Abstract
Pak2 is a serine/threonine kinase that participates in the cellular response to stress. Among the potential substrates for Pak2 is the protein Myc, encoded by the proto-oncogene MYC. Here we demonstrate that Pak2 phosphorylates Myc at three sites (T358, S373, and T400) and affects Myc functions both in vitro and in vivo. Phosphorylation at all three residues reduces the binding of Myc to DNA, either by blocking the requisite dimerization with Max (through phosphorylation at S373 and T400) or by interfering directly with binding to DNA (through phosphorylation at T358). Phosphorylation by Pak2 inhibits the ability of Myc to activate transcription, to sustain cellular proliferation, to transform NIH 3T3 cells in culture, and to elicit apoptosis on serum withdrawal. These results indicate that Pak2 is a negative regulator of Myc, suggest that inhibition of Myc plays a role in the cellular response to stress, and raise the possibility that Pak2 may be the product of a tumor suppressor gene.
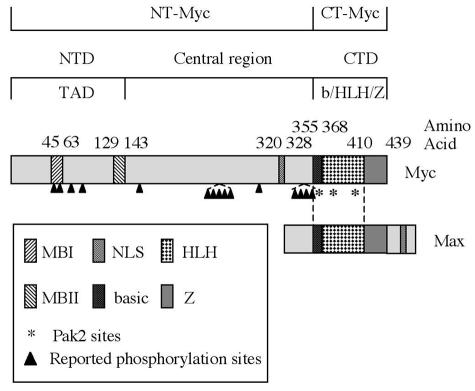
The nuclear protein Myc is a central regulator of cellular proliferation and growth. Deregulated expression of MYC induces genomic instability, can elicit both cell transformation and apoptosis, and has been found in many types of cancer (17, 33, 46). The MYC gene encodes a basic helix-loop-helix leucine zipper (b/HLH/Z) protein, which forms dimers with the ubiquitously expressed protein Max (11, 52) (see Fig. 1). The Myc-Max heterodimer serves as a transcription factor by binding to the specific DNA sequence CACGTG known as an E box (9, 11) and recruiting accessory factors (61). The pleiotropic effects of Myc can be explained by the large number of genes under its control (16, 26). Other topographical features of Myc include an amino-terminal transactivation domain and, within that domain, two conserved sequences known as Myc boxes (MB1 and MB2) (see Fig. 1).
FIG. 1.
Functional domains of Myc. The amino-terminal domain (NTD) of Myc contains two conserved sequences, Myc Box 1 (MBI) and Myc Box II (MBII), that are required for transcriptional activation activity. The carboxyl-terminal domain (CTD) contains the b/HLH/Z domain, which is responsible for dimerization with Max and binding to E-box DNA. A nuclear localization signal (NLS) is present in the central region of the protein. The arrowheads point to multiple phosphorylation sites reported previously. The Myc fragments used in the following experiments are peptide 1 to 352 (NT-Myc) and peptide 353 to 439 (CT-Myc).
Multiple sites of phosphorylation have been identified in Myc (Fig. 1). The functional consequences of these phosphorylations have not been fully elucidated. The known effects include changes in the stability of the protein (6, 27, 62) and in its ability to elicit either transformation (12, 53) or apoptosis (12, 47). Several mutations that eliminate phosphorylation sites in the amino-terminal domain increase the ability of MYC to transform cells in culture (12, 53) and also occur in certain human tumors (5). In addition, phosphorylation of Myc occurs in response to certain forms of cellular stress, but only one of the responsive enzymes (Jun N-terminal kinase) has been identified, and it cannot account for all of the observed phosphorylation (1, 47).
Pak2 is a serine/threonine protein kinase that is activated by cellular stress, such as hyperosmolarity, ionizing radiation, and DNA-damaging agents, all of which result in cell cycle arrest (57). The activity of Pak2 is normally constrained by an intramolecular autoinhibitory domain (AID) that occludes the catalytic site of the enzyme. Activation by the small G-protein Rac1/Cdc42(GTP), which binds within the AID, relieves the inhibitory effect and precipitates autophosphorylation of Pak2 (57). Pak2 can also be activated by the caspase 3 protease (60, 73) and by other stress signals such as sphingosine (58). The activity of Pak2 can inhibit cellular proliferation in a variety of species (35, 59), but the responsible substrates have not been identified.
The serine and threonine sites of phosphorylation by Pak2 reside in a characteristic peptide sequence containing (K/R)RX(S/T), or one basic residue with upstream basic amino acids (70; P. T. Tuazon, J. Quijano, and J. A. Traugh, unpublished data). Myc contains three such sites in the b/HLH/Z domain (T358, S373, and T400) (Fig. 1). Phosphorylation at these residues has not been reported previously. Pak2 and Myc have opposite effects on the cell cycle. It seemed possible, therefore, that the biological effects of Pak2 might be mediated, at least in part, by inactivation of Myc.
Here we show that Pak2 phosphorylates the three potential target sites in the b/HLH/Z domain of Myc but not those elsewhere in the protein. The phosphorylations can be elicited by cellular stress. Phosphorylation at S373 and T400 interferes with the formation of Myc-Max dimers, whereas phosphorylation at T358 directly impedes the binding of the dimers to DNA. Phosphorylation of the three residues reduces the ability of Myc to activate transcription and to elicit cellular proliferation, transformation, and apoptosis. We conclude that Pak2 executes at least part of the cellular response to stress by inhibiting the activity of Myc, and we propose that Pak2 may be the product of a tumor suppressor gene.
MATERIALS AND METHODS
Cell culture.
Human embryonic kidney 293T and mouse NIH 3T3 cells were from the American Type Culture Collection. Cells were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% (vol/vol) fetal calf serum and penicillin-streptomycin. Fibroblasts were infected with preparations of the pMIG retrovirus containing various alleles of MYC as described previously (71), and 293T cells were transfected with Superfect as described by the manufacturer (Qiagen).
Construction of expression and retroviral vectors and production of retroviruses.
The retroviral expression vector pMIG was a kind gift of Y. Refaeli (71). cDNAs encoding wild-type and mutant MYC were cloned into this vector to produce Myc-expressing retrovirus. Human Pak2 tagged with hemagglutinin (HA) was prepared and inserted into the vector pcDNA3.1+ as described previously (35). Full-length Myc (FL-Myc), a carboxyl-terminal fragment (amino acids 353 to 439) (CT-Myc), and an amino-terminal fragment (amino acids 1 to 352) (NT-Myc) were cloned as poly(His)-tagged proteins into pcDNA3.1+ between the KpnI and XbaI restriction sites. To replace T358, S373, and T400 with alanine or aspartic acid in MYC, site-directed mutagenesis was conducted using the QuikChange site-directed mutagenesis kit as described by the manufacturer (Stratagene). A chimeric protein consisting of HA-tagged Pak2 at the amino terminus and the hormone binding domain of the human estrogen receptor (ER) at the carboxyl terminus was prepared by inserting the sequences of HA-Pak2 (cleaved with BglII-BamHI) and ER (cleaved with BamHI-EcoRI) into the pMSCV-puro retroviral vector (Clontech). Retroviruses expressing the resulting chimeric protein (Pak2ER) were generated by transfecting Bosc 23 cells with 10 μg of plasmid DNA as described previously (71). Cell supernatants were collected 48 and 72 h after transfection and used to infect NIH 3T3 cells. Infections were performed by centrifuging at 300 × g for 1 h at 30°C as described previously (55). The efficiency of infection was assessed by flow cytometry for green fluorescent protein (GFP) and immunoblotting for expressed protein. For coexpression of Pak2ER and Myc, NIH 3T3 cells were infected with Pak2ER-expressing retrovirus and selected with puromycin for 48 h. NIH 3T3 Pak2ER cells were then infected with Myc-expressing retrovirus and used for further experiments.
In vitro kinase assay.
Recombinant Cdc42 and Pak2 proteins were prepared as previously described (73). FL-Myc, CT-Myc and NT-Myc were produced by translation in vitro, using the TNT Quick Coupled transcription-translation systems (Promega). The product was purified with Ni-nitrilotriacetate-agarose beads (Qiagen). Pak2 was activated in the presence of Cdc42(GTPγS) (0.1 μg) as described previously (73). Myc phosphorylation by Pak2 was carried out in 70-μl reaction mixtures containing Pak2 (0.1 μg), Myc (0.5 μg), 20 mM Tris-HCl (pH 7.4), 10 mM MgCl2, 15 mM 2-mercaptoethanol, and 0.2 mM [γ-32P]ATP (500 to 2,000 dpm/pmol). Incubation was carried out for 15 min at 30°C. The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Autophosphorylation of Pak2 and phosphorylation of Myc were analyzed by autoradiography. 32P-labeled CT-Myc was eluted from the gel and subjected to acid hydrolysis. The phosphorylated amino acids were separated by electrophoresis on a thin-layer cellulose gel plate as described previously (70).
In vivo 32P labeling.
Myc-, Pak2-, and Pak2-AID-expressing plasmids were cotransfected or transfected individually into 293T cells using Superfect (Qiagen). For 32P labeling, cells were incubated for 12 h in phosphate-free DMEM containing 0.25 mCi of [32P]orthophosphoric acid per ml and 10% dialyzed newborn calf serum, and then sorbitol (400 mM), cisplatin (50 mM), or cytosine-β-d-arabinofuranoside (50 mM) was added. After another 2 h, the cells were washed three times with phosphate-free DMEM, lysed in 500 μl of lysis buffer containing 50 mM Tris-HCl (pH 7.5), 5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 10 mM β-mercaptoethanol, phosphatase inhibitors (50 mM NaF, 5 mM Na4P2O7, 2 mM Na3VO4), and protease inhibitors (40 μg of leupeptin per ml, 40 μg of pepstatin per ml, 40 μg of aprotinin per ml, 0.5 mM phenylmethylsulfonyl fluoride), and stored at −70°C.
Immunoprecipitation.
Immunoprecipitation of endogenous Myc and Pak2 was performed with polyclonal antibodies sc-764 and sc-1872 (Santa Cruz), respectively. The cell lysates (200 μg of protein) were adjusted to 500 μl with lysis buffer and incubated with 2 μg of antibody for 30 min at 4°C. Immune complexes were collected on protein G-Sepharose beads by incubation for 1 h at 4°C. Immunocomplexes were subjected to SDS-PAGE and, when necessary, autoradiography. The amounts of radioactivity in 32P-labeled proteins were quantified with a PhosphorImager (Molecular Dynamics).
To assess coimmunoprecipitation of Max with Myc, complexes formed with polyclonal antibody sc-764 were subjected to SDS-PAGE and immunoblotted with monoclonal anti-Max antibody sc-8011 (Santa Cruz). For in vitro kinase assays of immunoprecipitated Pak2, immunocomplexes were used in the in vitro kinase assay as described above, with histone 4 (2 μg) as the substrate. His-tagged FL-Myc and CT-Myc in cell lysates were purified with Ni-nitriloacetate-agarose beads as specified by the manufacturer (Qiagen).
Glutathione S-transferase pulldown assay.
The Max coding sequence was inserted into the pGEX-2T plasmid (Pharmacia Biotech Inc.) between the BamHI and EcoRI restriction sites to create a glutathione S-transferase-Max fusion protein. The fusion protein was expressed in Escherichia coli containing the plasmid by isopropyl-β-d-thiogalactoside (IPTG) induction and purified with glutathione-Sepharose beads (Pharmacia). FL-Myc and CT-Myc (1 μg each) were produced by in vitro translation and phosphorylated with Pak2 as described above. The phosphorylated proteins were incubated with 15 μl of glutathione (GSH)-Sepharose beads loaded with glutathione S-transferase-Max (4 μg) in 500 μl of binding buffer containing 10% glycerol, 20 mM Tris (pH 7.5), 50 mM KCl, 3 mM MgCl2, 1 mM EDTA, 0.5 mM dithiothreitol, and 0.1% NP-40. After 2 h at 4°C, the beads were washed with binding buffer and the proteins retained on beads were analyzed by SDS-PAGE and immunoblotting with anti-Myc antibody 9E10 (22). One-fifth of the amount of Myc protein loaded for the pulldown assay was immunoblotted for Myc as the loading control.
Mammalian two-hybrid analysis.
Interactions between Myc and Max in vivo were analyzed by using the Mammalian two-hybrid assay kit (Stratagene). The NF-κB transcription activation domain (AD) was fused with MYC or MYC mutants. The Gal4 DNA binding domain (BD) was fused with Max. Constructs encoding fusion proteins were created by cloning PCR-generated fragments into BamHI-XbaI-digested vectors. All constructs were sequenced and found to express proteins of the expected molecular weights. A 1-μg portion of AD-Myc or AD-Myc mutants and 1 μg of BD-Max were cotransfected with 0.5 μg of reporter luciferase plasmid pFR-Luc into 293T cells, using SuperFect. Pak2 expression plasmid (1 μg) was cotransfected into the same cells as necessary. At 48 h after transfection, the cells were harvested and luciferase activities were measured. The Renilla luciferase expression plasmid was cotransfected to provide a background measurement. The experiments were performed in triplicate, and the results were recorded as the relative luciferase activity (i.e., the ratio of firefly luciferase activity to Renilla luciferase activity).
Oligonucleotide precipitation.
Biotinylated oligonucleotides were precipitated using 293T cell extracts overexpressing the Myc constructs. Approximately 200 μg of cell lysates was incubated at 25°C for 10 min in 500 μl of reaction solution containing 10% glycerol, 20 mM Tris (pH 7.5), 50 mM KCl, 3 mM MgCl2, 1 mM EDTA, 0.5 mM dithiothreitol, 0.1% NP-40, 0.1 mg of salmon sperm DNA per ml, and 1 μg of biotinylated E box oligonucleotide (5′-CCCCCACCACGTGGTGCCACGTGAGTG-3′). Oligonucleotide-protein complexes were collected on streptavidin-conjugated agarose beads by incubation for 1 h at 4°C. After three washes with reaction solution, the DNA-protein complexes were subjected to SDS-PAGE and immunoblotting with anti-Myc and anti-Max antibodies. A 40-μg portion of cell lysates (20% of the loading for precipitation) was also used for immunoblotting of Myc as loading control.
BrdU incorporation.
The bromodeoxyuridine (BrdU) incorporation assay was performed using a BrdU labeling kit from Roche Molecular Biochemicals as specified by the manufacturer. NIH 3T3 cells infected with Myc retrovirus were grown to 100% confluency for 48 h. The cells were then labeled with BrdU (1 μM) for 30 min and fixed in 70% ethanol followed by 2 N HCl with 0.5% Triton X-100 for 30 min at 25°C, incubated in phosphate-buffered saline with 1% fetal bovine serum for 1 h at 25°C, and then probed with anti-BrdU monoclonal antibodies for 30 min at 25°C. Antigen-antibody complexes were detected by fluorescein isothiocyanate-conjugated sheep anti-mouse immunoglobulin G antibodies in a fluorescence-activated cell sorter analysis.
BrdU incorporation was also performed to examine cell cycle progression during cisplatin treatment. NIH 3T3 cells infected with Myc retrovirus were treated with 40 μM cisplatin for 24 h. They were then labeled with BrdU (1 μM) for 4 h and processed and analyzed as described above.
Reporter assays.
The Myc promoter-luciferase reporter construct pGL2M4-Luc was kindly provided by Robert N. Eisenman (Fred Hutchinson Cancer Research Center) and was described previously (38). Transfections were performed using SuperFect (Qiagen). At 48 h after transfection, the cells were lysed in 0.25 M Tris (pH 7.8), with three freeze-thaw cycles. Luciferase assays were carried out using the Promega luciferase assay kit. A Renilla luciferase plasmid (Promega) was included to control for transfection efficiency. Normalized values are reported as the mean and standard deviation from triplicate transfections.
Cellular transformation.
NIH 3T3 cells infected with Myc-expressing retrovirus were grown for 6 days and photographed under phase-contrast microscopy to view focus formation and under fluorescence microscopy to view enhanced green fluorescent protein (EGFP) coexpressed with Myc (71). NIH 3T3 cells expressing Pak2ER were also infected with MYC retrovirus. At 24 h after infection, 40 nM 4-hydroxyltamoxifen (4-HT) was added to induce Pak2 activity. The cells were grown for 6 days, and focus formation was photographed as above. The cells were fixed in 75% (vol/vol) ethanol for 12 h at 4°C and then stained in phosphate-buffered saline containing 1% fetal bovine serum, 5 μg of propidium iodide per ml, and 1 μg of RNase A per ml. The stained cells were subjected to fluorescence-activated cell sorter analysis. For measuring the activation of Pak2ER, cells were labeled in vivo with [32P]orthophosphate in the presence of 4-HT and immunoprecipitated with anti-HA monoclonal antibody 101R (Covance) as described above. The immunoprecipitated Pak2ER protein was subjected to SDS-PAGE followed by autoradiography and immunoblotting with polyclonal anti-Pak2 antibody and a polyclonal antibody 2601 (Cell Signaling Technology) against phospho-T402, an indicator of Pak2 activation (57).
Soft-agar assays were performed as described previously (15). One thousand cells were plated in 35-mm dishes containing 2 ml of medium incorporating 0.35% agar, which was overlaid onto 2 ml of solidified medium containing 0.7% agar. The medium used for soft-agar assays was DMEM-10% fetal calf serum in the presence of 100 nM 4-HT as necessary. The plates were incubated for 4 weeks at 37°C. Anchorage-independent growth was assessed by scoring the number of colonies larger than 125 μm in diameter.
Apoptosis.
NIH 3T3 cells expressing Pak2ER were infected with Myc retrovirus. Infected cells were maintained in serum-free medium in the presence or absence of 4-HT for 4 days and analyzed by photomicroscopy. The percentage of apoptotic cells was assessed by staining with 7-aminoactinomycin D (5 μg/ml) and flow cytometry.
Statistical analyses.
The statistical analyses were performed using Student's t test. A P value of ≤0.05 was considered to be significant. All of the data are reported as the means and standard errors.
RESULTS
Phosphorylation of Myc protein by Pak2 in vitro.
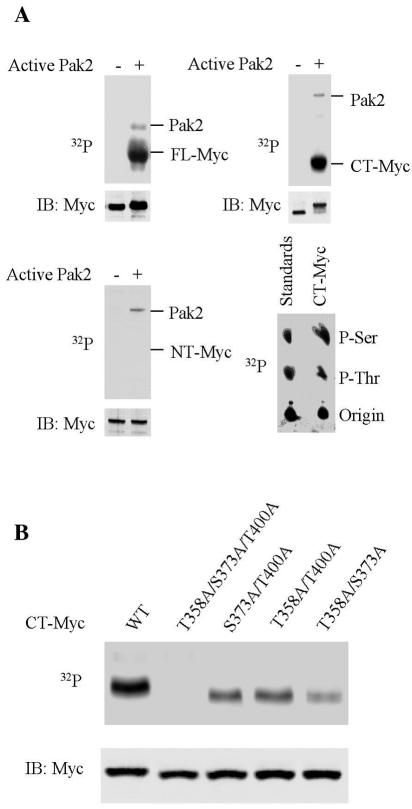
We first examined the ability of Pak2 to phosphorylate Myc protein in vitro. As a substrate, we used recombinant protein for FL-Myc, NT-Myc, or CT-Myc (Fig. 1). Recombinant Pak2 was activated in vitro by treatment with Cdc42(GTP). The activated enzyme phosphorylated both FL-Myc and CT-Myc protein at serine and threonine (Fig. 2A). The phosphorylation reduced the electrophoretic mobility of both Myc substrates. There was no apparent phosphorylation of NT-Myc by Pak2. Autophosphorylation of Pak2 was also evident.
FIG. 2.
Pak2 phosphorylates Myc in vitro. Enzymatic reactions were performed as described in Materials and Methods. Reaction products were fractionated by electrophoresis in polyacrylamide gels, followed by immunoblotting (IB) and autoradiography (32P). (A) Phosphorylation of Myc. Recombinant FL-Myc, CT-Myc, and NT-Myc were used as substrates (upper left, upper, right and bottom left panels, respectively). Analysis of phosphorylated amino acids is illustrated on the lower right. (B) Identification of phosphorylated residues. Various alleles of recombinant CT-Myc were used as substrates, as indicated. WT, wild type.
We identified the residues phosphorylated by the use of mutations that converted the three potential Pak2 sites in Myc to alanine (T358A, S373A, and T400A). In combination, the three mutations prevented the phosphorylation of CT-Myc by Pak2 (Fig. 2B). Each combination of two mutations partially reduced the phosphorylation. We can conclude that Myc is phosphorylated by Pak2 in vitro at T358, S373, and T400. In contrast, the two potential sites elsewhere in Myc were apparently not phosphorylated (Fig. 2A, lower left). We conclude that phosphorylation of Myc by Pak2 is restricted to the b/HLH/Z domain.
Phosphorylation of Myc by Pak2 in vivo.
We next examined the ability of Pak2 to phosphorylate Myc in vivo. The work was facilitated by the fact that overexpression of exogenous Pak2 in cells leads to activation of the enzyme (35). Pak2 and Myc proteins were coexpressed by transfection into 293T cells. The exogenous Myc was tagged with a poly(His) tag to facilitate its recovery from cellular extracts. Phosphorylation was detected by metabolic labeling with [32P]orthophosphate. The results revealed phosphorylation of both FL-Myc and CT-Myc (Fig. 3A). As before, the phosphorylation was also manifested by electrophoretic retardation of the Myc proteins in immunoblots; both forms of Myc were fully shifted in the gels, indicating that the bulk of the protein had been phosphorylated. The FL-Myc displayed a modest amount of phosphorylation even in the absence of exogenous Pak2. This background phosphorylation was barely detectable with CT-Myc and thus occurred mainly in a more amino-terminal region of Myc and was presumably due to kinases other than Pak2. There was some constitutive phosphorylation of endogenous Myc (Fig. 3A) as well, but additional phosphorylation by Pak2 was apparent in the appearance of a second radiolabeled isomorph with lower electrophoretic mobility.
FIG. 3.
Pak2 phosphorylates Myc in vivo. (A) Phosphorylation of Myc following overexpression of exogenous Pak2. Molecular clones of Pak2 and various alleles of MYC were transfected into 293T cells. At 24 h later, the cells were labeled with [32P]orthophosphate for 12 h and then harvested for analysis. Immunoprecipitation for endogenous or exogenously expressed Myc was performed and analyzed as described in Materials and Methods. Immunoblots for Myc protein are designated by IB, and autoradiograms for labeled Myc are designated by 32P. WT, wild type. The relative amount of radiolabeled Myc was determined by using ImageQuant (Molecular Dynamics). Immunoblotting was used to ascertain the relative mass of Myc precipitated. (B) Identification of the phosphorylated residues in Myc. In vivo phosphorylation of mutant CT-Myc was performed and analyzed as in panel A. (C) Activation of Pak2 by cellular stress. Exposure of 293T cells to stress and assay for Pak2 activity were performed as described in Materials and Methods. The activity of Pak2 was ascertained from the labeling of the substrate H4 and the relative mass of Pak2 in the reactions by immunoblotting (IB). Pak2-AID was expressed in 293T cells by transfection. AraC, cytosine arabinoside; CisP, cisplatin. (D) Phosphorylation of Myc by Pak2 in response to cellular stress. FL-Myc, CT-Myc, and Pak2-AID were expressed in 293T cells by transfection. The cells were exposed to stress as described in Materials and Methods and analyzed as in panel A. (E) Residues phosphorylated in Myc in response to cellular stress. Various alleles of CT-MYC were transfected into 293T cells. The cells were exposed to hyperosmolarity as described in Materials and Methods and analyzed as in panel A.
We used the alanine substitution mutants of MYC described above to identify the phosphorylated residues. In combination, the three mutations reduced the phosphorylation of both FL-Myc and CT-Myc to the background levels observed in the absence of Pak2 (Fig. 3A). In contrast, each of the three possible double mutants was phosphorylated (Fig. 3B). We conclude that all three potential target residues in CT-Myc were phosphorylated by Pak2 in vivo.
The preceding experiments relied on expression of Pak2 in considerable excess of the usual levels within the cell. To determine whether endogenous Pak2 could phosphorylate Myc, we exploited the response of the enzyme to stress (57). We found that Pak2 could be activated about fourfold above background in 293T cells by hyperosmolarity, cytosine arabinoside, or cisplatin (Fig. 3C). The enzymatic activity was detected with the substrate histone 4 (57) and could be inhibited to background by the exogenously expressed autoinhibitory domain of Pak2 (Pak2-AID), thus demonstrating the specificity of the activity.
Activation of Pak2 by the stress conditions led to the phosphorylation of endogenous Myc, as well as exogenously expressed FL-Myc and CT-Myc (Fig. 3D). The phosphorylation could be inhibited by Pak2-AID. Phosphorylation was again reflected in the electrophoretic shift of the target proteins. As anticipated, the triple-alanine mutant of CT-Myc was not phosphorylated in response to stress (Fig. 3E). Each combination of two mutations was phosphorylated under the stress condition, indicating again that all three sites were phosphorylated in vivo.
We conclude that activation of Pak2 in vivo leads to the phosphorylation of Myc. These findings raise the possibility that Pak2 can regulate both the biochemical and physiological actions of Myc. We pursued this possibility by examining the effect of phosphorylation by Pak2 on canonical properties of Myc, including its interaction with Max, its binding to DNA, its activation of transcription, and its effects on the cellular phenotype.
Phosphorylation by Pak2 inhibits the interaction of Myc with Max.
The participation of Myc in transcription requires that it dimerize with the protein Max (10, 50). The dimerization is facilitated by the HLH domain in Myc (10, 50), and substitution mutations in either the first or second helix abolish dimerization with Max (18). Two of the target residues for Pak2 in Myc reside within the helices: S373 in helix 1, and T400 in helix 2. It seemed reasonable to suspect that phosphorylation of these residues might affect the interaction of Myc with Max.
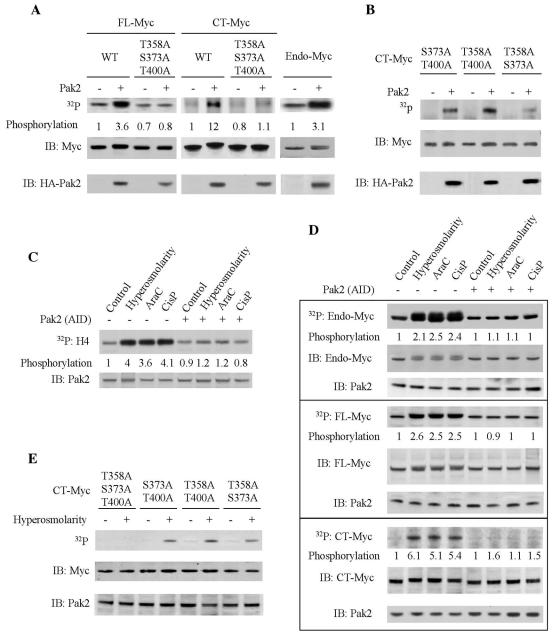
We first explored this possibility with a series of experiments in vitro, using coprecipitation as a measure of Myc-Max interaction. Phosphorylation of either FL-Myc or CT-Myc eliminated coprecipitation (Fig. 4A). Quantitative phosphorylation of the Myc proteins was again apparent as electrophoretic retardation. The inhibition by Pak2 could be prevented by combined alanine substitutions at the three target sites. To explore the effect of phosphorylation at the individual amino acid residues, we substituted aspartate at the sites, either individually or in combination, to mimic phosphorylation. The aspartate substitution mutants allowed the roles of individual sites to be examined. Substitutions at all three sites inhibited the Myc-Max interaction, as did a double mutation affecting only the substrate sites in helix 1 and helix 2 of Myc (S373D, T400D). S373D alone had a major effect on Myc-Max interaction, whereas T400D alone had no apparent effect. Aspartate substitution in the basic region (T358D) did not affect the Myc-Max interaction, in accord with previous reports that this region is not involved in Myc-Max interaction (10). The mimicry of phosphorylation extended to electrophoretic retardation of the mutant Myc proteins. We conclude that the aspartate substitutions apparently serve as surrogates for phosphorylation of Myc and can be used to examine the effects of phosphoryation at individual sites.
FIG. 4.
Phosphorylation by Pak2 inhibits the interaction of Myc with Max. (A) Analysis in vitro. Various forms of Myc were produced by translation in vitro. The ability of the Myc substrate to interact with Max after phosphorylation with activated Pak2 was evaluated as described in Materials and Methods. The relative amount of binding was determined by ImageQuant. Aliquots of cell lysates used in the binding assays were subjected directly to SDS-PAGE and immunoblotting for Myc. WT, wild type. (B) Analysis in vivo. The interaction between Myc and Max was evaluated by a two-hybrid assay with 293T cells, as described in Materials and Methods. Interaction was manifested as activation of a luciferase reporter. The expression of the Myc participant in the reaction was determined by immunoblotting. The data shown are the mean and standard deviation of three independent experiments (*, P < 0.005; **, P < 0.03). (C) Activation of Pak2 by stress blocks the interaction of Myc with Max in vivo. The stress conditions were applied to 293T cells as described in the legend to Fig. 3C. After stress treatment, endogenous Myc was immunoprecipitated from cellular extracts. The immunoprecipitates (IP) were examined for Myc and Max by immunoblotting (IB). Where required, the analyses were performed with cells expressing Pak2-AID that had been introduced by transfection. AraC, cytosine arabinoside; CisP, cisplatin.
Having demonstrated an effect of Pak2 on Myc-Max dimerization in vitro, we sought evidence that a similar effect occurs in vivo. We used a two-hybrid assay with 293T cells, in which interaction between FL-Myc and Max was manifested as activation of a luciferase reporter (Fig. 4B). Once again, a combination of the three aspartate substitutions at the Pak2 sites in Myc or the double mutation affecting only the residues in helix 1 and 2 fully blocked the interaction. Aspartate substitution for individual residues was partially effective at T400 and even more so at S373 but had no effect at T358.
We used stress to demonstrate directly that phosphorylation of Myc by Pak2 affects dimerization with Max in vivo. Stress imposed by hyperosmolarity, cytosine arabinoside, or cisplatin reduced the interaction between Max and Myc in 293T cells (Fig. 4C). The effect could be blocked by Pak2-AID. We conclude that phosphorylation of Myc by Pak2 can prevent the interaction with Max in vivo. The effect is mediated by phosphorylation at single residues within helix 1 and helix 2 of the Myc HLH domain.
Phosphorylation by Pak2 inhibits the binding of Myc-Max complexes to DNA.
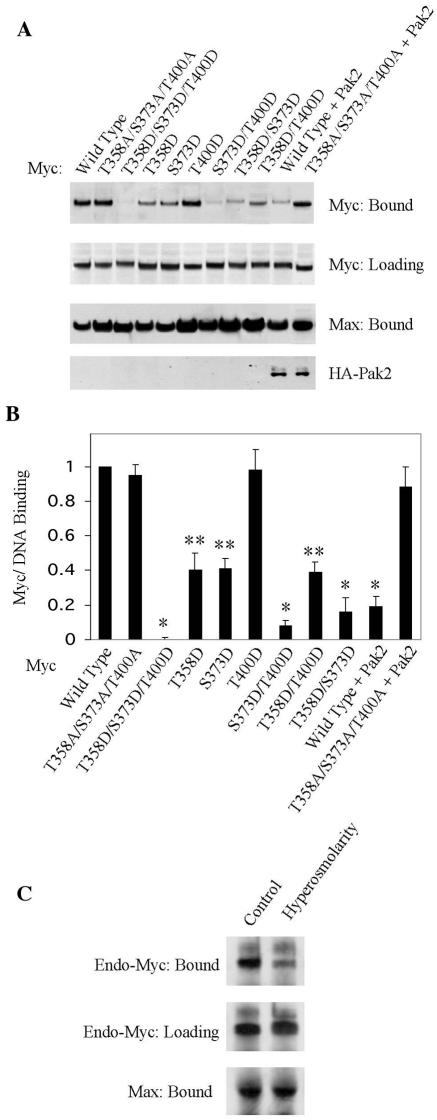
To assess the binding of Myc-Max dimers to DNA, we introduced biotinylated oligonucleotides representing the E-box into cellular extracts, precipitated the oligonucleotide, and examined the precipitate for Myc and Max (63). We prepared extracts from 293T cells expressing various exogenous alleles of MYC and examined the coprecipitation of the exogenous Myc and endogenous Max with E-box oligonucleotide. As anticipated, wild-type Myc bound to the E-box DNA in the presence of Max (Fig. 5). Alanine substitution mutations at the three Pak2 sites had no effect on the binding of Myc, whereas aspartate substitutions eliminated the binding. Aspartate substitution at either T358 or S373 partially reduced the binding, and the two in combination had an even greater effect. The T400D mutation alone or in combination with T358D had no effect, but it augmented the effect of S373D to the same extent as did T358D. In contrast, Max bound to the E-box DNA under all circumstances. We attribute this to the formation of Max-Max homodimers, which can bind DNA in the absence of Myc (36). We conclude that mimicry of phosphorylation at the Pak2 sites in Myc interferes with binding to DNA.
FIG. 5.
Phosphorylation inhibits the binding of Myc to DNA. Pak2 and various alleles of Myc were expressed by transfection into 293T cells. Analysis of cellular extracts for the binding of Myc and endogenous Max to E-box oligonucleotide was performed as described in Materials and Methods. (A) Coprecipitation of Myc and Max with biotinylated E-box oligonucleotide. Precipitated Myc and Max were detected by immunoblotting. Aliquots of cell lysates used in the binding assays were subjected to SDS-PAGE and immunoblotting for Myc. (B) Quantification of results. The histogram illustrates the mean and standard deviation of three independent experiments, quantified with ImageQuant (*, P < 0.005; **, P < 0.02). (C) Coprecipitation of endogenous Myc and Max with E-box DNA was detected by immunoblotting as in panel A.
To implicate Pak2 directly in an effect on DNA binding, we coexpressed exogenous Pak2 with alleles of MYC in 293T cells and performed the assay for binding to E-box DNA. The binding of wild-type Myc was greatly reduced, whereas Pak2 had no effect on the binding of a triple mutant that prevents phosphorylation at the Pak2 substrate sites (Fig. 5A and B). We also assessed the effect of Pak2 on endogenous Myc by using stress to activate the enzyme. The results again demonstrated a reduction in binding of Myc to DNA (Fig. 5C).
We conclude that phosphorylation of Myc by Pak2 impedes the binding of Myc-Max complexes to E-box DNA. Phosphorylation at each of the Pak2 sites can affect the binding, but the effect appears to be maximal only if all three sites are phosphorylated. At two of the sites (S373 and T400), the effect can be explained by the failure of dimerization between Myc and Max. However, phosphorylation at S358 has no effect on dimerization (see above), and so its effect on DNA binding may be more direct (see Discussion).
Phosphorylation by Pak2 reduces the ability of Myc to activate transcription.
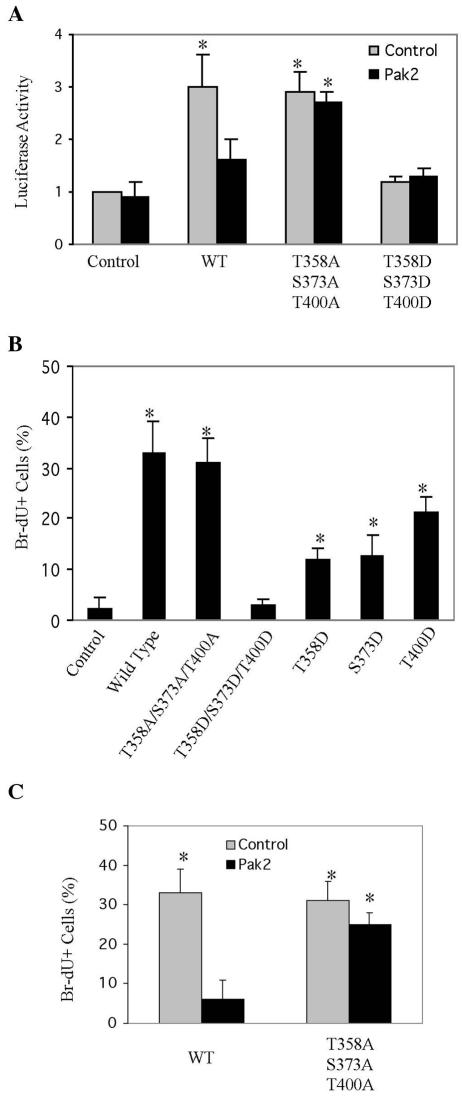
To assess the effect of phosphorylation by Pak2 on the activation of transcription by Myc, we performed luciferase reporter gene assays, using a promoter containing 4 E-boxes (38). As expected, expression of Myc induced a threefold increase in reporter gene transcription (Fig. 6A). The three alanine substitution mutations had no effect on the activation, whereas the three aspartate substitutions eliminated it. When coexpressed in the assay, Pak2 also inhibited activation of transcription by wild-type Myc but had no effect on the triple-alanine substitution mutant.
FIG. 6.
Phosphorylation by Pak2 reduces activation of transcription and stimulation of cellular proliferation by Myc. (A) Transactivation assay. Pak2 and various alleles of Myc were expressed by transfection into 293T cells, with a reporter plasmid as described previously (38). At 24 h later, the cells were harvested and assayed for luciferase activity as described in Materials and Methods. The histogram shows the mean and standard deviation of three independent experiments (*, P < 0.03). WT, wild type. (B) Effect on proliferation by mutations of Myc that mimic phosphorylation by Pak2. To evaluate the impact on cellular proliferation, NIH 3T3 cells expressing various mutant alleles of Myc were propagated to confluence, held for 48 h, and then labeled with BrdU for 30 min and analyzed as described in Materials and Methods. The mean and standard deviation of the percentage of BrdU-positive cells are shown (*, P < 0.04). (C) Effect of Pak2 on Myc-induced proliferation. NIH 3T3 cells expressing Pak2 and various alleles of Myc were treated and analyzed as in panel B. The mean and standard deviation of the percentage of BrdU-positive cells are shown (*, P < 0.01).
Phosphorylation by Pak2 inhibits the activation of cellular proliferation by Myc.
The action of Myc is required for progression of various cells through the division cycle, and, if expressed in excess, Myc can sustain the cycle under conditions that would otherwise not be permissive (2, 4, 34). We used the latter property of Myc to assess the physiological effects of phosphoryation by Pak2. When propagated to confluence and held there for 48 h, NIH 3T3 cells ceased to synthesize DNA (Fig. 6B). If the cells had been infected with a retrovirus expressing wild-type Myc, however, one-third of the population was labeled by a 30-min pulse with BrdU. Alanine substitutions at the three Pak2 sites in Myc had no effect on this stimulation of cellular proliferation. In contrast, combined aspartate substitutions at the three sites fully inhibited the stimulatory activity of Myc. Each of these substitutions in isolation partially suppressed stimulation, indicating that mimicry of phosphorylation on each site contributes to the inhibition of Myc in the proliferation assay.
The preceding experiments were predicated on the assumption that the aspartate substitutions in Pak2 sites act solely as surrogates of phosphorylation. We sought more direct evidence that phosphorylation by Pak2 can affect the physiological activity of Myc. Retroviral vectors were used to coexpress Pak2 and alleles of Myc in NIH 3T3 cells. The cells were then propagated to confluence, held for 48 h, and evaluated as before. As anticipated, exogenous FL-Myc alone sustained a substantial level of cellular proliferation (Fig. 6C). In contrast, coexpression of Pak2 with FL-Myc virtually eliminated the stimulatory activity of Myc. The triple alanine substitutions described above rendered Myc resistant to the effect of Pak2. We conclude that phosphorylation by Pak2 can control the stimulatory action of Myc in the cell cycle.
Phosphorylation by Pak2 inhibits cellular transformation by Myc.
Myc was first encountered as a cellular progenitor of a retroviral oncogene (72) and has since been implicated in the genesis of diverse human tumors (16). Accordingly, overexpression of Myc can transform certain established lines of cells in tissue culture (8, 24, 66). We used transformation of NIH 3T3 cells as a means of assessing the effect of Pak2 on this property of Myc.
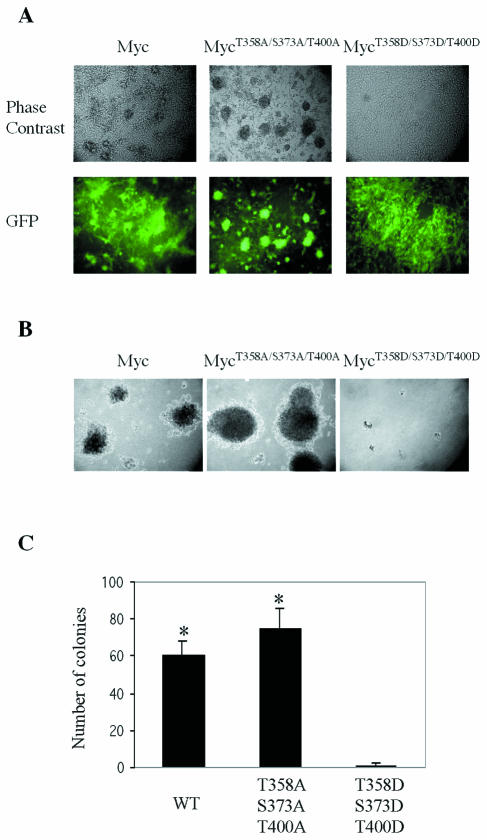
We first examined the effect of various alleles of MYC on the cells. FL-Myc gave rise to morphological transformation and anchorage-independent growth (Fig. 7A and B). Alanine substitutions at the three Pak2 sites in Myc had no effect on transformation, whereas the combination of aspartate substitutions at the three sites eliminated the transforming activity of Myc (Fig. 7).
FIG. 7.
Mutants that mimic phosphorylation by Pak2 reduce cellular transformation by Myc. Retroviral vectors were used to express wild-type and mutant alleles of Myc in NIH 3T3 cells. EGFP was coexpressed with Myc by using the pMIG retroviral vector as described in Materials and Methods. (A) Morphological transformation. Photomicroscopy was performed at a magnification of ×200. (B) Anchorage-independent growth. Assays with soft agar were performed as described in Materials and Methods. Photomicroscopy was performed at a magnification of ×40. (C) Quantification of anchorage-independent growth. Colonies were counted in 10 random fields for each specimen. The histograms represent the mean of three experiments, with error bars indicating standard deviation (*, P < 0.01). WT, wild type.
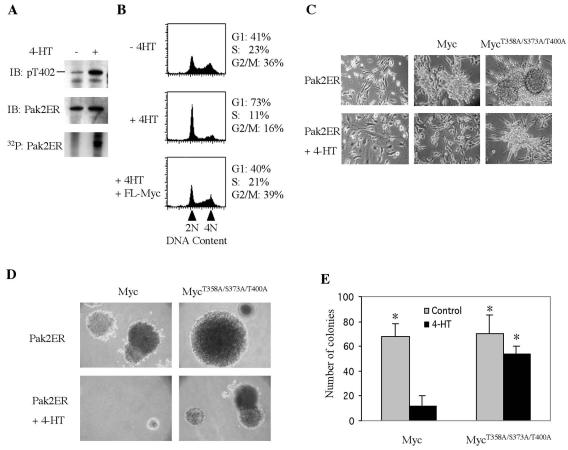
We then explored directly the effect of Pak2 on transformation by Myc. For this purpose, we fused Pak2 to the hormone binding domain of the human ER in an effort to obtain a conditional version of the enzyme (21). In the absence of the ligand tamoxifen, Pak2ER displayed no sign of the autophosphorylation that signifies activation of the enzyme (Fig. 8A): the protein could not be appreciably labeled with 32P and did not react with an antibody directed against phosphorylated T402, a crucial modification in Pak2 activation (13, 57). In the presence of tamoxifen, however, both parameters indicated that Pak2 had been activated.
FIG. 8.
Phosphorylation by Pak2 reduces cellular transformation by Myc. Retroviral vectors were used to express the Pak2ER construct and various alleles of Myc in NIH 3T3 cells. The enzymatic activity of the Pak2ER protein was elicited by exposure of the cells to 4-HT, as described in Materials and Methods. (A) Induction of enzymatic activity by 4-HT. Cells were incubated with 4-HT for 2 h, then labeled with [32P]orthophosphate for 12 h, and harvested for analysis by immunoprecipitation of the Pak2ER protein. The precipitates were analyzed by immunoblotting (IB) for phosphorylation of T402 in Pak2 and for the total mass of Pak2ER protein. Labeling of the protein by 32P was detected by autoradiography. (B) Induction of Pak2 activity arrests cells in G1. Cells were exposed to 4-HT for 2 days and then analyzed by fluorescence-activated cell sorting after labeling with propidium iodide, gating on Myc-positive cells with GFP as the indicative parameter. (C) Induction of Pak2 activity reduces morphological transformation by Myc. Cells were exposed to 4-HT for 6 days and then subjected to photomicroscopy at a magnification of ×200. (D) Induction of Pak2 reduces anchorage-independent growth elicited by Myc. Colonies in soft agar were photographed at a magnification of ×40 after 21 days in either the absence or presence of 4-HT. (E) Quantification of anchorage-independent growth. Colonies were counted in 10 random fields. The histograms represent the mean from three experiments, with error bars indicating standard deviation (*, P < 0.02).
Having demonstrated the conditional nature of Pak2ER, we used the construct to assess the effect of Pak2 in vivo. Activation of Pak2ER in NIH 3T3 cells caused the large majority of cells to arrest in G1 (Fig. 8B). The Pak2-induced arrest could be prevented by coexpression of FL-Myc, presumably by overwhelming the activity of Pak2 with a surfeit of target. Similarly, activation of Pak2ER reduced both morphological transformation and anchorage-independent growth elicited by overexpression of Myc (Fig. 8C to E). Combined alanine substitutions at the three Pak2 sites in Myc at least partially blocked the effect of Pak2 (Fig. 8E). These findings support our conclusion that phosphorylation by Pak2 can regulate the activity of Myc in vivo, both acutely and in a sustained manner. The effect on cellular transformation by Myc raises the possibility that Pak2 might serve as a tumor suppressor gene (see Discussion).
Phosphorylation by Pak2 inhibits apoptosis by Myc.
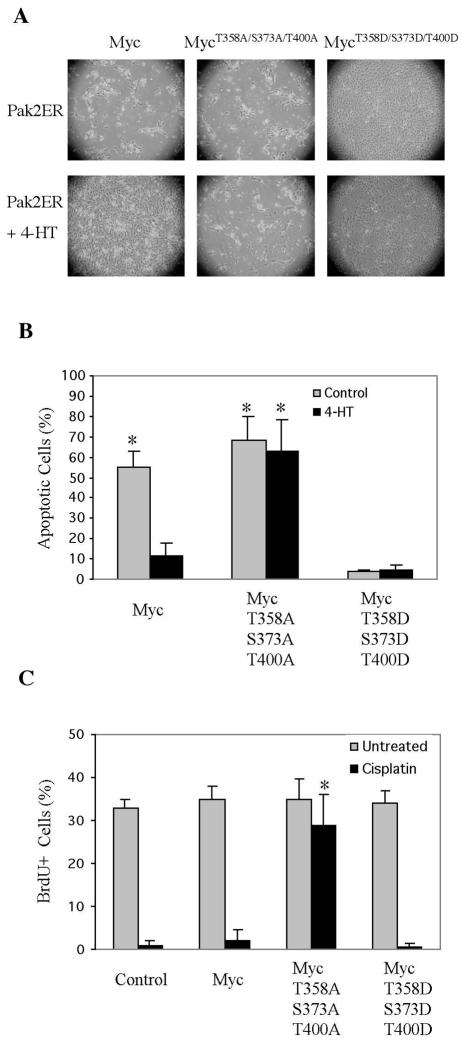
Sustained expression of Myc induces apoptosis in fibroblasts deprived of serum (23). We explored the ability of Pak2 to inhibit this effect of Myc. Various alleles of MYC were introduced into NIH 3T3 cells that were already expressing Pak2ER. In the absence of 4-HT, cells expressing FL-Myc became apoptotic following withdrawal of serum (Fig. 9A and B). Activation of Pak2 with 4-HT prevented this response, as did aspartate mutations at the three Pak2 sites in Myc. Myc with alanine substitutions at the three Pak2 sites induced apoptosis irrespective of whether Pak2 had been activated. We conclude that phosphorylation of Myc by Pak2 inhibits the ability of Myc to foster apoptosis.
FIG. 9.
Activation of Pak2 reduces apoptosis induced by Myc. (A) NIH 3T3 cells expressing Pak2ER were infected with a retroviral vector expressing various alleles of MYC. At 24 h after infection, cells were treated for 12 h with 4-HT. They were then kept in serum-free medium with 4-HT for 4 days. Induction of Pak2 activity reduced apoptosis induced by Myc. Photomicroscopy was performed at a magnification of ×40. (B) Quantification of apoptotic cells in panel A. Apoptotic cells were labeled by 7-aminoactinomycin D staining and analyzed by flow cytometry. The histograms represent the mean of three experiments, with error bars indicating standard deviation (*, P < 0.01). (C) A Myc mutant that cannot be phosphorylated by Pak2 drives cell cycle progression under stress. NIH 3T3 cells expressing various alleles of Myc were treated for 24 h with cisplatin, labeled with BrdU for 30 min, and analyzed as described in Materials and Methods. The histograms represent averages from three experiments, with error bars indicating standard deviation (*, P < 0.01).
Phosphorylation of Myc by Pak2 is required for cell cycle arrest in response to cellular stress.
The ability of phosphorylation by Pak2 to inhibit the activity of Myc raises the possibility that such inhibition is required to allow cells to enter a quiescent state in response to stress. We explored this possibility with the use of cisplatin, which causes vertebrate cells to arrest in the G1 phase of the cell cycle (31). Treatment of NIH 3T3 cells with cisplatin for 24 h caused complete cessation of DNA synthesis, as measured by incorporation of BrdU (Fig. 9C). The cells had become quiescent but did not undergo apopotosis during the 24-h course of the experiment (data not shown). Ectopic expression of either wild-type Myc or Myc carrying aspartate substitutions at the three Pak2 target sites could not rescue the cells from arrest. In contrast, Myc that cannot be phosphorylated by Pak2 because of alanine substitutions at the Pak2 target sites rendered the cells almost completely resistant to cisplatin-induced arrest. We conclude that inactivation of Myc by Pak2 is essential to the initial physiological response of cells to a characteristic form of stress.
DISCUSSION
Regulation of MYC and its protein product.
The expression of MYC is regulated at multiple points. These include the initiation and elongation of transcription (19, 44, 68), the stability of mRNA (19, 68), and the turnover of Myc protein (30, 54). The activity of Myc can also be controlled by direct modification of the protein. For example, phosphorylation of particular residues in the amino-terminal half of the protein modulates the activation of transcription from target genes (29, 64) and cellular transformation by MYC (32, 42, 53).
The carboxyl-terminal half of Myc also contains multiple sites of phosphorylation (41, 43), but less is known about the functional consequences of these modifications. A number of these phosphorylations have been attributed to protein kinase CKII (41). However, none have been implicated in functional changes, even though several lie close to the basic region that is vital to the binding of Myc to DNA (69). There are hints, however, that phosphorylation of Myc can affect functions that involve the b/HLH/Z domain. First, the binding of Myc to DNA declines during mitosis, a change that has been attributed to Myc phosphorylation at unidentified sites (40). Second, phorbol ester and gamma interferon may affect the phosphorylation of Myc at unidentified sites and, concomitantly, reduce its ability to bind DNA (7). Here we provide the first direct demonstration of phosphorylations that affect the function of the b/HLH/Z domain and, accordingly, can regulate the biochemical and biological activities of Myc.
Phosphorylation of Myc by Pak2.
The canonical site for phosphorylation by Pak2 occurs at three positions in Myc (T358, S373, and T400). They lie within the b/HLH/Z domain and are phosphorylated by Pak2 but not by the closely related enzyme, Pak1 (data not shown). Inactive Pak2 is located in the cytoplasm (35, 56), whereas Myc is a nuclear protein (3). However, the two proteins encounter one another because they can be coimmunoprecipitated from cellular extracts (data not shown). We suggest that the encounter occurs when Pak2 translocates to the endoplasmic reticulum following activation by stress (35, 44a), placing it in the vicinity of nascent Myc. Although only a small fraction of Myc (about 5% of the total Myc protein) was coimmunoprecipitated with Pak2 (data not shown), the fast turnover of Myc would allow the phosphorylated population to replace the unphosphorylated population rapidly. We think that the phosphorylations by Pak2 are physiologically significant because they occur in vivo following activation of Pak2 by cellular stress, the principal context in which the enzyme is known to function (57).
We used two sorts of mutations to explore the phosphorylation of Myc by Pak2. The first involved substitutions of alanine at the target sites to preclude phosphorylation at those positions. These mutations allowed us to demonstrate that phosphorylation occurs at each of the three candidate sites in the b/HLH/Z domain, both in vitro and in vivo. The second sort of mutations involved aspartate substitutions, intended to imitate the structural effects of phosphorylation. We were able to show that these mutations do indeed replicate the effect of phosphorylation at each of the target sites. The authenticity of the mimicry in turn allowed us to assess the specificity and relative magnitude of the effect of phosphorylation at each of the target sites.
Molecular structure and the control of Myc function by phosphorylation.
The b/HLH/Z domain is responsible for the ability of Myc to bind at specific sites in DNA (51). For that binding to occur, Myc must first heterodimerize with Max (10, 11, 50). The dimers form between HLH/Z domains in the two proteins and display the basic region of Myc in a manner that permits binding to DNA (10, 11, 50). The effects of phosphorylation by Pak2 are in accord with the location of the target sites within the three-dimensional structure of Myc (45). S373 is located in the first helix of the HLH domain, and T400 is located in the second (45). Accordingly, phosphorylation of either site reduces dimerization with Max. The effect of phosphorylation at S373 is much stronger than that at T400 when assessed in vivo, but the combination of the two is required to completely eliminate dimerization. The crippling of dimerization by phosphorylation is presumably due to interference with the hydrophobic interactions between the helices of Myc and Max (45). The T400 residue is in a more flexible region of the molecule than is S373. The flexibility may allow compensation for the impact of phosphorylation at T400, which might account for the lesser effect on dimerization. It has been reported that point mutations in the leucine zipper of Myc lead to homodimerization of the protein (67). However, we have not observed this effect with the substitution mutants used in the present study (data not shown).
T358 lies in the middle of the basic region. Phosphorylation of this residue has no effect on dimerization with Max, as might be expected, but appreciably reduces the binding of Myc to DNA. Residue 358 does not make direct contact with the DNA binding site. However, the adjacent amino acid residues do make contact, with K355 and R356 interacting with the phosphate backbone and H359 interacting with the N-7 constituent of a guanine in the E-box duplex (45). Hence, it is easy to imagine that phosphorylation of T358 could interfere with binding to DNA by means of charge repulsion. A previous report that mutation of R357 to threonine greatly reduces the transforming ability of MYC further dramatizes the critical nature of the amino acid identity and configuration in this portion of Myc (48).
The phosphorylation of three sites in the Myc b/HLH/Z domain by Pak2 works cooperatively. Dimerization with Max is eliminated completely only by the combined phosphorylation of S373 and T400. Binding to DNA, in turn, is fully prevented only by the combined phosphorylation of all three sites. The residual binding that occurs with the double mutant S373D/ T400D, which blocks dimerization, can be attributed to the limited ability of Myc to bind DNA unilaterally (9), which would be impeded by phosphorylation at T358. Cooperativity of this sort might facilitate fine-tuning of the control over Myc by Pak2, either alone or in combination with other protein kinases with target sites elsewhere in Myc.
Myc in the response to cellular stress.
Pak2 apparently plays a vital role in the cellular response to diverse forms of stress (57). In all likelihood, that role would be best served by pleiotropism on the part of the proteins on which Pak2 acts. The stressed cell is arrested in its growth and division cycle, which could conserve metabolic resources and avoid genetic damage. Survival of the cell would also require neutralization of programmed cell death that might be triggered by any of several consequences of stress, including genomic damage.
Myc is an excellent example of these expectations. The protein is exceptionally pleiotropic, participating in the transcriptional control of numerous genes with diverse functions (16, 25, 39). Inactivating Myc diminishes the call on metabolic resources by shutting down the genetic program required for cell growth (4); halts the cell division cycle, much as if a checkpoint control had been activated; and disables at least one effector of apoptosis, in the form of Myc itself. Accordingly, cells expressing wild-type Myc undergo growth arrest on cisplatin treatment, whereas those expressing the Ala substitution mutant, which cannot be phosphorylated by Pak2, still grow, as shown by BrdU incorporation. In essence, the repression of Myc by Pak2 leads to a state in some ways analogous to sporulation by yeast and bacteria—a state of quiescence or idling, designed to weather a period of stress.
Pak2 as a potential tumor suppressor protein.
Overexpression of Myc can transform a variety of cells in culture (14) and occurs widely among human tumors (19, 44, 68). The ability of Pak2 to repress the functions of Myc that lead to cellular transformation raises the possibility that Pak2 can serve as a tumor suppressor. The Pak2 gene resides at a chromosomal location (3q29) that is frequently affected by rearrangements in hematological malignancies, such as chronic myeloid leukemia and B-cell lymphoma, and in cutaneous neuroendocrine tumors (20, 37, 49, 65). It is also provocative that Pak2 is among the genes subject to repression by Myc in its role as a transcription factor (28). If overexpressed, Myc would have an advantage in its regulatory interplay with Pak2. These considerations suggest that it might be profitable to examine human tumors more closely for evidence that Pak2 is indeed a tumor suppressor protein.
Acknowledgments
We thank Peter Hwang and Kevan M. Shokat for helpful discussion and Anthony L. Defranco and David O. Morgan for critical reading of the manuscript. We also thank Polygena T. Tuazon for initial work on Myc phosphorylation by Pak2.
This work was supported by National Institutes of Health grant CA44338 (to J.M.B.) and the G. W. Hooper Research Foundation.
REFERENCES
- 1.Alarcon-Vargas, D., W. P. Tansey, and Z. Ronai. 2002. Regulation of c-myc stability by selective stress conditions and by MEKK1 requires aa 127-189 of c-myc. Oncogene 21:4384-4391. [DOI] [PubMed] [Google Scholar]
- 2.Alexandrow, M. G., M. Kawabata, M. Aakre, and H. L. Moses. 1995. Overexpression of the c-Myc oncoprotein blocks the growth-inhibitory response but is required for the mitogenic effects of transforming growth factor beta 1. Proc. Natl. Acad. Sci. USA 92:3239-3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alitalo, K., G. Ramsay, J. M. Bishop, S. O. Pfeifer, W. W. Colby, and A. D. Levinson. 1983. Identification of nuclear proteins encoded by viral and cellular myc oncogenes. Nature 306:274-277. [DOI] [PubMed] [Google Scholar]
- 4.Amati, B., K. Alevizopoulos, and J. Vlach. 1998. Myc and the cell cycle. Front. Biosci. 3:D250-D268. [DOI] [PubMed] [Google Scholar]
- 5.Axelson, H., M. Henriksson, Y. Wang, K. P. Magnusson, and G. Klein. 1995. The amino-terminal phosphorylation sites of C-MYC are frequently mutated in Burkitt's lymphoma lines but not in mouse plasmacytomas and rat immunocytomas. Eur. J. Cancer 31A:2099-2104. [DOI] [PubMed] [Google Scholar]
- 6.Bahram, F., N. von der Lehr, C. Cetinkaya, and L. G. Larsson. 2000. c-Myc hot spot mutations in lymphomas result in inefficient ubiquitination and decreased proteasome-mediated turnover. Blood 95:2104-2110. [PubMed] [Google Scholar]
- 7.Bahram, F., S. Wu, F. Oberg, B. Luscher, and L. G. Larsson. 1999. Posttranslational regulation of Myc function in response to phorbol ester/interferon-gamma-induced differentiation of v-Myc-transformed U-937 monoblasts. Blood 93:3900-3912. [PubMed] [Google Scholar]
- 8.Bechade, C., G. Calothy, B. Pessac, P. Martin, J. Coll, F. Denhez, S. Saule, J. Ghysdael, and D. Stehelin. 1985. Induction of proliferation or transformation of neuroretina cells by the mil and myc viral oncogenes. Nature 316:559-562. [DOI] [PubMed] [Google Scholar]
- 9.Blackwell, T. K., L. Kretzner, E. M. Blackwood, R. N. Eisenman, and H. Weintraub. 1990. Sequence-specific DNA binding by the c-Myc protein. Science 250:1149-1151. [DOI] [PubMed] [Google Scholar]
- 10.Blackwood, E. M., and R. N. Eisenman. 1991. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science 251:1211-1217. [DOI] [PubMed] [Google Scholar]
- 11.Blackwood, E. M., B. Luscher, and R. N. Eisenman. 1992. Myc and Max associate in vivo. Genes Dev. 6:71-80. [DOI] [PubMed] [Google Scholar]
- 12.Chang, D. W., G. F. Claassen, S. R. Hann, and M. D. Cole. 2000. The c-Myc transactivation domain is a direct modulator of apoptotic versus proliferative signals. Mol. Cell. Biol. 20:4309-4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chong, C., L. Tan, L. Lim, and E. Manser. 2001. The mechanism of PAK activation. Autophosphorylation events in both regulatory and kinase domains control activity. J. Biol. Chem. 276:17347-17353. [DOI] [PubMed] [Google Scholar]
- 14.Claassen, G. F., and S. R. Hann. 1999. Myc-mediated transformation: the repression connection. Oncogene 18:2925-2933. [DOI] [PubMed] [Google Scholar]
- 15.Clark, G. J., A. D. Cox, S. M. Graham, and C. J. Der. 1995. Biological assays for Ras transformation. Methods Enzymol. 255:395-412. [DOI] [PubMed] [Google Scholar]
- 16.Dang, C. V. 1999. c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol. Cell. Biol. 19:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dang, C. V., L. M. Resar, E. Emison, S. Kim, Q. Li, J. E. Prescott, D. Wonsey, and K. Zeller. 1999. Function of the c-Myc oncogenic transcription factor. Exp. Cell Res. 253:63-77. [DOI] [PubMed] [Google Scholar]
- 18.Davis, L. J., and T. D. Halazonetis. 1993. Both the helix-loop-helix and the leucine zipper motifs of c-Myc contribute to its dimerization specificity with Max. Oncogene 8:125-132. [PubMed] [Google Scholar]
- 19.DePinho, R. A., N. Schreiber-Agus, and F. W. Alt. 1991. myc family oncogenes in the development of normal and neoplastic cells. Adv. Cancer Res. 57:1-46. [DOI] [PubMed] [Google Scholar]
- 20.Dierlamm, J., C. Rosenberg, M. Stul, S. Pittaluga, I. Wlodarska, L. Michaux, M. Dehaen, G. Verhoef, J. Thomas, W. de Kelver, T. Bakker-Schut, J. J. Cassiman, A. K. Raap, C. De Wolf-Peeters, H. Van den Berghe, and A. Hagemeijer. 1997. Characteristic pattern of chromosomal gains and losses in marginal zone B cell lymphoma detected by comparative genomic hybridization. Leukemia 11:747-758. [DOI] [PubMed] [Google Scholar]
- 21.Eilers, M., D. Picard, K. R. Yamamoto, and J. M. Bishop. 1989. Chimaeras of myc oncoprotein and steroid receptors cause hormone-dependent transformation of cells. Nature 340:66-68. [DOI] [PubMed] [Google Scholar]
- 22.Evan, G. I., G. K. Lewis, G. Ramsay, and J. M. Bishop. 1985. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol. Cell. Biol. 5:3610-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evan, G. I., A. H. Wyllie, C. S. Gilbert, T. D. Littlewood, H. Land, M. Brooks, C. M. Waters, L. Z. Penn, and D. C. Hancock. 1992. Induction of apoptosis in fibroblasts by c-myc protein. Cell 69:119-128. [DOI] [PubMed] [Google Scholar]
- 24.Falcone, G., I. C. Summerhayes, H. Paterson, C. J. Marshall, and A. Hall. 1987. Partial transformation of mouse fibroblastic and epithelial cell lines with the v-myc oncogene. Exp. Cell Res. 168:273-284. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez, P. C., S. R. Frank, L. Wang, M. Schroeder, S. Liu, J. Greene, A. Cocito, and B. Amati. 2003. Genomic targets of the human c-Myc protein. Genes Dev. 17:1115-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grandori, C., and R. N. Eisenman. 1997. Myc target genes. Trends Biochem. Sci. 22:177-181. [DOI] [PubMed] [Google Scholar]
- 27.Gregory, M. A., and S. R. Hann. 2000. c-Myc proteolysis by the ubiquitin-proteasome pathway: stabilization of c-Myc in Burkitt's lymphoma cells. Mol. Cell. Biol. 20:2423-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo, Q. M., R. L. Malek, S. Kim, C. Chiao, M. He, M. Ruffy, K. Sanka, N. H. Lee, C. V. Dang, and E. T. Liu. 2000. Identification of c-myc responsive genes using rat cDNA microarray. Cancer Res 60:5922-5928. [PubMed] [Google Scholar]
- 29.Gupta, S., A. Seth, and R. J. Davis. 1993. Transactivation of gene expression by Myc is inhibited by mutation at the phosphorylation sites Thr-58 and Ser-62. Proc. Natl. Acad. Sci. USA 90:3216-3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hann, S. R., and R. N. Eisenman. 1984. Proteins encoded by the human c-myc oncogene: differential expression in neoplastic cells. Mol. Cell. Biol. 4:2486-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harder, H. C., and B. Rosenberg. 1970. Inhibitory effects of anti-tumor platinum compounds on DNA, RNA and protein syntheses in mammalian cells in vitro. Int. J. Cancer 6:207-216. [DOI] [PubMed] [Google Scholar]
- 32.Henriksson, M., A. Bakardjiev, G. Klein, and B. Luscher. 1993. Phosphorylation sites mapping in the N-terminal domain of c-myc modulate its transforming potential. Oncogene 8:3199-3209. [PubMed] [Google Scholar]
- 33.Henriksson, M., and B. Luscher. 1996. Proteins of the Myc network: essential regulators of cell growth and differentiation. Adv. Cancer Res. 68:109-182. [DOI] [PubMed] [Google Scholar]
- 34.Hermeking, H., J. O. Funk, M. Reichert, J. W. Ellwart, and D. Eick. 1995. Abrogation of p53-induced cell cycle arrest by c-Myc: evidence for an inhibitor of p21WAF1/CIP1/SDI1. Oncogene 11:1409-1415. [PubMed] [Google Scholar]
- 35.Huang, Z., J. Ling, and J. A. Traugh. 2003. Localization of p21-activated protein kinase gamma-PAK/Pak2 in the endoplasmic reticulum is required for induction of cytostasis. J. Biol. Chem. 278:13101-13109. [DOI] [PubMed] [Google Scholar]
- 36.Kretzner, L., E. M. Blackwood, and R. N. Eisenman. 1992. Myc and Max proteins possess distinct transcriptional activities. Nature 359:426-429. [DOI] [PubMed] [Google Scholar]
- 37.Lafage-Pochitaloff, M., M. Courcoul, J. Simonetti, D. Sainty, N. Dastugue, A. Tabilio, A. Hagemeijer, and F. Birg. 1992. Expression of the ETS2 and transferrin receptor genes in Philadelphia-positive chronic myeloid leukemia patients with a reciprocal t(3;21). Genes Chromosomes Cancer 5:1-13. [DOI] [PubMed] [Google Scholar]
- 38.Laherty, C. D., W. M. Yang, J. M. Sun, J. R. Davie, E. Seto, and R. N. Eisenman. 1997. Histone deacetylases associated with the mSin3 corepressor mediate mad transcriptional repression. Cell 89:349-356. [DOI] [PubMed] [Google Scholar]
- 39.Li, Z., S. V. Calcar, C. Qu, W. K. Cavenee, M. Q. Zhang, and B. Ren. 2003. A global transcriptional regulatory role for c-Myc in Burkitt's lymphoma cells. Proc. Natl. Acad. Sci. USA 100:8164-8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luscher, B., and R. N. Eisenman. 1992. Mitosis-specific phosphorylation of the nuclear oncoproteins Myc and Myb. J. Cell Biol. 118:775-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luscher, B., E. A. Kuenzel, E. G. Krebs, and R. N. Eisenman. 1989. Myc oncoproteins are phosphorylated by casein kinase II. EMBO J. 8:1111-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lutterbach, B., and S. R. Hann. 1994. Hierarchical phosphorylation at N-terminal transformation-sensitive sites in c-Myc protein is regulated by mitogens and in mitosis. Mol. Cell. Biol. 14:5510-5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lutterbach, B., and S. R. Hann. 1997. Overexpression of c-Myc and cell immortalization alters c-Myc phosphorylation. Oncogene 14:967-975. [DOI] [PubMed] [Google Scholar]
- 44.Marcu, K. B., S. A. Bossone, and A. J. Patel. 1992. myc function and regulation. Annu. Rev. Biochem. 61:809-860. [DOI] [PubMed] [Google Scholar]
- 44a.Miah, S. M. S., K. Sada, P. T. Tuazon, J. Ling, K. Maeno, S. Kyo, X. Qu, Y. Tohyama, J. A. Traugh, and H. Yamamura. 2004. Activation of Syk protein tyrosine kinase in response to osmotic stress requires interaction with p21-activated protein kinase Pak2/γ-PAK. Mol. Cell. Biol. 24:71-83. [DOI] [PMC free article] [PubMed]
- 45.Nair, S. K., and S. K. Burley. 2003. X-ray structures of Myc-Max and Mad-Max recognizing DNA. Molecular bases of regulation by proto-oncogenic transcription factors. Cell 112:193-205. [DOI] [PubMed] [Google Scholar]
- 46.Nesbit, C. E., J. M. Tersak, and E. V. Prochownik. 1999. MYC oncogenes and human neoplastic disease. Oncogene 18:3004-3016. [DOI] [PubMed] [Google Scholar]
- 47.Noguchi, K., C. Kitanaka, H. Yamana, A. Kokubu, T. Mochizuki, and Y. Kuchino. 1999. Regulation of c-Myc through phosphorylation at Ser-62 and Ser-71 by c-Jun N-terminal kinase. J. Biol. Chem. 274:32580-32587. [DOI] [PubMed] [Google Scholar]
- 48.O'Hagan, R. C., N. Schreiber-Agus, K. Chen, G. David, J. A. Engelman, R. Schwab, L. Alland, C. Thomson, D. R. Ronning, J. C. Sacchettini, P. Meltzer, and R. A. DePinho. 2000. Gene-target recognition among members of the myc superfamily and implications for oncogenesis. Nat. Genet. 24:113-119. [DOI] [PubMed] [Google Scholar]
- 49.Perlman, E. J., J. A. Lumadue, A. L. Hawkins, K. Cohen, P. Colombani, and C. A. Griffin. 1995. Primary cutaneous neuroendocrine tumors. Diagnostic use of cytogenetic and MIC2 analysis. Cancer Genet. Cytogenet. 82:30-34. [DOI] [PubMed] [Google Scholar]
- 50.Prendergast, G. C., D. Lawe, and E. B. Ziff. 1991. Association of Myn, the murine homolog of Max, with c-Myc stimulates methylation-sensitive DNA binding and ras cotransformation. Cell 65:395-407. [DOI] [PubMed] [Google Scholar]
- 51.Prendergast, G. C., and E. B. Ziff. 1991. Methylation-sensitive sequence-specific DNA binding by the c-Myc basic region. Science 251:186-189. [DOI] [PubMed] [Google Scholar]
- 52.Prendergast, G. C., and E. B. Ziff. 1992. A new bind for Myc. Trends Genet. 8:91-96. [DOI] [PubMed] [Google Scholar]
- 53.Pulverer, B. J., C. Fisher, K. Vousden, T. Littlewood, G. Evan, and J. R. Woodgett. 1994. Site-specific modulation of c-Myc cotransformation by residues phosphorylated in vivo. Oncogene 9:59-70. [PubMed] [Google Scholar]
- 54.Rabbitts, P. H., A. Forster, M. A. Stinson, and T. H. Rabbitts. 1985. Truncation of exon 1 from the c-myc gene results in prolonged c-myc mRNA stability. EMBO J. 4:3727-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Refaeli, Y., L. Van Parijs, C. A. London, J. Tschopp, and A. K. Abbas. 1998. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity 8:615-623. [DOI] [PubMed] [Google Scholar]
- 56.Roig, J., Z. Huang, C. Lytle, and J. A. Traugh. 2000. p21-activated protein kinase gamma-PAK is translocated and activated in response to hyperosmolarity. Implication of Cdc42 and phosphoinositide 3-kinase in a two-step mechanism for gamma-PAK activation. J. Biol. Chem. 275:16933-16940. [DOI] [PubMed] [Google Scholar]
- 57.Roig, J., and J. A. Traugh. 2001. Cytostatic p21 G protein-activated protein kinase gamma-PAK. Vitam. Horm. 62:167-198. [DOI] [PubMed] [Google Scholar]
- 58.Roig, J., P. T. Tuazon, and J. A. Traugh. 2001. Cdc42-independent activation and translocation of the cytostatic p21-activated protein kinase gamma-PAK by sphingosine. FEBS Lett. 507:195-199. [DOI] [PubMed] [Google Scholar]
- 59.Rooney, R. D., P. T. Tuazon, W. E. Meek, E. J. Carroll, J. J. Hagen, E. L. Gump, C. A. Monnig, T. Lugo, and J. A. Traugh. 1996. Cleavage arrest of early frog embryos by the G protein-activated protein kinase PAK I. J. Biol. Chem. 271:21498-21504. [DOI] [PubMed] [Google Scholar]
- 60.Rudel, T., and G. M. Bokoch. 1997. Membrane and morphological changes in apoptotic cells regulated by caspase-mediated activation of PAK2. Science 276:1571-1574. [DOI] [PubMed] [Google Scholar]
- 61.Sakamuro, D., and G. C. Prendergast. 1999. New Myc-interacting proteins: a second Myc network emerges. Oncogene 18:2942-2954. [DOI] [PubMed] [Google Scholar]
- 62.Sears, R., F. Nuckolls, E. Haura, Y. Taya, K. Tamai, and J. R. Nevins. 2000. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 14:2501-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seoane, J., C. Pouponnot, P. Staller, M. Schader, M. Eilers, and J. Massague. 2001. TGFbeta influences Myc, Miz-1 and Smad to control the CDK inhibitor p15INK4b. Nat. Cell Biol. 3:400-408. [DOI] [PubMed] [Google Scholar]
- 64.Seth, A., E. Alvarez, S. Gupta, and R. J. Davis. 1991. A phosphorylation site located in the NH2-terminal domain of c-Myc increases transactivation of gene expression. J. Biol. Chem. 266:23521-23524. [PubMed] [Google Scholar]
- 65.Slavutsky, I., M. L. de Vinuesa, I. Larripa, J. Dupont, and S. B. de Salum. 1986. Translocation (2;3) in hematologic malignancies. Cancer Genet. Cytogenet. 21:335-342. [DOI] [PubMed] [Google Scholar]
- 66.Small, M. B., N. Hay, M. Schwab, and J. M. Bishop. 1987. Neoplastic transformation by the human gene N-myc. Mol. Cell. Biol. 7:1638-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soucek, L., M. Helmer-Citterich, A. Sacco, R. Jucker, G. Cesareni, and S. Nasi. 1998. Design and properties of a Myc derivative that efficiently homodimerizes. Oncogene 17:2463-2472. [DOI] [PubMed] [Google Scholar]
- 68.Spencer, C. A., and M. Groudine. 1991. Control of c-myc regulation in normal and neoplastic cells. Adv. Cancer Res 56:1-48. [DOI] [PubMed] [Google Scholar]
- 69.Street, A. J., E. Blackwood, B. Luscher, and R. N. Eisenman. 1990. Mutational analysis of the carboxy-terminal casein kinase II phosphorylation site in human c-myc. Curr. Top. Microbiol. Immunol. 166:251-258. [DOI] [PubMed] [Google Scholar]
- 70.Tuazon, P. T., W. C. Spanos, E. L. Gump, C. A. Monnig, and J. A. Traugh. 1997. Determinants for substrate phosphorylation by p21-activated protein kinase (gamma-PAK). Biochemistry 36:16059-16064. [DOI] [PubMed] [Google Scholar]
- 71.Van Parijs, L., Y. Refaeli, J. D. Lord, B. H. Nelson, A. K. Abbas, and D. Baltimore. 1999. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity 11:281-288. [DOI] [PubMed] [Google Scholar]
- 72.Vennstrom, B., D. Sheiness, J. Zabielski, and J. M. Bishop. 1982. Isolation and characterization of c-myc, a cellular homolog of the oncogene (v-myc) of avian myelocytomatosis virus strain 29. J. Virol. 42:773-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walter, B. N., Z. Huang, R. Jakobi, P. T. Tuazon, E. S. Alnemri, G. Litwack, and J. A. Traugh. 1998. Cleavage and activation of p21-activated protein kinase gamma-PAK by CPP32 (caspase 3). Effects of autophosphorylation on activity. J. Biol. Chem. 273:28733-28739. [DOI] [PubMed] [Google Scholar]