Abstract
Objective
To study the incidence, clinical presentation and outcome of intracranial hemorrhagic complications in adult patients with community associated bacterial meningitis.
Methods
Nationwide prospective cohort study from all hospitals in the Netherlands, from 1 March 2006, through 31 December 2010.
Results
Of the 860 episodes of bacterial meningitis that were included, 24 were diagnosed with intracranial hemorrhagic complications: 8 upon presentation and 16 during clinical course. Clinical presentation between patients with or without intracranial hemorrhage was similar. Causative bacteria were Streptococcus pneumoniae in 16 patients (67%), Staphylococcus aureus in 5 (21%), Pseudomonas aeruginosa and Listeria monocytogenes both in 1 patient (4%). Occurrence of intracranial hemorrhage was associated with death (63% vs. 15%, P<0.001) and unfavorable outcome (94% vs. 34%, P<0.001). The use of anticoagulants on admission was associated with a higher incidence of intracranial hemorrhages (odds ratio 5.84, 95% confidence interval 2.17–15.76).
Conclusion
Intracranial hemorrhage is a rare but devastating complication in patients with community-associated bacterial meningitis. Since anticoagulant therapy use is associated with increased risk for intracranial hemorrhage, physicians may consider reversing or temporarily discontinuing anticoagulation in patients with bacterial meningitis.
Introduction
Bacterial meningitis is a life threatening disease with an incidence of 2 cases per 100,000 adults [1], [2]. Streptococcus pneumoniae and Neisseria meningitidis together cause 80% of all cases, leading to a mortality of up to 37% and 13%, respectively [1], [3], [4]. Of those patients who survive, up to 50% have neurological sequelae, including hearing loss and neuropsychological deficits [1], [4], [5]. One of the major causes of mortality and neurological sequelae is the development of cerebrovascular complications, of which cerebral ischemia is most frequently reported [1], [6], [7], [8]. Intracranial hemorrhages have been described as an uncommon complication of meningitis occurring in 2–9% of cases [6], [7], [9]. In this study, we investigated the prevalence, characteristics and outcome of patients who develop hemorrhages as a complication of community-associated bacterial meningitis.
Methods
In this prospective nationwide cohort study, patients older than 16 years were included who were listed in the database of the Netherlands Reference Laboratory for Bacterial Meningitis (NRLBM) in the period from March 2006 through December 2010. Ninety percent of all patients with cerebrospinal fluid (CSF) culture-positive bacterial meningitis in the Netherlands are registered by the NRLBM, which supplied daily updates of the names of the hospitals where patients had been admitted with bacterial meningitis during the previous 2–6 days. The treating physician was contacted and informed consent was obtained from all participating patients or their legally authorized representatives. Patients with bacterial meningitis who were not registered with the NRLBM could also be included if physicians contacted us directly. Patients with negative CSF cultures were only included if the clinical presentation was consistent with bacterial meningitis and the CSF analysis demonstrated at least 1 individual predictor of bacterial meningitis (defined as a glucose level of less than 34 mg/dL [1.9 mmol/L], a ratio of CSF glucose to blood glucose of less than 0.23, a protein level of more than 220 mg/dL, or a leukocyte count of more than 2,000/mm3) [10]. Patients with negative CSF cultures but positive CSF gram stains were also included. All patients with a hospital associated meningitis, recent neuro-trauma or neurosurgical procedure were excluded from analysis. These cases of meningitis have different pathophysiological mechanisms than cases associated with the community setting, and are caused by a different spectrum of microorganisms [11].
Upon discharge, patients underwent a neurological examination and outcome was assessed using the Glasgow Outcome Scale, a well validated measurement scale with scores ranging from 1 (death) to 5 (good recovery) [12]. A score of 1 on this scale indicates death; a score of 2, a vegetative state (the patient is unable to interact with the environment); a score of 3, severe disability (the patient is unable to live independently but can follow commands); a score of 4, moderate disability (the patient is capable of living independently but unable to return to work or school); and a score of 5, mild or no disability (the patient is able to return to work or school). A favorable outcome was defined as a score of 5, and an unfavorable outcome was defined as a score of 1 to 4. The study was approved by the ethical review committee of participating hospitals.
Patients' data was collected by means of a digital Clinical Record From (CRF) by the treating physician. Additional information, including the use of anticoagulant or platelet aggregation therapy, was gathered from discharge letters. Patients were classified as having an intracranial hemorrhagic complication if reported by the treating physician and cranial imaging confirmed the presence of intracranial blood. Neuroimaging was obtained from all patients with an intracranial hemorrhage and was reassessed by two investigators (B.M-K., D.F.) to determine the presence, type and distribution of the hemorrhage. To check for underreporting by physicians, we evaluated 150 consecutive patients included in the cohort who underwent cranial imaging and were not reported to have intracranial hemorrhages by the physician; none of these patients had cerebral hemorrhages.
Population description was performed using medians and interquartile ranges. Differences between episodes of bacterial meningitis with and without hemorrhages were assessed using a Mann-Whitney U-test regarding continuous variables, and a χ2-test or Fisher's exact test regarding dichotomous variables. We used logistic regression analysis to calculate odds ratio (OR) and 95% confidence interval (CI) to assess the strength of any observed associations. All statistical tests were 2-tailed, and a p value of <0.05 was considered to be significant. All analyses were executed using SPSS software, version 16.0.
Results
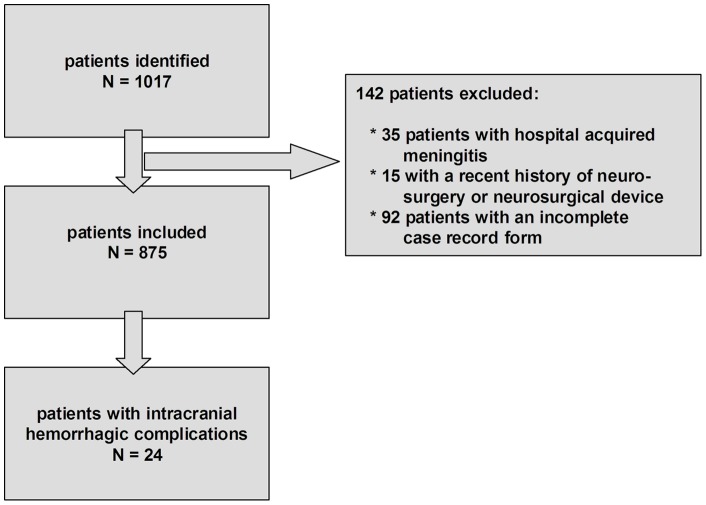
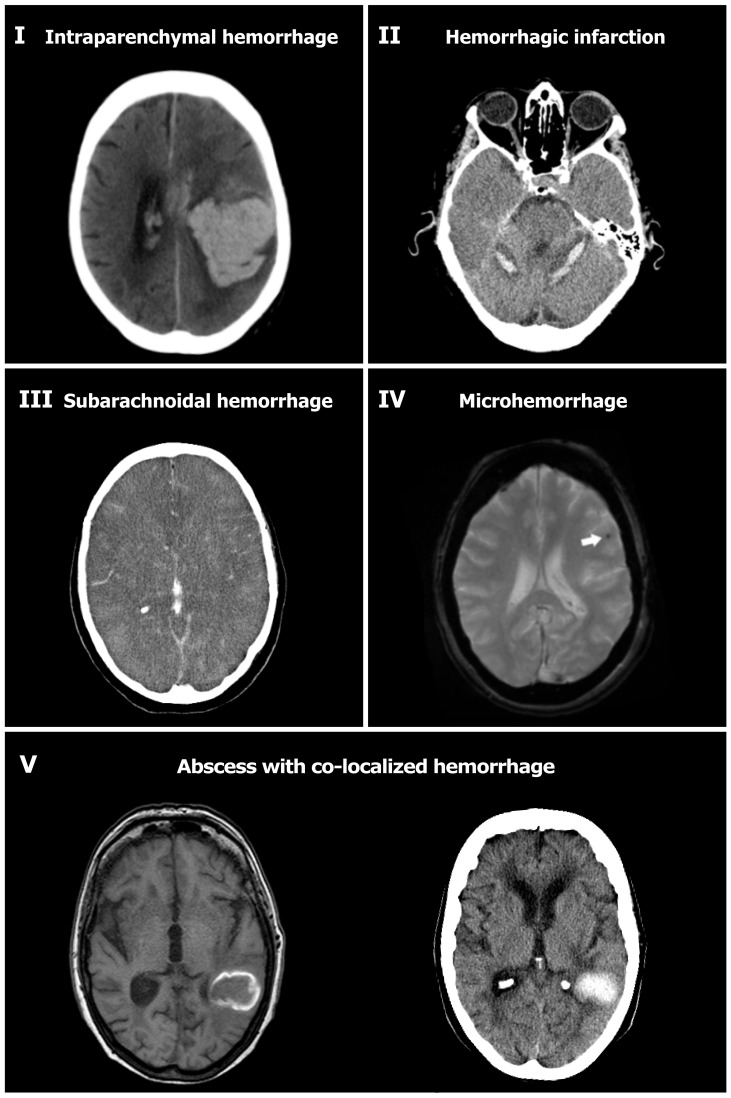
One-thousand-and-two patients with bacterial meningitis were identified by the national reference laboratory (Figure 1). Of the initial 1002, 142 were excluded –35 with hospital associated bacterial meningitis infection; 15 with recent neurosurgical procedure or neurosurgical device; 92 with an incomplete case record form. Thus, a total of 860 patients were included in the final analysis. The causative species were S. pneumoniae in 576 episodes (67%), N. meningitidis in 96 episodes (11%), and other bacteria in 188 episodes (22%). Cerebral hemorrhage was diagnosed in 24 of 860 (2.8%) patients (Table 1). Eight patients were diagnosed with cerebral hemorrhage on presentation and 16 during clinical course (median time to detection of hemorrhage 8 days; range, 1–48 days). Hemorrhages were classified as parenchymal in 10 patients, subarachnoid in 5, microhemorrhages in 3, secondary hemorrhagic transformation following cerebral infarction in 4, and hemorrhages co-localizing with a cerebral abscess in 2 (Figure 2). Predisposing conditions for bacterial meningitis were present in 16 of the 24 (67%) patients, of which the most common were an immunocompromised state in 42% patients, otitis/sinusitis and infective endocarditis each in 17%, and pneumonia in 13%.
Figure 1. Selection of patients.
Table 1. Clinical and laboratory characteristics in bacterial meningitis episodes complicated by intracranial hemorrhagea.
| Clinical characteristics | n/N (%) | Clinical characteristics | n/N (%) |
| Median age, y | 64 (50–74) | Focal neurologic deficits | 11/20 (55) |
| Male | 13/24 (54) | Cranial nerve palsy | 4/23 (17) |
| Predisposing factors for meningitis | 16/24 (67) | Aphasia | 3/13 (23) |
| Otitis or sinusitis | 4/24 (17) | Hemiparesis | 4/20 (20) |
| Pneumonia | 3/24 (13) | Blood values | |
| Endocarditis | 4/24 (17) | Trombopenia (<150x109/L) | 2/7 (29) |
| Immunocompromised stateb | 10/24 (42) | CSF values | |
| Medication on admission | White blood cells (cells/mm3) | 889 (267–2716) | |
| Anticoagulant therapyc | 6/24 (25) | <1000/mm3 | 16 (67) |
| Platelet aggregation inhibitorsd | 5/24 (21) | Protein, g/L | 2.99 (1.20–5.62) |
| Symptoms and signs on admission | CSF: blood glucose ratio | 0.03 (0.00–0.43) | |
| Symptoms <24 h | 8/20 (40) | Microbiological findings | |
| Headache | 11/19 (58) | Positive Gram staine | 16/23 (70) |
| Neck stiffness | 17/23 (74) | Positive blood culture | 18/22 (82) |
| Seizures | 1/22 (5) | CSF culture | |
| Temperature ≥38oC | 17 (71) | Streptococcus pneumoniae | 16 (67) |
| Triad (neck-stiffness, fever,altered mental status) | 13 (54) | Staphylococcus aureus | 5 (21) |
| Score on Glasgow Coma Scale (GCS) | 12 (10–13) | Pseudomonas aeruginosa | 1 (4) |
| Altered mental status (GCS <14) | 20 (83) | Listeria monocytogenes | 1 (4) |
| Coma (GCS <8) | 1 (4) | Negative | 1 (4) |
Data are number/number evaluated (%) or median (interquartile range).
Immunocompromise was defined by the use of immunosuppressive drugs (1), a history of splenectomy (1), or the presence of diabetes mellitus (4) or alcoholism (4), as well as patients infected with the human immunodeficiency virus (HIV)(1).
Five patients were using oral anticoagulants (coumarin derivatives); 2 were using subcutaneous low molecular weight heparin (nadroparin) in therapeutic doses.
Four patients were using acetylsalicylic acid; 1 was using clopidogrel.
Gram-positive cocci in 14 (58%), Gram-positive rods in 0, Gram-negative cocci in 1 (4%), and Gram-negative rods in 1 (4%).
Figure 2. Types of intracranial hemorrhagic complications encountered in bacterial meningitis patients.
Intraparenchymal hemorrhage in left parietal lobe (I); hemorrhagic infarction (II); subarachnoidal hemorrhage (III); micro-hemorrhages (IV, arrow depicts location of hemorrhage; MRI-gradient echo); Abscess formation and subsequent hemorrhagic transformation; panel V depicts MRI T-1 with gadolinium, and a CT-scan 5 days later).
Of the eight patients diagnosed with intracranial hemorrhagic upon admission, focal neurological deficits were present in only one patient. Clinical presentation did not differentiate between patients with or without intracranial hemorrhage, although patients presenting with intracranial hemorrhage were more likely to have an extensor plantar reflex (3 of 7 [43%] vs. 110 of 735 [15%]; P = 0.041). During admission an additional 16 patients were diagnosed with cerebral hemorrhage. In these patients the cerebral hemorrhage was identified on cranial imaging after the patients developed a progressive impairment of consciousness (in 13 patients), developed focal neurological deficit (in 3 patients), showed no clinical improvement (in 2 patients), or developed a status epilepticus (in 1 patient).
Of the 639 patients of which information regarding the use of anticoagulant therapy could be obtained, 41 patients (6%) were using anticoagulant therapy upon admission. Of these patients, 6 patients (15%) developed intracranial hemorrhage (Table 2). Indication for anticoagulation therapy in these 6 patients were history of venous thrombotic events in 3 patients, antithrombin-III deficiency and factor V leiden-mutations in 1 patient each, prosthetic heart-valves in 2 patients, atrial fibrillation in 3 patients. At the time of developing the intracranial hemorrhage all six patients had prolonged coagulation times and/or documented use of anticoagulant therapy. Two patients were diagnosed with hemorrhages in the first week of admission, 1 patient in the second week and 3 patients after more than 2 weeks after admission. Anticoagulant therapy was converted and stopped in all patients after intracranial hemorrhage was diagnosed. The risk of an intracranial hemorrhagic complication in patients using anticoagulants was significantly higher compared to patients who did not use anticoagulants (odds ratio 5.84, 95% confidence interval 2.17–15.76). The risk of intracranial hemorrhagic complications was not increased in patients using platelet aggregation therapy or patients with a thrombocytopenia (odds ratio 2.00, 95% confidence interval 0.72–5.54).
Table 2. Clinical characteristics of patients with bacterial meningitis with intracranial hemorrhagic complications vs. patients without hemorrhagic complicationsa.
| Clinical characteristics | Patients with brain hemorrhage (n = 24) | Patients without brain hemorrhage (n = 836) | p-value |
| Median age, y | 64 (50–74) | 60 (44–69) | 0.207 |
| Predisposing conditions | 16/24 (67) | 494/831 (59) | 0.477 |
| Endocarditis | 4/24 (17) | 8/808 (1) | <0.001c |
| Medication upon admission | |||
| Anticoagulant therapyb | 6/23 (25) | 35/615 (6) | 0.002c |
| Platelet aggregation inhibitors | 5/24 (21) | 75/615 (12) | 0.193c |
| Symptoms and signs on admission | |||
| Seizures | 1/22 (5) | 48/810 (6) | 1.00c |
| Score on Glasgow Coma Scale | 12 (10–13) | 11 (9–14) | 0.482 |
| Altered mental status (GCS <14) | 20/24 (83) | 602/836 (72) | 0.222 |
| Coma (GCS <8) | 1/24 (4) | 111/836 (13) | 0.350c |
| Focal neurologic deficits | 9/24 (38) | 224/836 (27) | 0.245 |
| Cranial nerve palsy | 4/24 (17) | 63/769 (8) | 0.122c |
Data are number/number evaluated (%) or median (interquartile range).
Five patients were using oral anticoagulants (coumarin derivatives); 2 were using subcutaneous low molecular weight heparin (nadroparin) in therapeutic doses.
Fisher exact test.
A lumbar puncture was performed in all 24 patients. The CSF of all patients had either a positive culture and/or at least one individual CSF finding predictive of bacterial meningitis (glucose level less that 34 mg/dL [1.9 mmol/L], a ratio of CSF glucose to blood glucose of less than 0.23, a protein level of more than 220 mg/dL, or a leukocyte count of more than 2,000/mm3) [10]. The CSF leukocyte count was lower in patients with intracranial hemorrhage upon or during admission than in patients without intracranial hemorrhagic complications (67% of patients under 1000 leukocytes/mm3 vs. 30%, P<0.001). Other CSF parameters did not significantly differ. CSF cultures revealed S. pneumoniae in 16 of 24 patients (67%), Staphylococcus aureus in 5 (21%), Pseudomonas aeruginosa and Listeria monocytogenes both in 1 patient (4%). One patient had a negative CSF culture but had a positive Gram stain. Four patients fulfilled the Dukes criteria for an infective endocarditis (17%) – in all cases the causative agents was S. aureus.
Neurosurgical interventions were performed in 2 patients – one HIV–positive patient with S. aureus meningitis, which was complicated by a subarachnoidal hemorrhage, underwent a bilateral frontotemporal craniotomy and died 10 days after the procedure following rebleeding from a mycotic aneurysm. Another patient with a S. pneumoniae meningitis and microhemorrhage in the left hemisphere developed a hydrocephalus and deteriorating consciousness. An external ventricular drain was placed and she partially recovered with persisting cranial nerve palsies and ataxia.
Occurrence of intracranial hemorrhage was associated with a higher rate of mortality (63% vs. 15s P<0.001) and unfavorable outcome (96% vs. 34%, P<0.001) than in patients without intracranial hemorrhagic complication (Table 3). There were significantly more neurological sequelae among the survivors (78% vs. 14%, P<0.001).
Table 3. Complications and outcome in adults with vs. without intracranial hemorrhagic complicating bacterial meningitisa.
| Characteristic | Episodes with Brain Hemorrhage (n = 24) | Episodes without Brain Hemorrhage (n = 836) | p-value |
| Complications | |||
| Impairment of consciousness | 23/24 (96) | 447/825 (54) | <0,001 |
| Focal neurologic deficits | 11/20 (56) | 163/796 (21) | <0.001 |
| Systemic complicationsb | 20/24 (83) | 302/824 (37) | <0.001 |
| Glasgow Outcome Scale | |||
| 1. Death | 15/24 (63) | 129/836 (15) | <0.001d |
| 2. Vegetative state | 0/24 | 1/836 (0.1) | 0.99d |
| 3. Severely disabled | 2/24 (8) | 35/836 (4) | 0.561d |
| 4. Moderately disabled | 6/24 (25) | 116/836 (14) | 0.135d |
| 5. Good recovery | 1/24 (4) | 555/836 (66) | <0.001d |
| Neurologic sequelae | 7/9 (78)c | 99/707 (14) | <0.001d |
Data are number/number evaluated (%).
Systemic complications included respiratory failure and circulatory shock (58% and 67%, respectively).
Neurologic sequelae in patients with intracranial hemorrhage include hemiparesis (4), cognitive impairment (3), cranial nerve palsy (3) and ataxia (3).
Fisher exact test.
Discussion
Our study shows that intracranial hemorrhages are a severe complication of bacterial meningitis, occurring in about 3% of adults. Two previous case series from tertiary referral centers have reported rates varying from 2% to 9% [7], [13]. Our study was performed nationwide and, therefore, we are able to study a representative sample of adults with acute bacterial meningitis. Patients with intracranial hemorrhages were at high risk for unfavorable outcome (96%) and death (67%).
A high proportion of the patients who developed intracranial hemorrhagic complications were using anticoagulant therapy when they developed meningitis (25%), of which most were diagnosed with intracranial hemorrhages during admission. Conversely, our data shows a 5-fold increased risk of developing intracranial hemorrhage if the patient with bacterial meningitis uses anticoagulant therapy. Therefore, the question is whether anticoagulant therapy should be discontinued once a patient has been diagnosed with bacterial meningitis. Or, practically speaking, after a lumbar puncture is performed upon admission (for which prior reversal of anticoagulant therapy is necessary), is it advisable to immediately resume anticoagulant therapy? Previous studies on the effect of anticoagulant therapies in bacterial meningitis showed a higher rate of hemorrhagic complications and mortality [14], [15]. In a retrospective analysis of several open-label, placebo controlled and compassionate-use trials of activated protein C (APC), intracranial hemorrhage was seen in 6% of patients treated with APC compared to 3% of patients receiving placebo or no APC. Adjunctive treatment with heparin was examined in a clinical trial in which 15 patients were randomized to a heparin or control treatments; 4 of 7 patients (57%) receiving heparin died compared to 2 of 8 patients (25%) receiving control treatment. No studies have investigated the risk or benefit of discontinuing anticoagulation therapy once bacterial meningitis has been diagnosed. In the light of our present findings, we suggest that physicians weigh the risks of intracranial hemorrhage against the risks of the pro-thrombotic state for which the anticoagulant was prescribed, and consider discontinuation of therapeutic anticoagulant treatment until the patient has recovered from the acute phase of the bacterial meningitis episode (e.g., 2–3 weeks).
A relative high proportion of patients with cerebral hemorrhage had S. aureus meningitis. S. aureus is a rare cause of community-associated bacterial meningitis and has previously been associated with endocarditis and cerebral abscesses [16], [17]. In our cohort of 860 patients 13 patients (1.5%) had S. aureus meningitis, five of whom developed intracranial hemorrhages (38%). Of these five patients with S. aureus meningitis and intracranial hemorrhagic complications, four fulfilled the Dukes criteria for having infective endocarditis, two had cerebral abscesses, and two were using anticoagulant therapy. The occurrence of ischemic stroke in patients in S. aureus infective endocarditis has previously been shown to be between 22% and 34%.[18], [19] Intracranial hemorrhages in S. aureus infective endocarditis are thought to be caused by hemorrhagic transformations of cerebral ischemia and occur in 3–17% of patients [18], [19]. Debate is still ongoing regarding the benefits and potential risks of anticoagulant therapy in these patients [20], [21], [22]. The higher rate of intracranial hemorrhages in patients with S. aureus meningitis and infective endocarditis (vs. those patients with infective endocarditis alone), suggests that anticoagulant therapy should not be prescribed in these patients. The increased risk for intracranial hemorrhage in patients with bacterial meningitis using anticoagulant therapy remains marked (odds ratio 4.62, 95% confidence interval 1.45–14.71) even when patients with endocarditis are excluded from the analysis. Two patients with S. aureus meningitis had secondary haemorrhages that co-localized to the site of the abscess.
The rate of mortality and unfavorable outcome in patients with bacterial meningitis and intracranial hemorrhage is high (65% and 95% respectively). However, it is improbable that all observed intracranial hemorrhages were symptomatic and contributed equally to the clinical outcome, which is likely to be also determined by the severity of the meningitis itself, as well as the occurrence of other systemic or neurologic complications. Three of the patients with intracranial hemorrhage had a intracranial abscess, four patients had cerebral infarctions and two had a hydrocephalus, which are all associated with higher mortality and unfavorable outcome [8], [16], [23]. The underlying pathophysiology of cerebrovascular complications during bacterial meningitis (infarct, venous thrombosis and hemorrhages) is largely unknown but may share common pathophysiological mechanisms: first, there is a dysregulation of both the coagulation and fibrinolytic pathways, not only systemically but also locally in the central nervous system compartment [24], [25], [26]. Second, vascular endothelial cell swelling and activation is a common finding in bacterial meningitis, which leads to release of procoagulant factors and pro-inflammatory cytokines, causing further endothelial activation and swelling [27]. Finally, vasculitis has been proposed as a possible mechanism of cerebral infarction, largely supported by autopsy studies from the 1930′s through 1960′s and angiographic description of segmental arterial narrowing in patients with pneumococcal meningitis, although a recent series of autopsies suggested that vascular complications may also occur in non-vasculitic areas [28]. Massive clotting hypothetically may result in the local depletion of coagulation factors, which together with microvascular damage, vasculitis and cerebral infarction, might lead to the observed manifest cerebral hemorrhages [28].
Our study has several limitations. First, not all patients in the cohort underwent cranial neuroimaging (14% did not undergo imaging). Thus, intracranial hemorrhagic complications may have been missed. Second, only patients who underwent a lumbar puncture and fulfilled CSF criteria for bacterial meningitis were included in the cohort. Patients presenting with a space occupying lesion such as a large intracerebral hemorrhage on initial neuroimaging are not likely to have undergone a lumbar puncture and will therefore not have been included in this cohort. Likewise, those patients whose anticoagulation therapy was not reversed also are unlikely to have undergone a lumbar puncture and are not included in the cohort. These factors may have led to an underestimation of the incidence of intracranial hemorrhage and the use of anticoagulant therapy in patients with bacterial meningitis.
We conclude that intracranial hemorrhage is a rare but devastating complication in patients with community-associated bacterial meningitis. Since anticoagulant therapy use is associated with increased risk for intracranial hemorrhage, physicians may consider reversing or temporarily discontinuing anticoagulation in these patients.
Acknowledgments
We are indebted to all Dutch physicians who participated in the study.
Funding Statement
This study has been funded by grants from the European Research Council (ERC Starting Grant [Proposal/Contract no. 281156] to DB), Netherlands Organization for Health Research and Development (ZonMw; NWOVeni grant 2006 [Proposal/Contract no. 916.76.023], NWO-Vidi grant 2010 [Proposal/Contract no. 016.116.358] to DB), the Academic Medical Center (AMC Fellowship 2008 to DB). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. van de Beek D, de Gans J, Tunkel AR, Wijdicks EF (2006) Community-acquired bacterial meningitis in adults. N Engl J Med 354: 44–53. [DOI] [PubMed] [Google Scholar]
- 2. Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Lynfield R, et al. (2011) Bacterial meningitis in the United States, 1998–2007. N Engl J Med 364: 2016–2025. [DOI] [PubMed] [Google Scholar]
- 3. Brouwer MC, Tunkel AR, van de Beek D (2010) Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev 23: 467–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, et al. (2004) Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med 351: 1849–1859. [DOI] [PubMed] [Google Scholar]
- 5. Hoogman M, van de Beek D, Weisfelt M, de Gans J, Schmand B (2007) Cognitive outcome in adults after bacterial meningitis. J Neurol Neurosurg Psychiatry 78: 1092–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weisfelt M, van de Beek D, Spanjaard L, Reitsma JB, de Gans J (2006) Clinical features, complications, and outcome in adults with pneumococcal meningitis: a prospective case series. Lancet Neurol 5: 123–129. [DOI] [PubMed] [Google Scholar]
- 7. Pfister HW, Feiden W, Einhaupl KM (1993) Spectrum of complications during bacterial meningitis in adults. Results of a prospective clinical study. Arch Neurol 50: 575–581. [DOI] [PubMed] [Google Scholar]
- 8. Schut ES, Lucas MJ, Brouwer MC, Vergouwen MD, van der Ende A, et al. (2012) Cerebral infarction in adults with bacterial meningitis. Neurocrit Care 16: 421–427. [DOI] [PubMed] [Google Scholar]
- 9. Kastenbauer S, Pfister HW (2003) Pneumococcal meningitis in adults: spectrum of complications and prognostic factors in a series of 87 cases. Brain 126: 1015–1025. [DOI] [PubMed] [Google Scholar]
- 10. Spanos A, Harrell FE Jr, Durack DT (1989) Differential diagnosis of acute meningitis. An analysis of the predictive value of initial observations. JAMA 262: 2700–2707. [PubMed] [Google Scholar]
- 11. van de Beek D, Drake JM, Tunkel AR (2010) Nosocomial bacterial meningitis. N Engl J Med 362: 146–154. [DOI] [PubMed] [Google Scholar]
- 12.Jennett B, Teasdale G (1981) Management of head injuries. Philadelphia: F.A. Davis Co. vii, 361 p.
- 13. Kastenbauer S, Pfister HW (2003) Pneumococcal meningitis in adults: spectrum of complications and prognostic factors in a series of 87 cases. Brain 126: 1015–1025. [DOI] [PubMed] [Google Scholar]
- 14. Vincent JL, Nadel S, Kutsogiannis DJ, Gibney RT, Yan SB, et al. (2005) Drotrecogin alfa (activated) in patients with severe sepsis presenting with purpura fulminans, meningitis, or meningococcal disease: a retrospective analysis of patients enrolled in recent clinical studies. Crit Care 9: R331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. MacFarlane JT, Cleland PG, Attai ED, Greenwood BM (1977) Failure of heparin to alter the outcome of pneumococcal meningitis. Br Med J 2: 1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jim KK, Brouwer MC, van der Ende A, van de Beek D (2012) Cerebral abscesses in patients with bacterial meningitis. J Infect 64: 236–238. [DOI] [PubMed] [Google Scholar]
- 17. Brouwer MC, Keizerweerd GD, De Gans J, Spanjaard L, Van De Beek D (2009) Community acquired Staphylococcus aureus meningitis in adults. Scand J Infect Dis 41: 375–377. [DOI] [PubMed] [Google Scholar]
- 18. Rasmussen RV, Snygg-Martin U, Olaison L, Buchholtz K, Larsen CT, et al. (2009) Major cerebral events in Staphylococcus aureus infective endocarditis: is anticoagulant therapy safe? Cardiology 114: 284–291. [DOI] [PubMed] [Google Scholar]
- 19. Tornos P, Almirante B, Mirabet S, Permanyer G, Pahissa A, et al. (1999) Infective endocarditis due to Staphylococcus aureus: deleterious effect of anticoagulant therapy. Arch Intern Med 159: 473–475. [DOI] [PubMed] [Google Scholar]
- 20. Rasmussen RV (2011) Anticoagulation in patients with stroke with infective endocarditis is safe. Stroke 42: 1795–1796. [DOI] [PubMed] [Google Scholar]
- 21. Molina CA, Selim MH (2011) Anticoagulation in patients with stroke with infective endocarditis: the sword of Damocles. Stroke 42: 1799–1800. [DOI] [PubMed] [Google Scholar]
- 22. Sila C (2011) Anticoagulation should not be used in most patients with stroke with infective endocarditis. Stroke 42: 1797–1798. [DOI] [PubMed] [Google Scholar]
- 23. Kasanmoentalib ES, Brouwer MC, van der Ende A, van de Beek D (2010) Hydrocephalus in adults with community-acquired bacterial meningitis. Neurology 75: 918–923. [DOI] [PubMed] [Google Scholar]
- 24. Kowalik MM, Smiatacz T, Hlebowicz M, Pajuro R, Trocha H (2007) Coagulation, coma, and outcome in bacterial meningitis–an observational study of 38 adult cases. J Infect 55: 141–148. [DOI] [PubMed] [Google Scholar]
- 25. Weisfelt M, Determann RM, de Gans J, van der Ende A, Levi M, et al. (2007) Procoagulant and fibrinolytic activity in cerebrospinal fluid from adults with bacterial meningitis. J Infect 54: 545–550. [DOI] [PubMed] [Google Scholar]
- 26. Winkler F, Kastenbauer S, Koedel U, Pfister HW (2002) Role of the urokinase plasminogen activator system in patients with bacterial meningitis. Neurology 59: 1350–1355. [DOI] [PubMed] [Google Scholar]
- 27. Mook-Kanamori BB, Geldhoff M, van der Poll T, van de Beek D (2011) Pathogenesis and pathophysiology of pneumococcal meningitis. Clin Microbiol Rev 24: 557–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vergouwen MD, Schut ES, Troost D, van de Beek D (2010) Diffuse cerebral intravascular coagulation and cerebral infarction in pneumococcal meningitis. Neurocrit Care 13: 217–227. [DOI] [PubMed] [Google Scholar]