Abstract
Insulators are DNA elements that establish independent transcriptional domains within eukaryotic genomes. The Drosophila scs and scs′ insulators localize near the borders of a structural domain in the polytene chromosomes, known as a puff, produced by transcription of the 87A heat shock protein (hsp) genes. It has been suggested that scs and scs′ are boundary elements that delimit this decondensed chromatin domain, reflecting the mechanism by which these sequences act to constrain regulatory interactions. This model was tested using transposons that carried a yellow gene to assess enhancer blocking and an hsp70-lacZ gene to examine the structure of a heat shock puff in the presence and absence of insulators. We found that although scs and scs′ blocked enhancer function, these sequences did not prevent the spread of decondensation resulting from hsp70-lacZ transcription. Further analysis of the endogenous 87A locus demonstrated that scs and scs′ reside within, not at, the borders of the puff. Taken together, our studies suggest that scs and scs′ are not boundary elements that block the propagation of an altered chromatin state associated with puff formation. We propose that these insulators may have a direct role in limiting regulatory interactions in the gene-dense 87A region.
Eukaryotic genomes are assembled into chromatin to establish the platform for accurate transcriptional regulation. Chromatin is not uniformly organized along the length of the chromosome but is folded into regions of distinct higher-order structure. For example, the Drosophila polytene chromosomes display a reproducible dark and light banding pattern, reflective of an underlying structural organization that aligns the 500 to 1,000 chromatids (49, 62). Specialized DNA elements, known as boundary elements, are proposed to generate topologically independent structural domains by assembling protein complexes that interact with each other or nuclear substructures to form loop domains (16, 43, 46). Structural domains have been linked to independent gene expression, postulating that higher-order chromatin structures constrain regulatory interactions such that they occur only within, but not between, domains (reviewed in references 18 and 36). While recent studies suggest that 20 to 30 percent of genes in the Drosophila genome are organized into coexpressed clusters, a correlation between coregulated domains and the banding pattern of polytene chromosomes was not found (7, 13, 60). These observations suggest that a more detailed analysis of independent structural domains is required to fully understand the connection between structural and functional chromosomal domains.
One well-characterized structural domain in the polytene chromosomes resides in the 87A region of the third chromosome (Fig. 1). This 15-kb region contains a pair of divergently transcribed heat shock protein 70 (hsp70) genes (Fig. 1). Under non-heat shock conditions, histones H3 and H4 within the 87A region are acetylated, suggesting that the locus is primed for transcriptional activation (44). Upon heat shock, robust transcription of the hsp70 genes produces a defined structural domain of decondensation, referred to as a puff. Formation of the heat shock puff requires poly(ADP)-ribose polymerase, which ribosylates chromatin proteins. Poly(ADP)-ribose polymerase activity promotes decondensation and unwinding of the chromatin fiber and is accompanied by an increase in histone H3 phosphorylation (44, 63). At the borders of the 87A heat shock puff are two regions of specialized chromatin structures, known as scs and scs′ (64). Each element contains two nuclease-hypersensitive sites flanking a nuclease-resistant core and topoisomerase II cleavage sites that redistribute upon heat shock (64, 65). These properties suggested that scs and scs′ are boundary elements of the 87A puff domain that control the long-range topological transitions and regulate the limits of decondensation associated with transcription of this heat shock locus (33, 64, 65).
FIG. 1.
Cytological region 87 of the Drosophila polytene chromosomes. Shown is the genomic organization of the 87A puff region. This 15-kb region contains five genes, indicated by black arrows: a pair of divergently transcribed hsp70 genes, CG31211 (previously annotated as CG14732), CG3281, and aurora. The dashed arrow designates a possible transcript originating from a previously identified cryptic promoter (30). The locations of scs (white box) and scs′ (gray box) are shown, with more detail provided for the fragments included in the puffing assay. The Zw5 binding site within scs is indicated by a black rectangle. Positions of the CGATA motifs within scs′ are shown as arrowheads, with the arrow indicating the direction of the motif (5′-CGATA-3′). Clusters of three CGATA motifs form low- and high-affinity binding sites for the BEAF protein (68). Restriction site designations are as follows: V, PvuII; R, EcoRI; B, BamHI.
Characterization of the functional properties of scs and scs′ demonstrated that these sequences are insulators (12, 15, 34, 35, 56, 66). Insulators are a class of regulatory elements that establish independent domains of transcriptional activity within eukaryotic genomes (19, 36, 67). Two properties define insulators. First, insulators provide a position-dependent block of enhancer or silencer action. Insulators prevent enhancers and silencers from communicating with a promoter only when inserted between regulatory elements and a promoter (22, 32, 34, 41, 56). Second, insulators protect gene expression from positive and negative chromatin position effects (5, 14, 35, 51, 52). Insulator effects are orientation independent and block regulatory interactions without inactivation of any of the control elements (9, 22, 54). The observation that the scs and scs′ insulators reside at the borders of the heat shock puff provided the first link between an insulator-defined domain and a structural domain, forming the foundation of a model that proposes that insulators are domain boundary elements (20, 64, 66).
Two proteins involved in the insulator effects of scs and scs′ have been identified. The zinc finger Zeste-white 5 (Zw5) protein binds scs, while the boundary element-associated factor (BEAF) proteins bind scs′ (Fig. 1) (17, 28, 68). Zw5 and BEAF localize at opposite borders of the 87A heat shock puff in the polytene chromosomes, with additional sites of association throughout the chromosome arms (6, 17, 68). Non-scs′ BEAF binding regions have been identified that possess insulator properties, suggesting that BEAF localization may define functional domains throughout the genome (11, 12).
The Drosophila genome contains many types of insulators (reviewed in references 19, 21, and 61). These insulators have been identified in a variety of ways, such as by effects on chromosome structure, transcriptional regulation, or the presence of binding sites for an insulator protein. The properties of these diverse insulators suggest that regulatory isolation may be established in mechanistically distinct ways (31, 45). Drosophila insulators are widely distributed, consistent with the proposal that chromosomes are divided into independent functional domains that are important for correct elaboration of transcriptional programs.
The role of the scs and scs′ insulators in defining the limits of the structural domain of the 87A heat shock puff has not been tested. To this end, we developed a polytene chromosome assay that addressed whether the insulator function of scs and scs′ was coupled to formation of puff boundaries. Our studies used the previously characterized hsp70-lacZ transgene to produce large heat shock puffs randomly throughout the genome (40). We used in situ hybridization to determine the extent of decondensation that occurred in the presence and absence of insulators. We find that the scs, scs′, and gypsy insulators do not delimit the borders of a heat shock puff, even though these elements demonstrate enhancer-blocking activity. A reevaluation of the structure of the 87A heat shock puff supports our hsp70-lacZ transgene findings, showing that scs and scs′ reside within, not at, the borders of the heat shock puff. Our results suggest that scs and scs′ do not regulate the structural domain of decondensation at 87A and imply that the endogenous role of these insulators may be restricted to controlling regulatory interactions in this gene-dense region.
MATERIALS AND METHODS
DNA constructions.
Three plasmids were made to facilitate cloning of P[wyp] and derivative transposons: loxP, y/w, and p70ZF. The loxP plasmid (EK710) contained direct repeats of the target site for the bacteriophage P1 Cre recombinase, flanking a unique EcoRI restriction site. The 35-bp loxP sites were isolated from p[SFL] (55). Each insulator was inserted into the EcoRI site, and the loxP-insulator-loxP fragment was removed as a NotI fragment. The y/w plasmid (EK1027) contained the mini-yellow and mini-white genes. The intronless mini-yellow gene was 5.2 kb in size and contained the wing and body enhancers and coding region of the yellow gene, including 2.8 kb of 5′ and 0.13 kb of 3′ flanking DNA (23). The Eco47III site at position −893 relative to the transcription start site was changed to a NotI site, into which the loxP-insulator-loxP fragments were inserted. Modified mini-yellow genes were inserted into an XbaI site within the transformation vector pCaSpeRW15, which carries the mini-white gene with most of the first intron deleted and includes 305 bp of 5′ and 500 bp of 3′ flanking DNA (47). The p70ZF plasmid was derived from cp70ZT, which contained a fusion of the 87C1 hsp70 gene with the bacterial lacZ, lacY, and lacA genes (58). The lacZ, lacY, and lacA genes were inserted into the BamHI site within hsp70, with the eighth codon of lacZ joined in frame to hsp70 (40). The hsp70 gene contained 194 bp of 5′ DNA and extended to 79 bp beyond an AATAAA site at the 3′ end (58). Two 114-bp FRT sites, targets for the yeast FLP recombinase, were PCR amplified from pBSloxFRT (a derivative of p[SFL] [55]) and inserted at position +86 within the hsp70 5′ UTR. A unique NotI site was engineered between the FRTs to allow insertion of the insulators. The total distance from the start site of hsp70 transcription to the 5′ end of the insulator was approximately 200 bp.
Four P[Ins-Ins] transposons were made. These were P[scs′-scs′], P[2scs′-2scs′], P[gyp-gyp], and P[scs-2scs′]. Attempts to make a P[scs-scs] transposon were unsuccessful, because the scs insulator was unstable when inserted within the hsp70-lacZ UTR.
The scs′ insulator corresponded to an approximately 500-bp fragment, numbered 1 to 501 in the scs′ GenBank sequence (accession number X63732). This fragment contains high- and low-affinity BEAF binding sites (Fig. 1) (68). The scs insulator corresponded to a 990-bp PvuII fragment, numbered 510 to 1503 in the scs GenBank sequence (accession number X63731). This fragment has enhancer-blocking activity similar to the full-length scs and contains the Zw5 binding site (17, 66). The gypsy insulator contained 12 Su(Hw) protein binding sites, corresponding to bp 647 to 1077 in the gypsy retrotransposon (42).
Germ line transformation and genetic manipulations.
The P[wyp] and P[Ins-Ins] transposons were introduced by germ line transformation into the host strain y1w67c23, which carries a point mutation in the yellow translation start codon and a deletion of a portion of the white gene. Transposons were coinjected with “wings clipped” helper plasmid pπ25.7 at concentrations of 1 mg/ml and 200 μg/ml, respectively. Transformants were recognized by a change in eye phenotype and used to establish stocks. Southern analysis determined the number of inserts and analyzed the integrity of the transposon and the presence of insulators in all lines. Only lines with single insertions were analyzed further.
Derivative lines lacking the upstream insulator (P[ΔIns-Ins]) were obtained by crossing males that carried the original P[Ins-Ins] transposons to females that carried a constitutively active Cre recombinase transgene (y1w; P[w+ Cre] CyO/Sco, provided by Dan Hartl and Mark Siegal, Harvard University). Male y1w/Y; P[Ins-Ins]/+; P[w+ Cre] CyO/+ progeny with dark wing and body pigmentation were selected. This phenotype suggested that somatic excision of the insulator occurred, allowing the wing and body enhancers to activate the yellow promoter, and suggested that these males also carried a deletion of the insulator in the germ line. These males were backcrossed to the parental y1w67c23 stock. Male y1w67c23/Y; P[ΔIns-Ins] progeny lacking the P[w+ Cre] transgene were selected and backcrossed to y1w67c23 females to establish a stock. Southern analysis confirmed excision of the 5′ insulator.
P[Ins-ΔIns] derivative lines lacking the downstream insulator were obtained by crossing males that carried the original P[Ins-Ins] transposons to females that carried a Flp transgene under the control of a heat shock promoter (y−ac−w1118 P[70Flp 3F]; P[70Flp 3F] flies provided by Kent Golic, University of Utah). Embryos were heat shocked for 1 h at 37°C at approximately 24 h after egg laying to activate the Flp transgene. Male y−ac−w1118 P[70Flp 3F]/Y; P[Ins-Ins]/+ progeny were selected and backcrossed to the parental y1w67c23 stock. Male y1w67c23/Y; P[Ins-ΔIns] progeny lacking the P[70Flp 3F] transgene were backcrossed to y1w67c23 females to establish a stock. Southern analysis confirmed excision of the 3′ insulator.
Polytene chromosome analyses.
In situ hybridization was performed as described previously (39), using a lacZ-hsp70 probe (4.6-kb EcoRI/SalI fragment from p70ZF) or a white probe (the 4.5-kb mini-white gene from pCaSpeRW15 [47]). Analysis of the patterns of scs and scs′ hybridization at the 87A region used a 990-bp PvuII fragment containing the minimal scs insulator and a 500-bp fragment containing the minimal scs′ insulator, described above. The approximately 1-kb 5′scs probe was obtained by PCR amplification of Canton S genomic DNA, using the primers 5′-AGTTTGCTTGCCGCAGGATATG-3′ (forward) and 5′-GTCGCATCAGTTGGTCTACACG-3′ (reverse). The reverse primer anneals approximately 5 kb upstream of the PvuII fragment containing the minimal scs insulator. For heat shock studies, larvae were incubated at 37°C for 20 min in a circulating water bath. Glands were dissected and fixed within 15 min following heat shock.
Puffs hybridized with the lacZ-hsp70 probe were classified into four different size categories: none (tight band, 1 to 10% the size of the 87C puff), small (11 to 40% 87C puff), intermediate (41 to 75% 87C puff), and large (>100% 87C puff). For each line, at least 50 puffs were classified, with the predominant sizes listed in Tables 1 and 2.
TABLE 1.
Categorization of heat shock puff size in P[wyp] and P[Ins-Ins] transgenic linesa
| Construct | No. of lines
|
|||
|---|---|---|---|---|
| None | Small | Intermediate | Large | |
| P[wyp] | 0 | 0 | 0 | 5 |
| P[scs′-scs′] | 1 | 0 | 0 | 6 |
| P[2scs′-2scs′] | 2 | 1 | 2 | 0 |
| P[scs-2scs′] | 2 | 2 | 0 | 0 |
| P[gyp-gyp] | 0 | 0 | 1 | 3 |
Scale based on size relative to 87C puff: none, 1 to 10%; small, 11 to 40%; intermediate, 41 to 75%; large, 76 to 100+%.
TABLE 2.
Sizes of heat shock puffs formed by P[Ins-Ins] transgenic lines and derivativesa
| Construct | Location | Size of puffs
|
||
|---|---|---|---|---|
| P[Ins-Ins] | P[Ins-ΔIns] | P[ΔIns-Ins] | ||
| P[2scs′-2scs′] | 19D | Small | ND | Small |
| 26A | None | None | None | |
| 79E | None | Int | None | |
| 95C | Int | Large | ND | |
| 99B | Sm/Int | Large | Int | |
| P[scs-2scs′] | 26A | Small | ND | ND |
| 42E | None | Large | None | |
| 60A | None | Large | Small | |
| 83E | Small | Large | Small | |
| P[gyp-gyp] | 19F | Int/Large | Int/Large | ND |
| 25C | Large | Large | Large | |
| 71E | Large | Large | Int/Large | |
Int, intermediate; Sm, small. Lines listed as Sm/Int or Int/Large had an approximately equal number of puffs in the small and intermediate or intermediate and large categories, respectively. ND, not determined.
Puffs hybridized with the white probe were analyzed to determine the extent to which white sequences were included within the puff. At least 50 puffs were examined per line. The majority of puffs observed in P[wyp], P[2scs′-Δ2scs′], P[scs-Δ2scs′], and P[gyp-Δgyp] lines had white hybridization in the middle or throughout at least 50% of the puff, with some lines displaying punctate hybridization at the edge of the puff. In the cases tested, removal of the 5′ insulator in lines with hybridization close to the edge of the puff did not change the appearance of hybridization.
Northern analysis.
Total RNA was isolated from adult flies heat shocked for 45 min in a 37°C warm air incubator. Samples were run on a 1.2% formaldehyde agarose gel, with approximately 15 μg of RNA loaded per lane. Northern analysis was performed using standard methods (8), using a lacZ probe corresponding to an approximately 1-kb EcoRI/SacI fragment containing the 3′ end of the lacZ gene and a loading control probe containing the rp49 gene. Radioactive counts were recorded using a Packard instant imager. Counts for the hsp70-lacZ transcript in each lane were normalized to the amount of rp49 counts in each lane. The factor of change in hsp70-lacZ RNA accumulation was determined by dividing the normalized hsp70-lacZ value in the absence of the insulator by the normalized value in the presence of insulator.
RESULTS
Our chromosome-puffing assay used the hsp70-lacZ fusion gene to generate ectopic heat shock puffs at random sites in the genome. This fusion gene was constructed by insertion of the lacZ operon into the hsp70 coding sequences (40, 58). The hsp70-lacZ transgene establishes a large transcription unit that generates a puff that spreads both upstream and downstream of the hsp70 promoter (57).
The hsp70-lacZ gene was cloned into a P element transformation vector that carried the mini-white and mini-yellow genes, called P[white yellow puff] or P[wyp] (Fig. 2). The mini-white gene encodes a protein required for eye pigmentation, allowing transformed flies to be identified by a change in eye phenotype. The mini-yellow gene encodes a protein responsible for dark cuticle pigmentation and contains two of the tissue-specific enhancers, the upstream wing and body enhancers (23). If a functional insulator is inserted between these upstream enhancers and the promoter, then yellow expression is lost in the wing and body cuticle and pigmentation is brown. Analysis of yellow expression in the presence of insulators served as a control for the influence of genomic context on insulator function and therefore possible position effects on puff boundary formation (10).
FIG. 2.
Large heat shock puffs are produced by the P[wyp] transposon. (A) Shown is the structure of the P[wyp] transposon that contains three transgenes, indicated by rectangles (red = mini-white; yellow = mini-yellow; white = hsp70; blue = lacZ). The arrows indicate the direction of transcription. The yellow ovals represent the wing and body enhancers for the yellow gene. The heat shock elements are shown as white rectangles. Black arrowheads represent loxP sites for recognition by the Cre recombinase. White arrows represent FRT sites for recognition by the FLP recombinase. Note that P[wyp] is not drawn to scale. Black bars indicate the extent of the probes used for in situ hybridization (white and lacZ-hsp70). Chromosomes from a transgenic line containing the P[wyp] transposon at cytological position 49F were hybridized with the white probe (left) or the lacZ-hsp70 probe (right). For the lacZ-hsp70 probe, both non-heat-shocked (no HS) and heat shocked (HS) chromosomes are shown. (B) Shown is a larger region of heat-shocked chromosomes from a larva containing the 49F P[wyp] transposon hybridized with the lacZ-hsp70 probe. Hybridization occurs at the endogenous hsp70 puffs at 87A and 87C, as well as the transgene puff.
To confirm that the hsp70-lacZ transgene produced large puffs in the context of P[wyp], five transgenic lines were established. P[wyp] flies had dark wing and body pigmentation (data not shown). Analysis of chromosomes from heat-shocked and non-heat-shocked third-instar larvae was done by in situ hybridization. Initial analyses used the lacZ-hsp70 probe (Fig. 2). Hybridization with this probe detected the transgene, as well as the natural 87A and 87C heat shock puffs, because it contained sequences from the 3′ end of the hsp70 gene. The sizes of the heat shock puffs produced by P[wyp] were compared to those of the endogenous hsp70 puffs to control for the degree of heat shock and amount of squashing of the chromosomes. The non-heat-shocked P[wyp] chromosomes showed a tight band of lacZ-hsp70 hybridization at the site of the P[wyp] insertion in all five lines tested (Fig. 2A). In contrast, the heat-shocked P[wyp] chromosomes had a large puff at the transgene site, with hybridization of the probe occurring throughout the puff. The size of the P[wyp] puffs differed slightly between transgenic lines, with all lines showing puffs that were the size of the endogenous 87C puff or larger and were classified as large (Fig. 2B, Table 1). Production of a puff of this dimension was not surprising because the size of a heat shock puff depends, in part, upon the length of the transcription unit (58). The hsp70-lacZ transcript is nearly four times larger than the endogenous hsp70 genes.
We determined whether the decondensation associated with the P[wyp] heat shock puffs extended upstream of the hsp70 promoter using in situ hybridization with the mini-white gene as a probe (Fig. 2A). In all five lines, the white probe hybridized throughout the puff in the majority of chromosomes analyzed (60 to 70%), while the remaining puffs showed hybridization at a puff edge that reached towards the puff center, covering 25% or more of the interior. The pattern of white hybridization differed from that observed with the lacZ-hsp70 probe, displaying a more punctate pattern. We note that the lacZ-hsp70 probe includes sequences that are transcribed, while the white probe does not. These observations suggest that the distinct pattern of hybridization associated with these probes may reflect different levels of chromatin decondensation in the puff, with the lacZ-hsp70 region in a highly extended and decondensed conformation due to the passage of RNA polymerase, while the white region is less decondensed. These data demonstrate that the P[wyp] transposon produces large puffs that spread at least 5.5 kb upstream of the hsp70 promoter. This spread is in the range of decondensation associated with the endogenous 87A heat shock puff that encompasses sequences over an approximately 15-kb region. The properties of the P[wyp] transposon provided a system for analyzing whether insulators delimit the boundaries of a heat shock puff.
A puffing assay to test whether scs and scs′ are domain boundaries.
A series of transposons were constructed that carried insulators inserted both upstream and downstream of the hsp70 promoter in P[wyp] to make P[Ins-Ins] (Fig. 3 and 4, see Fig. 6). The upstream insulator in P[Ins-Ins] was inserted between the enhancers and the promoter of the yellow gene (3.4 kb upstream of the hsp70-lacZ promoter). We reasoned that if an insulator were active at a given genomic location, then transcriptional activation by the wing and body enhancers would be lost, as assessed by the level of adult cuticle pigmentation. A demonstration of enhancer-blocking activity was necessary to confirm that each sequence tested for puff boundary activity functioned as an insulator. The 5′ limits of the heat shock puff were defined using the mini-white gene as a probe. These sequences were 2 kb upstream of the yellow gene, providing distance between the insulator and the white probe to ensure that this probe was outside of an insulator-defined domain.
FIG. 3.
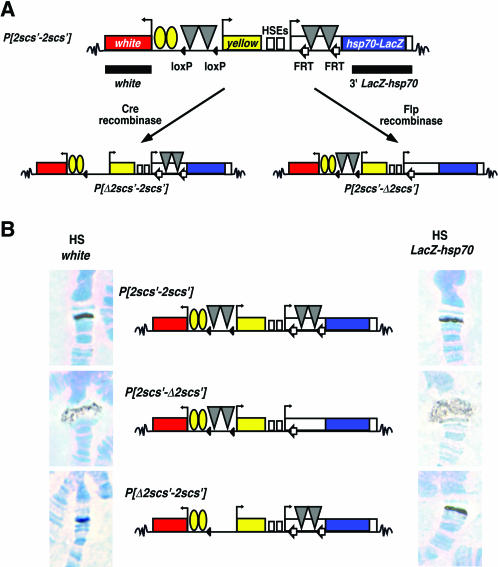
Effects of the 2scs′ insulator on the structure of a heat shock puff. (A) Shown are the P[2scs′-2scs′] transposon and its derivatives, P[Δ2scs′-2scs′] and P[2scs′-Δ2scs′]. Symbols are as described in Fig. 2. A pair of scs′ insulators (gray triangles) was inserted between the wing and body enhancers and yellow promoter at position −900 relative to the yellow transcription start site and at position +86 relative to the hsp70-lacZ transcription start site. Derivative lines lacking the upstream 2scs′ (P[Δ2scs′-2scs′]) were generated by crossing flies that carried P[2scs′-2scs′] to flies expressing a source of Cre recombinase (left arrow). Derivative lines lacking the downstream 2scs′ (P[2scs′-Δ2scs′]) were generated by crossing flies that carried the P[2scs′-2scs′] transposon to flies expressing a source of FLP recombinase (right arrow). (B) The structures of the P[2scs′-2scs′] transposon and its derivatives are shown in the center, with the corresponding heat-shocked chromosomes hybridized with either the white probe (left) or lacZ-hsp70 probe (right) isolated from transposons at position 79E.
FIG. 4.
Effects of the gypsy insulator on the structure of a heat shock puff. Structures of the P[gyp-gyp] transposon and derivative transposons are shown. Symbols were described previously in Fig. 2. The gypsy insulators are represented by black triangles. All chromosomes were isolated from heat-shocked larvae containing the indicated transposon at cytological position 71E. Chromosomes were hybridized with either the white probe (left) or the lacZ-hsp70 probe (right).
FIG. 6.
Effect of the scs insulator on the structure of a heat shock puff. (A) Structures of the P[scs-2scs′] transposon and derivative transposons are shown. Symbols were previously described in the legend to Fig. 2. The white triangle represents the scs insulator. All chromosomes shown are from heat-shocked larvae with the indicated transposon at cytological position 42E. Chromosomes were hybridized with either the white probe (left) or the lacZ-hsp70 probe (right). ND, not determined. (B) Shown are chromosomes isolated from heat-shocked larvae from cytological position 83E hybridized with either the white probe (left) or the lacZ-hsp70 probe (right). These puffs represent exceptional cases in which intermediate puffs were formed, allowing visualization of the pattern of hybridization of the white and lacZ-hsp70 probes.
The downstream insulator was positioned at +86 bp relative to the start site of hsp70 transcription, within the hsp70-lacZ untranslated region. This placed the transposon insulator closer to the hsp70 promoter than the natural location of the scs and scs′ insulators in the 87A locus. The +86 site was chosen to ensure that if the insulator defined a puff boundary, a dramatically decreased puff size would be observed. This insertion site was outside of regulatory sequences required for hsp70 transcription, including the site of the paused polymerase (25, 38, 48). We reasoned that transcription through the insulator would not affect its function, since insulators block enhancer-activated transcription when located within an intron (4, 22). The 3′ limits of the puff were defined by the lacZ-hsp70 probe, located 4.2 kb away from the downstream insulator.
The upstream and downstream insulators in the P[Ins-Ins] transposons were placed in cassettes that contained direct repeats of the target sites for the site-specific Cre and FLP recombinases, respectively (Fig. 3A). In this way, independent removal of the insulators was possible, without a change in genomic location. In each case, derivative lines of P[Ins-Ins] produced by the site-specific recombinases were examined by Southern analysis to confirm the structure and unchanged location of the insulator-deleted transposon. The generation of insulator-deleted derivatives of P[Ins-Ins] at a given genomic site allowed us to attribute changes in the size and structure of heat shock puffs to the presence of insulators and not chromosomal position effects.
The model that scs and scs′ regulate the boundaries of the 87A structural domain that corresponds to the heat shock puff leads to two predictions. First, the size of the puff in the P[Ins-Ins] transgenic lines should be smaller than found in the P[wyp] lines. Second, the puff found in the P[Ins-Ins] lines should be limited to the transposon sequences located between the insulators, such that the white and lacZ-hsp70 probes would not be in the puff. Fulfillment of either prediction would be demonstrated by in situ hybridization results that were distinct from those obtained from P[wyp] lines.
The downstream 2scs′ insulator restricts puff formation.
The P[scs′-scs′] transposon contained a single scs′ insulator positioned upstream and downstream of the hsp70 promoter. This 500-bp fragment includes both the weak and strong BEAF binding sites of scs′ (Fig. 1). Eight P[scs′-scs′] transgenic lines were obtained. Analysis of the yellow phenotype demonstrated that the single scs′ produced only a partial block of the wing and body enhancers, a finding consistent with previous data (37, 66). Heat-shocked chromosomes isolated from larvae of seven of the P[scs′-scs′] transgenic lines were hybridized with the lacZ-hsp70 probe. These studies showed that hybridization was throughout a large puff in six of the lines, with one line showing no puff (Table 1). These data suggest that a single scs′ is a weak insulator that does not form a domain boundary.
A second transposon, P[2scs′-2scs′], was generated, which carried a pair of scs′ insulators, referred to as 2scs′, inserted both upstream and downstream of the hsp70 promoter (Fig. 3). Nine lines were established that showed a greater block of the yellow enhancers than was seen with a single scs′. These results imply that two scs′ elements establish a stronger insulator than one (37). We studied the boundary activity of the stronger 2scs′ insulator because structural models of insulator function predict that the strength of an insulator reflects its capacity to establish a boundary of a structural domain (18, 66).
Effects of the 2scs′ insulator were evaluated by in situ hybridization to polytene chromosomes isolated from larvae of five P[2scs′-2scs′] lines. In all cases, hybridization of the lacZ-hsp70 or white probes to non-heat-shocked chromosomes produced a single, tight band of hybridization at the transgene site, whereas hybridization of these probes to heat-shocked chromosomes produced a range of puff sizes, from no puff to an intermediate puff (Fig. 3B; Table 1). These data are distinct from the large puffs obtained from P[wyp] lines and suggest that the 2scs′ insulator restricted puffing, perhaps by forming a boundary to the spread of decondensation caused by hsp70 transcription.
The downstream 2scs′ was positioned close to the hsp70 promoter to produce a dramatic reduction of puff size, if these sequences established a domain boundary. To test whether the downstream 2scs′ restricted puff formation, flies from four P[2scs′-2scs′] transgenic lines were crossed to flies carrying a transgene that expressed FLP recombinase. Progeny from these crosses were genotyped and those that carried a deletion of the downstream 2scs′ insulator were used to establish a stock. Polytene chromosome analysis was undertaken for all four P[2scs′-Δ2scs′] deletion lines. Chromosomes from heat-shocked larvae of three P[2scs′-Δ2scs′] lines had larger puffs than the corresponding P[2scs′-2scs′] chromosomes, with hybridization of the lacZ-hsp70 probe throughout the puff (Fig. 3; Table 2). The size of the puff in the fourth line did not change, probably reflecting a negative position effect (Table 2). These data demonstrate that the downstream 2scs′ was responsible for limiting puff formation in P[2scs′-2scs′] heat-shocked chromosomes. These results were consistent with a model that proposes that 2scs′ is the boundary of a puff domain, with removal causing an increase in puff size.
The downstream 2scs′ has negative effects on heat shock transcription.
The effects of the upstream 2scs′ on puff formation were examined. We predicted that if 2scs′ were a boundary element, then removal of the upstream 2scs′ would produce a large puff, allowing decondensation to include white sequences. P[2scs′-2scs′] flies were crossed to flies carrying a Cre recombinase transgene, and P[Δ2scs′-2scs′] derivative lines were identified by dark pigmentation of the wing and body. These flies were genotyped, and four P[Δ2scs′-2scs′] lines were established. Chromosomes isolated from heat-shocked larvae were analyzed by in situ hybridization with the lacZ-hsp70 and white probes. To our surprise, we did not observe any change in decondensation in the heat-shocked chromosomes, with both probes showing a tight band of hybridization (Fig. 3B, Table 2). These results imply that restriction of puff formation in the heat shocked P[2scs′-2scs′] chromosomes resulted from the downstream 2scs′.
We wondered whether insertion of any insulator at +86 bp relative to the hsp70 promoter blocked puff formation. We chose the gypsy insulator for study because this insulator shares no sequence motifs or protein partners with scs and scs′ (17, 24, 59, 64, 68). Four independent P[gyp-gyp] lines were established and analyzed. Flies from these lines had low levels of wing and body cuticle pigmentation (data not shown), demonstrating strong enhancer blocking (22, 37). The lacZ-hsp70 probe hybridized throughout the intermediate (one line) to large (three lines) puffs that were formed in heat-shocked chromosomes isolated from the four P[gyp-gyp] lines (Fig. 4; Table 1). The size of the puffs changed only minimally, if at all, when one of the gypsy insulators was removed, as demonstrated by analyses of P[gyp-Δgyp] and P[Δgyp-gyp] heat-shocked chromosomes (Fig. 4; Table 2). These results are distinct from those obtained with P[2scs′-2scs′], implying that the gypsy insulator does not form boundaries of a heat shock puff and that restriction of puff formation by the insertion of an insulator at +86 in the hsp70-lacZ gene is not a general effect.
Previous studies demonstrated that there was a correlation between transcription and puff size (58). We reasoned that restriction of puff formation by the downstream 2scs′ may result because these sequences negatively affected hsp70 transcription. To address this possibility, we undertook Northern analyses of RNA isolated from heat-shocked P[2scs′-2scs′] and P[2scs′-Δ2scs′] adults. We found that hsp70-lacZ RNA accumulation in heat-shocked P[2scs′-2scs′] adults was low and correlated with the size of the larval puff (Fig. 5A; Table 2). In the 79E P[2scs′-2scs′] transgenic line, hsp70-lacZ RNA was barely detectable, consistent with the lack of ectopic puff produced in heat-shocked polytene chromosomes (Figs. 3 and 5A; Table 2). In the 95C and 99B P[2scs′-2scs′] transgenic lines, the observed level of hsp70-lacZ RNA was lower than that found in P[wyp] lines, matching observations that these lines had small to intermediate puffs in heat-shocked polytene chromosomes (Fig. 5A; Table 2). Deletion of the downstream 2scs′ increased accumulation of hsp70-lacZ RNA in all three transgenic lines relative to the parental line at the same genomic position (Fig. 5A). These data demonstrate that 2scs′ negatively impacts heat shock transcription when inserted close to the hsp70 promoter. As a control, the effects of the downstream gypsy insulator on hsp70-lacZ RNA accumulation were determined. We found that the level of hsp70-lacZ RNA was unaffected by the presence of a downstream gypsy insulator, consistent with observations that this insertion did not alter the size or structure of the heat shock puff (Fig. 4 and 5B; Table 2). Taken together, these results indicate that insertion of an insulator near the hsp70 promoter does not generally interfere with transcription, suggesting that the transcriptional repression observed in P[2scs′-2scs′] lines reflects properties of 2scs′.
FIG. 5.
Effects of the 2scs′ and gypsy insulators on hsp70-lacZ transcription. (A) Shown is a Northern blot of total RNA isolated from heat-shocked adults carrying the P[wyp] or P[2scs′-2scs′] transposon. The yw lane contains RNA isolated from the y1w67c23 parental stock that does not carry a P[wyp] transposon. Blots were hybridized with a lacZ probe, as well as rp49, which served as a loading control. The cytological position of transposon insertion in each line is indicated. The presence or absence of a 2scs′ insulator within the hsp70-lacZ UTR is shown by a + or −, respectively. Numbers at the bottom of the blots represent the fold increase in hsp70-lacZ transcript in the P[2scs′-Δ2scs′] derivatives relative to the original P[2scs′-2scs′] lines. (B) Shown is a Northern blot with total RNA isolated from heat-shocked adult flies carrying the P[gyp-gyp] (+) or P[gyp-Δgyp] (−) transposons. Probes and symbols are the same as described above. NA, not applicable.
Sequences upstream of the 2scs′ insulator are included in the heat shock puff.
The versatility of our transgene structure allowed an evaluation of the puff boundary effects of 2scs′. Although heat-shocked chromosomes isolated from the P[2scs′-2scs′] lines had smaller puffs than P[wyp] lines, removal of the downstream 2scs′ increased the size of the puff (Table 2). We predicted that if 2scs′ defined a puff boundary, then the heat shock puffs produced in the P[2scs′-Δ2scs′] lines should not include the white gene sequences. Heat-shocked chromosomes isolated from two P[2scs′-Δ2scs′] lines were hybridized with the white probe. We found that hybridization was throughout the puff in the majority of chromosomes analyzed for each line (>80%) (Fig. 3B). In a third line, half of the chromosomes showed white hybridization throughout the puff while the other half showed hybridization that was nearer the edge. Patterns of white hybridization in P[2scs′-Δ2scs′] puffs were punctate, similar to those found in P[wyp] chromosomes (Fig. 2). We conclude that 2scs′ does not function as a structural boundary of the puff, similar to results with a single scs′, even though 2scs′ had better enhancer-blocking activity.
Effects of scs on the limits of the heat shock puff.
To assess whether scs established a boundary of a heat shock puff, we generated P[scs-2scs′] lines, where an approximately 1-kb scs insulator was inserted upstream of the hsp70 promoter (Fig. 6). This fragment of scs contains the Zw5 binding site (Fig. 1) and has enhancer-blocking activity similar that of to the full-length element (17, 66). The four transgenic P[scs-2scs′] lines showed a strong block of the yellow wing and body enhancers, indicating that the scs insulator was functional at all of the genomic sites sampled (data not shown). Heat-shocked polytene chromosomes were isolated from P[scs-2scs′] larvae from each of the transgenic lines and hybridized with the lacZ-hsp70 probe. In two lines, a tight band of hybridization was observed, while in two other lines, hybridization occurred throughout a small puff (Fig. 6A; Table 1). These results are consistent with data from the P[2scs′-2scs′] lines, in which the downstream 2scs′ restricted formation of a large puff.
Derivatives of three P[scs-2scs′] lines were generated by crossing these flies to flies carrying FLP recombinase. Chromosomes were isolated from heat-shocked larvae of these P[scs-Δ2scs′] lines and hybridized with the lacZ-hsp70 probe. Large heat shock puffs were observed in all lines, with lacZ-hsp70 hybridization throughout the puff (Fig. 6A; Table 2). The presence of puffs in the heat-shocked chromosomes of P[scs-Δ2scs′] lines allowed an investigation of whether the scs insulator established a puff boundary. Chromosomes of heat shocked P[scs-Δ2scs′] larvae were hybridized with the white probe. In two lines, this probe hybridized throughout the puff in the majority of chromosomes analyzed (Fig. 6A). In the third line, half the chromosomes had white hybridization throughout at least 50% of the puff, with the other half of the chromosomes showing hybridization near the edge of the puff. This pattern of white hybridization was similar to that observed in P[wyp] and P[2scs′-Δ2scs′] chromosomes, indicating that the puff spreads beyond the scs insulator. These data suggest that scs is not at the border of the puff.
To determine whether the upstream scs insulator in the P[scs-2scs′] lines affected the size of the puff, transgenic P[Δscs-2scs′] derivatives were made. Three lines were produced by crossing P[scs-2scs′] flies to flies expressing Cre recombinase. Heat-shocked P[Δscs-2scs′] chromosomes were analyzed by in situ hybridization with the lacZ-hsp70 and white probes (Fig. 6A; Table 2). We found that there was no change in puff size in two lines, while in the third line, a change from no puff to a small puff was observed. Based on the findings that two of the three lines showed no change, we conclude that the upstream scs has only minimal, if any, effects on defining the borders of the heat shock puff.
The scs insulator does not establish a puff boundary in the presence of 2scs′.
We considered the possibility that the spread of decondensation upstream of scs that we observed in the P[scs-Δ2scs′] chromosomes reflected a requirement of an interaction between scs and scs′ (6). To address whether both insulators were needed to define the puff boundaries, we examined the extent of puffing in two P[scs-2scs′] lines (26A and 83E). These lines produced small puffs upon heat shock, with the occasional chromosome puff showing an intermediate size (Table 2). Chromosomes were isolated from heat-shocked larvae of the P[scs-2scs′] 26A and 83E lines and hybridized with the white probe. We found that white hybridization was throughout the small puff in the 83E line (Fig. 6B), while in the 26A line hybridization began near the puff edge and extended inwards. Importantly, these patterns of white hybridization did not change in the corresponding derivative P[Δscs-2scs′] lines that were deleted for the upstream scs insulator, as would be expected if scs defined the limits of the puff. These observations strongly suggest that scs insulator activity is independent of the formation of a puff boundary, even in the presence of a nearby 2scs′ insulator.
Sequences upstream of scs are included within the endogenous 87A heat shock puff.
To determine whether the properties of scs and scs′ insulators in our hsp70-lacZ transgenes were reflective of those at the 87A region, we reevaluated the structure of the endogenous heat shock puff. In these studies, we carried out in situ hybridization with three probes to chromosomes isolated from Canton S larvae, including a 1-kb fragment located 5 kb upstream of the scs insulator (5′scs), the 1-kb scs, and 0.5-kb scs′ insulators. Hybridization of the 5′scs probe to chromosomes isolated from non-heat-shocked larvae showed a single, tight band of hybridization at the 87A locus (Fig. 7A). We reasoned that if the scs and scs′ insulators were boundaries of the puff, then these sequences would hybridize at the edge of the puff, whereas the 5′scs probe would remain hybridized in a tight band, since these sequences are located outside of the putative structural domain defined by scs and scs′. In contrast to these predictions, we found the 5′scs, scs, and scs′ probes hybridized within the puff found in chromosomes isolated from heat-shocked larvae (Fig. 7B). Interestingly, the pattern of hybridization of the 5′scs probe was diffuse and nearly identical to that found with scs, even though these sequences are located 5 kb away from scs (Fig. 7B). These data imply that the boundaries of the puff are not coincident with the positions of the scs and scs′ insulators. Hybridization of the 5′scs, scs, and scs′ probes occupies a smaller portion of the puff than is seen for the white and lacZ probes in the hsp70-lacZ transgene puffs. We attribute this difference to the sizes of DNA used in the hybridization experiments, with the white and lacZ probes containing 4 to 5 kb of DNA relative to 1 kb or less for the 5′scs, scs, and scs′ probes. Our results demonstrate that the scs and scs′ insulators are not boundaries of the 87A puff. In addition, these experiments confirm that results obtained using hsp70-lacZ transgenes correspond to those of scs and scs′ at their endogenous location.
FIG. 7.
Analysis of the positions of scs and scs′ within the 87A puff. (A) Chromosomes isolated from a Canton S larva under non-heat shock conditions, hybridized with the 5′scs probe, which anneals 5 kb upstream of the scs insulator. (B) Cytological region 87 of chromosomes isolated from Canton S larvae are shown, under non-heat shock or heat shock conditions. The positions of probes used for in situ hybridization (5′scs, scs, and scs′) are indicated on a map of the 87A locus. Symbols were described previously in the legends to Fig. 3 and 5.
DISCUSSION
We used two approaches to test the hypothesis that scs and scs′ are boundary elements of the domain of decondensation associated with transcription of the 87A hsp70 genes. First, we developed a transgene system that evaluated boundary activity in conjunction with enhancer blocking function. We found that scs and 2scs′ did not prevent the upstream spread of decondensation associated with transcription of the hsp70-lacZ transgenes, even though these insulators blocked the yellow enhancers (Fig. 3 and 6). Second, we reexamined the properties of scs and scs′ at the endogenous 87A locus. Surprisingly, we found that the heat shock puff extended beyond scs and scs′ and included DNA sequences 5 kb upstream of scs (Fig. 7). Taken together, these data demonstrate that scs and scs′ do not define the boundaries of the heat shock puff and suggest that these insulators do not block the propagation of altered chromatin states associated with puffing.
Several explanations may account for the difference between our studies and those done previously (64). First, different heat shock conditions may have been used. In our experiments, heat shock chromosomes were isolated prior to regression of the puff, which allowed us to observe the full extent of decondensation that occurs. Second, conditions used to prepare chromosomes may have differed. In our transgene studies, the sizes of the heat shock puffs produced by hsp70-lacZ were compared to those of the endogenous hsp70 puffs to control for the degree of heat shock and amount of squashing of the chromosomes. However, it is possible that our conditions produced some puff distortion, resulting in a more diffuse hybridization pattern. Third, the hybridization methods used in the two studies differed significantly. The previous study used autoradiography, whereas our experiments used biotin-labeled probes, coupled with a peroxidase detection system. The higher resolution of our technique provided a more accurate determination of the positions of specific DNA sequences relative to the Giemsa banding pattern. With this new technology, we find that scs and scs′ are within the 87A puff.
In the course of our studies, we found that the dimerized 2scs′ repressed hsp70-lacZ transcription 2.5- to 3.5-fold (Fig. 5). It is possible that these negative effects were caused by transcriptional interference between the closely opposed hsp70 promoter and the promoters in scs′, where transcription from these promoters under non-heat shock conditions could influence establishment of a paused polymerase. However, we do not favor this proposal because a single scs′ insulator contains the same promoters yet did not restrict puff formation (Table 1). A second possibility is that proteins bound to 2scs′ may interfere with transcription when positioned close to a promoter. For example, adjacent to the BEAF binding sites in an scs′-related insulator, BE28, is an AT-rich sequence that binds the D1 AT hook protein, a protein that localizes to transcriptionally inactive regions of chromosomes (2, 11, 50). BEAF and D1 interact in vitro, suggesting that an association between these two proteins may play a role in BE28 insulator function (11). In a similar way, 2scs′ may recruit proteins that interfere with hsp70 transcription. It is unknown whether the repressive effects of 2scs′ reflect the natural properties of a single scs′ insulator or are a novel consequence of generating a dimer. We propose that dimerization may intensify the endogenous properties of scs′ in the same way that insulator effectiveness is increased.
A recent study using the chromosome conformation capture assay found evidence that the scs and scs′ insulator binding proteins, Zw5 and BEAF, are in close physical proximity in embryonic nuclei (6). These observations indicate that the scs and scs′ insulators may interact to form a loop domain. However, such interactions may not occur in polytene chromosomes, since the Zw5 and BEAF proteins localize only to scs and scs′, respectively (6, 68). Our studies suggest that even if such interactions occur in polytene tissues, they do not restrict the spread of the heat shock puff.
The role of scs/scs′ at the borders of the 87A domain.
The 87A region is genetically complex (3). Between scs and scs′ are four genes, the two coordinately regulated hsp70 genes and the CG31211 and CG3281 genes (Fig. 1). The scs insulator includes the promoter for CG31211 and a cryptic divergent promoter (3, 30). CG31211 RNA accumulates throughout development, with the highest levels of RNA in embryos and larvae (1) (http://genome.med.yale.edu/Lifecycle). The scs′ insulator includes the promoters of CG3281 and aurora, which encodes a serine/threonine kinase required for mitotic spindle function (26). CG3281 RNA is present predominantly in early embryos, with low levels found in larvae and pupae (1). The developmental expression pattern of aurora has not been determined, although it is predicted that its expression is highest in tissues undergoing mitosis. These RNA patterns suggest that the non-hsp70 genes in the 87A region are not coordinately regulated and imply that this structural domain is not a functional domain.
The role of scs and scs′ in the 87A locus remains unclear. Our findings imply that these elements do not limit the extent of heat shock-induced chromatin decondensation. Instead, the localization of these two insulators in such a gene-dense region suggests that scs and scs′ maintain transcriptional fidelity in the region. That the insulator proteins, Zw5 and BEAF, bind within 100 bp of transcription start sites suggests that these proteins may have a direct role in maintaining appropriate levels of transcription of the non-hsp70 genes. These proteins may form a loop that limits enhancer action. Alternatively, the scs/scs′ insulator proteins may control the nature of the proteins bound at the locus. For example, BEAF and a transcriptional activator, DREF, have overlapping DNA binding specificities (27, 29). DREF regulates several genes involved in DNA replication and cell proliferation (29, 53). Using DREF as a paradigm, it is possible that BEAF competes with transcriptional activators, such as DREF, for scs′ binding, to prevent activation of aurora. The formation of a loop, through interactions between Zw5 and BEAF, may help to maintain BEAF association and repression of aurora expression. A better understanding of the specific roles of the scs and scs′ insulators at this locus will require a more detailed analysis of this interesting chromosomal region.
Acknowledgments
We thank Jeff Simon, who catalyzed these experiments and provided thoughtful and insightful comments. We are grateful to Judy Kassis, Timothy Parnell, Lori Wallrath, and Oya Yazgan for their critical reading of the manuscript and helpful comments on the experimental design and interpretation.
This work was supported by a National Institutes of Health grant to P.K.G. (GM42539).
REFERENCES
- 1.Arbeitman, M. N., E. E. Furlong, F. Imam, E. Johnson, B. H. Null, B. S. Baker, M. A. Krasnow, M. P. Scott, R. W. Davis, and K. P. White. 2002. Gene expression during the life cycle of Drosophila melanogaster. Science 297:2270-2275. [DOI] [PubMed]
- 2.Aulner, N., C. Monod, G. Mandicourt, D. Jullien, O. Cuvier, A. Sall, S. Janssen, U. K. Laemmli, and E. Kas. 2002. The AT-hook protein D1 is essential for Drosophila melanogaster development and is implicated in position-effect variegation. Mol. Cell. Biol. 22:1218-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avramova, Z., and A. Tikhonov. 1999. Are scs and scs' ′neutral' chromatin boundaries of the 87A7 locus in vivo? Trends Genet. 15:138-139. [DOI] [PubMed] [Google Scholar]
- 4.Belozerov, V. E., P. Majumder, P. Shen, and H. N. Cai. 2003. A novel boundary element may facilitate independent gene regulation in the Antennapedia complex of Drosophila. EMBO J. 22:3113-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bi, X., and J. R. Broach. 1999. UASrpg can function as a heterochromatin boundary element in yeast. Genes Dev. 13:1089-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanton, J., M. Gaszner, and P. Schedl. 2003. Protein:protein interactions and the pairing of boundary elements in vivo. Genes Dev. 17:664-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boutanaev, A. M., A. I. Kalmykova, Y. Y. Shevelyov, and D. I. Nurminsky. 2002. Large clusters of co-expressed genes in the Drosophila genome. Nature 420:666-669. [DOI] [PubMed] [Google Scholar]
- 8.Brown, T. 1991. Analysis of RNA by Northern and slot blot hybridization, p. 4.9.1-4.9.8. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 1. Wiley Interscience, New York, N.Y. [DOI] [PubMed]
- 9.Cai, H., and M. Levine. 1995. Modulation of enhancer-promoter interactions by insulators in the Drosophila embryo. Nature 376:533-536. [DOI] [PubMed] [Google Scholar]
- 10.Cai, H. N., Z. Zhang, J. R. Adams, and P. Shen. 2001. Genomic context modulates insulator activity through promoter competition. Development 128:4339-4347. [DOI] [PubMed] [Google Scholar]
- 11.Cuvier, O., C. M. Hart, E. Kas, and U. K. Laemmli. 2002. Identification of a multicopy chromatin boundary element at the borders of silenced chromosomal domains. Chromosoma 110:519-531. [DOI] [PubMed] [Google Scholar]
- 12.Cuvier, O., C. M. Hart, and U. K. Laemmli. 1998. Identification of a class of chromatin boundary elements. Mol. Cell. Biol. 18:7478-7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Gregorio, E., P. T. Spellman, P. Tzou, G. M. Rubin, and B. Lemaitre. 2002. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. EMBO J. 21:2568-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donze, D., C. R. Adams, J. Rine, and R. T. Kamakaka. 1999. The boundaries of the silenced HMR domain in Saccharomyces cerevisiae. Genes Dev. 13:698-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunaway, M., J. Y. Hwang, M. Xiong, and H. L. Yuen. 1997. The activity of the scs and scs' insulator elements is not dependent on chromosomal context. Mol. Cell. Biol. 17:182-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fransz, P., J. H. De Jong, M. Lysak, M. R. Castiglione, and I. Schubert. 2002. Interphase chromosomes in Arabidopsis are organized as well defined chromocenters from which euchromatin loops emanate. Proc. Natl. Acad. Sci. USA 99:14584-14589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaszner, M., J. Vazquez, and P. Schedl. 1999. The Zw5 protein, a component of the scs chromatin domain boundary, is able to block enhancer-promoter interaction. Genes Dev. 13:2098-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerasimova, T. I., and V. G. Corces. 1996. Boundary and insulator elements in chromosomes. Curr. Opin. Genet. Dev. 6:185-192. [DOI] [PubMed] [Google Scholar]
- 19.Gerasimova, T. I., and V. G. Corces. 2001. Chromatin insulators and boundaries: effects on transcription and nuclear organization. Annu. Rev. Genet. 35:193-208. [DOI] [PubMed] [Google Scholar]
- 20.Gerasimova, T. I., and V. G. Corces. 1998. Polycomb and trithorax group proteins mediate the function of a chromatin insulator. Cell 92:511-521. [DOI] [PubMed] [Google Scholar]
- 21.Geyer, P. K., and I. Clark. 2002. Protecting against promiscuity: the regulatory role of insulators. Cell. Mol. Life Sci. 59:2112-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geyer, P. K., and V. G. Corces. 1992. DNA position-specific repression of transcription by a Drosophila zinc finger protein. Genes Dev. 6:1865-1873. [DOI] [PubMed] [Google Scholar]
- 23.Geyer, P. K., and V. G. Corces. 1987. Separate regulatory elements are responsible for the complex pattern of tissue-specific and developmental transcription of the yellow locus in Drosophila melanogaster. Genes Dev. 1:996-1004. [DOI] [PubMed] [Google Scholar]
- 24.Geyer, P. K., M. M. Green, and V. G. Corces. 1988. Mutant gene phenotypes mediated by a Drosophila melanogaster retrotransposon require sequences homologous to mammalian enhancers. Proc. Natl. Acad. Sci. USA 85:8593-8597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilmour, D. S., and J. T. Lis. 1986. RNA polymerase II interacts with the promoter region of the noninduced hsp70 gene in Drosophila melanogaster cells. Mol. Cell. Biol. 6:3984-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glover, D. M., M. H. Leibowitz, D. A. McLean, and H. Parry. 1995. Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell 81:95-105. [DOI] [PubMed] [Google Scholar]
- 27.Hart, C. M., O. Cuvier, and U. K. Laemmli. 1999. Evidence for an antagonistic relationship between the boundary element-associated factor BEAF and the transcription factor DREF. Chromosoma 108:375-383. [DOI] [PubMed] [Google Scholar]
- 28.Hart, C. M., K. Zhao, and U. K. Laemmli. 1997. The scs' boundary element: characterization of boundary element-associated factors. Mol. Cell. Biol. 17:999-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirose, F., M. Yamaguchi, H. Handa, Y. Inomata, and A. Matsukage. 1993. Novel 8-base pair sequence (Drosophila DNA replication-related element) and specific binding factor involved in the expression of Drosophila genes for DNA polymerase alpha and proliferating cell nuclear antigen. J. Biol. Chem. 268:2092-2099. [PubMed] [Google Scholar]
- 30.Hogga, I., and F. Karch. 2002. Transcription through the iab-7 cis-regulatory domain of the bithorax complex interferes with maintenance of Polycomb-mediated silencing. Development 129:4915-4922. [DOI] [PubMed] [Google Scholar]
- 31.Hogga, I., J. Mihaly, S. Barges, and F. Karch. 2001. Replacement of Fab-7 by the gypsy or scs insulator disrupts long-distance regulatory interactions in the Abd-B gene of the bithorax complex. Mol. Cell 8:1145-1151. [DOI] [PubMed] [Google Scholar]
- 32.Holdridge, C., and D. Dorsett. 1991. Repression of hsp70 heat shock gene transcription by the suppressor of hairy-wing protein of Drosophila melanogaster. Mol. Cell. Biol. 11:1894-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jupe, E. R., R. R. Sinden, and I. L. Cartwright. 1995. Specialized chromatin structure domain boundary elements flanking a Drosophila heat shock gene locus are under torsional strain in vivo. Biochemistry 34:2628-2633. [DOI] [PubMed] [Google Scholar]
- 34.Kellum, R., and P. Schedl. 1992. A group of scs elements function as domain boundaries in an enhancer-blocking assay. Mol. Cell. Biol. 12:2424-2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kellum, R., and P. Schedl. 1991. A position-effect assay for boundaries of higher order chromosomal domains. Cell 64:941-950. [DOI] [PubMed] [Google Scholar]
- 36.Kuhn, E. J., and P. K. Geyer. 2003. Genomic insulators: connecting properties to mechanism. Curr. Opin. Cell Biol. 15:259-265. [DOI] [PubMed] [Google Scholar]
- 37.Kuhn, E. J., M. M. Viering, K. M. Rhodes, and P. K. Geyer. 2003. A test of insulator interactions in Drosophila. EMBO J. 22:2463-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee, H., K. W. Kraus, M. F. Wolfner, and J. T. Lis. 1992. DNA sequence requirements for generating paused polymerase at the start of hsp70. Genes Dev. 6:284-295. [DOI] [PubMed] [Google Scholar]
- 39.Lim, J. K. 1993. Protocol for in situ hybridization with biotinylated DNA. Dros. Inf. Serv. 72:73-76. [Google Scholar]
- 40.Lis, J. T., J. A. Simon, and C. A. Sutton. 1983. New heat shock puffs and beta-galactosidase activity resulting from transformation of Drosophila with an hsp70-lacZ hybrid gene. Cell 35:403-410. [DOI] [PubMed] [Google Scholar]
- 41.Mallin, D. R., J. S. Myung, J. S. Patton, and P. K. Geyer. 1998. Polycomb group repression is blocked by the Drosophila suppressor of Hairy-wing [su(Hw)] insulator. Genetics 148:331-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marlor, R. L., S. M. Parkhurst, and V. G. Corces. 1986. The Drosophila melanogaster gypsy transposable element encodes putative gene products homologous to retroviral proteins. Mol. Cell. Biol. 6:1129-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mirkovitch, J., M. E. Mirault, and U. K. Laemmli. 1984. Organization of the higher-order chromatin loop: specific DNA attachment sites on nuclear scaffold. Cell 39:223-232. [DOI] [PubMed] [Google Scholar]
- 44.Nowak, S. J., and V. G. Corces. 2000. Phosphorylation of histone H3 correlates with transcriptionally active loci. Genes Dev. 14:3003-3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parnell, T. J., and P. K. Geyer. 2000. Differences in insulator properties revealed by enhancer blocking assays on episomes. EMBO J. 19:5864-5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paulson, J. R., and U. K. Laemmli. 1977. The structure of histone-depleted metaphase chromosomes. Cell 12:817-828. [DOI] [PubMed] [Google Scholar]
- 47.Pirrotta, V. 1988. Vectors for P-mediated transformation in Drosophila. Butterworths, Boston, Mass. [DOI] [PubMed]
- 48.Rasmussen, E. B., and J. T. Lis. 1993. In vivo transcriptional pausing and cap formation on three Drosophila heat shock genes. Proc. Natl. Acad. Sci. USA 90:7923-7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodman, T. C. 1967. DNA replication in salivary gland nuclei of Drosophila melanogaster at successive larval and prepupal stages. Genetics 55:375-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez Alfageme, C., G. T. Rudkin, and L. H. Cohen. 1980. Isolation, properties and cellular distribution of D1, a chromosomal protein of Drosophila. Chromosoma 78:1-31. [DOI] [PubMed] [Google Scholar]
- 51.Roseman, R. R., E. A. Johnson, C. K. Rodesch, M. Bjerke, R. N. Nagoshi, and P. K. Geyer. 1995. A P element containing suppressor of hairy-wing binding regions has novel properties for mutagenesis in Drosophila melanogaster. Genetics 141:1061-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roseman, R. R., V. Pirrotta, and P. K. Geyer. 1993. The su(Hw) protein insulates expression of the Drosophila melanogaster white gene from chromosomal position-effects. EMBO J. 12:435-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ryu, J. R., T. Y. Choi, E. J. Kwon, W. H. Lee, Y. Nishida, Y. Hayashi, A. Matsukage, M. Yamaguchi, and M. A. Yoo. 1997. Transcriptional regulation of the Drosophila-raf proto-oncogene by the DNA replication-related element (DRE)/DRE-binding factor (DREF) system. Nucleic Acids Res. 25:794-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scott, K. S., and P. K. Geyer. 1995. Effects of the su(Hw) insulator protein on the expression of the divergently transcribed Drosophila yolk protein genes. EMBO J. 14:6258-6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siegal, M. L., and D. L. Hartl. 1996. Transgene coplacement and high efficiency site-specific recombination with the Cre/loxP system in Drosophila. Genetics 144:715-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sigrist, C. J., and V. Pirrotta. 1997. Chromatin insulator elements block the silencing of a target gene by the Drosophila polycomb response element (PRE) but allow trans interactions between PREs on different chromosomes. Genetics 147:209-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simon, J. A., C. A. Sutton, and J. T. Lis. 1985. Localization and expression of transformed DNA sequences within heat shock puffs of Drosophila melanogaster. Chromosoma 93:26-30. [DOI] [PubMed] [Google Scholar]
- 58.Simon, J. A., C. A. Sutton, R. B. Lobell, R. L. Glaser, and J. T. Lis. 1985. Determinants of heat shock-induced chromosome puffing. Cell 40:805-817. [DOI] [PubMed] [Google Scholar]
- 59.Spana, C., D. A. Harrison, and V. G. Corces. 1988. The Drosophila melanogaster suppressor of Hairy-wing protein binds to specific sequences of the gypsy retrotransposon. Genes Dev. 2:1414-1423. [DOI] [PubMed] [Google Scholar]
- 60.Spellman, P. T., and G. M. Rubin. 2002. Evidence for large domains of similarly expressed genes in the Drosophila genome. J. Biol. 1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun, F. L., and S. C. Elgin. 1999. Putting boundaries on silence. Cell 99:459-462. [DOI] [PubMed] [Google Scholar]
- 62.Swift, H. 1962. Nucleic acids and cell morphology in dipteran salivary glands, p. 75-125. In J. Allen (ed.), The molecular control of cellular activity. McGraw-Hill, New York, N.Y.
- 63.Tulin, A., and A. Spradling. 2003. Chromatin loosening by poly(ADP)-ribose polymerase (PARP) at Drosophila puff loci. Science 299:560-562. [DOI] [PubMed] [Google Scholar]
- 64.Udvardy, A., E. Maine, and P. Schedl. 1985. The 87A7 chromomere. Identification of novel chromatin structures flanking the heat shock locus that may define the boundaries of higher order domains. J. Mol. Biol. 185:341-358. [DOI] [PubMed] [Google Scholar]
- 65.Udvardy, A., and P. Schedl. 1993. The dynamics of chromatin condensation: redistribution of topoisomerase II in the 87A7 heat shock locus during induction and recovery. Mol. Cell. Biol. 13:7522-7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vazquez, J., and P. Schedl. 1994. Sequences required for enhancer blocking activity of scs are located within two nuclease-hypersensitive regions. EMBO J. 13:5984-5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.West, A. G., M. Gaszner, and G. Felsenfeld. 2002. Insulators: many functions, many mechanisms. Genes Dev. 16:271-288. [DOI] [PubMed] [Google Scholar]
- 68.Zhao, K., C. M. Hart, and U. K. Laemmli. 1995. Visualization of chromosomal domains with boundary element-associated factor BEAF-32. Cell 81:879-889. [DOI] [PubMed] [Google Scholar]