S171A data, mutants Y173A and Q174A showed a significant increase in IC50 compared with native hSAP, suggesting that the mutants have reduced activity (Fig. 2E). However, when these residues were changed to alanine in a triple 171/173/174 mutant, the bioactivity of the mutant was similar to that of native hSAP (Fig. 2E). Additionally, the IC50 of R38A was similar to that of hSAP (Fig. 2E). The data suggest that hSAP residues 173 and 174 may mediate some but not all of SAP bioactivity.
Murine FcRγ and FcγRI mediate sensitivity to SAP
As altering key residues of hSAP at the predicted FcγRIIa binding site [27] had only a modest effect on SAP bioactivity, other FcγRs may bind SAP. The observation that FcRγ appears to be necessary for SAP to inhibit fibrosis in vivo (2 mg/kg for lung or 20 mg/kg for kidney) [30, 46] suggests that SAP might activate FcγRI or FcγRIII. To test this, we measured the inhibition of fibrocyte differentiation by SAP using cells from mice lacking specific FcγRs. Culturing murine fibrocytes can require multiple mice to obtain enough circulating monocytes for a single experiment [55]. Therefore, we used the more plentiful splenic reservoir of monocytes [62, 63] to test for SAP bioactivity [47].
With respect to fibrocyte differentiation, the bioactivity of mSAP and hSAP on FcRγ KO cells was reduced significantly compared with the bioactivity on WT cells (Figs. 3A and B and 4). These data suggest that fibrocyte differentiation is regulated by activating FcγRs and that the mouse spleen in vitro fibrocyte differentiation assay is a viable method for studying this effect in KO mice.
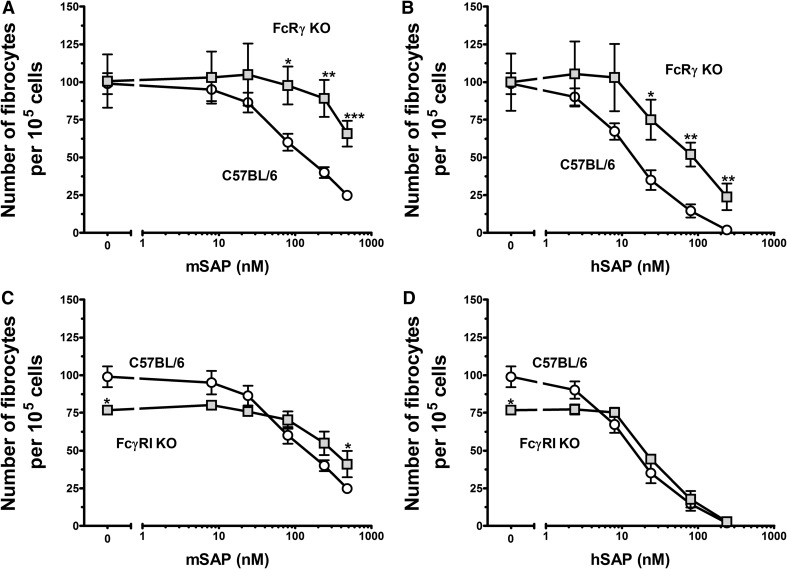
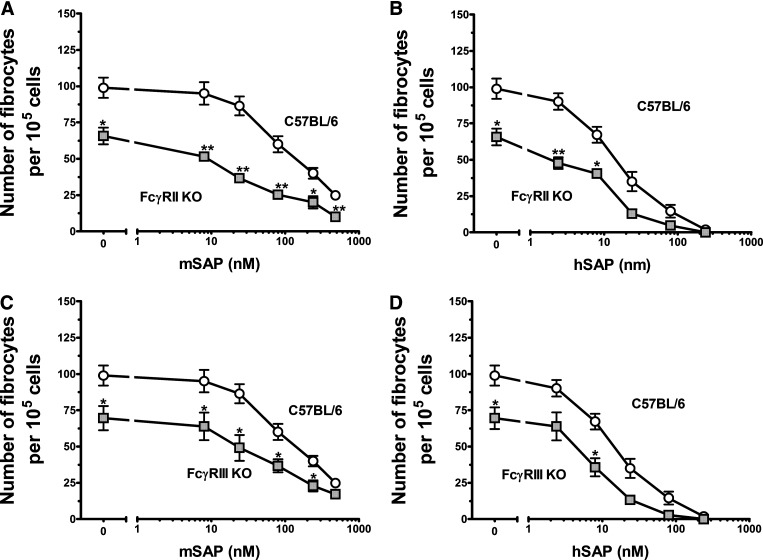
Figure 3. Murine FcRγ KO and FcγRI KO cells are less sensitive to SAP than WT C57BL/6 cells.
Cells were cultured in the indicated concentrations of mSAP (A and C) or hSAP (B and D). After a 5-day incubation, cells were air-dried, fixed, and stained, and the number of fibrocytes was counted. Results are mean ± sem (n=5 for C57BL6; n=4 for FcRγ KO; n=3 for FcγRI). (A and B) At concentrations above 24 nM for mSAP and concentrations above 8 nM for hSAP, SAP bioactivity was reduced significantly for the FcRγ KO cells compared with WT cells, as determined by t test; *P < 0.05; **P < 0.01; ***P < 0.001. (C and D) At 480 nM, mSAP bioactivity was reduced significantly for the FcγRI KO cells compared with WT spleen cells, as determined by t test. FcγRI KO spleen cells had significantly fewer fibrocytes in the serum-free control wells compared with WT spleens (t test).
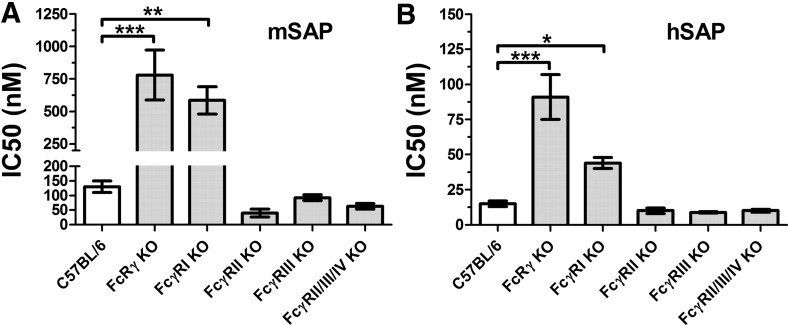
Figure 4. SAP IC50 values for the inhibition of fibrocyte differentiation in FcγR KO cell cultures.
Spleen cells from the indicated strains of mice were cultured in the presence of (A) mSAP or (B) hSAP. Cells were air-dried, fixed, stained, and enumerated by morphology. Using fibrocyte counts normalized to SFM controls, IC50 levels were calculated by fitting SAP bioactivity to a sigmoidal dose response curve with a variable slope. Values are mean ± sem (n=5 for C57BL/6; n=3 for FcγRIIb KO and FcγRIIIa KO; n=4 for FcRγ KO, FcγRI KO, and FcγRIIb/IIIa/IV KO). FcRγ KO and FcγRI KO spleen cells were significantly less responsive to SAP compared with the WT strain (one-way ANOVA, Dunnett's test); *P < 0.05; **P < 0.01; ***P < 0.001. If the data are also examined by t test, then FcγRIIb KO and FcγRIIb/IIIa/IV KO spleen cells were significantly more responsive to mouse SAP compared with WT cells (P<0.05).
Compared with WT cells, FcγRI KO cells were significantly less sensitive to mSAP and hSAP (Figs. 3C and D and 4). The shift in bioactivity was obfuscated by an overall reduction in total fibrocyte number for the FcγRI KO cells compared with WT cells, as indicated by a significant reduction in fibrocyte number in the absence of SAP (Fig. 3C and D). These data suggest that FcγRI mediates some of the effect of SAP on fibrocyte differentiation.
Murine FcγRIIb reduces sensitivity to SAP
FcγRIIb KO and FcγRIII KO cells differentiated into an abnormally low number of fibrocytes (Fig. 5A–D). FcγRIIb KO cells were more sensitive to mSAP than WT cells, whereas FcγRIII KO cells showed a normal sensitivity (Fig. 4A). These data indicate that neither FcγRIIb nor FcγRIII is critical for the ability of SAP to inhibit fibrocyte differentiation.
Figure 5. FcγRIIb KO cells have increased sensitivity to SAP, whereas FcγRIIIa KO cells have normal sensitivity.
Cells from C57BL/6, FcγRIIb KO, and FcγRIIIa KO mice were cultured in the indicated concentrations of mSAP (A and C) or hSAP (B and D). Values are mean ± sem (n=3, except n=5 for C57BL/6). (A and B) FcγRIIb KO spleen cells had significantly fewer fibrocytes in the serum-free controls compared with WT spleen cells and had an increased sensitivity to (A) mSAP and (B) hSAP, as determined by t test; *P < 0.05; **P < 0.01. (C and D) FcγRIIIa KO spleen cells had significantly fewer fibrocytes in the serum-free control wells compared with WT spleens (t test).
Cells from FcγRIIb/III/IV KO mice have normal sensitivity to SAP
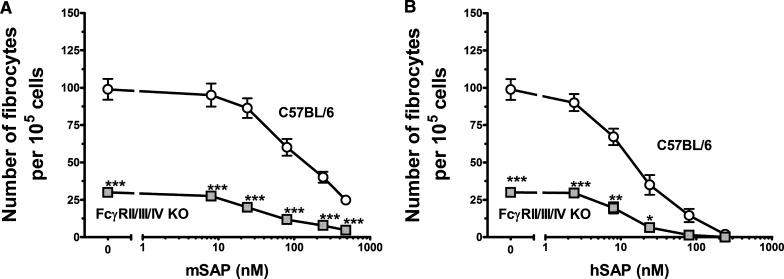
As SAP inhibited fibrocyte differentiation from spleen cells of FcγRIIb KO and FcγRIII KO mice, we determined whether the deletion of multiple FcγRs affects the ability of SAP to inhibit fibrocyte differentiation. Spleen cells from FcγRIIb/FcγRIII/FcγRIV triple-KO mice also allowed us to examine whether FcγRIV regulates fibrocyte differentiation, which we did not have access to as an individual KO. The triple-KO cells differentiated into an abnormally low number of fibrocytes (Fig. 6A and B). However, the IC50 of hSAP in the triple-KO spleen cells was comparable with that of WT mice (Fig. 4B), although the triple-KO spleen cells were about twice as sensitive to mSAP as WT cells (Fig. 4A). The spleen cells from the triple-KO mice responded to mSAP and hSAP to the same degree or with increased sensitivity compared with spleen cells from WT mice, suggesting that these receptors are not essential for the ability of SAP to inhibit fibrocyte differentiation. To determine whether the reduced fibrocyte number was a result of a decrease in the number of monocytes in the spleen, we analyzed spleen cells for cell markers by flow cytometry. Spleens from the triple-KO mice had normal numbers of total cells, monocytes (CD45+, CD11b+, Gr-1+ cells), and other leukocytes (Supplemental Table 2). This suggests that the low-fibrocyte yield was not a result of a reduced number of monocytes in the spleen. These data indicate that SAP is still able to reduce fibrocyte differentiation in the absence of FcγRII, FcγRIII, and FcγRIV, at least to the same degree as normal controls; however, the lack of these receptors does affect the development of fibrocytes in culture.
Figure 6. FcγRIIb/IIIa/IV KO spleen cells produce significantly fewer fibrocytes compared with WT C57BL/6 cells.
Cells from WT C57BL/6 and FcγRIIb/IIIa/IV KO mice were cultured in the indicated concentrations of mSAP (A) or hSAP (B). Values are mean ± sem (n=4 for KO; n=5 for C57BL/6). (A and B) The FcγRIIb/IIIa/IV KO spleen cells had a significantly lower number of fibrocytes in the serum-free control wells compared with WT spleen cells, as determined by t test; *P < 0.05; **P < 0.01; ***P < 0.001.
Reduction of FcRγ and FcγRI in human cells significantly reduces sensitivity to SAP
Because of the differences in biology between mouse and human FcγRs, we also examined how reducing FcγR expression in human cells using siRNA (Supplemental Table 3) affects the inhibition of fibrocyte differentiation by SAP. The siRNAs were transfected into human PBMCs, using concentrations up to 1.25 nM, as concentrations above 1.25 nM (scrambled or specific siRNA) nonspecifically inhibited fibrocyte differentiation (data not shown). The addition of hSAP to the PBMCs was delayed 24 h to give time for the siRNAs to act. Reduction of levels of FcRγ reduced sensitivity to hSAP (Figs. 7A and 8A and Supplemental Fig. 1C and D). These effects were not observed with a scrambled siRNA control (Figs. 7A and B and 8A). This suggests that hSAP acts through activating FcγRs in regulating human fibrocyte differentiation, which corresponds with the data from FcRγ KO mice (Figs. 3A and 4). Reducing the levels of FcγRI also significantly reduced sensitivity to hSAP (Figs. 7A and C and 8B). This suggests that hSAP acts, at least in part, through FcγRI to inhibit human fibrocyte differentiation.
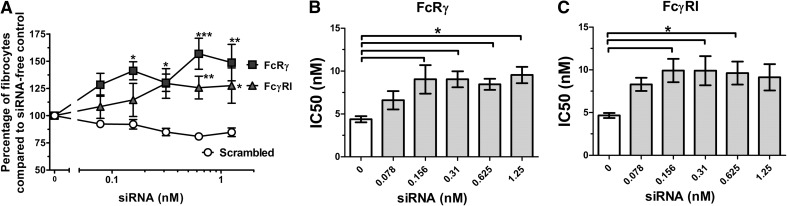
Figure 7. siRNA knockdown of FcRγ and FcγRI (CD64) in human PBMC significantly reduces hSAP bioactivity.
Human PBMCs were cultured in the presence of siRNA or an equal volume of SFM. After 24 h, hSAP was added to the cultures at 2, 1, and 0.5 μg/ml. After 5 days, fibrocytes were counted. Results are normalized to the number of fibrocytes in the absence of siRNA. Values are mean percentage ± sem (n=5 FcRγ; n=6 for FcγRI). (A) PBMCs were treated with 0.5 μg/ml hSAP, and the change in SAP bioactivity was measured through fibrocyte counts. siRNA knockdown of FcRγ and FcγRI significantly increased the number of fibrocytes compared with scrambled controls (t test); *P < 0.05; **P < 0.01; ***P < 0.001. (B and C) IC50 levels were measured for the ability of hSAP to inhibit fibrocyte differentiation. siRNA knockdown of FcRγ and FcγRI significantly increased the IC50 levels of hSAP compared with siRNA-free controls (one-way ANOVA, Dunnett's test); *P < 0.05.
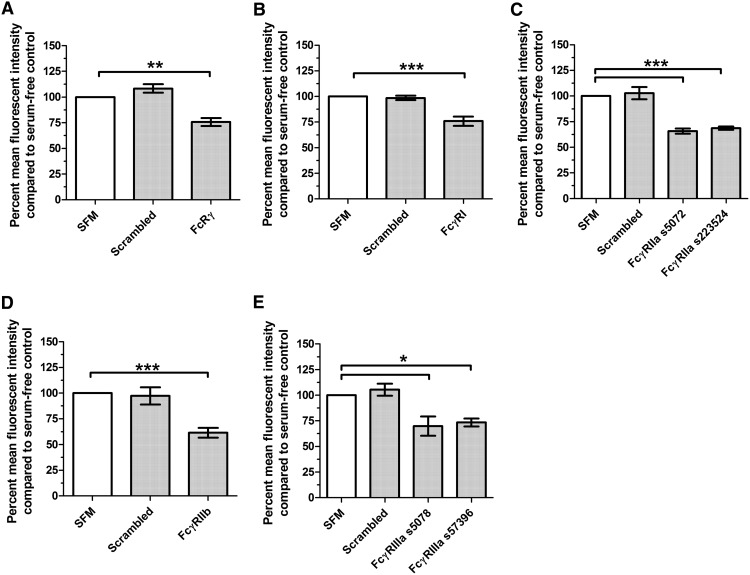
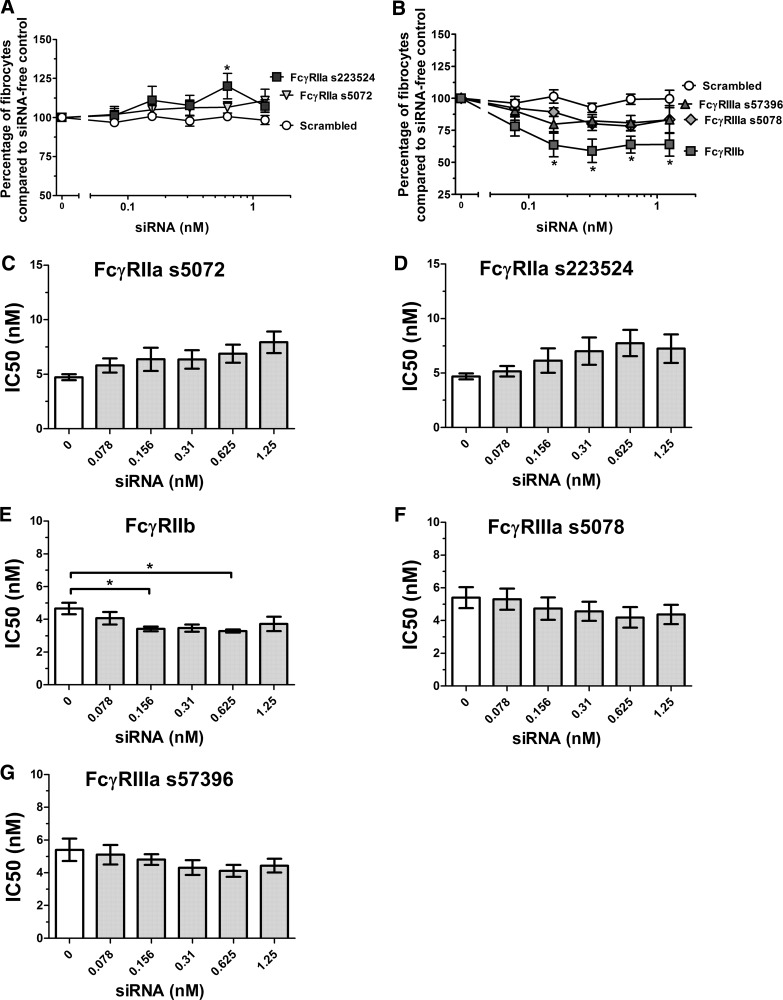
Figure 8. siRNA knockdown of FcRγ, FcγRI, and FcγRIIa, -IIb, and -IIIa significantly reduces the corresponding protein levels.
Human PBMCs were cultured for 3 days in the presence of siRNA or an equal volume of SFM and were then stained for the associated receptor. Results are normalized to the mean fluorescent intensity of cells in the serum-free control wells, and the results are expressed as the mean percentage ± sem (n=3; n=4 for FcγRI). A minimum of 100 cells was counted/condition. The levels of (A) FcRγ, (B) FcγRI, (C) FcγRIIa, (D) FcγRIIb, and (E) FcγRIIIa were reduced significantly in cell cultures treated with siRNA (one-way ANOVA, Dunnett's test); *P < 0.05; **P < 0.01; ***P < 0.001.
Reduction of FcγRIIb in human cells increases sensitivity to SAP
Reducing the levels of of FcγRIIa (Fig. 8C) did not significantly affect sensitivity to hSAP (Fig. 9A, C, and D). However, an increase in fibrocyte number was observed (Fig. 9A), suggesting that this receptor could have a modest effect on fibrocyte differentiation. Reduction of FcγRIIb levels (Fig. 8D) resulted in a significant decrease in the number of fibrocytes, even in the absence of hSAP (Fig. 9B), and an increase in sensitivity to hSAP (Fig. 9E). This result is similar to the increased sensitivity observed in FcγRIIb KO mice (Figs. 4 and 5). Reduction of FcγRIIIa levels (Fig. 8E) did not significantly alter sensitivity to hSAP (Fig. 9B, F, and G). These data suggest that FcγRIIa and FcγRIIb may affect fibrocyte differentiation and that FcγRIIb appears to counteract the ability of SAP to inhibit fibrocyte differentiation.
Figure 9. siRNA knockdown of FcγRIIa and FcγRIIIa does not significantly alter the bioactivity of hSAP, whereas knockdown of FcγRIIb increases sensitivity to hSAP.
Human PBMCs were cultured for 5 days in the presence of siRNA or an equal volume of SFM. At 24 h, hSAP was added to the cultures at 2, 1, and 0.5 μg/ml. Results are normalized to the number of fibrocytes in siRNA-free wells, and the results are expressed as the mean percentage ± sem (n=3; n=6 for FcγRIIa). (A and B) PBMCs were treated with 0.5 μg/ml hSAP, and the change in SAP bioactivity was measured through fibrocyte counts. siRNA knockdown of FcγRIIb significantly decreased the number of fibrocytes compared with scrambled controls (t test); *P < 0.05. (C–G) IC50 levels were measured for the ability of hSAP to inhibit fibrocyte differentiation. siRNA knockdown of FcγRIIb significantly decreased the IC50 levels of hSAP compared with siRNA-free controls (one-way ANOVA, Dunnett's test); *P < 0.05.
DISCUSSION
The plasma protein SAP inhibits the differentiation of monocytes into fibrocytes. SAP binds to FcγRs, and FcγRs are present on monocytes. As there are multiple FcγRs, we determined how the different FcγRs affect the ability of SAP to inhibit fibrocyte differentiation. Deletion of FcγRI or the FcRγ chain in mice and reduction of FcγRI or the FcRγ chain levels in human cells significantly decreased but did not abolish the sensitivity of cells to SAP. This indicates that FcγRI and the FcRγ chain play a major role in the signal transduction pathway that SAP uses to inhibit fibrocyte differentiation. However, the ability of SAP to inhibit fibrocyte differentiation in cells lacking FcγRI or FcRγ suggests that SAP activates receptors in addition to FcγRI and additionally activates a signaling pathway that does not require FcRγ. In WT mice, SAP injections inhibit fibrosis, whereas in FcRγ−/− mice, injections of the same amounts of SAP do not inhibit fibrosis [30, 46]. Based on our observation that in cultures of FcRγ−/− cells, only very high concentrations of SAP inhibit fibrocyte differentiation, we would predict that in FcRγ−/− mice, injections of high concentrations of SAP may inhibit fibrosis.
Deletion of FcγRIII in mice and reduction of FcγRIIa or FcγRIIIa levels in human cells (mice do not have FcγRIIa) had no significant effect on the sensitivity of cells to SAP. In addition, altering SAP residues at the SAP-FcγRIIa crystal structure interaction site had only a modest effect on the inhibition of fibrocyte differentiation by SAP. Together, this suggests that FcγRIIa and FcγRIIIa do not play a major role in the effect of SAP on fibrocyte differentiation.
Deletion of FcγRIIb in mice and reduction of FcγRIIb levels in human cells increased the sensitivity of cells to SAP. This suggests that FcγRIIb may activate a pathway that counteracts the ability of SAP to inhibit fibrocyte differentiation. An intriguing possibility is that signals that promote fibrocyte differentiation may be sensed by this receptor. In the absence of added SAP, deletion of FcγRIIb in mice and reduction of FcγRIIb in human cells both decreased the number of fibrocytes observed in cultures. Deletion of other FcγRs in mice or reduction of their levels in human cells also affected fibrocyte numbers, but there was no correlation between the mouse and human data.
The FcγRIIb/IIIa/IV triple-KO spleen cells retained full sensitivity to SAP. We did not have access to the single FcγRIV KO, but the SAP activity in the triple-KO mice suggests that FcγRIV is not essential for SAP bioactivity. However, the triple-KO cells differentiated into an abnormally low number of fibrocytes. This suggests that fibrocyte differentiation may be regulated by unknown factors that signal using one or more pathways, which interact with pathways regulated by FcγRIIb/IIIa/IV. Combined with the observations that cytokines, angiotensin II, leukotrienes, TLR agonists, and hyaluronan fragments regulate fibrocyte differentiation [15, 64–67], our results, showing that SAP uses FcγRI, as well as other unknown receptors, and FcRγ, as well as other intracellular signaling components, indicate that a complex network regulates fibrocyte differentiation.
Our experiments were performed in the absence of serum, resulting in the appearance of fibrocytes within 5 days, a time period closer to the 3–7 days, which fibrocytes take to appear within wounds and inflammatory sites [11, 12, 59, 68, 69], compared with the 10–14 days, which it takes when incubated in bovine serum in vitro [11, 12, 62, 70]. Therefore, these conclusions may not be applicable to studies performed in serum-containing conditions.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by U.S. National Institutes of Health (NIH) grant R01 HL083029 (to R.H.G.), U.S. NIH Biotechnology Research Training Program at Rice University (to J.R.C.), and U.S. NIH grant RO1-HL089792 (to Dr. Mark Entman). We thank Promedior for the generous donation of hSAP. We thank Dr. Sjef Verbeek and Jill Claassens at Leiden University Medical Center for the generous assistance with the donation of receptor KO spleens. Similarly, we thank Dr. Bryce Binstadt and Jennifer Auger at the University of Minnesota for additional spleens.
SEE CORRESPONDING EDITORIAL ON PAGE 693
- CTE
- constitutive transport element
- FcRγ
- FcR common γ-chain
- FDA
- U.S. Food and Drug Administration
- HEK
- human embryonic kidney
- hSAP
- human serum amyloid P
- KO
- knockout
- mSAP
- murine serum amyloid P
- rhSAP
- recombinant human SAP
- RT
- room temperature
- RTE
- RNA transport element
- SAP
- serum amyloid P
- SFM
- serum-free medium
- siRNA
- small interfering RNA
AUTHORSHIP
J.R.C. designed and performed the experiments, analyzed the data, and wrote the paper. D.P. and R.H.G. designed the experiments, analyzed the data, and wrote the paper.
REFERENCES
- 1. Kumar V., Abbas A. K., Fausto N., Kumar V., Abbas A. K., Fausto N. (2005) Tissue renewal and repair: regeneration, healing, and fibrosis. In Robbins and Cotran: Pathological Basis of Disease, vol. 7, Elsevier Saunders, Philadelphia, PA, 87–118 [Google Scholar]
- 2. Bouros D., Antoniou K. M. (2005) Current and future therapeutic approaches in idiopathic pulmonary fibrosis. Eur. Respir. J. 26, 693–703 [DOI] [PubMed] [Google Scholar]
- 3. Gomer R. H., Lupher M. L., Jr., (2010) Investigational approaches to therapies for idiopathic pulmonary fibrosis. Expert Opin. Investig. Drugs 19, 737–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cowper S. E. (2003) Nephrogenic fibrosing dermopathy: the first 6 years. Curr. Opin. Rheumatol. 15, 785–790 [DOI] [PubMed] [Google Scholar]
- 5. Phillips R. J., Burdick M. D., Hong K., Lutz M. A., Murray L. A., Xue Y. Y., Belperio J. A., Keane M. P., Strieter R. M. (2004) Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J. Clin. Invest. 114, 438–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haudek S. B., Xia Y., Huebener P., Lee J. M., Carlson S., Crawford J. R., Pilling D., Gomer R. H., Trial J., Frangogiannis N. G., Entman M. L. (2006) Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc. Natl. Acad. Sci. USA 103, 18284–18289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kisseleva T., Uchinami H., Feirt N., Quintana-Bustamante O., Segovia J. C., Schwabe R. F., Brenner D. A. (2006) Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J. Hepatol. 45, 429–438 [DOI] [PubMed] [Google Scholar]
- 8. Mehrad B., Burdick M. D., Zisman D. A., Keane M. P., Belperio J. A., Strieter R. M. (2007) Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem. Biophys. Res. Commun. 353, 104–108 [DOI] [PubMed] [Google Scholar]
- 9. Herzog E. L., Bucala R. (2010) Fibrocytes in health and disease. Exp. Hematol. 38, 548–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sakai N., Furuichi K., Shinozaki Y., Yamauchi H., Toyama T., Kitajima S., Okumura T., Kokubo S., Kobayashi M., Takasawa K., Takeda S., Yoshimura M., Kaneko S., Wada T. (2010) Fibrocytes are involved in the pathogenesis of human chronic kidney disease. Hum. Pathol. 41, 672–678 [DOI] [PubMed] [Google Scholar]
- 11. Bucala R., Spiegel L. A., Chesney J., Hogan M., Cerami A. (1994) Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol. Med. 1, 71–81 [PMC free article] [PubMed] [Google Scholar]
- 12. Abe R., Donnelly S. C., Peng T., Bucala R., Metz C. N. (2001) Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J. Immunol. 166, 7556–7562 [DOI] [PubMed] [Google Scholar]
- 13. Pilling D., Buckley C. D., Salmon M., Gomer R. H. (2003) Inhibition of fibrocyte differentiation by serum amyloid P. J. Immunol. 17, 5537–5546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gomperts B. N., Strieter R. M. (2007) Fibrocytes in lung disease. J. Leukoc. Biol. 82, 449–456 [DOI] [PubMed] [Google Scholar]
- 15. Shao D. D., Suresh R., Vakil V., Gomer R. H., Pilling D. (2008) Pivotal Advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J. Leukoc. Biol. 83, 1323–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pilling D., Fan T., Huang D., Kaul B., Gomer R. H. (2009) Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS ONE 4, e7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reilkoff R. A., Bucala R., Herzog E. L. (2011) Fibrocytes: emerging effector cells in chronic inflammation. Nat. Rev. Immunol. 11, 427–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Strieter R. M., Keeley E. C., Hughes M. A., Burdick M. D., Mehrad B. (2009) The role of circulating mesenchymal progenitor cells (fibrocytes) in the pathogenesis of pulmonary fibrosis. J. Leukoc. Biol. 86, 1111–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mathai S. K., Gulati M., Peng X., Russell T. R., Shaw A. C., Rubinowitz A. N., Murray L. A., Siner J. M., Antin-Ozerkis D. E., Montgomery R. R., Reilkoff R. A., Bucala R. J., Herzog E. L. (2010) Circulating monocytes from systemic sclerosis patients with interstitial lung disease show an enhanced profibrotic phenotype. Lab. Invest. 90, 812–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mehrad B., Burdick M. D., Strieter R. M. (2009) Fibrocyte CXCR4 regulation as a therapeutic target in pulmonary fibrosis. Int. J. Biochem. Cell Biol. 41, 1708–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gomer R. H., Pilling D. (2010) Fibrocytes and collagen producing cells of the peripheral blood. In Fibrocytes (Bucala, ed.), R. Singapore, World Scientific Publishing [Google Scholar]
- 22. Steel D. M., Whitehead A. S. (1994) The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunol. Today 15, 81–88 [DOI] [PubMed] [Google Scholar]
- 23. Gewurz H., Zhang X. H., Lint T. F. (1995) Structure and function of the pentraxins. Curr. Opin. Immunol. 7, 54–64 [DOI] [PubMed] [Google Scholar]
- 24. Pepys M. B., Booth D. R., Hutchinson W. L., Gallimore J. R., Collins P. M., Hohenester E. (1997) Amyloid P component. A critical review. Amyloid 4, 274–295 [Google Scholar]
- 25. Emsley J., White H. E., O'Hara B. P., Oliva G., Srinivasan N., Tickle I. J., Blundell T. L., Pepys M. B., Wood S. P. (1994) Structure of pentameric human serum amyloid P component. Nature 367, 338–345 [DOI] [PubMed] [Google Scholar]
- 26. Aquilina J. A., Robinson C. V. (2003) Investigating interactions of the pentraxins serum amyloid P component and C-reactive protein by mass spectrometry. Biochem. J. 375, 323–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lu J., Marnell L. L., Marjon K. D., Mold C., Du Clos T. W., Sun P. D. (2008) Structural recognition and functional activation of FcγR by innate pentraxins. Nature 456, 989–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Agrawal A., Singh P. P., Bottazzi B., Garlanda C., Mantovani A. (2009) Pattern recognition by pentraxins. Adv. Exp. Med. Biol. 653, 98–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pilling D., Roife D., Wang M., Ronkainen S. D., Crawford J. R., Travis E. L., Gomer R. H. (2007) Reduction of bleomycin-induced pulmonary fibrosis by serum amyloid P. J. Immunol. 179, 4035–4044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Castano A. P., Lin S. L., Surowy T., Nowlin B. T., Turlapati S. A., Patel T., Singh A., Li S., Lupher M. L., Jr., Duffield J. S. (2009) Serum amyloid P inhibits fibrosis through Fc γ R-dependent monocyte-macrophage regulation in vivo. Sci. Transl. Med. 1, 5ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moreira A. P., Cavassani K. A., Hullinger R., Rosada R. S., Fong D. J., Murray L., Hesson D. P., Hogaboam C. M. (2010) Serum amyloid P attenuates M2 macrophage activation and protects against fungal spore-induced allergic airway disease. J. Allergy Clin. Immunol. 126, 712–721.e7 [DOI] [PubMed] [Google Scholar]
- 32. Ravetch J. V., Bolland S. (2001) IgG Fc receptors. Annu. Rev. Immunol. 19, 275–290 [DOI] [PubMed] [Google Scholar]
- 33. Daeron M. (1997) Fc receptor biology. Annu. Rev. Immunol. 15, 203–234 [DOI] [PubMed] [Google Scholar]
- 34. Nimmerjahn F. (2006) Activating and inhibitory FcγRs in autoimmune disorders. Springer Semin. Immunopathol. 28, 305–319 [DOI] [PubMed] [Google Scholar]
- 35. Ravetch J. V., Lanier L. L. (2000) Immune inhibitory receptors. Science 290, 84–89 [DOI] [PubMed] [Google Scholar]
- 36. Nimmerjahn F., Ravetch J. V. (2006) Fcγ receptors: old friends and new family members. Immunity 24, 19–28 [DOI] [PubMed] [Google Scholar]
- 37. Ravetch J. V., Kinet J. P. (1991) Fc receptors. Annu. Rev. Immunol. 9, 457–492 [DOI] [PubMed] [Google Scholar]
- 38. Pilling D., Tucker N. M., Gomer R. H. (2006) Aggregated IgG inhibits the differentiation of human fibrocytes. J. Leukoc. Biol. 79, 1242–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nimmerjahn F., Ravetch J. V. (2008) Fcγ receptors as regulators of immune responses. Nat. Rev. Immunol. 8, 34–47 [DOI] [PubMed] [Google Scholar]
- 40. Hughes A. L. (1996) Gene duplication and recombination in the evolution of mammalian Fc receptors. J. Mol. Evol. 43, 4–10 [DOI] [PubMed] [Google Scholar]
- 41. Bharadwaj D., Mold C., Markham E., Du Clos T. W. (2001) Serum amyloid P component binds to Fc γ receptors and opsonizes particles for phagocytosis. J. Immunol. 166, 6735–6741 [DOI] [PubMed] [Google Scholar]
- 42. Grage-Griebenow E., Flad H. D., Ernst M. (2001) Heterogeneity of human peripheral blood monocyte subsets. J. Leukoc. Biol. 69, 11–20 [PubMed] [Google Scholar]
- 43. Chi M., Tridandapani S., Zhong W., Coggeshall K. M., Mortensen R. F. (2002) C-reactive protein induces signaling through Fc γ RIIa on HL-60 granulocytes. J. Immunol. 168, 1413–1418 [DOI] [PubMed] [Google Scholar]
- 44. Mold C., Baca R., Du Clos T. W. (2002) Serum amyloid P component and C-reactive protein opsonize apoptotic cells for phagocytosis through Fcγ receptors. J. Autoimmun. 19, 147–154 [DOI] [PubMed] [Google Scholar]
- 45. Macdonald S. L., Kilpatrick D. C. (2006) Human serum amyloid P component binds to peripheral blood monocytes. Scand. J. Immunol. 64, 48–52 [DOI] [PubMed] [Google Scholar]
- 46. Haudek S. B., Trial J., Xia Y., Gupta D., Pilling D., Entman M. L. (2008) Fc receptor engagement mediates differentiation of cardiac fibroblast precursor cells. Proc. Natl. Acad. Sci. 105, 10179–10184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Crawford J. R., Pilling D., Gomer R. H. (2010) Improved serum-free culture conditions for spleen-derived murine fibrocytes. J. Immunol. Methods 363, 9–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Murray L. A., Rosada R., Moreira A. P., Joshi A., Kramer M. S., Hesson D. P., Argentieri R. L., Mathai S., Gulati M., Herzog E. L., Hogaboam C. M. (2010) Serum amyloid P therapeutically attenuates murine bleomycin-induced pulmonary fibrosis via its effects on macrophages. PLoS ONE 5, e9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Murray L. A., Chen Q., Kramer M. S., Hesson D. P., Argentieri R. L., Peng X., Gulati M., Homer R. J., Russell T., van Rooijen N., Elias J. A., Hogaboam C. M., Herzog E. L. (2011) TGF-β driven lung fibrosis is macrophage dependent and blocked by serum amyloid P. Int. J. Biochem. Cell Biol. 43, 154–162 [DOI] [PubMed] [Google Scholar]
- 50. Murray L., Kramer M., Hesson D., Watkins B., Fey E., Argentieri R., Shaheen F., Knight D., Sonis S. (2010) Serum amyloid P ameliorates radiation-induced oral mucositis and fibrosis. Fibrogenesis Tissue Repair 3, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Smulevitch S., Bear J., Alicea C., Rosati M., Jalah R., Zolotukhin A. S., von Gegerfelt A., Michalowski D., Moroni C., Pavlakis G. N., Felber B. K. (2006) RTE and CTE mRNA export elements synergistically increase expression of unstable, Rev-dependent HIV and SIV mRNAs. Retrovirology 3, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pepys M. B., Dash A. C., Munn E. A., Feinstein A., Skinner M., Cohen A. S., Gewurz H., Osmand A. P., Painter R. H. (1977) Isolation of amyloid P component (protein AP) from normal serum as a calcium-dependent binding protein. Lancet 1, 1029–1031 [DOI] [PubMed] [Google Scholar]
- 53. Gomer R. H., Pilling D., Kauvar L., Ellsworth S., Pissani S., Real L., Ronkainen S. D., Roife D., Ma F., Davis S. C. (2009) A serum amyloid P-binding hydrogel speeds healing of partial thickness wounds in pigs. Wound Repair Regen. 17, 397–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. De Beer F. C., Pepys M. B. (1982) Isolation of human C-reactive protein and serum amyloid P component. J. Immunol. Methods 50, 17–31 [DOI] [PubMed] [Google Scholar]
- 55. Pilling D., Vakil V., Gomer R. H. (2009) Improved serum-free culture conditions for the differentiation of human and murine fibrocytes. J. Immunol. Methods 351, 62–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hazenbos W. L., Gessner J. E., Hofhuis F. M., Kuipers H., Meyer D., Heijnen I. A., Schmidt R. E., Sandor M., Capel P. J., Daeron M., van de Winkel J. G., Verbeek J. S. (1996) Impaired IgG-dependent anaphylaxis and Arthus reaction in Fc γ RIII (CD16) deficient mice. Immunity 5, 181–188 [DOI] [PubMed] [Google Scholar]
- 57. Takai T., Ono M., Hikida M., Ohmori H., Ravetch J. V. (1996) Augmented humoral and anaphylactic responses in Fc γ RII-deficient mice. Nature 379, 346–349 [DOI] [PubMed] [Google Scholar]
- 58. Ioan-Facsinay A., de Kimpe S. J., Hellwig S. M., van Lent P. L., Hofhuis F. M., van Ojik H. H., Sedlik C., da Silveira S. A., Gerber J., de Jong Y. F., Roozendaal R., Aarden L. A., van den Berg W. B., Saito T., Mosser D., Amigorena S., Izui S., van Ommen G. J., van Vugt M., van de Winkel J. G., Verbeek J. S. (2002) FcγRI (CD64) contributes substantially to severity of arthritis, hypersensitivity responses, and protection from bacterial infection. Immunity 16, 391–402 [DOI] [PubMed] [Google Scholar]
- 59. Naik-Mathuria B., Pilling D., Crawford J. R., Gay A. N., Smith C. W., Gomer R. H., Olutoye O. O. (2008) Serum amyloid P inhibits dermal wound healing. Wound Repair Regen. 16, 266–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Santhiago M. R., Singh V., Barbosa F. L., Agrawal V., Wilson S. E. (2011) Monocyte development inhibitor PRM-151 decreases corneal myofibroblast generation in rabbits. Exp. Eye Res. 93, 810–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hutchinson W. L., Hohenester E., Pepys M. B. (2000) Human serum amyloid P component is a single uncomplexed pentamer in whole serum. Mol. Med. 6, 482–493 [PMC free article] [PubMed] [Google Scholar]
- 62. Niedermeier M., Reich B., Rodriguez Gomez M., Denzel A., Schmidbauer K., Gobel N., Talke Y., Schweda F., Mack M. (2009) CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes. Proc. Natl. Acad. Sci. USA 106, 17892–17897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Swirski F. K., Nahrendorf M., Etzrodt M., Wildgruber M., Cortez-Retamozo V., Panizzi P., Figueiredo J. L., Kohler R. H., Chudnovskiy A., Waterman P., Aikawa E., Mempel T. R., Libby P., Weissleder R., Pittet M. J. (2009) Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vannella K. M., McMillan T. R., Charbeneau R. P., Wilke C. A., Thomas P. E., Toews G. B., Peters-Golden M., Moore B. B. (2007) Cysteinyl leukotrienes are autocrine and paracrine regulators of fibrocyte function. J. Immunol. 179, 7883–7890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Haudek S. B., Cheng J., Du J., Wang Y., Hermosillo-Rodriguez J., Trial J., Taffet G. E., Entman M. L. (2010) Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. J. Mol. Cell. Cardiol. 49, 499–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Maharjan A. S., Pilling D., Gomer R. H. (2010) Toll-like receptor 2 agonists inhibit human fibrocyte differentiation. Fibrogenesis Tissue Repair 3, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Maharjan A. S., Pilling D., Gomer R. H. (2011) High and low molecular weight hyaluronic acid differentially regulate human fibrocyte differentiation. PLoS ONE 6, e26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schmidt M., Sun G., Stacey M. A., Mori L., Mattoli S. (2003) Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J. Immunol. 171, 380–389 [DOI] [PubMed] [Google Scholar]
- 69. Mori L., Bellini A., Stacey M. A., Schmidt M., Mattoli S. (2005) Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp. Cell. Res. 304, 81–90 [DOI] [PubMed] [Google Scholar]
- 70. Quan T. E., Bucala R. (2007) Culture and analysis of circulating fibrocytes. Methods Mol. Med. 135, 423–434 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.