Abstract
Background
Recipient NK cells may detect the lack of recipient's (i.e., self) HLA antigens on donor renal tissue by means of their killer cell immunoglobulin-like receptors (KIRs). KIR genes are differently distributed in individuals, possibly contributing to differences in response to allogeneic graft.
Methodology/Principal Findings
We compared frequencies of 10 KIR genes by PCR-SSP in 93 kidney graft recipients rejecting allogeneic renal transplants with those in 190 recipients accepting grafts and 690 healthy control individuals. HLA matching results were drawn from medical records. We observed associations of both a full-length KIR2DS4 gene and its variant with 22-bp deletion with kidney graft rejection. This effect was modulated by the HLA-B,-DR matching, particularly in recipients who did not have glomerulonephritis but had both forms of KIR2DS4 gene. In contrast, in recipients with glomerulonephritis, HLA compatibility seemed to be much less important for graft rejection than the presence of KIR2DS4 gene. Simultaneous presence of both KIR2DS4 variants strongly increased the probability of rejection. Interestingly, KIR2DS5 seemed to protect the graft in the presence of KIR2DS4fl but in the absence of KIR2DS4del.
Conclusions/Significance
Our results suggest a protective role of KIR2DS5 in graft rejection and an association of KIR2DS4 with kidney rejection, particularly in recipients with glomerulonephritis.
Introduction
Acute or chronic rejection of solid organ grafts such as kidney is mediated by alloreactive T lymphocytes recognizing major (HLA) and minor histocompatibility antigens by means of antigen-specific T cell receptors (TCR) [1], [2]. However, a contribution of natural killer (NK) cells has also been postulated. Thus, infiltration of renal allografts by NK cells [3]–[5], increased numbers of NK cells in peripheral blood of patients acutely rejecting kidney graft [6], and increased cytotoxicity of recipient NK cells against donor peripheral blood cells in vitro were described [7].
NK cells recognize the presence of HLA class I (HLA I) molecules on the surface of potential target cells using several types of the receptors, among them polymorphic killer cell immunoglobulin-like receptors (KIRs). Normal cells of an individual are spared by his or her NK cells because they express normal level of cell surface HLA I molecules detected by NK cell inhibitory receptors. However, virus-infected or neoplastic cells tend to lose HLA I expression, and may be eliminated by NK cells [8].
Due to HLA and KIR polymorphism, in some combinations of the graft donor and recipient, recipient NK cell's inhibitory KIRs may not bind HLA I molecules present on donor cells, leading to NK cell alloreactivity against the transplanted organ, similarly to the reaction in opposite direction in hematopoietic cell transplantation [9]–[11]. In addition, KIRs are expressed also on some T lymphocytes, particularly on special subpopulation of CD4+CD28− cytotoxic T cells involved in autoimmune vasculitis [12]–[14], potentially influencing their activity in graft rejection.
Human KIRs are encoded by genes located in the chromosomal region 19q14. KIR genetics is characterized by both allelic (up to more than 50 alleles for some KIRs) and haplotypic (i.e., different numbers of inhibitory and activating genes on individual chromosomes) polymorphism [8], [15]. As a result, above 97% of unrelated persons differ by their KIR genotype [16]–[17]. Two categories of KIR haplotypes were described: A-type haplotypes containing mostly inhibitory KIRs, and only KIR2DS4 and KIR2DL4 as activating ones, and B-type haplotypes, characterized by one or more of other activating KIRs in addition to inhibitory ones. For this reason, people may differ substantially in their NK and T cell responses, depending on KIR genotype. We have recently published results showing a contribution of KIR2DS5 gene to a tolerance of kidney graft as well as to other clinical situations [18]. Here, we focused on kidney graft rejection and compared frequencies of 10 KIR genes in recipients rejecting the allogeneic renal transplant with those in recipients accepting such a graft. Our study is the first report on different HLA and KIR genetic associations of kidney graft acute rejection in recipients whose pre-transplant renal failure resulted from glomerulonephritis versus those whose renal failure was a result of other disease.
Materials and Methods
Kidney graft recipients and controls
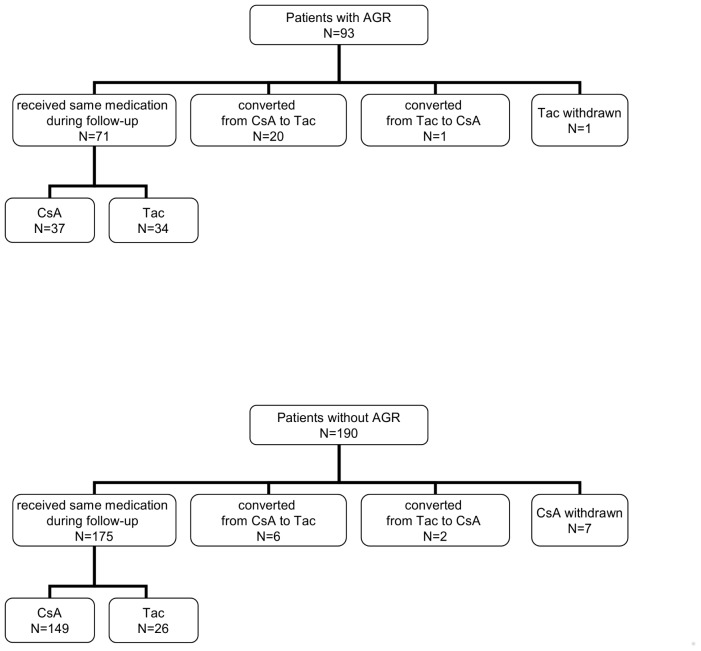
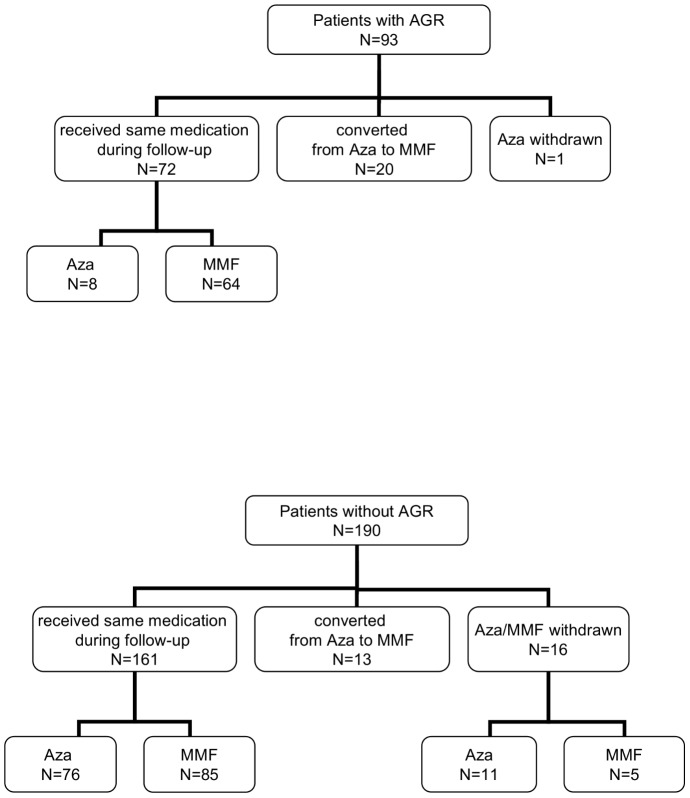
All individuals, including kidney graft recipients, donors, and healthy controls, were Polish Caucasians. Two hundred eighty-three kidney patients (clinical data presented in Table 1 ) underwent first transplantation and received deceased donor kidney between 1989 and 2008 (166 patients after 2000). All patients were treated with triple-therapy ( Figure 1 ) as initial immunosuppression that incorporated cyclosporine (n = 219) or tacrolimus (n = 64, beginning in 2000) in combination with azathioprine (n = 129) or mycofenolate mofetil (n = 154, since 1998) ( Figure 2 ) and steroids. No induction with antibodies was used. During the follow up (mean time was 7 years) there were 246 (87%) patients who were treated with the same calcineurin inhibitor. Among 29 patients who changed the type of calcineurin inhibitor, 20 patients were converted from CsA to tacrolimus after an episode of rejection treated with methylprednisolone. Calcineurin inhibitor was withdrawn in 8 individuals. There were 233 patients who received the same type of purine metabolism inhibitor during follow up: azathioprine (n = 84) and mycofenolate mofetil (n = 149). Azathioprine was replaced by mycofenolate mofetil in 33 patients (in 20 patients after an episode of acute rejection) or stopped in 12 patients. The frequency of a change in treatment regimen was almost 2-fold higher in patients who suffered a rejection (refers to 38% and 21% of patients with and without rejection, respectively, p = 0.0063).
Table 1. Clinical characteristics of patients.
| Patients with AGR | Patients without AGR | P | OR | 95%CI | ||
| N = 93 | N = 190 | |||||
| Age | Mean +/− SD | 43.65±11.31 | 43.36±11.46 | 0.8 | ||
| min-max | 15–67 | 16–72 | ||||
| Sex | Females/Males | 32/61 | 92/98 | 0.03 | 0.56 | 0.33–0.93 |
| % of Females | 34.4% | 48.4% | ||||
| N (%) | N (%) | |||||
| Cause | Glomerulonephritis | 58 (62.4) | 74 (39.0) | 0.0002 | 2.60 | 1.56–4.33 |
| of | Interstitial nephritis | 10 (10.7) | 27 (14.2) | 0.46 | 0.73 | 0.34–1.58 |
| renal | Cystic kidney | 10 (10.7) | 24 (12.6) | 0.7 | 0.83 | 0.38–1.82 |
| failure | Hypertensive nephropathy | 3 (3.2) | 13 (6.8) | 0.28 | 0.45 | 0.13–1.63 |
| Diabetic nephropathy | 5 (5.4) | 7 (3.7) | 0.54 | 1.49 | 0.46–4.81 | |
| Other | 7 (7.6) | 45 (23.7) | 0.0009 | 0.26 | 0.11–0.61 |
Figure 1. Patient disposition according to initial calcineurine inhibitor use.
AGR, acute graft rejection; CsA, cyclosporine A; Tac, Tacrolimus.
Figure 2. Patient disposition according to purine metabolism inhibitor use.
AGR, acute graft rejection; Aza, azathioprine; MMF, mycophenolate mofetil.
93 recipients exhibited symptoms of acute graft rejection based on clinical criteria (an increase in serum creatinine level of at least 20% above the baseline measurements not attributable to another cause) confirmed by histopathological examination according to Banff criteria [19]. Apart from three patients, all had a biopsy-confirmed acute rejection episode. Remaining 190 recipients experienced stable graft function during long-term follow-up. 31 (33%) patients who suffered acute rejection subsequently lost their grafts in comparison to 22 (12%) patients without an episode of rejection. During the follow-up, 5 out of 93 (5.4%) patients with AGR and 13 out of 190 (6.8%) patients without AGR died for different reasons.
Six hundred and ninety unrelated healthy volunteers, constituting a basic control group in KIR studies performed in our laboratory, were recruited in the years 2001–2008 by the Regional Center of Blood Transfusion, Wroc3aw, as well as by clinics of the Wroc3aw Medical University, the Medical University of Warsaw, and the Pomeranian Medical University, Szczecin.
The same cohorts of patients and controls have already been used to describe a protective effect of KIR2DS5 gene on kidney graft rejection and some other clinical situations [18].
The Bioethics Committee of the Wroc3aw Medical University specifically approved this study. Signed written informed consent was given by all participants.
DNA isolation and KIR typing
DNA was isolated from venal blood as described [20], [21]. The presence or absence of KIR genes was detected by either individual [20]–[22] or multiplex [23] polymerase chain reactions (PCR) which, when tested on the same samples, gave virtually identical results. Our KIR typing has been validated three times per year by the International KIR Exchange program organized by the Immunogenetics Center of the University of California at Los Angeles.
HLA-A, -B, and -DR typing of donors and recipients has been routinely done before transplantation either in Non-Public Tissue Typing Facility at our Institute or in other transplant centers in Poland, and it was drawn from the clinical histories of the patients. Tissue samples were available for only 42 donors, therefore their HLA-C typing was possible only in these instances, had statistically insufficient power, and therefore its results are not presented here. Recipient HLA-C variants encoding C1 and C2 epitopes were described and discussed earlier [18].
Statistical analysis
General linear model (GLM) with binomial errors was used to investigate relationship between clinical and genetic variables and probability of rejecting the transplanted kidney ( Table 2 ). Frequencies of KIR genes in recipients and HLA matching between donors and recipients were explanatory variables. Clinical characteristics (age, sex, creatinine, course of transplantation and time of observation) was concomitant variables. Akaike's information criterion (AIC) was used as a measure of fit of models. Bootstrap approach was employed to estimate model's coefficients and 95% confidence intervals. Chi-squared test with Yates' continuity correction was used to test hypothesis that rejection and type of genotype were independent. To test differences in KIR's distribution among patients and control, a group permutation test was employed. This procedure was based on Mahalanobis distance (DM) between two groups and test performed in 10 000 permutations. Odds ratio (OR) was computed as a measure of effect size. Probability that graft is not rejected at a given time, S(t), was computed according to the Kaplan-Meier method, comparing KIR genotypes. Haplotype frequencies (HFs) among two KIRs: 2DS4 (full-length or deletion variant) and 2DS5 were estimated with maximum likelihood function [24].
Table 2. Variables significantly associated with probability of graft rejection.
| Variable | OR | 95% CI | P | |
| HLA-B+DR match | 0.46 | 0.30 | 0.70 | 0.0003 |
| KIR2DS4fl | 2.02 | 1.05 | 3.90 | 0.03 |
| KIR2DS4del | 2.61 | 1.19 | 5.75 | 0.02 |
| HLA-B+DR match×GN | 2.01 | 1.46 | 2.76 | 0.0000 |
Abbreviations: CI, confidence interval; GN, glomerulonephritis; HLA, human leukocyte antigen; KIR, killer immunoglobulin-like receptor; KIR2DS4fl, KIR2DS4 full length gene; KIR2DS4del, KIR2DS4 22-base pair deletion variant of the KIR2DS4 gene; OR, odds ratio.
Measures for the estimation of linkage disequilibrium (LD) were the correlation of two alleles frequencies, r, global squared correlation between two loci, R2 and Kullback-Leibler divergency two loci from LE [24], [25]. For two loci 2DS4 and 2DS5, r and R2 obtained as: 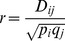 , where
, where 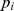 and
and 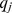 are the population allele frequencies of the
are the population allele frequencies of the 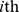 allele on locus 2DS4 and the
allele on locus 2DS4 and the 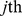 allele on locus 2DS5,
allele on locus 2DS5, 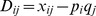 , and
, and 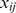 is the frequency of the haplotype with alleles
is the frequency of the haplotype with alleles  and
and 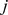 on loci 2DS4 and 2DS5, respectively.
on loci 2DS4 and 2DS5, respectively. 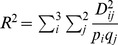 . Kullback-Leibler divergence [26], [27],
. Kullback-Leibler divergence [26], [27], 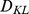 , is a measure of distance between the observed haplotype distribution and the expected distribution assuming LE:
, is a measure of distance between the observed haplotype distribution and the expected distribution assuming LE: 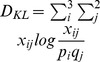 . Chi-square statistic was calculated to test that all of the
. Chi-square statistic was calculated to test that all of the 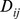 's between 2DS4 and 2DS5 are zeros:
's between 2DS4 and 2DS5 are zeros: 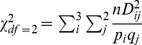 . Likelihood ratio statistic, LRS, was used to test for differences in haplotype frequencies between rejectors, non-rejectors and controls.
. Likelihood ratio statistic, LRS, was used to test for differences in haplotype frequencies between rejectors, non-rejectors and controls.
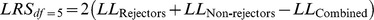 , where log likelihoods were produced based on haplotype frequencies and
, where log likelihoods were produced based on haplotype frequencies and 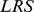 is approximately a χ2. Results were regarded as statistically significant at p<0.05. All data were analyzed using R version 2.2.1.
is approximately a χ2. Results were regarded as statistically significant at p<0.05. All data were analyzed using R version 2.2.1.
Results
HLA and KIR2DS4 gene effects on acute kidney graft rejection
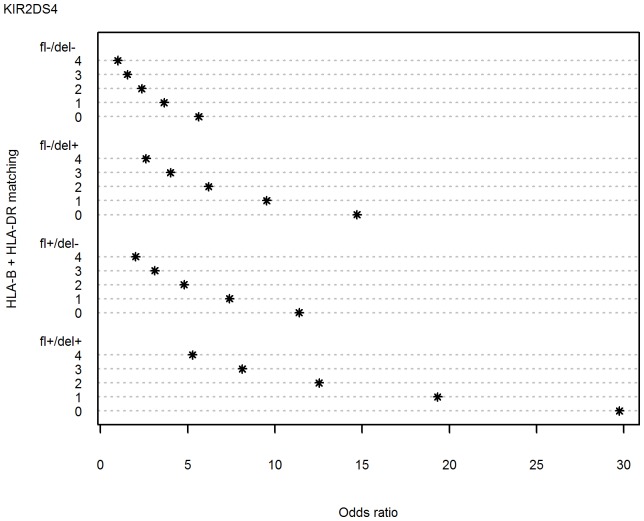
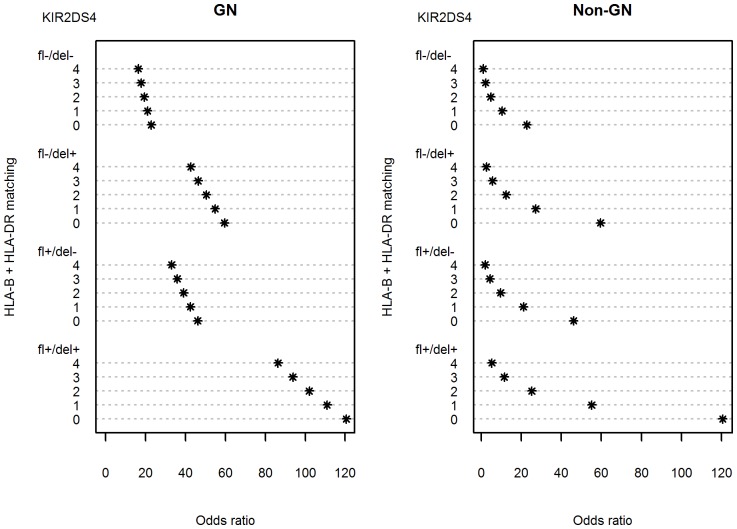
Frequencies of KIR genes were not different between patients and controls ( Table 3 ). However, there were some differences between patients with acute graft rejection (AGR) (determined by Banff criteria) and patients without AGR. First, the frequency of the KIR2DS5 gene in patients with AGR was two times lower than in control individuals (p = 0.0056). This protective effect of KIR2DS5 gene on kidney graft rejection has already been published recently on the same cohorts of patients and controls [18]. Multivariate analysis indicated significant protective effect of HLA-B,-DR matching ( Table 2 ) but HLA-A did not affect graft fate (data not shown). We also observed that the presence of both KIR2DS4 full-length (KIR2DS4fl) and 22-base pair deletion variant (KIR2DS4del) gene was increasing a probability of rejection at least twofold ( Table 2 ). This effect was amplified to a great extent by the HLA-B,-DR mismatching (matching = 0, Figure 3 ).
Table 3. KIR gene frequencies in controls and patients.
| KIR | ||||||||||||
| Group | 2DL1 | 2DL2 | 2DL3 | 2DS1 | 2DS2 | 2DS3 | 2DS4fl | 2DS4del | 2DS5 | 3DL1 | 3DS1 | |
| Patients | Present | 268 | 142 | 257 | 105 | 143 | 81 | 82 | 236 | 65 | 256 | 93 |
| N = 283 | % | 94.70 | 50.18 | 90.81 | 37.10 | 50.53 | 28.62 | 28.97 | 83.39 | 22.97 | 90.46 | 32.86 |
| Control | Present | 665 | 374 | 623 | 299 | 369 | 214 | 194 | 561 | 205 | 642 | 264 |
| N = 690 | % | 96.38 | 54.20 | 90.29 | 43.33 | 53.48 | 31.01 | 28.12 | 81.30 | 29.71 | 93.04 | 38.26 |
| p | 0.3 | 0.3 | 0.9 | 0.07 | 0.4 | 0.5 | 0.8 | 0.5 | 0.03 | 0.2 | 0.1 | |
| OR | 0.67 | 0.85 | 1.06 | 0.77 | 0.89 | 0.89 | 1.04 | 1.55 | 0.71 | 0.71 | 0.79 | |
| 95%CI | 0.35–1.29 | 0.65–1.12 | 0.66–1.71 | 0.58–1.03 | 0.67–1.17 | 0.66–1.21 | 0.77–1.42 | 0.80–1.67 | 0.51–0.97 | 0.43–1.16 | 0.59–1.06 |
Abbreviations: CI, confidence interval; KIR, killer immunoglobulin-like receptor; KIR2DS4fl, KIR2DS4 full length gene; KIR2DS4del, KIR2DS4 22-base pair deletion variant of the KIR2DS4 gene; OR, odds ratio.
Figure 3. Dependence of odds ratio for kidney graft rejection on HLA-B,-DR matching, KIR2DS4 full length (KIR2DS4fl) and/or KIR2DS4 deletion variant (KIR2DS4del) gene presence.
For odds ratio calculations, the recipient group with complete (n = 4) HLA-B,-DR match with the donor and a lack of any KIR2DS4 variant (KIR2DS4fl and KIR2DS4del negative: fl-/del-) was taken as 1.
Effects of KIR2DS4 gene variants and KIR2DS5 gene on the probability of graft rejection
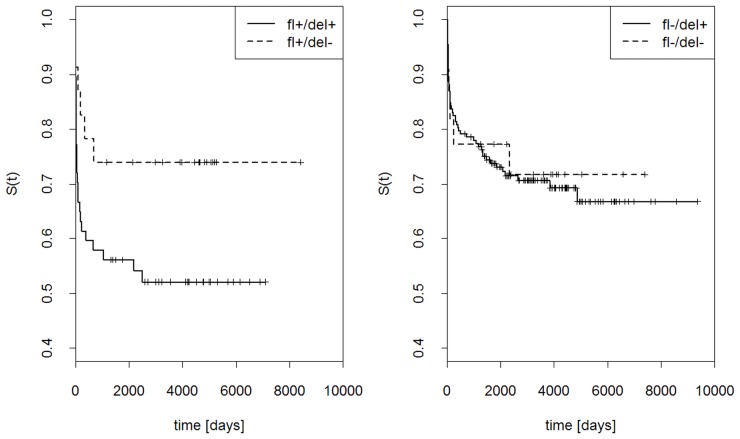
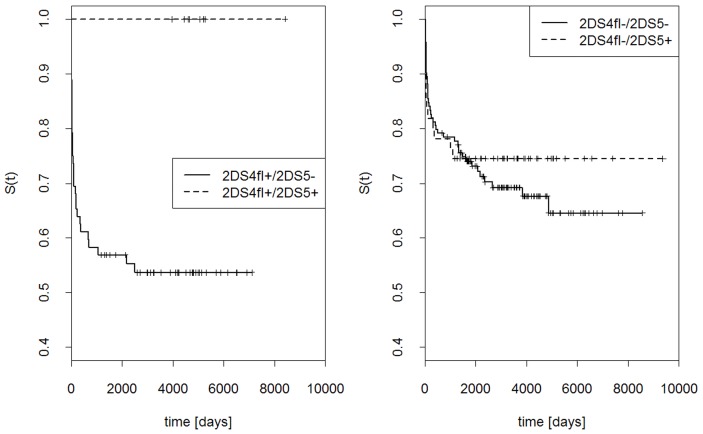
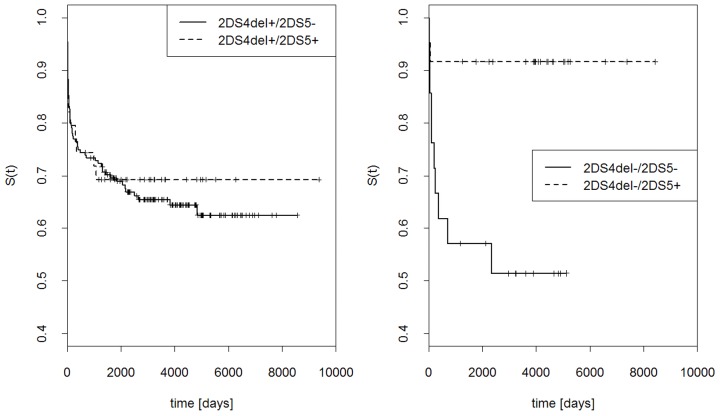
Probability that graft was not rejected at a given time, S(t), was computed for the presence or absence of KIR2DS4fl, KIR2DS4del or both ( Figure 4 ). Simultaneous presence of both gene variants strongly increased the probability of graft rejection, whereas the presence of only KIR2DS4fl, only KIR2DS4del, or none of them gave much lower probability of rejection. Interestingly, the two variants of KIR2DS4 gene had opposite influence on the effect of KIR2DS5 gene: KIR2DS5 seemed to protect the graft stronger in the presence of KIR2DS4fl than in its absence ( Figure 5 ), but stronger in the absence than presence of KIR2DS4del ( Figure 6 ).
Figure 4. Effects of full-length KIR2DS4 gene (fl) and its deletion variant (del) on the outcome of renal transplantation.
Kaplan Kaplan-Meier estimations of probability that graft is not rejected at a given time, S(t). Left panel: KIR2DS4fl present, KIR2DS4del present or absent; right panel: KIR2DS4fl absent, KIR2DS4del present or absent.
Figure 5. Effects of KIR2DS5 and full-length (fl) KIR2DS4 gene on the outcome of renal transplantation.
Kaplan Kaplan-Meier estimations as in Figure 4. Left panel: KIR2DS4fl present, KIR2DS5 present or absent; right panel: KIR2DS4fl absent, KIR2DS5 present or absent.
Figure 6. Effects of KIR2DS5 and KIR2DS4 deletion variant (KIR2DS4del) gene on the outcome of renal transplantation.
Kaplan Kaplan-Meier estimations as in Figure 4. Left panel: KIR2DS4del present, KIR2DS5 present or absent; right panel: KIR2DS4del absent, KIR2DS5 present or absent.
Combinations of KIR genes gave 133 different genotypes present in patients and/or controls (data not shown). These genotypes were divided into AA and BX genotypes, containing two A haplotypes or at least one B haplotype, respectively (for a definition of A and B haplotypes, see Introduction). Two individual AA genotypes were distributed significantly differently between patient subgroups: among patients with the genotype No. 1 acute graft rejection (AGR) was nearly four times less frequent than lack of AGR (21.9% vs 78.1%), whereas in those with the genotype No. 2 the ratio of AGR and non-AGR was 1∶1 ( Table 4 ). Interestingly, these two genotypes differed only by the absence of full-length KIR2DS4 gene in the genotype No. 1 and its presence in the genotype No. 2, and both were devoid of KIR2DS5 by definition, as AA genotypes. An additional AA genotype containing KIR2DS4fl but no KIR2DS4del gene was extremely rare (one individual among patients and 8 in controls), therefore it could not play any role in rejection and was omitted from our calculations. Within BX (i.e., non-AA) genotype group, fraction of patients with AGR was nearly two times less frequent than these without AGR. These differences between genotype groups were not accidental (χ2 = 6.675, df = 2, p = 0.035).
Table 4. Distribution of KIR genotype groups in patients with and without acute graft rejection.
| Genotype | KIR | Pts with AGR | Pts without AGR | |||||||||||||
| No. | 2DL1 | 2DL2 | 2DL3 | 2DS1 | 2DS2 | 2DS3 | 2DS4 fl | 2DS4 del | 2DS5 | 3DL1 | 3DS1 | N | % | N | % | |
| 1 | AA/2DS4fl− | + | − | + | − | − | − | − | + | − | + | − | 14 | 21.9 | 50 | 78.1 |
| 2 | AA/2DS4fl+ | + | − | + | − | − | − | + | + | − | + | − | 11 | 50.0 | 11 | 50.0 |
| 3 | BX | 68 | 34.5 | 129 | 65.5 | |||||||||||
Differences between frequencies of three genotype groups: χ2 = 6.675, df = 2, p = 0.035.
Abbreviations: AA, homozygote for the killer immunoglobulin-like receptor A haplotype (for a definition of A and B haplotypes, see Introduction); AGR, acute graft rejection; BX, a group of the killer immunoglobulin-like receptor genotypes containing at least one B haplotype; as they contain different combinations of KIR genes, the presence or absence of particular genes could not been shown here; KIR, killer immunoglobulin-like receptor; KIR2DS4fl, KIR2DS4 full length gene; KIR2DS4del, KIR2DS4 22-base pair deletion variant of the KIR2DS4 gene; Pts, patients.
KIR2DS4 and KIR2DS5 are in negative linkage disequilibrium (LD) in all populations tested so far [28]. Table 5 shows that they are in negative LD also in our population. KIR2DS4/KIR2DS5 haplotype frequencies for non-rejectors and controls were very similar, and these two groups were combined in the following calculations. Likelihood ratio statistics (LRS) for patients with AGR versus combined group of patients without AGR and controls was high, which suggests that haplotype frequencies in the former were different from those in the latter (p = 0.05). We see, for example, that a haplotype KIR2DS4−/KIR2DS5+ (−/2DS5) was three times less frequent in patients with AGR than in other groups. This seems to confirm a protective role of KIR2DS5 in graft rejection shown above.
Table 5. Linkage disequilibrium between KIR2DS4 gene variants and KIR2DS5 gene in patients and controls.
| Group | Haplotypes | 2DS4full/2DS5 | 2DS4del/2DS5 | —/DS25 | 2DS4full/— | 2DS4del/— | —/— |
| Controls | HFs | 0.000 | 0.006 | 0.155 | 0.152 | 0.558 | 0.129 |
| r | −0.157 | −0.282 | 0.512 | 0.069 | 0.123 | −0.224 | |
| R2 = 0.436 | DKL = 0.306 | ?2 = 300 | df = 2 | p<0.00001 | |||
| Patients with AGR | HFs | 0.000 | 0.041 | 0.048 | 0.214 | 0.636 | 0.061 |
| r | −0.138 | −0.079 | 0.39 | 0.043 | 0.025 | −0.122 | |
| R2 = 0.194 | DKL = 0.102 | ?2 = 17.49 | df = 2 | p = 0.00016 | |||
| Patients without AGR | HFs | 0.005 | 0.000 | 0.136 | 0.131 | 0.586 | 0.142 |
| r | −0.138 | −0.287 | 0.489 | 0.042 | 0.116 | −0.198 | |
| R2 = 0.395 | DKL = 0.278 | ?2 = 77.06 | df = 2 | p<0.00001 |
Abbreviations: AGR, acute graft rejection; HFs, haplotype frequencies; DKL, Kullback-Leibler divergence from linkage equilibrium; KIR, killer immunoglobulin-like receptor; KIR2DS4fl, KIR2DS4 full length gene; KIR2DS4del, KIR2DS4 22-base pair deletion variant of the KIR2DS4 gene; R2, global squared correlation coefficient for two loci; r, correlation coefficient for alleles frequencies. Likelihood ratio statistics (LRS): {Pts with AGR} vs {Controls+Pts without AGR}; LRS = 11.01; df = 5; p = 0.0513.
Difference between patients with glomerulonephritis and those with other kidney diseases in association of acute graft rejection with KIR2DS4 and HLA genotype
Multivariate analysis revealed also a difference between patients whose end stage renal failure was caused by glomerulonephritis and those with other nephropathies. Namely, in the non-glomerulonephritis group, HLA-B,-DR matching seemed to be much more important for acute graft rejection than the presence or absence of KIR2DS4 gene variants ( Figure 7 , right panel). Thus, in the case of perfect HLA-B,-DR matching (matching = 4), the presence or absence of KIR2DS4fl and KIR2DS4del genes only very weakly influenced graft fate. Recipients of completely HLA-B,-DR incompatible grafts (matching = 0) possessing both forms of KIR2DS4 gene had only 6 times higher chance of kidney rejection than recipients of similarly HLA-B,-DR-incompatible grafts negative for KIR2DS4 ( Figure 7 , right panel).
Figure 7. Effect of glomerulonephritis on the dependence of odds ratio for kidney graft rejection on HLA-B,-DR matching, KIR2DS4 full length (KIR2DS4fl) and/or KIR2DS4 deletion variant (KIR2DS4del) gene presence.
For odds ratio calculations, the non-GN group with complete (n = 4) HLA-B,-DR match and lack of any KIR2DS4 variant (KIR2DS4fl and KIR2DS4del negative: fl-/del) was taken as 1.
In contrast, in recipient group with glomerulonephritis, HLA incompatibility seemed to be much less important than KIR2DS4 for graft rejection. For example, completely HLA-mismatched KIR2DS4-negative recipients had only about 1.4 times higher chance of acute rejection than perfectly HLA-matched KIR2DS4-negative recipients ( Figure 7 , left panel). On the other hand, the presence of both forms of KIR2DS4 gene had strong effect on graft rejection in glomerulonephritis group, as even perfect HLA matching did not reduce a chance of rejection below odds ratio of 85 ( Figure 7 , left panel). Individuals from the glomerulonephritis group possessing both variants of KIR2DS4 and perfect HLA-B,-DR matching had about 15 times higher chance of rejection than analogous persons from the non-glomerulonephritis group (see Figure 7 , both panels).
Discussion
We compared the distribution of KIR genes in patients rejecting and non-rejecting kidney graft as well as in healthy controls. Among individual KIR genes, only KIR2DS4 (both full-length and deletion variants) was remarkably more frequent in patients with AGR than in patients with stable graft function and controls. Moreover, this effect was particularly strong in the absence of KIR2DS5 gene which exerted opposite effect, i.e., its presence decreased the chance of graft rejection as published already on the same cohorts of patients and controls [18]. It is interesting in this context that KIR2DS4 molecule was expressed on remarkable proportion of CD4+CD28− T cell clones isolated from an acute coronary syndrome patient [29]. Also, dialyzed patients exhibited an increased number of circulating CD4+CD28− T cells [30]. In addition, CMV positivity is universal in our transplant population (data not shown), and the association of CMV infection and the presence of CD4+CD28− cells is well documented (ref.30 and references therein). CD4+CD28− T cells, virtually absent from peripheral blood of healthy individuals but present in acute coronary syndrome and rheumatoid vasculitis [29], multiple sclerosis [14] and, most important here, in end-stage renal disease [30], [31], were found to be resistant to immunoregulation [14] and therefore postulated to play a role in autoimmune diseases and aging [12]. KIR2DS4fl encodes an activating receptor which might possibly be involved in stimulation of effector cells (e.g., CD4+CD28− T cells) contributing to transplant rejection, whereas KIR2DS4del potentially codes for a soluble molecule [32]. It might be that, in kidney graft recipients, this soluble KIR2DS4 molecule is masking a ligand for membrane-bound KIR2DS4 or another receptor of some regulatory cells (T, NK, or other). The ligand for KIR2DS5 receptor is not known, however it has been observed that the simultaneous presence of KIR2DS5 gene and HLA-C-encoded epitopes for both KIR2DL1 and KIR2DL2/3 receptors significantly decreased leukemia-free survival of hematopoietic stem cell-transplanted patients [33], which could suggest KIR2DS5 interaction with HLA-C. Thus, in the case of renal transplant recipients, soluble KIR2DS4 might block interaction of KIR2DS5 with its ligand which otherwise would protect a graft from rejection. In recipients negative for KIR2DS4del gene the presence of KIR2DS5 seems to favor the acceptance of the graft, particularly in the presence of KIR2DS4fl gene. The reason for the latter effect is not clear; either both KIR2DS5 and KIR2DS4fl act in the same direction, e.g. expressed on the same or on two different regulatory cells, or, alternatively, the presence of KIR2DS4fl gene, by excluding the presence of its allele, KIR2DS4del, from the same chromosome, decreases its frequency in KIR2DS4fl-positive patient population.
KIR2DS4 molecule differs from KIR2DS1 and KIR2DS2/3 by weaker interaction with HLA-C and by binding to HLA-A*11 [34]. As mentioned in Material and Methods section, blood, lymphoid tissue or DNA samples of majority of donors were not available for our study, and their HLA-C typing was not possible here. However, in a recent study, Hanvesakul and coworkers [35] have not detected any association between donor HLA-C-encoded KIR ligand and acute rejection of kidney allograft. On the other hand, these authors have reported an effect of recipient HLA-C on the kidney graft rejection, i.e., a protective effect of C2 on allograft survival. They observed donor-derived NK cells in the allograft at the time of transplantation. These NK cells could stimulate maturation of dendritic cells which would then be capable of indirect stimulation of adaptive immune system for alloreactivity. In vitro studies of Hanvesakul et al. [35] have shown that donor-derived, interleukin-15-activated NK cells promoted efficiently the maturation of recipient dendritic cells only when these were C2-negative. They propose that, after kidney transplantation, donor NK cells interacting with recipient C2-positive dendritic cells do not stimulate them for maturation as efficiently as in C2-negative recipients, and this is beneficial for graft survival. In our earlier study, we have not observed such an effect of recipient C2 on graft survival; on the contrary, we have rather seen some, albeit weak and non-significant, association of C2 with kidney rejection [18]. The reason for this discrepancy may lie in numbers: our sample (283 patients) was 2.7 times less numerous than that of the British group (760 individuals). Alternatively, the British and our patients might have differed in a cause of renal failure: we have found a substantial genetic difference between recipients with glomerulonephritis and those with other conditions, whereas Hanvesakul et al. [35] did not show a clinical status of their patients before transplantation.
HLA-A*11 allele (a KIR2DS4 ligand, see above) frequency in the Polish population is 6.239%, as established by Schmidt and colleagues [36]; therefore, its effect on kidney graft survival, if any, could not be strong. Nevertheless, we have noticed that a majority of HLA-A11-positive graft-rejecting recipients (4 out of 5) possessed KIR2DS4fl gene, whereas only one fifth of A11-positive patients with stable graft function (4 out of 20) typed positive for KIR2DS4fl (p = 0.02). However, the numbers were far too small for any conclusion. No difference between distribution of KIR2DS4del variant in A11-positive patients with and without AGR was seen (data not shown).
So far, only few publications dealt with KIR gene associations with kidney graft rejection. Tran et al. [37] studied an effect of KIR ligand (i.e., C1, C2, and Bw4) matching on graft survival in 1416 recipients of cadaver kidney (both first grafts and regrafts) from all inhabited continents, but did not detect any effect. Similarly negative result was obtained by Kreijveld et al. [38] who tested not only KIR ligands, but also KIR genes themselves as well as combinations of both in Dutch population. On the other hand, upon testing a KIR polymorphism in HLA-identical recipient-donor pairs from U.S.A., Cirocco et al. [39] obtained a result suggesting protective effect of KIR2DL2 and KIR2DS2 genes; however, their study was based on extremely low number of individuals (only 12 recipient-donor pairs). Nevertheless, this finding was confirmed by Kunert et al. [40] on 105 graft recipients and 119 controls in Germany. Finally, van Bergen et al. [41] observed an effect of KIR-KIR ligand mismatch between recipient and donor in Dutch population, but only in HLA-A,-B,-DR-compatible donor-recipient pairs, i.e., when HLA-identical partners differed in their KIR gene repertoire, resulting in lack of a ligand in donor for a KIR present in recipient. Notably, the effect of KIRs in HLA-matched pairs was as strong as that of HLA-A,-B mismatch in pairs matched only for HLA-DR [41], [42]. In our study, we observed a strong effect of KIR2DS4 variants in patients with glomerulonephritis, where HLA-B,-DR mismatch exerted much weaker influence on the graft fate, whereas in recipients without glomerulonephritis the effect of HLA-mismatch was predominant. The striking finding of this study was the stronger association of KIR2DS4 polymorphism than HLA incompatibility in GN patients. We can speculate that engagement of KIRs in inflammatory pathway activation defined by their polymorphism may represent a common link between autoimune and alloimmune response. The possibility that KIR-ligand interaction may aggravate both the natural history of glomerulonephritis sustaining immune injury leading to end-stage renal disease and influence alloimmune response causing acute rejection cannot be ruled out. In this setting, KIR2DS4 polymorphism can provoke the immune response as it can modulate autoimmunity. We can hypothesize that GN and non-GN patients may differ in KIR2D receptor expression on NK and T cells, particularly in those rejecting vs. non-rejecting the graft, as it has recently been shown in liver transplantation [43].
In summary, our results suggest that typing of the recipient for KIR2DS4 and KIR2DS5 genes may help to predict the outcome of renal transplantation. We show here, for the first time, that the effect of KIR genotype on the fate of kidney graft in recipients with glomerulonephritis seems to be stronger than that of HLA matching, whereas opposite is true for patients with other causes of end-stage renal disease. The lack of strong association of graft rejection with HLA-B,-DR mismatching in recipients with glomerulonephritis could not have been observed in earlier studies done without stratification for the presence or absence of KIR2DS4 gene. However, a small number of data that was collected over a long time period is a limitation to the reliability of our findings. Therefore, more definitive studies would require data input from much higher number of patients and, preferably, from more than one institution.
Acknowledgments
We wish to thank our patients for blood donation and agreement to participate in this study. We are also grateful to Mr. Maciej Sobczyñski, M. Sci., from the Faculty of Biotechnology, University of Wroc3aw, for statistical analysis.
Funding Statement
This work was supported by the Ministry of Science and High Education grant No. N401077 31/1819 and by the Ludwik Hirszfeld Institute of Immunology and Experimental Therapy grant no. 14/2011. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Womer KL, Kaplan B (2009) Recent developments in kidney transplantation – a critical assessment. Am J Transplant 9: 1265–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nankivell BJ, Alexander SI (2010) Rejection of the kidney allograft. N Engl J Med 363: 1451–1462. [DOI] [PubMed] [Google Scholar]
- 3. Andersen CB, Ladefoged SD, Larsen S (1994) Acute kidney graft rejection. A morphological and immunohistochemical study on ‘zero-hour’ and follow-up biopsies with special emphasis on cellular infiltrates and adhesion molecules. APMIS 102: 23–37. [PubMed] [Google Scholar]
- 4. Hancock WW, Gee D, De Moerloose P, Rickles FR, Ewan VA, et al. (1985) Immunohistological analysis of serial biopsies taken during human renal allograft rejection. Changing profile of infiltrating cells and activation of the coagulation system. Transplantation 39: 430–438. [DOI] [PubMed] [Google Scholar]
- 5. Totterman TH, Hanas E, Bergstrom R, Larsson E, Tufveson G (1989) Immunologic diagnosis of kidney rejection using FACS analysis of graft-infiltrating functional and activated T and NK cell subsets. Transplantation 47: 817–823. [DOI] [PubMed] [Google Scholar]
- 6. Cooksey G, Robins RA, Blamey RW (1984) Natural killer cells in renal allograft rejection. Br J Surg 71: 874–877. [DOI] [PubMed] [Google Scholar]
- 7. Vampa ML, Norman PJ, Burnapp L, Vaughan RW, Sacks SH, et al. (2003) Natural killer-cell activity after human renal transplantation in relation to killer immunoglobulin-like receptors and human leukocyte antigen mismatch. Transplantation 76: 1220–1228. [DOI] [PubMed] [Google Scholar]
- 8. Parham P (2005) MHC class I molecules and KIRs in human history, health and survival. Nature Rev Immunol 5: 201–214. [DOI] [PubMed] [Google Scholar]
- 9. Moretta L, Locatelli F, Pende D, Marcenaro E, Mingari MC, et al. (2011) Killer Ig-like receptor-mediated control of natural killer cell alloreactivity in haploidentical hematopoietic stem cell transplantation. Blood 117: 764–771. [DOI] [PubMed] [Google Scholar]
- 10. Velardi A, Ruggeri L, Mancusi A, Aversa F, Christiansen FT (2009) Natural killer cell allorecognition of missing self in allogeneic hematopoietic transplantation: a tool for immunotherapy of leukemia. Curr Opin Immunol 21: 525–530. [DOI] [PubMed] [Google Scholar]
- 11. Pegram HJ, Ritchie DS, Smyth MJ, Wiernik A, Prince HM, et al. (2011) Alloreactive natural killer cells in hematopoietic stem cell transplantation. Leuk Res 35: 14–21. [DOI] [PubMed] [Google Scholar]
- 12. Weng N-p, Akbar AN, Goronzy J (2009) CD28− T cells: their role in the age-associated decline of immune function. Trends Immunol 30: 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Snyder MR, Nakajima T, Leibson PJ, Weyand CM, Goronzy JJ (2004) Stimulatory killer Ig-like receptors modulate T cell activation through DAP12-dependent and DAP12-independent mechanisms. J Immunol 173: 3725–3731. [DOI] [PubMed] [Google Scholar]
- 14. Thewissen M, Somers V, Hellings N, Fraussen J, Damoiseaux J, et al. (2007) CD4+CD28null T cells in autoimmune disease: Pathogenic features and decreased susceptibility to immunoregulation. J Immunol 179: 6514–6523. [DOI] [PubMed] [Google Scholar]
- 15. Middleton D, Meenagh A, Moscoso J, Arnaiz-Villena A (2008) Killer immunoglobulin receptor gene and allele frequencies in Caucasoid, Oriental and Black populations from different continents. Tissue Antigens 71: 105–113. [DOI] [PubMed] [Google Scholar]
- 16. Middleton D, Gonzelez F (2009) The extensive polymorphism of KIR genes. Immunology 129: 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shilling HG, Young N, Guethlein LA, Cheng NW, Gardiner CM, et al. (2002) Genetic control of human NK repertoire. J Immunol 169: 239–247. [DOI] [PubMed] [Google Scholar]
- 18. Nowak I, Majorczyk E, Wiśniewski A, Pawlik A, Magott-Procelewska M, et al. (2010) Does the KIR2DS5 gene protect from some human diseases? PLoS ONE 5: e12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, et al. (1999) The Banff 97 working classification of renal allograft pathology. Kidney International 55: 713–723. [DOI] [PubMed] [Google Scholar]
- 20. Majorczyk E, Pawlik A, Łuszczek W, Nowak I, Wiśniewski A, et al. (2007) Associations of killer cell immunoglobulin-like receptor genes with complications of rheumatoid arthritis. Genes Immunity 8: 678–683. [DOI] [PubMed] [Google Scholar]
- 21. Łuszczek W, Mańczak M, Cisło M, Nockowski P, Wiśniewski A, et al. (2004) Gene for the activating natural killer cell receptor, KIR2DS1, is associated with susceptibility to psoriasis vulgaris. Hum Immunol 65: 758–766. [DOI] [PubMed] [Google Scholar]
- 22. Vilches C, Castaño J, Gómez-Lozano N, Estefanía E (2007) Facilitation of KIR genotyping by a PCR-SSP method that amplifies short DNA fragments. Tissue Antigens 70: 415–422. [DOI] [PubMed] [Google Scholar]
- 23. Sun JY, Gaidulis L, Miller MM, Goto RM, Rodriguez R, et al. (2004) Development of a multiplex PCR-SSP method for killer cell immunoglobulin-like receptor genotyping. Tissue Antigens 64: 462–468. [DOI] [PubMed] [Google Scholar]
- 24. Excoffier L, Slatkin M (1995) Maximum-likelihood estimation of molecular haplotype frequencies in a diploid population. Mol Biol Evol 12: 921–927. [DOI] [PubMed] [Google Scholar]
- 25. Abdallah JM, Goffinet B, Cierco-Ayrolles C, Pérez-Enciso M (2003) Linkage disequilibrium fine mapping of quantitative trait loci: a simulation study. Genet Sel Evol 35: 513–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bhasi K, Zhang L, Brazeau D, Zhang A, Ramanathan M (2006) Information-theoretic identification of predictive SNPs and supervised visualization of genome-wide association studies. Nucleic Acids Res 34: e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu Z, Lin S (2005) Multilocus LD measure and tagging SNP selection with generalized mutual information. Genet Epidemiol 29: 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Single RM, Martin MP, Meyer D, Gao X, Carrington M (2008) Methods for assessing gene content diversity of KIR with examples from a global set of populations. Immunogenetics 60: 711–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yen JH, Moore BE, Nakajima T, Scholl D, Schaid DJ, et al. (2001) Major histocompatibility complex class I-recognizing receptors are disease risk genes in rheumatoid arthritis. J Exp Med 193: 1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yadav AK, Jha V (2011) CD4+CD28null cells are expanded and exhibit a cytolytic profile in end-stage renal disease patients on peritoneal dialysis. Nephrol Dial Transplant 26: 1689–1694. [DOI] [PubMed] [Google Scholar]
- 31. Betjes MGH, Huisman M, Weimar W, Litjens NHR (2008) Expansion of cytolytic CD4+CD28− T cells in end-stage renal disease. Kidney International 74: 760–767. [DOI] [PubMed] [Google Scholar]
- 32. Middleton D, Gonzalez A, Gilmore PM (2007) Studies on the expression of the deleted KIR2DS4*003 gene product and distribution of KIR2DS4 deleted and nondeleted versions in different populations. Hum Immunol 68: 128–134. [DOI] [PubMed] [Google Scholar]
- 33. van der Meer A, Schaap NPM, Schattenberg AVMB, van Cranenbroek B, Tijssen HJ, Joosten I (2998) KIR2DS5 is associated with leukemia free survival after HLA identical stem cell transplantation in chronic myeloid leukemia patients. Molec Immunol 45: 3631–3638. [DOI] [PubMed] [Google Scholar]
- 34. Graef T, Moesta AK, Norman PJ, Abi-Rached L, Vago L, et al. (2009) KIR2DS4 is a product of gene conversion with KIR3DL2 that introduced specificity for HLA-A*11 while diminishing avidity for HLA-C. J Exp Med 206: 2557–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hanvesakul R, Kubal C, Moore J, Neil D, Cook M, Ball S, et al. (2011) KIR and HLA-C interactions promote differential dendritic cell maturation and is a major determinant of graft failure following kidney transplantation. PLoS ONE 6: e23631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schmidt AH, Solloch UV, Pingel J, Baier D, Böhme I, et al. (2011) High resolution human leukocyte antigen allele and haplotype frequencies of the Polish population based on 20,653 stem cell donors. Hum Immunol 72: 558–565. [DOI] [PubMed] [Google Scholar]
- 37. Tran TH, Mytilineos J, Scherer S, Laux G, Middleton D, et al. (2005) Analysis of KIR ligand incompatibility in human renal transplantation. Transplantation 80: 1121–1123. [DOI] [PubMed] [Google Scholar]
- 38. Kreijveld E, van der Meer A, Tijssen HJ, Hilbrands LB, Joosten I (2007) KIR gene and KIR ligand analysis to predict graft rejection after renal transplantation. Transplantation 84: 1045–1051. [DOI] [PubMed] [Google Scholar]
- 39. Cirocco RE, Mathew JM, Burke GW 3rd, Esquenazi V, Miller J (2007) Killer cell immunoglobulin-like receptor polymorphisms in HLA-identical kidney transplant recipients: lack of 2DL2 and 2DS2 may be associated with poor graft function. Tissue Antigens 69 Suppl 1:S123–S124. [DOI] [PubMed] [Google Scholar]
- 40. Kunert K, Seiler M, Mashreghi MF, Klippert K, Schönemann C, et al. (2007) KIR/HLA ligand incompatibility in kidney transplantation. Transplantation 84: 1527–1533. [DOI] [PubMed] [Google Scholar]
- 41. van Bergen J, Thompson A, Haasnoot GW, Roodnat JI, de Fijter JW, et al. (2011) KIR-ligand mismatches are associated with reduced long-term graft survival in HLA-compatible kidney transplantation. Am J Transplant 11: 1959–1964. [DOI] [PubMed] [Google Scholar]
- 42. Rajalingam R, Gebel HM (2011) KIR-HLA mismatching in human renal allograft transplantation: Emergence of a new concept. Am J Transplant 11: 1771–1772. [DOI] [PubMed] [Google Scholar]
- 43. López-Álvarez MR, Campillo JA, Legaz I, Blanco-García RM, Salgado-Cecilia G, et al. (2011) Divergences in KIR2D+ natural killer and KIR2D+ CD8+ T-cell reconstitution following liver transplantation. Hum Immunol 72: 229–237. [DOI] [PubMed] [Google Scholar]