Abstract
Blood glutamate scavengers have been shown to effectively reduce blood glutamate concentrations and improve neurological outcome after traumatic brain injury and stroke in rats. This study investigates the efficacy of blood glutamate scavengers oxaloacetate and pyruvate in the treatment of subarachnoid hemorrhage (SAH) in rats. Isotonic saline, 250 mg/kg oxaloacetate, or 125 mg/kg pyruvate was injected intravenously in 60 rats, 60 minutes after induction of SAH at a rate of 0.1 ml/100 g/min for 30 minutes. There were 20 additional rats that were used as a sham-operated group. Blood samples were collected at baseline and 90 minutes after SAH. Neurological performance was assessed at 24 h after SAH. In half of the rats, glutamate concentrations in the cerebrospinal fluid were measured 24 h after SAH. For the remaining half, the blood brain barrier permeability in the frontal and parieto-occipital lobes was measured 48 h after SAH. Blood glutamate levels were reduced in rats treated with oxaloacetate or pyruvate at 90 minutes after SAH (p < 0.001). Cerebrospinal fluid glutamate was reduced in rats treated with pyruvate (p < 0.05). Neurological performance was significantly improved in rats treated with oxaloacetate (p < 0.05) or pyruvate (p < 0.01). The breakdown of the blood brain barrier was reduced in the frontal lobe in rats treated with pyruvate (p < 0.05) and in the parieto-occipital lobes in rats treated with either pyruvate (p < 0.01) or oxaloacetate (p < 0.01). This study demonstrates the effectiveness of blood glutamate scavengers oxaloacetate and pyruvate as a therapeutic neuroprotective strategy in a rat model of SAH.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-012-0129-6) contains supplementary material, which is available to authorized users.
Keywords: Glutamate, Neurological outcome, Scavengers, Subarachnoid hemorrhage, Oxaloacetate, Pyruvate
Introduction
Subarachnoid hemorrhage (SAH) is a common condition that is associated with significant mortality and morbidity. As many as 30,000 of North Americans suffer from SAH annually, accounting for 5 to 10 % of all stroke cases [1–3]. The mortality rate for SAH approaches 45 % at 30 days, and 10 to 15 % of cases are fatal before hospitalization [4–7]. At the time of the initial bleed, there is a critical reduction in cerebral blood flow as the regional intracranial pressure increases and approaches the systemic arterial pressure. The persistent lack of blood flow results in cerebral vasospasm and subsequent swelling of perivascular astrocytes, neuronal cells, and capillary endothelium [8]. Although a great deal of knowledge exists regarding the delayed effects of SAH, the pathophysiology of early brain injury has yet to be fully understood and essential early treatment remains to be a challenge [9].
There are several rat models of SAH, including endovascular perforation of the internal carotid artery [9–11], blood injection into pre-chiasmatic cistern [11], and blood injection into the cisterna magna [11–17]. The last model has 2 modifications: a single hemorrhagic model [16, 18] and a double hemorrhagic model [13, 19], which are intended to simulate the late complication of vasospasm commonly seen after SAH [13, 19]. All models have their own advantages and disadvantages [11, 12, 19]. We have chosen the single hemorrhagic model of SAH in this study due to its low mortality rate, simplicity, effectiveness, and high reproducibility.
There is a growing body of evidence suggesting that elevated glutamate levels in the interstitial fluid and cerebrospinal fluid (CSF) of the brain may play a significant role in the mechanism for various acute brain insults [20–23] and chronic disease states [24–26]. After many neurodegenerative processes, elevated glutamate concentrations in both CSF and blood have been shown to correlate with a worse neurological outcome [23, 27, 28].
The brain has several mechanisms by which excess glutamate is eliminated to prevent neurotoxicity. In addition to astroglial and neuronal membrane glutamate transporters, glutamate transporters are also present on the anti-luminal side of the brain capillary endothelial cells [29, 30]. These transporters provide an additional pathway for glutamate elimination from the brain via a brain-to–blood efflux of glutamate [29, 30–33].
Gottlieb et al. [34] demonstrated the rapid appearance of radiolabeled glutamate in blood after being injected into rat brain lateral ventricles. These authors [34] further showed that the rate of brain-to-blood efflux of glutamate could be increased by creating a larger concentration gradient between the brain extracellular fluid (ECF)/CSF and blood [34]. The efflux of glutamate into the blood resulted in increased concentrations of radiolabeled glutamate in the blood and its disappearance from the CSF [34].
Thus, one of the proposed mechanisms of reducing blood glutamate levels involved the metabolism of glutamate into its inactive form: α-ketoglutarate [34]. This naturally occurring enzymatic process is mediated by the activation of resident plasma enzymes glutamate-oxaloacetate transaminase (GOT) and glutamate-pyruvate transaminase in the presence of their respective co-enzymes oxaloacetate and pyruvate [34]. Reducing blood glutamate concentrations with blood glutamate scavengers creates a new, more favorable glutamate concentration gradient between the extracellular fluids and blood of the brain. This in turn facilitates the brain-to-blood glutamate efflux, thereby limiting the neurotoxic effects of glutamate. Treatments with blood glutamate scavengers oxaloacetate, pyruvate, GOT, and glutamate-pyruvate transaminase have been shown to effectively improve neurological recovery after traumatic brain injury (TBI) [35–38] and stroke [39–43], with a concomitant decrease in blood glutamate levels [30, 34].
The primary goal of this study was to investigate the efficacy of blood glutamate scavengers oxaloacetate and pyruvate in the treatment of SAH in rats. We further examined whether any neuroprotective effects of blood glutamate scavengers were mediated via a glutamate-scavenging mechanism.
Materials and Methods
The experiments were conducted in accordance with the recommendations of the Declarations of Helsinki and Tokyo and to the Guidelines for the Use of Experimental Animals of the European Community. The experiments were approved by the Animal Care Committee of Ben-Gurion University of the Negev, Israel.
Drugs and Doses
Oxaloacetate and pyruvate were purchased from Sigma Israel Chemicals (Rehovot, Israel). Drugs were stored at -30 ° C until their use and dissolved in isotonic saline immediately prior to intravenous administration of the animals. Doses of 250 mg/kg oxaloacetate or 125 mg/kg pyruvate were injected intravenously at a rate of 0.1 ml/min.
Animals
A total of 80 male Sprague-Dawley rats (Harlan Laboratories, Israel) were used in this experiment. Rats had no overt pathology and weighed between 300 and 350 g each. Rats were kept in cages, with 3 rats per cage for at least 3 days after arrival to allow adaptation. Purina Chow and water were available ad libitum.
Experimental Design
Under general anesthesia with 2 % isoflurane, baseline blood glutamate samples were drawn. Immediately afterward, SAH was inflicted in a manner described as follows. Rats were allowed to awaken after the procedure. At 60 minutes after the induction of SAH, the rats were re-anesthetized and received treatment according to their previously assigned experimental group. Saline, oxaloacetate, or pyruvate were injected intravenously at a rate of 0.1 ml/min for 30 minutes. The second blood sample was collected at 90 minutes after SAH (immediately after the cessation of treatment). Neurological performance was assessed at 24 h after SAH. At this point, the rats were divided into 2 subgroups. The first subgroup was used for the measurement of glutamate concentrations in CSF, collected under general anesthesia at 24 h after SAH (immediately after the neurological assessment). The second subgroup was used for the determination of the blood brain barrier (BBB) permeability at 48 hours after SAH.
Experimental Groups
Eighty rats were randomly divided into 1 of the 4 groups listed as follows. The number of animals in each group and subgroup is listed in Table 1. In group 1 (sham group), 0.3 ml of saline was injected into the cisterna magna of the rates. Unlike the remaining 3 groups in which autologous arterial blood was injected into the cisterna magna simulating SAH, this group did not undergo SAH induction. In group 2 (SAH control saline treatment group), rats were treated with isotonic saline at a rate of 0.1 ml/100 g/min for a duration of 30 minutes. In group 3 (SAH oxaloacetate treatment group), rats were treated with 250 mg/kg oxaloacetate at a rate of 0.1 ml/100 g/min for 30 minutes. In group 4 (SAH pyruvate treatment group), rats were treated with 125 mg/kg pyruvate at a rate of 0.1 ml/100 g/min for 30 minutes. The chosen doses of oxaloacetate and pyruvate were based on the available data on their blood glutamate-reducing activity in TBI and stroke previously shown in rats [36, 37, 44].
Table 1.
The number of animals in each experimental group and subgroup
| The total number of rats in each of the different groups | |||
|---|---|---|---|
| Animal Group | Total | NSS and CSF collection | Determination BBB breakdown |
| Sham-operated group | 20 | 10 | 10 |
| Control SAH | 20 | 10 | 10 |
| SAH and Oxal. 250 mg/kg | 20 | 10 | 10 |
| SAH and Pyr. 125 mg/kg | 20 | 10 | 10 |
BBB = blood brain barrier; CSF = cerebrospinal fluid; NSS = neurological severity score; Oxal. = oxaloacetate; Pyr. = pyruvate; SAH = subarachnoid hemorrhage
Induction of Subarachnoid Hemorrhage
SAH was induced by injecting autologous arterial blood into the cisterna magna, as previously described [14]. The procedure was performed together by 2 experimenters to reduce experimental error. Rats were anesthetized with a mixture of 2 % isoflurane in oxygen. The cranium was fixed in a stereotactic device, with the head flexed 90 degrees in relation to the cervical spine. The tail vein was cannulated using a Neoflon (Becton Dickinson, Helsingborg, Sweden) plastic cannula and 0.3 ml of blood was collected into an insulin syringe. Cisterna magna was cannulated via the atlanto-occipital membrane using a 26-guage needle, and proper position of the catheter was validated via the appearance of CSF in the small internal volume tubing connected to the needle. Immediately after cannulation, 0.3 ml of fresh autologous blood was injected into the cisterna magna for 15 seconds. After injection, the anesthesia was discontinued and rats were returned to their cages for recovery.
Determination of Neurological Performance
Severity of neurological injury was assessed at 24 h after the induction of SAH using the Feldman neurological severity score (NSS), previously and successfully used for neurological assessment after SAH [45]. Timing for the NSS assessment was based on observations that maximal neurological impairment is seen 24 h after SAH, becoming nonsignificant by 72 h [46]. The NSS of the rats was determined by 2 blinded observers, independent of one another Points were assigned for alterations of motor functions and behavior, such that the maximal score of 25 represents greatest neurological dysfunction, whereas a score of 0 indicates an intact neurological condition. Specifically, the following were assessed: ability to exit from a circle (3-point scale), gait on a wide surface (3-point scale), gait on a narrow surface (4-point scale), effort to remain on a narrow surface (2-point scale), reflexes (5-point scale), seeking behavior (2-point scale), beam walking (3-point scale), and beam balance (3-point scale).
Blood Sample Collection
Blood was collected from the tail vein for the determination of glutamate levels prior to the induction of SAH (as a baseline measurement) and immediately after the cessation of treatment (at 90 minutes after SAH induction) via a 24-guage Neoflon (Becton Dickinson, Helsingborg, Sweden) catheter. After the blood sample collection, the catheter was removed from the vein.
CSF Sample Collection
CSF was collected for glutamate measurement at 24 h after SAH, immediately after neurological assessment. Rats were anesthetized and the cisterna magna was cannulated as previously described. There was 0.1 to 0.2 ml of CSF gently aspirated. After CSF collection, rats were euthanized by deepening the anesthesia with isoflurane.
Determination of Blood Glutamate
Whole blood (200 μl aliquot) was de-proteinized by adding an equal volume of ice-cold 1 M perchloric acid and then centrifuging at 10,000 × g for 10 minutes at 4 ° C. The pellet was discarded and the supernatant was collected, adjusted to pH 7.2, with 2 M K2CO3, and stored at -80 ° C for later analysis, if needed [34]. Glutamate concentration was measured using the fluorometric method of Graham and Aprison [47]. A 60 μl aliquot from the perchloric acid supernatant was added to 90 μl of a 0.3 M glycine, 0.25 M hydrazine hydrate buffer adjusted to pH 8.6 with 1 M H2SO4 and containing 11.25 U of glutamate dehydrogenase in 10 mM Nicotinamide adenine dinucleotide (NAD). After incubation for 30 to 45 minutes at room temperature, the fluorescence was measured at 460 nm with excitation at 350 nm. A glutamate standard curve was established with concentrations ranging from 0 to 6 μM. All determinations were done at least in duplicates.
Determination of Glutamate Concentration in CSF
Fresh CSF (110 μl) was mixed with perchloric acid (25 μl) of 0.3 M, and then centrifuged at 10,000 × g for 10 minutes at 4 ° C. The pellet was discarded and the supernatant was collected, adjusted to pH 7.2 with 12.5 μl of 2 M K2CO3 and stored at -80 ° C for later analysis.
BBB Breakdown Evaluation Protocol
An increase in the permeability of the BBB has been shown to follow SAH and correlate with a worsened neurological outcome [48]. In the current study, the timing for determining BBB disruption was chosen based on the observation of a maximal disruption of BBB 48 h after SAH [48].
Evans Blue 2 % in saline (4 ml/kg) was administered intravenously through the cannulated tail vein as a blood-brain permeability tracer and was allowed to circulate for 60 minutes. To remove the intravascularly localized dye, the rats' chests were opened and the animals were perfused with cooled saline through the left ventricle at a pressure of 110 mm Hg until colorless perfusion fluid was obtained from the right atrium. The whole brain was removed, and measurements of vascular permeability were made by comparing its weight with pre-weighed loci in the frontal and parieto-occipital lobes.
Each brain area was weighted and homogenized in 1 ml of 50 % trichloroacetic acid (weight/volume), and was centrifuged at 10,000 × g for 20 minutes. One milliliter of the supernatant was added to 1.5 ml of the solvent (50 % trichloroacetic acid/96 % ethanol, 1:3). A fluorescence detector (model Infinite 200 PRO multimode reader; Tecan, Männedorf Switzerland) was used at an excitation wavelength of 620 nm (bandwidth 10 nm) and an emission wavelength of 680 nm (bandwidth 10 nm). Calculations were based on external standards in the solvent (10 ± 500 ng/ml). Data are expressed as mean ± SD (in mg/g of protein) of extravasated Evans Blue dye per gram of brain tissue.
Statistical Analysis
Statistical evaluation of the results was done with the SPSS 17 package (SPSS Inc., Chicago, IL). The significance of comparisons between groups (glutamate blood and CSF levels, BBB permeability, and NSS) was determined using the Kruskal-Wallis test followed by Mann–Whitney U test. Normally distributed data and continuous variables (glutamate concentrations and BBB breakdown) are presented as an average ± SEM. Nonparametric data was presented as a median ± inner quartile range. Results were considered statistically significant when p < 0.05, and highly significant when p < 0.01.
Results
Blood Glutamate Levels
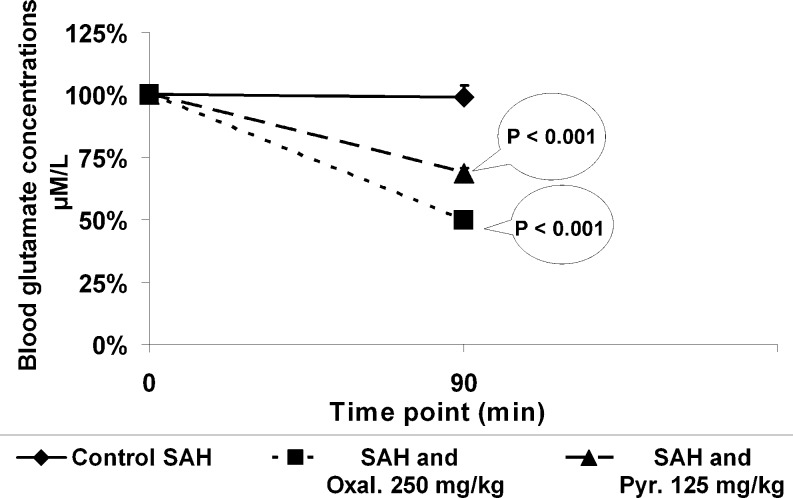
There was a significant decrease in blood glutamate levels compared to baseline in rats treated with oxaloacetate (by 50 %) or pyruvate (by 30 %) at 90 minutes after SAH (p < 0.001). In contrast, blood glutamate levels did not change in time with the rats treated with isotonic saline (Fig. 1).
Fig. 1.
Blood glutamate concentrations for a duration of time. There was a significant decrease in blood glutamate levels compared to baseline in rats treated with oxaloacetate or pyruvate at 90 minutes after subarachnoid hemorrhage (SAH) (p < 0.001). Blood glutamate levels did not change with a period of time in rats treated with isotonic saline
Concentration of Glutamate in CSF
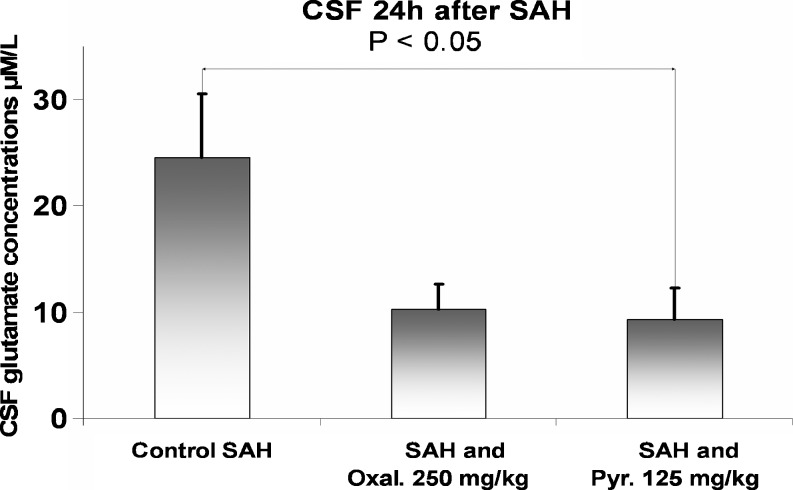
Concentrations of glutamate in CSF 24 h after SAH were significantly reduced in rats treated with pyruvate compared to rats treated with isotonic saline (p < 0.05) (Fig. 2). In rats treated with oxaloacetate, CSF glutamate levels were reduced, but they did not reach statistical significance (p = 0.06).
Fig. 2.
Concentrations of glutamate in cerebrospinal fluid (CSF) 24 h after subarachnoid hemorrhage (SAH). CSF glutamate levels were significantly reduced in rats treated with pyruvate 24 h after SAH compared with rats treated with isotonic saline (p < 0.05)
NSS
NSS was significantly improved in rats treated with oxaloacetate 1.5 (0 to 3.25) or pyruvate 0 (0 to 0) compared to rats treated with isotonic saline 9 (0 to 10) (p < 0.05 and p < 0.01, respectively) (Table 2).
Table 2.
Values for the NSS measured 24 H after SAH
| NSS values of the various groups at 24 h after SAH | ||
|---|---|---|
| Animal group | N | NSS 24 h after SAH (range) |
| Sham-operated group | 10 | 0 (0–0)** |
| Control SAH | 10 | 9 (0–10) |
| SAH and Oxal. 250 mg/kg | 10 | 1.5 (0–3.25)* |
| SAH and Pyr. 125 mg/kg | 10 | 0 (0–0)** |
NSS = neurological severity score; Oxal. = oxaloacetate; Pyr. = pyruvate; SAH = subarachnoid hemorrhage
BBB Breakdown
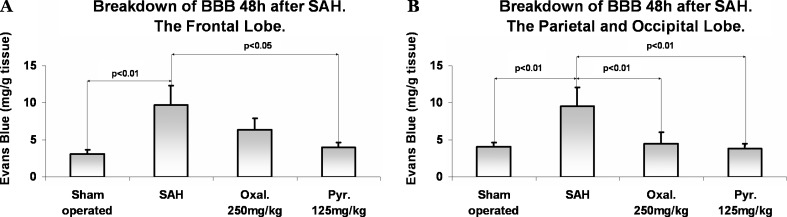
The breakdown of the BBB measured in the frontal lobe at 48 h after SAH was significantly reduced in rats treated with pyruvate compared to rats treated with isotonic saline (p < 0.05) (Fig. 3a). The breakdown of the BBB measured in the parietal and occipital lobes at 48 h after SAH was significantly reduced in rats treated with either pyruvate or oxaloacetate (p < 0.01) (Fig. 3b). As expected, there was an elevated BBB breakdown observed in the rats that underwent SAH induction and were given isotonic saline compared to the sham-operated group in the frontal, parietal, and occipital lobes (p < 0.01).
Fig. 3.
(a) Blood brain barrier (BBB) breakdown 48 h after subarachnoid hemorrhage (SAH) measured in the frontal lobe. The breakdown of the BBB measured in the frontal lobe at 48 h after SAH was significantly reduced in rats treated with pyruvate compared to rats treated with isotonic saline (p < 0.05). (b) BBB breakdown 48 h after SAH measured in the parietal and occipital lobes. The breakdown of the BBB measured in the parietal and occipital lobes at 48 h after SAH was significantly reduced in rats treated with either pyruvate (Pyr.) (p < 0.01) or oxaloacetate (p < 0.01). Data are expressed as mean ± SD (in mg/g of brain tissue) of extravasated Evans Blue dye per gram of tissue
Discussion
The principle finding of this study was that the intravenous administration of the blood glutamate scavengers oxaloacetate and pyruvate led to an improved neurological outcome in a rat model of SAH. Moreover, we demonstrated that the observed neuroprotective effects were mediated via a blood glutamate-scavenging mechanism, which in turn led to the reduction of CSF glutamate levels.
Many neurodegenerative disorders are associated with pathologically elevated ECF glutamate levels, including stroke [20], TBI [23], intracerebral hemorrhage [49], meningitis brain hypoxia [22], amyotrophic lateral sclerosis [50], glaucoma [51], human immunodeficiency virus dementia [52], glioma [53], and many other conditions. Preventing glutamate-induced neurotoxicity could potentially prevent neuronal death and improve neurological outcomes. Thus, much research has been focused on different approaches for limiting the neurotoxic effects of glutamate. Currently, investigated strategies include inhibiting glutamate synthesis, blocking its release from presynaptic terminals, antagonizing its actions on postsynaptic receptors, and accelerating its reuptake from the synaptic cleft.
Glutamate receptor antagonists have been previously shown to provide neuroprotection in animal models of ischemia [54]. Moreover, it has been shown that the N-Methyl-D-aspartic (NMDA) receptors antagonist, Felbamate, leads to an improved neurological performance and limits the BBB breakdown in the single-injection rat model of SAH [55]. This finding is consistent with the results of the current study. However, clinical trials using NMDA receptor antagonists after stroke and TBI have not lived up to the expectations from the experimental data in animals. Clinical results failed to provide any neurological benefit, and in some instances were shown to be harmful, leading to worsened neurological outcomes and increased mortality rates [56–58]. Maintaining a minimal glutamate level is known to be critical for normal neuronal function. NMDA receptor antagonists do not discriminate between the diverse actions of the receptor, and thus interfere with both the negative and positive effects of this signaling [58, 59]. Furthermore, NMDA antagonists affect glutamate transporters that reside in many extracerebral peripheral tissues, such as the pancreas, which play an important role in the metabolic regulation of glutamate [60–64].
The remaining lack of an effective treatment for these neurodegenerative conditions inspired the investigation of new treatment modalities, which would focus on eliminating excess toxic glutamate. This strategy used blood glutamate scavengers oxaloacetate, pyruvate, GOT, glutamate-pyruvate transaminase, estrogen, progesterone, and activation of β2-adrenergic agonists and different kinds of stress. Gottlieb et al. [34] showed that the rate of brain-to-blood efflux of glutamate could be increased by creating a larger concentration gradient between the brain ECF/CSF and the blood. The efflux of glutamate into the blood resulted in increased concentrations of radiolabeled glutamate in the blood and its disappearance from the CSF [34]. Magnetic resonance spectroscopy confirmed that blood glutamate scavengers oxaloacetate and GOT are capable of decreasing ECF glutamate concentrations via blood glutamate elimination in a rat model of experimental focal ischemia [41–43]. Teichberg [65] and Teichberg et al. [30] used microdialysis probes in rats to demonstrate that the artificial decrease of blood glutamate with blood glutamate scavengers decreases the concentration of glutamate in rats’ ECF [30, 65]. Campos et al. further showed that lower blood glutamate levels and higher levels of GOT were associated with better neurological outcome in patients after ischemic stroke [41–43].
Considering the close relationship between low blood and CSF glutamate levels, the results of the current study suggest that oxaloacetate and pyruvate provide their neuroprotective properties via a glutamate-scavenging mechanism. Thus, the data available to date collectively support the idea that blood glutamate scavengers promote their neuroprotective properties via a universal mechanism for each of the different pathological brain conditions previously mentioned. Therefore, the data is promising for examining blood glutamate scavengers as a possible new therapeutic strategy for a wide spectrum of conditions in which elevated brain glutamate levels play a principal role in the pathogenesis.
The chosen parameters for the assessment of the neurological outcome in this study were based on previous studies in which SAH was similarly induced by the injection of autologous blood into the cisterna magna [48]. Numerous parameters have been previously used to measure neurological damage after SAH in rats, including but not limited to examining mortality rats, behavioral and motor scores, neuronal count, brain edema, and BBB permeability. As with many studies in the past, we explored the BBB breakdown as an indicator for brain injury after SAH. An increase in the permeability of the BBB has been shown to follow SAH in both human [66] and experimental settings [67–70], and it correlates with a worsened neurological outcome [48, 67–70]. The BBB breakdown develops even in the acute stage of SAH [66, 67], and the indicator has been shown to be independent of influences, such as increased intracranial pressure or brain edema that may disrupt the BBB itself [48]. On the contrary, histological examination, including neuronal count, is usually used in models of SAH, which was induced by endovascular perforation of the internal carotid artery and is very rarely used in a model of SAH induced by the injection of blood into the cisterna magna [46]. In contrast to endovascular perforation, induction of SAH by the injection of blood into the cisterna magna is associated with neurological damage that is much more subtle. While developing the protocol of this study, we measured brain water content in small pilot groups of ~5 animals per group, and did not find any significant difference in brain edema between the sham and SAH control group. This finding was in line with existing literature, thus we decided to measure BBB disruption after SAH instead of neuronal count or brain edema, together with motor tests and concentrations of glutamate in the CSF and blood. The timing for determining BBB disruption in this study was chosen based on the observation of a maximal disruption of BBB 48 h after SAH [48].
The authors understand and recognize that the use of blood glutamate scavengers may have time limitations in clinical practice, given the short therapeutic window for reducing glutamate toxicity. In this study, treatment was initiated 60 minutes after SAH induction. In rat models of TBI, blood glutamate scavengers oxaloacetate and pyruvate have been shown to be an effective treatment when administered immediately prior to TBI infliction, 30 minutes and 60 minutes after TBI [36, 37]. In these studies, treatment was not effective if initiated 120 minutes after TBI [36, 37]. Thus, one can predict that scavengers will be effective in removing excess brain glutamate only when it is administered before the brain injury or during a specific time window of glutamate elevation. However, previous studies have shown that the elevation of glutamate in the ECF of the brain in rats after TBI and stroke is usually short-lasting, rarely extending to 120 minutes [71–74], whereas in humans it continues for several hours and even days [75–77]. Considering that the elevation of glutamate lasts longer in humans than in rats, blood glutamate scavengers may theoretically be effective, even if given later in the course of the condition. Additional experiments in the clinical settings are still warranted to investigate the therapeutic window for blood glutamate scavengers as a potential treatment strategy.
In conclusion, for the first time, this study demonstrates the effectiveness of blood glutamate scavengers oxaloacetate and pyruvate as a therapeutic neuroprotective strategy in a rat model of SAH. The data suggest that the observed neuroprotection with treatment of oxaloacetate and pyruvate is mediated via their blood glutamate scavenging effect.
Electronic supplementary material
(PDF 510 kb)
Acknowledgments
This work was supported by the grant awarded from the European Society of Anesthesiologists in 2010 (to A.Z.). We thank Sarah Boyko and Anastasia Zlotnik for their outstanding help as laboratory assistants. We thank Valeria Frishman, laboratory assistant at the Department of Clinical Biochemistry, Soroka Medical Center, Ben-Gurion University of the Negev, for her help with the biochemical analysis. We thank A. Alir and the staff at the Critical Care Unit, Soroka Medical Center, for their support and helpful discussions.
The authors state that no competing financial or other conflicts of interests exist. Full conflict of interest disclosures are available in the electronic supplementary material for this article.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Summary
For the first time, this study demonstrates the effectiveness of blood glutamate scavengers oxaloacetate and pyruvate as a therapeutic neuroprotective strategy in a rat model of subarachnoid hemorrhage.
Matthew Boyko and Israel Melamed contributed equally to this article.
References
- 1.King JT., Jr Epidemiology of aneurysmal subarachnoid hemorrhage. Neuroimaging Clin N Am. 1997;7:659–668. [PubMed] [Google Scholar]
- 2.Graf CJ, Nibbelink DW. Cooperative study of intracranial aneurysms and subarachnoid hemorrhage. Report on a randomized treatment study. 3. Intracranial surgery. Stroke. 1974;5:557–601. doi: 10.1161/01.STR.5.4.557. [DOI] [PubMed] [Google Scholar]
- 3.Feigin VL, Rinkel GJ, Lawes CM, et al. Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke. 2005;36:2773–2780. doi: 10.1161/01.STR.0000190838.02954.e8. [DOI] [PubMed] [Google Scholar]
- 4.Cross DT, 3rd, Tirschwell DL, Clark MA, et al. Mortality rates after subarachnoid hemorrhage: variations according to hospital case volume in 18 states. J Neurosurg. 2003;99:810–817. doi: 10.3171/jns.2003.99.5.0810. [DOI] [PubMed] [Google Scholar]
- 5.Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369:306–318. doi: 10.1016/S0140-6736(07)60153-6. [DOI] [PubMed] [Google Scholar]
- 6.Harmsen P, Tsipogianni A, Wilhelmsen L. Stroke incidence rates were unchanged, while fatality rates declined, during 1971–1987 in Goteborg, Sweden. Stroke. 1992;23:1410–1415. doi: 10.1161/01.STR.23.10.1410. [DOI] [PubMed] [Google Scholar]
- 7.Stegmayr B, Eriksson M, Asplund K. Declining mortality from subarachnoid hemorrhage: changes in incidence and case fatality from 1985 through 2000. Stroke. 2004;35:2059–2063. doi: 10.1161/01.STR.0000138451.07853.b6. [DOI] [PubMed] [Google Scholar]
- 8.Barash PG. Clinical anesthesia. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 9.Sehba FA, Bederson JB. Mechanisms of acute brain injury after subarachnoid hemorrhage. Neurol Res. 2006;28:381–398. doi: 10.1179/016164106X114991. [DOI] [PubMed] [Google Scholar]
- 10.Veelken JA, Laing RJ, Jakubowski J. The Sheffield model of subarachnoid hemorrhage in rats. Stroke. 1995;26:1279–1284. doi: 10.1161/01.STR.26.7.1279. [DOI] [PubMed] [Google Scholar]
- 11.Prunell GF, Mathiesen T, Svendgaard NA. Experimental subarachnoid hemorrhage: cerebral blood flow and brain metabolism during the acute phase in three different models in the rat. Neurosurgery. 2004;54:426–437. doi: 10.1227/01.NEU.0000103670.09687.7A. [DOI] [PubMed] [Google Scholar]
- 12.Prunell GF, Mathiesen T, Diemer NH, Svendgaard NA. Experimental subarachnoid hemorrhage: subarachnoid blood volume, mortality rate, neuronal death, cerebral blood flow, and perfusion pressure in three different rat models. Neurosurgery. 2003;52:165–175. doi: 10.1097/00006123-200301000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Meguro T, Clower BR, Carpenter R, Parent AD, Zhang JH. Improved rat model for cerebral vasospasm studies. Neurol Res. 2001;23:761–766. doi: 10.1179/016164101101199144. [DOI] [PubMed] [Google Scholar]
- 14.Solomon RA, Antunes JL, Chen RY, Bland L, Chien S. Decrease in cerebral blood flow in rats after experimental subarachnoid hemorrhage: a new animal model. Stroke. 1985;16:58–64. doi: 10.1161/01.STR.16.1.58. [DOI] [PubMed] [Google Scholar]
- 15.Germano A, d’Avella D, Cicciarello R, Hayes RL, Tomasello F. Blood–brain barrier permeability changes after experimental subarachnoid hemorrhage. Neurosurgery. 1992;30:882–886. doi: 10.1227/00006123-199206000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Marzatico F, Gaetani P, Rodriguez y Baena R, Silvani V, Paoletti P, Benzi G. Bioenergetics of different brain areas after experimental subarachnoid hemorrhage in rats. Stroke. 1988;19:378–384. doi: 10.1161/01.STR.19.3.378. [DOI] [PubMed] [Google Scholar]
- 17.Germano A, Caruso G, Caffo M, et al. Does subarachnoid blood extravasation per se induce long-term neuropsychological and cognitive alterations? Acta Neurochir (Wien) 1998;140:805–812. doi: 10.1007/s007010050182. [DOI] [PubMed] [Google Scholar]
- 18.Jackowski A, Crockard A, Burnstock G, Russell RR, Kristek F. The time course of intracranial pathophysiological changes following experimental subarachnoid haemorrhage in the rat. J Cereb Blood Flow Metab. 1990;10:835–849. doi: 10.1038/jcbfm.1990.140. [DOI] [PubMed] [Google Scholar]
- 19.Gules I, Satoh M, Clower BR, Nanda A, Zhang JH. Comparison of three rat models of cerebral vasospasm. Am J Physiol Heart Circ Physiol. 2002;283:H2551–H2559. doi: 10.1152/ajpheart.00616.2002. [DOI] [PubMed] [Google Scholar]
- 20.Castillo J, Davalos A, Naveiro J, Noya M. Neuroexcitatory amino acids and their relation to infarct size and neurological deficit in ischemic stroke. Stroke. 1996;27:1060–1065. doi: 10.1161/01.STR.27.6.1060. [DOI] [PubMed] [Google Scholar]
- 21.Castillo J, Davalos A, Noya M. Progression of ischaemic stroke and excitotoxic aminoacids. The Lancet. 1997;349:79–83. doi: 10.1016/S0140-6736(96)04453-4. [DOI] [PubMed] [Google Scholar]
- 22.Johnston MV, Trescher WH, Ishida A, Nakajima W. Neurobiology of hypoxic-ischemic injury in the developing brain. Pediatr Res. 2001;49:735–741. doi: 10.1203/00006450-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Zauner A, Bullock R, Kuta AJ, Woodward J, Young HF. Glutamate release and cerebral blood flow after severe human head injury. Acta Neurochir Suppl. 1996;67:40–44. doi: 10.1007/978-3-7091-6894-3_9. [DOI] [PubMed] [Google Scholar]
- 24.Ferrarese C, Aliprandi A, Tremolizzo L, et al. Increased glutamate in CSF and plasma of patients with HIV dementia. Neurology. 2001;57:671–675. doi: 10.1212/WNL.57.4.671. [DOI] [PubMed] [Google Scholar]
- 25.Shaw PJ, Forrest V, Ince PG, Richardson JP, Wastell HJ. CSF and plasma amino acid levels in motor neuron disease: elevation of CSF glutamate in a subset of patients. Neurodegeneration. 1995;4:209–216. doi: 10.1006/neur.1995.0026. [DOI] [PubMed] [Google Scholar]
- 26.Spranger M, Krempien S, Schwab S, Maiwald M, Bruno K, Hacke W. Excess glutamate in the cerebrospinal fluid in bacterial meningitis. J Neurol Sci. 1996;143:126–131. doi: 10.1016/S0022-510X(96)00197-9. [DOI] [PubMed] [Google Scholar]
- 27.Koura SS, Doppenberg EM, Marmarou A, Choi S, Young HF, Bullock R. Relationship between excitatory amino acid release and outcome after severe human head injury. Acta Neurochir Suppl. 1998;71:244–246. doi: 10.1007/978-3-7091-6475-4_70. [DOI] [PubMed] [Google Scholar]
- 28.Zhang H, Zhang X, Zhang T, Chen L. Excitatory amino acids in cerebrospinal fluid of patients with acute head injuries. Clin Chem. 2001;47:1458–1462. [PubMed] [Google Scholar]
- 29.O’Kane RL, Martinez-Lopez I, DeJoseph MR, Vina JR, Hawkins RA. Na(+)-dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) of the blood-brain barrier. A mechanism for glutamate removal. J Biol Chem. 1999;274:31891–31895. doi: 10.1074/jbc.274.45.31891. [DOI] [PubMed] [Google Scholar]
- 30.Berl S, Lajtha A, Waelsch H. Amino acid and protein metabolism of the brain. VI. Cerebral compartments of glutamic acid metabolism. J Neurochem. 1961;7:186–197. doi: 10.1111/j.1471-4159.1961.tb13503.x. [DOI] [PubMed] [Google Scholar]
- 31.Berl S, Takagaki G, Clarke DD, Waelsch H. Metabolic compartments in vivo. Ammonia and glutamic acid metabolism in brain and liver. J Biol Chem. 1962;237:2562–2569. [PubMed] [Google Scholar]
- 32.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/S0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 33.Teichberg VI, Cohen-Kashi-Malina K, Cooper I, Zlotnik A. Homeostasis of glutamate in brain fluids: An accelerated brain-to-blood efflux of excess glutamate is produced by blood glutamate scavenging and offers protection from neuropathologies. Neuroscience 2009;158:301–308. [DOI] [PubMed]
- 34.Gottlieb M, Wang Y, Teichberg VI. Blood-mediated scavenging of cerebrospinal fluid glutamate. J Neurochem. 2003;87:119–126. doi: 10.1046/j.1471-4159.2003.01972.x. [DOI] [PubMed] [Google Scholar]
- 35.Zlotnik A, Gruenbaum SE, Artru AA, et al. The neuroprotective effects of oxaloacetate in closed head injury in rats is mediated by its blood glutamate scavenging activity: evidence from the use of maleate. J Neurosurg Anesthesiol. 2009;21:235–241. doi: 10.1097/ANA.0b013e3181a2bf0b. [DOI] [PubMed] [Google Scholar]
- 36.Zlotnik A, Gurevich B, Cherniavsky E, et al. The contribution of the blood glutamate scavenging activity of pyruvate to its neuroprotective properties in a rat model of closed head injury. Neurochem Res. 2008;33:1044–1050. doi: 10.1007/s11064-007-9548-x. [DOI] [PubMed] [Google Scholar]
- 37.Zlotnik A, Gurevich B, Tkachov S, Maoz I, Shapira Y, Teichberg VI. Brain neuroprotection by scavenging blood glutamate. Exp Neurol. 2007;203:213–220. doi: 10.1016/j.expneurol.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 38.Zlotnik A, Sinelnikov I, Gruenbaum BF, et al. Effect of glutamate and blood glutamate scavengers oxaloacetate and pyruvate on neurological outcome and pathohistology of the hippocampus after traumatic brain injury in rats. Anesthesiology. 2012;116:73–83. doi: 10.1097/ALN.0b013e31823d7731. [DOI] [PubMed] [Google Scholar]
- 39.Boyko M, Zlotnik A, Gruenbaum BF, et al. Pyruvate’s blood glutamate scavenging activity contributes to the spectrum of its neuroprotective mechanisms in a rat model of stroke. Eur J Neurosci. 2011;34:1432–1441. doi: 10.1111/j.1460-9568.2011.07864.x. [DOI] [PubMed] [Google Scholar]
- 40.Fanne RA, Nassar T, Heyman SN, Hijazi N, Higazi AA. Insulin and glucagon share the same mechanism of neuroprotection in diabetic rats: role of glutamate. Am J Physiol Regul Integr Comp Physiol. 2011;301:R668–R673. doi: 10.1152/ajpregu.00058.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campos F, Sobrino T, Ramos-Cabrer P, et al. High blood glutamate oxaloacetate transaminase levels are associated with good functional outcome in acute ischemic stroke. J Cereb Blood Flow Metab 2011;31:1387–1393. [DOI] [PMC free article] [PubMed]
- 42.Campos F, Sobrino T, Ramos-Cabrer P, et al. Neuroprotection by glutamate oxaloacetate transaminase in ischemic stroke: an experimental study. J Cereb Blood Flow Metab 2011;31:1378–1386. [DOI] [PMC free article] [PubMed]
- 43.Campos F, Rodriguez-Yanez M, Castellanos M, et al. Blood levels of glutamate oxaloacetate transaminase are stronger associated with good outcome in acute ishcemic stroke than glutamate pyruvate transaminase. Clin Sci (Lond) 2011;121:11–17. [DOI] [PubMed]
- 44.Boyko M, Zlotnik A, Gruenbaum BF, et al. Pyruvate’s blood glutamate scavenging activity contributes to the spectrum of its neuroprotective mechanisms in a rat model of stroke. Eur J Neurosci 2011;34:1432–1441. [DOI] [PubMed]
- 45.Feldman Z, Gurevitch B, Artru AA, et al. Effect of magnesium given 1 hour after head trauma on brain edema and neurological outcome. J Neurosurg. 1996;85:131–137. doi: 10.3171/jns.1996.85.1.0131. [DOI] [PubMed] [Google Scholar]
- 46.Jeon H, Ai J, Sabri M, et al. Neurological and neurobehavioral assessment of experimental subarachnoid hemorrhage. BMC Neurosci. 2009;10:103. doi: 10.1186/1471-2202-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graham LT, Jr, Aprison MH. Fluorometric determination of aspartate, glutamate, and gamma-aminobutyrate in nerve tissue using enzymic methods. Anal Biochem. 1966;15:487–497. doi: 10.1016/0003-2697(66)90110-2. [DOI] [PubMed] [Google Scholar]
- 48.Germano A, d’Avella D, Imperatore C, Caruso G, Tomasello F. Time-course of blood-brain barrier permeability changes after experimental subarachnoid haemorrhage. Acta Neurochir. 2000;142:575–581. doi: 10.1007/s007010050472. [DOI] [PubMed] [Google Scholar]
- 49.Castillo J, Davalos A, Alvarez-Sabin J, et al. Molecular signatures of brain injury after intracerebral hemorrhage. Neurology. 2002;58:624–629. doi: 10.1212/WNL.58.4.624. [DOI] [PubMed] [Google Scholar]
- 50.Andreadou E, Kapaki E, Kokotis P, et al. Plasma glutamate and glycine levels in patients with amyotrophic lateral sclerosis. In Vivo. 2008;22:137–141. [PubMed] [Google Scholar]
- 51.Bunting H, Still R, Williams DR, Gravenor M, Austin MW. Evaluation of plasma glutamate levels in normal tension glaucoma. Ophthalmic Res. 2010;43:197–200. doi: 10.1159/000272024. [DOI] [PubMed] [Google Scholar]
- 52.Espey MG, Basile AS, Heaton RK, Ellis RJ. Increased glutamate in CSF and plasma of patients with HIV dementia. Neurology. 2002;58:1439–1440. doi: 10.1212/WNL.58.9.1439. [DOI] [PubMed] [Google Scholar]
- 53.Takano T, Lin JH, Arcuino G, Gao Q, Yang J, Nedergaard M. Glutamate release promotes growth of malignant gliomas. Nat Med. 2001;7:1010–1015. doi: 10.1038/nm0901-1010. [DOI] [PubMed] [Google Scholar]
- 54.Buchan AM, Lesiuk H, Barnes KA, et al. AMPA antagonists: do they hold more promise for clinical stroke trials than NMDA antagonists? Stroke. 1993;24(12 suppl):1148–1152. [PubMed] [Google Scholar]
- 55.Germanò A, Caffo M, Angileri FF, et al. NMDA receptor antagonist felbamate reduces behavioral deficits and blood-brain barrier permeability changes after experimental subarachnoid hemorrhage in the rat. J Neurotrauma. 2007;24:732–744. doi: 10.1089/neu.2006.0181. [DOI] [PubMed] [Google Scholar]
- 56.Morris GF, Juul N, Marshall SB, Benedict B, Marshall LF. Neurological deterioration as a potential alternative endpoint in human clinical trials of experimental pharmacological agents for treatment of severe traumatic brain injuries. Executive Committee of the International Selfotel Trial. Neurosurgery. 1998;43:1369–1374. [PubMed] [Google Scholar]
- 57.Muir KW. Glutamate-based therapeutic approaches: clinical trials with NMDA antagonists. Curr Opin Pharmacol. 2006;6:53–60. doi: 10.1016/j.coph.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 58.Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002;1:383–386. doi: 10.1016/S1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 59.Hardingham GE, Bading H. The Yin and Yang of NMDA receptor signalling. Trends Neurosci. 2003;26:81–89. doi: 10.1016/S0166-2236(02)00040-1. [DOI] [PubMed] [Google Scholar]
- 60.Bertrand G, Gross R, Puech R, Loubatieres-Mariani MM, Bockaert J. Evidence for a glutamate receptor of the AMPA subtype which mediates insulin release from rat perfused pancreas. Br J Pharmacol 1992;106:354–359. [DOI] [PMC free article] [PubMed]
- 61.Gonoi T, Mizuno N, Inagaki N, et al. Functional neuronal ionotropic glutamate receptors are expressed in the non-neuronal cell line MIN6. J Biol Chem. 1994;269:16989–16992. [PubMed] [Google Scholar]
- 62.Molnar E, Varadi A, McIlhinney RA, Ashcroft SJ. Identification of functional ionotropic glutamate receptor proteins in pancreatic beta-cells and in islets of Langerhans. FEBS Lett. 1995;371:253–257. doi: 10.1016/0014-5793(95)00890-L. [DOI] [PubMed] [Google Scholar]
- 63.Inagaki N, Kuromi H, Gonoi T, et al. Expression and role of ionotropic glutamate receptors in pancreatic islet cells. FASEB J. 1995;9:686–691. [PubMed] [Google Scholar]
- 64.Weaver CD, Yao TL, Powers AC, Verdoorn TA. Differential expression of glutamate receptor subtypes in rat pancreatic islets. J Biol Chem. 1996;271:12977–12984. doi: 10.1074/jbc.271.51.32551. [DOI] [PubMed] [Google Scholar]
- 65.Teichberg VI. From the liver to the brain across the blood-brain barrier. Proc Natl Acad Sci U S A. 2007;104:7315–7316. doi: 10.1073/pnas.0702450104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Doczi T. The pathogenetic and prognostic significance of blood-brain barrier damage at the acute stage of aneurysmal subarachnoid haemorrhage. Clinical and experimental studies. Acta Neurochir. 1985;77:110–132. doi: 10.1007/BF01476215. [DOI] [PubMed] [Google Scholar]
- 67.Dóczi T, Joó F, Ádám G, Bozóky B, Szerdahelyi P. Blood-brain barrier damage during the acute stage of subarachnoid hemorrhage, as exemplified by a new animal model. Neurosurgery. 1986;18:733. doi: 10.1227/00006123-198606000-00010. [DOI] [PubMed] [Google Scholar]
- 68.Germanó A, d'Avella D, Cicciarello R, Hayes RL, Tomasello F. Blood-brain barrier permeability changes after experimental subarachnoid hemorrhage. Neurosurgery. 1992;30:882. doi: 10.1227/00006123-199206000-00011. [DOI] [PubMed] [Google Scholar]
- 69.Germanò A, Imperatore C, d'Avella D, Costa G, Tomasello F. Antivasospastic and brain-protective effects of a hydroxyl radical scavenger (AVS) after experimental subarachnoid hemorrhage. J Neurosurg 998;88:1075–1081. [DOI] [PubMed]
- 70.Imperatore C, Germanó A, d’Avella D, Tomasello F, Costa G. Effects of the radical scavenger AVS on behavioral and BBB changes after experimental subarachnoid hemorrhage. Life Sciences. 2000;66:779–790. doi: 10.1016/S0024-3205(99)00651-7. [DOI] [PubMed] [Google Scholar]
- 71.Palmer AM, Marion DW, Botscheller ML, Swedlow PE, Styren SD, DeKosky ST. Traumatic brain injury-induced excitotoxicity assessed in a controlled cortical impact model. J Neurochem. 1993;61:2015–2024. doi: 10.1111/j.1471-4159.1993.tb07437.x. [DOI] [PubMed] [Google Scholar]
- 72.Faden AI, Demediuk P, Panter SS, Vink R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science. 1989;244:798–800. doi: 10.1126/science.2567056. [DOI] [PubMed] [Google Scholar]
- 73.Guyot LL, Diaz FG, O’Regan MH, McLeod S, Park H, Phillis JW. Real-time measurement of glutamate release from the ischemic penumbra of the rat cerebral cortex using a focal middle cerebral artery occlusion model. Neurosci Lett. 2001;299:37–40. doi: 10.1016/S0304-3940(01)01510-5. [DOI] [PubMed] [Google Scholar]
- 74.Phillis JW, Smith-Barbour M, O’Regan MH. Changes in extracellular amino acid neurotransmitters and purines during and following ischemias of different durations in the rat cerebral cortex. Neurochem Int. 1996;29:115–120. doi: 10.1016/0197-0186(95)00154-9. [DOI] [PubMed] [Google Scholar]
- 75.Baker AJ, Moulton RJ, MacMillan VH, Shedden PM. Excitatory amino acids in cerebrospinal fluid following traumatic brain injury in humans. J Neurosurg. 1993;79:369–372. doi: 10.3171/jns.1993.79.3.0369. [DOI] [PubMed] [Google Scholar]
- 76.Dávalos A, Castillo J, Serena J, Noya M. Duration of glutamate release after acute ischemic stroke. Stroke. 1997;28:708–710. doi: 10.1161/01.STR.28.4.708. [DOI] [PubMed] [Google Scholar]
- 77.Bullock R, Zauner A, Woodward J, Young H. Massive persistent release of excitatory amino acids following human occlusive stroke. Stroke. 1995;26:2187–2189. doi: 10.1161/01.STR.26.11.2187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 510 kb)