Abstract
The serine/threonine kinase protein kinase B (PKB)/Akt plays a central role in many cellular processes, including cell growth, glucose metabolism, and apoptosis. However, the identification and validation of novel regulators or effectors is key to future advances in understanding the multiple functions of PKB. Here we report the identification of a novel PKB binding protein, called Ft1, from a cDNA library screen using a green fluorescent protein-based protein-fragment complementation assay. We show that the Ft1 protein interacts directly with PKB, enhancing the phosphorylation of both of its regulatory sites by promoting its interaction with the upstream kinase PDK1. Further, the modulation of PKB activity by Ft1 has a strong effect on the apoptosis susceptibility of T lymphocytes treated with glucocorticoids. We demonstrate that this phenomenon occurs via a PDK1/PKB/GSK3/NF-ATc signaling cascade that controls the production of the proapoptotic hormone Fas ligand. The wide distribution of Ft1 in adult tissues suggests that it could be a general regulator of PKB activity in the control of differentiation, proliferation, and apoptosis in many cell types.
The serine/threonine protein kinase protein kinase B (PKB)/Akt has been demonstrated to play a central role in a number of cellular responses to growth factors and insulin, which include cell growth, protein synthesis, and antiapoptotic and survival signals (6, 7, 13, 23, 27). Various orthologues of PKB have also been shown to play key roles in embryogenesis, particularly in the regulation of apoptosis during normal development, and in programmed cell death throughout the lifetime of an organism (13, 27). Considerable efforts to map out the link between growth factor receptors and PKB have resulted in a model in which activation of phosphatidylinositol 3-kinase (PI3K) by insulin and growth factor receptors results in the production of the lipid derivative phosphatidylinositol (3,4,5)-triphosphate (PIP3). PIP3 acts as a receptor for the pleckstrin homology (PH) domains of PKB and the 3-phosphoinositide-dependent protein kinase 1 (PDK1), directing their localization to the plasma membrane, where PKB is phosphorylated and activated by PDK1 (for a review, see references 5, 37, and 48) and perhaps by autophosphorylation (46). This initiation process and the subsequent phosphorylation of substrates are regulated by a number of proteins that have been identified and demonstrated to be bona fide mediators of PKB activity. However, a key to future advances in understanding the multiple functions of PKB rests in identifying and validating novel substrates and regulators (7).
In the studies described here, we sought to identify new PKB-interacting proteins that could be involved in modulating or integrating signaling pathways that impinge upon PKB activation. To achieve this aim, we applied a novel expression cloning strategy based on a protein-fragment complementation assay (PCA) using green fluorescent protein (GFP) as a reporter to screen a human brain cDNA library, using PKB as bait. We identified a human gene encoding a protein highly similar to the mouse protein Ft1. The corresponding Ft1 gene was found to be deleted in a Ft/+ mouse mutant characterized by developmental abnormalities, including fused toes on the fore limbs and thymic hyperplasia, and a reduced sensitivity of thymocytes to dexamethasone-induced apoptosis (29, 47). We demonstrate here that the Ft1 protein binds directly to PKB, enhancing the phosphorylation of both of its regulatory sites by promoting its interaction with the upstream kinase PDK1. Further, and consistent with the Ft/+ phenotype, the enhancement of PKB activity by Ft1 dramatically increased the apoptosis susceptibility of T lymphocytes treated with glucocorticoids. We demonstrate that this phenomenon occurs via a PDK1/PKB/GSK3/NF-ATc signaling cascade, resulting in increased production of the proapoptotic hormone Fas ligand. Activation of the transcription factor NF-ATc can lead to the production of antiapoptotic (interleukin-2 [IL-2]) as well as proapoptotic (Fas ligand) factors, depending on the balance of the total inputs applied to the cell, to preserve the homeostasis of T-cell populations (elimination of autoreactive or excess T cells) (1, 26, 28). Our studies demonstrate that Ft1 (via a PKB-dependent pathway) can alter this balance by inducing the production of Fas ligand, leading to profound apoptosis in the cell population, when the pathway is made dominant over IL-2 signaling by treatment of the cells with glucocorticoids.
MATERIALS AND METHODS
DNA constructs.
The full-length cDNAs encoding PKB and PDK1 were amplified by PCR and subcloned 5′ or 3′ of the F[1] (amino acids [aa]1 to 158) and F[2] (aa 159 to 239) fragments of GFP (pCMS-EGFP; Clontech, Palo Alto, Calif.) into the eukaryotic expression vector pMT3 (24). The PKB-F[2] fusion was also inserted in a pMT3 vector where the ampicillin resistance gene had been replaced by a chloramphenicol resistance gene (pMT3-chloramphenicol) for the purpose of the cDNA library screen. In all cases, a 10-amino-acid flexible linker consisting of (Gly-Gly-Gly-Gly-Ser)2 was inserted between the cDNA and the GFP fragments to ensure that the orientation and arrangement of the fusions in space were optimal to bring the GFP fragments into close proximity. The F[1]-GCN4 and GCN4-F[2] constructs consisted of fusions with GCN4 leucine zipper-forming sequences used as controls. For the GFP PCA-based cDNA library screen, a human brain cDNA library was excised from the vector pEXP1 (ClonCapture cDNA library; Clontech) using SfiI restriction sites and inserted into the pMT3 vector, 3′ of the F[1] fragment of GFP and a 10-amino-acid flexible linker. The PCA-cDNA library fusion expression vectors were divided into several pools (according to the size of the inserted cDNAs, from 0.5 to 4.6 kb) and amplified at 30°C in liquid medium. After the human Ft1 (hFt1) gene was isolated, a Myc tag-hFt1 fusion was also constructed. The GSK3β (Ser9Ala) point mutant was generated using the QuikChange site-directed mutagenesis kit (Stratagene).
Cell lines.
COS-1, HEK293, and HEK293T cells were grown in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, Calif.) supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, Utah). The human Tag-Jurkat cell line was grown in RPMI 1640 (Invitrogen) supplemented with 10% FBS, 1 mM sodium pyruvate, and 10 mM HEPES. The Tag-Jurkat cells express the simian virus 40 large T antigen and harbor an integrated β-galactosidase reporter plasmid where three tandem copies of the NF-AT binding site direct transcription of the lacZ gene (15).
PCA-based cDNA library screen.
COS-1 cells were plated in 150-mm dishes 24 h before transfection. Cells were transfected (10 μg of total DNA/dish) using Lipofectamine reagent (Invitrogen), at around a 60% confluence, with pMT3 vector harboring the human brain cDNA library fused to the F[1] fragment of GFP (F[1]-cDNA library) and the pMT3-chloramphenicol vector containing the full-length PKB fused to the F[2] fragment of GFP (PKB-F[2]). The F[1]-cDNA library fusions were transfected in several pools, according to their size. At 48 h after transfection, positive clones (folding and reconstitution of GFP from its fragments) were collected on a fluorescence-activated cell sorter (FACS) analyzer (FACScalibur; Becton Dickinson, Franklin Lakes, N.J.). The total DNA from each pool of positive cells was extracted (DNeasy tissue kit; Qiagen, Chatsworth, Calif.), transformed in DH5α bacterial cells, and plated on Luria-Bertani (LB) agar containing 100 μg of ampicillin/ml (no propagation of the chloramphenicol-resistant vector harboring the PKB-F[2] fusion). DNA plasmids containing the F[1]-cDNA fusions were extracted from individual clones and retransfected separately with PKB-F[2] or the F[2] fragment alone (negative control) to discard negative clones that entered the pool during the cell sorting. After this second round of selection, the DNA plasmids corresponding to the positive clones were submitted to sequence analysis.
Cell transfection and fluorometric analysis.
COS-1 and HEK293T cells were split in 12-well plates 24 h before transfection. Cells were transfected, at around 60% confluence, with different pairs of the GFP PCA fusion expression vectors (1 μg of total DNA/well), using Lipofectamine reagent (Invitrogen) according to the manufacturer's instructions. For the microplate measurements, 48 h after transfection cells were washed one time with phosphate-buffered saline (PBS), gently trypsinized, and resuspended in 200 μl of PBS. The total cell suspensions were transferred to 96-well black microtiter plates (Dynex Technologies, Chantilly, Va.) and subjected to fluorometric analysis (Spectra MAX GEMINI XS; Molecular Devices, Sunnyvale, Calif.). Afterwards, the data were normalized to total protein concentration in cell lysates (Bio-Rad protein assay; Bio-Rad, Hercules, Calif.). The background fluorescence intensity corresponding to nontransfected cells was subtracted from the fluorescence intensities of all of the samples. For the fluorescence microscopy, 48 h after transfection cells were washed two times with PBS, incubated for 5 h in serum-free medium, and untreated or treated with 300 nM wortmannin (Calbiochem, San Diego, Calif.) for the last hour. Afterwards, cells were stimulated for 30 min with 10% serum (FBS; HyClone). Cells were washed two times with PBS and mounted on glass slides. Fluorescence microscopy was performed on live cells (Zeiss Axiophot microscope; 100× objective lens).
Preparation of cell lysates and immunoblot analysis.
HEK293T and Tag-Jurkat cells were cotransfected with PDK1 and PKB expression vectors, or with PKB and GSK3β expression vectors, in the presence or absence of hFt1, in 12-well plates as described above. The amounts of DNA transfected in each experiment were kept constant by adding empty vector. HEK293T cells were serum starved for 16 h and stimulated for 15 min with 10% serum (FBS; HyClone) prior to lysis. Tag-Jurkat cells were stimulated for 30 min with 5 μg of phytohemagglutinin (PHA)/ml plus 500 nM phorbol-12-myristate-13-acetate (PMA; Calbiochem) prior to lysis. Cells were lysed 48 h posttransfection (lysis buffer, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate [SDS], 150 mM NaCl, 20 mM Tris [pH 8.0], 20 μg of aprotinin/ml, 5 mM 4-(2-aminoethyl)benzenesulfonyl fluoride, 10 mM NaF, and 5 mM sodium vanadate). Equal amounts of total proteins were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene difluoride membranes, and immunoblotted with antibodies according to the manufacturer's instructions. Anti-PKB, anti-PKB-Thr308-p, anti-PKB-Ser473-p, and anti-GSK3β-Ser9-p were from Cell Signaling (Beverly, Mass.). Anti-GSK3β was from BD Biosciences (San Diego, Calif.). Anti-hFt1 is a custom-made antibody (Washington Biotechnology, Baltimore, Md.). Bound antibodies were detected using horseradish peroxidase-conjugated anti-immunoglobulin G (anti-IgG; Cell Signaling) and a chemiluminescence detection system (NEN Life Science Products, Boston, Mass.).
In vitro kinase assay.
HEK293T cells were cotransfected with PDK1 and PKB expression vectors, in the presence or absence of hFt1, in 12-well plates as described above. Cells were serum starved for 16 h and stimulated for 15 min with 10% serum (FBS) prior to lysis. PKB was immunoprecipitated with immobilized anti-PKB antibodies (Cell Signaling). Immunoprecipitates were washed four times, and the pellets were incubated in kinase buffer (25 mM Tris [pH 7.5], 5 mM β-glycerolphosphate, 2 mM dithiothreitol, 0.1 mM sodium vanadate, 10 mM MgCl2) containing 200 μM ATP and 1 μg of a pure protein substrate (paramyosin fused to GSK-3α/β cross-tide, corresponding to 20 residues surrounding GSK-3α/β-Ser21/9) (Akt kinase assay kit; Cell Signaling) in each reaction mixture. Phosphorylation of the substrate was detected by immunoblotting using anti-GSK3α/β-Ser21/9-p antibodies (Cell Signaling). Expression levels of PKB and hFt1 were determined by immunoblotting total cell lysates with their corresponding antibodies.
In vitro binding assay.
Recombinant PKB and PDK1 were purified from Escherichia coli as His tag fusion proteins (pQE vector; Qiagen). Approximately 5 μg of glutathione S-transferase (GST) or the GST-hFt1 fusions (GST-hFt1-N [aa 1 to 116], GST-hFt1-Ub [aa 117 to 217] and GST-hFt1-C [aa 218 to 292]) was incubated with 3 μg of recombinant PKB or PDK1 for 2 h at 4°C in binding buffer (20 mM Tris [pH 8.0], 150 mM NaCl, 1% Nonidet P-40). Proteins were eluted from glutathione Sepharose using 10 mM glutathione in 50 mM Tris, pH 8.0 (Amersham Pharmacia Biotech, Piscataway, N.J.). PKB or PDK1 that associated with the GST-hFt1 fragment fusions was detected by immunoblotting with anti-PKB (Cell Signaling) and anti-PDK1 (Biosource International, Camarillo, Calif.) antibodies, respectively. Expression levels of the GST-hFt1 fragment fusions were detected by immunoblotting with anti-GST antibodies (Cell Signaling). The same strategy was used to study the association of hFt1 with the GST-PKB fusions (GST-PKB-N [aa 1 to 149], GST-PKB-CAT [aa 150 to 408], and GST-PKB-C [aa 409 to 480]) and the GST-PDK1 fusions (GST-PDK1-N [aa 1 to 81], GST-PDK1-CAT [aa 82 to 342], GST-PDK1-C [aa 343 to 456], and GST-PDK1-PH [aa 457 to 556]).
Coimmunoprecipitation.
Endogenous PKB and PDK1 were immunoprecipitated from HEK293 cell lysates with anti-PKB (Cell Signaling) and anti-PDK1 (Biosource International) antibodies, respectively, and a protein G Plus-protein A-agarose suspension (Calbiochem). Immunoprecipitates were washed four times with lysis buffer (described above), separated on SDS-PAGE, and transferred to a polyvinylidene difluoride membrane. The membrane was immunoblotted with an anti-hFt1 antibody (custom-made antibody; Washington Biotechnology). Expression levels of the endogenous proteins were detected by immunoblotting total lysates (cell extracts before immunoprecipitations) with their corresponding antibodies. For the PKB-PDK1 coimmunoprecipitation experiments, HEK293T cells were cotransfected with PKB and PDK1 expression vectors, in the presence of hFt1 or a truncated version of hFt1 lacking its C-terminal domain (aa 218 to 292) (hFt1ΔCT). PKB was immunoprecipitated, and the amount of PDK1 in the immune complexes was determined by immunoblotting with anti-PDK1 antibodies.
Apoptosis detection.
Tag-Jurkat cells were transfected at 106 cells/well in 12-well plates (2 μg of total DNA/well) using DMRIE-C reagent (Invitrogen) with different combinations of expression vectors (as indicated). The next day, 1 μg of PHA/ml and 50 ng of PMA/ml were added to the growth medium to enhance promoter activity and gene expression. At 48 h after transfection, cells were harvested and incubated for 7 to 8 h in serum-free medium containing 1 μM dexamethasone (Calbiochem). Another set of experiments was also performed by simultaneously adding 5 μg of neutralizing anti-human Fas ligand NOK-1 mouse antibody (BD Biosciences)/ml or control mouse anti-IgG antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.). Cells were washed one time with PBS and kept overnight at 4°C in the same buffer. The next day, cells were stained with annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) according to the manufacturer's instructions (BD Biosciences) and analyzed by flow cytometry (FACScalibur; Becton Dickinson). The transition between early and late apoptosis was very sharp in these experiments. Thus, simultaneous detection of annexin V (early apoptosis) and propidium iodide (late apoptosis) staining was performed.
NF-AT transcription reporter assay.
Tag-Jurkat cells were transfected and treated with dexamethasone as described for the apoptosis detection experiment except that the cells were incubated with dexamethasone for only 4 h, with or without the addition of 200 nM FK506 (Calbiochem). After this incubation, cells were spun down and resuspended in 400 μl of deionized water. One quarter of this cell suspension was mixed with 100 μl of 1 mM fluorescein digalactoside (FDG; Molecular Probes, Eugene, Oreg.) prepared in deionized water and transferred to a 96-well black microtiter plate (Dynex Technologies). The resulting hypotonic solution permeabilizes the cells, allowing the FDG to enter. The plate was incubated at 37°C for 30 min, and the conversion of FDG to fluorescein was detected by fluorometry (Spectra MAX GEMINI XS; Molecular Devices). The background fluorescence intensity corresponding to cells not treated with FDG was subtracted from the fluorescence intensities of all of the samples.
RESULTS
Identification of Ft1 as a PKB binding protein.
The identification of specific substrates or regulatory proteins for many interesting classes of enzymes such as kinases, phosphatases, and proteases is difficult, because they can have very broad substrate specificity and bind to many proteins or protein domains when studied out of the context of other proteins with which they interact in intact living cells (36, 50). It was with this problem in mind that we developed PCAs. The principle of the PCA strategy is that cells simultaneously expressing complementary fragments of an enzyme (F[1] and F[2]) fused to test proteins will produce a colorimetric or fluorescent signal only if the fused proteins physically interact and then bring the complementary fragments of the enzyme or reporter protein into proximity where it can fold and reassemble into its active form (Fig. 1A). In a PCA, protein-protein interactions are detected directly and between full-length proteins expressed in cells in which they normally function, ensuring that subcellular targeting, posttranslational modifications, and interactions with other proteins needed for their correct functioning can occur. We and others have described several PCAs that use different enzyme reporters, including one based on GFP (17, 22, 32, 43).
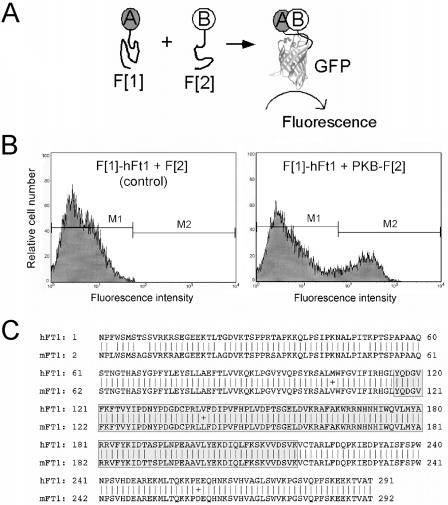
FIG. 1.
Identification of hFt1 as a binding partner of PKB in a GFP PCA-based cDNA library screen. (A) The GFP PCA strategy. The principle of the GFP PCA is that cells simultaneously expressing complementary fragments of GFP (F[1] and F[2]) fused to test proteins (A and B) will produce a fluorescent signal only if the fused proteins physically interact and then bring the complementary fragments of GFP into proximity where it can fold and reassemble into its active form, generating fluorescence. For the GFP PCA-based cDNA library screen, a human brain cDNA library was fused to fragment 1 of GFP (F[1]-cDNA library) and the full-length PKB cDNA was fused to fragment 2 (PKB-F[2]). (B) Interaction of hFt1 with PKB was confirmed by transiently cotransfecting COS-1 cells with the isolated F[1]-cDNA fusion coding for full-length hFt1 (F[1]-hFt1) and the PKB-F[2] fusion, followed by FACS analysis. The physical interaction between PKB and hFt1 induced the folding and reconstitution of GFP from its fragments, generating a fluorescent signal (gate window M2). Cotransfection of cells with the F[1]-hFt1 fusion and free F[2] expression vectors was used as a negative control. (C) Alignment of predicted amino acid sequences of Ft1 from human (accession number NM022476) and mouse (accession number Z67963) origins. The ubiquitin-ligase homology domain (aa 116 to 217) is represented by a gray box.
For the GFP PCA-based library screening, a human brain cDNA library was fused to the F[1] fragment of GFP (F[1]-cDNA library) and the full-length PKB cDNA was fused to the F[2] fragment (PKB-F[2]) (Fig. 1A). A physical interaction between a cDNA-expressed protein and the bait induced the reconstitution of GFP from its fragments, and positive clones could be collected by FACS analysis. Out of 107 to 108 clones screened, several hundred positive clones were obtained and 100 were sequenced. Among these, three corresponded to a human hypothetical protein similar to the mouse fused toes protein-1 (Ft1) (29). The physical interaction between PKB and Ft1 was reconfirmed by transiently cotransfecting the isolated F[1]-Ft1 cDNA fusion with the bait fusion (PKB-F[2]), or with the F[2] fragment alone as a negative control, and analysis by FACS (Fig. 1B). Technical details of the GFP PCA library screening strategy are reported elsewhere (41).
hFt1 is 96% identical to the mouse protein (mFt1) (Fig. 1C). mFt1 transcripts were found during development and in adult mouse organs, with the highest expression in kidney, testis, and brain (29). The mouse mutant Fused toes (Ft) is phenotypically characterized by fused toes on the fore limbs and thymic hyperplasia in heterozygous animals (29, 47). Homozygous animals die around day 10 of development. The Ft mouse mutation consists of a deletion of several hundred kilobases of mouse chromosome 8; consequently, several genes (including Ft1) could be involved in these phenotypes. It has been proposed that a defect in the regulation of programmed cell death could be at the origin of these morphological abnormalities (47). Analysis of the hFt1 sequence revealed an open reading frame of 292 aa with a predicted molecular mass of 32 kDa which does not contain consensus sequences for any known domain except for weak homology with a ubiquitin ligase homology domain encompassing aa 116 to 217 (Fig. 1C). However, Ft1 is unlikely a ubiquitin ligase enzyme, since its domain lacks a conserved cysteine residue to which ubiquitin becomes covalently linked (29). Motif search analysis (PROSITE database [21]) on the hFt1 amino acid sequence revealed one potential phosphorylation site for PKA and five for PKC, but none for PKB.
PKB interacts directly with hFt1 through its C-terminal noncatalytic domain.
We next confirmed the interaction between PKB and hFt1 by coimmunoprecipitating the endogenous proteins using specific antibodies in HEK293 cells (Fig. 2A). To determine if the binding of hFt1 to PKB disrupts its normal cellular translocation following growth factor stimulation, we used the GFP PCA to monitor the location of hFt1/PKB complexes by fluorescence microscopy in intact living cells (Fig. 2B). We have previously demonstrated that induction or inhibition of protein interactions by pathway-specific hormones and inhibitors and also their cellular location can be detected with the PCA strategy (42). The hFt1/PKB protein complexes are predominantly observed at the plasma membrane in serum-stimulated cells, and they appear to be both disrupted and to dissociate from the membrane after treatment of cells with the PI3K inhibitor wortmannin (Fig. 2B). Wortmannin inhibits the activity of PI3K and, consequently, the production of PIP3, preventing the binding of PKB and of its upstream kinase PDK1 at the plasma membrane via their PH domains. These patterns of stimulation and inhibition are consistent with PKB activation through PI3K-associated signaling pathways and, consequently, suggest that the binding of hFt1 to PKB does not alter its normal cellular localization.
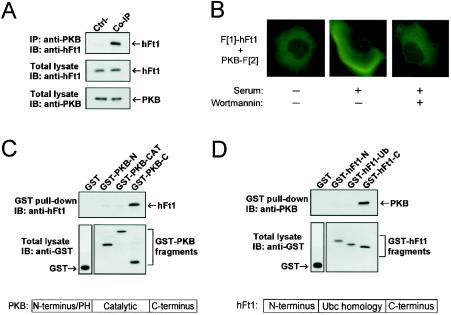
FIG. 2.
Characterization of the interaction between PKB and hFt1. (A) Coimmunoprecipitation of the endogenous interacting partners. Endogenous PKB was immunoprecipitated from HEK293 cells with anti-PKB antibodies, and the immunoprecipitate was run on SDS-PAGE. The presence of hFt1 in the immune complexes was detected by immunoblotting with an anti-hFt1 antibody (upper panel). Expression levels of hFt1 and PKB were detected by immunoblotting total lysates with their corresponding antibodies (middle and lower panels). Immunoprecipitation of a sample with anti-IgG instead of anti-PKB antibodies was used as a control (Ctrl-). (B) Cellular location of the PKB/hFt1 complexes with GFP PCA. COS-1 cells were cotransfected with vectors expressing F[1]-hFt1 or PKB-F[2], serum starved, and untreated or treated with 300 nM wortmannin for 1 h. Afterwards, cells were stimulated for 30 min with 10% serum. Fluorescence microscopy was performed on live cells. (C) hFt1 directly interacts with the C-terminal noncatalytic domain of PKB. Bacterially purified hFt1 was incubated with immobilized GST-PKB fragments (N terminus, catalytic domain, and C terminus). The PKB-bound hFt1 was eluted with glutathione and detected by immunoblotting with anti-hFt1. Levels of the GST fusions were determined by immunoblotting with anti-GST antibodies. (D) PKB directly interacts with the C-terminal domain of hFt1. Bacterially purified PKB was incubated with immobilized GST-hFt1 fragments (N terminus, ubiquitin-ligase homology domain, and C terminus). The hFt1-bound PKB was eluted with glutathione and detected by immunoblotting with anti-PKB antibodies. Levels of the GST fusions were determined by immunoblotting with anti-GST antibodies.
In order to determine what are the regions of specific interaction between PKB and hFt1, deletion mutants of PKB were tested for binding to hFt1 (Fig. 2C). The minimal region of PKB capable of binding hFt1 corresponded to its C-terminal noncatalytic domain (aa 409 to 480). Deletion mutant studies of hFt1 showed that the protein bound to PKB through its C-terminal region (Fig. 2D). The fact that recombinant hFt1 and a PKB fragment purified from E. coli interacted with each other in vitro also indicated that the interaction is direct. The C-terminal domain of PKB contains a key site (Ser473) that must be phosphorylated for its full activation (2, 3, 44). Knowing that the C-terminal domain of PKB is involved in the regulation of its kinase activity, we set out to study the effect of hFt1 on PKB phosphorylation and activation.
hFt1 enhances the phosphorylation and activity of PKB by promoting its interaction with the upstream kinase PDK1.
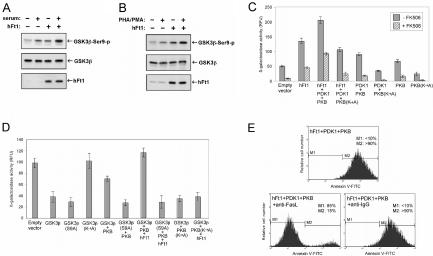
We examined the effect of hFt1 overexpression on PKB phosphorylation and activation in HEK293T and Tag-Jurkat T cells. Results indicated that hFt1 enhanced both basal and stimulus-induced phosphorylation of PKB on both of its regulatory sites (Thr308 and Ser473) (Fig. 3A and C). An in vitro kinase assay was also performed to confirm that this hFt1-dependent increase of PKB phosphorylation correlated with an increase of its kinase activity. As shown in Fig. 3B, PKB kinase activity increased significantly in the presence of hFt1, both in stimulated and nonstimulated cells. These results may suggest that hFt1 acts as a scaffold, mediating efficient interaction of PKB with its upstream kinase PDK1, and serves to increase the efficiency with which PDK1 phosphorylates PKB. To test this hypothesis, we examined the effect of hFt1 overexpression on the formation of PKB/PDK1 complexes in cells. Coimmunoprecipitation experiments in HEK293T cells indicated that hFt1 increased the formation of PKB/PDK1 complexes and that a truncated mutant of hFt1 lacking its C-terminal region (the region shown to interact with PKB) (Fig. 2D) failed to produce the same effect (Fig. 3D). As observed in the coimmunoprecipitation experiments, assays with the GFP PCA also indicated that hFt1 increased the interaction between PKB and PDK1 (Fig. 3E). Consistent with these results, we were able to coimmunoprecipitate hFt1 and PDK1 in HEK293 cells (Fig. 4A), and we localized the hFt1/PDK1 protein complexes at the plasma membrane in serum-stimulated cells (Fig. 4B). We identified the minimal region of PDK1 capable of binding hFt1 as its N-terminal domain (aa 1 to 81) (Fig. 4C), and deletion mutant studies of hFt1 showed that the protein bound to PDK1 through its C-terminal region (Fig. 4D). Interestingly, hFt1 bound to both PKB and PDK1 in noncatalytic domains at opposite ends of the proteins from their respective PH domains. Since the orientation of the proteins at the membrane are defined by the interaction of their PH domains with PIP3, hFt1 should not and did not (as seen in the cellular location of the complexes) disrupt interactions of PKB and PDK1 with the plasma membrane. Indeed, our results suggested that hFt1 promotes the activation of PKB by PDK1 at the plasma membrane following growth factor receptor activation, perhaps by acting as a scaffold.
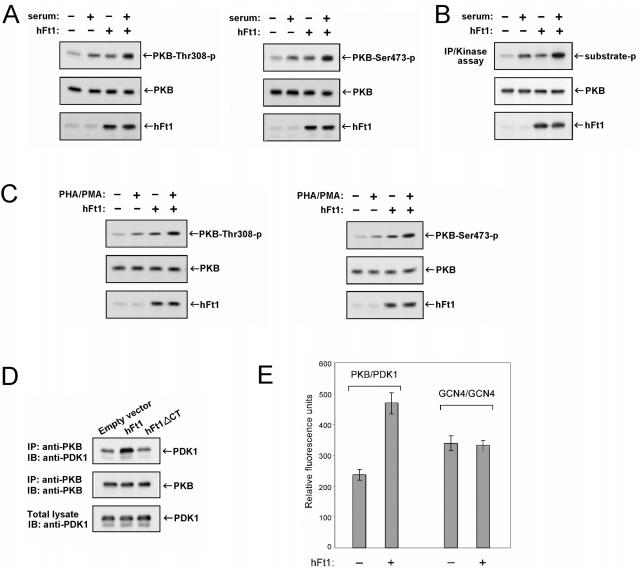
FIG. 3.
hFt1 enhances the phosphorylation and activation of PKB and the binding to its upstream kinase PDK1. (A) HEK293T cells were cotransfected with PKB and PDK1 expression vectors, in the presence (+) or absence (−) of hFt1, and stimulated with serum (+) or not stimulated (−). The phosphorylation status of PKB on both of its regulatory sites was analyzed by immunoblotting using the corresponding phospho-specific antibodies (upper panels). Expression levels of PKB and hFt1 were also determined using anti-PKB and anti-hFt1 antibodies (middle and lower panels). (B) In vitro kinase assay to confirm that the hFt1-dependent increase of PKB phosphorylation correlates with an enhancement of its kinase activity. HEK293T cells, transfected and treated as for panel A, were lysed and PKB immunoprecipitated. PKB kinase activity in the immunoprecipitates was measured using a pure substrate (paramyosin fused to a 20-amino-acid GSK-3α/β segment). Phosphorylation of the substrate (substrate-p) was detected by immunoblotting using anti-GSK3α/β-Ser21/9-p antibodies (upper panel). Expression levels of PKB and hFt1 were also determined using anti-PKB and anti-hFt1 antibodies (middle and lower panels). (C) Tag-Jurkat cells were cotransfected with PKB and PDK1 expression vectors, in the presence (+) or absence (−) of hFt1, and stimulated with PHA and PMA (+) or not stimulated (−). The phosphorylation status of PKB on both of its regulatory sites was analyzed as for panel A. (D) hFt1 enhances the formation of PKB/PDK1 complexes. HEK293T cells were cotransfected with PKB and PDK1 expression vectors, in the presence of hFt1 or a truncated form of hFt1 lacking its C-terminal domain (hFt1ΔCT; negative control). PKB was immunoprecipitated using anti-PKB antibodies, and the amount of PDK1 bound to PKB was determined by immunoblotting with anti-PDK1 antibodies (upper panel). The amount of PKB in the immune complexes was detected by immunoblotting with anti-PKB antibodies (middle panel). The expression level of PDK1 was also determined by immunoblotting total lysates with anti-PDK1 antibodies (lower panels). (E) Formation of the PKB/PDK1 complexes was also studied using the GFP PCA in HEK293T cells. PDK1 was fused to the F[1] fragment and PKB was fused to the F[2] fragment of GFP, and the complementary pairs were transiently coexpressed in cells in the presence of hFt1 (without fusion) expression vector (+) or an empty vector (−). The relative amount of reconstituted GFP, a measure of the interaction between the fused protein partners, was detected by fluorometric analysis in intact cells. The constitutive dimerization of the GCN4 leucine zipper fused to the F[1] and F[2] fragments was used as a control. Error bars represent standard errors of the means calculated from three independent samples.
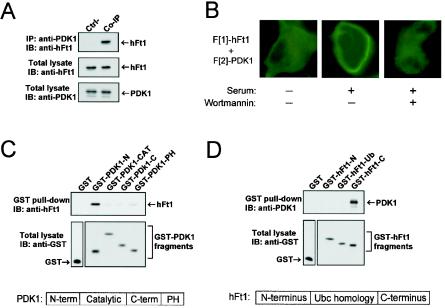
FIG. 4.
PDK1 interacts with hFt1 at the plasma membrane in activated cells through its N-terminal noncatalytic domain. (A) Coimmunoprecipitation of endogenous PDK1 and hFt1. Endogenous PDK1 was immunoprecipitated from HEK293 cells with anti-PDK1 antibodies, and the presence of hFt1 in the immune complexes was detected by immunoblotting with an anti-hFt1 antibody (upper panel). Expression levels of hFt1 and PDK1 were detected by immunoblotting total lysates with their corresponding antibodies (middle and lower panels). Immunoprecipitation of a sample with anti-IgG instead of anti-PDK1 antibodies was used as a control (Ctrl-). (B) Cellular location of the PDK1/hFt1 complexes with GFP PCA. COS-1 cells were cotransfected with F[1]-hFt1 and F[2]-PDK1 expression vectors, serum starved, and untreated or treated with 300 nM wortmannin for 1 h. Afterwards, cells were stimulated for 30 min with 10% serum. Fluorescence microscopy was performed on live cells. (C and D) The interaction between hFt1 and PDK1 occurs through the N-terminal domain of PDK1 and the C-terminal domain of hFt1. These studies were carried out as described in the legend for Fig. 2C and D.
hFt1-mediated enhancement of PKB activity dramatically increases dexamethasone-induced apoptosis in T lymphocytes.
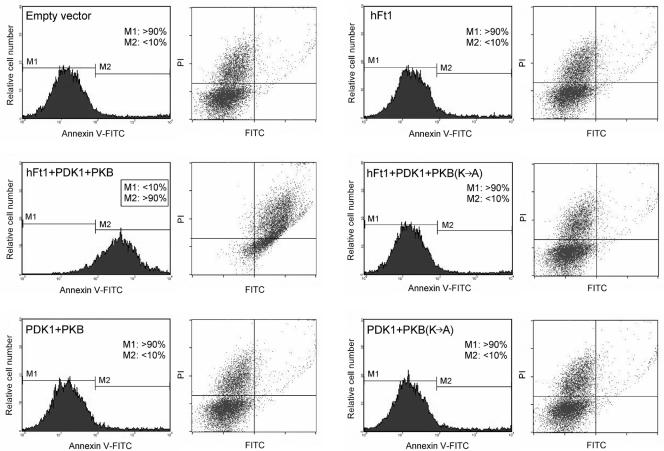
Having established that hFt1 interacts directly with PKB and increases its activity, we set out to determine if the reduction of Ft1 in the Ft/+ mouse could account for the Ft mouse mutant phenotypes. Specifically, it has been shown that thymocytes in heterozygous Ft/+ mutant mice were more resistant to dexamethasone (cortisone)- and antigen-induced apoptosis (47). To determine whether hFt1, and more specifically the interaction between hFt1 and PKB, could be involved in this phenotype, Tag-Jurkat T cells were transfected with different combinations of hFt1, PDK1, and PKB expression vectors and treated with the glucocorticoid dexamethasone, and the induction of apoptosis was analyzed by annexin V-FITC and PI staining (Fig. 5). Under our experimental conditions, cells expressing hFt1 alone did not show more susceptibility to apoptosis than control cells expressing an empty vector (less than 10% of cells undergoing apoptosis in both cases). However, the coexpression of hFt1 with PKB and PDK1 was able to induce a strong susceptibility to apoptosis in the cell population (over 90% of cells undergoing apoptosis). The replacement of wild-type PKB with a kinase-dead mutant [PKB(K→A)] completely reversed this susceptibility to apoptosis, indicating that the activity of PKB is essential for this phenomenon to occur (Fig. 5). Cells expressing hFt1 and PKB without PDK1 did not give rise to this phenotype, supporting the idea that the active form of PKB was required (data not shown). We next sought to determine through which mechanism hFt1 was able to produce profound apoptosis in T cells.
FIG. 5.
Modulation of PKB activity by hFt1 dramatically enhances the apoptosis susceptibility of Tag-Jurkat cells treated with dexamethasone. Cells were transfected with different combinations of hFt1, PDK1, PKB, or PKB(K→A) expression vectors, as indicated. Cells were treated with 1 μM dexamethasone for 7 to 8 h (in the absence of serum), stained with annexin V-FITC and PI, and analyzed by flow cytometry. Cells that stain positive for annexin V-FITC are undergoing apoptosis (M2; x axis). Cells that stain positive for both annexin V-FITC and PI (upper right square) are either in the late stages of apoptosis or are already dead.
hFt1 increases NF-AT activity via the PDK1/PKB/GSK3β signaling cascade.
Stimulation of T lymphocytes via their TCR (T-cell antigen receptor) can lead to proliferation and differentiation as well as programmed cell death (1). TCR-mediated apoptosis is an important mechanism to maintain the homeostasis of T-cell populations (28). The NF-AT transcription complex plays a critical role in the TCR-regulated fate of T lymphocytes by controlling the expression of hormones that induce growth and proliferation (such as IL-2) or apoptosis (Fas ligand). Stimulation of the TCR increases the intracellular Ca2+ concentration, leading to the activation of the serine/threonine phosphatase calcineurin and subsequent dephosphorylation of the cytoplasmic component of the NF-AT complex (NF-ATc) required for its translocation to the nucleus (9, 10, 38). A countermechanism to calcineurin-mediated dephosphorylation of NF-ATc is the specific phosphorylation of NF-ATc by the glycogen synthase kinase 3β (GSK3β) (4). PKB has been demonstrated to specifically phosphorylate and inhibit GSK3β activity in several cell types, including T cells (12). Moreover, it has recently been shown that PKB is activated upon TCR stimulation via a pathway involving the GTPase Rac1 and PI3K (16). In T cells, glucocorticoids thwart the normal balance between growth and proapoptotic signals by inhibiting the expression of both IL-2 and components of the IL-2 receptor and other growth factor receptors, and also of JAK3 and Stat5, proteins necessary for intracellular signaling by IL-2 receptor activation (8, 14, 18, 19, 33, 34). We reasoned, therefore, that PKB-dependent inhibition of GSK3β and subsequent induction of Fas ligand could become a dominant pathway, and thus augmentation of PKB activity by hFt1 would explain our observations. An alternative hypothesis, inhibition of PKB-mediated antiapoptotic pathways, can be excluded on the basis that, first, this would be contradictory to the positive effect of hFt1 on PKB activity and second, antiapoptotic pathways mediated by PKB phosphorylation of, for instance, Bad and forkhead transcription factors have not been shown to be implicated in cell survival signals in T cells or other cells of hematopoietic origin (11, 20). Thus, to understand the proapoptotic effect of hFt1 on T cells, we needed first to connect the enhancement of PKB activity by hFt1 to the modulation of GSK3β by PKB.
The effects of this hFt1-dependent increase of PKB activity on the phosphorylation or inactivation of GSK3β and on NF-ATc activity were then analyzed. We observed that overexpression of hFt1 enhanced both basal and stimulus-induced phosphorylation of GSK3β by PKB in HEK293T and Tag-Jurkat T cells (Fig. 6A and B). We directly quantified NF-ATc activity in Tag-Jurkat T cells transfected with hFt1 alone or in combination with PDK1 and PKB or PKB(K→A) and treated with dexamethasone (Fig. 6C). A significant augmentation of NF-AT activity was observed when cells expressed hFt1 alone (2.5-fold augmentation compared to cells transfected with an empty vector). However, the highest activity was observed when hFt1 was coexpressed with PKB and PDK1 (fourfold augmentation), consistent with the results of the apoptosis experiment. Also as predicted, coexpression of hFt1 with PDK1 and the kinase-dead mutant (K→A) of PKB did not show the same capacity to increase NF-AT activity (Fig. 6C). Knowing that the activity of the NF-ATc phosphatase calcineurin can be specifically inhibited by the immunosuppressive drugs FK506 and cyclosporine (30), we then asked whether hFt1 could compete against the effects of FK506, with the assumption that the augmentation of the phosphorylation (and then inactivation) of GSK3β via hFt1 would compensate for the inhibition of calcineurin activity by FK506. NF-AT activity was considerably reduced when cells were treated with FK506, but to a lesser extent when cells expressed hFt1 alone or in combination with PKB and PDK1 (Fig. 6C). To determine more directly the effect of hFt1 on GSK3β phosphorylation and inactivation, we also quantified NF-ATc activity in cells transfected with GSK3β alone [we tested three forms of GSK3β, wild type, kinase dead (K→A), or a mutant resistant to phosphorylation by PKB, GSK3β(S9A)] and in combination with PKB or PKB(K→A), with or without hFt1 (Fig. 6D). As predicted, overexpression of GSK3β in cells inhibited NF-ATc activity, and this inhibition could be partially reversed by coexpressing PKB. However, this GSK3β-mediated inhibition of NF-AT activity was completely reversed by coexpressing PKB with hFt1. Coexpression of hFt1 with kinase-dead (K→A) PKB failed to produce the same effect (Fig. 6D). Consistent with these results, coexpression of PKB and hFt1 had no effect on the GSK3β-mediated inhibition of NF-AT activity when GSK3β was replaced by a mutant resistant to PKB phosphorylation [GSK3β(S9A)] (Fig. 6D). These results confirmed that hFt1 increased NF-AT activity via PKB and GSK3β. The key step then was to connect activation of NF-AT to the control of genes that could mediate the observed massive apoptosis induced by hFt1, specifically with Fas ligand.
FIG. 6.
hFt1 increases production of the proapoptotic hormone Fas ligand via the PKB/GSK3/NF-ATc signaling cascade. (A) hFt1 enhances the PKB-dependent phosphorylation and inactivation of GSK3β. HEK293T cells were cotransfected with GSK3β and PKB expression vectors, in the presence (+) or absence (−) of hFt1, and stimulated with serum (+) or not stimulated (−). The phosphorylation status of GSK3β was analyzed by immunoblotting with the corresponding phospho-specific antibody (upper panel). Expression levels of the proteins were also determined using specific antibodies (middle and lower panels). (B) Tag-Jurkat cells were cotransfected with GSK3β and PKB expression vectors, in the presence (+) or absence (−) of hFt1, and stimulated with PHA and PMA (+) or not stimulated (−). The phosphorylation status of GSK3β was analyzed as for panel A. (C) hFt1 increases NF-AT activity. NF-AT activity was directly quantified in Tag-Jurkat cells with an NF-AT transcription reporter assay. The Tag-Jurkat cell line harbors an integrated β-galactosidase reporter plasmid where three tandem copies of the NF-AT binding site direct transcription of the lacZ gene (15). Cells transfected with the indicated expression vectors were treated with 1 μM dexamethasone, with or without the addition of 300 nM FK506. The conversion of FDG to fluorescein by β-galactosidase was detected by fluorometry. Fluorescence intensity, representing the β-galactosidase activity, is given in relative fluorescence units (y axis). Error bars represent standard errors for the means calculated from three independent samples. (D) Tag-Jurkat cells were transfected with the indicated expression vectors and treated with 1 μM dexamethasone. NF-AT activity was measured as described for panel C. (E) hFt1 increases the production of Fas ligand. Tag-Jurkat cells were transfected with hFt1, PDK1, and PKB expression vectors and treated with 1 μM dexamethasone, with or without the addition of 5 μg of neutralizing anti-human Fas ligand antibody/ml (anti-FasL) or control anti-IgG antibody. Cells were stained with annexin V-FITC and PI and analyzed by flow cytometry.
hFt1 induces apoptosis by increasing the production of the proapoptotic hormone Fas ligand.
NF-AT is a key regulator of Fas ligand expression (FasL/CD95L), and FasL-induced apoptosis is an important mechanism for maintaining the homeostasis of a cell population (26, 28). FasL can initiate programmed cell death in T cells by binding to its cell surface receptor (Fas/CD95) (49). An upregulation of FasL expression induces apoptosis via autocrine suicide and paracrine action on neighboring cells (45). Thus, we set out to test whether hFt1 could act through this mechanism. To test this hypothesis, we treated Tag-Jurkat T cells transfected with hFt1, PDK1, and PKB with dexamethasone and a neutralizing anti-human FasL antibody (Fig. 6E). Incubation of cells with 5 μg of the neutralizing anti-FasL antibody/ml reversed the apoptosis induced by hFt1, while a control anti-IgG antibody failed to block apoptosis, demonstrating that an increased secretion of FasL was at the origin of this phenomenon (Fig. 6E). The paracrine action of FasL on neighboring cells explains the massive cell death (over 90% of the cell population) observed in our apoptosis experiments, in which between only 20 and 30% of cells were cotransfected.
DISCUSSION
In the studies described here, we sought to identify novel regulators or effectors of the serine/threonine kinase PKB/Akt. We have identified a novel PKB binding protein, called hFt1, in a cDNA library screen using PKB as bait. We then asked how hFt1 might participate in PKB signaling and what are the consequences for the regulatory role of PKB in apoptosis in T cells. We therefore set out to determine whether the effects of hFt1 on PKB activity could be mapped into a known mechanism of apoptosis in these cells and whether the actions of hFt1 on these pathways would be consistent with the abnormalities characteristic of Ft/+ mice.
We first demonstrated that hFt1 interacted directly with PKB, via its C-terminal regulatory domain, and enhanced PKB phosphorylation and activation by PDK1. The C-terminal domain of PKB has a peculiar tertiary structure, a largely unstructured polypeptide that binds along the surface of the substrate- and ATP-binding domains (51, 52). The function of the C-terminal domain of PKB and other AGC kinases that contain this domain is poorly understood, but with the discovery of both negative and positively acting binding proteins it is beginning to appear that it may serve a very general regulatory function. The recently discovered CTMP protein also interacts with the C-terminal domain of PKB but has the opposite effect from hFt1, preventing its phosphorylation at positions 308 and 473 and thereby inhibiting its kinase activity (31). Although both CTMP and hFt1 bind to the C-terminal domain of PKB, CTMP might somehow prevent access of PDK1 to PKB, perhaps by stabilizing the inactive form of PKB or sterically preventing access of PDK1 to its phosphorylation site. Detailed structural studies of PKB-regulatory protein complexes should reveal the alternative mechanisms by which binding proteins regulate PKB function. Identification of more regulatory proteins using mass spectroscopic approaches may provide further insights into how PKB functions, specificities, cellular localization, and turnover are controlled in different cellular contexts.
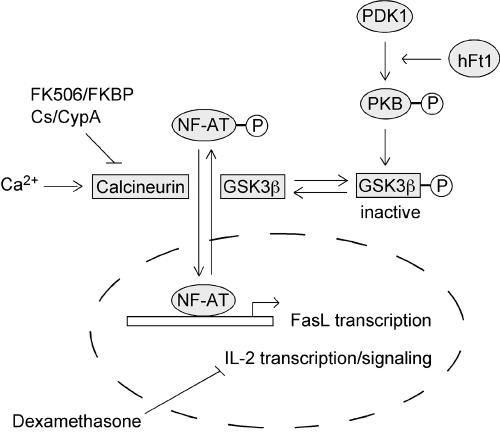
In spite of the generally accepted antiapoptotic function of PKB in epithelial cells and fibroblasts, the role of PKB in cell survival signals in T cells or other cells of hematopoietic origin is not clear (11, 20, 25, 35, 39, 40). Indeed, in lymphocytes, PKB is activated by both cytokines that promote cell growth and survival and by antigen receptors which trigger activation-induced cell death. Consequently, PKB is activated by receptors that produce opposite signals for cell life and death and, therefore, the function of PKB in these cells cannot be simply reduced to a survival regulator. Our results indicate that hFt1, via activation of a PKB-dependent pathway, can induce profound apoptosis in a T-cell population when the pathway is made dominant over IL-2 signaling by treatment of the cells with glucocorticoids. We demonstrated that this phenomenon occurs via a PDK1/PKB/GSK3 signaling cascade, leading to the activation of the transcription factor NF-ATc and the production of the proapoptotic hormone Fas ligand (Fig. 7).
FIG. 7.
Proposed model for the mode of action of hFt1 in T cells. hFt1 enhances the phosphorylation and activation of PKB by promoting its interaction with the upstream kinase PDK1. This hFt1-induced activation of PKB increases the phosphorylation, and thus inactivation, of the NF-ATc kinase GSK3β. In addition to this, an active phosphatase calcineurin (Ca2+ dependent) dephosphorylates NF-ATc. The immunosuppressive drugs FK506 and cyclosporine (Cs) specifically inhibit the activity of calcineurin. The unphosphorylated form of NF-ATc translocates to the nucleus, where it participates in the activation of early immune response genes, including Fas ligand (FasL/CD95L) and IL-2. Treatment of T cells with glucocorticoids downregulates IL-2 expression, leading to an increase of the FasL/IL-2 ratio. This ratio of proliferative versus antiproliferative factors constitutes an important mechanism to preserve the homeostasis of T-cell populations. Consequently, the hFt1-mediated upregulation of Fas ligand expression, via the PDK1/PKB/GSK3/NF-ATc signaling cascade, leads to a Fas ligand-dependent massive cell death in glucocorticoid-treated T cells.
Our results support a function of hFt1 as a regulator of PKB activity that controls apoptotic signals mediated by this kinase. Determining how, when and through which receptors these pathways modulate hFt1 will be crucial to understanding its general role in mediating apoptosis during development and/or cellular homeostasis. Further, results may also provide a molecular link between PKB regulation and the switch from proliferation to the elimination of autoreactive or excess T cells. A switch from proliferation to the elimination of autoreactive cells (negative selection) or removal of excess T cells after the eradication of a pathogen may be regulated, in part, by hFt1. hFt1 may alter the balance between expression of Fas ligand versus growth factors such as IL-2, resulting in apoptosis in T-cell populations. Again, the loss of sensitivity of thymocytes to glucocortocoid and antigen-induced apoptosis in Ft/+ mice would be consistent with this argument. The wide distribution of hFt1 in adult tissues (29) suggests that it could be a general regulator of PKB activity in the control of differentiation, proliferation, and apoptosis in many cell types.
Acknowledgments
We gratefully acknowledge Serge Sénéchal for his technical assistance in FACS analysis. We also thank Monique Davies for the gift of the pMT3 vector, Patricia Mark and Stuart Schreiber for the Tag-Jurkat cell line, Jing Jin and Jim Woodgett for PKB and GSK3β cDNAs, and Michael Scheid for PDK1.
This work was supported by the Canadian Institute of Health Research (MOP-42477).
REFERENCES
- 1.Alberola-Ila, J., S. Takaki, J. D. Kerner, and R. M. Perlmutter. 1997. Differential signaling by lymphocyte antigen receptors. Annu. Rev. Immunol. 15:125-154. [DOI] [PubMed] [Google Scholar]
- 2.Alessi, D. R., M. Deak, A. Casamayor, F. B. Caudwell, N. Morrice, D. G. Norman, P. Gaffney, C. B. Reese, C. N. MacDougall, D. Harbison, A. Ashworth, and M. Bownes. 1997. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr. Biol. 7:776-789. [DOI] [PubMed] [Google Scholar]
- 3.Alessi, D. R., S. R. James, C. P. Downes, A. B. Holmes, P. R. Gaffney, C. B. Reese, and P. Cohen. 1997. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol. 7:261-269. [DOI] [PubMed] [Google Scholar]
- 4.Beals, C. R., C. M. Sheridan, C. W. Turck, P. Gardner, and G. R. Crabtree. 1997. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 275:1930-1934. [DOI] [PubMed] [Google Scholar]
- 5.Belham, C., S. Wu, and J. Avruch. 1999. Intracellular signalling: PDK1—a kinase at the hub of things. Curr. Biol. 9:R93-R96. [DOI] [PubMed] [Google Scholar]
- 6.Brazil, D. P., and B. A. Hemmings. 2001. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem. Sci. 26:657-664. [DOI] [PubMed] [Google Scholar]
- 7.Brazil, D. P., J. Park, and B. A. Hemmings. 2002. PKB binding proteins. Getting in on the Akt. Cell 111:293-303. [DOI] [PubMed] [Google Scholar]
- 8.Chen, C. Y., F. Del Gatto-Konczak, Z. Wu, and M. Karin. 1998. Stabilization of interleukin-2 mRNA by the c-Jun NH2-terminal kinase pathway. Science 280:1945-1949. [DOI] [PubMed] [Google Scholar]
- 9.Crabtree, G. R. 1999. Generic signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-AT. Cell 96:611-614. [DOI] [PubMed] [Google Scholar]
- 10.Crabtree, G. R., and E. N. Olson. 2002. NFAT signaling: choreographing the social lives of cells. Cell 109:S67-S79. [DOI] [PubMed] [Google Scholar]
- 11.Craddock, B. L., E. A. Orchiston, H. J. Hinton, and M. J. Welham. 1999. Dissociation of apoptosis from proliferation, protein kinase B activation, and BAD phosphorylation in interleukin-3-mediated phosphoinositide 3-kinase signaling. J. Biol. Chem. 274:10633-10640. [DOI] [PubMed] [Google Scholar]
- 12.Cross, D. A., D. R. Alessi, P. Cohen, M. Andjelkovich, and B. A. Hemmings. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785-789. [DOI] [PubMed] [Google Scholar]
- 13.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 14.Fessler, B. J., F. Paliogianni, N. Hama, J. E. Balow, and D. T. Boumpas. 1996. Glucocorticoids modulate CD28 mediated pathways for interleukin 2 production in human T cells: evidence for posttranscriptional regulation. Transplantation 62:1113-1118. [DOI] [PubMed] [Google Scholar]
- 15.Fiering, S., J. P. Northrop, G. P. Nolan, P. S. Mattila, G. R. Crabtree, and L. A. Herzenberg. 1990. Single cell assay of a transcription factor reveals a threshold in transcription activated by signals emanating from the T-cell antigen receptor. Genes Dev. 4:1823-1834. [DOI] [PubMed] [Google Scholar]
- 16.Genot, E. M., C. Arrieumerlou, G. Ku, B. M. Burgering, A. Weiss, and I. M. Kramer. 2000. The T-cell receptor regulates Akt (protein kinase B) via a pathway involving Rac1 and phosphatidylinositide 3-kinase. Mol. Cell. Biol. 20:5469-5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh, I., A. D. Hamilton, and L. Regan. 2000. Antiparallel leucine zipper-directed protein reassembly: application to the green fluorescent protein. J. Am. Chem. Soc. 2000:5658-5659. [Google Scholar]
- 18.Goleva, E., K. O. Kisich, and D. Y. Leung. 2002. A role for STAT5 in the pathogenesis of IL-2-induced glucocorticoid resistance. J. Immunol. 169:5934-5940. [DOI] [PubMed] [Google Scholar]
- 19.Helmberg, A., N. Auphan, C. Caelles, and M. Karin. 1995. Glucocorticoid-induced apoptosis of human leukemic cells is caused by the repressive function of the glucocorticoid receptor. EMBO J. 14:452-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinton, H. J., and M. J. Welham. 1999. Cytokine-induced protein kinase B activation and Bad phosphorylation do not correlate with cell survival of hemopoietic cells. J. Immunol. 162:7002-7009. [PubMed] [Google Scholar]
- 21.Hofmann, K., P. Bucher, L. Falquet, and A. Bairoch. 1999. The PROSITE database, its status in 1999. Nucleic Acids Res. 27:215-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu, C. D., Y. Chinenov, and T. K. Kerppola. 2002. Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol. Cell 9:789-798. [DOI] [PubMed] [Google Scholar]
- 23.Kandel, E. S., and N. Hay. 1999. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp. Cell Res. 253:210-229. [DOI] [PubMed] [Google Scholar]
- 24.Kaufman, R. J., M. V. Davies, V. K. Pathak, and J. W. Hershey. 1989. The phosphorylation state of eucaryotic initiation factor 2 alters translational efficiency of specific mRNAs. Mol. Cell. Biol. 9:946-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lafont, V., E. Astoul, A. Laurence, J. Liautard, and D. Cantrell. 2000. The T cell antigen receptor activates phosphatidylinositol 3-kinase-regulated serine kinases protein kinase B and ribosomal S6 kinase 1. FEBS Lett. 486:38-42. [DOI] [PubMed] [Google Scholar]
- 26.Latinis, K. M., L. L. Carr, E. J. Peterson, L. A. Norian, S. L. Eliason, and G. A. Koretzky. 1997. Regulation of CD95 (Fas) ligand expression by TCR-mediated signaling events. J. Immunol. 158:4602-4611. [PubMed] [Google Scholar]
- 27.Lawlor, M. A., and D. R. Alessi. 2001. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J. Cell Sci. 114:2903-2910. [DOI] [PubMed] [Google Scholar]
- 28.Lenardo, M., K. M. Chan, F. Hornung, H. McFarland, R. Siegel, J. Wang, and L. Zheng. 1999. Mature T lymphocyte apoptosis—immune regulation in a dynamic and unpredictable antigenic environment. Annu. Rev. Immunol. 17:221-253. [DOI] [PubMed] [Google Scholar]
- 29.Lesche, R., A. Peetz, F. van der Hoeven, and U. Ruther. 1997. Ft1, a novel gene related to ubiquitin-conjugating enzymes, is deleted in the Fused toes mouse mutation. Mamm. Genome 8:879-883. [DOI] [PubMed] [Google Scholar]
- 30.Liu, J., J. Farmer, Jr., W. S. Lane, J. Friedman, I. Weissman, and S. L. Schreiber. 1991. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66:807-815. [DOI] [PubMed] [Google Scholar]
- 31.Maira, S. M., I. Galetic, D. P. Brazil, S. Kaech, E. Ingley, M. Thelen, and B. A. Hemmings. 2001. Carboxyl-terminal modulator protein (CTMP), a negative regulator of PKB/Akt and v-Akt at the plasma membrane. Science 294:374-380. [DOI] [PubMed] [Google Scholar]
- 32.Michnick, S. W., I. Remy, F.-X. Campbell-Valois, A. Vallee-Belisle, and J. N. Pelletier. 2000. Detection of protein-protein interactions by protein fragment complementation strategies. Methods Enzymol. 328:208-230. [DOI] [PubMed] [Google Scholar]
- 33.Nakajima, H., X. W. Liu, A. Wynshaw-Boris, L. A. Rosenthal, K. Imada, D. S. Finbloom, L. Hennighausen, and W. J. Leonard. 1997. An indirect effect of Stat5a in IL-2-induced proliferation: a critical role for Stat5a in IL-2-mediated IL-2 receptor alpha chain induction. Immunity 7:691-701. [DOI] [PubMed] [Google Scholar]
- 34.Paliogianni, F., A. Raptis, S. S. Ahuja, S. M. Najjar, and D. T. Boumpas. 1993. Negative transcriptional regulation of human interleukin 2 (IL-2) gene by glucocorticoids through interference with nuclear transcription factors AP-1 and NF-AT. J. Clin. Investig. 91:1481-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parry, R. V., K. Reif, G. Smith, D. M. Sansom, B. A. Hemmings, and S. G. Ward. 1997. Ligation of the T cell co-stimulatory receptor CD28 activates the serine-threonine protein kinase protein kinase B. Eur. J. Immunol. 27:2495-2501. [DOI] [PubMed] [Google Scholar]
- 36.Pawson, T., and P. Nash. 2000. Protein-protein interactions define specificity in signal transduction. Genes Dev. 14:1027-1047. [PubMed] [Google Scholar]
- 37.Peterson, R. T., and S. L. Schreiber. 1999. Kinase phosphorylation: keeping it all in the family. Curr. Biol. 9:R521-R524. [DOI] [PubMed] [Google Scholar]
- 38.Rao, A., C. Luo, and P. G. Hogan. 1997. Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 15:707-747. [DOI] [PubMed] [Google Scholar]
- 39.Reif, K., B. M. Burgering, and D. A. Cantrell. 1997. Phosphatidylinositol 3-kinase links the interleukin-2 receptor to protein kinase B and p70 S6 kinase. J. Biol. Chem. 272:14426-14433. [DOI] [PubMed] [Google Scholar]
- 40.Reif, K., S. Lucas, and D. Cantrell. 1997. A negative role for phosphoinositide 3-kinase in T-cell antigen receptor function. Curr. Biol. 7:285-293. [DOI] [PubMed] [Google Scholar]
- 41.Remy, I., and S. W. Michnick. A cDNA library functional screening strategy based on fluorescent protein complementation assays to identify novel components of signaling pathways. Methods, in press. [DOI] [PubMed]
- 42.Remy, I., and S. W. Michnick. 2001. Visualization of biochemical networks in living cells. Proc. Natl. Acad. Sci. USA 98:7678-7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Remy, I., J. N. Pelletier, A. Galarneau, and S. W. Michnick. 2001. Protein interactions and library screening with protein fragment complementation strategies, p. 449-475. In E. A. Golemis (ed.), Protein-protein interactions: a molecular cloning manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 44.Stephens, L., K. Anderson, D. Stokoe, H. Erdjument-Bromage, G. F. Painter, A. B. Holmes, P. R. Gaffney, C. B. Reese, F. McCormick, P. Tempst, J. Coadwell, and P. T. Hawkins. 1998. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science 279:710-714. [DOI] [PubMed] [Google Scholar]
- 45.Su, X., J. Cheng, W. Liu, C. Liu, Z. Wang, P. Yang, T. Zhou, and J. D. Mountz. 1998. Autocrine and paracrine apoptosis are mediated by differential regulation of Fas ligand activity in two distinct Jurkat T cell populations. J. Immunol. 160:5288-5293. [PubMed] [Google Scholar]
- 46.Toker, A., and A. C. Newton. 2000. Akt/Protein kinase B is regulated by autophosphorylation at the hypothetical PDK-2 site. J. Biol. Chem. 275:8271-8274. [DOI] [PubMed] [Google Scholar]
- 47.van der Hoeven, F., T. Schimmang, A. Volkmann, M. G. Mattei, B. Kyewski, and U. Ruther. 1994. Programmed cell death is affected in the novel mouse mutant Fused toes (Ft). Development 120:2601-2607. [DOI] [PubMed] [Google Scholar]
- 48.Vanhaesebroeck, B., and D. R. Alessi. 2000. The PI3K-PDK1 connection: more than just a road to PKB. Biochem. J. 3:561-576. [PMC free article] [PubMed] [Google Scholar]
- 49.Wallach, D., E. E. Varfolomeev, N. L. Malinin, Y. V. Goltsev, A. V. Kovalenko, and M. P. Boldin. 1999. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu. Rev. Immunol. 17:331-367. [DOI] [PubMed] [Google Scholar]
- 50.Weston, C. R., and R. J. Davis. 2001. Signal transduction: signaling specificity—a complex affair. Science 292:2439-2440. [DOI] [PubMed] [Google Scholar]
- 51.Yang, J., P. Cron, V. M. Good, V. Thompson, B. A. Hemmings, and D. Barford. 2002. Crystal structure of an activated Akt/protein kinase B ternary complex with GSK3-peptide and AMP-PNP. Nat. Struct. Biol. 9:940-944. [DOI] [PubMed] [Google Scholar]
- 52.Yang, J., P. Cron, V. Thompson, V. M. Good, D. Hess, B. A. Hemmings, and D. Barford. 2002. Molecular mechanism for the regulation of protein kinase B/Akt by hydrophobic motif phosphorylation. Mol. Cell 9:1227-1240. [DOI] [PubMed] [Google Scholar]