Abstract
Background
Unlike mammals, zebrafish exhibits extensive neural regeneration after injury in adult stages of its lifetime due to the neurogenic activity of the radial glial cells. However, the genes involved in the regenerative neurogenesis response of the zebrafish brain are largely unknown. Thus, understanding the underlying principles of this regeneration capacity of the zebrafish brain is an interesting research realm that may offer vast clinical ramifications.
Results
In this paper, we characterized the expression pattern of cxcr5 and analyzed the function of this gene during adult neurogenesis and regeneration of the zebrafish telencephalon. We found that cxcr5 was upregulated transiently in the RGCs and neurons, and the expression in the immune cells such as leukocytes was negligible during both adult neurogenesis and regeneration. We observed that the transgenic misexpression of cxcr5 in the ventricular cells using dominant negative and full-length variants of the gene resulted in altered proliferation and neurogenesis response of the RGCs. When we knocked down cxcr5 using antisense morpholinos and cerebroventricular microinjection, we observed outcomes similar to the overexpression of the dominant negative cxcr5 variant.
Conclusions
Thus, based on our results, we propose that cxcr5 imposes a proliferative permissiveness to the radial glial cells and is required for differentiation of the RGCs to neurons, highlighting novel roles of cxcr5 in the nervous system of vertebrates. We therefore suggest that cxcr5 is an important cue for ventricular cell proliferation and regenerative neurogenesis in the adult zebrafish telencephalon. Further studies on the role of cxcr5 in mediating neuronal replenishment have the potential to produce clinical ramifications in efforts for regenerative therapeutic applications for human neurological disorders or acute injuries.
Keywords: Adult zebrafish telencephalon, cxcr5, Radial glia, Proliferation, Regenerative neurogenesis, Adult neurogenesis, Differentiation
Background
The zebrafish, unlike mammals, can regenerate its adult brain and therefore has become an excellent system to study adult neurogenesis and brain regeneration in recent years [1-6]. The adult neurogenic capacity of zebrafish is in part due to widespread stem cell niches and the neurogenic regions located in the brain [1,2,6-14]. The zebrafish has sixteen ventricular stem cell zones along the entire anterior-posterior brain axis [8]. One of these proliferation zones is located dorsally along the ventricle in the telencephalon [7,8]. The dorsal telencephalic progenitors, which show radial glial cell (RGC) morphology and are positive for glia markers such as S100β and her4.1, display constitutive neurogenic activity during the life-span of the fish [12,15]. In addition to the constitutive levels of adult neurogenesis, zebrafish brain can also induce a regenerative response after traumatic injury [3-6]. For instance stab wounds in the adult fish brain induce cell death, inflammation, and proliferation of RGCs [3]. Using a Cre-lox lineage tracing strategy, these cells were shown to give rise to newborn neurons, which migrate to the lesioned site and integrate into the existing brain circuitry [3]. Therefore, zebrafish offers a unique opportunity for understanding the molecular basis of adult neurogenesis and brain regeneration in vertebrates. However, the molecular programs and the genes involved in these processes are largely unknown in zebrafish.
In order to identify genes involved in adult neurogenesis and regeneration response of the adult zebrafish telencephalon, we performed an in situ hybridization (ISH) screen. We identified that the gene cxcr5 was expressed in the ventricular region of the zebrafish telencephalon. cxcr5 encodes for a G-protein-coupled chemokine receptor with seven transmembrane domains [16]. The extracellular domain at the N-terminus is critical for ligand binding whereas the intracellular part is involved in G-protein interaction and activation [16,17]. The receptor was first isolated from human Burkitt’s lymphoma and is also known as the Burkitt lymphoma receptor-1 (BLR-1) [18,19]. The ligand for cxcr5 is the B-cell-attracting chemokine 1 (BCA-1), also known as Cxcl13 [20,21]. In mammals, cxcr5 is expressed by mature B cells and in a subset of T cells in the immune system [22,23]. CXCR5 and CXCL13 are responsible for the organization of B cell follicles and for directing T-helper cells to the lymphoid follicles in humans [24,25]. Furthermore, the chemokine CXCL13 attracts and maintains B and T cells during inflammation [23]. Besides the immune system, cxcr5 is found in the central nervous system in adult mice in the granule and Purkinje cell layer of the cerebellum [26-28]. The receptor is also expressed in human neuronal precursor cells, which migrate across the blood vessels in the brain upon exposure to CXCL13 [29]. However, the role of cxcr5 gene in the adult neurogenesis and the regeneration of the central nervous system in vertebrates is unknown.
In our study, we analyzed the expression pattern and the function of cxcr5 during adult neurogenesis and regeneration of the zebrafish telencephalon. We show that cxcr5 is detectable in the RGCs and neurons, during both adult neurogenesis and regeneration. We observed that the transgenic misexpression of cxcr5 in the ventricular cells using dominant negative and full-length variants of the gene resulted in reduced and increased proliferation and neurogenesis response of the RGCs, respectively. When we knocked down cxcr5 using antisense morpholinos and cerebroventricular microinjection [30], we also observed reduction of regenerative neurogenesis - an outcome similar to the overexpression of the dominant negative cxcr5 variant using transgenic tools. We propose that cxcr5 is an essential cue for ventricular cell proliferation and regenerative neurogenesis in the adult zebrafish telencephalon after injury.
Results and discussion
cxcr5 is expressed in radial glial cells and neurons in the adult zebrafish telencephalon
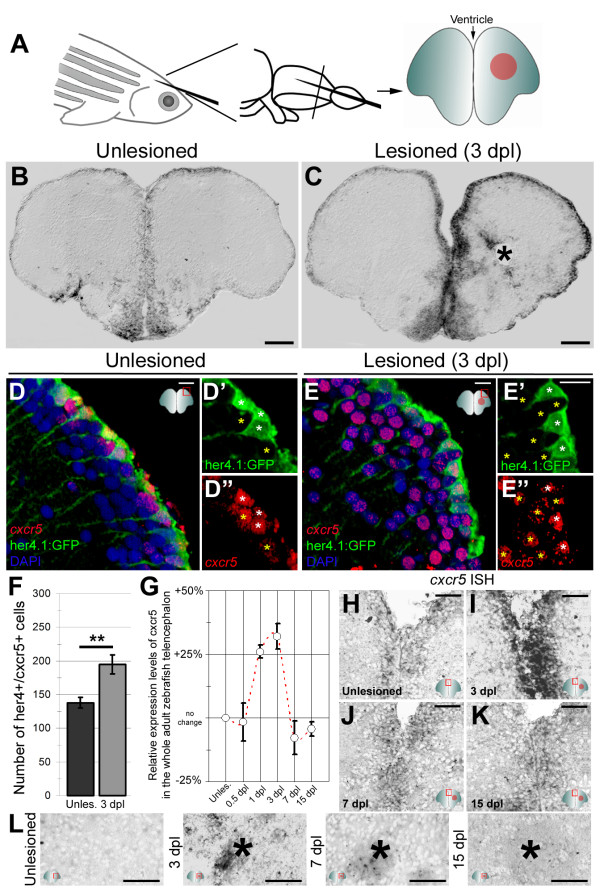
In situ hybridization on cross sections of the telencephalon (Figure 1A) showed that cxcr5 is expressed in cells along the ventricular zone and close to the ventricular surface in the homeostatic state (Figure 1B). A stab lesion enhances cxcr5 expression in the ventricular and periventricular zone, predominantly in the lesioned hemisphere (Figure 1C). We also observed cxcr5 expression in a small number of cells close to the lesion site (Figure 1C, asterisk). These results indicate that cxcr5 is present in the adult zebrafish telencephalon during homeostasis, and its expression is significantly enhanced at the ventricular region after traumatic injury, suggesting that cxcr5 may be involved in adult neurogenesis and regenerative response of the telencephalon.
Figure 1.
cxcr5 is expressed in radial glial cells (RGCs) and neurons in the adult zebrafish telencephalon. (A) Schematic representation of an adult zebrafish telencephalon. A stab lesion is performed in one hemisphere (red circle on the cross section scheme). (B)cxcr5 is expressed along the ventricular region in the unlesioned telencephalon. (C)cxcr5 expression after a lesion (asterisk) is stronger in the lesioned hemisphere along the ventricular region. (D)cxcr5 fluorescent in situ hybridization (FISH) coupled to green fluorescent protein (GFP) immunohistochemistry in Tg(her4.1:GFP) transgenics in unlesioned the adult zebrafish telencephalon; counterstained with 4,6-diamidino-2-phenylindole (DAPI). (D’) Individual channel for her4.1:GFP. (D”) Individual channel for cxcr5. Radial glial cells (white asterisks) and periventricular cells (yellow asterisks) express cxcr5. (E)cxcr5 FISH coupled to GFP staining in Tg(her4.1:GFP) transgenics in the 3 day post-lesion adult zebrafish telencephalon; counterstained with DAPI. (E’) Individual channel for her4.1:GFP. (E”) Individual channel for cxcr5. Radial glial cells (white asterisks) and periventricular cells (yellow asterisks) express cxcr5. Note the number of cxcr5-positive periventricular cells increased in comparison to the unlesioned region. (F) Graph indicating the number of her4-cxcr5 double-positive cells before and after inducing the lesion. (G) Quantitative real-time PCR analysis for cxcr5 expression at different time points after the lesion. (H-K) Time-course cxcr5 in situ hybridization analyses on the unlesioned region (H), 3 dpl (I), 7 dpl (J) and 15 dpl (K) telencephalons. (L)cxcr5 expression around the lesion site. Lesion site is denoted by an asterisk; n ≥ 3 telencephalons for every analysis. Scale bars 50 μm (B, C, H-L), and 10 μm (D-E”).
The expression of cxcr5 cells along the ventricle suggests that these are progenitor cells [1,2,6]. Therefore, we analyzed whether the cxcr5 gene is expressed in her4.1-positive RGCs, which act as neurogenic progenitors during adult neurogenesis and regeneration [3,12,15,31]. We performed fluorescent in situ hybridization (FISH) for cxcr5 coupled to immunohistochemistry to detect the reporter activity in RGCs of the transgenic line Tg(her4.1:GFP)[32]. In the unlesioned (Figure 1D-D”) and lesioned (Figure 1E-E”) telencephalons, cxcr5 is expressed in the her4.1-positive RGCs located along the dorsal ventricle (white asterisks) and the total number of cxcr5-positive radial glial cells increase significantly after inducing the lesion (Figure 1F). We also observed cxcr5 expression in her4.1-negative cells (Figure 1D-E”, yellow asterisks), which are located close to the ventricle in the periventricular region, suggesting that cxcr5 is also expressed in neurons. Based on our quantitative real-time PCR (qRT-PCR) analyses, we found that upregulated cxcr5 expression reduces back to unlesioned levels after 3 dpl (Figure 1G), as the expression pattern of cxcr5 at 7 dpl and 15 dpl resembles the unlesioned brains (Figure 1H-K). Additionally, the expression of cxcr5 around the lesion site diminishes after 7 dpl (Figure 1L). These results suggest that cxcr5 might have a transient early role during the regeneration response of the adult zebrafish telencephalon.
The RGCs of the adult zebrafish telencephalon are the proliferative neurogenic progenitor cells during adult neurogenesis and regeneration [1,2,6]. Additionally, from in situ hybridizations and BrdU immunostainings, we observed that cxcr5 expression overlaps with the ventricular cell proliferation (Additional file 1: Figure S1). Thus, to determine whether cxcr5 is expressed in proliferating RGCs, we treated the Tg(her4.1:GFP) animals with BrdU for 6 hours before sacrifice (Figure 2A) and performed cxcr5 FISH coupled to double immunohistochemistry for her4.1:GFP and BrdU (Figure 2B-E). In the unlesioned telencephalons, there are few proliferating cells at the ventricular region, reflecting the constitutive neurogenesis of the adult zebrafish brain (Figure 2B). We found that in unlesioned telencephalons, BrdU-positive RGCs express cxcr5 (Figure 2C, white asterisk). After injury, the proliferating RGCs dramatically increase in number (Figure 2D), and they were also cxcr5-positive (Figure 2E, white asterisks). These results indicate that in both the unlesioned and the lesioned telencephalons, BrdU-labeled her4.1-positive RGCs express cxcr5, suggesting a role of cxcr5 in proliferating radial glial cells.
Figure 2.
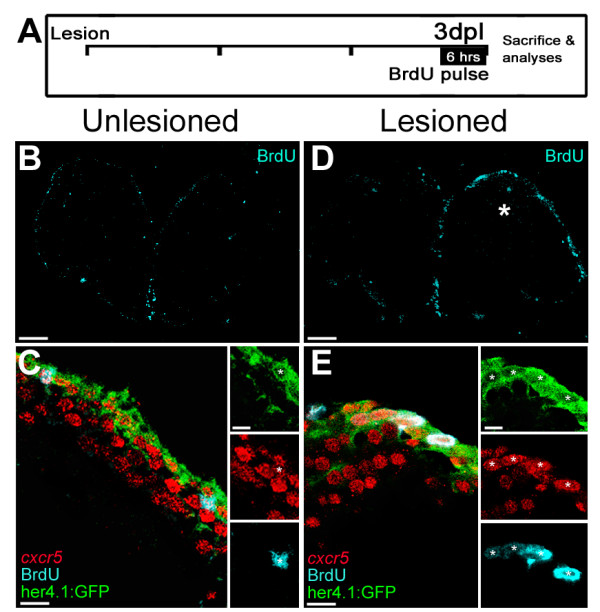
cxcr5 is expressed in proliferating radial glial cells (RGCs). (A) Scheme for experimental setup. At 3 days after a lesion or a sham operation, bromo-deoxyuridine (BrdU) is applied for 6 hours before sacrificing the animals. (B) BrdU immunohistochemistry on the unlesioned (sham-operated) adult zebrafish telencephalon section showing the proliferating cells. (C)cxcr5 fluorescent in situ hybridization (FISH) coupled to BrdU and GFP immunohistochemistry on unlesioned Tg(her4.1:GFP) transgenics. Insets show single channel images. BrdU-positive RGCs express cxcr5 (white asterisk). (D) BrdU immunohistochemistry on the 3 days post-lesion (dpl) adult zebrafish telencephalon section. Asterisk indicates the lesion site. (E)cxcr5 FISH coupled to BrdU and GFP immunohistochemistry on 3 dpl Tg(her4.1:GFP) transgenics. Insets show single channel images. BrdU-positive RGCs increase in number and they express cxcr5 (white asterisks). Scale bars 50 μm in B and D, and 10 μm in C and E; n = 3 telencephalons.
ISH for cxcr5 showed that in addition to the RGCs, cxcr5 was also expressed close to the ventricular surface in cells that are not radial glia (Figure 1, Figure 2C, 2E). To test whether those cxcr5-positive cells are neurons, we used the transgenic line Tg(HuC:GFP)[33] in combination with the FISH for cxcr5. We observed cxcr5 expression in HuC-positive neuronal cells in unlesioned (Additional file 2: Figure S2A) and lesioned telencephalons at 3 days post-lesion (dpl) (Additional file 2: Figure S2B), and a significant increase in the number of cxcr5-positive neurons upon lesion (Additional file 2: Figure S2C). The expression of cxcr5 in non-glial periventricular cells (Figure 1, Figure 2) suggests that cxcr5 is present in precursor cells that have committed to differentiate into neurons. These cells express the transcription factor NeuroD [34,35]. To determine whether the cxcr5 is present in these cells, we performed a chromogenic in situ for cxcr5 and immunohistochemistry for NeuroD (Additional file 2: Figure S2C, D). We found that cxcr5 can be detected in the vast majority of NeuroD cells in the unlesioned and lesioned telencephalons (white asterisks in Additional file 2: Figure S2C and S2D), indicating that cxcr5 is also expressed in differentiating neurons and thus might have a role in neuronal differentiation.
Cxcr5 is expressed in mature B cells and in a subset of T helper memory cells in the immune system of mammals [26] and traumatic injury in the vertebrate brain induces acute inflammation that includes these cells [36-39], suggesting that cxcr5 might be expressed in immune cells in the telencephalon. To address this, we performed ISH for cxcr5 and L-Plastin (a marker for microglia and leukocytes, [40] and GFP immunohistochemistry on telencephlons of Tg(her4.1:GFP) transgenic fish before and after lesioning. In unlesioned and lesioned telencephalons, we found that L-Plastin-positive cells do not express cxcr5 (Additional file 3: Figure S3), indicating that cxcr5 is not expressed in leukocytes in the adult zebrafish telencephalon. These findings suggest that cxcr5 is present only in the RGCs and the neurons in the adult zebrafish telencephalon.
cxcr5 regulates the proliferative capacity of RGCs and neuronal differentiation after injury
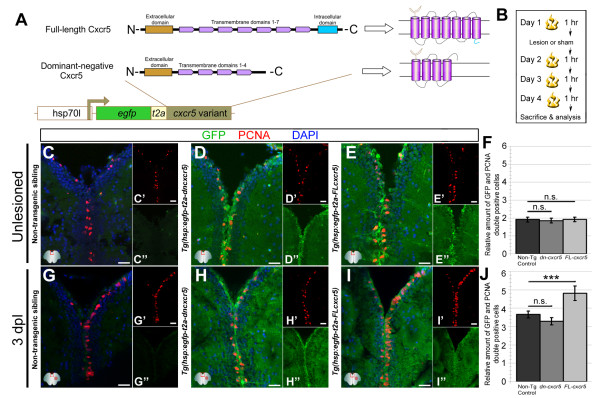
Characterization of cxcr5-expressing cells indicated that cxcr5 is expressed in proliferating RGCs and differentiating neurons of the unlesioned and lesioned adult zebrafish telencephalons (Figures 1 and 2). These results suggested that cxcr5 may either be required for initiation of cell proliferation in RGCs (instructive function) or may be a part of a competency mechanism that imposes a proliferative potential to the RGCs (permissive function). In order to analyze the function of cxcr5 in adult zebrafish telencephalon, we generated two transgenic lines Tg(hsp:EGFP-T2A-dncxcr5) and Tg(hsp:EGFP-T2A-FL-cxcr5), which express a dominant-negative and a full-length variant of the gene, respectively (Figure 3A). Both of these transgenics are inducible and result in larval pigment migration phenotypes upon heat-shock (Additional file 4: Figure S4A-E). Overexpression of cxcr5-variants also results in alterations in the expression levels of genes known to be regulated by the cxcr5 signaling pathway (Additional file 4: Figure S4F), suggesting that the two lines are functional. We found ubiquitous expression of the transgene in the embryos (data not shown), while the sections from the telencephalon of the heat-shocked transgenic animals (Additional file 4: Figure S4G) showed GFP-labeled cells mostly along the ventricular cells (Additional file 4: Figure S4H-J). This is either due to a possible relative silencing of the transgene in periventricular and parenchymal cells, or a positional effect of an enhancer influencing the transgene expression in the adult brain. However, the stronger expression of the transgene in the ventricular cells was advantageous for our study because it allowed us to misexpress cxcr5 variants, particularly in the ventricular cells containing the RGCs. We also determined that the expression of the transgene after the heat-shock returns back to the levels comparable to the non-heat-shocked animals after 24 hours (Additional file 4: Figure S4K-N) suggesting that the effect of transgenic misexpression of cxcr5 is observed only during the heat-shock period without a profound latency.
Figure 3.
cxcr5 is sufficient to increase ventricular cell proliferation after injury in the adult zebrafish telencephalon. (A) Cxcr5 is a seven-span transmembrane protein with an extracellular receptor domain, seven transmembrane domains and a C-terminus intracellular domain. We generated full-length and dominant-negative versions of Cxcr5. Both variants are inserted into a transgenesis cassette containing the enhanced green fluorescent protein (EGFP) reporter and self-cleaving T2A. The whole cassette is expressed under heat-inducible hsp70l promoter. (B) Heat shock scheme. Four heat shocks, three of which are after the lesion or sham operation, were given before sacrifice and analysis. (C) Proliferating cell nuclear antigen (PCNA) and green fluorescent protein (GFP) immunohistochemistry (IHC) in telencephalons of unlesioned (sham-operated) non-transgenic animals. C’ and C” are PCNA and GFP. (D) PCNA and GFP IHC in telencephalons of unlesioned (sham-operated) Tg(hsp:egfp-t2a-dncxcr5) animals. D’ and D” are PCNA and GFP. (E) PCNA and GFP IHC in telencephalons of unlesioned (sham-operated) Tg(hsp:egfp-t2a-FLcxcr5) animals. E’ and E” are PCNA and GFP. (F) Quantification graph for the relative amounts of GFP and PCNA double-positive cells in unlesioned telencephalons, where the transgenic misexpression of cxcr5 does not effect cell proliferation. (G) PCNA and GFP IHC in telencephalons of 3 days post-lesion (dpl) non-transgenic animals. G’ and G” are PCNA and GFP. (H) PCNA and GFP IHC in telencephalons of 3 dpl Tg(hsp:egfp-t2a-dncxcr5) animals. H’ and H” are PCNA and GFP. (I) PCNA and GFP IHC in telencephalons of 3 dpl Tg(hsp:egfp-t2a-FLcxcr5) animals. I’ and I” are PCNA and GFP. (J) Quantification graph for the relative amounts of GFP and PCNA double-positive in 3 dpl telencephalons, where transgenic misexpression of the full-length cxcr5 increases but the dominant negative variant does not affect the ventricular cell proliferation. Scale bars 25 μm; n = 4 telencephalons for each set of analyses.
Since cxcr5 is expressed in proliferating RGCs, we tested whether cxcr5 has a role in proliferation of these cells in the telencephalon by using the two transgenic conditional misexpression lines: Tg(hsp:EGFP-T2A-dncxcr5) and Tg(hsp:EGFP-T2A-FL-cxcr5). We analyzed proliferating cells in the homeostatic and regenerating telencephalons after one heat shock per day over four days (one before and three after the lesion or sham operations) (Figure 3B). In unlesioned animals, proliferating cell nuclear antigen (PCNA) and GFP immunohistochemistry (Figure 3C-E”) showed that in comparison to the non-transgenic siblings (Figure 3C), transgenic fish for both variants of cxcr5 show no significant difference in the number of proliferating cells at the ventricle (Figure 3D-F). Following injury (Figure 3G-I), overexpression of full-length cxcr5 significantly increased the ventricular cell proliferation (25.1 ± 8.3%; mean ± SD) (Figure 3I,J). However, overexpression of the dominant-negative cxcr5 did not alter the number of PCNA-positive cells (Figure 3H,J). These results indicate that cxcr5 is sufficient to promote cell proliferation of radial glial cells but may not be required, suggesting that the lack of alteration in cell proliferation in the dominant negative transgenic line might be an indication of the specificity of the effect after overexpression of the full length cxcr5. It is therefore possible that whether or not an RGC expressing cxcr5 proliferates, depends not solely on cxcr5 but also on other contextual factors (such as hypothetically, the prevalence of mitogenic factors, alleviation of suppressive mechanisms on neurogenesis, or other molecular programs activated upon the need for newborn neurons). Thus, we suggest a permissive role for cxcr5 in RGC proliferation. This hypothesis is supported by our observations that when the full-length cxcr5 is overexpressed, the proliferation of the RGCs increases only after injury but not during homeostasis. In such a case, even though the cxcr5 overexpression may endow RGCs the ability to proliferate more, the prevailing signaling in unlesioned conditions is not sufficient to enhance the cell proliferation. However, after an injury, the telencephalon induces molecular programs that ultimately converge on the enhanced proliferation of the RGCs [3]. Consequently, when cxcr5 is overexpressed following a lesion, it can increase the cell proliferation because the competent RGCs will be influenced by the injury-dependent mitogenic signals. Alternatively, the temporal dynamics of constitutive neurogenesis may be different, possibly slower, than that of the injury conditions, and this may prevent detection of significant changes in cell proliferation and neurogenesis in unlesioned brains, due to the time window we have used in our analyses. Overall, given that we observed cxcr5 expression in proliferating RGCs and also showed that when overexpressed, cxcr5 can enhance the proliferation of these cells context-dependently, we argue that cxcr5 might be a permissive but not an instructive factor involved in maintaining the regenerative neurogenic capacity of the radial glial cells, by priming them for proliferation as cxcr5 expression is sufficient but not required for proliferation of ventricular progenitors.
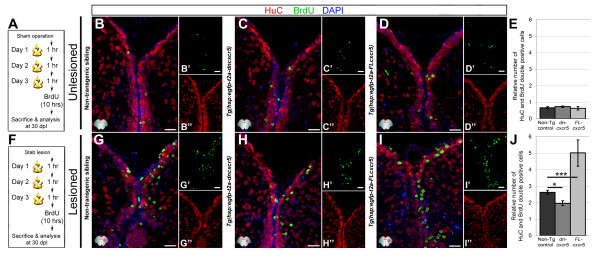
We found that the cxcr5 is expressed in differentiating neurons in addition to RGCs, which suggested a role for this gene in neurons. Thus, we hypothesized that cxcr5 might be involved in production of neurons from the RGCs. To address this question, we performed a BrdU pulse-chase experiment using the two transgenic lines: Tg(hsp:EGFP-T2A-dncxcr5) and Tg(hsp:EGFP-T2A-FL-cxcr5) and applied four heat shocks on consecutive days, three of which were after the sham or the lesion operation. We treated the animals with BrdU at 3 dpl or 3 days post-sham (dps) and sacrificed the fish 30 days after BrdU treatment (Figure 4A). When we performed HuC (neuronal marker) and BrdU immunohistochemistry (IHC) on unlesioned non-transgenic animals (Figure 4B), Tg(hsp:EGFP-T2A-dncxcr5) (Figure 4C) and Tg(hsp:EGFP-T2A-FL-cxcr5) (Figure 4D), we found that the overall levels of neurogenesis remain unchanged upon transgenic overexpression in the unlesioned animals (Figure 4E). However, after the lesion and BrdU treatment (Figure 4F), in comparison to the non-transgenic animals (Figure 4G), overexpression of the dominant negative cxcr5 variant significantly reduces (by 33.4 ± 5.2%, Figure 4H, J), while overexpression of full-length cxcr5 significantly increases the number of newborn neurons (by 47.7 ± 9.4%, Figure 4I,J). These results indicate that cxcr5 is required and sufficient for production of newborn neurons only after injury in the adult zebrafish telencephalon.
Figure 4.
cxcr5 is required and sufficient for regenerative neurogenesis. (A) Heat shock and BrdU scheme for neurogenesis assay in the unlesioned telencephalon. After a sham operation, three daily heat shocks were given before a 10-hour BrdU pulse and sacrifice at 30 days after the sham operation. (B-D) HuC and BrdU immunohistochemistry (IHC) and 4,6-diamidino-2-phenylindole (DAPI) counterstaining on unlesioned telencephalons from non-transgenic (B), Tg(hsp:egfp-t2a-dncxcr5)(C) and Tg(hsp:egfp-t2a-FLcxcr5)(D) animals. Primed and double-primed images are single channels for BrdU and HuC. (E) Quantification graph for relative numbers of HuC and BrdU double positive cells (newborn neurons) in unlesioned telencephalons. Transgenic misexpression of cxcr5 does not alter the constitutive levels of neurogenesis in the adult zebrafish telencephalon. (F) Heat shock and BrdU scheme for neurogenesis assay in lesioned telencephalons. After the lesion, three daily heat shocks were given before a 10-hour BrdU pulse and sacrifice at 30 days after lesioning. (G-I) HuC and BrdU IHC and DAPI counterstaining on unlesioned telencephalons from non-transgenic (G), Tg(hsp:egfp-t2a-dncxcr5)(H) and Tg(hsp:egfp-t2a-FLcxcr5)(I) animals. Primed and double-primed images are single channels for BrdU and HuC. (J) Quantification graph for relative numbers of newborn neurons in lesioned telencephalons. Transgenic misexpression of dominant negative variant of cxcr5 significantly reduces, and full-length cxcr5 significantly increases, the number of newborn neurons after lesioning in the adult zebrafish telencephalon. Scale bars 25 μm; n = 4 telencephalons for each analysis.
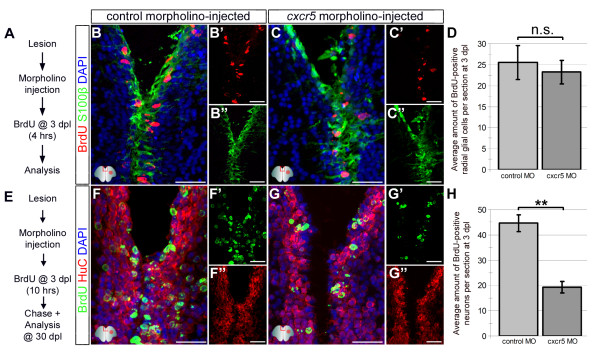
We found that following a lesion in the adult zebrafish brain, overexpression of the dominant negative form of cxcr5 (dn-cxcr5) does not alter ventricular cell proliferation (Figure 3J) but later reduces regenerative neurogenesis (Figure 4J). These results suggest that either the expression levels of the dn-cxcr5 was not sufficient to exert an effect on cell proliferation or cxcr5 was not required for ventricular cell proliferation. To distinguish between these two possibilities, we took advantage of cerebroventricular microinjection (CVMI) of morpholinos [30] as an efficient tool for gene knockdown. We designed and injected cxcr5 antisense morpholinos to the adult telencephalon after lesioning and analyzed the proliferation levels of the radial glial cells (RGC) (Figure 5A). These morpholinos are functional and the knockdown phenotypes can be rescued by cxcr5 mRNA (Additional file 5: Figure S5). We found that the knockdown of cxcr5 after lesion did not alter the levels of RGC proliferation (Figure 5B-D), which was consistent with the outcome of the overexpression of dn-cxcr5 (Figure 3J). When we analyzed the neurogenic response after lesioning and cerebroventricular microinjection (CVMI) of morpholinos (Figure 5E), we observed that the knockdown of cxcr5 reduced the regenerative neurogenesis significantly (55.3 ± 7.1%, Figure 5F-H), confirming the results of overexpression of dn-cxcr5 (Figure 4J). Additionally, we found that misexpression of cxcr5 variants did not affect the cell survival before and after lesioning (Additional file 6: Figure S6), hence, the reduction in the regenerative neurogenesis response is not due to cell death. These findings indicate that cxcr5 is not required for ventricular cell proliferation but is required for the regenerative neurogenesis in the adult zebrafish telencephalon.
Figure 5.
Knocking down cxcr5 with cerebroventricular microinjection (CVMI) results in reduced regenerative neurogenesis but not radial glial cell proliferation. (A) Lesion, CVMI and BrdU treatment scheme. (B) BrdU and S100β immunohistochemistry (IHC) with 4,6-diamidino-2-phenylindole (DAPI) counterstaining on control morpholino-injected brains. (B’) BrdU channel. (B”) S100β channel. (C) BrdU and S100β IHC with DAPI counterstaining on cxcr5 antisense morpholino-injected brains. (C’) BrdU channel. (C”) S100β channel. (D) Graph showing the average number of proliferating glial cells. Knocking down cxcr5 does not alter the levels of proliferating radial glial cells. (E) Lesion, CVMI, BrdU treatment and pulse-chase scheme. (F) BrdU and HuC IHC with DAPI counterstaining on control morpholino-injected brains. (F’) BrdU channel. (F”) HuC channel. (G) BrdU and HuC IHC with DAPI counterstaining on cxcr5 antisense morpholino-injected brains. (G’) BrdU channel. (G”) HuC channel. (H) Graph showing the average number of newborn neurons. Knocking down cxcr5 significantly reduces the levels of regenerative neurogenesis.
We also detected cxcr5 in non-proliferating RGCs (Figure 3). This suggests that either the expression of cxcr5 in non-proliferating glia reflects a physiological stage of the RGC at which it is competent to proliferate but is not actually in the cell cycle, or cxcr5 has a proliferation-independent role in those glial cells. In adult brains, glial cells are known to be heterogeneous in terms of their proliferation and progenitor characteristics [1,2,6,12,15]. Therefore, cxcr5 may either be expressed in glial cells with different inherent propensities to become neurogenic and proliferative or it can be a part of the mechanism that establishes the functional heterogeneity. It is not clear whether the constitutive neurogenesis and the injury-induced neurogenesis use the same progenitor cells to form neurons by simply enhancing the rate of cell divisions, or whether there are reserve stem cells that are activated after injury. Given that we observed an effect of cxcr5 misexpression on proliferation and neurogenesis only after the injury, it is possible that the roles of cxcr5 we identified are associated with the regenerative response. This suggests that the expression of cxcr5 in unlesioned brains may either be relevant to a permissive proliferative and neurogenic capacity of the RGCs or be a physiological function independent of RGC proliferation. However, it is currently not possible to distinguish between these alternatives with our current level of understanding. Long-term single-cell lineage tracing experiments with a cxcr5-positive non-proliferative RGC will help us understand whether under homeostatic conditions, cxcr5-positive glial cells initiate cell division and form neurons, or whether they are required for an as-yet unidentified role in the glial cells.
The dominant negative variant of cxcr5 did not affect cell proliferation in unlesioned or lesioned telencephalons. Similarly, Tg(hsp:EGFP-T2A-dncxcr5) animals did not alter their neurogenesis levels after heat shocks in unlesioned conditions. However, when overexpressed, this variant reduced the production of neurons after the lesion. These findings pose four possible explanations. First, cxcr5 may not be required for cell proliferation but may be required for differentiation of RGCs to neurons. Second, the different effects of dominant-negative cxcr5 could be due to the expression levels of the Tg(hsp:EGFP-T2A-dncxcr5) transgenic cassette. The dose of the transgene expression might not be sufficient to compete with the endogenous levels of cxcr5, which could be adequate to fulfil the cognate function in the cells and obscure any loss-of-function effect. However, we believe that this option is unlikely as by injecting cxcr5 morpholinos using CVMI to the adult fish brain, we ruled out the dosage effect. Third, cxcr5 may have a redundant function in RGC proliferation and therefore loss of cxcr5 function can be compensated. Finally, the transgene may not be expressed in all cxcr5-expressing cells. This situation would cause limitations if cxcr5 had a paracrine effect on other cells. Thus, inability to target all cxcr5 cells would cause a hypomorphic effect, which might hinder an overt phenotype. Identifying other factors relevant to radial glial cell proliferation and neurogenesis in adult fish brain and transgenic misexpression studies using endogenous cxcr5 promoter may be helpful in the future to address such reservations. Nevertheless, our results currently indicate that cxcr5 is functionally involved in regulation of progenitor cell proliferation and neuronal differentiation only after injury in the adult zebrafish telencephalon (Figure 6).
Figure 6.
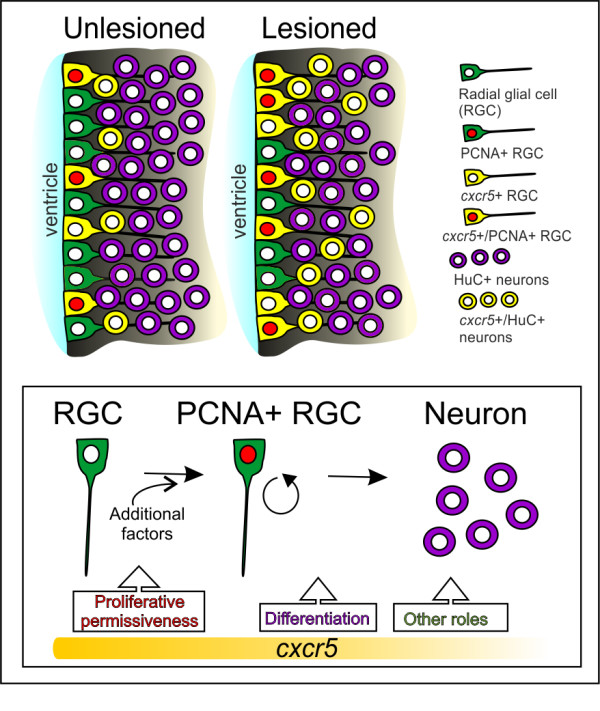
Summary and working model for cxcr5 expression and function in unlesioned and lesioned adult zebrafish telencephalons. In unlesioned adult zebrafish telencephalons, cxcr5 is expressed in proliferating and non-proliferating radial glial cells (RGCs), as well as neurons in the periventricular region. After a lesion, proliferating cxcr5-positive RGCs and cxcr5-positive periventricular neurons increase in number. cxcr5 expression is strongest at the ventricular surface and weakens towards the deeper layers of parenchyma. Based on the transgenic misexpression of the dominant negative and the full-length variants of cxcr5 gene in the ventricular zone cells, we propose that cxcr5 is a permissive cue for proliferative competency of the RGCs. cxcr5 is sufficient to enhance proliferation of RGCs not before but after injury, possibly due to additional injury-induced factors required in combination with cxcr5 to enhance cell proliferation. Based on the expression of cxcr5 in differentiating neurons and long-term neurogenesis experiments upon transgenic misexpression, we suggest that cxcr5 is also required for differentiation of RGCs to neurons. Since cxcr5 is also expressed in neurons, it might have other roles in neurons, such as migration, which are yet to be identified.
Conclusions
We demonstrated that cxcr5 is expressed at different physiological states of glial cells - during quiescence, proliferation and differentiation into neurons. We also demonstrated that cxcr5 function is biologically relevant to the proliferation of the neurogenic progenitor cells and the regenerative neurogenesis response of the adult zebrafish brain (Figure 6). Therefore, we present cxcr5 as an intriguing factor relevant to the proliferative status and differentiation capacity of the RGCs after lesion in the adult zebrafish telencephalon. In mammals, after traumatic lesions in the central nervous system, glial cells initiate proliferation but cannot replenish the lost neurons [37,41-47]. One of the detrimental cues for neurogenesis is believed to be the acute inflammation [38,48-50]. Given that zebrafish can regenerate traumatic injuries in the brain and the fish RGCs expresses cxcr5, which we showed to be relevant to RGC proliferation and neuronal differentiation, we believe that cxcr5 can be an interesting candidate that may be involved in the permissive link between the immune and the nervous systems in zebrafish. Further studies on the role of cxcr5 in mediating neuronal replenishment have the potential to produce clinical ramifications in efforts for regenerative therapeutic applications for human neurological disorders or acute injuries.
Methods
Ethics statement
All animal experiments were carried out in accordance with the recommendations and permits of the Landesdirektion Dresden (Permit numbers: AZ 24D-9168.11-1/2008-2, 4 and 14). All surgery was performed under anesthesia, and all efforts were made to minimize suffering. Fish were raised and kept at 28°C under a 14-hour light, 10 hours dark cycle, and fed with brine shrimp artemia daily as described [51]. Wildtype experimental animals were adult fish from the gol-b1 line with AB genetic background [52].
Tissue preparation and cryosectioning
Fish were sacrificed with 1% 3-amino-benzoic acid ethyl ester (MESAB, Sigma, Schnelldorf, Germany) and the skull was opened at the hindbrain area with sharp forceps to enable a better fixation of the tissue. The skull was partly removed without damaging or relocating the telencephalon. The head was cut off behind the pectoral fins with a scalpel and the brains were fixed in 2% paraformaldehyde (PFA, Sigma, Schnelldorf, Germany) at 4°C overnight. Heads were washed twice with 0.1 M phosphate buffer, transferred in 20% sucrose-ethylene-diamine-tetra-acetic acid (EDTA, Sigma, Schnelldorf, Germany) solution (in 0.1 M phosphate buffer, pH: 7.5) and incubated overnight at 4°C. Brains were then frozen in 7.5% gelatin/20% sucrose in 0.1 M phosphate buffer and cryosectioned at 14-μm thickness using a cryostat. Heads were sectioned for the telencephalon between olfactory bulb and diencephalon with sequential distribution to three slides, therefore one slide contained every third section. Sections were stored at −20°C.
In situ hybridization, immunohistochemistry, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining
A fragment of the zebrafish cxcr5 gene [NCBI Accession ID: XM_003200482; Ensembl Accession ID: ENSDARG00000010514] was isolated from 2 days post-fertilization (dpf) embryonic total cDNA using the following primers: forward primer: 5′-TACATCTGGCAGTGGCTGAC-3′ and reverse primer: 5′-CAGCAGGTCTCTTCGGAAAC-3′. The amplicon (726 bp) was subcloned into pGEM T-Easy (Promega). A DIG or fluorescein-labeled mRNA probe was generated using an in vitro transcription kit (Roche, Mannheim, Germany). In situ hybridization was performed as described [12,53]. To combine ISH with IHC, the primary antibody for the immunohistochemistry was added with the anti-DIG or anti-Fluorescein antibody. After the in situ staining reaction, the secondary antibody was applied. IHC was performed as described [3,8,11,12,30]. Antigen retrieval was performed with Tris–HCl buffer, pH: 8.0 at 100°C for 5 minutes, 10 mM sodium citrate buffer, pH: 6.0 at 85°C for 6 minutes or 2 M HCl for 20 minutes at 37°C. The following antibodies were used: primary antibodies: anti-BrdU (rat IgG2a, Serotec, Düsseldorf, Germany, 1:500), anti-GFP (rabbit, Molecular Probes, Darmstadt, Germany, 1:1,000); anti-GFP (chicken, Abcam, Cambridge, UK, 1:3,000); anti-HuC/D (mouse IgG2b, Molecular Probes, 1:50), anti-neuroD (mouse IgG2a, Abcam, 1:750), Anti-Lcp1 (rabbit, 1:10,000, gift from Michael Redd, University of Utah, USA), anti-PCNA (mouse IgG2a, Dako Cyto, Hamburg, Germany, 1:500), anti-DIG (sheep, Roche, Mannheim, Germany, 1:4,000), anti-fluorescein (sheep, Roche, 1:5,000); secondary antibodies: goat anti-rat, goat anti-rabbit, goat anti-mouse (IgG, IgG2a, IgG2b), goat anti-chicken (coupled to Alexa-488, Alexa-555; Alexa-633, Molecular Probes, 1:500). TUNEL staining was performed using ApopTag® Red in situ apoptosis detection kit (Millipore, Darmstadt, Germany) according to the manufacturer’s instructions.
BrdU labeling
To label cells in S-phase of the cell cycle, zebrafish were immersed in 10 mM BrdU (Sigma) solution [8]. BrdU was dissolved in E3 medium, the zebrafish were incubated for desired periods indicated throughout the text.
Brain lesions and cerebroventricular microinjection (CVMI)
Fish were anesthetized using MESAB (0.01%). A 30-gauge cannula (Becton Dickinson Biosciences, Heidelberg, Germany), which corresponds to an outer diameter of 200 μm, was inserted through the fish nostril and stabbed along the rostro-caudal axis through the olfactory bulb until reaching the caudal part of the telencephalon as described [3]. CVMI was performed as described [30], fish were anesthetized using MESAB (0.01%). With the help of a fine needle (Becton Dickinson Biosciences) an incision was made through the skull to the cerebroventricular fluid (CVF) of the adult zebrafish above the optic tectum. This incision generates a slit through the skull with a diameter of approximately 200 μm without damaging the optic tectum underneath. Injected liquid disperses through the CVF rostrally to the forebrain. We generated con-trol (5′-CCTCTTACCTCAGTTACAATTTATA-3′) and translation-blocking antisense (5′-AACCCCTCTCCTCAAGGACTGGCAT-3′) morpholinos for cxcr5, tested these morpholinos in embryos for functionality and specificity (Additional file 5: Figure S5), and used them for CVMI into adult zebrafish brain.
qRT-PCR
Quantitative RT-PCR analyses were performed as described [53] using the primers listed in Additional file 7: Table S1.
Generation of conditional transgenic lines for cxcr5 gene variants
For full-length cxcr5 transgenics, complete open reading frame of the zebrafish cxcr5 gene [NCBI accession: XM_003200482, Cxcr5: 402 aminoacids] was used (Figure 5A). For the dominant negative variant, the 5th, 6th and the 7th transmembrane domains and the intracellular C-terminal domain were deleted (Δ243-402-Cxcr5 (dnCxcr5), 243 aminoacids) (Figure 5A). The transgenesis cassette contains two flanking Tol2 elements [54,55], and in between these are the heat-inducible zebrafish hsp70 promoter, the EGFP-T2A-dnCxcr5 or EGFP-T2A-FL-Cxcr5 gene sequence (See Figure 5). To integrate the construct into the zebrafish genome, transgenesis constructs were co-injected with Tol2 transposase mRNA to the one-cell stage of the embryos [54-57]. Founder animals were outcrossed to wild type strains and the progeny was screened for GFP fluorescence after single heat shock at early gastrulation stages. The fish carrying the transgenes heterozygously were outcrossed for two generations to segregate multiple insertions. The nomenclature for the transgenic animals are as follows: Tg(hsp:EGFP-T2A-dncxcr5) for the dominant-negative variant, and Tg(hsp:EGFP-T2A-FL-Cxcr5) for the full-length variant. We also used Tg(her4.1:GFP)[32] and Tg(HuC:GFP)[33] transgenic lines, which were kindly provided by the relevant laboratories.
Heat shock paradigm
Heat-shock experiments were performed on embryos and adults. Embryos (at least 50% epiboly stage) were transferred into 2 ml tubes. After removal of the excess embryo medium (E3, 5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2.2H2O, 0.33 mM MgSO4.7H2O, 0.0002% methylene blue, pH: 6.5), E3 preheated to 39°C was added in the tubes, which were incubated for 30 minutes at 38°C. After the heat shock, embryos were returned to Petri dishes filled with E3 and placed in a 28°C incubator. The embryos were analyzed with a fluorescent microscope at least 4 hours after the heat induction. Adult fish were transferred into the heat shock aquarium and received in total, four heat shocks on four consecutive days. Each day the water was heated to 37°C, then the heating elements switched off and the water passively cooled down to 28°C (this takes approximately 5 to 6 hours after the heat shock). The fish were analyzed with an Olympus MVX10 fluorescent microscope after anesthetization with MESAB (0.01%). Embryos were sorted and used for desired purposes.
Imaging, quantification and statistical analysis
Fluorescence images were taken using a structured illumination microscope (Zeiss Apotome AxioImager.Z1) or a laser scanning confocal microscope (Zeiss 510 META). Quantifications were performed in an unbiased manner by counting the absolute number of cells in a minimum of eight different sections of the brain of every fish. For simplicity, results are depicted as the relative amount of cells. For statistical analysis, two-way analysis of variance (ANOVA) and Tukey’s post hoc test or paired t-test were used after determining whether the sample datasets conform to a normal distribution. P-values are indicated as follows: *P ≤ 0.05, **P ≤ 0.005, ***P ≤ 0.001. The number of fish used for every experiment is indicated in the corresponding figure legends.
Abbreviations
ANOVA, analysis of variance; BCA-1, B-cell-attracting chemokine 1; BLR-1, Burkitt lymphoma receptor-1; BrdU, bromo-deoxyuridine; CVMI, cerebroventricular microinjection; cxcr5, chemokine (C-X-C motif) receptor 5; DAPI, 4,6-diamidino-2-phenylindole; dpf, days post-fertilization; dn, dominant negative; dpl, days post-lesion; dps, days post-sham; EDTA, ethylene-diamine-tetra-acetic acid; fl, full-length; EGFP, enhanced green fluorescent protein; FISH, fluorescent in situ hybridization; GFP, green fluorescent protein; her4.1, hairy-related 4.1 basic helix-loop-helix transcription factor; IHC, immunohistochemistry; ISH, in situ hybridization; MESAB, 3-amino-benzoic acid ethyl ester; PCNA, proliferating cell nuclear antigen; PFA, paraformaldehyde; qRT-PCR, quantitative real-time polymerase chain reaction; RGC, radial glial cell; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
Competing of interests
The authors declare that there is no competing of interest.
Authors’ contributions
CK conceived and designed the experiments; CK, SD and AM generated the transgenic animals; CK, SD, NK, AM, JB carried out the experiments; VK contributed to the initial screen; CK wrote the manuscript; CK and MB edited the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Figure S1. Title: cxcr5 expression is overlapping to the proliferating ventricular cells in adult zebrafish telencephalon. (A) Lesion and bromo-deoxyuridine (BrdU) treatment paradigm. Fish were treated with BrdU 3 days post lesion (dpl) for 6 hours before the sacrifice. (B) cxcr5 in situ hybridization on lesioned telencephalon. (B’) High magnification of the dorsomedial region in (B). (B”) High-magnification of the dorsolateral region in (B). (C) BrdU immunohistochemistry on lesioned telencephalon. (C’) High-magnification of the dorsomedial region in (C). (C”) High-magnification of the dorsolateral region in (C). (D) Merged image of (B) and (C). (D’) Merged image of (B’) and (C’). (D”) Merged image of (B”) and (C”).
Figure S2. Title: Neurons express cxcr5. (A) cxcr5 fluorescent in situ hybridization (FISH) coupled to HuC immunohistochemistry (IHC) on a section of unlesioned adult zebrafish telencephalon. Inset is magnified image. (B)cxcr5 FISH coupled to HuC IHC on a section of 3 days post lesion (dpl) adult zebrafish telencephalon. Inset is magnified image. cxcr5 is expressed in neurons before and after injury. (C) Quantification graph for cxcr5-expressing HuC-positive cells. (D)cxcr5 chromogenic in situ hybridization (ISH) coupled to NeuroD IHC in unlesioned telencephalon. NeuroD-positive differentiating neurons, which are several cell diameters away from the ventricle express cxcr5 (white asterisks). cxcr5 expression is weaker in NeuroD-positive neurons in comparison to cxcr5-positive cells closer to the ventricle (yellow asterisks). In a transition zone between strong NeuroD-positive (white asterisks) and NeuroD-negative cells (yellow asterisks), cells express NeuroD and cxcr5 weakly (blue asterisks). (E)cxcr5 chromogenic ISH coupled to NeuroD IHC in 3 dpl telencephalon. NeuroD-positive differentiating neurons are more numerous, dispersed inside the parenchyma distantly in comparison to unlesioned telencephalons and express cxcr5 (white asterisks). cxcr5 expression is weaker in NeuroD-positive neurons in comparison to cxcr5-positive cells closer to the ventricle (yellow asterisks). (F) Quantification graph for cxcr5-positive NeuroD-expressing cells. The number of NeuroD/cxcr5 double-positive cells increase upon injury in adult zebrafish telencephalon. Scale bars 25 μm; n = 4 telencephalons for every set of analyses.
Figure S3. Title: cxcr5 is not expressed in L-Plastin-positive cells in the adult zebrafish telencephalon. (A) cxcr5 in situ hybridization coupled to immunohistochemistry for L-Plastin (red, marking macrophages and microglia) and her4.1:green fluorescent protein (GFP) (green, marking the radial glial cells) in the unlesioned telencephalons. White asterisks indicate the L-Plastin cells. (A1-A3) Individual channels for L-Plastin, her4.1:GFP and cxcr5, respectively. (B) cxcr5 in situ hybridization coupled to immunohistochemistry for L-Plastin and her4.1:GFP in the lesioned telencephalons (dorsolateral region). White asterisks indicate the L-Plastin cells. (B1-B3) Individual channels for L-Plastin, her4.1:GFP and cxcr5, respectively. (C) cxcr5 in situ hybridization coupled to immunohistochemistry for L-Plastin and her4.1:GFP in the lesioned telencephalons (dorsomedial region). White asterisks indicate the L-Plastin cells. (C1-C3) Individual channels for L-Plastin, her4.1:GFP and cxcr5, respectively.
Figure S4. Title: Larval phenotypes upon cxcr5 misexpression; and transgene activity in the adult zebrafish telencephalon. (A) Cxcr5 is a seven-span transmembrane protein with an extracellular receptor domain, seven transmembrane domains and a C-terminus intracellular domain. We generated full-length and dominant-negative versions of Cxcr5. Dominant negative variant lacks the last three transmembrane domains and the intracellular domain. Both variants are inserted into a transgenesis cassette that contains the coding sequence for enhanced green fluorescent protein (EGFP) and self-cleaving T2A peptide. The whole cassette is expressed under heat-inducible hsp70l promoter. (B) Non-transgenic sibling at 3 days post-fertilization (dpf). (B1) High-magnification image of the yolk-sac in B. (B2) High-magnification image of the yolk-tube extension in B. (B3) High-magnification image of the ventral fin fold in B. (C)Tg(hsp:egfp-T2A-dncxcr5) transgenic animals at 3 dpf after two heat shocks in gastrula. (C1) High-magnification image of the yolk-sac in C. (C2) High-magnification image of the yolk-tube extension in C. (C3) High-magnification image of the ventral fin fold in C. (D)Tg(hsp:egfp-T2A-FLcxcr5) transgenic animals at 3 dpf after two heat shocks in gastrula. (D1) High-magnification image of the yolk-sac in D. (D2) High-magnification image of the yolk-tube extension in D. (D3) High-magnification image of the ventral fin fold in D. (E) Quantification graph for the number of pigment cells in different regions of the non-transgenic, Tg(hsp:egfp-T2A-dncxcr5) and Tg(hsp:egfp-T2A-FLcxcr5) larvae. Note that dominant negative variant of cxcr5 significantly reduces while full-length cxcr5 significantly increases the number of pigment cells in comparison to the non-transgenic siblings. (F) Quantitative real-time PCR analyses at 3 dpf after the misexpression of cxcr5 with two heat shocks at gastrula. (G) Heat-shock scheme for adult zebrafish telencephalon expression. (H) GFP and DAPI staining in heat-shocked non-transgenic animals. (I) GFP and DAPI staining in heat-shocked Tg(hsp:egfp-T2A-dncxcr5) animals. (J) GFP and DAPI staining in heat-shocked Tg(hsp:egfp-T2A-FLcxcr5) animals. (K-M) GFP IHC on Tg(hsp:egfp).(K) Olfactory bulb at 1 day after heat-shock. (L) Olfactory bulb at 2 days after heat-shock. (M) Telencephalon at 1 day after heat-shock. (N) Telencephalon at 2 days after heat-shock. GFP protein as a result of heat shock paradigm reduces significantly after 24 hours after heat shock.
Figure S5. Title: cxcr5 translation-blocking antisense morpholino is functional. (A) Uninjected 2 day post-fertilization (dpf) embryos. (B) Control morpholino-injected embryos show no morphological phenotypes at 2 dpf. (C)cxcr5 antisense morpholinoinjected embryos display severe anomalies in axial extension and head development. (D)cxcr5 mRNA rescues the knockdown phenotypes when co-injected with antisense morpholinos. (E) Quantitative real-time PCR analysis of genes regulated by cxcr5, upon control and antisense morpholino injections. All the genes tested are upregulated after knocking down cxcr5. Percentages in A-D represent the ratio of embryos with gross morphological defects to the whole clutch size.
Figure S6. Title: Misexpression of cxcr5 does not lead to cell death. (A-I) TUNEL staining to detect the apoptotic cells in non-transgenic siblings, Tg(hsp:egfpt2a-FLcxcr5) and Tg(hsp:egfp-t2a-dncxcr5) animals pre-lesion, 3 days post-lesion (dpl) and 15 dpl time points. Red nuclei indicate the apoptotic cells. (J) Quantification graph shows the number of apoptotic nuclei in the lesioned hemisphere. Misexpression of cxcr5 does not alter the levels of cell death. Two telencephalons were used for every time point.
Table S1. Title: qRT-PCR primers.
Contributor Information
Caghan Kizil, Email: caghan.kizil@crt-dresden.de.
Stefanie Dudczig, Email: stefanie.dudczig@biotec.tu-dresden.de.
Nikos Kyritsis, Email: nikos.kyritsis@biotec.tu-dresden.de.
Anja Machate, Email: anja.machate@biotec.tu-dresden.de.
Juliane Blaesche, Email: juliane.blaesche@biotec.tu-dresden.de.
Volker Kroehne, Email: volker.kroehne@biotec.tu-dresden.de.
Michael Brand, Email: michael.brand@biotec.tu-dresden.de.
Acknowledgements
We would like to thank to Dr Stefan Hans and Dr. Christopher L Antos for discussion and critical reading of the manuscript; and to Dr. Michael Redd for the L-Plastin antibody. This work was supported by research grants to MB from Deutsche Forschungsgemeinschaft SFB-655, European Union (ZF Health, EC Grant Agreement HEALTH-F4-2010-242048) and Technical University of Dresden.
References
- Chapouton P, Jagasia R, Bally-Cuif L. Adult neurogenesis in non-mammalian vertebrates. Bioessays. 2007. pp. 745–757. [DOI] [PubMed]
- Kaslin J, Ganz J, Brand M. Proliferation, neurogenesis and regeneration in the non-mammalian vertebrate brain. Philos Trans R Soc Lond B Biol Sci. 2008;363:101–122. doi: 10.1098/rstb.2006.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroehne V, Freudenreich D, Hans S, Kaslin J, Brand M. Regeneration of the adult zebrafish brain from neurogenic radial glia-type progenitors. Development. 2011;138:4831–4841. doi: 10.1242/dev.072587. [DOI] [PubMed] [Google Scholar]
- Baumgart EV, Barbosa JS, Bally-Cuif L, Gotz M, Ninkovic J. Stab wound injury of the zebrafish telencephalon: A model for comparative analysis of reactive gliosis. Glia. 2011;60:343–357. doi: 10.1002/glia.22269. [DOI] [PubMed] [Google Scholar]
- Kishimoto N, Shimizu K, Sawamoto K. Neuronal regeneration in a zebrafish model of adult brain injury. Dis Model Mech. 2011;5:200–209. doi: 10.1242/dmm.007336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizil C, Kaslin J, Kroehne V, Brand M. Adult neurogenesis and brain regeneration in zebrafish. Dev Neurobiol. 2012;72:429–461. doi: 10.1002/dneu.20918. [DOI] [PubMed] [Google Scholar]
- Adolf B, Chapouton P, Lam CS, Topp S, Tannhäuser B, Strähle U, Götz M, Bally-Cuif L. Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev Biol. 2006;295:278–293. doi: 10.1016/j.ydbio.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Grandel H, Kaslin J, Ganz J, Wenzel I, Brand M. Neural stem cells and neurogenesis in the adult zebrafish brain: origin, proliferation dynamics, migration and cell fate. Dev Biol. 2006;295:263–277. doi: 10.1016/j.ydbio.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Stigloher C, Chapouton P, Adolf B, Bally-Cuif L. Identification of neural progenitor pools by E(Spl) factors in the embryonic and adult brain. Brain Res Bull. 2008;75:266–273. doi: 10.1016/j.brainresbull.2007.10.032. [DOI] [PubMed] [Google Scholar]
- Zupanc GKH. Adult neurogenesis and neuronal regeneration in the brain of teleost fish. J Physiol. 2008;102:357–373. doi: 10.1016/j.jphysparis.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Kaslin J, Ganz J, Geffarth M, Grandel H, Hans S, Brand M. Stem cells in the adult zebrafish cerebellum: initiation and maintenance of a novel stem cell niche. J Neurosci. 2009;29:6142–6153. doi: 10.1523/JNEUROSCI.0072-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz J, Kaslin J, Hochmann S, Freudenreich D, Brand M. Heterogeneity and Fgf dependence of adult neural progenitors in the zebrafish telencephalon. Glia. 2010;58:1345–1363. doi: 10.1002/glia.21012. [DOI] [PubMed] [Google Scholar]
- Antos CL, Brand M. In: Regeneration of Organs and Appendages in Zebrafish: A Window into Underlying Control Mechanisms. Zheng Y, editor. Wiley Ltd, Chichester; 2010. Encyclopedia of Life Sciences. [Google Scholar]
- Rinkwitz S, Mourrain P, Becker TS. Zebrafish: an integrative system for neurogenomics and neurosciences. Prog Neurobiol. 2011;93:231–243. doi: 10.1016/j.pneurobio.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Rothenaigner I, Krecsmarik M, Hayes JA, Bahn B, Lepier A, Fortin G, Gotz M, Jagasia R, Bally-Cuif L. Clonal analysis by distinct viral vectors identifies bona fide neural stem cells in the adult zebrafish telencephalon and characterizes their division properties and fate. Development. 2011;138:1459–1469. doi: 10.1242/dev.058156. [DOI] [PubMed] [Google Scholar]
- Müller G, Lipp M. Signal transduction by the chemokine receptor CXCR5: structural requirements for g protein activation analyzed by chimeric CXCR1/CXCR5 molecules. Biol Chem. 2001;382:1387–1397. doi: 10.1515/BC.2001.171. [DOI] [PubMed] [Google Scholar]
- Wess J. G-Protein coupled receptors: molecular mechanisms involved in receptor activation and selectivity of G-protein recognition. FASEB J. 1997;11:346–354. [PubMed] [Google Scholar]
- Dobner T, Wolf I, Emrich T, Lipp M. Differentiation-specific expression of a novel G protein-coupled receptor from Burkitt’s lymphoma. Eur J Immunol. 1992;22:2795–2799. doi: 10.1002/eji.1830221107. [DOI] [PubMed] [Google Scholar]
- Xu QQ, Chang MX, Sun RH, Xiao FS, Nie P. The first non-mammalian CXCR5 in a teleost fish: molecular cloning and expression analysis in grass carp (Ctenopharyngodon idella) BMC Immunol. 2010;11:25. doi: 10.1186/1471-2172-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt’s lymphoma receptor-1. Nature. 1998;391:799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O. Chemokines: A new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/S1074-7613(00)80165-X. [DOI] [PubMed] [Google Scholar]
- Müller G, Höpken UE, Lipp M. The impact of CCR7 and CXCR5 on lymphoid organ development and systemic immunity. Immunol Rev. 2003;195:117–135. doi: 10.1034/j.1600-065X.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- Krumbholz M, Theil D, Cepok S, Hemmer B, Kivisäkk P, Ransohoff RM, Hofbauer M, Farina C, Derfuss T, Hartle C, Newcombe J, Hohlfeld R, Meinl E. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain. 2006;129:200–211. doi: 10.1093/brain/awh680. [DOI] [PubMed] [Google Scholar]
- Brunn A, Montesinos-Rongen M, Strack A, Reifenberger G, Mawrin C, Schaller C, Deckert M. Expression pattern and cellular sources of chemokines in primary central nervous system lymphoma. Acta Neuropathol. 2007;114:271–276. doi: 10.1007/s00401-007-0258-x. [DOI] [PubMed] [Google Scholar]
- Bürkle A, Niedermeier M, Schmitt-Gräff A, Wierda WG, Keating MJ, Burger JA. Overexpression of the CXCR5 chemokine receptor, and its ligand, CXCL13 in B-cell chronic lymphocytic leukemia. Blood. 2007;110:3316–3325. doi: 10.1182/blood-2007-05-089409. [DOI] [PubMed] [Google Scholar]
- Förster R, Emrich T, Kremer E, Lipp M. Expression of the G-protein-coupled receptor BLR1 defines mature, recirculating B Cells and a subset of t-helper memory cells. Blood. 1994;84:830–840. [PubMed] [Google Scholar]
- Förster R, Wolf I, Kaiser E, Lipp M. Selective expression of the murine homologue of the G-protein-coupled receptor BLR1 in B cell differentiation, B cell neoplasia and defined area of the cerebellum. Cell Mol Biol. 1994;40:381–387. [PubMed] [Google Scholar]
- Kaiser E, Förster R, Wolf I, Ebensperger C, Kuehl WM, Lipp M. The G protein-coupled receptor BLR1 is involved in murine B cell differentiation and is also expressed in neuronal tissues. Eur J Immunol. 1993;23:2532–2539. doi: 10.1002/eji.1830231023. [DOI] [PubMed] [Google Scholar]
- Weiss N, Deboux C, Chaverot N, Miller F, Baron-Van Evercooren A, Couraud PO, Cazaubon S. IL8 and CXCL13 are potent chemokines for the recruitment of human neural precursor cells across brain endothelial cells. J Neuroimmunol. 2010;223:131–134. doi: 10.1016/j.jneuroim.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Kizil C, Brand M. Cerebroventricular microinjection (CVMI) into adult zebrafish brain is an efficient misexpression method for forebrain ventricular cells. PLoS One. 2011;6:e27395. doi: 10.1371/journal.pone.0027395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- März M, Chapouton P, Diotel N, Vaillant C, Hesl B, Takamiya M, Lam CS, Kah O, Bally-Cuif L, Strähle U. Heterogeneity in progenitor cell subtypes in the ventricular zone of the zebrafish adult telencephalon. Glia. 2010;58:87–888. doi: 10.1002/glia.20971. [DOI] [PubMed] [Google Scholar]
- Yeo SY, Kim M, Kim HS, Huh TL, Chitnis AB. Fluorescent protein expression driven by her4 regulatory elements reveals the spatiotemporal pattern of Notch signaling in the nervous system of zebrafish embryos. Dev Biol. 2007;301:555–567. doi: 10.1016/j.ydbio.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Park HC, Kim CH, Bae YK, Yeo SY, Kim SH, Hong SK, Shin J, Yoo KW, Hibi M, Hirano T, Miki N, Chitnis AB, Huh TL. Analysis of upstream elements in the HuC promotor leads to the establishment of transgenic zebrafish with fluorescent neurons. Dev Biol. 2000;227:279–293. doi: 10.1006/dbio.2000.9898. [DOI] [PubMed] [Google Scholar]
- Korzh V, Sleptsova I, Liao J, He J, Gong Z. Expression of zebrafish bHLH genes ngn1 and nrd defines distinct stages of neuronal differentiation. Dev Dyn. 1998;213:92–104. doi: 10.1002/(SICI)1097-0177(199809)213:1<92::AID-AJA9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Yan RT, Ma W, Liang L, Wang SZ. bHLH genes and retinal cell fate specification. Mol Neurobiol. 2005;32:157–171. doi: 10.1385/MN:32:2:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls A, Shechter R, Schwartz M. The bright side of the glial scar in CNS repair. Nat Rev Neurosci. 2009;10:235–241. doi: 10.1038/nrn2591. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: The dual role of microglia. Neuroscience. 2009;158:1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- Russo I, Barlati S, Bosetti F. Effects of neuroinflammation on the regenerative capacity of brain stem cells. J Neurochem. 2011;116:947–956. doi: 10.1111/j.1471-4159.2010.07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redd M, Kelly G, Dunn G, Way M, Martin P. Imaging macrophage chemotaxis in vivo: studies of microtubule function in zebrafish wound inflammation. Cell Motil Cytoskel. 2006;63:415–422. doi: 10.1002/cm.20133. [DOI] [PubMed] [Google Scholar]
- Morrens J, Van Den Broeck W, Kempermann G. Glial cells in adult neurogenesis. Glia. 2011;60:159–174. doi: 10.1002/glia.21247. [DOI] [PubMed] [Google Scholar]
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Götz M. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc Natl Acad Sci USA. 2008;105:3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Emsley JG, Mitchell BD, Kempermann G, Macklis JD. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75:321–341. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G. Adult Neurogenesis 2: Stem Cells and Neuronal Development in the Adult Brain. 2. Oxford University Press, Oxford; 2010. [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje M, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Whitney NP, Eidem TM, Peng H, Huang Y, Zheng JC. Inflammation mediates varying effects in neurogenesis: relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem. 2009;108:1343–1359. doi: 10.1111/j.1471-4159.2009.05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M, Granato M, Nüsslein-Volhard C. In: Keeping and raising zebrafish. Nüsslein-Volhard C, Dahm R, editor. Oxford University Press, Oxford; 2002. Zebrafish: A practical approach; pp. 7–39. [Google Scholar]
- Streisinger G, Walker C, Dower N, Nauber D, Singer F. Production of clones of homozygous diploid zebra fish (Brachydanio rerio) Nature. 1981;291:293–296. doi: 10.1038/291293a0. [DOI] [PubMed] [Google Scholar]
- Kizil C, Otto GW, Geisler R, Nusslein-Volhard C, Antos CL. Simplet controls cell proliferation and gene transcription during zebrafish caudal fin regeneration. Dev Biol. 2009;325:329–340. doi: 10.1016/j.ydbio.2008.09.032. [DOI] [PubMed] [Google Scholar]
- Kawakami K, Shima A. Identification of the Tol2 transposase of the medaka fish Oryzias latipes that catalyzes excision of a nonautonomous Tol2 element in zebrafish Danio rerio. Gene. 1999;240:239–244. doi: 10.1016/S0378-1119(99)00444-8. [DOI] [PubMed] [Google Scholar]
- Kawakami K. Transposon Tools and Methods in Zebrafish. Dev Dyn. 2005;234:244–245. doi: 10.1002/dvdy.20516. [DOI] [PubMed] [Google Scholar]
- Hans S, Kaslin J, Freudenreich D, Brand M. Temporally-controlled site-specific recombination in zebrafish. PLoS One. 2009;4:e4640. doi: 10.1371/journal.pone.0004640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans S, Freudenreich D, Geffarth M, Kaslin J, Machate A, Brand M. Generation of a non-leaky heat shock-inducible Cre line for conditional Cre/Lox strategies in zebrafish. Dev Dyn. 2011;240:108–115. doi: 10.1002/dvdy.22497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Title: cxcr5 expression is overlapping to the proliferating ventricular cells in adult zebrafish telencephalon. (A) Lesion and bromo-deoxyuridine (BrdU) treatment paradigm. Fish were treated with BrdU 3 days post lesion (dpl) for 6 hours before the sacrifice. (B) cxcr5 in situ hybridization on lesioned telencephalon. (B’) High magnification of the dorsomedial region in (B). (B”) High-magnification of the dorsolateral region in (B). (C) BrdU immunohistochemistry on lesioned telencephalon. (C’) High-magnification of the dorsomedial region in (C). (C”) High-magnification of the dorsolateral region in (C). (D) Merged image of (B) and (C). (D’) Merged image of (B’) and (C’). (D”) Merged image of (B”) and (C”).
Figure S2. Title: Neurons express cxcr5. (A) cxcr5 fluorescent in situ hybridization (FISH) coupled to HuC immunohistochemistry (IHC) on a section of unlesioned adult zebrafish telencephalon. Inset is magnified image. (B)cxcr5 FISH coupled to HuC IHC on a section of 3 days post lesion (dpl) adult zebrafish telencephalon. Inset is magnified image. cxcr5 is expressed in neurons before and after injury. (C) Quantification graph for cxcr5-expressing HuC-positive cells. (D)cxcr5 chromogenic in situ hybridization (ISH) coupled to NeuroD IHC in unlesioned telencephalon. NeuroD-positive differentiating neurons, which are several cell diameters away from the ventricle express cxcr5 (white asterisks). cxcr5 expression is weaker in NeuroD-positive neurons in comparison to cxcr5-positive cells closer to the ventricle (yellow asterisks). In a transition zone between strong NeuroD-positive (white asterisks) and NeuroD-negative cells (yellow asterisks), cells express NeuroD and cxcr5 weakly (blue asterisks). (E)cxcr5 chromogenic ISH coupled to NeuroD IHC in 3 dpl telencephalon. NeuroD-positive differentiating neurons are more numerous, dispersed inside the parenchyma distantly in comparison to unlesioned telencephalons and express cxcr5 (white asterisks). cxcr5 expression is weaker in NeuroD-positive neurons in comparison to cxcr5-positive cells closer to the ventricle (yellow asterisks). (F) Quantification graph for cxcr5-positive NeuroD-expressing cells. The number of NeuroD/cxcr5 double-positive cells increase upon injury in adult zebrafish telencephalon. Scale bars 25 μm; n = 4 telencephalons for every set of analyses.
Figure S3. Title: cxcr5 is not expressed in L-Plastin-positive cells in the adult zebrafish telencephalon. (A) cxcr5 in situ hybridization coupled to immunohistochemistry for L-Plastin (red, marking macrophages and microglia) and her4.1:green fluorescent protein (GFP) (green, marking the radial glial cells) in the unlesioned telencephalons. White asterisks indicate the L-Plastin cells. (A1-A3) Individual channels for L-Plastin, her4.1:GFP and cxcr5, respectively. (B) cxcr5 in situ hybridization coupled to immunohistochemistry for L-Plastin and her4.1:GFP in the lesioned telencephalons (dorsolateral region). White asterisks indicate the L-Plastin cells. (B1-B3) Individual channels for L-Plastin, her4.1:GFP and cxcr5, respectively. (C) cxcr5 in situ hybridization coupled to immunohistochemistry for L-Plastin and her4.1:GFP in the lesioned telencephalons (dorsomedial region). White asterisks indicate the L-Plastin cells. (C1-C3) Individual channels for L-Plastin, her4.1:GFP and cxcr5, respectively.
Figure S4. Title: Larval phenotypes upon cxcr5 misexpression; and transgene activity in the adult zebrafish telencephalon. (A) Cxcr5 is a seven-span transmembrane protein with an extracellular receptor domain, seven transmembrane domains and a C-terminus intracellular domain. We generated full-length and dominant-negative versions of Cxcr5. Dominant negative variant lacks the last three transmembrane domains and the intracellular domain. Both variants are inserted into a transgenesis cassette that contains the coding sequence for enhanced green fluorescent protein (EGFP) and self-cleaving T2A peptide. The whole cassette is expressed under heat-inducible hsp70l promoter. (B) Non-transgenic sibling at 3 days post-fertilization (dpf). (B1) High-magnification image of the yolk-sac in B. (B2) High-magnification image of the yolk-tube extension in B. (B3) High-magnification image of the ventral fin fold in B. (C)Tg(hsp:egfp-T2A-dncxcr5) transgenic animals at 3 dpf after two heat shocks in gastrula. (C1) High-magnification image of the yolk-sac in C. (C2) High-magnification image of the yolk-tube extension in C. (C3) High-magnification image of the ventral fin fold in C. (D)Tg(hsp:egfp-T2A-FLcxcr5) transgenic animals at 3 dpf after two heat shocks in gastrula. (D1) High-magnification image of the yolk-sac in D. (D2) High-magnification image of the yolk-tube extension in D. (D3) High-magnification image of the ventral fin fold in D. (E) Quantification graph for the number of pigment cells in different regions of the non-transgenic, Tg(hsp:egfp-T2A-dncxcr5) and Tg(hsp:egfp-T2A-FLcxcr5) larvae. Note that dominant negative variant of cxcr5 significantly reduces while full-length cxcr5 significantly increases the number of pigment cells in comparison to the non-transgenic siblings. (F) Quantitative real-time PCR analyses at 3 dpf after the misexpression of cxcr5 with two heat shocks at gastrula. (G) Heat-shock scheme for adult zebrafish telencephalon expression. (H) GFP and DAPI staining in heat-shocked non-transgenic animals. (I) GFP and DAPI staining in heat-shocked Tg(hsp:egfp-T2A-dncxcr5) animals. (J) GFP and DAPI staining in heat-shocked Tg(hsp:egfp-T2A-FLcxcr5) animals. (K-M) GFP IHC on Tg(hsp:egfp).(K) Olfactory bulb at 1 day after heat-shock. (L) Olfactory bulb at 2 days after heat-shock. (M) Telencephalon at 1 day after heat-shock. (N) Telencephalon at 2 days after heat-shock. GFP protein as a result of heat shock paradigm reduces significantly after 24 hours after heat shock.
Figure S5. Title: cxcr5 translation-blocking antisense morpholino is functional. (A) Uninjected 2 day post-fertilization (dpf) embryos. (B) Control morpholino-injected embryos show no morphological phenotypes at 2 dpf. (C)cxcr5 antisense morpholinoinjected embryos display severe anomalies in axial extension and head development. (D)cxcr5 mRNA rescues the knockdown phenotypes when co-injected with antisense morpholinos. (E) Quantitative real-time PCR analysis of genes regulated by cxcr5, upon control and antisense morpholino injections. All the genes tested are upregulated after knocking down cxcr5. Percentages in A-D represent the ratio of embryos with gross morphological defects to the whole clutch size.
Figure S6. Title: Misexpression of cxcr5 does not lead to cell death. (A-I) TUNEL staining to detect the apoptotic cells in non-transgenic siblings, Tg(hsp:egfpt2a-FLcxcr5) and Tg(hsp:egfp-t2a-dncxcr5) animals pre-lesion, 3 days post-lesion (dpl) and 15 dpl time points. Red nuclei indicate the apoptotic cells. (J) Quantification graph shows the number of apoptotic nuclei in the lesioned hemisphere. Misexpression of cxcr5 does not alter the levels of cell death. Two telencephalons were used for every time point.
Table S1. Title: qRT-PCR primers.