Abstract
The Ogt gene encodes a glycosyltransferase that links N-acetylglucosamine to serine and threonine residues (O-GlcNAc) on nuclear and cytosolic proteins. Efforts to study a mammalian model of Ogt deficiency have been hindered by the requirement for this X-linked gene in embryonic stem cell viability, necessitating the use of conditional mutagenesis in vivo. We have extended these observations by segregating Ogt mutation to distinct somatic cell types, including neurons, thymocytes, and fibroblasts, the latter by an approach developed for inducible Ogt mutagenesis. We show that Ogt mutation results in the loss of O-GlcNAc and causes T-cell apoptosis, neuronal tau hyperphosphorylation, and fibroblast growth arrest with altered expression of c-Fos, c-Jun, c-Myc, Sp1, and p27. We further segregated the mutant Ogt allele to parental gametes by oocyte- and spermatid-specific Cre-loxP mutagenesis. By this we established an in vivo genetic approach that supports the ontogeny of female heterozygotes bearing mutant X-linked genes required during embryogenesis. Successful production and characterization of such female heterozygotes further indicates that mammalian cells commonly require a functional Ogt allele. We find that O-GlcNAc modulates protein phosphorylation and expression among essential and conserved cell signaling pathways.
Intracellular protein glycosylation is a ubiquitous and highly regulated form of posttranslational modification. Many cytosolic and nuclear proteins are modified by the addition of a single monosaccharide, β-N-acetylglucosamine (GlcNAc), in O linkage to serine and threonine residues (reviewed in reference 51). These include c-Myc, c-Fos, c-Jun, p53, Sp1, RNA polymerase II, tubulin, vinculin, tau, and the heat shock proteins. The sites of O-GlcNAc linkage are sometimes otherwise modified by protein kinases in a reciprocal relationship, suggesting a role for O-GlcNAc in blocking cellular protein phosphorylation events during signal transduction, transcriptional regulation, nuclear import, and protein turnover (4, 8, 19, 25, 27, 40). O-GlcNAc levels on cell proteins are regulated during cell differentiation, growth, and activation and are aberrant in some disease states. This regulation can include the reversible nature of the O-GlcNAc linkage due to the presence of an intracellular β-N-acetylglucosaminidase (16).
The enzyme UDP-GlcNAc: polypeptide O-β-N-acetylglucosaminyltransferase (Ogt) produces the O-GlcNAc linkage and has been genetically isolated and characterized initially from vertebrate sources. Mammalian Ogt structure includes a tetratricopeptide repeat domain and is itself glycosylated with O-GlcNAc, as well as tyrosine phosphorylated in some contexts (29, 35). The Ogt gene resides on the mammalian X chromosome and is required for the viability of a male embryonic stem (ES) cell line normally bearing the XY sex chromosome karyotype. Conditional mutagenesis, previously applied by Cre-loxP recombination, was necessary to obtain viable ES cells and mice bearing the loxP-flanked (F) Ogt allele (46). In breeding schemes that engaged maternal Zp3-Cre transgenic mice, no surviving offspring were obtained that had inherited the recombined and partially deleted Ogt (Δ) allele. These findings indicated that the Ogt gene mutation produced is lethal in ES cell culture and mouse embryogenesis. However, it was not possible to determine whether the Ogt gene is responsible for O-GlcNAc formation in vivo and whether the mutation generated results in a null allele with a deficiency of cellular O-GlcNAc.
No mammalian cells with a deficiency of O-GlcNAc have been described, and our previous findings suggested that cell-type-specific and inducible Ogt mutagenesis may provide the approaches needed to further characterize Ogt function and investigate the effects of O-GlcNAc deficiency on mammalian cells. We have now characterized intact mice and differentiated cells in which the Ogt mutation was segregated to distinct lineages, including fibroblasts, neurons, oocytes, spermatids, and thymocytes. We further compared the embryologic consequences of Ogt disruption engineered specifically in either paternal or maternal germ cells, and in this way succeeded in establishing a method for obtaining viable female heterozygotes bearing a null allele of an X-linked gene that is essential in embryogenesis. These studies show that the Ogt gene is responsible for the O-GlcNAc formation and that the O-GlcNAc modification is an essential component in the function of various cell types.
MATERIALS AND METHODS
Animals.
Mice bearing the Ogt mutation and the Zp3-Cre transgene were previously described (46). Other strains used included those bearing the lck-Cre (24), Syn1-Cre (11, 55), or prm-Cre (42) transgenes.
Lymphoid cellularity, cytometry, and apoptosis.
Cells from the lymphoid tissues indicated were isolated and analyzed with a FACSCalibur flow cytometer and CELLQUEST software (Becton Dickinson) with antibodies to various cell surface markers as previously described (44).
Brain and spinal cord analyses.
Tissue and cell fractionation was modified slightly from that previously described (2). Brain and spinal cord were isolated from 8- to 9-day-old mice and homogenized in 5 mM Tris-HCl (pH 7.4) containing 0.32 M sucrose, various protease inhibitors (Complete; Boehringer Mannheim), and phosphatase inhibitors (1 mM EDTA, 20 mM sodium fluoride, 5 mM sodium vanadate, and 5 mM sodium molybdate). The homogenate was centrifuged at 800 × g for 10 min to remove nuclei. The supernatant was centrifuged at 10,200 × g for 30 min to generate a synaptosomal fraction (P2) containing synapsin-1 and a supernatant (S2) fraction containing tau and microtubule-associated protein 2 (MAP2). Extracts were then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose for immune blotting using antibodies specific for tau, MAP2 (Sternberger Monoclonal Antibodies Inc., Lutherville, Md.), and synapsin-1 (Cell Signaling Technologies, Beverly, Mass.). Antibodies were used at suggested dilutions of 1/1,000 to 1/5,000. The blots were scanned and protein levels were determined using NIH Image 1.6 software.
Cre retrovirus production.
The Cre recombinase gene was cloned into the EcoRI site of the pLXSN Moloney murine leukemia virus retroviral vector. Vesicular stomatitis virus G (VSV-G)-pseudotyped retrovirus was generated by transient transfection of 293GP cells that stably express VSV-G envelope protein with pLXSN derivatives as described previously (54). The concentrated pseudotyped virus stocks generated (VSV and VSV+Cre) were used to infect NIH 3T3 fibroblast targets, and titers were determined to be between 1 × 108 and 3 × 108 retroviral CFU/ml (data not shown).
Fibroblast derivation, mutagenesis, and characterization.
Embryos were harvested at postfertilization embryonic day 11.5 following matings of female mice homozygous for the OgtF allele and male mice hemizygous for the OgtF allele. Primary embryonic fibroblasts (PEFs) were derived and cultured in Dulbecco's modified Eagle medium (DMEM; low glucose) containing 10% fetal calf serum (FCS). Immortalized PEFs were obtained by transfection with the simian virus 40 (SV40) large-T-antigen expression plasmid, pOT (20). Fibroblasts were genotyped for Ogt allele structure (46), SV40 large-T-antigen sequence (15), and markers of X- and Y-chromosome sequence (references 32 and 31, respectively).
Fibroblasts were maintained at subconfluent densities and at 75% confluence were replated at a 1:5 dilution 6 h prior to addition of retrovirus stocks at a 10:1 multiplicity of infection. During retroviral infection, the medium was supplemented with 8 μg of polyvinylpyrrolidone/ml (Sigma). Cells were replated 24 h later, and G418 (1 mg/ml) was added 36 h postinfection. Noninfected cells died from G418 toxicity within 3 days of G418 treatment. Serum withdrawal in cell culture was accomplished using DMEM with 0.1% FCS for 16 h, prior to stimulation with the addition of DMEM containing 10% FCS. Cells were analyzed at the time points indicated following lysis and protein extract preparation in 1% NP-40, 50 mM Tris (pH 6.8), 150 mM NaCl, 1 mM EDTA, 20 mM sodium fluoride, 5 mM sodium vanadate, and 5 mM sodium molybdate or with the abovementioned buffer conditions except that 0.5% Triton X-100 was used instead of NP-40. Both conditions included protease inhibitors as indicated above (Boehringer-Mannheim).
Metabolic labeling of cells with inorganic 32P- or [35S]methionine and determination of label incorporation into protein were done as previously described (18). Succinylated wheat germ agglutinin (Vector, Burlingame, Calif.) lectin precipitations were performed as described previously (7). The antibodies used were specific for c-Myc and phospho-Myc (c-MycP; Cell Signaling Technologies), Sp1 (Sigma, St. Louis, Mo.), c-Jun and c-Fos (Santa Cruz Biotechnology, Santa Cruz, Calif.), Ogt (29), and O-GlcNAc (CTD110.6) (7). Protein blots were scanned and protein levels were determined as indicated above.
Embryo isolation and histology.
Timed mating of mice bearing the indicated Ogt genotypes and Cre transgenes was used to derive embryos for isolation and further histochemical analysis as previously described (37).
Ogt activity assay.
The 30% ammonium sulfate pellet of organs was analyzed for Ogt activity as previously reported (17). Assays contained 0.5 mM casein kinase II peptide (PGGSTPVSSANMM) (30).
RESULTS
T-cell-specific mutation of the Ogt gene.
T cells express and regulate O-GlcNAc levels upon immune activation, suggesting functional roles in this mature lymphoid lineage (26). To promote normal embryogenesis and attempt to derive a nonessential cell lineage lacking the Ogt glycosyltransferase for further characterization, in vivo mutagenesis of the Ogt allele was specifically restricted to T lymphocytes in vivo by using the lck-Cre transgene (24, 38). Cre recombination controlled by the lck promoter in these transgenic mice occurs at highest frequencies as T-cell development proceeds from cortical CD4+ CD8+ thymocytes into mature thymic T cells.
Female mice bearing the Ogt F allele were bred with males transgenic for lck-Cre, and male offspring with or without the lck-Cre transgene were analyzed further. Genotyping of isolated thymocytes, splenocytes, or lymph node cells revealed the presence of the Ogt Δ allele in thymocyte samples (Fig. 1A). The Ogt Δ allele was not detected, however, in the peripheral splenic and lymph node tissues, indicating that thymocytes lacking a wild-type (WT) or F Ogt allele failed to contribute to the peripheral T-cell repertoire.
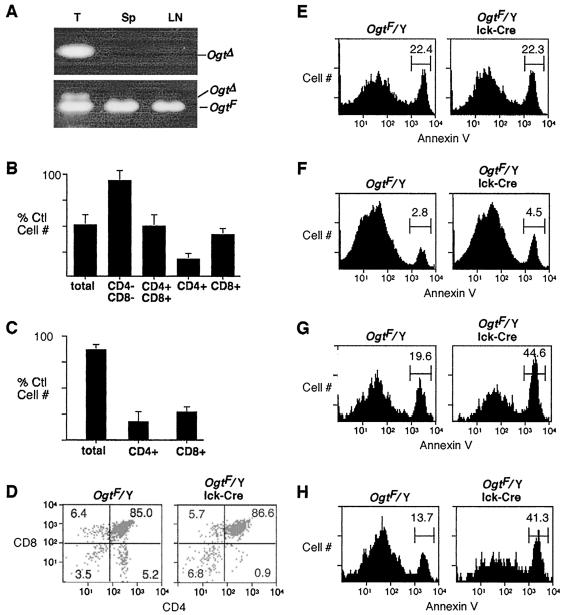
FIG. 1.
T-cell-specific Ogt mutagenesis. (A) PCR analysis of genomic DNA derived from thymocytes (T), splenocytes (Sp), and lymph node cells (LN) of an OgtF/Y mouse bearing the lck-Cre transgene. (B and C) T-cell levels in the thymus (B) and spleen (C) of OgtF/Y lck-Cre transgenic mice (n = 10), expressed as a percentage of cell number found among littermate controls (OgtF/Y) lacking the lck-Cre transgene. (D to H) Cytometric analyses of thymocyte developmental subpopulations (D) and their level of annexin V antibody binding (E to H). Panels: E, CD4− CD8−; F, CD8+ CD4+; G, CD8+; H, CD4+.
Production of the Ogt Δ allele in thymocytes correlated with a 50% reduction in cell number and a reduction of up to 75% in T cells in the lymph nodes and spleen among OgtF/Y lck-Cre mice compared with littermates lacking the lck-Cre transgene (Fig. 1B and C). The most immature CD4− CD8− thymocyte subpopulation was unaffected, while the cortical CD4+ CD8+ thymocytes were reduced by 50% and the mature CD8+ or CD4+ thymic T cells were reduced by 50 and 75%, respectively. The overall thymic developmental profile obtained by plotting CD4 and CD8 expression indicated a significant decrease in the frequency of CD4+ thymocytes (Fig. 1D). These findings are consistent with the death of cells that have undergone Cre recombination and have lost Ogt function. The presence of a reduced peripheral T-lymphoid population reflected a subset of T cells that failed to undergo Cre-mediated recombination during development in the thymus and contained the OgtF allele (Fig. 1A).
To further examine the timing and mechanism of this cell deficit, flow cytometry was performed of thymocyte subpopulations with the apoptotic indicator antibody to annexin-V (Fig. 1D to H). The cortical CD4+ CD8+ thymocyte population in OgtF/Y lck-Cre mice contained a slight but significant increase in annexin-V binding compared to what was seen with littermate controls. However, a two- to threefold increase in annexin V was observed in the more mature CD4+ and CD8+ single-positive populations of thymic T cells. Acquisition of the Ogt Δ allele results in thymocyte apoptosis prior to mutant-T-cell emigration and appearance in the periphery. Additional studies were hindered by an inability to support Ogt mutant cell growth in vitro.
Neuron-specific Ogt mutagenesis.
The O-GlcNAc modification is found on neuronal proteins, including synapsin-1, MAP2, and tau (1, 6, 12). We next mutagenized Ogt specifically in neuronal cells of intact mice by using the previously characterized Syn1-Cre transgene that restricts Cre recombinase expression to neurons and during synaptogenesis (11, 55). Offspring bearing the Syn1-Cre transgene and also possessing a WT Ogt allele appeared normal and were produced as expected. However, OgtF/Y Syn1-Cre mice were present among littermates at only 50% of the expected frequency. Newborn mice bearing the OgtF/Y or OgtF/F genotypes and the Syn1-Cre transgene were considerably smaller than littermates lacking the Syn1-Cre transgene and failed to develop normal locomotor activity. They rarely nursed, and none survived for longer than 10 days (Fig. 2A and B and data not shown). DNA was extracted from the brain tissue, and genomic analysis confirmed the production of the mutant Ogt Δ allele in the presence of the Syn1-Cre transgene (Fig. 2C). The frequency of recombination, approximately 10 to 20%, is similar to the frequency of neuronal cells present among other brain cell types.
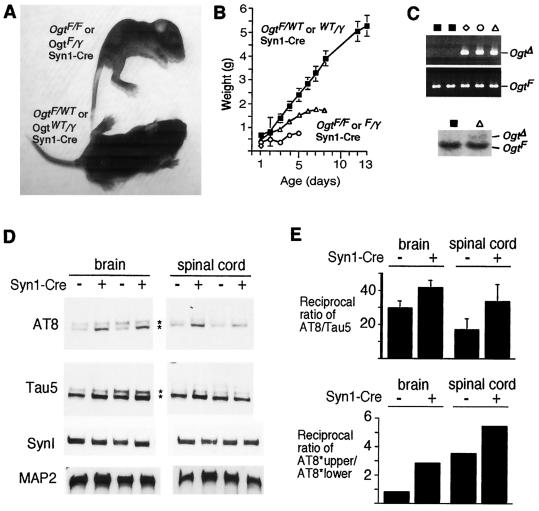
FIG. 2.
Neuron-specific Ogt mutagenesis. (A) Photograph of 9-day-old OgtF/Y (upper) and OgtF/Y Syn1-Cre (lower) littermates. (B and C) Growth rates (B) and PCR and Southern blot analyses (C) (upper and lower panels, respectively) of brain DNA among offspring derived from an OgtF/Y parent bred with an OgtF/WT Syn1-Cre mate. Open symbols represent individual OgtF/Y Syn1-Cre mice, and black squares represent littermates bearing at least one WT Ogt allele and the Syn1-Cre transgene. (D) Representative immunoblot analyses of brain and spinal cord protein using anti-tau antibodies AT8 (hyperphosphorylated tau) and Tau5 (total tau). (E) Ratios of phosphorylated tau to total tau (upper panel) and of the low-molecular-weight form of phosphorylated tau to the high-molecular-weight form (marked with asterisks in panel D) (lower panel). Immunoblotting with antibodies to synapsin I (Syn1) and MAP2 are also shown.
Brain and spinal cord protein extracts were prepared from littermates 9 to 10 days of age. Anti-tau antibodies that recognized total tau protein, or hyperphosphorylated tau, were used to analyze these extracts. An increase in tau expression appeared in the brain and spinal cord of OgtF/Y Syn1-Cre mice, while levels of synapsin I and MAP2 were unaffected (Fig. 2D). Moreover, levels of hyperphosphorylated tau were also increased. When normalized to the increase in tau protein levels, the phosphorylation state of tau remained significantly enhanced (Fig. 2E, upper panel). The major change in tau occurred on the predominant lower-molecular-weight form, with ratios in the brain of 1:0.7 and 1:2.6 in OgtF/Y and OgtF/Y Syn1-Cre animals, respectively. Spinal cord ratios were 1:3.1 and 1:5.2 in OgtF/Y and OgtF/Y Syn1-Cre animals, respectively (Fig. 2E, lower panel).
These findings indicate that neuronal Ogt function is essential for mouse survival and that neurons lacking an intact Ogt allele accumulate hyperphosphorylated tau. Interestingly, early mouse death and neuronal apoptosis have been previously associated with tau hyperphosphorylation (3, 28). The early timing of postnatal death among mice bearing a neuronal Ogt Δ allele also reduced possibilities for additional studies of O-GlcNAc expression and function by this approach.
Inducible Ogt gene inactivation in fibroblasts.
An inducible and in vitro approach for Ogt mutagenesis seemed necessary to produce a system more amenable to the study of cellular Ogt function and the biochemical events occurring upon O-GlcNAc deficiency. High-titer pseudotyped recombinant retroviruses were therefore developed using VSV-G envelope protein in the context of a recombinant murine retroviral expression vector containing the neomycin phosphotransferase selectable marker (54). Virus stocks with titers between 1 × 108 to 3 × 108 CFU/ml were obtained for the parental vector (VSV) and the derivative expressing the Cre recombinase (VSV+Cre). These VSV-pseudotyped retroviral particles were added to cultures of PEFs derived from OgtF/Y embryos or a polyclonal derivative of these PEFs transformed by the SV40 large T antigen. The significant viral titer and multiplicity of infection ratio promoted high integration and expression frequencies of more than 85% of cells treated, as indicated by results with subsequent G418 selection (data not shown).
Subsequent to retroviral infection, fibroblasts were replated 24 h later and their proliferation rate was monitored (Fig. 3A). Comparison of growth kinetics indicated a slight antiproliferative effect of Cre expression itself, as has been reported previously (10). More significantly, only OgtF/Y fibroblasts receiving the VSV+Cre retrovirus appeared to senesce and die by day 12 as judged by cell number and vital dye staining. When plated as single cells at day 1 postinfection, these fibroblasts did not form colonies of more than 10 cells, indicating a failure to undergo four or more further cell divisions (data not shown). Prolonged culture of large polyclonal populations did not lead to surviving clones and the rare post-day 12 viable clones obtained all contained the OgtF/Y genotype. Measurements of total protein synthesis and turnover at postinfection days 3 to 6 indicated that fibroblasts bearing the Ogt Δ allele continue to synthesize protein, and rates of total protein degradation were not substantially altered (data not shown). Fibroblasts bearing the OgtF/Y genotype and transformed by SV40 large T antigen were studied further within this brief viable time period post-Ogt mutation.
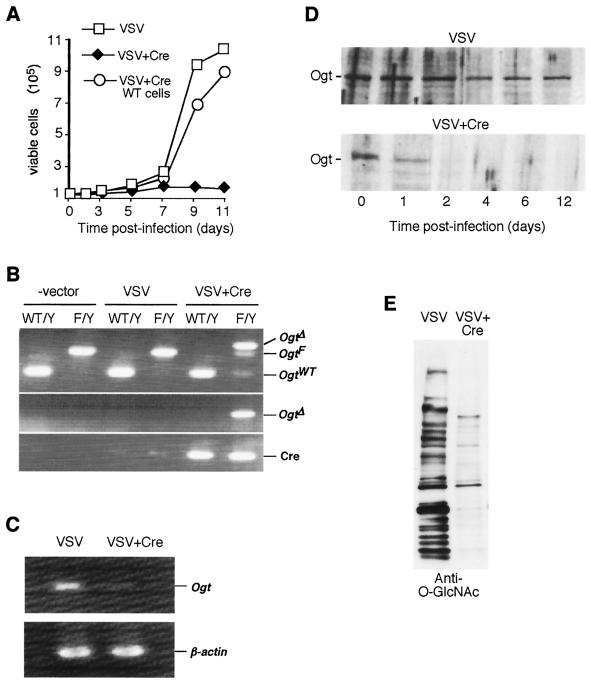
FIG. 3.
Retrovirally induced Ogt mutagenesis in vitro and effect on fibroblasts bearing the OgtF/Y or OgtWT/Y genotype. (A) Viable cell numbers in culture at the times indicated following retroviral infections of OgtF/Y fibroblasts with indicated recombinant virus stocks. (B) PCR analysis of Ogt allele structure in genomic DNA 4 days after either mock infection (-vector), infection with VSV parental retrovirus, or infection with recombinant VSV+Cre retrovirus. The PCR primer combination used in the upper panel amplifies OgtF, Ogt Δ, and OgtWT alleles; the PCR primers used in the middle panel specifically amplify the Ogt Δ allele; and the primers used in the lower panel detect the presence of Cre DNA sequences. (C) Reverse transcription-PCR detection of Ogt expression (upper panel) and β-actin (lower panel) using OgtF/Y fibroblast mRNA at postinfection day 4. (D) Ogt protein levels detected by anti-Ogt antibody blot of total protein extracts at various times postinfection with either VSV or VSV+Cre retrovirus. (E) O-GlcNAc levels detected using anti-O-GlcNAc antibody and total cell protein extracts prepared 4 days postinfection with either VSV or VSV+Cre.
Genomic DNA obtained from viable fibroblasts revealed an almost complete loss of the OgtF allele with the appearance of the Ogt Δ allele by 4 days postinfection with VSV+Cre (Fig. 3B). Ogt RNA expression was also deficient at this time point (Fig. 3C). Analyses of cellular proteins showed a reduction in Ogt protein level as early as day 1 postinfection and a total absence by day 4 (Fig. 3D). The presence of the O-GlcNAc modification was monitored similarly among total cell protein extracts, and by day 4 postinfection, the levels of O-GlcNAc were reduced to background (Fig. 3E), indicating that the Ogt gene appears to be solely responsible for O-GlcNAc formation in fibroblasts.
Defective serum response in fibroblasts lacking O-GlcNAc.
The expression patterns of Ogt substrates c-Myc, c-Fos, and c-Jun characteristically change upon induction by exogenous factors such as serum. Deprivation of serum followed by serum stimulation was applied to fibroblasts bearing the OgtF allele to monitor the early serum response that typically involves up-regulation of c-Fos, c-Jun, and c-Myc. Fibroblasts deficient in Ogt and O-GlcNAc survived the serum deprivation period but failed to normally induce expression of these nuclear proteins, and this included a reduction in the phosphorylated form of c-Myc (Fig. 4A and B). This defect was not due to reduced levels of RNA encoding these proteins; in contrast, steady-state mRNA levels of c-Fos, c-Jun, and c-Myc were increased (Fig. 4C).
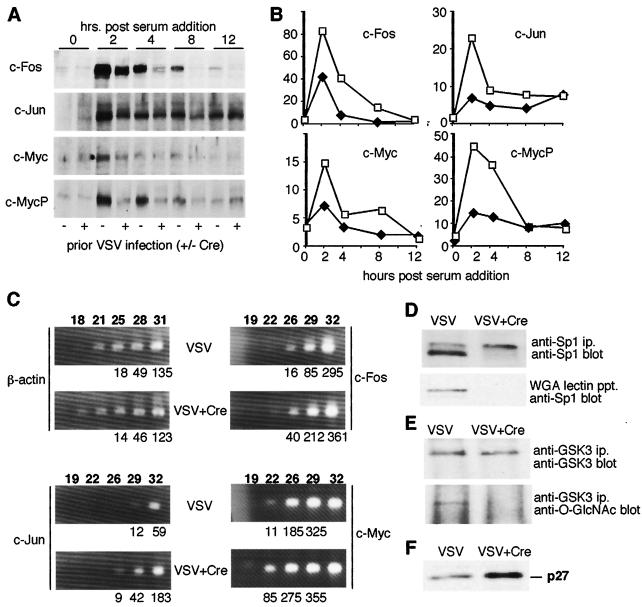
FIG. 4.
Altered serum stimulation response with defects in expression of transcription and cell cycle factors among Ogt-deficient fibroblasts. (A) Four days postretroviral infection, overnight serum-starved fibroblasts were treated with serum and cell protein extracts were analyzed at various times by immunoblotting with antibodies to c-Fos, c-Jun, c-Myc, and c-Myc phosphorylated at threonine 58 (c-MycP). (B) Relative protein levels plotted from a representative experiment shown in panel A. Open squares indicate prior VSV infection, and black diamonds denote prior VSV+Cre infection. (C) Semiquantitative reverse transcription-PCR on mRNA prepared from fibroblasts 4 days postinfection with VSV or VSV+Cre. The number of PCR cycles is indicated above the sample lanes in bold, and arbitrary intensity unit values are denoted below the sample lanes. (D) Sp1 levels and O-GlcNAc modification are reduced by fourfold upon Ogt deficiency at day 4 post-VSV+Cre infection. A twofold increase in the presumptive phosphorylated form of Sp1 also occurs. (E) GSK-3 levels remain normal in the absence of the O-GlcNAc linkage. (F) p27 protein levels are increased fourfold in Ogt-deficient fibroblasts by day 4 post-VSV+Cre infection.
Even without serum deprivation in normal cell culture conditions, some Ogt substrates, including the transcription factor Sp1, were significantly affected. A fourfold decrease in Sp1 protein levels was observed and an absence of O-GlcNAc was noted on remaining Sp1 (Fig. 4D). This remaining Sp1 represented a twofold increase in the higher-molecular-weight species that appears to be the nonglycosylated form in these fibroblasts.
Not all proteins modified by Ogt are altered in expression in the absence of O-GlcNAc. The glycogen synthase kinase 3 (GSK-3) is a reported substrate of Ogt (33, 36). In Ogt-deficient fibroblasts, GSK-3 lacked O-GlcNAc and its expression level was unchanged (Fig. 4E). However, levels of the cyclin kinase inhibitor p27 were increased by fourfold in the absence of O-GlcNAc (Fig. 4F). Although p27 has not been reported to be a substrate of Ogt, p27 expression correlates with cyclin-based mechanisms that control cell proliferation (5, 49).
While inducible Ogt mutagenesis provided a temporally restricted opportunity to study the function(s) of O-GlcNAc in vitro, the multiplicity of abnormalities observed in fibroblast culture requires considerable further study in order to refine the etiology of the abnormal phenotype. We reasoned that other opportunities to investigate O-GlcNAc function may exist if one or more mammalian cell types were identified as being less reliant upon Ogt and O-GlcNAc.
Characterization of female heterozygote offspring produced by male gamete-specific Ogt mutagenesis.
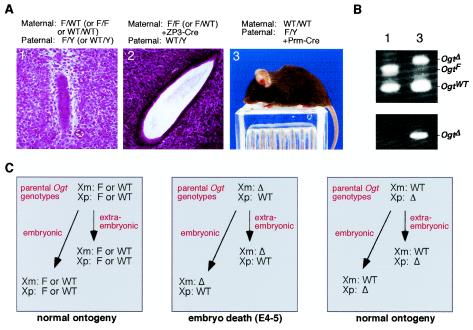
Mice inheriting the mutant Ogt Δ allele through maternal oocyte-specific Zp3-Cre recombination failed to survive due to a defect in oocytes or perhaps from abnormal embryogenesis (46). We have more recently found that Zp3-Cre transgenic females bearing the OgtF/F germ line genotype are infertile, even when mated with WT males (data not shown). Plausible hypotheses for the absence of female heterozygous OgtWT/Δ offspring would take into account the pattern of X-chromosome inactivation during mammalian embryonic development. Although cells comprising the embryo itself undergo random X inactivation, extraembryonic cells develop soon after fertilization, in which the paternal X chromosome is preferentially inactivated (21). This extraembryonic compartment expresses the maternal X chromosome almost exclusively and represents a cell lineage needed for development of the early placenta. This interface between embryo and mother is essential to supply nutrients shortly after embryo implantation on the uterine epithelium. Indeed, a rapid failure in early postimplantation embryogenesis resulting in death by embryonic day 5 was observed upon inheritance of the maternal Ogt Δ allele (Fig. 5A, panels 1 and 2).
FIG. 5.
Parental origin of the mutant Ogt allele determines female viability among OgtΔ/WT heterozygotes. (A) Postimplantation stage (embryonic day 5.0) embryo development from parental genotypes is indicated above each panel (panels 1 and 2). Early postimplantation death at day 4 or 5 occurs when the mutant Ogt allele is derived as a result of Cre recombination in maternal oocytes bearing the F allele in the presence of the Zp3-Cre transgene. In comparison, a paternal source of the mutant Ogt allele, engineered by the presence of the protamine (prm)-Cre transgene in males bearing the F allele, allows for normal development and viability of female heterozygotes (panel 3). (B) PCR analysis of Ogt genotypes present among viable offspring produced by maternal OgtWT/WT and paternal OgtF/Y genotypes, the latter in the presence of prm-Cre transgene. (C) Model of paternal mutant Ogt allele origin and effect among embryonic and extraembryonic cell lineages. Random inactivation of the X chromosome in embryonic cell types in the presence of nonrandom and maternal X inactivation among extraembryonic lineages permits heterozygous Ogt-null females to develop normally with paternal gamete-specific Ogt mutagenesis.
By segregating Ogt mutagenesis to male and not female gametes, it was theoretically possible to generate viable female heterozygotes. Such heterozygotes would be valuable for detecting cell types that may not require Ogt and O-GlcNAc. Embryonic cells should undergo random X inactivation, thereby reducing specific activity levels of cellular Ogt when compared with WT and control mice. However, a cellular requirement for Ogt would result in the developmental loss of cells in which the maternal X chromosome is inactivated, leading to normal Ogt levels in such tissues. We therefore bred WT females with OgtF/Y males bearing a Cre recombinase transgene in which Cre expression is controlled by the mouse protamine-1 promoter and is male gamete specific (42).
Female heterozygotes bearing the Ogt Δ allele were successfully produced by breeding WT females to males in which the paternal genome contained the prm-Cre transgene and the OgtF allele (Fig. 5A, panel 3). These female OgtΔ/WT mice could not be distinguished from control littermates and were found to be fertile. As expected, however, offspring produced by these females did not contain the Ogt Δ allele. Ogt enzyme activity was assessed among multiple organs and tissue types of female OgtΔ/WT mice, including the brain, liver, heart, thymus, spleen, kidney, and skeletal muscle. Normal levels of Ogt-specific activity were obtained in all samples analyzed (Table 1). These findings suggest that the tissue and cell types surveyed had undergone inactivation of the mutant paternal Ogt allele due to a requirement for a functional Ogt gene (Fig. 5C). The presence of specific cell populations comprising a low percentage of these tissues and which do not require Ogt function may nevertheless exist beyond the sensitivity of the assay used here. These findings also provide a general method of creating viable female heterozygotes bearing a mutant allele of an essential X-linked gene.
TABLE 1.
Ogt activity in tissues of female mice bearing F/WT or Δ/WT Ogt genotype
| Tissue | Ogt activitya
|
|
|---|---|---|
| F/WT | Δ/WT | |
| Brain | 187.6 ± 8.7 | 176.9 ± 11.0 |
| Liver | 21.9 ± 4.1 | 26.1 ± 3.8 |
| Heart | 46.4 ± 5.3 | 49.8 ± 4.1 |
| Thymus | 13.0 ± 2.4 | 11.9 ± 3.0 |
| Spleen | 63.5 ± 5.5 | 57.3 ± 6.9 |
| Kidney | 181.1 ± 10.5 | 177.9 ± 5.2 |
| Skeletal muscle | 38.2 ± 2.4 | 33.3 ± 4.4 |
Ogt activity (counts per minute per microgram of extract) was measured from four mice of each genotype. Averages were calculated and are provided with standard deviations.
DISCUSSION
The O-GlcNAc modification is a form of intracellular protein glycosylation that we find to be essential for mammalian embryogenesis by promoting the function and viability of various cell types, including thymocytes, neurons, oocytes, fibroblasts, and ES cells. By applying conditional mutagenesis involving Cre-loxP recombination of the Ogt allele, we were able to segregate Ogt deficiency to these and other cell lineages and demonstrated the requirement for the Ogt gene in O-GlcNAc formation among fibroblasts. The position of the Ogt locus on the mammalian X chromosome, and the apparent reliance upon Ogt function for cell and organism viability, indicates that the previous inability to produce female heterozygotes was likely due to the essential role of Ogt among extraembryonic cells comprising trophoblasts and early endoderm (46). For example, mutation of the X-linked glucose-6-phosphate dehydrogenase (G6PD) gene, although not lethal to ES cells, results in placental failure and embryo death (34). Only mice chimeric for the G6PD mutation are able to generate viable female heterozygotes, and as expected their viable offspring did not carry the mutant allele. This appears to reflect the preferential inactivation of the paternal X chromosome in extraembryonic trophoblast and primitive endoderm lineages (14, 21).
A reproducible and genetic approach to acquiring viable female heterozygotes bearing a null allele of an essential X-linked gene required a novel strategy and was theoretically possible by employing male gamete-specific mutagenesis. This approach does not require random chimerism and is not duplicated by the use of tetraploid embryo aggregation techniques involving ES cells. Viable female heterozygotes bearing the paternally derived Ogt Δ allele and the maternally derived OgtWT allele were successfully produced and were phenotypically normal, providing a source of tissues in which random X-chromosome inactivation would be expected to reduce the specific activity levels of Ogt by approximately 50% among cell types not requiring O-GlcNAc. As no such finding was obtained, we infer that a strong selection exists in early development against embryonic cells that have inactivated the maternal allele, have lost Ogt function, and are deficient in O-GlcNAc. We cannot, however, rule out the possibility of a low level of mosaicism that is not detected in our enzymatic analyses, which would indicate the survival of some cells, as well as the presence of Ogt-null cell types that were not isolated or analyzed.
Directing somatic cell mosaicism for the mutant Ogt allele in vivo was promoted by Cre recombination specifically among thymocytes and neurons. In thymocytes the result was cell death by apoptosis upon acquisition of the Ogt Δ allele and in the absence of either the WT or F Ogt allele. Apoptosis increased in frequency during maturation and in dependence upon thymic Cre expression, resulting in a failure to contribute to the peripheral T-lymphocyte repertoire. Only T cells not having undergone thymic Cre recombination were observed in the periphery. The high efficiency of Cre recombination and the rapidity of the apoptotic process resulted in a significant decrease in thymocytes and peripheral T-cell numbers.
Neuron-specific Ogt mutation resulted in neuronal dysfunction, and although animals were born, they were severely affected with deficient locomotor activity, failed to nurse, and died within 10 days after birth. The tau protein was increased in total amount and found to be hyperphosphorylated. Previous studies have found a close association of hyperphosphorylated tau with neuronal apoptosis and early lethality in the mouse (3, 28). Although no genetic linkage specifically to the Ogt allele has been made as yet in X-linked disease states among humans and mice, a rare neuralgic disorder classified as a form of Parkinson-dystonia has been mapped to the chromosomal region that includes the Ogt locus (41).
Derivation of a viable cell lineage lacking an intact Ogt gene and O-GlcNAc expression was not possible among the various cell types analyzed in this study. However, an inducible method of Ogt gene mutagenesis, using a VSV-pseudotyped recombinant retrovirus that expressed the Cre gene, enabled a short time span in which Ogt-null fibroblasts were viable and amenable for further study in vitro. Reciprocity of O-GlcNAc linkage with sites of protein phosphorylation has suggested a role in transcription, protein synthesis, and protein phosphorylation signaling cascades, including those involved in elongation factor phosphorylation (8, 25; reviewed in reference 51). Remarkably, we did not observe a significant change in total protein phosphorylation level in metabolic labeling studies of fibroblasts deficient in O-GlcNAc and prior to cell death when protein synthesis was still ongoing (data not shown). This is consistent with several possibilities, including a more restricted role for O-GlcNAc in the modulation of some signal transduction pathways in fibroblasts. It is also possible that the O-GlcNAc-deficient state alters protein degradation rates among some Ogt substrates or perhaps that O-GlcNAc deficiency activates cellular protein phosphatase enzymes. Nevertheless, we have found that Ogt-dependent O-GlcNAc formation does alter protein phosphorylation as well as levels of protein expression involving key regulators of cell growth and survival.
The serum stimulation response failed to normally induce Ogt substrates c-Fos, c-Jun, and c-Myc, and this occurred in the presence of increased steady-state levels of c-Jun, c-Fos, and c-Myc RNA. This is consistent with a role for O-GlcNAc in transcriptional repression, modulation of protein degradation, and regulation of protein synthesis. There are reasons to believe that O-GlcNAc is involved in all of these processes. Phosphorylated forms of RNA polymerase II are associated with active transcription and they specifically lack O-GlcNAc while nonphosphorylated forms are not actively transcribing (8, 22, 27). More recently, Ogt binding to the transcriptional corepressor mSin3A has been reported as a mechanism by which the O-GlcNAc linkage is targeted to a histone deacetylase complex involved in transcriptional silencing (53). Multiple roles of O-GlcNAc in modulating transcription appear evident, and we observed that the Sp1 transcription factor was significantly reduced in protein level coincident with a loss of O-GlcNAc. These findings are consistent with a proposed role for O-GlcNAc in blocking some protein interactions and protecting Sp1 from degradation by the ubiquitin-proteosome pathway (19, 45, 48, 52). The eukaryotic initiation factor 2 (eIF2)-associated protein p67 is also an Ogt substrate in which the O-GlcNAc modified form has been proposed to reduce eIF-2 phosphorylation and promote protein synthesis (9). The reduced level of phosphorylated c-Myc we observed may reflect a decrease in de novo synthesis of some cellular proteins in the O-GlcNAc-deficient serum response.
Considering the relationship between glucose abundance, UDP-GlcNAc levels, and protein O-GlcNAc formation, it has been suggested that Ogt functions in hexose metabolism by integrating nutritional status signals that control cell function and proliferation. For example, chemical inhibition of intracellular β-N-acetylglucosaminidase activity or transgenic overexpression of the Ogt gene are associated with increased O-GlcNAc levels, insulin resistance, and increased leptin production (39, 50). These and other studies show that increased O-GlcNAc levels may be pathological but can occur without abolishing cell proliferation or viability (18). In contrast, our studies show that a deficiency of O-GlcNAc leads to more severe consequences. Cell dysfunction and apoptosis are observed, with fibroblasts failing to undergo four cell division cycles and appearing to prematurely senesce, as occurs with fibroblasts deficient in components of the AP1 transcriptional complex, such as c-Fos, c-Jun, and c-Myc (reviewed in references 13 and 47). The increased levels of p27 observed are also consistent with reduced activity of the ubiquitin-proteosome pathway and a failure to progress through the cell cycle (5, 23, 43). Preliminary data suggest that fibroblasts lacking O-GlcNAc do not transit through cell cycle stages in parallel with cells bearing the Ogt F allele (not shown).
Although we succeeded in generating in vivo cell-type-specific mutants of the mammalian Ogt gene and observed a deficiency of the O-GlcNAc linkage, these were transient successes as our characterization of these fleeting model systems indicates an essential role for O-GlcNAc in the viability of several and possibly all mammalian cell types. Our findings of specific molecular defects in the absence of O-GlcNAc support multiple but restricted roles of O-GlcNAc in essential and conserved signaling pathways. Nevertheless, we more fully succeeded in developing genetic tools that can be used to further investigate Ogt-dependent O-GlcNAc functions. The derivation of an in vitro cell system for the rapid and efficient ablation of O-GlcNAc formation provides opportunities to further elaborate upon the cellular roles of this ubiquitous and essential form of intracellular protein glycosylation.
Acknowledgments
We thank our colleagues Steve Dowdy, Daniel Chui, and Lesley Ellies for helpful discussions.
This research was funded by the National Institutes of Health (DK48247 to J.D.M. and HD13563 to G.W.H.) and the Howard Hughes Medical Institute (J.D.M.). J.D.M. acknowledges support as an Investigator of the Howard Hughes Medical Institute.
Under a licensing agreement between Covance Research Products and Hoffman-La Roche and The Johns Hopkins University, G.W.H. receives a share of royalties on sales of the CTD110.6 antibody. The terms of this arrangement are being managed by The Johns Hopkins University in accordance with its conflict of interest policies.
This research adhered to all federal guidelines and institutional policies for the care of the mice used in these experiments.
REFERENCES
- 1.Arnold, C. S., R. N. Johnson, D. L. Cole, M. Lee, and G. W. Hart. 1996. The microtubule-associated protein tau is extensively modified with O-linked N-acetylglucosamine. J. Biol. Chem. 271:28741-28742. [DOI] [PubMed] [Google Scholar]
- 2.Berton, F., V. Cornet, C. Iborra, J. Garrido, B. Dargent, M. Fukuda, M. Seagar, and B. Marqueze. 2000. Synaptotagmin I and IV define distinct populations of neuronal transport vesicles. Eur. J. Neurosci. 12:1294-1302. [DOI] [PubMed] [Google Scholar]
- 3.Brich, J., F.-S. Shie, B. W. Howell, R. Li, K. Tus, E. K. Wakeland, L.-W. Jin, M. Mumby, G. Churchill, J. Herz, and J. A. Cooper. 2003. Genetic modulation of tau phosphorylation in the mouse. J. Neurosci. 23:187-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou, T. Y., C. V. Dang, and G. W. Hart. 1995. Glycosylation of the c-Myc transactivation domain. Proc. Natl. Acad. Sci. USA 92:4417-4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coats, S., W. M. Flanagan, J. Nourse, and J. M. Roberts. 1996. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science 272:877-880. [DOI] [PubMed] [Google Scholar]
- 6.Cole, R. N., and G. W. Hart. 1999. Glycosylation sites flank phosphorylation sites on synapsin I: O-linked N-acetylglucosamine residues are localized within domains mediating synapsin I interactions. J. Neurochem. 73:418-428. [DOI] [PubMed] [Google Scholar]
- 7.Comer, F. I., K. Vosseller, L. Wells, M. A. Accavitti, and G. W. Hart. 2001. Characterization of a mouse monoclonal antibody specific for O-linked N-acetylglucosamine. Anal. Biochem. 293:169-177. [DOI] [PubMed] [Google Scholar]
- 8.Comer, F. I., and G. W. Hart. 2001. Reciprocity between O-GlcNAc and O-phosphate on the carboxyl terminal domain of RNA polymerase II. Biochemistry 40:7845-7852. [DOI] [PubMed] [Google Scholar]
- 9.Datta, R., P. Choudhury, A. Gosh, and B. Datta. 2003. A glycosylation site, 60SGTS63, of p67 is required for its ability to regulate the phosphorylation and activity of eukaryotic initiation factor 2α. Biochemistry 42:5453-5460. [DOI] [PubMed] [Google Scholar]
- 10.de Alboran, I. M., R. C. O'Hagan, F. Gärtner, B. Malynn, L. Davidson, R. Rickert, K. Rajewsky, R. A. DePinho, and F. W. Alt. 2001. Analysis of C-MYC function in normal cells via conditional gene-targeted mutation. Immunity 14:45-55. [DOI] [PubMed] [Google Scholar]
- 11.DeFalco, J., M. Tomishima, H. Liu, C. Zhao, X. Cai, J. D. Marth, L. Enquist, and J. M. Friedman. 2001. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science 291:2608-2613. [DOI] [PubMed] [Google Scholar]
- 12.Ding, M., and D. D. Vandre. 1996. High molecular weight microtubule-associated proteins contain O-linked-N-acetylglucosamine. J. Biol. Chem. 271:12555-12561. [DOI] [PubMed] [Google Scholar]
- 13.Eisenman, R. N. 2001. Deconstructing Myc. Genes Dev. 15:2023-2030. [DOI] [PubMed] [Google Scholar]
- 14.Frels, W. I., and V. M. Chapman. 1980. Expression of the maternally derived X chromosome in the mural trophoblast of the mouse. J. Embryol. Exp. Morphol. 56:179-190. [PubMed] [Google Scholar]
- 15.Gabriel, A., T. Helman, and A. Wilks. 1993. A PCR based assay for the murine multiple intestinal neoplasia (Min) mutation. Mouse Genome 91:316-320.
- 16.Gao, Y., L. Wells, F. I. Comer, G. J. Partker, and G. W. Hart. 2001. Dynamic O-glycosylation of nuclear and cytosolic proteins: cloning and characterization of a neutral, cytosolic β-N-acetylglucosaminidase from human brain. J. Biol. Chem. 276:9838-9845. [DOI] [PubMed] [Google Scholar]
- 17.Haltiwanger, R. S., M. A. Blomberg, and G. W. Hart. 1992. Glycosylation of nuclear and cytoplasmic proteins. Purification and characterization of a uridine diphospho-N-acetylglucosamine:polypeptide β-N-acetylglucosaminyltransferase. J. Biol. Chem. 267:9005-9013. [PubMed] [Google Scholar]
- 18.Haltiwanger, R. S., K. Grove, and G. A. Philipsberg. 1998. Modulation of O-linked N-acetylglucosamine levels on nuclear and cytoplasmic proteins in vivo using the peptide O-GlcNAc-β-N-acetylglucosaminidase inhibitor O-(2-acetamido-2-deoxy-d-glucopyranosylidene)amino-N-phenylcarbamate. J. Biol. Chem. 273:3611-3617. [DOI] [PubMed] [Google Scholar]
- 19.Han, I., and J. E. Kudlow. 1997. Reduced O glycosylation of Sp1 is associated with increased proteasome susceptibility. Mol. Cell. Biol. 17:2550-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanahan, D., D. Lane, L. Lipsich, M. Wigler, and M. Botchan 1980. Characteristics of an SV40-plasmid recombinant and its movement into and out of the genome of a murine cell. Cell 21:127-139. [DOI] [PubMed] [Google Scholar]
- 21.Harper, M., M. Fosten, and M. Monk. 1982. Preferential paternal X inactivation in extra-embryonic tissues of early mouse embryos. J. Embryol. Exp. Morphol. 67:127-135. [PubMed] [Google Scholar]
- 22.Hart, G. W. 1997. Dynamic O-linked glycosylation of nuclear and cytoskeletal proteins. Annu. Rev. Biochem. 66:315-335. [DOI] [PubMed] [Google Scholar]
- 23.Hengst, L., and S. I. Reed. 1996. Translational control of p27Kip1 accumulation during the cell cycle. Science 271:1861-1864. [DOI] [PubMed] [Google Scholar]
- 24.Hennet, T., F. K. Hagen, L. A. Tabak, and J. D. Marth. 1995. T-cell-specific deletion of a polypeptide N-acetylgalactosaminyl-transferase gene by site-directed recombination. Proc. Natl. Acad. Sci. USA 92:12070-12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamemura, K., B. K. Hayes, F. I. Comer, and G. W. Hart. 2002. Dynamic interplay between O-glycosylation and O-phosphorylation of nucleocytoplasmic proteins: alternative glycosylation/phosphorylation of THR-58, a known mutational hot spot of c-Myc in lymphomas, is regulated by mitogens. J. Biol. Chem. 277:19229-19235. [DOI] [PubMed] [Google Scholar]
- 26.Kearse, K. P., and G. W. Hart. 1991. Lymphocyte activation induces rapid changes in nuclear and cytoplasmic glycoproteins. Proc. Natl. Acad. Sci. USA 88:1701-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly, W. G., M. E. Dahmus, and G. W. Hart. 1993. RNA polymerase II is a glycoprotein. Modification of the COOH-terminal domain by O-GlcNAc. J. Biol. Chem. 268:10416-10424. [PubMed] [Google Scholar]
- 28.Kobayashi, K., H. Nakano, M. Hayashi, M. Shimazaki, Y. Fukutani, K. Sasaki, K. Sugimori, and Y. Koshino. 2003. Association of phosphorylation site of tau protein with neuronal apoptosis in Alzheimer's disease. J. Neurol. Sci. 208:17-24. [DOI] [PubMed] [Google Scholar]
- 29.Kreppel, L. K., M. A. Blomberg, and G. W. Hart. 1997. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J. Biol. Chem. 272:9308-9315. [DOI] [PubMed] [Google Scholar]
- 30.Kreppel, L. K., and G. W. Hart. 1999. Regulation of a cytosolic and nuclear O-GlcNAc transferase. Role of the tetratricopeptide repeats. J. Biol. Chem. 274:32015-32022. [DOI] [PubMed] [Google Scholar]
- 31.Kunieda, T., M. Xian, E. Kobayashi, T. Imamichi, K. Moriwaki, and Y. Toyoda. 1992. Sexing of mouse preimplantation embryos by detection of Y chromosome-specific sequences using polymerase chain reaction. Biol. Reprod. 46:692-697. [DOI] [PubMed] [Google Scholar]
- 32.Lavrovsky, Y., C.-S. Song, B. Chatterjee, and A. K. Roy. 1998. A rapid and reliable PCR-based assay for gene transmission and sex determination in newborn transgenic mice. Transgenic Res. 7:319-320. [DOI] [PubMed] [Google Scholar]
- 33.Liu, F., K. Iqbal, I. Grundke-Iqbal, and C. X. Gong. 2002. Involvement of aberrant glycosylation in phosphorylation of tau by cdk5 and GSK-3 beta. FEBS Lett. 530:209-214. [DOI] [PubMed] [Google Scholar]
- 34.Longo, L., O. C. Vanegas, M. Patel, V. Rosti, H. Li, J. Waka, T. Merghoub, P. P. Pandolfi, R. Notaro, K. Manova, and L. Luzzatto. 2002. Maternally transmitted severe glucose 6-phosphate dehydrogenase deficiency is an embryonic lethal. EMBO J. 21:4229-4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lubas, W. A., D. W. Frank, M. Krause, and J. A. Hanover. 1997. O-linked GlcNAc transferase is a conserved nucleocytoplasmic protein containing tetratricopeptide repeats. J. Biol. Chem. 272:9316-9324. [DOI] [PubMed] [Google Scholar]
- 36.Lubas, W. A., and J. A. Hanover. 2000. Functional expression of O-linked GlcNAc transferase. Domain structure and substrate specificity. J. Biol. Chem. 275:10983-10988. [DOI] [PubMed] [Google Scholar]
- 37.Marek, K. W., I. Vijay, and J. D. Marth. 1999. A recessive deletion in the GlcNAc-1-phosphotransferase gene results in peri-implantation embryonic lethality. Glycobiology 9:1263-1271. [DOI] [PubMed] [Google Scholar]
- 38.Marth, J. D. 1996. Recent advances in gene mutagenesis by site-specific recombination. J. Clin. Investig. 97:1999-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McClain, D. A., W. A. Lubas, R. C. Cooksey, M. Hazel, G. J. Parker, D. C. Love, and J. A. Hanover. 2002. Altered glycan-dependent signaling induces insulin resistance and hyperleptinemia. Proc. Natl. Acad. Sci. USA 99:10695-10699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller, M. W., M. R. Caracciolo, W. K. Berlin, and J. A. Hanover. 1999. Phosphorylation and glycosylation of nucleoporins. Arch. Biochem. Biophys. 367:51-60. [DOI] [PubMed] [Google Scholar]
- 41.Nemeth, A. H., D. Nolte, E. Dunne, S. Niemann, M. Kostrzewa, U. Peters, E. Fraser, E. Bochukova, R. Butler, J. Brown, R. D. Cox, E. R. Levy, H. H. Ropers, A. P. Monaco, and U. Muller. 1999. Refined linkage disequilibrium and physical mapping of the gene locus for X-linked dystonia-parkinsonism (DYT3). Genomics 60:320-329. [DOI] [PubMed] [Google Scholar]
- 42.O'Gorman, S., N. A. Dagenais, M. Qian, and Y. Marchuk. 1997. Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc. Natl. Acad. Sci. USA 94:14602-14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pagano, M., S. W. Tam, A. M. Theodoras, P. Beer-Romero, G. De Sal, V. Chau, P. R. Yew, G. F. Draetta, and M. Rolfe. 1995. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science 269:682-685. [DOI] [PubMed] [Google Scholar]
- 44.Priatel, J. J., D. Chui, N. Hiraoka, C. J. T. Simmons, K. B. Richardson, D. M. Page, M. Fukuda, N. M. Varki, and J. D. Marth. 2000. The ST3Gal-l sialyltransferase controls CD8+ T lymphocyte homeostatis by modulating O-glycan biosynthesis. Immunity 12:273-283. [DOI] [PubMed] [Google Scholar]
- 45.Roos, M. D., S. Su, J. R. Baker, and J. E. Kudlow. 1997. O glycosylation of an Sp1-derived peptide blocks known Sp1 protein interactions. Mol. Cell. Biol. 17:6472-6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shafi, R., S. P. Iyer, L. G. Ellies, N. O'Donnell, K. W. Marek, D. Chui, G. W. Hart, and J. D. Marth. 2000. The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc. Natl. Acad. Sci. USA 97:5735-5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaulian, E., and M. Karin. 2001. AP-1 in cell proliferation and survival. Oncogene 20:2390-2400. [DOI] [PubMed] [Google Scholar]
- 48.Su, K., X. Yang, M. D. Roos, A. J. Paterson, and J. E. Kudlow. 2000. Human Sug1/p45 is involved in the proteasome-dependent degradation of Sp1. Biochem. J. 348:281-289. [PMC free article] [PubMed] [Google Scholar]
- 49.Toyoshima, H., and T. Hunter. 1994. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 78:67-74. [DOI] [PubMed] [Google Scholar]
- 50.Vosseller, K., L. Wells, M. D. Lane, and G. W. Hart. 2002. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc. Natl. Acad. Sci. USA 99:5313-5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wells, L., and G. W. Hart. 2003. O-GlcNAc turns twenty: functional implications for post-translational modification of nuclear and cytoplasmic proteins with a sugar. FEBS Lett. 546:154-158. [DOI] [PubMed] [Google Scholar]
- 52.Yang, X., K. Su, M. D. Roos, Q. Chang, A. J. Paterson, and J. E. Kudlow. 2001. O-linkage of N-acetylglucosamine to Sp1 activation domain inhibits its transcriptional capability. Proc. Natl. Acad. Sci. USA 98:6611-6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang, X., F. Zhang, and J. E. Kudlow. 2002. Recruitment of O-GlcNAc transferase to promoters by corepressor mSin3A: coupling protein O-GlcNAcylation to transcriptional repression. Cell 110:69-80. [DOI] [PubMed] [Google Scholar]
- 54.Yee, J.-K., A. Miyanohara, P. LaPorte, K. Bouic, J. C. Burns, and T. Friedman. 1994. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc. Natl. Acad. Sci. USA 91:9564-9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu, Y., M. I. Romero, P. Ghosh, Z. Ye, P. Charnay, E. J. Rushing, J. D. Marth, and L. F. Parada. 2001. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev. 15:859-876. [DOI] [PMC free article] [PubMed] [Google Scholar]