Abstract
Two novel nano-cages compounds, 8 and 9, were prepared by self-assembly of the ruthenium complexes 4 and 5, and the tripodal donor 1. The cytotoxicity of 8 was found to be considerably stronger than that of cisplatin. The complex 8 inhibited tumor cell proliferation by interfering into regulatory pathways of cell cycle via apoptosis.
The field of metallopharmaceuticals has emerged as an important new field of medicinal chemistry due to the therapeutic applications of metal-based drugs.1 The arrangements of organic ligands in three dimensional space from the wide range of coordination numbers and accessible redox states of tunable metal centers offers a wide spectrum of reactivity that can be utilized for medicinal purposes.2 Platinum complexes particularly cisplatin, carboplatin, and oxoplatin are currently among the most effective chemotherapeutic agents,3 despite their high toxicity and undesirable neuro-, hepato- and nephrotoxicity side effects.4 However, high systemic toxicity and resistance problems associated with platinum based drugs5 has opened up a new era of interest in the design and pharmacological evolution of alternative metal-based anti-tumor agents6 and the development of safer and more effective remedial agents. In particular, ruthenium complexes represent a new class of promising metal-based drugs with low toxicity and high activity in tumors that do not respond well to platinum drugs.7 Two ruthenium complexes, [ImH]trans-[RuCl4(DMSO)Im] or (NAMI-A) and KO1019, are the first mono-nuclear ruthenium based anticancer drugs to have successfully passed phase I clinical trials.8
Polynuclear drugs have also been designed to increase the range of treatable tumors. A number of macromolecular platinum compounds,9 such as the polymer linked diaminocyclohexyl platinum chemotherapeutic (AP5346) and Pt-coordinated hyperbranched polyglycerol polymer, could potentially be utilized to target specific types of tumor cells due to their distinctive extracellular environment which allows the drugs to selectively accumulate in cancer cells via an enhanced permeability and retention effect (EPR)10 and long range inter- and intrastrand DNA cross-links. Complexes derived from a combination of the polynuclear strategy and supramolecular self-assembly of ruthenium-arene complexes have also been evaluated as potential anticancer agents,11 but the scarcity of such reports is striking in contrast to the relative abundance of Pt-based macromolecules that have been documented.12 Herein, we describe the synthesis and characterization of two novel hexacationic ruthenium-arene nano-prismatic cage compounds and the proliferative activity of four arene-ruthenium cages that were tested in vitro against several cancer cell lines.
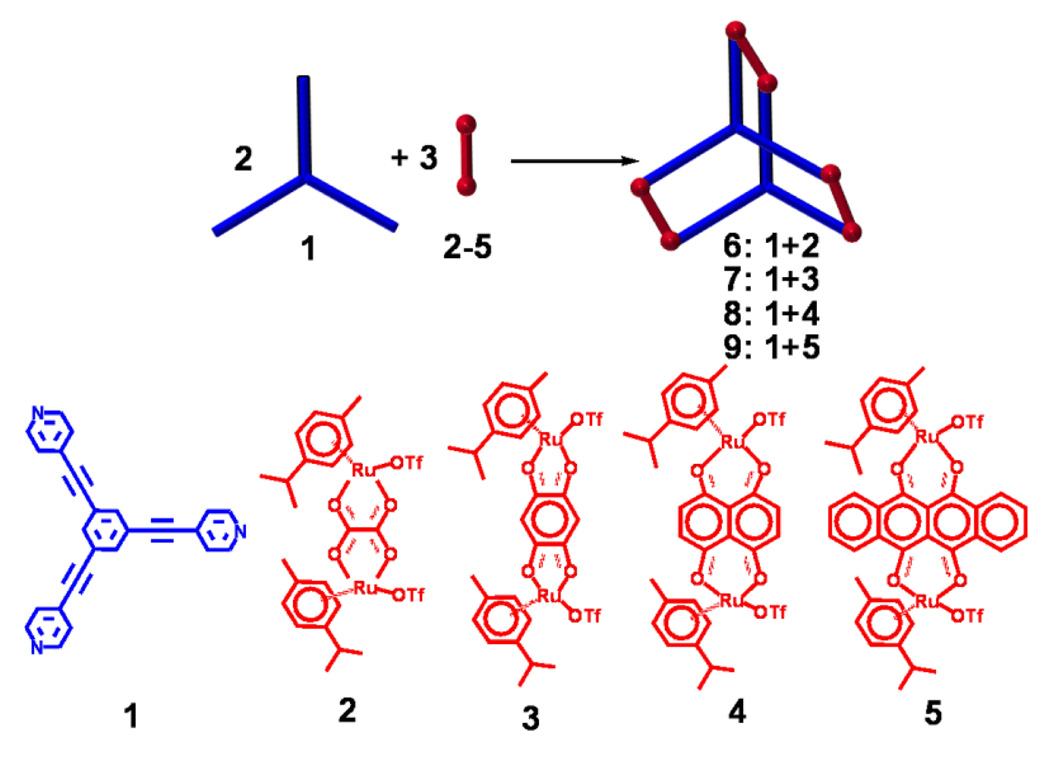
The new hexanuclear arene ruthenium prismatic cages 8 and 9 were readily prepared by reaction in methanol-dichloromethane solution of the half-sandwich arene ruthenium metalloacceptors 4 and 5 with tris(pyridylethynyl)benzene (1) in a 3:2 ratio. After the addition of diethyl ether to the concentrated reaction mixture, the pure self-assembled products 8 and 9 were isolated as sea green and green colored, solids, respectively. 3-D nano-prismatic cage structures were confirmed by 1H NMR and high resolution mass spectra. A single crystal x-ray structure also provided unequivocal evidence for the nano-cage 8. The synthesis and characterization of the nano-cages 6 and 7, and their application to the detection of nitro-aromatics have been communicated separately.13
The 1H NMR spectra of the nano-cages 8 and 9 show two doublets (δ 8.61 and 7.57 for 8, δ 8.77 and 7.44 for 9) for the pyridine protons after self-assembly along with a sharp singlet at δ 7.77 (8) and 7.97 (9) for the benzyl protons of the ligand, and p-cymene protons were observed at δ 6.00 and 5.81 for 8 and δ 6.20 and 5.98 ppm for 9 (Supporting information). The naphthoquinone protons of 8 were observed as a singlet at 7.31 ppm, and the naphthacenedione protons of 9 were observed as two multiplets at 8.85 and 7.99 ppm. 3-D cage structures for 8 and 9 were also supported by HR-ESI-MS spectra (Supporting information). Two charged states at m/z = 1667.2 and 1062.2 (8) and m/z = 1817.6 and 1162.3 (9) corresponding to [M − 2OTf]2+ and [M − 3OTf]3+ for the nano-prismatic cages were observed. These peaks were isotopically resolved and matched well with theoretical distributions.
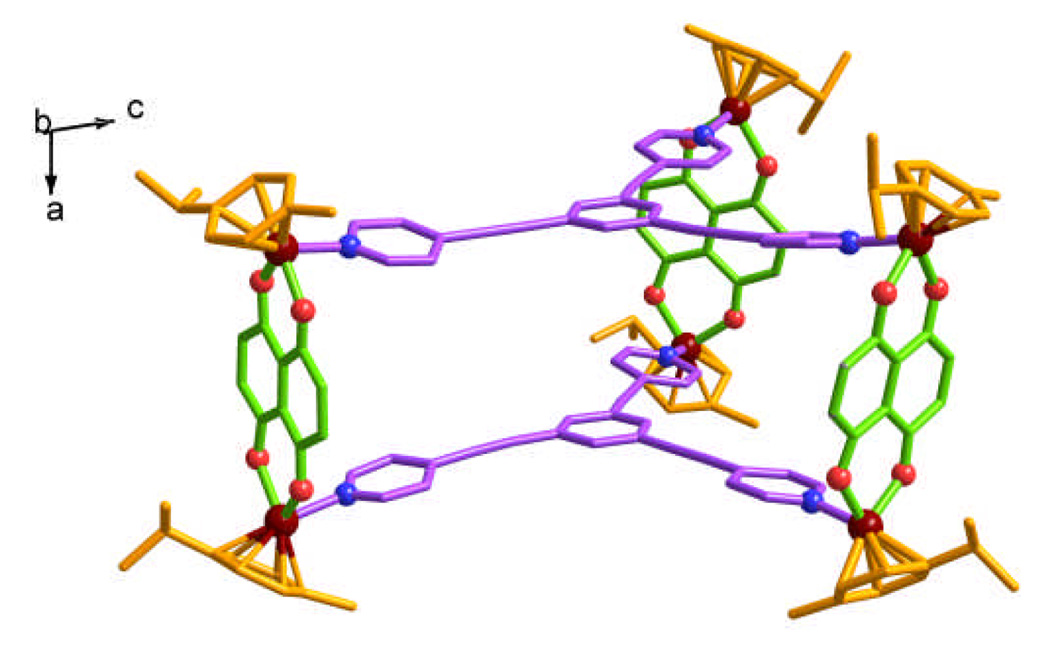
Slow vapor diffusion of diethyl ether into a dichloromethane – methanol solution of 8‡ provided crystals suitable for X-ray analysis using synchrotron radiation. X-ray analysis provided unequivocal proof of the trigonal prismatic nano-cage structure (Fig. 1), which featured two tritopic ligands 1 coordinated to three half-sandwich arene ruthenium metalloacceptors 4 with a height of approximately 7 Å and a distance of about 25 Å between the edges.
Fig. 1.
X-ray structures of the nano-prismatic cage 8 (Colour codes: Dark red = Ru, Red = O, Blue = N. H-atoms, counter anions and solvent molecules are omitted for clarity).
These multinuclear metal complexes could be of interest in investigations of new chemotherapeutics.1e,14 Thus, the synthesized hexanuclear ruthenium nano-cages with the π-electron rich system 1 linker were screened for growth inhibition activity in SK-hep-1 (liver cancer), HeLa (ovary cancer), HCT-15 (colon cancer), A549 (lung cancer), and MDA-MB-231 (breast cancer) tumor cell assays (Table 1).
Table 1.
Cytotoxicity of the complexes in human cancer cells.
| compound | IC50 µM[a] |
||||
|---|---|---|---|---|---|
| SK-hep-1 | HeLa | HCT-15 | A549 | MDA- MB-231 |
|
| 6 | 83.7 | 163.7 | 187.9 | >200 | >200 |
| 7 | >200 | >200 | >200 | >200 | >200 |
| 8 | 3.8 | 9.2 | 4.1 | 3.4 | 7.6 |
| 9 | >200 | >200 | >200 | >200 | >200 |
| Cisplatin | 6.3 | 10.5 | 5.6 | 2.4 | 2.7 |
IC50: drug concentration necessary for 50% inhibition of cell viability.
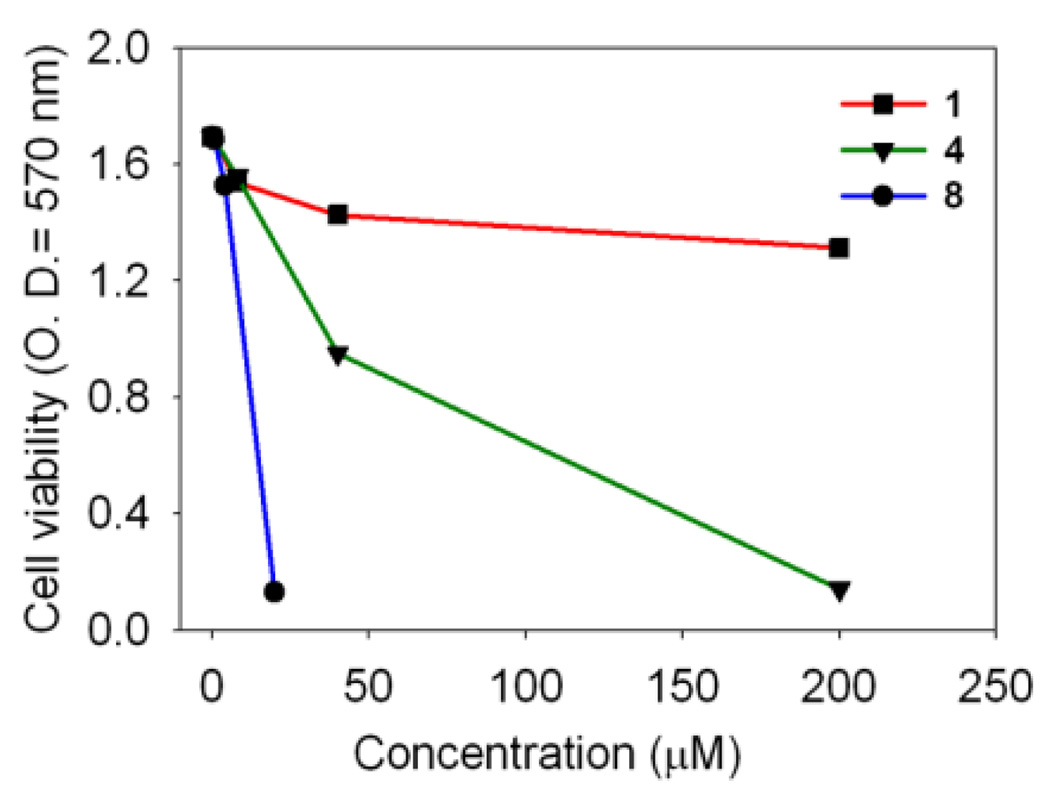
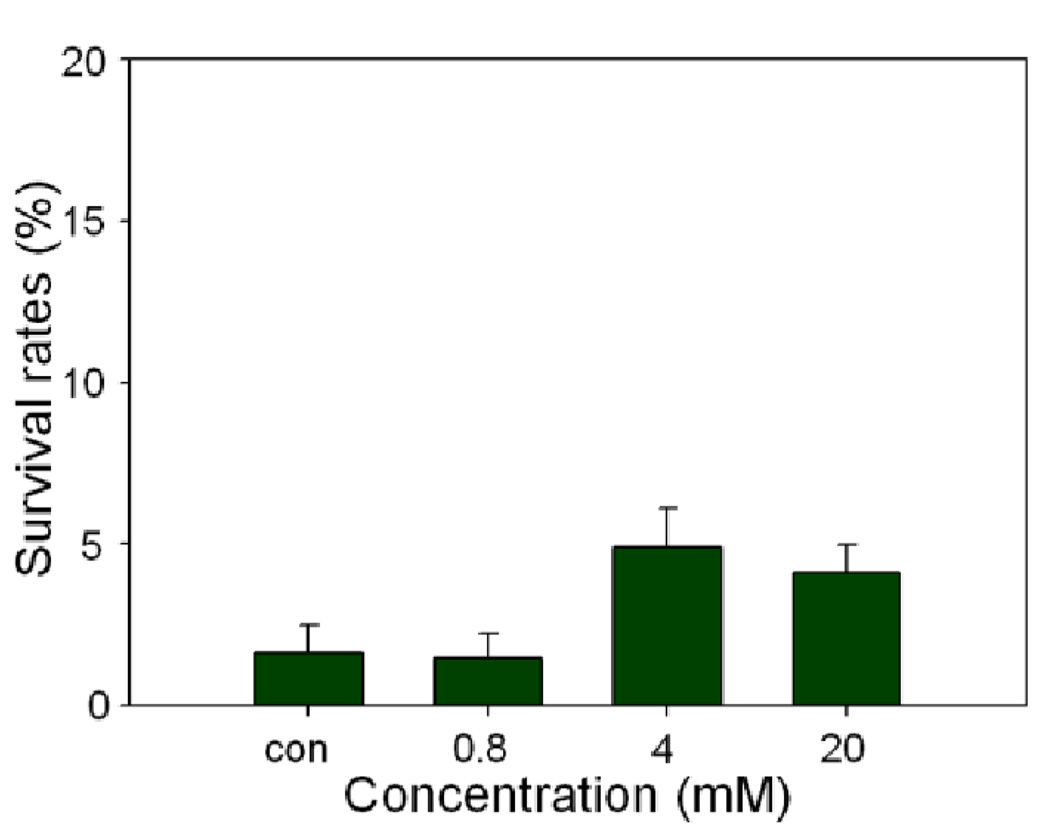
All cancer cells were exposed for 24 h to increasing concentrations of the compounds 6–9, and their survival was determined using the MTT assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide).11d The novel hexanuclear arene ruthenium prismatic cage 8 showed low micromolar inhibition against all cancer cell lines. In particular, the compound 8 was found to inhibit the proliferation of A549 cells with an IC50 of 3.4 µM, which is comparable to the effect seen with cisplatin (IC50 = 2.4 µM). For the SK-Hel-1 and HCT-15 cancer cell lines, the response was even better than the benchmark drug cisplatin as shown in Table 1. In a comparative study with the arene-ruthenium acceptor 4, the IC50 value of the cage complex 8 was much lower than 4, while the donor 1 alone did not have any cytotoxic effect on the A549 cell line, as shown in Fig. 2. To further define the mechanism of antiproliferative effect of the nano-cage 8, A549 cell lines were treated with the nano-cage 8 in a time-course manner to determine whether this bioactive compound 8 induced A-549 cell death via apoptosis. The apoptotic rates of the cage complex 8 on the A549 cells were analyzed by the calculation of the percentage of cells stained with propidiumiodide (PI) using FACS (Fluorescence-Activated Cell Sorting) flow cytometry assay. The apoptotic cell rates were 1.49±0.73 (0.8 mM), 4.88±1.21 (4 mM) and 4.11±0.86 (20 mM), respectively. Eventually, the nano-cage 8 showed apoptosis of about 2.5 times at dose of 4 mM, compared with control. In particular, the nano-cage 8 displayed the powerful cytotoxic effects through the arrest of G1 phase cell cycle (Fig. 3, See Supporting Information for more details). This finding suggests that the nano-cage 8 is able to interfere with many parts of the regulatory pathways via apoptosis, which control the cell growth cycle and supports that they could be useful for treating the tumours which are generally difficult to recover by chemotherapy.15 On the basis of previous literature and our results, we suggest the origin of cytotoxicity for these complexes. The anticancer activity through apoptosis of the dinuclear arene-ruthenium acceptor 4 originates from the interaction of the cytotoxic 5,8-dihydroxy-1,4-naphthoquinone16 with [(p-cymene)RuCl(μ-Cl)]2 which is enhanced in the nano-cage 8 due to increased nuclearity and by the presence of the extended π-conjugated system15.
Fig. 2.
Anticancer effects of the donor 1, the metal acceptor 4 and the ruthenium nano-cage 8 on the A549 cell line.
Fig. 3.
Apoptotic effects of the ruthenium nano-cage 8 for cell cycles on the A549 cell line.
In conclusion, we report the synthesis and anticancer activity of arene-ruthenium nano-prismatic cage compounds. The cage complex 8 showed low micromolar activity (IC50 = 3.4 µM) on the A549 cell line and is suitable as a lead for further SAR studies. Further studies to determine the more detailed apoptosis mechanism of this compounds are under investigation.
Supplementary Material
Scheme 1.
Self-assembled arene-ruthenium nano-cages 6–9.
Acknowledgment
This work was supported by the World Class University (WCU) program (R33-2008-000-10003) and Priority Research Centers program (2009-0093818) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology. X-ray diffraction experiments using synchrotron radiation were performed at the Pohang Accelerator Laboratory in Korea.
Footnotes
Electronic Supplementary Information (ESI) available: [detailed experimental data]. See DOI: 10.1039/b000000x/
The diffraction data from single crystals of 8 mounted on a loop were collected at 100 K on an ADSC Quantum 210 CCD diffractometer with synchrotron radiation (λ = 0.90000 Å) at the Macromolecular Crystallography Beamline 6B1, Pohang Accelerator Laboratory (PAL), Pohang, Korea. The raw data were processed and scaled using the program HKL2000. The structure was solved by direct methods, and the refinements were carried out with full-matrix least-squares on F2 with appropriate software implemented in the SHELXTL program package. C150.63H135.92F9.39N6O22.85Ru6S3.13, M = 3280.91, monoclinic, P21/n (No. 14), a = 14.548(3) Å, b = 34.324(7) Å, c = 34.518(7) Å, β = 99.43(3) °, V = 17004(6) Å3, Z = 4, T = 100 K, μ(synchrotron) = 0.619 mm 1, dcalc = 1.282 g·cm-3, 25687 reflections measured, 25687 unique (Rint = 0.0404), R1 = 0.1073, wR2 = 0.3022 for 12857 reflections (I > 2σ(I)), R1 = 0.1483, wR2 = 0.3349 (all data), GoF = 1.114, 1867 parameters and 885 restraints. All the non-hydrogen atoms were refined anisotropically, and hydrogen atoms were added to their geometrically ideal positions. CCDC 793778.
Notes and references
- 1.(a) Mascini M, Bagni G, Pietro MLD, Ravera M, Baracco S, Osella D. Bio Metals. 2006;19:409. doi: 10.1007/s10534-005-4340-3. [DOI] [PubMed] [Google Scholar]; (b) Hambley TW. Dalton Trans. 2007:4929. doi: 10.1039/b706075k. [DOI] [PubMed] [Google Scholar]; (c) Orvig C, Abrams MJ. Chem. Rev. 1999;99:2201. doi: 10.1021/cr980419w. [DOI] [PubMed] [Google Scholar]; (d) Fish RH, Jaouen G. Organometallics. 2003;22:2166. [Google Scholar]; (e) Kostova I. Recent Pat. Anti-Cancer Drug Discovery. 2006;1:1. doi: 10.2174/157489206775246458. [DOI] [PubMed] [Google Scholar]; (f) Farver O. Textbook of Drug Design and Discovery. 3rd ed. London, UK: Taylor & Francis Ltd.; 2002. p. 364. [Google Scholar]; (g) Guo Z, Sadler PJ. Advances in Inorganic Chemistry; Academic Press: San Diego. 2000;Vol. 49:183. [Google Scholar]; (h) Guo Z, Sadler PJ. Angew. Chem. Int. Ed. 1999;38:1512. doi: 10.1002/(SICI)1521-3773(19990601)38:11<1512::AID-ANIE1512>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 2.Schatzschneider U, Metzler-Nolte N. Angew. Chem., Int. Ed. 2006;45:1504. doi: 10.1002/anie.200504604. [DOI] [PubMed] [Google Scholar]
- 3.(a) Kelland L. Nat. Rev. Cancer. 2007;7:573. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]; (b) Reedijk J. Eur. J. Inorg. Chem. 2009:1303. [Google Scholar]; (c) Reedijk J. Proc. Natl. Acad. Sci. U. S. A. 2003;100:3611. doi: 10.1073/pnas.0737293100. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Siddik ZH. Oncogene. 2003;22:7265. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 4.(a) Jung Y, Lippard SJ. Chem. Rev. 2007;107:1387. doi: 10.1021/cr068207j. [DOI] [PubMed] [Google Scholar]; (b) Gianomenico C, Christen MUS. Patent 6413953. 2000; (c) Lippert B. Cisplatin: Chemistry and Biochemistry of a Leading Anticancer Drug. Weinheim: Wiley-VCH; 1999. [Google Scholar]; (d) Lippard SJ. Progress in Inorganic Chemistry: Bioinorganic Chemistry. Vol. 48. Sydney: Wiley; 1995. [Google Scholar]
- 5.(a) Siddik ZH. Oncogene. 2003;22:7265. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]; (b) Marzano C, Sbovata SM, Bettio F, Michelin RA, Seraglia R, Kiss T, Venzo A, Bertani R. J. Biol. Inorg. Chem. 2007;12:477. doi: 10.1007/s00775-006-0202-x. [DOI] [PubMed] [Google Scholar]
- 6.(a) Goss CHA, Henderson W, Wilkins AL, Evans CJ. J. Organomet. Chem. 2003;679:194. [Google Scholar]; (b) Quiroga AG, Ranninger CN. Coord. Chem. Rev. 2004;248:119. [Google Scholar]; (c) Henderson W, Nicholson BK, Tiekink ERT. Inorg. Chim. Acta. 2006;359:204. [Google Scholar]; (d) Liu H-K, Parkinson JA, Bella J, Wang F, Sadler PJ. Chem. Sci. 2010;1:258. [Google Scholar]
- 7.(a) Hartinger CG, Zorbas-Selfried S, Jakupee MA, Kynast B, Zorbas H, Keppler BK. J. Inorg. Biochem. 2006;100:891. doi: 10.1016/j.jinorgbio.2006.02.013. [DOI] [PubMed] [Google Scholar]; (b) Yan YK, Melchart M, Habtemariam A, Sadler PJ. Chem. Commun. 2005:4764. doi: 10.1039/b508531b. [DOI] [PubMed] [Google Scholar]; (c) Hotze ACG, Kariuki BM, Hannon MJ. Angew. Chem. 2006;118:4957. doi: 10.1002/anie.200601351. [DOI] [PubMed] [Google Scholar]; (d) Velders AH, van der Schilden K, Hotze ACG, Reedijk J, Kooijman H, Spek AL. Dalton Trans. 2004:448. doi: 10.1039/b313182c. [DOI] [PubMed] [Google Scholar]; (e) Habtemariam A, Melchart M, Fernandez R, Parsons S, Oswald IDH, Parkin A, Fabbiani FPA, Davidson JE, Dawson A, Aird RE. J. Med. Chem. 2006;49:6858. doi: 10.1021/jm060596m. [DOI] [PubMed] [Google Scholar]
- 8.(a) Sava G, Capozzi I, Bergamo A, Gagliardi R, Cocchietto M, Masiero L, Onisto M, Alessio E, Mestroni G, Garbisa S. Int. J. Cancer. 1996;68:60. doi: 10.1002/(SICI)1097-0215(19960927)68:1<60::AID-IJC12>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]; (b) Rademaker-Lakhai JM, Van Den Bongard D, Pluim D, Beijnen JH, Schellens JHM. Clin. Cancer Res. 2004;10:3717. doi: 10.1158/1078-0432.CCR-03-0746. [DOI] [PubMed] [Google Scholar]; (c) Groessl M, Hartinger CG, Polec-Pawlak K, Jarosz M, Dyson PJ, Keppler BK. Chem. Biodiversity. 2008;5:1609. doi: 10.1002/cbdv.200890148. [DOI] [PubMed] [Google Scholar]
- 9.(a) Yang Z, Wang X, Diao H, Zhang J, Li H, Sung H, Guo Z. Chem. Commun. 2007:3453. doi: 10.1039/b705326f. [DOI] [PubMed] [Google Scholar]; (b) Wheate NJ, Day AI, Blanch RJ, Arnold AP, Cullinane C, Collins JG. Chem. Commun. 2004:1424. doi: 10.1039/b404358h. [DOI] [PubMed] [Google Scholar]; (c) Rademaker-Lakhai JM, Terret C, Howell SB, Baud CM, de Boer RF, Pluim D, Beijnen JH, Schellens JHM, Droz JP. Clin. Cancer Res. 2004;10:3386. doi: 10.1158/1078-0432.CCR-03-0315. [DOI] [PubMed] [Google Scholar]; (d) Campone M, Rademaker-Lakhai JM, Bennouna J, Howell SB, Nowotnik DP, Beijnen JH, Schellens JHM. Cancer Chemother. Pharmacol. 2007;60:523. doi: 10.1007/s00280-006-0397-0. [DOI] [PubMed] [Google Scholar]; (e) Kam NWS, Jessop TC, Wender PA, Dai H. J. Am. Chem. Soc. 2004;126:6850. doi: 10.1021/ja0486059. [DOI] [PubMed] [Google Scholar]; (f) Wheate NJ, Taleb RI, Krause-Heuer AM, Cook RL, Wang S, Higgins VJ, Aldrich-Wright JR. Dalton Trans. 2007:5055. doi: 10.1039/b704973k. [DOI] [PubMed] [Google Scholar]
- 10.Matsumura Y, Maeda H. Cancer. Res. 1986;46:6387. [PubMed] [Google Scholar]
- 11.(a) Therrien B, Süss-Fink G, Govindaswamy P, Renfrew AK, Dyson PJ. Angew. Chem., Int. Ed. 2008;47:3773. doi: 10.1002/anie.200800186. [DOI] [PubMed] [Google Scholar]; (b) Zava O, Mattsson J, Dyson PJ, Therrien B. Chem.-Eur. J. 2010;16:1428. doi: 10.1002/chem.200903216. [DOI] [PubMed] [Google Scholar]; (c) Mattsson J, Zava O, Renfrew AK, Sei Y, Yamaguchi K, Dyson PJ, Therrien B. Dalton Trans. 2010;39:8248. doi: 10.1039/c0dt00436g. [DOI] [PubMed] [Google Scholar]; (d) Barry NPE, Zava O, Furrer J, Dyson PJ, Therrien B. Dalton Trans. 2010;39:5272. doi: 10.1039/c001521k. [DOI] [PubMed] [Google Scholar]; (e) Barry NPE, Abd Karim NH, Vilar R, Therrien B. Dalton Trans. 2009:10717. doi: 10.1039/b913642h. [DOI] [PubMed] [Google Scholar]
- 12.(a) Kostova I. Recents Pat. Anticancer Drug Discov. 2006;1:1. doi: 10.2174/157489206775246458. [DOI] [PubMed] [Google Scholar]; (b) Zehnulova J, Kasparkova J, Farrell N, Brabec V. J. Biol. Chem. 2001;276:22191. doi: 10.1074/jbc.M103118200. [DOI] [PubMed] [Google Scholar]; (c) Kasparkova J, Zehnulova J, Farrell N, Brabec V. J. Biol. Chem. 2002;277:48076. doi: 10.1074/jbc.M208016200. [DOI] [PubMed] [Google Scholar]
- 13.Wang M, Vajpayee V, Shanmugaraju S, Zheng Y-R, Pollock JB, Zhao Z-G, Kim H, Mukherjee PS, Chi K-W, Stang PJ. Inorg. Chem. 2011 doi: 10.1021/ic1020719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitt F, Govindaswamy P, Süss-Fink G, Ang WH, Dyson PJ, Juillerat-Jeanneret L, Therrien B. J. Med. Chem. 2008;51:1811. doi: 10.1021/jm701382p. [DOI] [PubMed] [Google Scholar]
- 15.Hartinger CG, Dyson PJ. Chem. Soc. Rev. 2009;38:391. doi: 10.1039/b707077m. [DOI] [PubMed] [Google Scholar]
- 16.Öllinger K, Brunmark A. J. Bio. Chem. 1991;266:21496. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.