Abstract
If environmental stress provides conditions under which positive relationships between plant species richness and productivity become apparent, then species that seem functionally redundant under constant conditions may add to community functioning under variable conditions. Using naturally co-occurring mosses and liverworts, we constructed bryophyte communities to test relationships between species diversity (1, 2, 4, 8, 16, 24, or 32 species) and productivity under constant conditions and when exposed to experimental drought. We found no relationship between species richness and biomass under constant conditions. However, when communities were exposed to experimental drought, biomass increased with species richness. Responses of individual species demonstrated that facilitative interactions rather than sampling effects or niche complementarity best explained results—survivorship increased for almost all species, and those species least resistant to drought in monoculture had the greatest increase in biomass. Positive interactions may be an important but previously underemphasized mechanism linking high diversity to high productivity under stressful environmental conditions.
The potential for loss of ecosystem functioning (physical and chemical processes occurring within ecosystems) with declining species diversity has prompted a number of recent studies that experimentally examine relationships between plant species richness and ecosystem processes, especially productivity (e.g., refs. 1–4). Although some studies have found that increased plant species richness resulted in increased productivity (1, 2, 4), the existence of positive relationships between diversity and productivity and the extent to which they are caused by simple “sampling effects” (increased probability of including highly productive dominant species in diverse communities), as opposed to more elaborate mechanisms such as niche complementarity (reduced interspecific compared with intraspecific competition), remain highly contentious (refs. 5–9; http://www.sciencemag.org/cgi/content/full/289/5483/1255a). However, most of these experiments were conducted under fairly constant conditions, where the value of high species richness may not be apparent. Instead, species that seem to be “redundant” under one set of conditions may provide additional services under different conditions. Such a scenario has been linked most often to the “insurance hypothesis” (10–14), a more complicated sampling-effects model suggesting that in communities with high levels of diversity at least some of the species will be highly productive after environmental disturbance (e.g., a drought or flood). However, this pattern also could arise through niche complementarity or even positive interactions among species in more diverse communities. Demonstrating compensatory abilities of apparently redundant species within a community would provide a powerful argument for the maintenance of high diversity in natural systems (12, 15), and also may be able to explain the inconsistent results of earlier experimental work.
We tested the value of high diversity under environmental variability by exposing experimentally assembled New Zealand bryophyte communities with different levels of diversity to constant or variable conditions and examining changes in the relationship between diversity and productivity. We then examined whether relationships between diversity and productivity could be explained by sampling effects (greater probability of, including large or drought-resistant species), or whether additional explanations were needed.
Methods
We created communities with 1, 2, 4, 8, 16, or 32 species (Tables 1 and 2) that came from similar habitats and were found growing in close proximity to one another in the field. Bryophyte diversity in New Zealand is extremely high at small spatial scales and these levels of diversity spanned those found in the field. The species pool increased with diversity (Table 1); using the same species pool throughout would have either resulted in communities that became increasingly similar in composition as diversity increased, or would have required an extremely large number of plots in order for all species to be included at all diversities. Of the 208 communities in our design, 7 could not be planted because of insufficient material (including monocultures of species 30 and 32 in Table 2). All other species were planted in monoculture with the exception of the eight species added to create the species pool for the highest diversity mixes. Half of the mixtures were composed of combinations of lower-diversity mixtures, whereas the other half consisted of random draws from the species pool. There were two replicates of each mixture.
Table 1.
Experimental design
| Diversity | Species pool | No. unique mixtures | No. plots |
|---|---|---|---|
| 1 | 32 | 32 | 64 |
| 2 | 24 | 16 | 32 |
| 4 | 24 | 16 | 32 |
| 8 | 24 | 16 | 32 |
| 16 | 32 | 8 | 16 |
| 24 | 32 | 8 | 16 |
| 32 | 40 | 8 | 16 |
Identical mixtures were used under control and drought conditions.
Table 2.
Species used in the experiment
| 1. Monoclea forsteri (l) | 21. Distichophyllum crispulum (m) |
| 2. Aneura orbiculata (l) | 22. Weymouthia cochlearifolia (m) |
| 3. Symphogyna hymenophyllum (l) | 23. Lopidium concinnum (m) |
| 4. Bazzania adnexa (l) | 24. Acrophyllum dentatum (m) |
| 5. Hypopterygium filiculaeforme (m) | 25. Fissidens asplenioides (m) |
| 6. Hypnodendron kerrii (m) | 26. Plagiochila stephensoniana (l) |
| 7. Trichocolea lanata (l) | 27. Hypopterygium rotulatum (m) |
| 8. Ptychomnion aciculare (m) | 28. Marchantia foliacea (l) |
| 9. Jungermannia sp. (l) | 29. Lepidolaera sp. (l) |
| 10. Thuidium furfurosum (m) | 30. Dicronoloma menziesii (m) |
| 11. Lepidozia microphylla (l) | 31. Rhizogonium novae-hollandiae (m) |
| 12. Dicranoloma billardierei (m) | 32. Papillaria flavo-limbata (m) |
| 13. Symphogyna prolifera (l) | 33. Racopilum convolutaceum (m) |
| 14. Shistochila nobilis (l) | 34. Telaranea praenitus (l) |
| 15. Trichocolea mollissima (l) | 35. Plagiochila sp. (l) |
| 16. Heteroscyphus coalitus (l) | 36. Wijkia extenuata (m) |
| 17. Leucobryum candidum (m) | 37. Pyrrhobryum bifarium (m) |
| 18. Hypnodendron menziesi (m) | 38. Porella elegantula (l) |
| 19. Riccardia sp. 1 (l) | 39. Campylopus introflexus (m) |
| 20. Riccardia sp. 2 (l) | 40. Hypnum cuppresiforme (m) |
Nomenclature follows Beever et al. (16) for mosses (indicated by “m”) and Allen and Child (17) for liverworts (indicated by “l”). In some cases, identification was only to genus because of a lack of identifying structures or poor taxonomic resolution. Species are listed in order of inclusion in the species pools (see Table 1). For example, only species 1–24 were used in two-species communities.
Communities were created by planting pieces of plants (“individuals,” ≈0.03 g each) in grid patterns (13 rows and 19 columns, individuals ≥ 1.5 cm apart) on a layer of peat in 0.4 m × 0.6 m plastic trays with drainage holes. The total density was equal for all plots (247 individuals per tray, ≈30 g/m2), and species were allocated randomly to each location with equal probabilities. Trays were covered with plastic, placed in the laboratory, and maintained at constant high humidity (relative humidity 95–100%) and low light conditions (<100 lumens per m2). After 1 year, 1 replicate of each mixture remained under these conditions (“control plots”) whereas the other replicate was exposed to 5 days of low humidity (20–30%) and higher light (100–200% increase over levels in control plots) by removal of the plastic covering. Soils remained moist to the touch at the end of the drought period. We harvested the biomass from half of each tray (0.3 m × 0.4 m) 3 months after the drought treatment. At this time, the total (live + dead) biomass of one-third of the trays was less than the initial biomass, whereas approximately half of the trays showed a ≥50% increase in biomass; in 20% of the trays, biomass had more than doubled. These are minimum estimates of productivity as they do not take tissue turnover into account.
A primary criticism of past diversity experiments has been the confounding effects of particular species in driving diversity results. Because species contributions were not identical at all diversity levels, we determined an expected biomass for each tray assuming there were no interspecific interactions. Expected biomass was calculated by averaging, for all species planted in the mix, the biomass in monoculture under the appropriate condition (i.e., control conditions for control plots, drought conditions for drought plots). The difference between the observed and expected values (DIFF) indicated whether each community had a greater (DIFF > 0) or lesser (DIFF < 0) biomass than predicted by the performance of the member species when grown in monoculture. For plots with the highest diversity (32 species), the expected values were calculated only for the species for which we had monocultures. For the additional species used at the highest level of diversity (a maximum of 8 per plot) we assumed no net difference between monoculture and the highest-diversity plots. In the unlikely event that they had much greater biomass or drought tolerance than the other 32 species, such a bias would either not influence this metric (if effects were purely additive) or would provide a conservative test for positive relationships (if other species were outcompeted). For the two species for which monocultures were part of the design but which could not be planted, values per individual from the lowest diversity level planted were used as expected values, making the test more conservative. Deleting trays that included these species or ignoring their contribution in all trays did not significantly alter results. We also calculated the difference between the observed biomass of individuals of each species in the community (biomass of each species in tray) and their expected biomass (biomass in monoculture divided by diversity level), which we term SDIFF.
We used regression to examine the effect of drought and diversity on total live biomass per tray and DIFF. Diversity was treated as a linear variable; using the log (base 2) did not improve model fit. All models included the date of planting (which was randomized across all treatments) as a continuous explanatory variable. When testing for interactions between drought and diversity (using ANOVA), we excluded 17 trays in which all plants had died before the start of the drought treatment. Biomass data were rank-transformed to meet model assumptions.
Results and Discussions
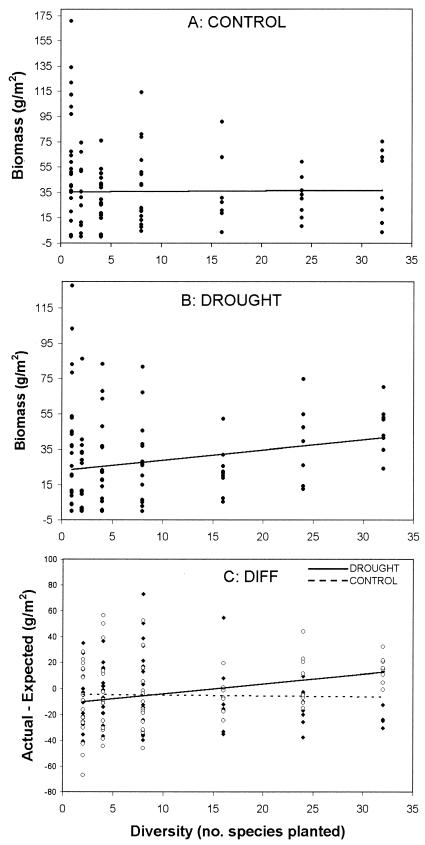
In the control plots, there was no relationship between species richness and live biomass (F(1, 98) = 0.37, P = 0.60; Fig. 1A). In contrast, in the drought plots there was a significant increase in live biomass with species richness (F(1, 97) = 10.48, P = 0.002; Fig. 1B), leading to a significant interaction between treatment and diversity (F(1, 179) = 5.47, P = 0.020). For control plots, the difference between the observed and the expected biomass (DIFF) did not change with species richness (F(1, 69) = 1.25, P = 0.27; Fig. 1C). For drought plots, the observed values were higher than expected at high diversity (F(1, 70) = 8.11, P = 0.006; Fig. 1C), again leading to a significant interaction between diversity and treatment (F(1, 139) = 7.69, P = 0.006). These comparisons demonstrate that the results were not caused by a simple species-sampling effect.
Figure 1.
Relationship between live biomass per plot and diversity (number of species planted). Points are values for individual trays. (A) Live biomass for control plots. (B) Live biomass for drought plots. (C) Difference in live biomass between actual plots and that expected based on the mean biomass in monoculture of all species in the mixture (“DIFF” in the text).
Both the sampling effect and niche complementarity (reduced interspecific compared with intraspecific competition) should result in increased biomass with increased species richness (5, 6, 8), yet in control plots there was no relationship between biomass and species richness. The sampling effect depends on a positive relationship between plant size in monoculture and dominance. Instead, our communities showed a “negative selection effect” (18, 19): there was a negative relationship between biomass per individual planted in monoculture and mean biomass (for diversities >1) per individual in mixture for the 30 species for which data were available (Pearson correlations range from 0.49 to 0.80; P < 0.01 for all), suggesting that species which were large in monoculture were poor competitors in mixture.
We examined the potential for increased niche complementarity with increased diversity by testing whether including more plant architectures resulted in greater biomass under control conditions. Plants were divided into seven growth forms: dendroid, tall turf, short turf, mat, thalloid, weft, and pendant (20). The number of growth forms did not explain any of the variation in live biomass in control plots (F(1, 67) = 0.00, P = 0.98). Plants of different growth forms did vary in their contribution to DIFF (F(6, 137) = 2.21, P = 0.045), but no growth form showed a consistent effect with changing diversity in control plots (P > 0.1 for all). Furthermore, although tall species contributed the most biomass (correlation between height of individual species and biomass: r = 0.60, P < 0.001), and thus greater mean plant height resulted in greater mean live biomass (F(1, 98) = 19.45, P < 0.0001), variation in plant height did not explain any of the variation in DIFF (P > 0.1). Thus, although the negative selection effect could be hiding positive effects of niche complementarity (resulting in no relationship between species richness and biomass overall), there is no evidence to support the notion that multiple growth forms lead to greater biomass under control conditions.
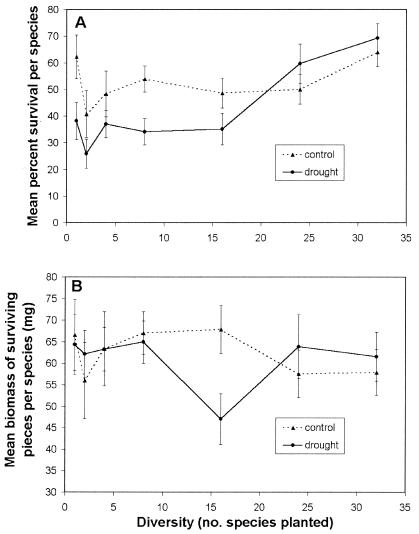
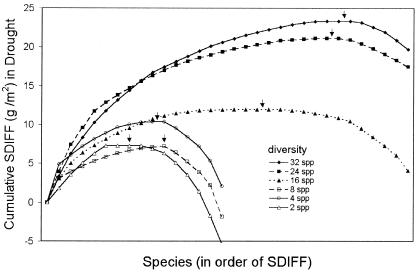
Results for total live biomass give the impression of being consistent with the insurance hypothesis: under control conditions, there was no advantage (in terms of biomass) of having many species, whereas under drought conditions, high diversity prevented the decrease in biomass found in low-diversity plots. However, responses of individual species do not support this hypothesis. Under the insurance hypothesis, increased biomass under drought as a result of increased diversity should occur through an increased probability of the community, including species that are capable of dominating under the changed environmental conditions. This hypothesis leads to three predictions: (i) increased biomass at high diversity can be explained primarily by increased biomass of surviving (drought-resistant) species at the expense of drought-sensitive species, resulting in greater variation between species than under control conditions; (ii) species that are the most resistant to drought will show the greatest increase in biomass with diversity; and (iii) the proportion of species that contribute positively to the higher-than-expected biomass will remain constant or decrease with diversity. Our data contradict all three predictions. First, variation in biomass between species under drought was similar to that under control conditions at all levels of diversity (ANOVA on absolute values of residuals: P > 0.25 for all diversity levels). Because there were very few new individuals (recruits) in our plots and they contributed a negligible amount of biomass (<1% for all species), the number initially planted and their survival and growth determined final biomass for each species in a tray. Increased biomass at high diversity was largely because of increased survivorship in most species (R2 = 0.80). Survivorship increased with increasing diversity under drought conditions but not in control plots (Fig. 2A). In contrast, biomass per surviving plant changed little (Fig. 2B), and explained little of the variation in total live biomass (R2 = 0.09). Second, species showing the greatest increase with diversity were least resistant to drought (Pearson correlation for SDIFF in polyculture and percent change across all plots in drought relative to control: r = −0.49, P = 0.005). Finally, for a given species pool the proportion of species that contributed positively (mean SDIFF > 0 at a given level of diversity) to the mean DIFF value increased with diversity (Fig. 3), with >75% of species showing a higher-than-expected biomass at high diversity (24 or 32 species).
Figure 2.
Responses of individual species to changes in diversity in control and drought plots. Means for 2, 4, and 8 species mixtures were calculated based on the 16 species found in those mixtures; all other means are for 32 species. Error bars are standard errors. (A) Change in mean survival per species at each level of diversity. (B) Means across species for biomass per surviving unit.
Figure 3.
Cumulative difference (“SDIFF” values) between observed biomass and expected biomass (based on mean biomass of species in the mix in monoculture under drought conditions) in drought plots. For each species, mean biomass per individual planted in monoculture under drought was subtracted from mean biomass per individual planted at each diversity level (symbols given in legend). These differences were ranked from high to low and presented cumulatively. The highest point on each curve (marked with an arrow) is the switch from species that have positive to those that have negative contributions to DIFF. The sums of these differences (the last point on each line) correspond to the mean DIFF across all plots at that diversity level (see Fig. 1C). For the 32-species plots, an additional 8 species were in the pool that could not be represented here because they were not planted in monoculture.
There is an alternative explanation for the increase in survival of most species at high diversity under drought: an increase in positive interactions between plants, possibly enhanced by niche complementarity under greater diversity. A recent study (21) has demonstrated positive density-dependent performance in some moss species under dry, high-light-level conditions. In our communities, positive interactions could have arisen from an increase in relative humidity resulting from transpiration of plants with different architectures, or through a decrease in photoinhibition in short plants protected by taller plants. As in control plots, growth forms varied in their contributions to DIFF (F(6, 137) = 7.14, P < 0.001, R2 = 0.24) but under drought, four growth forms (mat, short turf, tall turf, and dendroid) showed an increase in biomass with increased diversity (P < 0.05). The number of different growth forms in the plot explained a significant amount of variation in live biomass (F(1, 68) = 6.43, P = 0.013). However, these effects could not be separated from species richness effects because both explained a significant amount of the variation when entered into the model first (P < 0.01), but not when entered after the other variable was included (for species richness: F(1, 68) = 0.68, P = 0.78; for growth-form richness: F(1, 68) = 1.44, P = 0.23). Mean height of plants in drought plots did not explain variation in live biomass (F(1, 98) = 0.05, P = 0.83) or SDIFF (F(1, 69) = 1.08, P = 0.3). Tall plants were the most affected by drought when in monoculture (correlation between biomass in drought minus biomass in control vs. height: r = −0.34, P = 0.058), but they also showed the greatest positive response to increased diversity. These results suggest that tall plants are more affected by low humidity than short plants in greater contact with the soil, and transpiration by short species may increase humidity under the canopy and thus benefit taller plants. Variance in height among plants within a plot indeed did explain variation in DIFF (F(1, 69) = 8.87, P = 0.004), but this effect was again confounded with diversity (both species richness and variance in height were significant when entered into the model first, but not second; for variance in height: F(1, 68) = 2.03, P = 0.16).
By themselves, these results are consistent with both niche complementarity and positive interactions under drought conditions, both of which should increase with increased diversity of plant architectures. However, the evidence suggests that positive interactions rather than niche complementarity play the larger role. First, the large increase in survival but not biomass per individual for almost all species is more consistent with improved physical conditions than with reduced competition. Second, niche complementarity does not explain why species most affected by drought showed the greatest positive response to diversity. Finally, increasing the number of growth forms had a positive effect on biomass only under drought conditions. Facilitative interactions, particularly the maintenance of higher subcanopy humidity by species in full contact with the substrate, can explain all three observations.
A growing body of literature has demonstrated that facilitative effects are important under stressful conditions, at least when environmental harshness is ameliorated by the facilitator species (e.g., refs. 21–27), and our results are consistent with this idea. Although the particular mechanism that probably drives our results (greater humidity) may be specific to bryophytes, the evidence for interspecific facilitation in a wide variety of plant systems suggests that changes in diversity effects under environmental stress are unlikely to be unique to bryophytes. Although sampling effects and niche complementarity as mechanisms for generating positive relationships between biomass and diversity have been the subject of heated debate, the role of facilitative interactions has not been evaluated (28, 29). Our results reinforce the importance of high species richness for high productivity under fluctuating environmental conditions but suggest that positive interspecific interactions may play a greater role than previously thought. Theoretical models suggest that strong positive correlations in independent species' responses to environmental fluctuations may reduce stability–diversity relationships, whereas strong positive interactions, such those as we found, can create positive diversity effects (30). In this case, a loss of species from the community may result in a loss in both productivity and stability.
Our bryophyte communities responded differently (under control conditions) to changes in diversity than the grassland vascular plant species that most studies have used, demonstrating that relationships between diversity and ecosystem functioning need to be evaluated in multiple plant-community types. The lack of a relationship for bryophytes under constant conditions seems to be because of an inverse relationship between size in monoculture and dominance in polyculture. Yet a common assumption underlying discussions of sampling effects in diversity–productivity relationships is that size in monoculture and dominance are either positively related or not related (19). Variation in results from earlier experiments may be due in part to differences in this relationship in different communities. Finally, we find support for the notion that species that seem to play redundant roles in one condition may be complementary or facilitative of one another under other conditions, and that the value of diversity may be underestimated in short-term, constant condition experiments.
Acknowledgments
We thank J. Shulmeister for the initial suggestion to use bryophytes; M. Prebble and G. Straker for help with planting; B. Sneddon and B. Polly for identification of bryophytes; and A. Hector, M. Loreau, and D. Wardle for constructive comments on the manuscript.
Abbreviation
- DIFF
difference between the observed and expected values
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Naeem S, Håkansson K, Lawton J H, Crawley M J, Thompson L J. Oikos. 1996;76:259–265. [Google Scholar]
- 2.Tilman D, Wedin D, Knops J. Nature (London) 1996;379:718–719. [Google Scholar]
- 3.Hooper D U, Vitousek PM. Science. 1997;277:1302–1305. [Google Scholar]
- 4.Hector A, Schmid B, Beierkuhnlein C, Caldeira M C, Diemer M, Dimitrakopoulos P G, Finn J A, Freitas H, Giller P S, Good J, et al. Science. 1999;286:1123–1127. doi: 10.1126/science.286.5442.1123. [DOI] [PubMed] [Google Scholar]
- 5.Aarssen L W. Oikos. 1997;80:183–184. [Google Scholar]
- 6.Huston M A. Oecologia. 1997;110:449–460. doi: 10.1007/s004420050180. [DOI] [PubMed] [Google Scholar]
- 7.Van der Heijden M G A. Oikos. 1999;87:408–410. [Google Scholar]
- 8.Wardle D A. Oikos. 1999;87:403–407. [Google Scholar]
- 9.Huston M A, Aarssen L W, Austin M P, Cade B S, Fridley J D, Garnier E, Grime J P, Hodgson J, Lauenroth W K, Thompson K, et al. Science. 2000;289:1255. doi: 10.1126/science.289.5483.1255a. [DOI] [PubMed] [Google Scholar]
- 10.Walker B H. Cons Biol. 1992;6:18–23. [Google Scholar]
- 11.Lawton J H, Brown V K. In: Biodiversity and Ecosystem Function. Schulze E-D, Mooney H A, editors. Berlin: Springer; 1993. pp. 255–270. [Google Scholar]
- 12.Naeem S. Cons Biol. 1998;12:39–45. [Google Scholar]
- 13.Yachi S, Loreau M. Proc Natl Acad Sci USA. 1999;96:1463–1468. doi: 10.1073/pnas.96.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petchey O L, McPhearson P T, Casey T M, Morin P J. Nature (London) 1999;402:69–71. [Google Scholar]
- 15.Chapin F S, III, Sala O E, Burke I C, Grime J P, Hooper D V, Lauenroth W K, Lombard A, Mooney H A, Mosier A R, Naeem S, et al. Bioscience. 1998;48:45–53. [Google Scholar]
- 16.Beever J, Allison K W, Child J. The Mosses of New Zealand. Dunedin, NZ: Univ. of Otago Press; 1992. [Google Scholar]
- 17.Allen K W, Child J. Liverworts of New Zealand. Dunedin, NZ: Univ. of Otago Press; 1975. [Google Scholar]
- 18.Loreau M. Oikos. 2000;91:3–17. [Google Scholar]
- 19.Hector, A., Schmid, B., Beierkuhnlein, C., Caldeira, M. C., Diemer, M., Dimitrakopoulos, P. G., Finn, J. A., Freitas, H., Giller, P. S., Good, J., et al. (in press) in Functional Consequences of Biodiversity: Experimental Progress and Theoretical Extensions, eds. Kinzig, A., Tilman, D. & Pacala, S. (Princeton Univ. Press, Princeton).
- 20.Watson E V. The Structure and Life of Bryophytes. London: Hutchinson; 1964. [Google Scholar]
- 21.Pedersen B, Hanslin H M, Bakken S. Ecology. 2001;82:70–88. [Google Scholar]
- 22.Bertness M D. Ecology. 1991;72:125–137. [Google Scholar]
- 23.Bertness M D, Callaway R. Trends Ecol Evol. 1994;9:191–193. doi: 10.1016/0169-5347(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 24.Greenlee J, Callaway R M. Am Nat. 1996;148:386–396. [Google Scholar]
- 25.Callaway R M, Walker L R. Ecology. 1997;78:1958–1965. [Google Scholar]
- 26.Kitzberger T, Steinaker D F, Veblen T T. Ecology. 2000;81:1914–1924. [Google Scholar]
- 27.Tielbörger K, Kadmon R. Ecology. 2000;81:1544–1553. [Google Scholar]
- 28.Tilman D. Ecology. 1999;80:1455–1474. [Google Scholar]
- 29.Lehman C L, Tilman D. Am Nat. 2000;156:534–552. doi: 10.1086/303402. [DOI] [PubMed] [Google Scholar]
- 30.Doak D F, Bigger D, Harding E K, Marvier M A, O'Malley R E, Thomson D. Am Nat. 1998;151:264–276. doi: 10.1086/286117. [DOI] [PubMed] [Google Scholar]