Review on the viral entry process of HCMV and the potential role of receptor-ligand interactions in modulating monocyte biology.
Keywords: inflammation, transcriptome, integrin, EGFR, signaling
Abstract
HCMV pathogenesis is a direct consequence of the hematogenous dissemination of the virus to multiple host organ sites. The presence of infected monocytes in the peripheral blood and organs of individuals exhibiting primary HCMV infection have long suggested that these blood sentinels are responsible for mediating viral spread. Despite monocytes being “at the right place at the right time”, their short lifespan and the lack of productive viral infection in these cells complicate this scenario of a monocyte-driven approach to viral dissemination by HCMV. However, our laboratory has provided evidence that HCMV infection is able to induce a highly controlled polarization of monocytes toward a unique and long-lived proinflammatory macrophage, which we have demonstrated to be permissive for viral replication. These observations suggest that HCMV has evolved as a distinct mechanism to induce select proinflammatory characteristics that provide infected monocytes with the necessary tools to mediate viral spread following a primary infection. In the absence of viral gene products during the early stages of infection, the process by which HCMV “tunes” the inflammatory response in infected monocytes to promote viral spread and subsequently, viral persistence remains unclear. In this current review, we focus on the viral entry process of HCMV and the potential role of receptor-ligand interactions in modulating monocyte biology. Specifically, we examine the signaling pathways initiated by the distinct combination of cellular receptors simultaneously engaged and activated by HCMV during viral entry and how the acquisition of this distinct signalsome results in a nontraditional activation of monocytes leading to the induction of the unique, functional attributes observed in monocytes following HCMV infection.
Introduction
HCMV is a betaherpesvirus that infects 60–90% of the adult population and persists for the lifetime of the host [1]. HCMV persistence is maintained through chronic virus shedding and/or latent infection following an acute primary infection. Chronic infection is marked by the persistent shedding of virus at low levels from restricted sites (endothelial or epithelial cells) in the infected host [2]. In contrast to chronic infection, HCMV latency is maintained in hematopoietic cells and is defined by the lack of progeny virus, presence of viral genomic DNA, and expression of a highly restricted set of viral gene products [3, 4]. In addition, virus reactivation can occur during latent infection, which is a key event that distinguishes latency from an abortive infection. Chronic and latent HCMV infection is typically asymptomatic in immunocompentent hosts, although studies have linked HCMV infection to cancers, such as glioblastomas and several inflammatory diseases, including atherosclerosis and restenosis [5]. However, primary infection, chronic infection, or reactivation of latent virus in immunocompromised individuals, such as AIDS patients, transplant recipients, and neonates, can cause significant morbidity and mortality, including retinitis, pneumonitis, and end-organ disease [6]. The ability of HCMV to establish persistent infection is a direct consequence of the viral dissemination strategy following primary infection, which also leads to the wide range of organ pathologies associated with HCMV infection.
The onset of a cell-associated viremia early following the initial acute infection suggests that the transport of HCMV throughout the body is mediated by a cell-associated hematogenous mechanism [7, 8]. Monocytes are widely believed to be the primary cell type involved in the hematogenous dissemination of HCMV [7–10]. Analyzed blood samples from HCMV seropositive individuals primarily detected HCMV DNA in monocytes, not in B and T lymphocytes [11]. Additionally, monocytes are the primary cell type found in HCMV-infected organs and have been implicated in the transmission of HCMV to patients transfused with blood products [9, 12, 13]. Although there is a predominance of HCMV-infected monocytes in the blood and in organs of infected hosts, monocytes do not support HCMV replication [7, 10, 14, 15], suggesting that HCMV must somehow escape or alter the nonproductive environment of infected monocytes to successfully complete the viral life cycle. Further complicating this scenario, circulating monocytes have a short lifespan of 1–3 days [16]. This short lifespan coupled with the absence of viral protein expression to alter preprogrammed cellular responses, such as apoptosis, suggest that other virus-mediated events, such as cell surface engagement, may alter the physiology of the newly infected cell [10]. Our new studies have focused on gaining an understanding of the molecular events involved in allowing the virus to overcome these biological “hurdles” to disseminate throughout the host following a primary HCMV infection. Below, we present elements of these studies to document what we believe is a sophisticated HCMV strategy for viral dissemination and persistence in myeloid cells.
HCMV INDUCES A UNIQUE MONOCYTE PHENOTYPE FOLLOWING PRIMARY INFECTION
Our laboratory observes that HCMV induces changes in monocyte biology following initial infection; we argue that these changes allow the virus to use monocytes as “Trojan horses” to mediate viral spread. We have shown that HCMV infection of monocytes rapidly induced a proinflammatory response, which led to increased production of inflammatory cytokines/chemokines [17, 18], enhanced monocyte motility [10, 19, 20], increased expression of cellular adhesion molecules [10, 20], and promoted transendothelial migration [10, 19, 20], all of which would stimulate the extravasation of HCMV-infected monocytes into the surrounding tissues [10]. We suggest that HCMV has evolved to induce the proinflammatory response in infected monocytes to confer the necessary tools to mediate viral spread [10, 21]. In accord, knockout of a viral proinflammatory chemokine encoded by murine CMV blocked in vivo dissemination during primary infection, supporting the role of the proinflammatory response as a viable viral dissemination strategy [22]. Whereas many of the functional changes that occur following HCMV infection of monocytes are similar to the proinflammatory changes seen following infection with other pathogens, HCMV also induces a number of unique changes in monocytes that are not seen with other inflammatory activators. For example, HCMV infection of monocytes increased expression of N-WASP, an actin nucleator not normally expressed in myeloid cells. This virally induced N-WASP expression is critical for the induction of the “hyper” chemokinetic motility (high levels of nonvectorial/random motility) observed in infected monocytes when compared with PMA-treated monocytes, as knockdown of N-WASP inhibited HCMV- but not PMA-induced motility [10, 19, 20]. We propose that this hyper motility enhances HCMV-infected monocyte migration along blood vessel walls, resulting in the increased exit of these infected monocytes out of the bloodstream and into the peripheral tissue [10]. Additionally, HCMV induces proinflammatory and anti-inflammatory chemokines known to stimulate monocyte motility [17, 23], which we speculate would further promote the recruitment of monocytes to the site of infection. Finally, HCMV infection of monocytes drives the differentiation of infected monocytes into macrophages, which to date is the first example of a virally induced monocyte-to-macrophage differentiation process [10, 17]. Following differentiation, these macrophages become permissive for HCMV replication, indicating that this unique ability of HCMV to induce myeloid differentiation directly is necessary for the continuation of the viral life cycle during the dissemination process [10].
To better understand the HCMV-induced differentiation process, our laboratory examined HCMV-regulated transcriptional changes in infected monocytes. An analysis of the specific genes that were up-regulated by HCMV infection in monocytes revealed that many of the up-regulated genes were typically associated with a proinflammatory (M1), polarized macrophage [17]. However, we also observed that HCMV infection of monocytes led to the down-regulation of certain proinflammatory, M1-associated genes (i.e., endothelial cell growth factor 1 and chondroitin sulfate proteoglycan) and the up-regulation of select anti-inflammatory (M2) genes (i.e., chemokine ligands 18 and 23) [17]. These data indicate that HCMV selectively modulates M1- and M2- associated factors, resulting in a unique activation of infected monocytes, which phenotypically lies between the ends of the M1/M2 polarization spectrum. It remains to be elucidated whether the resultant M1/M2 phenotype following HCMV infection is sustained. However, these infected cells exhibit the same morphological characteristics for 3 months postinfection and produce progeny virus during this time period (data not shown), suggesting that the unique polarization of infected macrophages is maintained long term. Biologically, HCMV may preferentially up-regulate specific genes, which are necessary for the establishment of HCMV infection, whereas selectively down-regulating genes that may be detrimental to the virus, supporting our hypothesis that HCMV specifically controls components of the proinflammatory response during infection of monocytes that are necessary for the promotion of monocyte motility, migration, and differentiation.
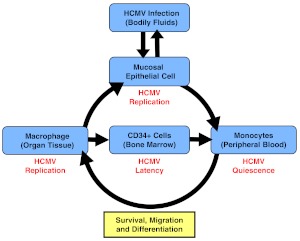
MODEL FOR HCMV INFECTION AND DISSEMINATION IN THE HOST
Based on the findings of our laboratory and others, we have developed a model of HCMV dissemination following primary infection where HCMV can exploit monocyte biology to spread throughout the infected host and establish productive infection (Fig. 1) [10]. Initial infection of epithelial cells occurs following contact of these cells with bodily fluids, such as saliva, breast milk, or urine, from an individual that is actively shedding virus [8]. As cell-free virus is rarely found in the peripheral blood following primary infection, monocytes likely acquire the virus directly from infected epithelial cells and then enter into peripheral blood [7, 8]. In the infected monocyte, HCMV enters a quiescent (nonreplicative) state; however, the process of viral entry induces the select biological changes in monocytes that lead to an increase in cellular motility and transendothelial migration to allow for the movement of these infected monocytes out of the peripheral blood into organ tissues. Concurrently, the infected monocytes begin a differentiation process that is completed following extravasation into organ tissue. The differentiation of monocytes into distinct, long-lived tissue macrophages has been shown in vitro [10, 24] and in vivo [25] to allow for their permissivity to viral replication, viral maturation, and release of mature virions. Productive HCMV infection in tissue macrophages would allow for spread of HCMV and the establishment of chronic HCMV infection within infected organs, consistent with long-term viral secretion observed in patients [26]. Moreover, migration of the infected monocytes into the bone marrow would lead to the establishment of viral latency within CD34+ myeloid progenitor cells. Reactivation of HCMV can occur when infected myeloid progenitor cells in the bone marrow differentiate into monocytes, and these newly infected monocytes are released into the peripheral blood [7, 27, 28]. Thus, HCMV enhances its own viral life cycle by regulating distinct changes in infected monocytes, which ensures effective dissemination and allows for the establishment of persistent (chronic and latent) infection.
Figure 1. A model for HCMV infection and dissemination in the host.
Epithelial cells of the host are infected by contact with HCMV-containing bodily fluids. HCMV then replicates and spreads to monocytes in the peripheral blood by an unknown mechanism, where HCMV appears to establish a “quiescent” infection. Our data suggest that primary infection of monocytes promotes their migration into host organ tissue and their survival and differentiation into long-lived, viral replication-permissive macrophages. These macrophages could become sites of persistent infection in the host tissue. The possibility also exists that HCMV-induced macrophages could migrate into the bone marrow and infect CD34+ myeloid progenitor cells, which are believed to be sites of HCMV latency.
HCMV FUNCTIONS AS A UNIQUE LIGAND ABLE TO ACTIVATE MULTIPLE CELLULAR SIGNALING PATHWAYS SIMULTANEOUSLY DURING VIRAL ENTRY INTO MONOCYTES
Recent work from our laboratory has focused on identifying the molecular mechanisms by which HCMV is able to induce the distinct biological changes in infected monocytes that are necessary for promoting viral dissemination. As HCMV cannot replicate in monocytes, the virus must promote these changes in the absence of viral gene expression [7, 10, 14, 15]. We have shown that HCMV infection can activate PI3K and NF-κB signaling pathways to regulate monocyte motility, transendothelial migration, and cytokine/chemokine production, suggesting that HCMV modulates signaling pathways that are required for viral dissemination [18, 20, 29–32]. In addition, the complex PI3K- and NF-κB- associated signalsome, initiated following HCMV infection, was responsible for the unique polarization of the infected monocytes during the virally induced differentiation process [23]. Specifically, NF-κB played a dominant role in the up-regulation of M1-associated genes in HCMV-infected monocytes, whereas PI3K and NF-κB activities were required for the HCMV-induced up-regulation of M2-associated genes in infected monocytes [23]. Overall, our data suggest that the HCMV-induced changes in monocytes occur by viral activation of multiple signaling pathways, which converge to promote specific alterations in monocyte function that aid in the establishment of viral spread.
The viral activation of monocyte signaling occurs rapidly following HCMV infection [19, 20, 29], suggesting that the virion itself is capable of activating cellular signaling pathways. In support, HCMV is able to induce monocyte motility, migration, differentiation, and cytokine production in the absence of viral replication and de novo viral gene expression, as UV-inactivated HCMV is able to promote the same changes seen in “live” HCMV-infected monocytes [10, 18, 33]. The induction of these changes seems to be dependent on viral engagement of cellular receptors, as neutralization of viral glycoproteins diminishes these changes, and the addition of soluble glycoprotein B can promote cytokine production [18] and motility (unpublished data), much like treatment with live HCMV. Therefore, our laboratory has also focused on the identification of the specific biological effects of the engagement of HCMV with the cellular receptors on monocytes to better define how the virus polarizes target monocytes rapidly and selectively.
CELLULAR RECEPTORS ENGAGED BY HCMV DURING VIRAL ENTRY
HCMV is able to infect a variety of biologically distinct cell types leading to disease pathogenesis in multiple organ systems within the infected host [34]. The promiscuous nature of HCMV infection suggests that a wide range of cellular receptors can be engaged and/or used during the viral entry process. HCMV has been shown to bind to several cellular receptors, including those that are not directly involved in mediating viral entry, such as MHC class 1 molecules [35], annexin II [36], CD13 [37], and TLR2 [38], and those that are required for viral entry, including HSPGs [39, 40], PDGFRα [41], EGFR [42], and integrin heterodimers (α2β1, α6β1, and αVβ3) [42, 43]. Although TLR2 has been shown to mediate the activation of monocytes in response to several pathogens, in this current review, we discuss only those cellular receptors that appear to be directly involved in HCMV entry because of our interest in identifying multifunctional cellular receptors that can concurrently mediate viral entry and trigger the unique biological changes observed in HCMV-infected monocytes.
The HCMV entry process begins with the tethering of the virion by ubiquitously expressed HSPGs to the cell surface [39, 40]. Although an essential step, binding HSPGs cannot in itself initiate the cellular signaling pathways required for the functional changes in HCMV-infected monocytes, as HSPGs require a signaling-competent coreceptor to induce cellular signaling cascades [44]. Indeed, the initial tethering of HCMV virions to HSPGs is reversible and is believed to function to stabilize the virus at the cell surface until engagement of secondary receptors can occur, at which time, signaling [19, 42, 45] and internalization [39] steps can take place.
PDGFRα, EGFR, and integrin heterodimers have been shown to mediate internalization of surface-bound HCMV into various target host cells [41–43, 45]. Inhibition of individual cellular binding receptors blocks HCMV internalization, whereas still allowing for signaling from the other receptors, suggesting that a coordination of multiple receptor-initiated signaling pathways is required for viral entry [45]. Indeed, a direct interaction and cross-talk communication between EGFR and integrins during HCMV entry trigger the functional changes needed to facilitate internalization [45]. However, notwithstanding the need for receptor-to-receptor signaling to mediate viral entry, other functional changes, such inflammatory cytokine secretion, does not appear to specifically require the HCMV-induced cross-talk between signaling cascades [46]. Thus, despite recent advances, the relationship among initial signaling, infection, and phenotypic changes following HCMV entry into target cells remains largely unknown and is complicated further by the cell-type specificity of many of these events.
HCMV entry receptors are widely expressed by a variety of different cell types, although none are expressed in all HCMV-infectable cells, suggesting that HCMV uses different cellular receptors to mediate entry functions in different cell types, contributing to the broad cellular tropism of the virus. We [19] and others [47] have shown that PDGFRα is not expressed on the surface of human primary monocytes, indicating that the functional changes in HCMV-infected monocytes cannot be attributed to this cellular receptor. Consequently, we concentrated our efforts into determining the potential role that integrin heterodimers and EGFR have in modulating monocyte biology to promote hematogenous dissemination of HCMV and subsequently, viral persistence.
HCMV TARGETS THE DIVERSE SIGNALING NATURE OF INTEGRINS
Integrins constitute a large family of cell surface receptors that play an important role in allowing cells to bind to ECM proteins and as cell–cell adhesion molecules for blood cells [48, 49]. As cell adhesion receptors, integrins typically bind their ligands with low affinity, and the strength of their interactions with specific ligands comes from multiple bonds created simultaneously, allowing at the same time, their ease of disassembly—a mechanism described as the “velcro” principle [49]. This mode of regulation enables prompt reversibility of integrin–ligand interactions and movement of cells [49]. The specific binding of integrins to a vast array of cellular targets is accomplished by the distinct way a functional receptor is created. Integrins are a family of heterodimeric receptors composed of a single α and a single β chain [49]. There are 24 α and nine β integrin chains known, which in turn, can form 25 individual receptors [49]. Each combination of α and β chain not only has its own binding properties but also has its own distinct downstream signaling characteristics. It is thought that the β chain possesses the signaling capacities, whereas the α chain has the modulatory function. The engagement of integrins to their natural ligands leads to the induction of specific signaling pathways using various protein tyrosine kinases, such as FAK, Src-family kinases (i.e., Src, Fyn), and the serine-threonine kinase, integrin-linked kinase [50]. The recruitment of different combinations of signaling kinases allows integrin heterodimers to initiate distinct signalsomes in cells [48, 50–54].
Integrins have been implicated in HCMV binding to and entry into human fibroblasts [45], an established in vitro model for HCMV infection. Two reports described that α2β1, α6β1 [43], or αvβ3 [45] integrin heterodimers were engaged on fibroblasts by HCMV. Our studies demonstrated that the use of integrins by HCMV is cell type-specific, as virus infection of endothelial cells and monocytes, two biologically relevant cell types in HCMV infection, simultaneously requires β1 and β3 integrins [55, 56] in contrast to single β integrin use in fibroblasts. We have also documented that HCMV interaction with integrins on the surface of monocytes resulted in the activation of integrin/Src-mediated signaling [56]. However, the kinetics of Src phosphorylation are different between fibroblasts and monocytes. Studies by Wang et al. [45] revealed that Src activation in fibroblasts was transient, occurring between 5 and 10 min postinfection, and quickly diminished thereafter to undetectable levels by 2 h postinfection. In contrast, Src was chronically activated at low levels in monocytes, with an increase between 15 and 30 min upon HCMV infection and then a return to low chronic levels afterward (ref. [56] and unpublished data). Taken together, these data suggest that HCMV may have evolved to target the monocyte-specific integrin signaling events to regulate the cellular, functional changes necessary for mediating viral spread.
INTEGRINS AND HCMV ENTRY INTO MONOCYTES
Taking into account the divergent signaling events triggered by different integrins and the use of different integrins by HCMV on biologically distinct cell types, we speculated that this difference would translate into distinct routes of entry into fibroblasts versus monocytes, as with fibroblasts versus endothelial and epithelial cells [57, 58]. In support, pH-independent fusion was demonstrated to be the entry pathway of HCMV into fibroblasts [59], whereas endocytosis was detected as the predominate mode of HCMV entry into epithelial and endothelial cells and monocytes [57, 58, 60]. In fibroblasts, integrins α2β1, α6β1, or αvβ3 appear to be required for HCMV entry [43, 45]. In trophoblasts, the blockage of infectivity was observed when virions were pretreated with soluble α1β1, α3β1, and αvβ3integrins [61]. We showed that HCMV internalization into target monocytes was inhibited significantly by blocking HCMV engagement with β1 and β3 integrins, as determined by a decrease in the amount of internalized viral genomic DNA in monocytes [56]. Together, these studies indicate that HCMV universally uses the signaling of β integrin subunits to directly modulate the entry process, although different combinations of β integrins are used in different cell types. There is also a distinct use of different sets of modulatory integrin α subunits among different cell types; in fibroblasts (α2, α6, or αV) versus in trophoblasts (α1, α3, and αV), suggesting that the modulatory capacity of α subunits may govern the specific mode of HCMV entry into and the downstream receptor-mediated changes in target host cells. Currently, our laboratory is in the process of identifying the specific integrin heterodimers used by HCMV to address the role that integrin α subunits play during infection of monocytes.
INTEGRINS AND HCMV-INDUCED MONOCYTE MOTILITY
Integrins are known for their regulation of cellular motility [50], as well as in the pathogenic deregulation of this process observed in metastasis of cancer cells [62, 63]. Our studies showed that mock-infected monocytes are characterized by a basal level of movement that is not inhibited when cells are treated with PP2 (a specific inhibitor of Src tyrosine kinase activity) or with blocking antibodies to integrins, as measured by the movement of monocytes on colloidal gold-coated coverslips [56]. In contrast, HCMV-infected monocytes are characterized by a heightened or hyper motility compared with mock-infected cells [10, 19, 20, 56], which is sensitive to pretreatment with PP2 or with neutralizing antibodies to selected β integrins (β1 or β3 integrins) prior to infection [56]. These studies indicate that integrin engagement and receptor-mediated signaling are critical for a hyper motility of monocytes following HCMV infection.
To determine the molecular mechanisms by which integrins regulate monocyte motility following infection, we performed a global transcriptome analysis. We found that expression of paxillin (a known scaffolding protein and signal transducer capable of modulating interactions among proteins involved in cell adhesion and movement) was regulated by cooperative action of integrin signaling, which controlled paxillin expression and activation, and EGFR signaling, which regulated only paxillin expression [56]. As paxillin has been shown to control a number of proteins associated with cellular motility and to be important for actin rearrangement [64] (a process showed to be involved in the viral entry process) [43, 45], we examined whether paxillin controlled cellular motility and viral entry. Indeed, we demonstrated that paxillin regulation is vital to induce efficient HCMV internalization and a hyper motility of target monocytes [56], strongly supporting the importance of HCMV-integrin engagement in shaping monocyte biology for its advantage during virus infection. Interestingly, it was found that paxillin expression was down-regulated in HCMV-infected fibroblasts [65] and that paxillin is apparently not involved in viral entry of fibroblasts (data not published), once again, advocating the idea of a very specific use of distinct combinations of cellular integrins by HCMV to induce unique biological outcomes on different cell types. This study also documented from a molecular standpoint how another HCMV entry receptor, EGFR, can cooperatively signal with integrins during the viral entry process to modulate monocyte function.
MONOCYTES EXRESS BONA FIDE SURFACE EGFR, WHICH IS ACTIVATED UPON HCMV INFECTION
Until recently, the presence of EGFR on the surface of human peripheral blood monocytes remained unclear. Expression studies performed on whole populations of PBMCs did not detect the presence of EGFR [66], suggesting that monocytes lack this receptor. However, the monocyte subpopulation constitutes only 5% of the total PBMC population [67]; thus, low-level EGFR expression may have been below the threshold of detection. Currently, there is growing evidence that cells of the myeloid lineage express EGFR. Primary peripheral blood monocytes isolated from rabbits were demonstrated to express low levels of EGFR, which could mediate chemotactic responses to EGF [68]. EGFR also appears to be involved in the polarization of monocytes toward a M1 macrophage in mice [69]. Model human monocytic cell lines have been shown to exhibit low levels of cell surface EGFR that can trigger downstream signaling pathways via the activation of JNK, ERK, and PI3K/Akt [70–72]. The activation of monocytic cell line surface EGFR and its downstream signaling cascades can stimulate the secretion of proinflammatory cytokines [70] and the acquisition of an antiapoptotic state [72]. Valente et al. [73] found that the EGFRK activity of primary human monocytes mediated the up-regulation of CD14 by steroid ouabain. In accord with these observations, we have shown recently, for the first time, low-level EGFR expression on the surface of primary human peripheral blood monocytes [19], which has been confirmed subsequently [74]. Treatment with the cognate ligand EGF or infection with endothelial/monocyte tropic HCMV strains Towne/E or TB40/E phosphorylated EGFR within 10 min postinfection [19]. However, infection of monocytes with HCMV resulted in the chronic activation of EGFR versus the transient activation of EGFR following EGF treatment. The chronic activation of EGFR was also observed in endothelial cells [55] but not in fibroblasts or trophoblasts [42, 75], indicating that HCMV is likely able to induce unique EGFR signaling events in biologically distinct cell types. The reasons for the disparity in EGFR induction between divergent cell types following HCMV infection remain to be elucidated; however, chronic induction of EGFR in monocytes and endothelial cells suggests that persistent EGFR activation following HCMV infection may be central to the functional outcomes of cells directly involved in mediating viral dissemination.
EGFR FUNCTIONS AS A VIRAL TROPISM RECEPTOR REQUIRED FOR EFFICIENT HCMV ENTRY INTO MONOCYTES
Integrins and PDGFRα have been reported to be required for efficient viral entry [41, 43, 45], but because of ubiquitous integrin expression on multiple PBMC subpopulations and the lack of PDGFRα expression on monocytes and macrophages [19, 47], it is unlikely that these receptors direct HCMV infection specifically toward the monocyte subpopulation. We [19] and others [76] have demonstrated that HCMV is unable to infect human primary peripheral blood T cells and B lymphocytes, which we have shown [19] do not to express EGFR. This relationship between EGFR expression and HCMV infection of the monocyte subpopulation of PBMCs indicates that EGFR could be an integral player in determining monocyte tropism for HCMV. In accord, EGFR was shown to act as a viral tropism receptor for targeting HCMV entry into biologically distinct populations of trophoblast cells [61]. Consequently, we asked if the expression of EGFR is responsible for the selective binding of virus to different populations of blood leukocytes or if it plays a different role in viral tropism, such as in the regulation of viral fusion. The presence of a blocking EGFR antibody prior to infection did not significantly affect the levels of HCMV associated with monocytes, indicating that EGFR expression does not alone dictate viral attachment to the monocyte cell surface [19]. In contrast, detection of internalized viral genomes revealed a 41% and 59% decrease in viral entry into monocytes in the presence of neutralizing EGFR antibody and AG1478 [19], respectively, which is consistent with the 63% reduction in infectivity observed in trophoblasts treated with an anti-EGFR antibody prior to HCMV infection [61]. Our data provide evidence that EGFR expression and the ensuing downstream signaling following viral engagement on monocytes provide cell-type specificity for efficient viral internalization.
It should also be pointed out that the role of EGFR during viral infection remains controversial, as others reported [77] that EGFR does not mediate entry into fibroblasts and that PDGFRα not EGFR was required for efficient viral entry [41]. The reasons for these conflicting results remain unresolved. Perhaps both receptors serve as viral entry receptors, depending on the cell type or infection condition. Although PDGFRα and EGFR are known to bind different ligands, they initiate similar molecular signaling events; thus, the possibility exists that HCMV has evolved to use multiple receptors with similar cellular activity to broaden its tropism. Furthermore, expression of distinct viral glycoproteins from endotheliotropic and clinical HCMV strains allows for viral infection of endothelial/epithelial cells, but not of fibroblasts, indicating the existence of cell type-specific receptors for different strains of HCMV [78]. Although more work is needed to address whether strain variation can account for the requirement of EGFR signaling during viral entry, our data demonstrated that the endotheliotropic stains Towne/E and TB40/E signal and enter through an EGFR-dependent mechanism on monocytes.
EGFR ACTIVATION MEDIATES KEY, EARLY FUNCTIONAL CHANGES IN HCMV-INFECTED MONOCYTES NECESSARY FOR THE VIRAL DISSEMINATION STRATEGY
Monocytes exhibit a short lifespan of ∼48 h upon entry into circulation from the bone marrow [16]. We have shown that HCMV infection rapidly induces a prosurvival state and the hyper chemokinetic movement of infected monocytes, which are two essential, early functional changes required for driving infected monocytes from the circulation into the peripheral tissue and allowing for differentiation of the infected monocyte into a long-lived macrophage. These HCMV-induced changes occurred within a timeframe where no new viral gene expression was observed [10]. Indeed, we found that EGFR activation during HCMV entry into monocytes was required for the acquisition of an antiapoptotic state and the induction of chemokinetic movement in infected monocytes. Transcriptome and protein analyses revealed that HCMV increased the cellular expression of Mcl-1, a member of the antiapoptotic protein B cell lymphoma 2 family, and N-WASP, an important regulator of actin cytoskeleton involved in cellular motility, in an EGFR-dependent manner. Using small interfering RNA knockdown, we confirmed the involvement of Mcl-1 in the rapid acquisition of a prosurvival state and of N-WASP in the induction of the hyper cellular motility following HCMV infection.
Further analysis of the EGFR-dependent, HCMV-infected monocyte transcriptome revealed a complex regulation of monocyte gene expression that originates from the engagement of and signaling through EGFR. Inhibition of the intracellular signaling of EGFR by AG1478 treatment regulated more genes during HCMV infection than the inhibition of the extracellular signaling domain of EGFR by anti-EGFR antibody [19]. The discrepancy between AG1478 and anti-EGFR antibody treatment is likely a result of the ability of AG1478 to block cross-talk phosphorylation of the cytoplasmic domain of EGFR by integrins activated during HCMV entry [43, 45, 79]. In support, the inhibition of EGFR by anti-EGFR antibody was 50% less effective than inhibition with AG1478 in blocking the up-regulation of Mcl-1 and N-WASP expression in monocytes following HCMV infection [19, 33]. We suggest that HCMV induces a unique multireceptor-mediated signalsome during viral entry that depends on the expression of cell-specific HCMV receptors on different cell types. In monocytes, communication between EGFR and integrins leads to the pathogenic changes that directly drive viral dissemination and persistence.
DO EGFR AND INTEGRINS COOPERATIVELY REGULATE THE FUNCTIONAL CHANGES INDUCED IN, AND HCMV ENTRY INTO, MONOCYTES?
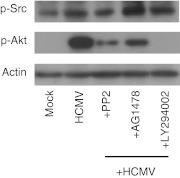
Wang et al. [45] showed in model cell types that significant cross-talk communication between the integrin/Src and EGFR/PI3K signaling pathways occurred following HCMV infection. A direct interaction between integrins and EGFR was essential for the cross-talk signaling to transpire and was demonstrated to occur within the lipid rafts of the target host cell during viral binding. The authors showed that the inhibition of the integrin/Src signaling pathway prior to infection with HCMV decreased the activation of the EGFR/PI3K cascade, and blocking EGFR or PI3K activities resulted in a substantial decrease in Src phosphorylation following infection, indicating that cross-talk signaling occurred in a bidirectional manner. Similarly, our results demonstrated that pretreatment with PP2 prior to infection decreased PI3K activity in HCMV-infected monocytes (Fig. 2). Furthermore, unlike pretreatment with LY294002 (a PI3K inhibitor), pretreatment with PP2 or AG1478 (an EGFR inhibitor) was not able to completely block the activation of PI3K following infection, indicating that a coordinated signaling between EGFR and integrins is required to fully activate downstream PI3K signaling. However, in contrast to fibroblasts, monocytes pretreated with AG1478 or LY294002 prior to infection had no effect on the HCMV-mediated phosphorylation of Src, indicating a unidirectional cross-talk from the integrin/Src to EGFR/PI3K signaling pathways. The unidirectional transmission between the integrin/Src and EGFR/PI3K signaling pathways in HCMV-infected monocytes may be a biological consequence of the expression levels of these receptors on the cell surface. Our data suggested that monocytes express low levels of EGFR and high levels of integrins [19, 56], which is consistent with other studies [71, 80]; thus, this stoichiometric ratio suggests to us that there is a lower probability of the abundantly expressed integrins being activated by the relatively low amounts of EGFR during viral binding. As a result, the limited effect that EGFR would have on integrin activation could be masked by the largely EGFR-independent activation of the integrin/Src signaling pathways during HCMV entry into monocytes. Overall, these observations indicate that despite HCMV activating EGFR and integrins, the engagement of these same receptors on monocytes leads to the acquisition of a distinctive signalsome following infection when compared with fibroblasts, which we argue results in the appropriate functional changes in monocytes that drive dissemination and persistence.
Figure 2. Unidirectional cross-talk from the integrin/Src to EGFR/PI3K signaling pathway occurs during HCMV infection of monocytes.
Purified peripheral blood monocytes were treated with AG1478 (1 μM), PP2 (1 μM), or LY294002 (25 μM) before infection with HCMV for 1 h. Following pretreatment, monocytes were infected with HCMV for 15 min. Phosphorylated (p) Src and phosphorylated Akt (immediate downsteam target of PI3K) were determined by immunoblotting. Membranes were reprobed with antibody against β-actin as a loading control.
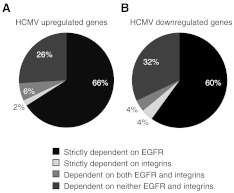
The unidirectional cross-talk from the integrin/Src cascade to the EGFR/PI3K signaling pathway suggests that EGFR plays a key regulatory role in the induction of the unique signalsome induced in monocytes following infection. In quiescent monocytes, Src appears to be chronically activated, at least at low basal levels, and is marginally induced following HCMV infection, whereas EGFR and PI3K activities are at or near undetectable levels until binding of the virus particle to the cell surface resulting in the strong induction of the EGFR/PI3K axis. However, it should be pointed out that these data indicate that the chronic low level of activated Src does not induce PI3K activity in the absence of infection, suggesting that there is some virus-specific effect on the pathway to allow the activated Src molecule to phosphorylate viral-required substrates. Moreover, our global transcriptome studies have shown that ∼70% of the HCMV-associated transcriptome is regulated in an EGFR-dependent manner, whereas only ∼8% was regulated via integrins (Fig. 3). Thus, despite integrins being expressed more abundantly on the surface of monocytes, EGFR appears to function as the “trigger” to initiate the distinct signaling in monocytes during HCMV entry, resulting in the cellular, functional changes necessary for hematogenous dissemination of the virus.
Figure 3. HCMV infection regulates the monocyte transcriptome in an EGFR- and integrin-dependent manner.
Purified peripheral blood monocytes were treated with DMSO, AG1478, or PP2 for 1 h. Pretreated monocytes were then mock- or HCMV-infected (MOI 5) for 24 h and total RNA harvested for Affymetric gene array analysis. A P value of ≤0.05 was used as the criteria for statistically significant genes among replicates. Genes with an average 1.5-fold (A) up-regulation or (B) down-regulation were considered to be regulated by infection. Cellular genes dependent on EGFR or Src activity were determined by comparing the genes in HCMV-infected replicates with those that were down-regulated or up-regulated an average of 1.5-fold or greater in AG1478- or PP2-pretreated, HCMV-infected samples. Data were derived from data published previously (see refs. [19] and [56]). Gene Expression Omnibus database accession numbers are GSE17948 and GSE24238.
Although the unidirectional cross-talk between EGFR and integrins is central to regulating the biological changes in monocytes following infection, our data suggest that this cross-talk between PI3K and Src signaling pathways is not required for HCMV entry into monocytes. In fibroblasts, the cross-talk communication between the integrin/Src cascade and the EGFR/PI3K cascade appears to be necessary for regulating viral entry. The inhibition of EGFR signaling in fibroblasts blocked virus infection by 97%, and the inhibition of integrin signaling blocked HCMV infection by 77% [45]. In contrast to fibroblasts, the blocking of EGFR on monocytes reduced HCMV entry by 45% [19], whereas blocking integrin decreased HCMV entry into monocytes by 50% [56]. Taken together, these results suggest that EGFR and integrins work independently to mediate HCMV entry into monocytes. The decreased efficiency of EGFR to mediate viral entry into monocytes versus fibroblasts may, in part, be a result of the lack of cross-talk signaling from the EGFR/PI3K axis to the integrin/Src axis, which occurs readily in fibroblasts. Moreover, unlike in fibroblasts, the activation of PI3K during viral entry was not required for HCMV entry into monocytes; thus, although cross-talk communication from the integrin/Src signaling pathway to the EGFR/PI3K signaling pathway occurs efficiently in monocytes, the activation of PI3K by the integrin/Src cascade would not lead to an increase in HCMV entry into monocytes. These data highlight the ability of HCMV to use divergent pathways originating from the same viral entry receptors in biologically distinct cell types to mediate HCMV entry, thus ensuring its self-survival in different cells types.
CONCLUSIONS
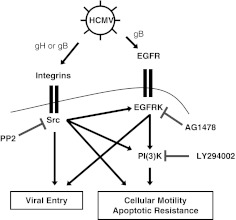
The clinical symptoms observed in patients with HCMV infection indicate that an inflammatory component is responsible for the progression of HCMV-associated diseases [2]. In vivo infection of proinflammatory mononuclear cells is closely associated with organ disease in patients with clinical manifestations of HCMV [2], yet the short lifespan of monocytes and the lack of productive infection created a puzzling scenario—monocytes were in the right place at the right time but were not able to produce infectious virus. Our laboratory now provides evidence for a molecular mechanism, involving viral ligand/cellular receptor interactions, which allows HCMV to modulate specific components of the proinflammatory response to promote viral spread. The lack of initial gene expression and viral replication in monocytes indicates that receptor/ligand-mediated signaling may play a larger role in regulating the biology of HCMV-infected monocytes when compared with other cell types. Our findings demonstrate that although HCMV has evolved to activate ubiquitously expressed viral entry cellular receptors found on monocytes, the activation of these receptors leads to a monocyte-specific and HCMV-specific signalsome, which in turn, promotes the unique, functional changes observed in monocytes. Indeed, functional studies directly link the activation of integrins and EGFR with key functional processes (the induction of motility, survival, and differentiation) in monocytes required for hematogenous dissemination of HCMV (Fig. 4). We further show a critical role of EGFR to function as a central “hub”, from which the complex HCMV-induced monocyte transcriptome network is initiated following infection. Overall, our work highlights the importance of deciphering the cell type-specific signaling systems associated with HCMV infection that distinctly modulate the cellular biology of divergent cell types necessary for lifelong persistence within the host.
Figure 4. A model of the HCMV-specific signalsome initiated following infection of peripheral blood monocytes.
HCMV virion particles (through gB and gH) bind to the surface of peripheral blood monocytes and engage EGFR and integrins leading to the induction of a complex HCMV-associated signalsome. The activation of EGFR and integrins is necessary for efficient viral entry into monocytes, but unlike in fibroblasts, the downstream activation of PI3K is not required. Furthermore, our data indicate that in monocytes, a unidirectional cross-talk from the integrin/Src signaling axis to the EGFR/PI3K signaling axis occurs following HCMV infection, which is in contrast to the bidirectional cross-talk that occurs during infection of fibroblasts. Consequently, whereas Src activity can directly regulate the expression of certain HCMV-induced genes, the position of the EGFR/PI3K signaling axis in the monocyte-specific, HCMV-stimulated signalsome places it as a central hub from where the HCMV-induced monocyte transcriptome network is initiated.
ACKNOWLEDGMENTS
This work was supported by a Malcolm Feist Cardiovascular Research Post-Doctoral Fellowship and American Heart Association Pre-Doctoral Fellowship (10PRE4200007) and U.S. National Institutes of Health grants (AI56077, HD051998, and GM103433).
Footnotes
- EGFRK
- EGFR kinase
- HCMV
- human CMV
- HSPG
- heparin sulfate proteoglycan
- Mcl-1
- myeloid cell leukemia sequence 1
- N-WASP
- neuronal Wiskott-Aldrich Syndrome protein
AUTHORSHIP
G.C., M.T.N., and A.D.Y. designed research and analyzed data. G.C. and M.T.N. performed research. G.C., M.T.N., E.V.S., and A.D.Y. wrote the paper.
REFERENCES
- 1. Mocarski E. S., Courcell C. T. (2001) Cytomegalovirus and their replication. In Fields Virology, Vol. 2 (Knipe D. M., Howley, eds.), P. M. Lippincott, Williams and Wilkins, Philadelphia, PA, USA, 2629–2673 [Google Scholar]
- 2. Britt W. (2008) Manifestations of human cytomegalovirus infection: proposed mechanisms of acute and chronic disease. In Current Topics in Microbiology and Immunology: Human Cytomegalovirus, Vol. 325 (Shenk T., Stinski, eds.), M. Springer-Verlag, Berlin and Heidelberg, Germany, 417–470 [DOI] [PubMed] [Google Scholar]
- 3. Reeves M. B., Sinclair J. H. (2010) Analysis of latent viral gene expression in natural and experimental latency models of human cytomegalovirus and its correlation with histone modifications at a latent promoter. J. Gen. Virol. 91, 599–604 [DOI] [PubMed] [Google Scholar]
- 4. Goodrum F., Caviness K., Zagallo P. (2012) Human cytomegalovirus persistence. Cell. Microbiol. 14, 644–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Soderberg-Naucler C. (2008) HCMV microinfections in inflammatory diseases and cancer. J. Clin. Virol. 41, 218–223 [DOI] [PubMed] [Google Scholar]
- 6. Crough T., Khanna R. (2009) Immunobiology of human cytomegalovirus: from bench to bedside. Clin. Microbiol. Rev. 22, 76–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sinclair J., Sissons P. (1996) Latent and persistent infections of monocytes and macrophages. Intervirology 39, 293–301 [DOI] [PubMed] [Google Scholar]
- 8. Sinzger C., Jahn G. (1996) Human cytomegalovirus cell tropism and pathogenesis. Intervirology 39, 302–319 [DOI] [PubMed] [Google Scholar]
- 9. Booss J., Dann P. R., Griffith B. P., Kim J. H. (1989) Host defense response to cytomegalovirus in the central nervous system. Predominance of the monocyte. Am. J. Pathol. 134, 71–78 [PMC free article] [PubMed] [Google Scholar]
- 10. Smith M. S., Bentz G. L., Alexander J. S., Yurochko A. D. (2004) Human cytomegalovirus induces monocyte differentiation and migration as a strategy for dissemination and persistence. J. Virol. 78, 4444–4453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taylor-Wiedeman J., Sissons J. G., Borysiewicz L. K., Sinclair J. H. (1991) Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J. Gen. Virol. 72, 2059–2064 [DOI] [PubMed] [Google Scholar]
- 12. Gerna G., Baldanti F., Revello M. G. (2004) Pathogenesis of human cytomegalovirus infection and cellular targets. Hum. Immunol. 65, 381–386 [DOI] [PubMed] [Google Scholar]
- 13. Weber B., Doerr H. W. (1994) Diagnosis and epidemiology of transfusion-associated human cytomegalovirus infection: recent developments. Infusionsther. Transfusionsmed. 21 (Suppl. 1), 32–39 [DOI] [PubMed] [Google Scholar]
- 14. Fish K. N., Depto A. S., Moses A. V., Britt W., Nelson J. A. (1995) Growth kinetics of human cytomegalovirus are altered in monocyte-derived macrophages. J. Virol. 69, 3737–3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ibanez C. E., Schrier R., Ghazal P., Wiley C., Nelson J. A. (1991) Human cytomegalovirus productively infects primary differentiated macrophages. J. Virol. 65, 6581–6588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Whitelaw D. M. (1972) Observations on human monocyte kinetics after pulse labeling. Cell Tissue Kinet. 5, 311–317 [DOI] [PubMed] [Google Scholar]
- 17. Chan G., Bivins-Smith E. R., Smith M. S., Smith P. M., Yurochko A. D. (2008) Transcriptome analysis reveals human cytomegalovirus reprograms monocyte differentiation toward an M1 macrophage. J. Immunol. 181, 698–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yurochko A. D., Huang E. S. (1999) Human cytomegalovirus binding to human monocytes induces immunoregulatory gene expression. J. Immunol. 162, 4806–4816 [PubMed] [Google Scholar]
- 19. Chan G., Nogalski M. T., Yurochko A. D. (2009) Activation of EGFR on monocytes is required for human cytomegalovirus entry and mediates cellular motility. Proc. Natl. Acad. Sci. USA 106, 22369–22374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith M. S., Bentz G. L., Smith P. M., Bivins E. R., Yurochko A. D. (2004) HCMV activates PI(3)K in monocytes and promotes monocyte motility and transendothelial migration in a PI(3)K-dependent manner. J. Leukoc. Biol. 76, 65–76 [DOI] [PubMed] [Google Scholar]
- 21. Mocarski E. S., Jr., (2002) Virus self-improvement through inflammation: no pain, no gain. Proc. Natl. Acad. Sci. USA 99, 3362–3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saederup N., Aguirre S. A., Sparer T. E., Bouley D. M., Mocarski E. S. (2001) Murine cytomegalovirus CC chemokine homolog MCK-2 (m131-129) is a determinant of dissemination that increases inflammation at initial sites of infection. J. Virol. 75, 9966–9976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chan G., Bivins-Smith E. R., Smith M. S., Yurochko A. D. (2009) NF-κB and phosphatidylinositol 3-kinase activity mediates the HCMV-induced atypical M1/M2 polarization of monocytes. Virus Res. 144, 329–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Soderberg-Naucler C., Streblow D. N., Fish K. N., Allan-Yorke J., Smith P. P., Nelson J. A. (2001) Reactivation of latent human cytomegalovirus in CD14(+) monocytes is differentiation dependent. J. Virol. 75, 7543–7554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sinzger C., Plachter B., Grefte A., The T. H., Jahn G. (1996) Tissue macrophages are infected by human cytomegalovirus in vivo. J. Infect. Dis. 173, 240–245 [DOI] [PubMed] [Google Scholar]
- 26. Noyola D. E., Demmler G. J., Williamson W. D., Griesser C., Sellers S., Llorente A., Littman T., Williams S., Jarrett L., Yow M. D. (2000) Cytomegalovirus urinary excretion and long term outcome in children with congenital cytomegalovirus infection. Congenital CMV Longitudinal Study Group. Ped. Infect. Dis. J. 19, 505–510 [DOI] [PubMed] [Google Scholar]
- 27. Kondo K., Kaneshima H., Mocarski E. S. (1994) Human cytomegalovirus latent infection of granulocyte-macrophage progenitors. Proc. Natl. Acad. Sci. USA 91, 11879–11883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maciejewski J. P., Bruening E. E., Donahue R. E., Mocarski E. S., Young N. S., St Jeor S. C. (1992) Infection of hematopoietic progenitor cells by human cytomegalovirus. Blood 80, 170–178 [PubMed] [Google Scholar]
- 29. Smith M. S., Bivins-Smith E. R., Tilley A. M., Bentz G. L., Chan G., Minard J., Yurochko A. D. (2007) Roles of phosphatidylinositol 3-kinase and NF-κB in human cytomegalovirus-mediated monocyte diapedesis and adhesion: strategy for viral persistence. J. Virol. 81, 7683–7694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yurochko A. D., Hwang E. S., Rasmussen L., Keay S., Pereira L., Huang E. S. (1997) The human cytomegalovirus UL55 (gB) and UL75 (gH) glycoprotein ligands initiate the rapid activation of Sp1 and NF-κB during infection. J. Virol. 71, 5051–5059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yurochko A. D., Kowalik T. F., Huong S. M., Huang E. S. (1995) Human cytomegalovirus upregulates NF-κB activity by transactivating the NF-κB p105/p50 and p65 promoters. J. Virol. 69, 5391–5400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yurochko A. D., Mayo M. W., Poma E. E., Baldwin A. S., Jr., Huang E. S. (1997) Induction of the transcription factor Sp1 during human cytomegalovirus infection mediates upregulation of the p65 and p105/p50 NF-κB promoters. J. Virol. 71, 4638–4648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chan G., Nogalski M. T., Bentz G. L., Smith M. S., Parmater A., Yurochko A. D. (2010) PI(3)K-dependent upregulation of Mcl-1 by human cytomegalovirus is mediated by epidermal growth factor receptor and inhibits apoptosis in short-lived monocytes. J. Immunol. 184, 3213–3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mocarski E. S., Shenk T., Pass R. F. (2007) Cytomegaloviruses. In Fields Virology, Vol. 2 (Knipe D. M., Howley, eds.), P. M. Lippincott, Williams and Wilkins, Philadelphia, PA, USA, 2701–2772 [Google Scholar]
- 35. Grundy J. E., McKeating J. A., Griffiths P. D. (1987) Cytomegalovirus strain AD169 binds β 2 microglobulin in vitro after release from cells. J. Gen. Virol. 68, 777–784 [DOI] [PubMed] [Google Scholar]
- 36. Wright J. F., Kurosky A., Wasi S. (1994) An endothelial cell-surface form of annexin II binds human cytomegalovirus. Biochem. Biophys. Res. Commun. 198, 983–989 [DOI] [PubMed] [Google Scholar]
- 37. Soderberg C., Giugni T. D., Zaia J. A., Larsson S., Wahlberg J. M., Moller E. (1993) CD13 (human aminopeptidase N) mediates human cytomegalovirus infection. J. Virol. 67, 6576–6585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boehme K. W., Guerrero M., Compton T. (2006) Human cytomegalovirus envelope glycoproteins B and H are necessary for TLR2 activation in permissive cells. J. Immunol. 177, 7094–7102 [DOI] [PubMed] [Google Scholar]
- 39. Compton T., Nowlin D. M., Cooper N. R. (1993) Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology 193, 834–841 [DOI] [PubMed] [Google Scholar]
- 40. Kari B., Gehrz R. (1992) A human cytomegalovirus glycoprotein complex designated gC-II is a major heparin-binding component of the envelope. J. Virol. 66, 1761–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Soroceanu L., Akhavan A., Cobbs C. S. (2008) Platelet-derived growth factor-α receptor activation is required for human cytomegalovirus infection. Nature 455, 391–395 [DOI] [PubMed] [Google Scholar]
- 42. Wang X., Huong S. M., Chiu M. L., Raab-Traub N., Huang E. S. (2003) Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 424, 456–461 [DOI] [PubMed] [Google Scholar]
- 43. Feire A. L., Koss H., Compton T. (2004) Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc. Natl. Acad. Sci. USA 101, 15470–15475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dreyfuss J. L., Regatieri C. V., Jarrouge T. R., Cavalheiro R. P., Sampaio L. O., Nader H. B. (2009) Heparan sulfate proteoglycans: structure, protein interactions and cell signaling. An. Acad. Bras. Cienc. 81, 409–429 [DOI] [PubMed] [Google Scholar]
- 45. Wang X., Huang D. Y., Huong S. M., Huang E. S. (2005) Integrin αβ3 is a coreceptor for human cytomegalovirus. Nat. Med. 11, 515–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Juckem L. K., Boehme K. W., Feire A. L., Compton T. (2008) Differential initiation of innate immune responses induced by human cytomegalovirus entry into fibroblast cells. J. Immunol. 180, 4965–4977 [DOI] [PubMed] [Google Scholar]
- 47. Inaba T., Shimano H., Gotoda T., Harada K., Shimada M., Ohsuga J., Watanabe Y., Kawamura M., Yazaki Y., Yamada N.., et al. (1993) Expression of platelet-derived growth factor β receptor on human monocyte-derived macrophages and effects of platelet-derived growth factor BB dimer on the cellular function. J. Biol. Chem. 268, 24353–24360 [PubMed] [Google Scholar]
- 48. Hynes R. O. (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 49. Albert B., Johnson A., Lewis J., Raff M., Roberts K., Walter P. (2002) Molecular Biology of the Cell. Garland Science, New York, NY, USA [Google Scholar]
- 50. Giancotti F. G., Ruoslahti E. (1999) Integrin signaling. Science 285, 1028–1032 [DOI] [PubMed] [Google Scholar]
- 51. Schlaepfer D. D., Hanks S. K., Hunter T., van der Geer P. (1994) Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature 372, 786–791 [DOI] [PubMed] [Google Scholar]
- 52. Wary K. K., Mainiero F., Isakoff S. J., Marcantonio E. E., Giancotti F. G. (1996) The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell 87, 733–743 [DOI] [PubMed] [Google Scholar]
- 53. Wary K. K., Mariotti A., Zurzolo C., Giancotti F. G. (1998) A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell 94, 625–634 [DOI] [PubMed] [Google Scholar]
- 54. Yamada K. M., Miyamoto S. (1995) Integrin transmembrane signaling and cytoskeletal control. Curr. Opin. Cell Biol. 7, 681–689 [DOI] [PubMed] [Google Scholar]
- 55. Bentz G. L., Yurochko A. D. (2008) Human CMV infection of endothelial cells induces an angiogenic response through viral binding to EGF receptor and β1 and β3 integrins. Proc. Natl. Acad. Sci. USA 105, 5531–5536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nogalski M. T., Chan G., Stevenson E. V., Gray S., Yurochko A. D. (2011) Human cytomegalovirus-regulated paxillin in monocytes links cellular pathogenic motility to the process of viral entry. J. Virol. 85, 1360–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bodaghi B., Slobbe-van Drunen M. E., Topilko A., Perret E., Vossen R. C., van Dam-Mieras M. C., Zipeto D., Virelizier J. L., LeHoang P., Bruggeman C. A., Michelson S. (1999) Entry of human cytomegalovirus into retinal pigment epithelial and endothelial cells by endocytosis. Invest. Ophthalmol. Vis. Sci. 40, 2598–2607 [PubMed] [Google Scholar]
- 58. Ryckman B. J., Jarvis M. A., Drummond D. D., Nelson J. A., Johnson D. C. (2006) Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion. J. Virol. 80, 710–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Compton T., Nepomuceno R. R., Nowlin D. M. (1992) Human cytomegalovirus penetrates host cells by pH-independent fusion at the cell surface. Virology 191, 387–395 [DOI] [PubMed] [Google Scholar]
- 60. Straschewski S., Patrone M., Walther P., Gallina A., Mertens T., Frascaroli G. (2011) Protein pUL128 of human cytomegalovirus is necessary for monocyte infection and blocking of migration. J. Virol. 85, 5150–5158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Maidji E., Genbacev O., Chang H. T., Pereira L. (2007) Developmental regulation of human cytomegalovirus receptors in cytotrophoblasts correlates with distinct replication sites in the placenta. J. Virol. 81, 4701–4712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Felding-Habermann B. (2003) Integrin adhesion receptors in tumor metastasis. Clin. Exp. Metastasis. 20, 203–213 [DOI] [PubMed] [Google Scholar]
- 63. Holzmann B., Gosslar U., Bittner M. (1998) α4 Integrins and tumor metastasis. Curr. Top. Microbiol. Immunol. 231, 125–141 [DOI] [PubMed] [Google Scholar]
- 64. Brown M. C., Turner C. E. (2004) Paxillin: adapting to change. Physiol. Rev. 84, 1315–1339 [DOI] [PubMed] [Google Scholar]
- 65. Stanton R. J., McSharry B. P., Rickards C. R., Wang E. C., Tomasec P., Wilkinson G. W. (2007) Cytomegalovirus destruction of focal adhesions revealed in a high-throughput Western blot analysis of cellular protein expression. J. Virol. 81, 7860–7872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Real F. X., Rettig W. J., Chesa P. G., Melamed M. R., Old L. J., Mendelsohn J. (1986) Expression of epidermal growth factor receptor in human cultured cells and tissues: relationship to cell lineage and stage of differentiation. Cancer Res. 46, 4726–4731 [PubMed] [Google Scholar]
- 67. Sipka S., Szanto S., Szucs K., Kovacs I., Kiss E., Antal-Szamas P., Lakos G., Aleksza M., Illes A., Gergely P., Szegedi G. (2001) Decreased arachidonic acid release in peripheral blood monocytes of patients with systemic lupus erythematosus. J. Rheumatol. 28, 2012–2017 [PubMed] [Google Scholar]
- 68. Lamb D. J., Modjtahedi H., Plant N. J., Ferns G. A. (2004) EGF mediates monocyte chemotaxis and macrophage proliferation and EGF receptor is expressed in atherosclerotic plaques. Atherosclerosis 176, 21–26 [DOI] [PubMed] [Google Scholar]
- 69. Prada P. O., Ropelle E. R., Mourao R. H., de Souza C. T., Pauli J. R., Cintra D. E., Schenka A., Rocco S. A., Rittner R., Franchini K. G., Vassallo J., Velloso L. A., Carvalheira J. B., Saad M. J. (2009) EGFR tyrosine kinase inhibitor (PD153035) improves glucose tolerance and insulin action in high-fat diet-fed mice. Diabetes 58, 2910–2919 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70. Cheon H., Woo Y. S., Lee J. Y., Kim H. S., Kim H. J., Cho S., Won N. H., Sohn J. (2007) Signaling pathway for 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced TNF-α production in differentiated THP-1 human macrophages. Exp. Mol. Med. 39, 524–534 [DOI] [PubMed] [Google Scholar]
- 71. Eales-Reynolds L. J., Laver H., Modjtahedi H. (2001) Evidence for the expression of the EGF receptor on human monocytic cells. Cytokine 16, 169–172 [DOI] [PubMed] [Google Scholar]
- 72. Moon D. O., Kim M. O., Lee J. D., Choi Y. H., Lee M. K., Kim G. Y. (2007) Molecular mechanisms of ZD1839 (Iressa)-induced apoptosis in human leukemic U937 cells. Acta Pharmacol. Sin. 28, 1205–1214 [DOI] [PubMed] [Google Scholar]
- 73. Valente R. C., Nascimento C. R., Araujo E. G., Rumjanek V. M. (2009) mCD14 expression in human monocytes is downregulated by ouabain via transactivation of epithelial growth factor receptor and activation of p38 mitogen-activated protein kinase. Neuroimmunomodulation 16, 228–236 [DOI] [PubMed] [Google Scholar]
- 74. El Zein N., D'Hondt S., Sariban E. (2010) Crosstalks between the receptors tyrosine kinase EGFR and TrkA and the GPCR, FPR, in human monocytes are essential for receptors-mediated cell activation. Cell. Signal. 22, 1437–1447 [DOI] [PubMed] [Google Scholar]
- 75. LaMarca H. L., Nelson A. B., Scandurro A. B., Whitley G. S., Morris C. A. (2006) Human cytomegalovirus-induced inhibition of cytotrophoblast invasion in a first trimester extravillous cytotrophoblast cell line. Placenta 27, 137–147 [DOI] [PubMed] [Google Scholar]
- 76. Soderberg C., Larsson S., Bergstedt-Lindqvist S., Moller E. (1993) Identification of blood mononuclear cells permissive of cytomegalovirus infection in vitro. Transplant. Proc. 25, 1416–1418 [PubMed] [Google Scholar]
- 77. Isaacson M. K., Feire A. L., Compton T. (2007) Epidermal growth factor receptor is not required for human cytomegalovirus entry or signaling. J. Virol. 81, 6241–6247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ryckman B. J., Chase M. C., Johnson D. C. (2008) HCMV gH/gL/UL128-131 interferes with virus entry into epithelial cells: evidence for cell type-specific receptors. Proc. Natl. Acad. Sci. USA 16, 14118–14123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Edick M. J., Tesfay L., Lamb L. E., Knudsen B. S., Miranti C. K. (2007) Inhibition of integrin-mediated crosstalk with epidermal growth factor receptor/Erk or Src signaling pathways in autophagic prostate epithelial cells induces caspase-independent death. Mol. Biol. Cell 18, 2481–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Klingemann H. G., Dedhar S. (1989) Distribution of integrins on human peripheral blood mononuclear cells. Blood 74, 1348–1354 [PubMed] [Google Scholar]