Abstract
The DNA damage-dependent poly(ADP-ribose) polymerase-2 (PARP-2) is, together with PARP-1, an active player of the base excision repair process, thus defining its key role in genome surveillance and protection. Telomeres are specialized DNA-protein structures that protect chromosome ends from being recognized and processed as DNA strand breaks. In mammals, telomere protection depends on the T2AG3 repeat binding protein TRF2, which has been shown to remodel telomeres into large duplex loops (t-loops). In this work we show that PARP-2 physically binds to TRF2 with high affinity. The association of both proteins requires the N-terminal domain of PARP-2 and the myb domain of TRF2. Both partners colocalize at promyelocytic leukemia bodies in immortalized telomerase-negative cells. In addition, our data show that PARP activity regulates the DNA binding activity of TRF2 via both a covalent heteromodification of the dimerization domain of TRF2 and a noncovalent binding of poly(ADP-ribose) to the myb domain of TRF2. PARP-2−/− primary cells show normal telomere length as well as normal telomerase activity compared to wild-type cells but display a spontaneously increased frequency of chromosome and chromatid breaks and of ends lacking detectable T2AG3 repeats. Altogether, these results suggest a functional role of PARP-2 activity in the maintenance of telomere integrity.
Among the immediate eukaryotic cellular responses to DNA damage is the modification of histones and nuclear proteins by ADP-ribose polymers catalyzed by poly(ADP-ribose) polymerases (PARPs). PARP enzymes now constitute a superfamily of 18 proteins, encoded by 18 different genes (J.-C. Amé et al., unpublished data). They all share homology with the catalytic domain of the founding member PARP-1 (113 kDa). PARP family members display complex patterns of subcellular localization, thus extending the biological relevance of poly(ADP-ribosyl)ation (50). Among them, PARP-1 and PARP-2 (62 kDa) are until now the only characterized nuclear proteins whose catalytic activity is stimulated by DNA strand breaks (1, 48). Both homologues were shown to homo- and heterodimerize and were found to be active players in DNA base excision repair (BER) by interacting with common partners, i.e., X-ray cross-complementing factor 1 (XRCC1), DNA polymerase β, and DNA ligase III (17, 48). As it was described for the PARP-1 knockout background, PARP-2-deficient mice and the derived cells are sensitive to both ionizing radiation and alkylating agents, thus supporting a role of both proteins in the cellular response to DNA damage (19, 39). Moreover, the double knockout PARP-1−/− PARP-2−/− leads to embryonic lethality at the onset of gastrulation, whereas the PARP-1+/− PARP-2−/− background displays specific female lethality associated with X chromosome instability (39). PARP-3 (60 kDa) was identified as a core component of the centrosome, the microtubule organizing center which also partly contains PARP-1 (3, 34). Tankyrase 1 is a telomeric PARP, originally identified through its interaction with the telomeric protein TRF1, a negative regulator of telomere length (52, 53). Tankyrase 1 was found to colocalize with TRF1 to human telomeres. In vitro poly(ADP-ribosyl)ation by tankyrase 1 inhibits the binding of TRF1 to telomeres, thus pointing out tankyrase 1 as a regulator of telomere dynamics (53). In addition, tankyrase 1 localizes to different subcellular compartments in a cell-cycle-dependent manner, including nuclear pore complexes, mitotic centrosomes, and the Golgi complex (14, 51). A closely related protein named tankyrase 2 was identified recently and was shown to interact and colocalize with tankyrase 1, thus suggesting that both proteins might perform overlapping functions (33, 47).
Telomeres are specialized nucleoprotein structures that protect chromosome ends from being recognized and processed as DNA breaks (7, 18). Telomeres contain long duplex arrays of T2AG3 repeats maintained by the reverse transcriptase telomerase and bound by two related DNA binding proteins, TRF1 and TRF2 (6, 52). Both proteins carry a C-terminal Myb-like helix-turn-helix DNA binding domain and a central conserved domain involved in homodimerization, but they differ at the N terminus, which is acidic in TRF1 and basic in TRF2 (10). TRF1 has been shown to regulate telomere length at each individual chromosome end. Overexpression of TRF1 results in progressive telomere shortening, whereas inhibition of TRF1 induces telomere elongation although telomerase activity is globally not affected (55, 59). Indeed, TRF1 acts in cis as a negative telomere length regulator (2). TRF2 has been shown to remodel telomeres into large duplex loops (t-loops), possibly formed by invasion of the 3′ strand overhang into the duplex array of TTAGGG repeats (26). The t-loop has been proposed to prevent telomeres from being recognized as damaged DNA and to contribute to the regulation of telomerase activity (26). The sequestration of the 3′ terminus into t-loops defines TRF2 as an additional regulator of telomere length dynamics. Furthermore, TRF2 plays a key role in the protection of chromosome ends. Inhibition of TRF2 by expression of a dominant-negative form of TRF2 results in loss of the G-strand overhang, induces end-to-end chromosome fusions generated by DNA ligase IV-dependent nonhomologous end joining, and rapidly initiates a p53- and ATM-dependent apoptotic pathway (35, 54, 60). Some additional regulators of telomere length dynamics have been identified in human cells: the TRF1 interacting factors PinX1 and TIN2, both shown to regulate telomere length in a telomerase-dependent manner, and hRAP1, which is recruited to telomeres by TRF2 (37, 38, 64).
Some immortalized human cell lines and some tumors maintain their telomeres in the absence of detectable telomerase activity by a recombination-based pathway termed the alternative lengthening of telomeres (ALT) pathway (11, 21, 28, 42). ALT cells are characterized by a heterogeneous telomere length phenotype and the presence of nuclear structures referred to as ALT-associated promyelocytic leukemia bodies (PML bodies), which contain in addition to the PML protein telomeric DNA, the telomeric factors TRF1 and TRF2, and several proteins involved in DNA repair and recombination, such as RPA, RAD51, RAD52, the RAD50/MRE11/NBS1 complex, and the RecQ family helicases WRN and BLM (61-63, 65).
Several studies have identified DNA repair proteins, such as the DNA-PK complex (Ku proteins and DNA-PKcs) as well as the RAD50-MRE11-NBS1 complex, as important components of a repair machinery required to protect telomeres from DNA damage (5, 30, 56, 65). Consistently, mice and cells with knockouts for repair proteins were found to display telomeric phenotypes (24, 31, 44). In addition, components of the DNA-PK complex have been shown to mediate both chromosomal fusions and apoptosis due to critically short telomeres, in agreement with the notion that a short telomere is processed and signaled as a double-strand break (22, 23, 35). Furthermore, uncapped telomeres achieved through TRF2 inhibition were shown to induce the formation of telomere dysfunction-induced foci containing several DNA repair factors (57). The role of PARP-1 in maintaining genomic integrity of telomeres has also been investigated but resulted in controversial conclusions (16, 43). Interestingly, PARP-1 as well as tankyrase, TRF2, and hRap1 have been shown to bind cooperatively to the dyad symmetry region of oriP containing nonamer sites (TTAGGGTTA) closely resembling telomeric repeats (20). However, the involvement of the DNA-dependent repair protein PARP-2 in telomere integrity has not yet been addressed.
Here, we present evidence for the possible participation of PARP-2 in the maintenance of telomere integrity. We show that PARP-2 physically binds to TRF2 with high affinity. The association of both proteins requires the N-terminal domain of PARP-2 and the myb domain of TRF2. Both partners colocalize at PML bodies in immortalized telomerase-negative cells. In addition, our data show that PARP activity regulates the DNA binding activity of TRF2 via both a covalent heteromodification of the dimerization domain of TRF2 and a noncovalent binding of poly(ADP-ribose) to the myb domain of TRF2. Parp-2−/− primary cells show normal telomere length as well as normal telomerase activity compared to wild-type cells but display a spontaneously increased frequency of chromosome and chromatid breaks and of ends lacking detectable T2AG3 repeats. These results suggest that PARP-2 activity functions at telomeres, possibly by modulating t-loop formation in response to DNA damage.
MATERIALS AND METHODS
Plasmids.
The HindIII/EcoRI fragment encoding full-length human TRF2 with an N-terminal Myc tag (Myc-hTRF2) was isolated from pBS-TRF2 and subcloned into EcoRV/EcoRI sites of the pBC vector (13) in frame with glutathione S-transferase (GST), allowing the expression of a GST-MycTRF2 fusion protein renamed GST-hTRF2. Truncated forms of human TRF2 (hTRF2) (as indicated in the figure legends) were generated by PCR and cloned in frame with GST in the pBC vector. The pCDNA3-MycTRF2 plasmid was kindly provided by Dominique Broccoli (Fox Chase Cancer Center, Philadelphia, Pa.). Plasmids encoding green fluorescent protein (GFP)- or GST-fused full-length or truncated versions of murine PARP-2 (mPARP-2) were described by Schreiber et al. (48).
Cell culture.
Cos1 and U2OS cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and gentamicin and maintained at 37°C in 5% CO2.
GST pull-down analyses.
Cos1 cells (5 × 105 cells) were transfected by calcium-phosphate coprecipitation with 10 μg of recombinant DNA. Forty-eight hours later, cells were lysed in 50 mM Tris-HCl (pH 8), 150 mM NaCl, 0.1% NP-40, 0.5 mM phenylmethylsulfonyl fluoride, and protease inhibitors (Complete Mini; Roche, Mannheim, Germany). Lysates were cleared by centrifugation and incubated for 2 h with gluthatione-Sepharose beads (Pharmacia, Uppsala, Sweden). Beads were washed three times with 50 mM Tris-HCl (pH 8), 150 to 500 mM NaCl, 0.1 to 0.5% NP-40, and 0.5 mM phenylmethylsulfonyl fluoride as indicated in the figure legends, and samples were resuspended in Laemmli buffer, boiled for 4 min, and analyzed by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting. Blots were subsequently incubated with mouse monoclonal anti-Myc antibody (1/250; 9E10; Santa Cruz Biotechnology), mouse monoclonal anti-GST antibody (1/10,000; IGBMC, Illkirch, France), and rabbit polyclonal antipolymer antibody (1/1,000; Biomol Research Labs). Blots where then probed with horseradish peroxidase-coupled secondary antibodies (goat anti-rabbit, 1/20,000; sheep anti-mouse, 1/20,000 [Sigma, St. Louis, Mo.]), and immunoreactivity was detected by enhanced chemiluminescence (Amersham) according to the manufacturer.
Immunofluorescence.
Cells grown on glass coverslips were transiently transfected with GFP-mPARP-2 and subsequently synchronized by double thymidine block in G1/S boundary as described previously (61), released in fresh medium, and processed for immunofluorescence 13 h later at G2/M. For poly(ADP-ribose) synthesis, cells were treated for 10 min with 5 mM H2O2 prior to staining. Cells were washed with ice-cold phosphate-buffered saline (PBS), fixed for 10 min with ice-cold methanol-acetone (1:1), followed by washing three times with ice-cold PBS-0.1% Triton-0.1% bovine serum albumin (BSA). Cells were then incubated overnight at 4°C with either rabbit polyclonal anti-PML antibody (1:100; H-238; Santa Cruz Biotechnology), goat polyclonal anti-TRF2 antibody (1:100; N-20; Santa Cruz Biotechnology), or mouse monoclonal antipolymer antibody (1:100; H10 [36]). After three washes with ice-cold PBS-0.1% Triton-0.1% BSA, cells were incubated for 1 h at 4°C with the appropriate conjugated secondary antibodies: an Alexa Fluor 568 goat anti-rabbit immunoglobulin G (1:1,500; Molecular Probes), an Alexa Fluor 594 donkey anti-goat immunoglobulin G (1:1,500; Molecular Probes), or a fluorescein isothiocyanate-conjugated sheep anti-mouse antiserum (1:400; Sigma). DNA was counterstained with 4′,6′-diamidino-2-phenylindole (DAPI). Immunofluorescence microscopy was performed using a Zeiss Axioplan equipped with a DP50 chilled charge-coupled device camera (Olympus) and the capture software ViewFinder Lite (Olympus).
Heteromodification of GST fusion proteins by PARP-2.
The protocol used was exactly that described by Schreiber et al. (48).
DNA binding assay.
For electrophoretic mobility shift assays, a 54-mer oligonucleotide was end labeled with polynucleotide kinase and [γ-32P]ATP and annealed with the complementary 48-mer oligonucleotide to produce the 5′-labeled six-nucleotide 3′ overhanging duplex named dsT4S1 (see Fig. 5A). Radiolabeled duplex (5 nM) was incubated at 20°C for 10 to 30 min as indicated in the binding buffer (20 mM HEPES [pH 7.9], 150 mM KCl, 0.1 mM EDTA, 1 mM dithiothreitol [DTT], 500 μg of BSA/ml) and with various concentrations of either mPARP-2 or hTRF2 in a final volume of 12 μl. To activate PARP-2, NAD+ was added at a final concentration of 400 μM in the same binding buffer. Supershift experiments were performed using either an affinity-purified rabbit polyclonal anti-mPARP-2 antibody (1:10; antibody 175; Oncogene) or a monoclonal anti-hTRF2 antibody (1:20; 4A794; Oncogene). After adding 2 μl of loading solution (Ficoll, 3% final concentration, with bromophenol blue and xylene cyanol), the samples were loaded onto nondenaturing 1% agarose gels prerun at 100 V for 30 min at 4°C. Electrophoresis was carried out at 150 V for 30 min to 1 h at 4°C in 0.5× Tris-borate-EDTA. Gels were dried and subjected to phosphorimaging analysis and quantification.
FIG. 5.
PARP-2 activity negatively regulates TRF2 DNA binding activity. (A) Schematic representation of the telomeric probe dsT4S1. The telomeric repeats are shown in bold. (B) Increasing amounts of either purified mPARP-2 (lanes 1 to 6) or purified hTRF2 (lanes 7 to 12) were incubated with the radiolabeled telomeric probe under binding conditions for 30 min at 20°C. Complexes were analyzed by electrophoresis at 4°C through a nondenaturing 1% agarose gel and phosphorimaging of the dried gel. The positions of free DNA and covalent complexes (mPARP-2-DNA and hTRF2-DNA) are indicated. (C) Purified mPARP-2 was incubated with the radiolabeled telomeric probe for various times as indicated. The complexes were analyzed on a nondenaturing 1% agarose gel as described for panel B. The positions of free DNA and covalent complexes (mPARP-2-DNA) are indicated. (D) Purified hTRF2 (100 nM) was preincubated with the radiolabeled telomeric probe for 10 min under binding conditions at 20°C, and purified mPARP-2 (100 nM) was subsequently added for 10 min (lanes 4 to 6), followed by the addition of the indicated antibodies (lanes 5 and 6). In lanes 1 to 3, the radiolabeled telomeric substrate was incubated with, respectively, no protein, hTRF2 (100 nM), and mPARP-2 (100 nM) for 30 min under binding conditions at 20°C. In lanes 7 to 10, purified hTRF2 (lanes 7 and 9) or purified mPARP-2 (lanes 8 and 10) was preincubated with the radiolabeled probe before the addition of the indicated antibodies. The binding products were analyzed on a nondenaturing 1% agarose gel as described for panel B. The positions of free DNA, covalent complexes (mPARP-2-hTRF2-DNA, hTRF2-DNA, and mPARP-2-DNA), and supershifts are indicated. (E) Purified hTRF2 (100 nM) was preincubated with the radiolabeled telomeric substrate for 10 min under binding conditions as described for panel D, and purified mPARP-2 (100 nM) was subsequently added for 10 min (lanes 4 to 10) followed by the addition of NAD+ for various times as indicated in the absence (lanes 4 to 9) or in the presence of 3-AB (lane 10). In lanes 1 to 3, the radiolabeled telomeric substrate was incubated with, respectively, no protein, mPARP-2 (100 nM), or hTRF2 (100 nM) for 30 min under binding conditions. The binding products were analyzed on a nondenaturing 1% agarose gel as described for panel B. The positions of free DNA, covalent complexes, and supershifts are indicated. Note that the migration of the mPARP-2-hTRF2-DNA complex was only slightly retarded compared to that of the hTRF2-DNA complex.
Synthesis and purification of poly(ADP-ribose).
A 100-μg aliquot of PARP-1 was incubated for 30 min at room temperature in 2 ml of reaction buffer (50 mM Tris-HCl [pH 8], 4 mM MgCl2, 0.2 mM DTT, 50 μg of BSA/ml, 2 μg of DNase I-activated DNA/ml, 200 μM NAD+) containing 2 μCi of [α-32P]NAD+ (3,000 Ci/mmol). The DNA was then digested for 1 h at 37°C by adding 4 μl of a 10-mg/ml DNase I solution and 4 μl of 1 M CaCl2. Proteins were digested for 4 h at 37°C by adding 15 μl of 20% SDS and 50 μl of a 10-mg/ml proteinase K solution. The solution was treated with 100 mM NaOH, 20 mM EDTA for 1 h at 60°C and neutralized with 100 mM HCl. The polymer was extracted with 1 volume of phenol-chloroform (1:1), washed three times with diethyl ether, precipitated twice with ethanol, and dissolved in water.
Polymer blot analysis.
Purified recombinant proteins were spotted directly onto nitrocellulose. Alternatively, GST, GST-hXRCC1141-572, GST-hTRF2, and GST-tagged deletion mutants of hTRF2 were expressed in Cos1 cells, extracted by GST pull down, separated on SDS-12% PAGE, and blotted onto nitrocellulose membrane. The blots were incubated in renaturation binding buffer (50 mM Tris-HCl [pH 8], 150 mM NaCl [TBS 1×], 1 mM DTT, 0.3% Tween) for 30 min at room temperature. The incubation with the radioactive polymer (final concentration, 2 μg/ml) was performed in the renaturation binding buffer overnight at 4°C. The blots were then washed with four changes of TBS-Tween buffer (TBS 1×, 0.3% Tween) and subjected to autoradiography. Subsequently, proteins were analyzed by either Coomassie staining or Western blotting with anti-GST antibody.
Telomere length analysis by Q-FISH.
Primary mouse embryonic fibroblasts (MEFs) were prepared from 13.5-day embryos derived from heterozygous crosses (39). First-passage MEFs were used for quantitative fluorescence in situ hybridization (Q-FISH) as described previously (44). To correct for lamp intensity and alignment, images from fluorescent beads (Molecular Probes) were analyzed using the TFL-Telo program (gift from Peter Lansdorp, Vancouver, Canada). Telomere fluorescence values were extrapolated from the telomere fluorescence of LY-R (R cells) and LY-S (S cells) lymphoma cell lines of known lengths of 80 and 10 kb (38a). There was a linear correlation (r2 = 0.999) between the fluorescence intensity of the R and S telomeres, with a slope of 38.6. The calibration-corrected telomere fluorescence intensity was calculated as described elsewhere (29). Images were recorded using a COHU charge-coupled device camera on a fluorescence microscope (Leitz DMRB; Leica). A mercury steam lamp (CS 100W-2; Philips) was used as the source. For capture of the images, the Leica Q-FISH software was used at a 400-ms integration time in a linear acquisition mode to prevent oversaturation of fluorescence intensity. TFL-Telo software was used to quantify the fluorescence intensity of telomeres from at least 20 metaphases per genotype. The images from littermate wild-type, PARP-2+/−, and PARP-2−/− metaphases were captured on the same day, in parallel and blindly. All images were captured over a 3-day period after the hybridization.
TRAP assay.
S-100 extracts were prepared as described previously (8) from wild-type, PARP-2−/−, and PARP-2+/− MEFs. Telomerase activity was measured by a modified version of the telomere repeat amplification protocol (TRAP) assay (9). An internal control for PCR efficiency was included (TRAPeze kit; Oncor).
Scoring of chromosomal abnormalities by Q-FISH.
The indicated numbers of metaphases from each MEF culture were scored for chromosomal aberrations by superimposing the telomere image on the DAPI chromosome image using the TFL-telo software (gift from Peter Lansdorp). The criteria applied were the same as those described by Samper et al. (44).
Scoring of chromosomal abnormalities.
Between 100 and 250 metaphases each of wild-type, PARP-2+/−, and PARP-2−/− were scored for telomere fusions, chromatid breaks, and chromosome fragments by superimposing the telomere image on the DAPI chromosome image in the TFL-telo program. The following criteria were applied: telomeric fusions were chromosomes fused by their telomeres showing two or three telomeric signals at the point of fusion; Robertsonian-like fusions were chromosomes fused by their p-arms (they may or may not show telomeres at the fusion point; all the Robertsonian-like fusions found in this study showed telomeres at the fusion point and were included in the group of telomeric fusions); telomere associations were chromosomes associated by their telomeres (but not fused), showing four telomeric signals at the point of association; breaks were gaps in a chromatid whose corresponding chromosome was identified; chromosome fragments were chromosome pieces (with two telomeres or less) whose corresponding chromosome was not easily identified.
Statistical analysis.
Statistical calculations were done using Microsoft Excel. For statistical significance, Student's t test values were calculated.
RESULTS
TRF2 interacts with DNA damage-dependent PARP-2.
To investigate the possible interaction between TRF2 and PARP-2, Cos1 cells were transfected with Myc-tagged hTRF2 together with either GST or GST-fused mPARP-2 (Fig. 1A). GST-fused proteins were trapped on glutathione-Sepharose beads, and copurifying Myc-tagged hTRF2 was assessed by Western blot analysis using anti-Myc antibody. The efficiency of the interaction between both partners was verified by increasing the stringency conditions of the washing steps after pull down. Figure 1A shows that Myc-hTRF2 coprecipitated with GST-mPARP-2 (lanes 2 to 5) but not with GST (lane 1), and the interaction between both partners was maintained throughout increasing stringency conditions up to 0.5% NP-40, 500 mM NaCl. Under similar conditions of pull down, a weaker interaction could be detected between Myc-hTRF2 and GST-hPARP-11-524 but not resisting high-stringency conditions over 0.1% NP-40, 120 mM NaCl (data not shown), thus supporting a higher specific interaction of TRF2 with PARP-2 than with PARP-1. Similarly, a weak interaction could be detected between Flag-hTRF1 and GST-mPARP-2, but only under low-stringency conditions (data not shown).
FIG. 1.
PARP-2 interacts with TRF2. (A) Lysates from Cos1 cells expressing Myc-hTRF2 fusion protein (lanes 1 to 5) together with either GST (lane 1) or GST-mPARP-2 fusion protein (lanes 2 to 5) were analyzed by GST pull down under increasing stringency conditions of washing buffers as indicated, followed by Western blotting using, successively, anti-Myc (top) and anti-GST (bottom) antibodies. (B) Conditions of interaction between PARP-2 and TRF2. Interaction of GST (lane 1) or GST-mPARP-2 fusion protein (lanes 2 to 5) with Myc-hTRF2 fusion protein (lanes 1 to 5) in Cos1 cells either untreated (lane 2) or treated with 4 mM N-nitroso-N-methylurea (lane 3), 2 mM 3-AB (lane 4), or 10 μg of ethidium bromide/ml (lane 5). Proteins were analyzed by GST pull down and Western blotting using, successively, anti-Myc, anti-GST, and antipolymer antibodies as indicated.
We next examined the conditions regulating the PARP-2-TRF2 association (Fig. 1B). To analyze the effect of poly(ADP-ribosyl)ation, cells overexpressing both partners were either left untreated (lane 2), treated for 30 min with N-nitroso-N-methylurea (4 mM) to stimulate poly(ADP-ribose) synthesis (lane 3), or preincubated for 2 h with 3-aminobenzamide (3-AB; 2 mM) to inhibit PARP activity (lane 4). Proteins were analyzed by pull-down experiments and Western blotting successively with the indicated antibodies. Figure 1B shows that the PARP-2-TRF2 association is not dependent on poly(ADP-ribosyl)ation (compare lanes 2 and 3 with lane 4). It should be noted that a poly(ADP-ribosyl)ation of GST-mPARP-2 was also observed in undamaged cells (as revealed by the antipolymer antibody), likely occurring during the lysis procedure (lane 2). To prevent any coprecipitation of either proteins through DNA, ethidium bromide was added throughout the pull down (lane 5). Our results showed that DNA is not involved in the interaction of PARP-2 with TRF2.
Identification of the domains involved in the association between PARP-2 and TRF2.
To map the TRF2 interaction domain within PARP-2, GST fusion proteins were generated expressing truncated versions of mPARP-2 (Fig. 2A): amino acids 1 to 69 (Nt, the DNA binding domain), amino acids 63 to 202 (domain E), and amino acids 203 to 559 (F, the catalytic domain) (48). These fusion proteins were overexpressed in Cos1 cells together with Myc-hTRF2. Coprecipitating proteins were analyzed by GST pull-down experiments followed by Western blot analyses. Copurification of Myc-hTRF2 was efficient with constructs containing either the full-length mPARP-2 (lane 2) or the DNA binding domain (lane 3).
FIG. 2.
The N-terminal domain of PARP-2 interacts with the myb domain of TRF2. (A) Upper panel: schematic representation of mPARP-2. DBD, DNA binding domain. Lower panel: GST (lane 1), GST-tagged mPARP-2 (lane 2), and GST-tagged deletion mutants of mPARP-2 (lanes 3 to 5) were expressed in Cos1 cells together with myc-hTRF2 fusion protein (lanes 1 to 5). Interacting proteins were analyzed by GST pull down and Western blotting with anti-Myc and, subsequently, anti-GST antibodies. (B) Upper panel: schematic representation of hTRF2. NLS, nuclear localization signal. Lower panel: lysates from Cos1 cells expressing either GFP (lanes 1, 3, and 5) or GFP-tagged mPARP-2 (lanes 2, 4, and 6 to 9) were mixed with lysates from Cos1 cells expressing either GST (lanes 3 and 4), GST-hTRF2 (lanes 5 and 6), or GST-tagged deletion mutants of hTRF2 (lanes 7 to 9). Proteins were analyzed by GST pull down and Western blotting with, respectively, anti-GFP and anti-GST antibodies.
In reciprocal experiments, GST fusion proteins expressing deletion mutants of hTRF2 were generated: amino acids 2 to 68 (the basic domain), amino acids 46 to 446 (the central region containing the dimerization domain), and amino acids 447 to 500 (the myb domain). Extracts from Cos1 cells expressing either GST, full-length hTRF2, or the GST-fused deletion mutants were mixed with extracts from Cos1 cells expressing either GFP or GFP-tagged full-length mPARP-2. Coprecipitating proteins were analyzed by GST pull-down experiments and Western blot analyses. As shown in Fig. 2B, copurification of GFP-mPARP-2 was efficient with full-length hTRF2 and with its myb domain (Fig. 2B, lanes 6 and 9, respectively).
Colocalization of PARP-2 and TRF2 in ALT cell lines.
We next monitored the colocalization of PARP-2 and TRF2 in human cells expressing GFP-mPARP-2. No colocalization of GFP-mPARP-2 and TRF2 could be detected in telomerase-positive cell lines, including HeLa, Cos1, and MEF cells (data not shown). However, in a few telomerase-negative (ALT) U2OS cells, GFP-mPARP-2 localized in both the nucleolus and in large nuclear foci resembling ALT-associated PML bodies (APB). Given that APB are enriched in the G2/M phase of the cell cycle, U2OS cells expressing GFP-mPARP-2 were fixed 13 h postrelease of a double thymidine block, a time release corresponding to G2/M as previously described (27), and stained for PML with a rabbit anti-PML antibody and for TRF2 with a goat anti-TRF2 antibody (Fig. 3). In a majority of these synchronized cells, GFP-mPARP-2 nuclear foci colocalized with large aggregates of both PML (Fig. 3a to c) and TRF2 (Fig. 3d to f), which are characteristic of ALT cell lines. In addition, when PARP activity was stimulated by exposure to H2O2, a colocalization of TRF2 nuclear foci with polymer foci (synthesized by either PARP-1 and/or PARP-2) could also be detected (Fig. 3g to i).
FIG. 3.
Colocalization of PARP-2 and TRF2 in U2OS cells. U2OS cells were transfected with GFP-mPARP-2 proteins (green), fixed 13 h postrelease of a double thymidine block, and stained red with Alexa 568-labeled rabbit anti-PML antibody (a to c) or Alexa 594-labeled goat anti-TRF2 antibody (d to i). To detect PARP activity, cells were treated with 5 mM H2O2 for 10 min before fixation and costained with Alexa 594-labeled anti-TRF2 (red) and fluorescein isothiocyanate-conjugated antipolymer antibodies (green) (g to i).
PARP-2 poly(ADP-ribosyl)ates TRF2 in vitro.
The ability of PARP-2 to poly(ADP-ribosyl)ate TRF2 was evaluated. Truncated versions of TRF2 fused to GST and expressed in Cos1 cells were isolated on glutathione-Sepharose beads as described above for Fig. 2B, except that the washing buffer used contained 0.5 M NaCl, 0.5% NP-40, and 3-AB. Trapped proteins on the beads were incubated for 4 min with either mPARP-2 or no protein, in the presence of [α-32P]NAD+ (1 μM) and DNase I-treated calf thymus DNA (Fig. 4). Autoradiography revealed that mPARP-2 was able to poly(ADP-ribosyl)ate TRF2 and its central domain, although less efficiently. Interestingly, hTRF2 was modified by mPARP-2 as observed for the BER factor XRCC1, also described to be a functional partner of mPARP-2 (48). In the presence of 3-AB, no poly(ADP-ribosyl)ation of TRF2 was observed, confirming that the radioactive labeling detected was due to polymer synthesis.
FIG. 4.
The central domain of hTRF2 is poly(ADP-ribosyl)ated. GST, GST-hXRCC1141-572, GST-hTRF2, and GST-tagged deletion mutants of hTRF2 were expressed in Cos1 cells, extracted by GST pull down, and incubated with or without mPARP-2 as indicated in activity buffer containing [α-32P]NAD+ and DNase I-activated DNA. Where indicated, 3-AB was added throughout the experiment. (Right panel) Autoradiography. (Left panel) Subsequently, fusion proteins were analyzed by Western blotting with anti-GST antibody.
The activity of PARP-2 negatively affects the DNA binding activity of TRF2.
To test whether the presence of PARP-2 has an effect on TRF2 DNA binding activity, gel retardation assays were performed under conditions where both TRF2 and PARP-2 bound to the same target DNA. The telomeric probe dsT4S1 contained four T2AG3 repetitions on the 5′ strand and only three C3TA2 repetitions on the 3′ strand, giving after annealing a double-stranded DNA with six nucleotides overhanging at the 3′ end, as depicted in Fig. 5A. In a gel retardation assay, increasing amounts of mPARP-2 (Fig. 5B, lanes 1 to 6) and hTRF2 (lanes 7 to 12) were incubated with 5 nM 32P-radiolabeled dsT4S1. Both proteins bound to the telomeric substrate, but TRF2 displayed a higher affinity than PARP-2, since 30 nM TRF2 was sufficient to bind to the majority of the DNA, in comparison to 100 nM PARP-2 (compare lane 11 with lane 6). When a double-stranded telomeric probe (with no 3′ overhang) was used, only a retardation with TRF2 was observed (data not shown). We next tested whether the activity of PARP-2 could affect its own binding to the telomeric dsT4S1 DNA (Fig. 5C). As expected, the incubation overtime of PARP-2 with the target DNA and under conditions where PARP-2 was activated affected the capacity of the protein to bind to the DNA. Only a small amount of PARP-2-DNA complex remained after 30 min of incubation. These results demonstrate that the auto-poly(ADP-ribosyl)ation of PARP-2 inhibits its binding to dsT4S1.
Next, we investigated whether the two proteins could bind simultaneously to the telomeric substrate (Fig. 5D). When mPARP-2 and hTRF2 were incubated sequentially with dsT4S1, an mPARP-2-hTRF2-DNA ternary complex was obtained (lane 4) that migrated slightly slower than the hTRF2-DNA complex (lane 3). The supershift experiments using both anti-PARP-2 and anti-TRF2 antibodies confirmed the presence of both proteins in the ternary complex (compare lanes 5 and 6 with lane 4). Control supershift studies showed that anti-PARP-2 antibody was able to displace the mPARP-2-DNA complex (lane 8) without affecting the hTRF2-DNA interaction (lane 9), whereas anti-TRF2 antibody shifted the hTRF2-DNA complex (lane 7) but not mPARP-2-DNA (lane 10).
The physical interaction of TRF2 with PARP-2 (Fig. 1) and its DNA-dependent poly(ADP-ribosyl)ation (Fig. 4) likely affect the ability of TRF2 to bind to its target DNA. To test this hypothesis, we incubated both proteins together with the dsT4S1 DNA under conditions where PARP-2 was active in the presence of NAD+ (Fig. 5E). After 30 min of incubation, a significant amount of TRF2 was released from the telomeric substrate, as reflected by the appearance of free DNA (compare lane 7 with lane 4). The mPARP-2-DNA complex was still present, as confirmed by the supershift obtained using an anti-PARP-2 antibody (lane 8), but not when using an anti-TRF2 antibody (lane 9). In the latter case, the formation of an mPARP-2-hTRF2-DNA anti-TRF2 complex could be observed, possibly due to the release of hTRF2 from poly(ADP-ribose) competing with the anti-TRF2 antibody. A release of mPARP-2 could be detected when incubation was maintained after 40 min (data not shown). These results thus indicate that poly(ADP-ribose) synthesis negatively affects the DNA binding activity of TRF2. This effect was severely reduced in the presence of the PARP inhibitor 3-AB (compare lane 10 with lane 7).
Poly(ADP-ribose) competes with the binding of TRF2 to its target DNA.
To test whether poly(ADP-ribose) could compete for the binding of TRF2 to its target DNA, gel retardation assays were performed under conditions where TRF2 was bound to dsT4S1 and challenged by adding increasing amounts of purified poly(ADP-ribose) to the binding reaction mixture. Figure 6A shows that small amounts of polymer (0.5 ng) were able to displace TRF2 from its DNA. These results indicate that the noncovalent binding of poly(ADP-ribose) to TRF2 regulates its DNA binding activity in addition to the covalent modification of TRF2 by PARP-2.
FIG. 6.
Poly(ADP-ribose) inhibits the DNA binding activity of hTRF2. (A) Purified hTRF2 (30 nM) was incubated with the radiolabeled telomeric probe and increasing amounts of poly(ADP-ribose) as indicated for 30 min at 20°C under binding conditions as described in the legend for Fig. 5. The binding products were analyzed by autoradiography after nondenaturing 1% agarose gel electrophoresis. The positions of free DNA and covalent complexes (hTRF2-DNA) are indicated. (B) Poly(ADP-ribose) binds to purified recombinant hTRF2. Upper panel: 1 μg of purified recombinant hTRF2, hXRCC1, or mPARP-2 was spotted onto nitrocellulose and incubated with [32P]poly(ADP-ribose) as described in Materials and Methods. Lower panel: the amount of protein loaded was controlled by Coomassie staining. (C) Poly(ADP-ribose) binds to the myb domain of hTRF2. GST (lane 1), GST-hXRCC1141-572 (lane 2), GST-hTRF2 (lane 3), and GST-tagged deletion mutants of hTRF2 (lanes 4 to 6) were expressed in Cos1 cells, extracted by GST pull down, separated on SDS-12% PAGE, blotted onto nitrocellulose membrane, renatured, and incubated with [32P]poly(ADP-ribose) as described for panel B. Lane 7, hTRF2 (1 μg). Upper panel: autoradiography. Lower panel: subsequently, fusion proteins were analyzed by Western blotting with anti-GST.
To test whether TRF2 could directly bind to poly(ADP-ribose), we spotted similar amounts (10 pmol) of TRF2, XRCC1, and PARP-2 onto nitrocellulose and incubated the renatured proteins with radioactive polymer (Fig. 6B). Detection of a radioactive signal bound to TRF2 demonstrated that the telomeric protein bound tightly and stably to the poly(ADP-ribose). Binding to poly(ADP-ribose) was also observed with XRCC1, as previously described (41), but not to PARP-2.
The fact that poly(ADP-ribose) directly competes with the binding of the protein to its DNA substrate suggests that the DNA binding domain of TRF2 interacts with the poly(ADP-ribose). To test this idea, we verified the noncovalent binding of poly(ADP-ribose) to the different domains of TRF2 (Fig. 6C). As expected, poly(ADP-ribose) bound to both purified hTRF2 (lane 7) and full-length hTRF2 fused to GST (lane 3) through its myb domain (lane 6). Again, polymer binding to XRCC1 was observed (lane 2). Altogether, these results indicate that PARP-2 regulates TRF2 DNA binding activity partly via noncovalent poly(ADP-ribose) binding.
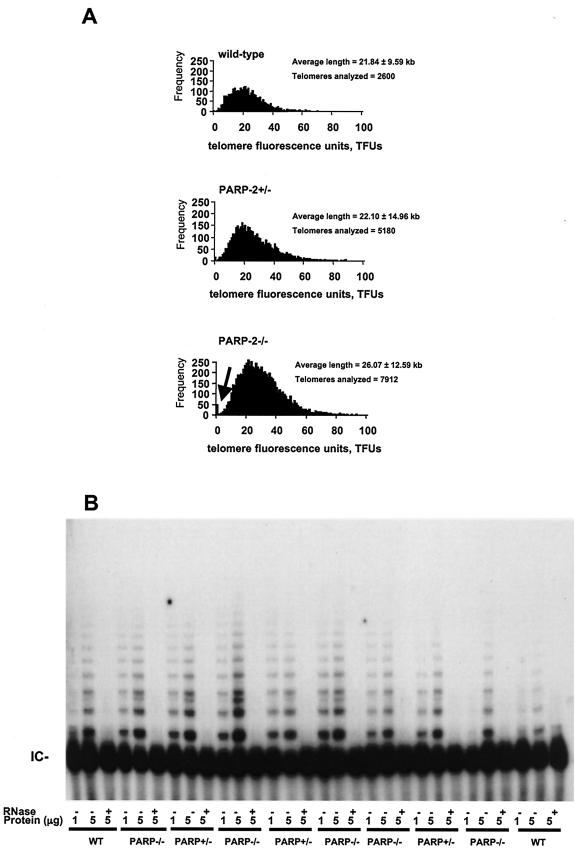
PARP-2 deficiency does not significantly alter telomere length.
To study whether the elimination of PARP-2 leads to changes in telomere length, we measured the length of TTAGGG repeats in individual metaphase chromosomes obtained from wild-type, PARP-2+/−, and PARP-2−/− passage 1 primary MEFs. To this end, we performed Q-FISH using a peptide nucleic acid telomere-specific probe on metaphases, which allowed us to obtain thousands of individual telomere length values per genotype. Q-FISH is a very reliable and powerful technique to detect significant differences in telomere length in mouse cells, which typically have very long and heterogeneous telomeres. Q-FISH indicated that the telomere length was similar for the three genotypes analyzed. The average (± standard deviation) telomere length was 21.84 ± 9.59, 22.10 ± 14.96, and 26.07 ± 12.59 kb for wild-type, PARP-2+/−, and PARP-2−/− primary MEFs, respectively (Fig. 7A). The differences between genotypes were not statistically significant after comparing thousands of individual telomere fluorescence values of each genotype (Student's t test; P > 0.01). These results indicate that PARP-2 deficiency in mice does not lead to significant differences in the distribution of telomere frequencies. There was, however, a significant increase in the signal-free ends in these cells (Fig. 7A), as well as in the heterogeneity of telomere lengths per genotype (Fig. 7A, see standard deviation values and histograms for telomere length frequencies).
FIG. 7.
PARP-2 deficiency does not alter telomere length nor telomerase activity. (A) Telomere length analysis by Q-FISH. Histograms show telomere length frequencies (i.e., the number of telomeres of a given length, indicated on the x axis) in primary MEFs from littermate wild-type, PARP-2+/−, and PARP-2−/− mice. The histograms indicate a similar distribution of telomere length frequencies in the different genotypes. One telomere-forming unit (TFU) corresponds to 1 kb of TTAGGG repeats. The increase in the number of signal-free ends (telomeres with undetectable TTAGGG signal) in PARP-2+/− and PARP-2−/− MEFs is indicated with an arrow. (B) Telomerase activity. S-100 extracts were prepared from primary MEFs of the indicated genotypes. Extracts were pretreated or not with RNase. The protein concentration used (in micrograms) is indicated. The internal control (IC) for PCR efficiency is indicated.
PARP-2 deficiency does not lead to changes in telomerase activity.
S-100 extracts were prepared from wild-type, PARP-2+/−, and PARP-2−/− primary MEFs as indicated (9) and used to perform a TRAP assay to measure the levels of telomerase activity in these cells (8). MEFs from the different genotypes were derived from littermate embryos. TRAP activity was similar in the different genotypes studied (Fig. 7B), indicating that PARP-2 was not modulating telomerase activity in the mice. This finding is in agreement with the fact that telomere length was not significantly different between genotypes (see above).
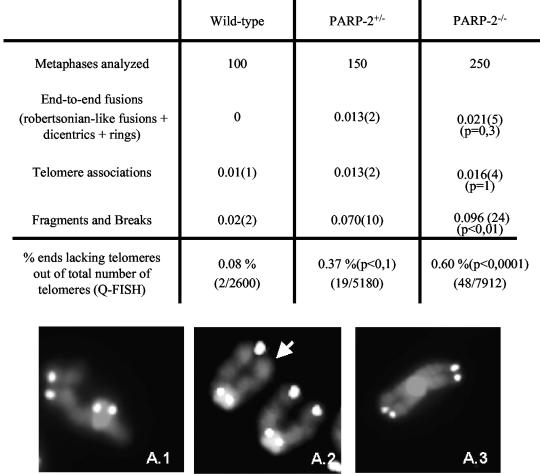
Increased chromosome and chromatid breaks and chromosome ends lacking telomere repeats in PARP-2-deficient MEFs.
To address whether elimination of PARP-2 leads to dysfunctional telomeres in mice, we determined the frequency of spontaneous chromosomal aberrations in primary MEFs of the indicated genotypes. For this, we scored >100 metaphases of each genotype after telomere Q-FISH, which allowed us to study the involvement of telomeres in the aberrations (i.e., end-to-end fusions). PARP-2−/− and PARP-2+/− primary MEFs showed an increased frequency of chromosome and chromatid breaks and fragments per metaphase when compared with the wild-type controls (Fig. 8), in agreement with the role of PARP-2 in the DNA damage response. A significantly higher frequency of signal-free chromosome ends was also observed in PARP-2+/− and PARP-2−/− MEFs when compared with the wild-type control (0.37 and 0.60% signal-free ends compared to 0.08%, respectively; Fig. 8A). These observations involving telomeres in PARP-2−/− and PARP-2+/− MEFs could be indicative of a telomere dysfunction in these cells.
FIG. 8.
Chromosomal instability in wild-type, PARP-2+/−, and PARP-2−/− primary MEFs. (Upper panel) Frequency of the indicated chromosomal aberrations per metaphase in the indicated number of metaphases in wild-type, PARP-2+/−, and PARP-2−/− primary MEFs. In parentheses is the number of aberrations in the total number of metaphases. The percentage of undetectable telomeric signals out of the total number of telomeres analyzed is also indicated. (Lower panel) Representative images of a chromosome break (A1), a signal-free end (as indicated by an arrow in A2), and an end-to-end Robertsonian-like fusion (A3).
DISCUSSION
In this study, we have presented evidence of a physical and functional association of PARP-2 with TRF2. We showed by GST pull-down analysis that PARP-2 binds TRF2 with high affinity. The complex formed between both proteins is very stable compared to the interactions of either PARP-2 with TRF1 or PARP-1 with TRF2, the latter complexes being observed only under low-stringency conditions (data not shown). The interaction between PARP-2 and TRF2 is mediated through their respective DNA binding domains, thus implying that the association could be mediated by DNA tethering. However, the addition of ethidium bromide throughout pull-down experiments did not disrupt the PARP-2-TRF2 complex, thus arguing against DNA tethering as the condition of their copurification. In addition, the interaction was maintained under high-salt conditions known to dislodge TRF2 from telomeric DNA (65).
Immunofluorescence analysis revealed a colocalization of TRF2 with both PARP-2 and poly(ADP-ribosyl)ation activity in APB in telomerase-negative cell lines. Although we could not detect PARP-2 colocalized with TRF2 in telomerase-positive cell lines, we cannot exclude its presence at telomeres, which could be masked by the strong homogeneous PARP-2 nuclear signal. Key players in DNA repair and recombination are localized to APB, including the RAD50/MRE11/NBS1 complex, RAD52, RPA, and the RecQ family helicases BLM and WRN (32, 40, 61, 65). It was originally proposed that the function of APB is to repair telomeric DNA that is recognized by the cell as it is being damaged. The identification of a population of PARP-2 at APB would suggest that PARP-2 could participate in a repair process maintaining telomere integrity in these structures. However, several recent studies have now demonstrated that these bodies represent sites of telomere maintenance through a recombinational mechanism (12, 21, 49). Our observation that PARP-2 and poly(ADP-ribosyl)ation activity colocalized with TRF2 at APB likely suggests that PARP-2 could regulate the recombination-driven telomere synthesis in ALT cells through its association with TRF2. In this regard, we have observed that even though PARP-2 deficiency does not lead to significant changes in the average telomere length, it results in a broader distribution of telomere length frequencies.
Although pull-down experiments suggest that poly(ADP-ribosyl)ation is not involved in the physical interaction of PARP-2 with TRF2, the in vitro bandshift studies clearly demonstrate a role of PARP-2 polymerizing activity in regulating the functional interaction of both partners. We found that PARP-2 activity was unfavorable to TRF2 DNA binding activity and resulted in the release of TRF2 from its telomeric substrate. The mechanism by which PARP-2 inhibits TRF2 DNA binding activity involves both a covalent heteromodification of TRF2 and a noncovalent binding of poly(ADP-ribose) to TRF2.
Basically, the effect of TRF2 poly(ADP-ribosyl)ation would be to alter its ability to bind telomeric DNA, owing to electrostatic repulsion of negatively charged ADP-ribose polymers present on TRF2 from DNA. The poly(ADP-ribosyl)ation of TRF2 occurs on its central domain containing the dimerization motif, which has been shown to mediate homotypic interactions (10). TRF2 binds to double-stranded T2AG3 repeats as a homodimer. Therefore, heteromodification of TRF2 could likely alter its DNA binding properties by affecting homodimerization. Alternatively, the modification of the dimerization domain could interfere with the recruitment of TRF2-interacting partners to the telomeres, i.e., hRap1 (38).
An additional way of regulation involves a noncovalent binding of TRF2 to poly(ADP-ribose). This has previously been described for several important DNA damage checkpoint proteins, including among others the TERT catalytic subunit of telomerase (41). Poly(ADP-ribose) regulates the functions of these proteins through binding to a poly(ADP-ribose)-binding sequence motif. Here poly(ADP-ribose) shows high affinity for the myb DNA binding domain of TRF2, but we have no evidence whether poly(ADP-ribose) and DNA binding sites are merged or not. This suggests that once PARP-2 is activated, the synthesized polymer competes with telomeric DNA for binding to TRF2 and consequently releases TRF2 from its substrate.
Altogether, the work described here identifies another step towards TRF2 regulation and possibly an understanding of telomere remodelling. TRF2 has been shown to remodel the telomere end into a large duplex looped configuration (t-loop) (25, 26). A t-loop hides the natural end of the chromosome to protect it from DNA damage checkpoint systems and machineries that scan DNA for broken ends (26). Additionally, t-loops may provide a mechanism of regulation of telomere homeostasis by telomerase and/or DNA replication.
Through poly(ADP-ribosyl)ation, PARP-2 provides two possible ways of regulation of TRF2 and may consequently act to resolve the t-loop structure. PARP-2 activated by strand breaks could facilitate opening of the t-loop structure and access of the repair machinery. As such, PARP-2 appears to be an additional putative candidate mediating DNA repair at telomeres. The high frequency of telomere loss in the PARP-2−/− cells (as previously observed for p53 −/− cells [58]) strongly supports a role of PARP-2 in the maintenance of telomere integrity. Furthermore, we identified an interaction of TRF2 with two essential players of the BER process, i.e., XRCC1 and DNA polymerase β (unpublished data). The role of PARP-2 in the regulation of a telomere capping structure is also supported by the fact that PARP-2 deficiency leads to increased frequencies of anaphase bridges (39), which in turn is coincidental with a slight increase in the number of undetectable telomeres.
Alternatively, PARP-2-mediated polymer synthesis may promote resolution of the telomeric structure to facilitate replication and/or telomere lengthening processes (4). However, this hypothesis is unlikely, as we have not found differences in telomerase activity nor telomere length between PARP-2−/− cells and wild-type cells.
In conclusion, the present study has identified PARP-2 as an additional contributor to telomere functions by regulating TRF2, whereas tankyrase 1 and 2 have been shown to target TRF1 (15). The identification of several PARP homologues regulating telomere protection and/or synthesis adds to the complexity but undoubtedly reinforces the increasing number of molecular processes needed for functional telomeres (7). In addition, the results of this and previous studies demonstrate the association of PARP-1 and PARP-2 with heterochromatin, centromeres (45, 46), telomeres (this study and reference 43), and X stability (39) and aid the comprehension of the contribution of PARP activity in the epigenetic information and its connection with a variety of chromatin transactions, such as chromatin remodeling, cell cycle progression, DNA repair and replication, apoptosis, and telomere protection.
Acknowledgments
We thank Titia de Lange (Rockefeller University) for anti-TRF2 antibodies.
This work was supported by the Association pour la Recherche Contre le Cancer, Ligue Nationale contre le Cancer et Comité Régional, Electricité de France, Commissariat à l'Energie Atomique, and Centre National de la Recherche Scientifique. I.J. is a predoctoral fellow of the Gulbenkian Foundation, Portugal. The M.B. laboratory is funded by The Swiss Bridge Cancer Research Award 2000, by the Josef Steiner Cancer Research Award 2003, by The Genome Special Action of the Ministry of Science and Technology of Spain (MCYT; GEN2001-4856-C13-08), by grant SAF2001-1869 from the MCYT, by grant 08.1/0054/01 from the CAM, by EU grants FIGH CT 1999-00009, FIGH CT 1999-00002, QLG1 1999-01341, and FIS5-2002-00078, and by the Department of Immunology and Oncology (DIO). The DIO is funded by the Spanish Council for Scientific Research and by Pharmacia Corporation.
REFERENCES
- 1.Ame, J. C., V. Rolli, V. Schreiber, C. Niedergang, F. Apiou, P. Decker, S. Muller, T. Hoger, J. Menissier-de Murcia, and G. de Murcia. 1999. PARP-2, a novel mammalian DNA damage-dependent poly(ADP-ribose) polymerase. J. Biol. Chem. 274:17860-17868. [DOI] [PubMed] [Google Scholar]
- 2.Ancelin, K., M. Brunori, S. Bauwens, C. E. Koering, C. Brun, M. Ricoul, J. P. Pommier, L. Sabatier, and E. Gilson. 2002. Targeting assay to study the cis functions of human telomeric proteins: evidence for inhibition of telomerase by TRF1 and for activation of telomere degradation by TRF2. Mol. Cell. Biol. 22:3474-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augustin, A., C. Spenlehauer, H. Dumond, J. Menissier-De Murcia, M. Piel, A. C. Schmit, F. Apiou, J. L. Vonesch, M. Kock, M. Bornens, and G. De Murcia. 2003. PARP-3 localizes preferentially to the daughter centriole and interferes with the G1/S cell cycle progression. J. Cell Sci. 116:1551-1562. [DOI] [PubMed] [Google Scholar]
- 4.Bailey, S. M., M. N. Cornforth, A. Kurimasa, D. J. Chen, and E. H. Goodwin. 2001. Strand-specific postreplicative processing of mammalian telomeres. Science 293:2462-2465. [DOI] [PubMed] [Google Scholar]
- 5.Bianchi, A., and T. de Lange. 1999. Ku binds telomeric DNA in vitro. J. Biol. Chem. 274:21223-21227. [DOI] [PubMed] [Google Scholar]
- 6.Bilaud, T., C. Brun, K. Ancelin, C. E. Koering, T. Laroche, and E. Gilson. 1997. Telomeric localization of TRF2, a novel human telobox protein. Nat. Genet. 17:236-239. [DOI] [PubMed] [Google Scholar]
- 7.Blackburn, E. H. 2001. Switching and signaling at the telomere. Cell 106:661-673. [DOI] [PubMed] [Google Scholar]
- 8.Blasco, M. A., H. W. Lee, M. P. Hande, E. Samper, P. M. Lansdorp, R. A. DePinho, and C. W. Greider. 1997. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91:25-34. [DOI] [PubMed] [Google Scholar]
- 9.Blasco, M. A., M. Rizen, C. W. Greider, and D. Hanahan. 1996. Differential regulation of telomerase activity and telomerase RNA during multi-stage tumorigenesis. Nat. Genet. 12:200-204. [DOI] [PubMed] [Google Scholar]
- 10.Broccoli, D., A. Smogorzewska, L. Chong, and T. de Lange. 1997. Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nat. Genet. 17:231-235. [DOI] [PubMed] [Google Scholar]
- 11.Bryan, T. M., A. Englezou, L. Dalla-Pozza, M. A. Dunham, and R. R. Reddel. 1997. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat. Med. 3:1271-1274. [DOI] [PubMed] [Google Scholar]
- 12.Cerone, M. A., J. A. Londono-Vallejo, and S. Bacchetti. 2001. Telomere maintenance by telomerase and by recombination can coexist in human cells. Hum. Mol. Genet. 10:1945-1952. [DOI] [PubMed] [Google Scholar]
- 13.Chatton, B., A. Bahr, J. Acker, and C. Kedinger. 1995. Eukaryotic GST fusion vector for the study of protein-protein associations in vivo: application to interaction of ATFa with Jun and Fos. BioTechniques 18:142-145. [PubMed] [Google Scholar]
- 14.Chi, N. W., and H. F. Lodish. 2000. Tankyrase is a Golgi-associated mitogen-activated protein kinase substrate that interacts with IRAP in GLUT4 vesicles. J. Biol. Chem. 275:38437-38444. [DOI] [PubMed] [Google Scholar]
- 15.Cook, B. D., J. N. Dynek, W. Chang, G. Shostak, and S. Smith. 2002. Role for the related poly(ADP-ribose) polymerases tankyrase 1 and 2 at human telomeres. Mol. Cell. Biol. 22:332-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.d'Adda di Fagagna, F., M. P. Hande, W. M. Tong, P. M. Lansdorp, Z. Q. Wang, and S. P. Jackson. 1999. Functions of poly(ADP-ribose) polymerase in controlling telomere length and chromosomal stability. Nat. Genet. 23:76-80. [DOI] [PubMed] [Google Scholar]
- 17.Dantzer, F., G. de La Rubia, J. Menissier-De Murcia, Z. Hostomsky, G. de Murcia, and V. Schreiber. 2000. Base excision repair is impaired in mammalian cells lacking poly(ADP-ribose) polymerase-1. Biochemistry 39:7559-7569. [DOI] [PubMed] [Google Scholar]
- 18.de Lange, T. 2002. Protection of mammalian telomeres. Oncogene 21:532-540. [DOI] [PubMed] [Google Scholar]
- 19.de Murcia, J. M., C. Niedergang, C. Trucco, M. Ricoul, B. Dutrillaux, M. Mark, F. J. Oliver, M. Masson, A. Dierich, M. LeMeur, C. Walztinger, P. Chambon, and G. de Murcia. 1997. Requirement of poly(ADP-ribose) polymerase in recovery from DNA damage in mice and in cells. Proc. Natl. Acad. Sci. USA 94:7303-7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng, Z., L. Lezina, C. J. Chen, S. Shtivelband, W. So, and P. M. Lieberman. 2002. Telomeric proteins regulate episomal maintenance of Epstein-Barr virus origin of plasmid replication. Mol. Cell 9:493-503. [DOI] [PubMed] [Google Scholar]
- 21.Dunham, M. A., A. A. Neumann, C. L. Fasching, and R. R. Reddel. 2000. Telomere maintenance by recombination in human cells. Nat. Genet. 26:447-450. [DOI] [PubMed] [Google Scholar]
- 22.Espejel, S., S. Franco, S. Rodriguez-Perales, S. D. Bouffler, J. C. Cigudosa, and M. A. Blasco. 2002. Mammalian Ku86 mediates chromosomal fusions and apoptosis caused by critically short telomeres. EMBO J. 21:2207-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Espejel, S., S. Franco, A. Sgura, D. Gae, S. M. Bailey, G. E. Taccioli, and M. A. Blasco. 2002. Functional interaction between DNA-PKcs and telomerase in telomere length maintenance. EMBO J. 21:6275-6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goytisolo, F. A., E. Samper, S. Edmonson, G. E. Taccioli, and M. A. Blasco. 2001. The absence of the DNA-dependent protein kinase catalytic subunit in mice results in anaphase bridges and in increased telomeric fusions with normal telomere length and G-strand overhang. Mol. Cell. Biol. 21:3642-3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greider, C. W. 1999. Telomeres do D-loop-T-loop. Cell 97:419-422. [DOI] [PubMed] [Google Scholar]
- 26.Griffith, J. D., L. Comeau, S. Rosenfield, R. M. Stansel, A. Bianchi, H. Moss, and T. de Lange. 1999. Mammalian telomeres end in a large duplex loop. Cell 97:503-514. [DOI] [PubMed] [Google Scholar]
- 27.Grobelny, J. V., A. K. Godwin, and D. Broccoli. 2000. ALT-associated PML bodies are present in viable cells and are enriched in cells in the G2/M phase of the cell cycle. J. Cell Sci. 113:4577-4585. [DOI] [PubMed] [Google Scholar]
- 28.Henson, J. D., A. A. Neumann, T. R. Yeager, and R. R. Reddel. 2002. Alternative lengthening of telomeres in mammalian cells. Oncogene 21:598-610. [DOI] [PubMed] [Google Scholar]
- 29.Herrera, E., E. Samper, J. Martin-Caballero, J. M. Flores, H. W. Lee, and M. A. Blasco. 1999. Disease states associated with telomerase deficiency appear earlier in mice with short telomeres. EMBO J. 18:2950-2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu, H. L., D. Gilley, E. H. Blackburn, and D. J. Chen. 1999. Ku is associated with the telomere in mammals. Proc. Natl. Acad. Sci. USA 96:12454-12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu, H. L., D. Gilley, S. A. Galande, M. P. Hande, B. Allen, S. H. Kim, G. C. Li, J. Campisi, T. Kohwi-Shigematsu, and D. J. Chen. 2000. Ku acts in a unique way at the mammalian telomere to prevent end joining. Genes Dev. 14:2807-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson, F. B., R. A. Marciniak, M. McVey, S. A. Stewart, W. C. Hahn, and L. Guarente. 2001. The Saccharomyces cerevisiae WRN homolog Sgs1p participates in telomere maintenance in cells lacking telomerase. EMBO J. 20:905-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaminker, P. G., S. H. Kim, R. D. Taylor, Y. Zebarjadian, W. D. Funk, G. B. Morin, P. Yaswen, and J. Campisi. 2001. TANK2, a new TRF1-associated poly(ADP-ribose) polymerase, causes rapid induction of cell death upon overexpression. J. Biol. Chem. 276:35891-35899. [DOI] [PubMed] [Google Scholar]
- 34.Kanai, M., M. Uchida, S. Hanai, N. Uematsu, K. Uchida, and M. Miwa. 2000. Poly(ADP-ribose) polymerase localizes to the centrosomes and chromosomes. Biochem. Biophys. Res. Commun. 278:385-389. [DOI] [PubMed] [Google Scholar]
- 35.Karlseder, J., D. Broccoli, Y. Dai, S. Hardy, and T. de Lange. 1999. p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science 283:1321-1325. [DOI] [PubMed] [Google Scholar]
- 36.Kawamitsu, H., H. Hoshino, H. Okada, M. Miwa, H. Momoi, and T. Sugimura. 1984. Monoclonal antibodies to poly(adenosine diphosphate ribose) recognize different structures. Biochemistry 23:3771-3777. [DOI] [PubMed] [Google Scholar]
- 37.Kim, S. H., P. Kaminker, and J. Campisi. 1999. TIN2, a new regulator of telomere length in human cells. Nat. Genet. 23:405-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, B., S. Oestreich, and T. de Lange. 2000. Identification of human Rap1: implications for telomere evolution. Cell 101:471-483. [DOI] [PubMed] [Google Scholar]
- 38a.McIlrath, J., S. D. Bouffler, E. Samper, A. Cuthbert, A. Wojcik, I. Szumiel, P. E. Bryant, A. C. Riches, A. Thompson, M. A. Blasco, R. F. Newbold, and P. Slijepcevic. 2001. Telomere length abnormalities in mammalian radiosensitive cells. Cancer Res. 61:912-915. [PubMed]
- 39.Ménissier-de Murcia, J., M. Ricoul, L. Tartier, C. Niedergang, A. Huber, F. Dantzer, V. Schreiber, J.-C. Amé, A. Dierich, M. LeMeur, L. Sabatier, P. Chambon, and G. de Murcia. 2003. Functional interaction between PARP-1 and PARP-2 in chromosome stability and embryonic development in mouse. EMBO J. 22:2255-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Opresko, P. L., C. von Kobbe, J. P. Laine, J. Harrigan, I. D. Hickson, and V. A. Bohr. 2002. Telomere-binding protein TRF2 binds to and stimulates the Werner and Bloom syndrome helicases. J. Biol. Chem. 277:41110-41119. [DOI] [PubMed] [Google Scholar]
- 41.Pleschke, J. M., H. E. Kleczkowska, M. Strohm, and F. R. Althaus. 2000. Poly(ADP-ribose) binds to specific domains in DNA damage checkpoint proteins. J. Biol. Chem. 275:40974-40980. [DOI] [PubMed] [Google Scholar]
- 42.Reddel, R. R., T. M. Bryan, L. M. Colgin, K. T. Perrem, and T. R. Yeager. 2001. Alternative lengthening of telomeres in human cells. Radiat. Res. 155:194-200. [DOI] [PubMed] [Google Scholar]
- 43.Samper, E., F. A. Goytisolo, J. Menissier-de Murcia, E. Gonzalez-Suarez, J. C. Cigudosa, G. de Murcia, and M. A. Blasco. 2001. Normal telomere length and chromosomal end capping in poly(ADP-ribose) polymerase-deficient mice and primary cells despite increased chromosomal instability. J. Cell Biol. 154:49-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samper, E., F. A. Goytisolo, P. Slijepcevic, P. P. van Buul, and M. A. Blasco. 2000. Mammalian Ku86 protein prevents telomeric fusions independently of the length of TTAGGG repeats and the G-strand overhang. EMBO Rep. 1:244-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saxena, A., R. Saffery, L. H. Wong, P. Kalitsis, and K. H. Choo. 2002. Centromere proteins Cenpa, Cenpb, and Bub3 interact with poly(ADP-ribose) polymerase-1 protein and are poly(ADP-ribosyl)ated. J. Biol. Chem. 277:26921-26926. [DOI] [PubMed] [Google Scholar]
- 46.Saxena, A., L. H. Wong, P. Kalitsis, E. Earle, L. G. Shaffer, and K. H. Choo. 2002. Poly(ADP-ribose) polymerase 2 localizes to mammalian active centromeres and interacts with PARP-1, Cenpa, Cenpb and Bub3, but not Cenpc. Hum. Mol. Genet. 11:2319-2329. [DOI] [PubMed] [Google Scholar]
- 47.Sbodio, J. I., H. F. Lodish, and N. W. Chi. 2002. Tankyrase-2 oligomerizes with tankyrase-1 and binds to both TRF1 (telomere-repeat-binding factor 1) and IRAP (insulin-responsive aminopeptidase). Biochem. J. 361:451-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schreiber, V., J. C. Ame, P. Dolle, I. Schultz, B. Rinaldi, V. Fraulob, J. Menissier-de Murcia, and G. de Murcia. 2002. Poly(ADP-ribose) polymerase-2 (PARP-2) is required for efficient base excision DNA repair in association with PARP-1 and XRCC1. J. Biol. Chem. 277:23028-23036. [DOI] [PubMed] [Google Scholar]
- 49.Smith, S. 2000. Recombination: a means to an end in human cells. Nat. Genet. 26:388-389. [DOI] [PubMed] [Google Scholar]
- 50.Smith, S. 2001. The world according to PARP. Trends Biochem. Sci. 26:174-179. [DOI] [PubMed] [Google Scholar]
- 51.Smith, S., and T. de Lange. 1999. Cell cycle dependent localization of the telomeric PARP, tankyrase, to nuclear pore complexes and centrosomes. J. Cell Sci. 112:3649-3656. [DOI] [PubMed] [Google Scholar]
- 52.Smith, S., and T. de Lange. 1997. TRF1, a mammalian telomeric protein. Trends Genet. 13:21-26. [DOI] [PubMed] [Google Scholar]
- 53.Smith, S., I. Giriat, A. Schmitt, and T. de Lange. 1998. Tankyrase, a poly(ADP-ribose) polymerase at human telomeres. Science 282:1484-1487. [DOI] [PubMed] [Google Scholar]
- 54.Smogorzewska, A., J. Karlseder, H. Holtgreve-Grez, A. Jauch, and T. de Lange. 2002. DNA ligase IV-dependent NHEJ of deprotected mammalian telomeres in G1 and G2. Curr. Biol. 12:1635-1644. [DOI] [PubMed] [Google Scholar]
- 55.Smogorzewska, A., B. van Steensel, A. Bianchi, S. Oelmann, M. R. Schaefer, G. Schnapp, and T. de Lange. 2000. Control of human telomere length by TRF1 and TRF2. Mol. Cell. Biol. 20:1659-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stellwagen, A. E., Z. W. Haimberger, J. R. Veatch, and D. E. Gottschling. 2003. Ku interacts with telomerase RNA to promote telomere addition at native and broken chromosome ends. Genes Dev. 17:2384-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takai, H., A. Smogorzewska, and T. de Lange. 2003. DNA damage foci at dysfunctional telomeres. Curr. Biol. 13:1549-1556. [DOI] [PubMed] [Google Scholar]
- 58.Tong, W. M., M. P. Hande, P. M. Lansdorp, and Z. Q. Wang. 2001. DNA strand break-sensing molecule poly(ADP-ribose) polymerase cooperates with p53 in telomere function, chromosome stability, and tumor suppression. Mol. Cell. Biol. 21:4046-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Steensel, B., and T. de Lange. 1997. Control of telomere length by the human telomeric protein TRF1. Nature 385:740-743. [DOI] [PubMed] [Google Scholar]
- 60.van Steensel, B., A. Smogorzewska, and T. de Lange. 1998. TRF2 protects human telomeres from end-to-end fusions. Cell 92:401-413. [DOI] [PubMed] [Google Scholar]
- 61.Wu, G., W. H. Lee, and P. L. Chen. 2000. NBS1 and TRF1 colocalize at promyelocytic leukemia bodies during late S/G2 phases in immortalized telomerase-negative cells. Implication of NBS1 in alternative lengthening of telomeres. J. Biol. Chem. 275:30618-30622. [DOI] [PubMed] [Google Scholar]
- 62.Yankiwski, V., R. A. Marciniak, L. Guarente, and N. F. Neff. 2000. Nuclear structure in normal and Bloom syndrome cells. Proc. Natl. Acad. Sci. USA 97:5214-5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yeager, T. R., A. A. Neumann, A. Englezou, L. I. Huschtscha, J. R. Noble, and R. R. Reddel. 1999. Telomerase-negative immortalized human cells contain a novel type of promyelocytic leukemia (PML) body. Cancer Res. 59:4175-4179. [PubMed] [Google Scholar]
- 64.Zhou, X. Z., and K. P. Lu. 2001. The Pin2/TRF1-interacting protein PinX1 is a potent telomerase inhibitor. Cell 107:347-359. [DOI] [PubMed] [Google Scholar]
- 65.Zhu, X. D., B. Kuster, M. Mann, J. H. Petrini, and T. Lange. 2000. Cell-cycle-regulated association of RAD50/MRE11/NBS1 with TRF2 and human telomeres. Nat. Genet. 25:347-352. [DOI] [PubMed] [Google Scholar]