Abstract
Protein kinase B (PKB)/Akt is known to promote cell migration, and this may contribute to the enhanced invasiveness of malignant cells. To elucidate potential mechanisms by which PKB/Akt promotes the migration phenotype, we have investigated its role in the endosomal transport and recycling of integrins. Whereas the internalization of αvβ3 and α5β1 integrins and their transport to the recycling compartment were independent of PKB/Akt, the return of these integrins (but not internalized transferrin) to the plasma membrane was regulated by phosphatidylinositol 3-kinases and PKB/Akt. The blockade of integrin recycling and cell spreading on integrin ligands effected by inhibition of PKB/Akt was reversed by inhibition of glycogen synthase kinase 3 (GSK-3). Moreover, expression of nonphosphorylatable active GSK-3β mutant GSK-3β-A9 suppressed recycling of α5β1 and αvβ3 and reduced cell spreading on ligands for these integrins, indicating that PKB/Akt promotes integrin recycling by phosphorylating and inactivating GSK-3. We propose that the ability of PKB/Akt to act via GSK-3 to promote the recycling of matrix receptors represents a key mechanism whereby integrin function and cell migration can be regulated by growth factors.
Engagement of integrins with the extracellular matrix (ECM) is known to be required for a range of biological processes, including cell proliferation and migration and, importantly, the prevention of apoptosis and anoikis (37). It is now clear that integrin function is influenced by the endosomal and receptor recycling pathways, and some of the Rab GTPase- and dynamin-dependent steps that are responsible for this have now been described (31, 35). Furthermore our laboratory has reported that growth factor stimulation influences the Rab-dependent recycling of integrins (35), but the intracellular signaling pathways that mediate this effect are unknown.
Growth factor-regulated, class I phosphatidylinositol 3-kinases [PI(3)Ks] regulate postendocytic trafficking and recycling of various receptors and transporters (19, 42), and it is now established that regulated recycling of the GLUT4 transporter requires the activity of p85/p110 PI(3)K in adipocytes and muscle cells (43, 45). Protein kinase B (PKB)/Akt is activated downstream of class I PI(3)Ks and is known to be involved in many cellular processes, including vesicular trafficking (25), and it is now apparent that PKB/Akt plays a key role in regulating GLUT4 recycling (16). A number of reports have shown that inactivation of PKB/Akt opposes the ability of insulin to activate GLUT4 translocation, and constitutively active mutant PKB/Akt proteins increase delivery of GLUT4 to the plasma membrane in the absence of insulin stimulation (13, 15, 46). It has been shown that GLUT4 is recycled to the plasma membrane via Rab4- and Rab11-dependent pathways (7, 8, 49), similar to those found for integrins (35), suggesting that GLUT4 and integrin recycling pathways may have similar mechanisms of regulation.
The various physiological functions of PKB/Akt are brought about by phosphorylation of several target molecules including Bad, caspase 9, Raf, and glycogen synthase kinase 3 (GSK-3) (25). GSK-3 is inactivated by phosphorylation of an N-terminal serine residue, Ser21 in GSK-3-α and Ser9 in GSK-3-β. Although GSK-3 was originally identified as a kinase that phosphorylates glycogen synthase, more recently it has become apparent that GSK-3 acts on a broader range of substrates including tau, β-catenin, eukaryotic initiation factor 2B (6), and the microtubular motor, kinesin (30). PKB/Akt and phospho-GSK have both been localized to the leading edge of migrating cells and have been shown to influence the motility of a number of cell types (5, 20). Inside-out activation of integrins is also thought to be key to the coordination of cell migration, and the activation of αvβ3, α5β1, αvβ5, and α2β1 integrin heterodimers is mediated by PKB/Akt (5). Activation of integrins is likely to involve the regulation of integrin recycling, and it is, therefore, possible that PKB/Akt and GSK-3 are involved in the transport of integrins in the endosomal pathway and/or the recycling pathway. Here we present evidence for the involvement of PI(3)K and PKB/Akt in integrin trafficking and cell spreading and show that these processes are likely to be promoted by inactivation of GSK-3.
MATERIALS AND METHODS
Expression plasmids.
αv, β3, α5, and β1 integrins and N121IRab4, wild-type Rab11 (wt-Rab11), N124IRab11, and S24NRab11 were expressed by pcDNA3 and have been described previously (35). Hemagglutinin (HA)-PKB-α, HA-PKB-AAA (K179/A, T308/A, S473/A), HA-membrane targeted PKB-α (memb-PKB-α), and HA-PKB kinase dead (K179/A) were expressed by the pCMV5 vector and have been described previously (50); they were a generous gift from D. Alessi (University of Dundee, Dundee, United Kingdom). memb-PKB-estrogen receptor (ER; conditionally active, membrane targeted) has been described (23, 27) and was a generous gift from M. McMahon (University of California). The green fluorescent protein (GFP)-early endosomal antigen 1 (EEA1) fusion (GFP-EEA1) was expressed by the pEGFP-C1 vector. The myc-tagged human transferrin receptor (myc-hTfn-R) was expressed by the pCB7 vector and was described previously (29). The mU6pro vector was used for the expression of RNA duplexes (48, 52). A gene-specific sequence identical in the human and murine genes encoding Akt1 and Akt2 was targeted (5′-GT CGC TGC CTG CAG TGG ACC AC-3′) (11). EcoRI/NotI fragments of human GSK-3β-A9 and -F216 cDNAs were subcloned from pcDNA3 (14) into EcoRI/HindIII sites in the pCMV5 expression vector (NotI and HindIII restriction sites were end filled to create blunt ends with T4 DNA polymerase). All plasmids were purified by CsCl banding prior to transfection.
Cell culture and transfection.
NIH 3T3 mouse fibroblasts were grown in Dulbecco's modified Eagle's medium (DMEM) as described previously (35). For assays of receptor internalization, integrin recycling, cell spreading, and receptor localization by immunofluorescence, NIH 3T3 fibroblasts were grown to 50% confluence, fed with fresh DMEM containing 10% fetal calf serum, and transfected with Fugene 6 (Roche) according to the manufacturer's instructions. The ratio of Fugene 6 to DNA was maintained at 3 μl of Fugene 6:1 μg of DNA.
For the 125I-transferrin recycling assays, transfections were carried out by using the Amaxa Nucleofector system. Briefly, cells were grown to 80% confluence, removed by trypsinization, washed in phosphate-buffered saline (PBS), and resuspended in Amaxa solution R together with 10 μg of DNA for either wt-PKB-α, PKB-AAA, Mu6pro, or Mu6pro-akt1/2. Following electroporation (in Nucleofector; program T-20), the cells were replated in eight-well dishes. All experiments were carried out 24 h posttransfection, and cells were serum starved for 30 min prior to labeling of cell surface receptors.
Internalization and recycling assays.
Receptor internalization and integrin recycling assays were performed as described previously (35). Detection of biotinylated receptors was by capture enzyme-linked immunosorbent assay (ELISA) as previously described (35) with microtiter wells coated with 5 μg of monoclonal anti-human β3 (Pharmingen; clone VI-PL2) and α5 (Pharmingen; clone VC5) integrins or anti-human transferrin receptor (CD71) (Pharmingen; clone M-A712)/ml. 125I-transferrin recycling assays were performed essentially as described previously (47) with some modifications. Briefly, serum-starved cells were incubated with 125I-labeled transferrin (NEN; NEX212; 0.1 μCi/well) for 1 h at 4°C in PBS with 1% (wt/vol) bovine serum albumin (BSA). The tracer was allowed to internalize for 15 min at 22°C (to label early endosomes) or 30 min at 37°C (to label the recycling compartment). Tracer remaining at the cell surface was removed by incubation with acid-PBS (corrected to pH 4.0 by the addition of HCl) at 4°C for 6 min, and the tracer was allowed to recycle at 37°C in serum-free DMEM supplemented with 1% BSA and 50 μM desferoxamine (Sigma; D9533). The quantity of 125I recycled into the medium is expressed as a percentage of the number of counts incorporated during the internalization period.
For internalization assays 100 nM wortmannin (Sigma; W1628) and 60 μM LY294002 (Calbiochem; 440202) were added during the last 10 min of the serum starvation period and maintained throughout the internalization period. For integrin recycling assays, these reagents were added for the last 10 min of the internalization period and maintained throughout the recycling period. For experiments involving the inhibition of GSK-3, 20 mM LiCl (Sigma; L-8895), 10 μM SB216763 (Affiniti; EI-312), and 30 μM SB415286 (Affiniti; EI-311) were included only during the recycling period.
Immunofluorescence.
For tracking the endocytic transport of receptors, NIH 3T3 fibroblasts were plated onto glass coverslips and grown to 50% confluence and then transfected with human αvβ3 integrin or human transferrin receptor in combination with either GFP-EEA1 or wt-Rab11 and either wt-PKB-α or dominant negative PKB-AAA by using Fugene 6. Under the transfection conditions employed the PKB constructs were found to be expressed in all integrin and transferrin receptor-expressing cells. Surface receptors were tagged by incubation with 2 μg of a mouse anti-β3 monoclonal antibody (Pharmingen; clone VI-PL2; dialyzed against PBS to remove sodium azide)/ml or 60 μg of Texas Red-conjugated transferrin (Molecular Probes; T-2875)/ml for 30 min at 4°C in PBS containing 0.1% (wt/vol) BSA. The tracer was allowed to internalize for 15 min at 22°C or for 30 min at 37°C, and the cells were rapidly cooled to 4°C. Tracer remaining at the cell surface was removed by a low-pH wash at 4°C as described above, and the cells were fixed in 4% paraformaldehyde and permeabilized in 0.2% Triton X-100. Internalized anti-β3 was visualized with Texas Red-conjugated anti-mouse antibody (Southern Biotechnology; 1030-07), and the cells were counterstained with rabbit anti-Rab11 (Zymed; 71-5300) followed by fluorescein isothiocyanate (FITC)-labeled anti-rabbit antibody (Southern Biotechnology, 4050-02) where appropriate.
Cell spreading assays.
Cell spreading assays were carried out on 24-well tissue culture plates which were coated overnight at 4°C with fibronectin (Sigma; F-1141) or vitronectin (Sigma; V-8379) at a concentration of 20 μg/ml and then blocked with 2% BSA. Cells were allowed to spread for 1 h and, where appropriate, 100 nM wortmannin, 60 mM LY294002 (Calbiochem; 440202), 20 mM LiCl, and 30 μM SB415286 (Affiniti; EI-311) were added 5 min after cell attachment and maintained throughout the spreading period. Attached cells were fixed for 1 min in 0.2% glutaraldehyde containing 5 mM EGTA, and β-galactosidase-expressing cells were visualized by incubation with 5 mM potassium ferricyanide and 1 mg of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) overnight at 37°C. To obtain an index of cell spreading, the area of cells expressing β-galactosidase was determined by delineation of the cell envelope using the National Institutes of Health Image software.
RESULTS
Integrin and transferrin receptor endocytosis does not require PI(3)K or PKB/Akt.
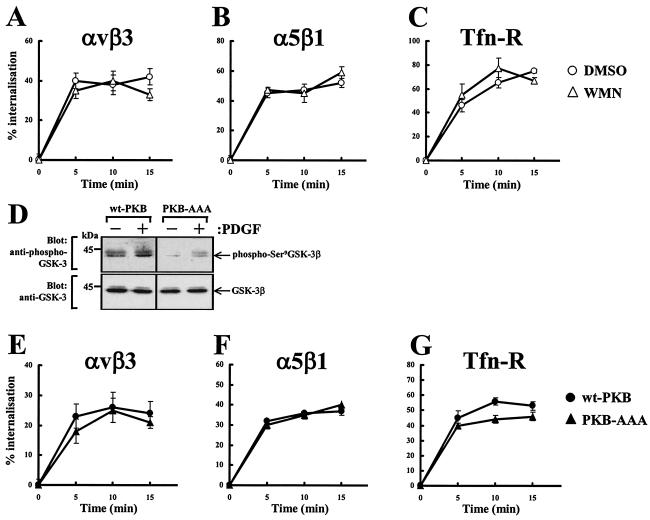
To determine whether the activity of PI(3)K is necessary for the endocytosis of αvβ3 and α5β1 integrins and Tfn-R, receptor internalization was measured following treatment of NIH 3T3 fibroblasts with 100 nM wortmannin. The assays were conducted in the presence of 0.6 mM primaquine in order to negate the effect that membrane recycling has on measurements of endocytosis. All three receptors were internalized rapidly following transfer of the cells to 37°C, and the rate and extent of endocytosis were unaffected by wortmannin (Fig. 1A to C). PKB/Akt is activated downstream of wortmannin-sensitive PI(3)Ks, so, to oppose the activity of this, we used a mutant PKB/Akt (PKB-AAA) in which the activator phosphorylation sites Thr308 and Ser473 as well as the Lys179 in the kinase domain have been replaced by alanine residues (50). As indicated in Fig. 1D, expression of PKB-AAA opposed both the basal and growth factor-induced phosphorylation of the well-characterized PKB/Akt substrate GSK-3β. Given the insensitivity of internalization to wortmannin treatment, it seemed unlikely that integrin internalization would be sensitive to the expression of PKB-AAA. Indeed, the dynamics of integrin and Tfn-R internalization were the same irrespective of overexpression of wild-type or dominant negative PKB constructs (Fig. 1E to G). These data indicate that integrin and Tfn-R endocytosis does not require the activity of PI(3)K or PKB/Akt.
FIG. 1.
Receptor internalization does not require PKB/Akt. NIH 3T3 fibroblasts were transfected with human αvβ3 (hαvβ3) (A and E), hα5β1 (B and F), or hTfn-R (C and G) together with wt-PKB or PKB-AAA. Cells were serum starved for 30 min at 37°C with the inclusion of 100 nM wortmannin (WMN) or dimethyl sulfoxide (DMSO) for the last 10 min of the starvation period and then surface labeled with 0.2 mg of N-hydroxysuccinimide-S-S-biotin/ml at 4°C for 30 min. Internalization was determined by warming the cells to 37°C for the times indicated in the presence of 0.6 mM primaquine. Biotin was released from proteins remaining at the cell surface by incubation with β-mercaptoethanesulfonic acid (MESNa), and biotinylated proteins were determined by capture ELISA with microtiter wells coated with monoclonal antibodies against hβ3 (A and E) and hα5 (B and F) integrins or human CD71 (C and G). Values are means ± standard errors of the means (n > 4). For analysis of cellular phospho-GSK-3 levels, cells were transfected with wt-PKB or PKB-AAA by using the Amaxa Nucleofector. Cells were then serum starved and incubated in the presence and absence of 10 ng of PDGF-BB/ml for 10 min. Cell lysates were then probed for the presence of GSK-3β phosphorylated at Ser9 and total GSK-3β by Western blotting (D).
Trafficking to early endosomes and the recycling compartment does not require PKB/Akt.
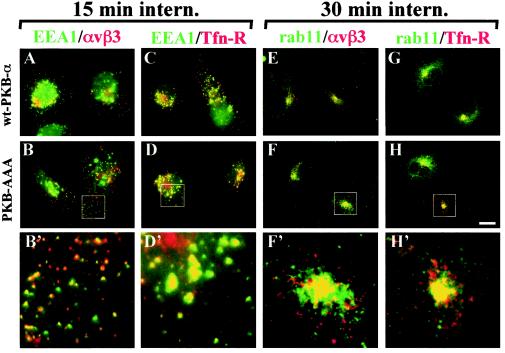
Following internalization, recycling receptors are known to pass through early endosomes and then to accumulate in the Rab11-positive recycling compartment prior to their return to the plasma membrane. To determine whether this was influenced by the activity of PKB/Akt, cells were pulse-labeled with an anti-β3 monoclonal antibody or Tfn and incubated at 22°C for 15 min or at 37°C for 30 min. Tracer remaining on the cell surface was removed by a low-pH wash at 4°C, and internalized tracer was visualized with respect to GFP-EEA1 (a marker for early endosomes) or Rab11 (a marker for the perinuclear recycling compartment). Following the shorter internalization period, both αvβ3 and Tfn-R became closely colocalized with EEA1 in early endosomes (Fig. 2A to D). Longer internalization times resulted in these receptors being transported out of early endosomes, and, after 30 min at 37°C, both αvβ3 and Tfn-R accumulated in the perinuclear recycling compartment and colocalized closely with Rab11 (Fig. 2E to H). Furthermore, both the transport of integrin and Tfn-R to the early endosomes and their subsequent accumulation in the recycling compartment were identical irrespective of the expression of wt-PKB-α or dominant negative PKB-AAA (Fig. 2).
FIG. 2.
Transport of αvβ3 and Tfn-R to early endosomes and the recycling compartment does not require PKB/Akt. NIH 3T3 fibroblasts were transfected with GFP-EEA1 in combination with human αvβ3 (hαvβ3; A and B) or hTfn-R (C and D) together with wt-PKB (A and C) or PKB-AAA (B and D). Alternatively, cells were transfected with Rab11 in combination with hαvβ3 (E and F) or hTfn-R (G and H) together with wt-PKB (E and G) or PKB-AAA (F and H). Surface receptors were tagged by incubation with a mouse anti-β3 monoclonal antibody (A, B, E, and F) or Texas Red-conjugated Tfn (C, D, G, and H), and the tracer was allowed to internalize for 15 min at 22°C (A to D) or 30 min at 37°C (E to H). Tracer remaining at the cell surface was removed by a low-pH wash at 4°C, and the cells were fixed and permeabilized with detergent. Internalized anti-β3 was visualized with Texas Red-conjugated anti-mouse antibody (A, B, E, and F), and the cells were counterstained with rabbit anti-Rab11, followed by detection with a FITC-conjugated anti-rabbit antibody (E to H). GFP-EEA1 and FITC fluorescence is shown in green, Texas Red fluorescence is shown in red, and yellow indicates colocalization of the two fluorophores. The boxed areas are shown enlarged by a factor of 3.9 in panels B′, D′, F′, and H′. Scale bar, 16 μm.
Integrin, but not Tfn-R, recycling requires PI(3)K and PKB/Akt.
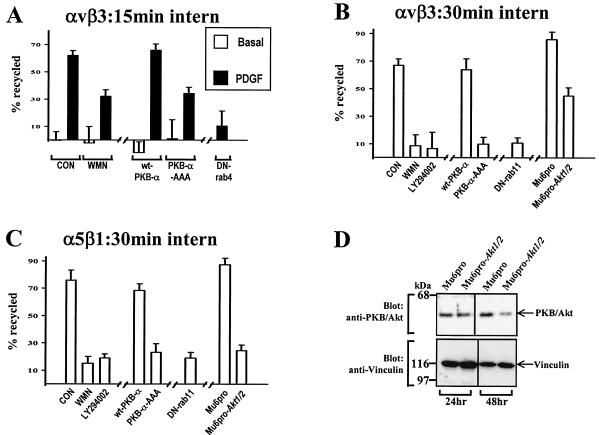
Having established that both integrin internalization and its transport through early endosomes to the recycling compartment were independent of PKB/Akt, we proceeded to determine whether the recycling of integrins from these compartments to the plasma membrane was regulated by PI(3)K and PKB/Akt. Initially we concentrated on the platelet-derived growth factor (PDGF)-regulated αvβ3 recycling that we have previously shown to be dependent on Rab4 (35). Cells were surface labeled, internalization was allowed to proceed for 15 min at 22°C to allow integrin to accumulate in early endosomes, and recycling was determined in the presence and absence of PDGF as described previously (35). Wortmannin treatment and expression of PKB-AAA both suppressed PDGF-stimulated αvβ3 recycling by ≈50% (Fig. 3A), indicating that growth factor-stimulated integrin recycling was dependent, at least in part, on the PI(3)K/PKB signaling axis.
FIG. 3.
The requirement for PI(3)K and PKB/Akt in integrin recycling. NIH 3T3 fibroblasts were transfected with human αvβ3 (hαvβ3; A and B) or hα5β1 (C) in combination with wt-PKB, PKB-AAA, Mu6pro, Mu6pro-akt1/2, N121IRab4 (DN-Rab4), or N124IRab11 (DN-Rab11). Serum-starved cells were surface labeled with 0.2 mg of N-hydroxysuccinimide-S-S-biotin/ml for 30 min at 4°C, and internalization was allowed to proceed for 15 min at 22°C (A) or 30 min at 37°C (B and C). Where appropriate 100 nM wortmannin (WMN) or 60 μM LY294002 was included in the last 10 min of the internalization period. Biotin remaining at the cell surface was removed by exposure to β-mercaptoethanesulfonic acid (MESNa) at 4°C, and internalized integrin was chased back to the cell surface at 37°C for 10 min in the absence (open bars; control) and presence (solid bars) of 10 ng of PDGF-BB/ml for 10 min (A) or for 30 min at 37°C in the absence of PDGF (B and C). Wortmannin and LY294002 were included in the recycling medium where indicated. Cells were then reexposed to MESNa, and biotinylated integrin was determined by capture ELISA using microtiter wells coated with anti-human β3 (A and B) or anti-human α5 (C) integrin monoclonal antibodies. The proportion of integrin recycled to the plasma membrane is expressed as a percentage of the pool of integrin labeled during the internalization period. Values are means ± standard errors of the means (n > 10). To test the ability of the Akt1/Akt2 hairpin RNA to suppress cellular PKB/Akt levels, cells were transfected with Mu6pro or Mu6pro-akt1/2 by using the Amaxa Nucleofector. Cells were lysed for 24 and 48 h following transfection, and the lysates were probed for the presence of PKB/Akt and vinculin (as a loading control) by Western blotting (D).
Recycling of αvβ3 and α5β1 integrins occurs from the perinuclear recycling compartment in the absence of growth factor addition. We wished, therefore, to determine the dependence of this basal recycling pathway on PKB/Akt. Cells were surface labeled, and internalization was allowed to proceed for 30 min at 37°C to accumulate integrin in the perinuclear recycling compartment. Recycling was then determined in the absence of growth factor. Dominant negative PKB-AAA profoundly inhibited the recycling of αvβ3 (Fig. 3B) and α5β1 (Fig. 3C) from the recycling compartment, as did wortmannin and the structurally unrelated inhibitor of PI(3)K, LY294002.
Overexpression of inactive forms of kinases can result in “sideways” inhibition of other pathways under the control of the same upstream kinases (25). For instance, overexpression of PKB-AAA may prevent phosphorylation of PKC-ζ by 3-phosphoinositide-dependent protein kinase 1 (2). RNA interference (RNAi) has previously been employed to reduce the cellular levels of PKB/Akt (11, 52), so we used this approach to corroborate results obtained with PKB-AAA. We constructed a U6 hairpin RNAi expression vector directed against a nucleotide sequence that is found in both the murine Akt1 and Akt2 genes (and that is also identical to the corresponding human sequences for these kinase genes). To test the efficacy of this vector in suppressing cellular levels of the PKB/Akt protein, we performed Western blot analysis on lysates from cells transfected with Mu6pro-akt1/2 and compared these lysates with lysates from cells transfected with a control vector (Mu6pro). Expression of Mu6pro-akt1/2 reduced cellular levels of PKB/Akt by approximately fourfold, and this suppression was evident 48 h following transfection (Fig. 3D). We therefore determined the effect of Mu6pro-akt1/2 on integrin recycling. Indeed, RNAi of PKB/Akt suppressed recycling of both αvβ3 (Fig. 3B) and α5β1 (Fig. 3C) integrins, indicating that endogenous PKB/Akt is required for delivery of these integrins from the recycling compartment to the plasma membrane.
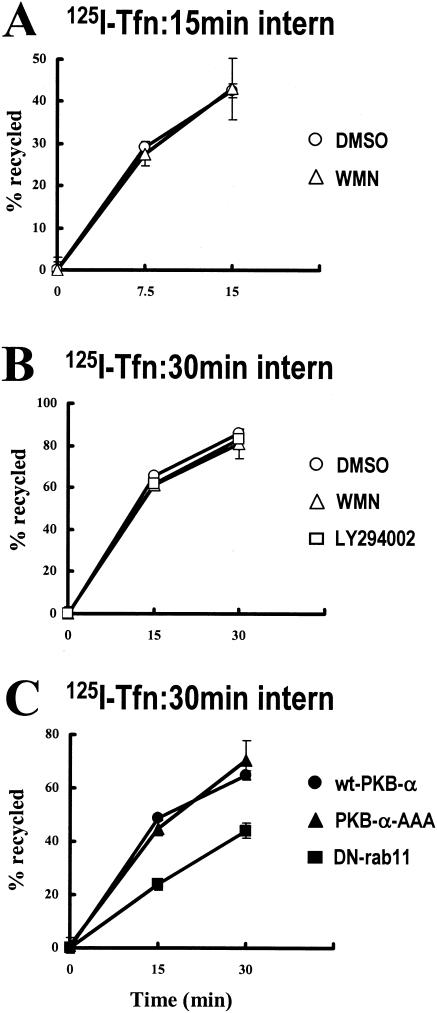
For comparison we also investigated the requirement for PI(3)K and PKB/Akt for the recycling of the Tfn-R. NIH 3T3 fibroblasts were transfected with the Amaxa Nucleofector system, which, in our hands, results in the expression of PKB-AAA in >95% of the cells (data not shown). Transfected cells were allowed to internalize 125I-labeled human transferrin for either 15 min at 22°C or 30 min at 37°C, and the return of the labeled transferrin into the medium was measured during a subsequent chase period at 37°C. Consistent with a previous report (34), Tfn-R recycling was partially inhibited by expression of dominant negative Rab11 (Fig. 4C). However, recycling of 125I-Tfn from either early endosomes (Fig. 4A) or the recycling compartment (Fig. 4B and C) was completely unaffected by inhibition of PI(3)K or expression of PKB-AAA. These data indicate that growth factor-regulated “short-loop” recycling and basal “long-loop” recycling of integrins are regulated by the PI(3)K/PKB signaling axis, whereas Tfn-R recycling is independent of the activity of PKB/Akt.
FIG. 4.
125I-Tfn recycles independently of PI(3)K and PKB/Akt. NIH 3T3 fibroblasts were transfected with wt-PKB, PKB-AAA, or S24NRab11 (DN-Rab11) by using the Amaxa Nucleofector. Cells were then serum starved and incubated with 125I-labeled transferrin for 1 h at 4°C. The tracer was allowed to internalize for 15 min at 22°C (A) or 30 min at 37°C (B and C), with 100 nM wortmannin (WMN) or 60 μM LY294002 being included in the incubation for the last 10 min of the internalization time. Tracer remaining at the cell surface was removed, and the internalized 125I-transferrin was allowed to recycle in the absence and presence of wortmannin or LY294002 for the times indicated. The quantity of 125I recycled into the medium is expressed as a percentage of the number of counts incorporated during the internalization period. Values are means ± standard errors of the means (n = 8).
Active PKB/Akt drives αvβ3 integrin recycling.
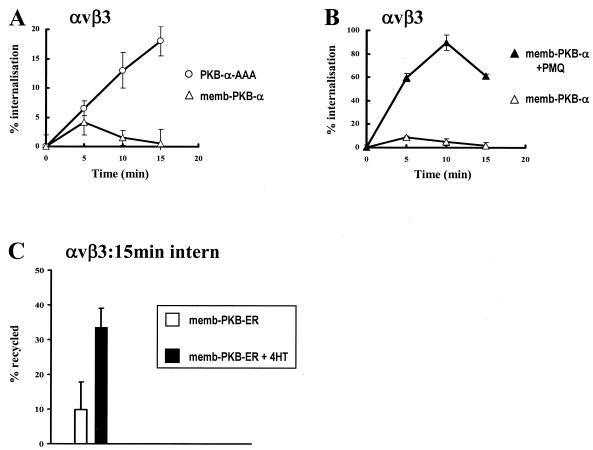
We wished to determine the effect of constitutively active memb-PKB on αvβ3 integrin recycling. Initially, attempts to measure this directly indicated that it was not possible to load αvβ3 integrin into early endosomes upon expression of memb-PKB. Indeed following expression of memb-PKB, αvβ3 appeared to remain on the cell surface (Fig. 5A). This could be due to either the ability of memb-PKB to stimulate recycling from the early endosome or conversely may represent a blockade of αvβ3 endocytosis. To distinguish these possibilities, we determined the internalization of αvβ3 in memb-PKB-transfected cells in the presence and absence of primaquine. Upon addition of primaquine, αvβ3 accumulated rapidly within the cell, indicating that memb-PKB does not oppose internalization but acts to drive the recycling of integrin such that its endosomal pool is too small to be detected easily at steady state (Fig. 5B).
FIG. 5.
Active PKB/Akt drives αvβ3 integrin recycling. (A and B) NIH 3T3 fibroblasts were transfected with human αvβ3 (hαvβ3) integrin in combination with constitutively active PKB (memb-PKB) or PKB-AAA. Cells were serum starved and surface labeled with 0.2 mg of N-hydroxysuccinimide (NHS)-S-S-biotin/ml for 30 min at 4°C. Internalization was initiated by warming to 37°C for the times shown in the absence (open symbols) and presence (closed symbols) of 0.6 mM primaquine. Biotin was released from proteins remaining at the cell surface, and biotinylated integrin was determined by capture ELISA using microtiter wells coated with an anti-human β3 integrin monoclonal antibody. (C) Cells were transfected with hαvβ3 in combination with the conditionally active fusion memb-PKB and the hormone-binding domain of the ER (memb-PKB-ER). Serum-starved cells were surface labeled with 0.2 mg of NHS-S-S-biotin/ml for 30 min at 4°C, and internalization was allowed to proceed for 15 min at 22°C. Biotin remaining at the cell surface was removed by exposure to β-mercaptoethanesulfonic acid (MESNa) at 4°C, and internalized integrin was chased back to the cell surface at 37°C for 15 min in the absence (open bars; control) and presence (solid bars) of 300 nM 4-HT. Cells were then reexposed to MESNa, and biotinylated integrin was determined by capture ELISA and expressed as for Fig. 3. Values are means ± standard errors of the means (n = 12).
To overcome the difficulty of labeling an endosomal pool of integrin following expression of memb-PKB, we explored the possibility of using a membrane-targeted, conditionally active form of PKB/Akt, memb-PKB-ER. This fusion protein, in which a membrane-targeted PKB kinase domain is fused to the hormone binding domain of the ER, is inactive in the absence of hormone ligand. However memb-PKB-ER becomes rapidly activated following the addition of 4-hydroxytamoxifen (4-HT) and elicits cellular responses known to be a function of the endogenous kinase (23, 27). Following expression of memb-PKB-ER, we were able to monitor the internalization of αvβ3 into early endosomes (not shown) and found that the integrin did not recycle appreciably from this compartment during the following 15-min chase period (Fig. 5C). However activation of memb-PKB-ER at the start of the chase period by addition of 300 nM 4-HT enhanced recycling of αvβ3 integrin by approximately threefold (Fig. 5C). Taken together these data show that, following expression of active memb-PKB, αvβ3 is returned to the plasma membrane from the early endosome without the need for growth factor stimulation, indicating that activation of PKB is necessary and sufficient to drive the recycling of αvβ3 integrin.
PKB/Akt is necessary for cell spreading.
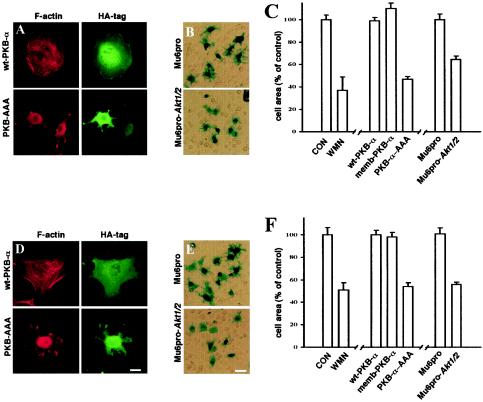
We have previously shown that inhibition of αvβ3 recycling by expression of dominant negative Rab4 results in reduction of cell spreading on vitronectin (a good ligand for αvβ3), whereas dominant negative Rab4 did not affect the recycling of α5β1 or the spreading of cells onto fibronectin (a good ligand for α5β1) (35). We hypothesized that, as expression of PKB-AAA and RNAi of endogenous PKB/Akt affect the recycling of both αvβ3 and α5β1, PKB activity would be necessary for cells to spread on vitronectin and fibronectin. Cells were transfected with wild-type, dominant negative, and constitutively active PKBs or the RNAi vector for PKB/Akt (Mu6pro-akt1/2), and allowed to adhere to coverslips coated with either vitronectin or fibronectin. Cells transfected with wt-PKB, memb-PKB, and the control Mu6pro vector spread normally on both matrices (Fig. 6). However, inhibition of PKB/Akt with PKB-AAA, treatment with wortmannin, or suppression of cellular PKB/Akt with Mu6pro-akt1/2 resulted in inefficient cell spreading on both matrix proteins (Fig. 6). The spread area of the cells was reduced by approximately 50% following inhibition of PKB/Akt signaling (Fig. 6C and F), and the cells spread abnormally without extending a properly organized lamella (Fig. 6A and D). These results are consistent with the idea that integrin recycling is indeed necessary for cell spreading on the ECM.
FIG. 6.
PKB/Akt is necessary for cell spreading on vitronectin and fibronectin. NIH 3T3 fibroblasts were transfected with wt-PKB, memb-PKB, PKB-AAA, Mu6pro, or Mu6pro-akt1/2 in combination with a β-galactosidase transfection marker. The cells were then briefly trypsinized and allowed to adhere to either vitronectin (A to C) or fibronectin (D to F) in the presence of 10 ng of PDGF-BB/ml and in the presence and absence of wortmannin (WMN; 100 nM). Attached cells were fixed and stained for β-galactosidase expression and then photographed with a digital camera. In panels C and F, the area of the transfected cells was then determined by delineation of the cell envelope using the National Institutes of Health Image software. Values are means ± standard errors of the means (n > 200 cells). CON, control. Cells expressing HA-tagged PKBs were visualized with a FITC-conjugated anti-HA antibody (green) and counterstained with Texas Red-conjugated phalloidin (red) (A and D). Scale bars, 16 (D) and 80 μm (E).
Active GSK-3β suppresses integrin recycling and cell spreading.
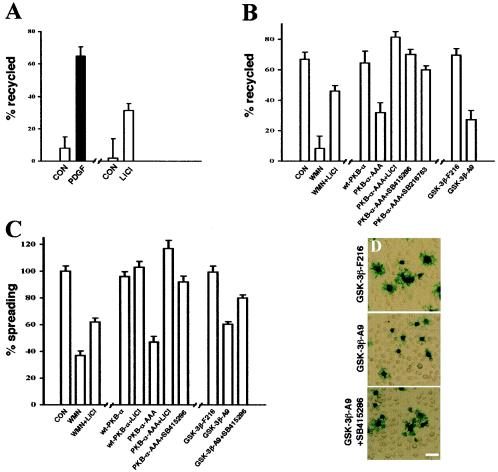
Inactivation of the serine/threonine kinase GSK-3 is now known to be required for polarized-cell migration (12), proliferation (32), and the suppression of apoptosis (10), all processes in which both αvβ3 and α5β1 integrins play a key role. It is well established that the PI(3)K signaling axis inactivates GSK-3 via PKB/Akt-dependent phosphorylation of Ser9 in GSK3-β and Ser21 in GSK3-α (6). Therefore, it is possible that the reduction in integrin recycling and cell spreading that we observe following addition of wortmannin or LY294002 or expression of PKB-AAA is due to increased cellular levels of desphosphorylated, active GSK-3. If so, pharmacological blockade of GSK3 would relieve the inhibition of integrin recycling effected by suppression of PKB/Akt signaling. To test this, we investigated the effect of LiCl and two other well-characterized inhibitors of GSK-3 (SB415286 and SB216763 [10]) in the recycling assay. Recycling of αvβ3 from early endosomes was significantly increased by addition of 20 mM LiCl, indicating that inactivation of GSK-3 is indeed sufficient in itself to drive recycling from this compartment (Fig. 7A). Furthermore, addition of LiCl was able partially to reverse the blockade of αvβ3 recycling effected by wortmannin, and LiCl, SB415286, and SB216763 restored integrin recycling from the Rab11 compartment in cells expressing PKB-AAA (Fig. 7B). We therefore tested the ability of GSK-3 inhibitors to restore cell spreading following suppression of PI(3)K and PKB/Akt. Interestingly, LiCl, SB415286, and SB216763 completely restored spreading in cells expressing PKB-AAA, and, even following treatment with wortmannin, the addition of LiCl partially rescued cell spreading (Fig. 7C).
FIG. 7.
Integrin recycling and cell spreading are suppressed by active GSK-3β. (A and B) NIH 3T3 fibroblasts were transfected with human αvβ3 (hαvβ3) in combination with wt-PKB, PKB-AAA, Mu6pro, Mu6pro-akt1/2, GSK-3β-F216, or GSK-3β-A9. Serum-starved cells were surface labeled with 0.2 mg of N-hydroxysuccinimide-S-S-biotin/ml for 30 min at 4°C, and internalization was allowed to proceed for 15 min at 22°C (A) or for 30 min at 37°C (B). Recycling was determined as for Fig. 3; recycling conditions were 10 min at 37°C in the absence (open bars; control) and presence (solid bars) of PDGF-BB (10 ng/ml) (A) or for 30 min at 37°C in the absence of PDGF (B). One hundred nanomolar wortmannin (WMN), 20 mM LiCl, 10 μM SB216763, and 30 μM SB415286 were included during the recycling period where indicated. Values are means ± standard errors of the means (SEM; n > 10). CON, control. (C and D) NIH 3T3 fibroblasts were transfected with wt-PKB, PKB-AAA, Mu6pro, Mu6pro-akt1/2, GSK-3β-F216, or GSK-3β-A9 in combination with a β-galactosidase transfection marker, and cell spreading on vitronectin was determined as for Fig. 6. Cells were spread in the presence of 100 nM wortmannin, 20 mM LiCl, and 30 μM SB415286 as indicated. Values are means ± SEM (n > 80 cells).
To extend these studies on the involvement of GSK-3 in the PKB/Akt regulation of integrin recycling, we employed two mutant versions of GSK-3β: GSK-3β-F216, which is less active due to lack of a key activation loop phosphorylation site, and GSK-3β-A9, which cannot be phosphorylated and inactivated by PKB/Akt. Following expression of GSK-3β-F216 the integrin recycling and cell spreading responses were normal, consistent with the notion that a relatively high level of inactive phospho-GSK-3 is present in cells to maintain integrin recycling. Conversely, expression of GSK-3β-A9, which would be expected to be active even in cells that express high levels of activated PKB/Akt, inhibited integrin recycling and cell spreading, and, furthermore, these responses were restored by pharmacological inhibition of GSK-3 (Fig. 7). These data indicate that PKB/Akt activates integrin recycling and cell spreading by suppressing the activity of GSK-3.
DISCUSSION
Here we show that PI(3)K and its downstream target PKB/Akt are regulators of integrin recycling events. Wortmannin treatment and blockade of PKB/Akt using dominant negative proteins and RNAi did not affect the internalization of integrins or the Tfn-R, nor was the activity of PKB/Akt required for the subsequent transport of these receptors to early endosomes or the perinuclear recycling compartment. We found no requirement for PKB/Akt in Tfn-R recycling but show that both αvβ3 and α5β1 integrins require active PKB/Akt to return to the plasma membrane. Experiments with mutant GSK-3βs indicate that PKB/Akt is likely to promote integrin recycling by inactivating GSK-3, and we present the finding that, if integrin recycling and cell spreading are suppressed by expression of dominant negative PKB-AAA or RNAi of PKB/Akt, this inhibition can be relieved by inhibition of GSK-3.
PKB/Akt and membrane recycling pathways.
A number of workers have reported that internalization of many receptors proceeds independently of PI(3)K or PKB/Akt activity (9, 39, 41), and in agreement with this we find that the internalization of both integrin and Tfn-R is unaffected by wortmannin treatment or blockade of PKB/Akt. In addition, our data indicate that PKB/Akt has no role to play in the transport of α5β1, αvβ3, and Tfn-R to early endosomes and their subsequent delivery to the Rab11-positive perinuclear recycling compartment. This indicates that the inhibitory effects of PKB-AAA on integrin recycling are not due to reduced “inward” transport of receptors or to overt disturbance of the structure and function of the early and recycling endosomes.
So how does PKB/Akt regulate integrin recycling? There is evidence to suggest that PI(3)K and PKB/Akt are involved in activating Rab proteins. The possibility that Rabs may be activated downstream of PI(3)K was first suggested by the observation that insulin can stimulate GTP exchange onto Rab4 in a fashion that was opposed by wortmannin (40). Additionally, other Rabs have been shown to be activated downstream of the PI(3)K/PKB/Akt axis. For instance, in Dictyostelium, following the sequential activation of PI(3)K and PKB/Akt, Rab7 is recruited to macropinosomes (36), and Rab5-mediated internalization events also require PKB/Akt activity (3). However, it is well established that Tfn-R recycling is a Rab4/Rab11-dependent event, and our data show that there is no requirement for PKB/Akt or for a wortmannin- or LY294002-sensitive PI(3)K in the return of 125I-Tfn to the plasma membrane in NIH 3T3 fibroblasts. This indicates that PI(3)K and PKB/Akt do not influence Rab function per se and are, therefore, more likely to regulate the transport of integrin recycling vesicles themselves, and it will be interesting to determine whether these are distinct from the organelles that recycle Tfn.
It is apparent from our data that the length of time that an integrin spends within the cell before returning to the plasma membrane depends on the cellular activity of PKB/Akt. For instance, if cells are expressing active PKB/Akt or if PDGF is added, αvβ3 integrin returns to the plasma membrane from early endosomes. If cells are serum starved for 30 min, the integrin is transported further into the cell (i.e., to the perinuclear recycling compartment) before being recycled. Furthermore, if PKB/Akt is strongly suppressed by wortmannin treatment, RNAi, or expression of PKB-AAA, or indeed if cells are serum starved for longer times (not shown), α5β1 and αvβ3 integrins are not recycled efficiently from either early endosomes or the recycling compartment and accumulate within the cell. These differences are likely to be due to changes in the balance between the inward and outward transport of integrins in the endocytic pathway. Interactions of endocytic cargo with microtubules and microtubular motors are necessary for movement of endocytic vesicles, and the nature of these is likely to define the cargo's direction of travel (38). α5β1 has recently reported to be associated with the kinesin-associated adaptor, kinectin (44), and kinesins mediate the transport of vesicles toward the plus ends of microtubules at the cell periphery and, therefore, favor recycling. Experiments with GSK-3 inhibitors and mutant GSK-3βs indicate that PI(3)K and PKB/Akt achieve their effects on integrin transport via the inactivation of GSK-3, and it is now clear that GSK-3 phosphorylates the kinesin light chain (30). This phosphorylation inhibits the ability of kinesin to transport vesicular cargo toward the plus ends of microtubules. Inactivation of GSK-3 by PKB/Akt may, therefore, favor the transport of integrin vesicles toward the cell periphery. Furthermore, it is now clear that GSK-3 plays a key role in the regulation of cell migration and the development of cell polarity by inducing the interaction of adenomatous polyposis coli (APC) protein with the plus ends of microtubules (12). A physical link between APC and kinesin has recently been reported (18), and it is possible that this is responsible for targeting integrins to particular cellular regions.
It is interesting that PDGF-dependent αvβ3 recycling was only partially inhibited by wortmannin treatment or expression of PKB-AAA (Fig. 3A). Studies under way in our laboratory indicate that the remaining component of growth factor-induced recycling is under the control of other PDGF-activated signaling pathways, and this will be the subject of further investigation.
The endocytic pathway and integrin function.
PI(3)K has long been known to be involved in inside-out regulation of integrin function (17), and a more recent report highlights the role that PKB/Akt may play in this process (5). PI(3)K activity may activate integrins via the recruitment of PH domain-containing proteins to the membrane, which then influence integrin cytoplasmic tails and cause subsequent heterodimer activation, as has been demonstrated for the activation of β2 integrins by cytohesin-1 (24). PKB/Akt may do this, although this is unlikely, as memb-PKB and memb-PKB-ER clearly promote αvβ3 recycling and these are targeted to the membrane not by a PH domain but by N-terminal myristoylation (27). Another possible route for direct regulation of integrin function by PKB/Akt is via direct phosphorylation of Thr753 of the β3 integrin cytodomain (22). However, in [32P]orthophosphate labeling experiments we have been unable to detect phosphorylation of either αvβ3 or α5β1 in NIH 3T3 fibroblasts (not shown), even following expression of constitutively active PKB/Akt.
Many of the questions surrounding integrin inside-out activation remain unsolved, and it is possible that the endosomal pathway contributes to this phenomenon. Indeed, a considerable proportion of active, high-affinity αvβ3 integrin has been observed within cytoplasmic vesicles in oligodendrocytes (4) and endothelial cells (21). The luminal pH of endosomes is lower than that of the extracellular medium, and it is well established that this dissociates lysosome-destined ligands from receptors that are recycled (1, 51). Therefore, if αvβ3 and α5β1 were internalized with their ligand-binding pockets occluded with fragments of ECM proteins, it is likely that integrin and ligand would separate in endosomes, and the integrin could then be returned to the plasma membrane competent to reengage the ECM. A number of signaling pathways result in increased endosome acidification (53), and recently a regulatory subunit of the V-ATPase E has been shown to bind to Sos1 (28). This association may form part of the mechanism whereby endosome acidification could be driven by receptor tyrosine kinase signaling. Miura et al. (28) have reported that overexpression of V-ATPase in COS cells enhanced the exchange of GTP onto Rac1. Given that we have found that the endosomal pathway modulates integrin function and that engagement of integrins is known to activate Rac1 (33), it would be interesting to determine whether integrin recycling mediates the influence of endosomal pH on Rac1 function.
Cell survival is correlated with the degree of spreading on fibronectin and vitronectin, and it is now well established that integrins can prevent anoikis by enhancing binding to the ECM (26). GSK-3 is also emerging as a key cell survival factor, and inhibitors of GSK-3 are known to protect against apoptosis (10). Our results are consistent with a mechanism whereby PKB/Akt-mediated inactivation of GSK-3 promotes cell survival by maintaining a plasma membrane pool of functional antiapoptotic integrins, and in this regard it would be interesting to investigate the requirement for endosomal recycling in the maintenance of cell survival.
Acknowledgments
This work was generously supported by grants from the Wellcome Trust.
We gratefully acknowledge Dave Critchley for critical reading and helpful comments on the manuscript. We thank Kul Sikand and Sam Wattam their invaluable assistance with the fluorescence microscopy. We thank Dario Alessi and Martin McMahon for the generous gifts of PKB constructs and Karl Matter for the RNAi vector Mu6pro.
REFERENCES
- 1.Bakkeren, D. L., C. M. de Jeu-Jaspars, M. J. Kroos, and H. G. van Eijk. 1987. Release of iron from endosomes is an early step in the transferrin cycle. Int. J. Biochem. 19:179-186. [DOI] [PubMed] [Google Scholar]
- 2.Balendran, A., R. M. Biondi, P. C. Cheung, A. Casamayor, M. Deak, and D. R. Alessi. 2000. A 3-phosphoinositide-dependent protein kinase-1 (PDK1) docking site is required for the phosphorylation of protein kinase Cζ (PKCζ) and PKC-related kinase 2 by PDK1. J. Biol. Chem. 275:20806-20813. [DOI] [PubMed] [Google Scholar]
- 3.Barbieri, M. A., A. D. Kohn, R. A. Roth, and P. D. Stahl. 1998. Protein kinase B/akt and rab5 mediate Ras activation of endocytosis. J. Biol. Chem. 273:19367-19370. [DOI] [PubMed] [Google Scholar]
- 4.Baron, W., S. J. Shattil, and C. ffrench-Constant. 2002. The oligodendrocyte precursor mitogen PDGF stimulates proliferation by activation of αvβ3 integrins. EMBO J. 21:1957-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byzova, T. V., C. K. Goldman, N. Pampori, K. A. Thomas, A. Bett, S. J. Shattil, and E. F. Plow. 2000. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol. Cell 6:851-860. [PubMed] [Google Scholar]
- 6.Cohen, P., and S. Frame. 2001. The renaissance of GSK3. Nat. Rev. Mol. Cell Biol. 2:769-776. [DOI] [PubMed] [Google Scholar]
- 7.Cormont, M., M. N. Bortoluzzi, N. Gautier, M. Mari, E. van Obberghen, and Y. Le Marchand-Brustel. 1996. Potential role of Rab4 in the regulation of subcellular localization of Glut4 in adipocytes. Mol. Cell. Biol. 16:6879-6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cormont, M., M. Mari, A. Galmiche, P. Hofman, and Y. Le Marchand-Brustel. 2001. A FYVE-finger-containing protein, Rabip4, is a Rab4 effector involved in early endosomal traffic. Proc. Natl. Acad. Sci. USA 98:1637-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cormont, M., E. Van Obberghen, M. Zerial, and Y. Le Marchand-Brustel. 1996. Insulin induces a change in Rab5 subcellular localization in adipocytes independently of phosphatidylinositol 3-kinase activation. Endocrinology 137:3408-3415. [DOI] [PubMed] [Google Scholar]
- 10.Cross, D. A., A. A. Culbert, K. A. Chalmers, L. Facci, S. D. Skaper, and A. D. Reith. 2001. Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurones from death. J. Neurochem. 77:94-102. [DOI] [PubMed] [Google Scholar]
- 11.Czauderna, F., M. Fechtner, H. Aygun, W. Arnold, A. Klippel, K. Giese, and J. Kaufmann. 2003. Functional studies of the PI(3)-kinase signalling pathway employing synthetic and expressed siRNA. Nucleic Acids Res. 31:670-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Etienne-Manneville, S., and A. Hall. 2003. Cdc42 regulates GSK-3β and adenomatous polyposis coli to control cell polarity. Nature 421:753-756. [DOI] [PubMed] [Google Scholar]
- 13.Foran, P. G., L. M. Fletcher, P. B. Oatey, N. Mohammed, J. O. Dolly, and J. M. Tavare. 1999. Protein kinase B stimulates the translocation of GLUT4 but not GLUT1 or transferrin receptors in 3T3-L1 adipocytes by a pathway involving SNAP-23, synaptobrevin-2, and/or cellubrevin. J. Biol. Chem. 274:28087-28095. [DOI] [PubMed] [Google Scholar]
- 14.Fraser, E., N. Young, R. Dajani, J. Franca-Koh, J. Ryves, R. S. Williams, M. Yeo, M. T. Webster, C. Richardson, M. J. Smalley, L. H. Pearl, A. Harwood, and T. C. Dale. 2002. Identification of the Axin and Frat binding region of glycogen synthase kinase-3. J. Biol. Chem. 277:2176-2185. [DOI] [PubMed] [Google Scholar]
- 15.Hajduch, E., D. R. Alessi, B. A. Hemmings, and H. S. Hundal. 1998. Constitutive activation of protein kinase B alpha by membrane targeting promotes glucose and system A amino acid transport, protein synthesis, and inactivation of glycogen synthase kinase 3 in L6 muscle cells. Diabetes 47:1006-1013. [DOI] [PubMed] [Google Scholar]
- 16.Hajduch, E., G. J. Litherland, and H. S. Hundal. 2001. Protein kinase B (PKB/Akt)—a key regulator of glucose transport? FEBS Lett. 492:199-203. [DOI] [PubMed] [Google Scholar]
- 17.Hughes, P. E., and M. Pfaff. 1998. Integrin affinity modulation. Trends Cell Biol. 8:359-364. [DOI] [PubMed] [Google Scholar]
- 18.Jimbo, T., Y. Kawasaki, R. Koyama, R. Sato, S. Takada, K. Haraguchi, and T. Akiyama. 2002. Identification of a link between the tumour suppressor APC and the kinesin superfamily. Nat. Cell Biol. 4:323-327. [DOI] [PubMed] [Google Scholar]
- 19.Joly, M., A. Kazlauskas, F. S. Fay, and S. Corvera. 1994. Disruption of PDGF receptor trafficking by mutation of its PI-3 kinase binding sites. Science 263:684-687. [DOI] [PubMed] [Google Scholar]
- 20.Kim, D., S. Kim, H. Koh, S. O. Yoon, A. S. Chung, K. S. Cho, and J. Chung. 2001. Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J. 15:1953-1962. [DOI] [PubMed] [Google Scholar]
- 21.Kiosses, W. B., S. J. Shattil, N. Pampori, and M. A. Schwartz. 2001. Rac recruits high-affinity integrin αvβ3 to lamellipodia in endothelial cell migration. Nat. Cell Biol. 3:316-320. [DOI] [PubMed] [Google Scholar]
- 22.Kirk, R. I., M. R. Sanderson, and K. M. Lerea. 2000. Threonine phosphorylation of the beta 3 integrin cytoplasmic tail, at a site recognized by PDK1 and Akt/PKB in vitro, regulates Shc binding. J. Biol. Chem. 275:30901-30906. [DOI] [PubMed] [Google Scholar]
- 23.Kohn, A. D., A. Barthel, K. S. Kovacina, A. Boge, B. Wallach, S. A. Summers, M. J. Birnbaum, P. H. Scott, J. C. Lawrence, Jr., and R. A. Roth. 1998. Construction and characterization of a conditionally active version of the serine/threonine kinase Akt. J. Biol. Chem. 273:11937-11943. [DOI] [PubMed] [Google Scholar]
- 24.Kolanus, W., W. Nagel, B. Schiller, L. Zeitlmann, S. Godar, H. Stockinger, and B. Seed. 1996. αLβ2 integrin/LFA-1 binding to ICAM-1 induced by cytohesin-1, a cytoplasmic regulatory molecule. Cell 86:233-242. [DOI] [PubMed] [Google Scholar]
- 25.Lawlor, M. A., and D. R. Alessi. 2001. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J. Cell Sci. 114:2903-2910. [DOI] [PubMed] [Google Scholar]
- 26.Liu, W., S. A. Ahmad, N. Reinmuth, R. M. Shaheen, Y. D. Jung, F. Fan, and L. M. Ellis. 2000. Endothelial cell survival and apoptosis in the tumor vasculature. Apoptosis 5:323-328. [DOI] [PubMed] [Google Scholar]
- 27.Mirza, A. M., A. D. Kohn, R. A. Roth, and M. McMahon. 2000. Oncogenic transformation of cells by a conditionally active form of the protein kinase Akt/PKB. Cell Growth Differ. 11:279-292. [PubMed] [Google Scholar]
- 28.Miura, K., S. Miyazawa, S. Furuta, J. Mitsushita, K. Kamijo, H. Ishida, T. Miki, K. Suzukawa, J. Resau, T. D. Copeland, and T. Kamata. 2001. The Sos1-Rac1 signaling. Possible involvement of a vacuolar H+-ATPase E subunit. J. Biol. Chem. 276:46276-46283. [DOI] [PubMed] [Google Scholar]
- 29.Mohrmann, K., L. Gerez, V. Oorschot, J. Klumperman, and P. van der Sluijs. 2002. Rab4 function in membrane recycling from early endosomes depends on a membrane to cytoplasm cycle. J. Biol. Chem. 277:32029-32035. [DOI] [PubMed] [Google Scholar]
- 30.Morfini, G., G. Szebenyi, R. Elluru, N. Ratner, and S. T. Brady. 2002. Glycogen synthase kinase 3 phosphorylates kinesin light chains and negatively regulates kinesin-based motility. EMBO J. 21:281-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng, T., D. Shima, A. Squire, P. I. Bastiaens, S. Gschmeissner, M. J. Humphries, and P. J. Parker. 1999. PKCα regulates β1 integrin-dependent cell motility through association and control of integrin traffic. EMBO J. 18:3909-3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohteki, T., M. Parsons, A. Zakarian, R. G. Jones, L. T. Nguyen, J. R. Woodgett, and P. S. Ohashi. 2000. Negative regulation of T cell proliferation and interleukin 2 production by the serine threonine kinase GSK-3. J. Exp. Med. 192:99-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Price, L. S., J. Leng, M. A. Schwartz, and G. M. Bokoch. 1998. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol. Biol. Cell 9:1863-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ren, M., G. Xu, J. Zeng, C. De Lemos-Chiarandini, M. Adesnik, and D. D. Sabatini. 1998. Hydrolysis of GTP on rab11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes. Proc. Natl. Acad. Sci. USA 95:6187-6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts, M., S. Barry, A. Woods, P. van der Sluijs, and J. Norman. 2001. PDGF-regulated rab4-dependent recycling of αvβ3 integrin from early endosomes is necessary for cell adhesion and spreading. Curr. Biol. 11:1392-1402. [DOI] [PubMed] [Google Scholar]
- 36.Rupper, A., K. Lee, D. Knecht, and J. Cardelli. 2001. Sequential activities of phosphoinositide 3-kinase, PKB/Aakt, and Rab7 during macropinosome formation in Dictyostelium. Mol. Biol. Cell 12:2813-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz, M. A., and V. Baron. 1999. Interactions between mitogenic stimuli, or, a thousand and one connections. Curr. Opin. Cell Biol. 11:197-202. [DOI] [PubMed] [Google Scholar]
- 38.Sheetz, M. P. 1999. Motor and cargo interactions. Eur. J. Biochem. 262:19-25. [DOI] [PubMed] [Google Scholar]
- 39.Shepherd, P. R., M. A. Soos, and K. Siddle. 1995. Inhibitors of phosphoinositide 3-kinase block exocytosis but not endocytosis of transferrin receptors in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 211:535-539. [DOI] [PubMed] [Google Scholar]
- 40.Shibata, H., W. Omata, and I. Kojima. 1997. Insulin stimulates guanine nucleotide exchange on Rab4 via a wortmannin-sensitive signaling pathway in rat adipocytes. J. Biol. Chem. 272:14542-14546. [DOI] [PubMed] [Google Scholar]
- 41.Shpetner, H., M. Joly, D. Hartley, and S. Corvera. 1996. Potential sites of PI-3 kinase function in the endocytic pathway revealed by the PI-3 kinase inhibitor, wortmannin. J. Cell Biol. 132:595-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siddhanta, U., J. McIlroy, A. Shah, Y. Zhang, and J. M. Backer. 1998. Distinct roles for the p110α and hVPS34 phosphatidylinositol 3′-kinases in vesicular trafficking, regulation of the actin cytoskeleton, and mitogenesis. J. Cell Biol. 143:1647-1659, 1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Somwar, R., W. Niu, D. Y. Kim, G. Sweeney, V. K. Randhawa, C. Huang, T. Ramlal, and A. Klip. 2001. Differential effects of phosphatidylinositol 3-kinase inhibition on intracellular signals regulating GLUT4 translocation and glucose transport. J. Biol. Chem. 276:46079-46087. [DOI] [PubMed] [Google Scholar]
- 44.Tran, H., R. Pankov, S. D. Tran, B. Hampton, W. H. Burgess, and K. M. Yamada. 2002. Integrin clustering induces kinectin accumulation. J. Cell Sci. 115:2031-2040. [DOI] [PubMed] [Google Scholar]
- 45.Tsakiridis, T., H. E. McDowell, T. Walker, C. P. Downes, H. S. Hundal, M. Vranic, and A. Klip. 1995. Multiple roles of phosphatidylinositol 3-kinase in regulation of glucose transport, amino acid transport, and glucose transporters in L6 skeletal muscle cells. Endocrinology 136:4315-4322. [DOI] [PubMed] [Google Scholar]
- 46.Ueki, K., R. Yamamoto-Honda, Y. Kaburagi, T. Yamauchi, K. Tobe, B. M. Burgering, P. J. Coffer, I. Komuro, Y. Akanuma, Y. Yazaki, and T. Kadowaki. 1998. Potential role of protein kinase B in insulin-induced glucose transport, glycogen synthesis, and protein synthesis. J. Biol. Chem. 273:5315-5322. [DOI] [PubMed] [Google Scholar]
- 47.van Dam, E. M., and W. Stoorvogel. 2002. Dynamin-dependent transferrin receptor recycling by endosome-derived clathrin-coated vesicles. Mol. Biol. Cell 13:169-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vojtek, A. B., J. Taylor, S. L. DeRuiter, J. Y. Yu, C. Figueroa, R. P. Kwok, and D. L. Turner. 2003. Akt regulates basic helix-loop-helix transcription factor-coactivator complex formation and activity during neuronal differentiation. Mol. Cell. Biol. 23:4417-4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vollenweider, P., S. S. Martin, T. Haruta, A. J. Morris, J. G. Nelson, M. Cormont, Y. Le Marchand-Brustel, D. W. Rose, and J. M. Olefsky. 1997. The small guanosine triphosphate-binding protein Rab4 is involved in insulin-induced GLUT4 translocation and actin filament rearrangement in 3T3-L1 cells. Endocrinology 138:4941-4949. [DOI] [PubMed] [Google Scholar]
- 50.Walker, K. S., M. Deak, A. Paterson, K. Hudson, P. Cohen, and D. R. Alessi. 1998. Activation of protein kinase B beta and gamma isoforms by insulin in vivo and by 3-phosphoinositide-dependent protein kinase-1 in vitro: comparison with protein kinase B alpha. Biochem. J. 331(Pt. 1):299-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamashiro, D. J., L. A. Borden, and F. R. Maxfield. 1989. Kinetics of alpha 2-macroglobulin endocytosis and degradation in mutant and wild-type Chinese hamster ovary cells. J. Cell. Physiol. 139:377-382. [DOI] [PubMed] [Google Scholar]
- 52.Yu, J. Y., S. L. DeRuiter, and D. L. Turner. 2002. RNA interference by expression of short-interfering RNAs and hairpin RNAs in mammalian cells. Proc. Natl. Acad. Sci. USA 99:6047-6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zen, K., J. Biwersi, N. Periasamy, and A. S. Verkman. 1992. Second messengers regulate endosomal acidification in Swiss 3T3 fibroblasts. J. Cell Biol. 119:99-110. [DOI] [PMC free article] [PubMed] [Google Scholar]