Summary
Psychiatric research applications of electroencephalography (EEG), the earliest approach to imaging human cortical brain activity, are attracting increasing scientific and clinical interest. For more than 40 years, EEG research has attempted to characterize and quantify the neurophysiology of attention-deficit/hyperactivity disorder (ADHD), most consistently associating it with increased frontocentral theta band activity and increased theta to beta (θ/β) power ratio during rest compared to non-ADHD controls. Recent reports suggest that while these EEG measures demonstrate strong discriminant validity for ADHD, significant EEG heterogeneity also exists across ADHD-diagnosed individuals. In particular, additional studies validating the use of the θ/β power ratio measure appear to be needed before it can be used for clinical diagnosis. In recent years, the number and the scientific quality of research reports on EEG-based neurofeedback (NF) for ADHD have grown considerably, although the studies reviewed here do not yet support NF training as a first-line, stand-alone treatment modality. In particular, more research is needed comparing NF to placebo control and other effective treatments for ADHD. Currently, after a long period of relative stasis, the neurophysiological specificity of measures used in EEG research is rapidly increasing. It is likely, therefore, that new EEG studies of ADHD using higher density recordings and new measures drawn from viewing EEG as a 3-dimensional functional imaging modality, as well as intensive re-analyses of existing EEG study data, can better characterize the neurophysiological differences between and within ADHD and non-ADHD subjects, and lead to more precise diagnostic measures and effective NF approaches.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-012-0131-z) contains supplementary material, which is available to authorized users.
Keywords: ADHD, Electroencephalography, EEG, Neurofeedback, EEG biofeedback, Diagnosis, Treatment
Background
Electroencephalography (EEG) was the earliest measure used to systematically examine human brain cortical activity. After a long period of decline in clinical interest, occasioned by the development of brain imaging using magnetic resonance, EEG is now attracting increasing scientific and clinical interest. This resurgence is made possible by ongoing advances in signal processing and visualization that increase the spatial resolution of EEG imaging and exploit its ability to image quick transient cortical events and more precise regional changes in cortical tone; these advances are discussed later in this review.
The EEG potential fluctuations measured on the scalp represent summed cortical potentials that arise from synchronous firing of large collections of neurons. Because the electrical potentials are recorded on the scalp, far from the cortical region or patch from which the signals originate, only electrical source potentials that are highly synchronous or spatially consistent are detectable in the scalp EEG signals. We refer to the locally-synchronous activities in the cortical regions from which these source signals originate as cortical EEG source processes. EEG scalp channel data sum these changing cortical source processes with excellent time resolution (1-10 ms), but relatively poor spatial resolution. Nonbrain source processes (from eye movements, scalp muscles, line noise, and so forth) also contribute to scalp EEG signals. Because of the broad spatial mixing by volume conduction of both cortical and nonbrain potentials in the scalp data, computer analysis is needed to separate the activities of the cortical brain and nonbrain artifact sources.
The use of EEG technology was first reported by Hans Berger [1] in the 1920s, who provided an extensive description of methodology and initial recordings made on his son. Subsequently, EEG was used to study children with behavioral problems (many of whom would be likely to receive a diagnosis of attention-deficit/hyperactivity disorder [ADHD] today), with first reports indicating that they exhibited frontocentral EEG slowing [2], a finding subsequently noted to predict a positive response to medication treatment. In the 1960s, technological advancements allowed processing of the EEG signal using fast Fourier transform (FFT) methods to study the mean power spectrum of the recording (sometimes referred to as quantitative EEG measurement). In addition, averaging across many data epochs or trials time-locked to a particular type of experimental event (giving stimulus-locked and response-locked event-related potentials [ERPs]) became possible. Quantification of the recorded signals allowed the application of statistical analysis to test for group differences in amplitudes and latencies of the signal measures, including peaks in ERP waveforms (time-domain), and peaks in the power spectra (frequency-domain).
More recent advances in recording hardware, data storage, and computer science have led to further expansion of EEG recording to high densities (as many as 256 electrodes), to concurrent recording of EEG and functional magnetic resonance imaging data, and to sophisticated signal processing approaches that allow modeling of the cortical sources of the recorded EEG signals and rejection or removal of nonbrain EEG artifacts. Recent research shows that (with proper analysis) high-density EEG recordings can give valuable, spatiotemporally precise information regarding dynamic aspects of cortical activation and intracortical communication. However, these new imaging capabilities are just beginning to be applied to clinical research in ADHD and other psychiatric conditions.
EEG Variables Typically Studied
For the last few decades, scalp channel EEG data have been analyzed principally either in the time domain via ERP trial averaging, or in the frequency domain using FFT that estimate spectral power within a given frequency (reported in hertz [Hz], the number of waveform cycles per second). Although phenomena and definitions may vary, EEG spectral power variations are typically dominated by distinct changes in power in a few frequency bands. The standard terminology for these is: delta (<4 Hz), theta (4-7 Hz), alpha (8-12 Hz), beta (13-25 Hz; often split into beta-1/sensorimotor rhythm (SMR), 13-16 Hz, and beta-2, 17-25 Hz), and gamma (25-50 Hz or even still higher frequency broadband activity extending to 200 Hz or greater) [3]. It is important to note that each EEG channel recording comprises a wide range of frequencies across the power spectrum. Thus, EEG frequency-band activities do not occur in isolation, but rather might be said to act in concert. Spectral power in the frequency bands has typically been examined at each electrode location. However, because signals at adjacent electrodes are generally highly correlated (because of common volume conduction from the active brain and nonbrain sources), average power estimates across spatially adjacent channels (e.g., frontal electrodes) are sometimes used to estimate regional amplitude or power (e.g., power over frontal scalp).
Problems with Current Measures
Both spectral power and ERP measures greatly reduce the complexity of EEG data, which is collected at rates ranging from a few hundred to thousands of samples per second. While reducing this high complexity and dimensionality is necessary for achieving a coherent result, the approaches typically used in the past come at a cost of (greatly) reducing the amount of information regarding brain state and dynamics that can be extracted from the data. Much of this information may be irrelevant to answering the particular question at hand; identifying the relevant information before reducing the data complexity could save the resulting measures from containing an admixture of relevant and irrelevant information. This may increase the effective signal-to-noise ratio of the information gleaned from the data.
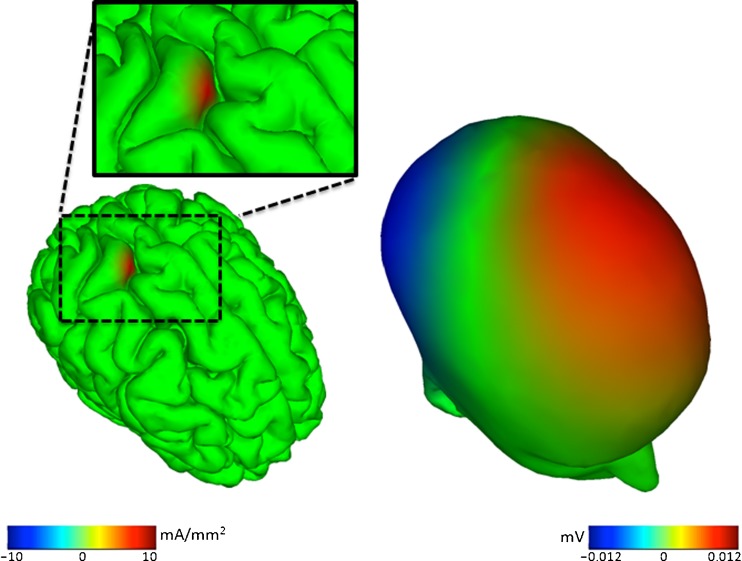
The primary difficulty in making sense of EEG data, as reflecting dynamics in specific cortical areas, is the very broad “point spread function” through which each of the EEG source signals spreads out via volume conduction to reach and contribute to nearly all the scalp electrode signals with varying strength and polarity (Fig. 1). The broad spatial projection of each cortical source signal means that the signal at each electrode sums the activities of many brain cortical (as well as nonbrain “artifactual”) signal sources.
Fig. 1.
Propagation of cortical electroencephalography (EEG) source potentials to scalp electrodes. A simulated (roughly cm2) cortical EEG source region (left) is shown, and its broad potentials projected to the scalp by volume conduction (right). For example, the source of the positive (red) scalp potential on the left frontal scalp is not a frontal brain source. Similar animations further illustrating the complexity of spatial relationships between brain source activities and scalp EEG activities are available at: http://www.youtube.com/playlist?list=PLDF9D201769ADC62D. (Image credit: Zeynep Akalin Acar [83])
Thus, the naïve assumption that each electrode is sensitive only to the cortical territory directly below the electrode scalp location is just that, although the implications of this well-established biophysical fact have been largely ignored in selecting and justifying the analysis methods used in much EEG research to date. The practical and mathematical difficulty in determining the source signals for EEG signals recorded at some time point has long thwarted efforts to develop EEG as a true functional brain imaging modality. However, new methods, described later in this review, are now bringing this goal within reach.
EEG Research Findings in ADHD
The frequency bands of most interest in ADHD research are theta, alpha, and beta, either alone (e.g., absolute or relative alpha power) or compared to each other (such as theta/beta power or amplitude ratio). In a resting state, (lower frequency) theta band activity can reflect drowsiness or “cortical slowing.” Alpha band activity is typically observed during eyes closed at rest, particularly in posterior regions, and it is negatively associated with central nervous system arousal. Beta band activity, by contrast, generally accompanies mental activity and concentration. A theta-to-beta power ratio measured at the vertex (Cz) during eyes-open or eyes-closed resting condition has been proposed to capture the relative contributions of two relevant frequency bands for diagnosing and monitoring ADHD [4]; however, the true functional significance of this measure remains unknown.
Theta Band EEG and Underarousal
In the 1970s, Satterfield et al. [5] conducted a series of EEG studies of children with ADHD and found EEG abnormalities, including excess slow-wave activity and increased epileptiform spike and wave activity. These findings were thought to suggest underarousal and maturational delay as underlying pathophysiologies in ADHD. Furthermore, children with ADHD who had greater excess slow-wave activity were more likely to have a positive response to stimulant medication [6], a finding that fit well with cortical underarousal theories. For the past 40 years, there have been numerous EEG studies in ADHD research that have helped to clarify and refine these early findings (for more detail see Barry et al [7] and Snyder and Hall [8] for review and meta-analysis, respectively).
Current research findings suggest that most children with ADHD display fairly consistent EEG differences in brain electrical activity as compared to normal children, particularly with respect to their increased frontocentral theta (4-7 Hz) activity during primarily resting state conditions [9–18], a difference indicating decreased cortical activity that may be associated with underarousal. A recent meta-analysis of 9 studies with a collective sample of 1,498 subjects found an effect size (ES) of 1.31 (95 % confidence interval [CI], 1.14-1.48) and an average excess of 32 % in theta band power for children with ADHD relative to controls [8]. An association of increased theta band power in ADHD has been found across the lifespan: both adolescents and adults with ADHD exhibit increased frontocentral theta band power when compared to non-ADHD populations [19]. Elevated theta power, however, may be a nonspecific marker of cortical dysfunction common to other disorders, such as epilepsy, bipolar disorder, and polysubstance abuse [20].
Theta/Beta Ratio
Given these findings of elevated theta levels, and the general association of beta band activity with attentional arousal, it is not surprising that the ratio of theta to beta (θ/β) power during (either eyes open or closed) resting conditions over the frontocentral scalp has also been reported to be higher among children, adolescents, and adults with ADHD by several [21], but not all [17, 22–25] independent research groups. A recent meta-analysis reported that this θ/β marker is remarkably robust with an ES of 3.08 [8], and highly stable over time with a 1-month reliability of 0.96 [21]. It is also reported to correlate precisely (0.99) with age-related changes in ADHD behavioral symptomatology over time [8]. The increases in both theta band activity and in the theta/beta power ratio are two of the most reliable EEG findings in ADHD to date.
The theta/beta ratio has been negatively correlated with mean reaction time in adults both with and without ADHD [27], indicating an increased θ/β ratio was associated with shorter, faster reaction time. This association coupled with the result that the ADHD group had increased omission errors suggests that the θ/β ratio may reflect increased impulsivity and difficulty negotiating the speed-accuracy tradeoff (faster speed but poorer performance) in ADHD. This finding was not replicated in a child sample in which the θ/β ratio was not significantly correlated with ADHD symptoms or any aspect of cognitive performance on a sustained attention task [17]. This measure has been proposed to reflect task-related cortical activation, but more research is needed to identify the range of conditions under which these differences appear and to understand the functional significance of these effects in terms of the underlying cortical processes that produce them.
Alpha and Beta Bands
Findings in ADHD studies involving alpha and beta band activities have been mixed, with the majority of studies reporting reduced activity in both bands, particularly in posterior regions, for those with ADHD compared to normal controls [12, 14, 28–30]. An ES of -0.51 (95 % CI, -0.65 to -0.35) has been reported for beta activity in ADHD, with a mean reduction of 6 % in beta band power relative to controls [8]. Across several independent studies, a subgroup (~10-15 %) of children with ADHD who exhibit increased (rather than decreased) frontal beta band power compared to controls has been identified [9, 31, 32]. This group appears more likely to be diagnosed with ADHD Combined Type, to have a lower (although still normal range) intelligence score, and to exhibit more delinquent behaviors relative to both controls and other children with ADHD who exhibit decreased beta band activity [31].
Findings for alpha band power have been more variable with the previously mentioned studies finding reduced alpha band power in ADHD, whereas others have reported alpha activity increases [9, 22, 31] or no significant differences [27, 33] when compared to controls. In a recent study that included both children and adults, no significant differences in alpha band power were reported for ADHD children relative to their non-ADHD siblings, however, adults with ADHD Combined Type exhibited significantly reduced alpha activity globally when compared to non-ADHD controls [18], suggesting that alpha activity may differ by age, ADHD subtype, and potentially psychiatric comorbidity.
Interpretation of Power Spectral Differences
Interpretation of EEG frequency band power in general remains difficult because of conflicting reports of its association with different physiological states and with cognitive phenomena (e.g., eyes closed resting versus working memory). For example, the aforementioned association between increased frontocentral theta band power and cortical slowing in ADHD is at odds with the well-replicated finding that increased attentional loading (i.e., during working memory [WM]) is also associated with increased frontal midline theta band activity [34]. This paradox is resolved by considering that the increased frontocentral theta band power association with ADHD is based on data collected during the resting state, whereas the increased theta band power during working memory appears as bursts at stimulus onset and during WM maintenance within eyes-open WM task trials. Thus, theta band activity can represent different phenomena depending on the recording circumstances, and this must be considered when trying to determine the functional significance of the EEG signals.
Confusion as to the functional association, significance, or “meaning” of EEG measures may also result from analyzing the data at the level of individual scalp channels rather than the cortical source level. Channel signals are, in fact, weighted mixtures of source signals originating from many different cortical regions supporting distinct cognitive functions (plus signals from nonbrain artifact sources). Thus, any single-channel based measure mixes potentials from several sources, not all of which contribute to the effect of interest, thereby constituting noise in the signal of interest.
To move the study of ADHD using EEG forward through correct interpretation of the EEG signals, we will need to capture more information from the data by incorporating more sophisticated data analysis that yields measures of its time-frequency characteristics, its cortical sources that project to and are mixed at the scalp, and time- and frequency-varying relationships between the sources.
EEG Heterogeneity
Despite fairly consistent mean results across many of the studies previously reviewed, several studies have demonstrated marked heterogeneity in EEG characteristics within the ADHD population. Because individuals with ADHD exhibit significant heterogeneity in behavioral, cognitive, neurobiological domains, and subsequent medication response, it is not surprising that they may exhibit neurophysiological heterogeneity as well. Several studies have examined whether different neurophysiologic profiles, as defined by EEG measures, may be delineated within subgroups of children with ADHD. For example, Clarke et al. [31, 32] suggest there are several different EEG subtypes, including maturationally delayed, cortical hypoarousal, and excess beta activity subgroups. In addition, discriminant function analyses identified an excess alpha band activity subgroup in ADHD [31], which was originally reported by Chabot and Serfontein [9]. Other groups have described as many as 9 EEG subtypes (including the 4 identified by Clarke et al. [31] and by Chabot and Serfontein [9]) in children with and without ADHD [35, 36]. Not surprisingly, the EEG subtypes do not correspond well to behaviorally defined ADHD subtypes. However, enhanced medication response, as measured by continuous performance task (CPT) improvement, was apparent only in the frontal slow (with excess theta band activity) and the frontal alpha subgroups [23]. The presentation (if confirmed) of EEG subtypes suggests there are different underlying neurophysiological substrates in ADHD that may represent variation in etiological factors, psychiatric comorbidity, and/or treatment response. Further work on EEG heterogeneity within ADHD is needed.
Diagnostic Utility of EEG in ADHD
Currently, no single diagnostic test for ADHD exists. A proper diagnostic evaluation for ADHD (and all other childhood psychiatric disorders) generally involves a process of collecting data on the history, course, and duration of symptoms, both at home and at school, using clinical interviews and behavior rating scales. Because inattention is pathognomonic to nearly all childhood psychiatric disorders, it is often difficult to make differential diagnoses between ADHD and other disorders that can have a similar presentation, including autism spectrum disorders, mood and anxiety disorders, and learning disabilities. Thus, a biologically based diagnostic test or biological marker (i.e., biomarker) that is sensitive and specific to ADHD would be of great assistance. Based on the findings previously reviewed, EEG measures have been viewed as a promising biomarker for ADHD.
The diagnostic utility of EEG is usually examined by comparing it to the gold standard for diagnosis (conclusions from a structured clinical interview) and calculating clinical group statistics, including sensitivity (the percent of people who have an ADHD diagnosis using clinical interview and abnormality on EEG marker [i.e., true positives]) and specificity (the percent of people without ADHD and normal EEG marker [i.e., true negatives]). Two additional measures are also examined: positive predictive power (PPP) (i.e., the percent of people who have an abnormal EEG marker and receive a diagnosis of ADHD) and negative predictive power (NPP) (i.e., the percent of people with a normal EEG marker and no diagnosed ADHD). The predictive power is perhaps the most important to examine because it more closely mirrors how EEG would be used in clinical practice in which the EEG measure would be used to predict the likelihood of an ADHD diagnosis. These 4 values, although slightly overlapping, determine the discriminant validity of the EEG measure. In a previous review by Loo and Barkley [37], studies that reported data on the discriminant validity of EEG measures in ADHD were reviewed. These studies demonstrated good sensitivity (86-97 %) and specificity (84-98 %) of EEG measures (i.e., θ/β ratio) for ADHD diagnosis [4, 21, 26, 38]. When using the 1.5 SD increase above the mean value for non-ADHD controls as a cutoff for ADHD, as recommended by the authors, the θ/β ratio resulted in a misclassification rate of 16 % [21], meaning that by using the EEG marker alone, 16 % of actual ADHD cases would be missed because they produce a normal EEG θ/β ratio.
In addition, several methodological issues limit the usefulness of these EEG findings for clinical practice. The first methodological issue is the need to use a proper non-ADHD control group. When the controls are typically developing peers with no psychiatric diagnoses, they are in theory easier to distinguish from ADHD or ADHD with additional comorbidities. In clinical practice, a child undergoes diagnostic evaluation with several possible outcomes. More specifically, the child could ultimately be diagnosed with ADHD alone or with additional comorbidities, given an alternative non-ADHD diagnosis, or given no diagnosis at all. All these clinical variants were not included in previous studies. In addition, all of the studies were retrospective in nature, which result in higher accuracy rates because cutoffs can be adjusted to maximize group differences. Finally, some early studies [38] did not use standardized diagnostic criteria; ADHD group status was instead based on behavior rating scales, which tends to inflate the rate of ADHD diagnosis. In the following, we focus on studies that have been published since the last review (see Table 1).
Table 1.
Studies examining the diagnostic utility of EEG in ADHD
| Author | Year | Age range | N | Condition | EEG/ ERP | Method | Measures | Accuracy | Findings |
|---|---|---|---|---|---|---|---|---|---|
| Theta/Beta (θ/β) ratio | |||||||||
| Ogrim et al. [17] | 2012 | 7-16 years | 62 ADHD 39 CON | EO fixed attn | EEG power | ROC analysis | Cz abs theta & beta power, θ/β ratio | θ/β 63 % ACC; theta 58 % ACC; GNG OE 85 % ACC | Cognitive tasks better at identifying ADHD than EEG |
| Snyder et al. [16] | 2008 | 6-18 years | Clinic: 97 ADHD 62 CON | EO fixed attn | EEG power | SEN SPEC | Cz, θ/β ratio | 89 % ACC, 87 % SEN, 94 % SPEC, 95 % PPP, 82 % NPP | EEG better than behavior rating scales at identifying ADHD; does not identify ADHD subtype or comorbidities |
| Quintana et al. [39] | 2007 | 6-21 years | Clinic: 16 ADHD 10 CON | EO fixed attn | EEG power | SEN SPEC | Cz, θ/β ratio | 96% ACC, 94% SEN, 100% SPEC | EEG better than behavior rating scales in identifying ADHD |
| Monastra et al. [21] | 2001 | 6-20 years | Exp 1: 96 ADHD 33 CON; Exp 2/3: 313 ADHD | EO fixed attn, reading, listening, drawing | EEG power | SEN SPEC | Cz, θ/β ratio | 91% ACC, 90% SEN, 94% SPEC, 98% PPP, 76% NPP | θ/β ratio is reliable and discriminates from non-clinical controls |
| ADHD discrimination using multiple measures or new methods | |||||||||
| Mueller et al. [41] | 2011 | 18-47 years | 75 ADHD 75 CON; 2nd sample 17 ADHD | GNG | ERP | ICA and SVM appliedto ERPs | 5 ERP features: 4 latency, 1 amplitude | 91 % ACC, 94 % predictive power | 5 ERP features 100 % ACC in validation sample: 17 ADHD subjects |
| Abibullaev and An [42] | 2011 | 7-12 years | 7 ADHD 3 CON | CPT | EEG power | SVM | Rel theta, beta power, θ/β and θ/α ratios | 97 % ACC | Relative theta power most accurate with semi-supervised method |
| Ahmadlou and Adeli [43] | 2010 | 8-13 years | 12 ADHD 12 CON | EC | EEG power | FSL, community analysis, partioning | Inter-electrode synchronization | 87.5 % ACC | Community pattern of anterior and posterior neuronal networks has discriminant validity |
| Magee [26] | 2005 | 7-13 years, boys only | 253 ADHD 67 CON | EC | EEG power | Cluster analysis, logistic regression | Abs and rel power | ADHD: 3 cluster vs. CON 87 % ACC 89 % SEN 80 % SPEC | Accuracy of resting EEG improved when comparing each ADHD cluster vs controls |
θ/β = theta/beta band power ratio; θ/α = theta/alpha band power ratio; abs = absolute; attn = attention; ACC = accuracy; ADHD = attention-deficit/hyperactivity disorder; clinic = clinic sample; CON = non-ADHD control; Cz = vertex; EC = eyes closed; EEG = electroencephalography; EO = eyes open; ERP = event-related potential; Exp = experiment; FSL = fuzzy synchronization likelihood; GNG = go/no-go; ICA = independent component analysis; N = number; NPP = negative predictive power; OE = omission errors; PPP = positive predictive power; rel = relative; ROC = receiver operating curve; SEN = sensitivity (true positive); SPEC = specificity (true negative); SVM = support vector machine
Diagnostic Utility of the Theta/Beta Power Ratio for ADHD
As previously mentioned, several previous studies have examined the discriminant validity of the θ/β ratio in ADHD. In their meta-analysis, Snyder and Hall [8] found an ES of 3.08, which predicts a sensitivity and specificity of 94 %. This estimate is largely consistent with a pilot study [39], as well as a large, multi-site, prospective study of the discriminant validity of the θ/β ratio in ADHD [16]. The total sample of the larger study consisted of 159 children and adolescents who presented to 4 different clinics with suspected ADHD, 61 % of whom received a diagnosis of ADHD. The majority also had additional psychiatric comorbidities. The overall accuracy rate in identifying ADHD was 89 %, with a PPP of 95 % and NPP of 82 %.
Although this suggests that an abnormally high θ/β ratio marker identifies almost all of the children who are subsequently given a diagnosis of ADHD, 18 % of those with a normal θ/β ratio also go on to receive an ADHD diagnosis. For clinical purposes, a misdiagnosis rate of 18 % is simply too high. In the Snyder et al. [16] study, EEG performed significantly better than parent and teacher behavior rating scales. Using these scales, the overall accuracy ranged from 47 to 58 %, the PPP ranged from 62 to 67 %, and the NPP ranged from 27 to 43 %. The θ/β power ratio demonstrated similarly high rates of diagnostic accuracy across demographic groups that varied according to age, gender, and ethnic background (range, 87-95 %), as well as in the presence or absence of comorbid psychiatric conditions (range, 87-96 %). These results are remarkably consistent with previous reported results using the θ/β power ratio [4, 21], and suggest that this measure exhibits similar accuracy rates among diverse clinical samples and age ranges. However, an increased θ/β power ratio, as previously reviewed, is not ubiquitous in ADHD [22, 23, 27, 40], and this accuracy rate was not replicated in a recent study, in which the θ/β power ratio identified ADHD subjects with only 58 % accuracy [26].
It is difficult to reconcile such disparate results regarding the reliability of the θ/β ratio marker. The Snyder et al. [16] study in 2008 was scientifically sound and it provides class 1 evidence that EEG may indeed be useful in confirming a diagnosis of ADHD as part of a multimodal assessment that includes clinical interviews, behavior rating scales, and neuropsychological tests for identification of comorbid learning disabilities and co-occurring psychiatric disorders. The inconsistencies across studies may be due to methodological issues, such as sampling, instrumentation, and data processing and analysis differences or actual EEG heterogeneity within the ADHD population.
In addition, a rarely mentioned fact is that there may be wide variation in EEG instrumentation that can make it extremely difficult to compare across datasets collected with different EEG hardware and software. Finally, as with any other clinical result, it would be reassuring to have independent replication of the positive θ/β measure findings by a research group without a potential conflict of interest (e.g., a positive report by researchers who are not stockholders of the company making the EEG instrumentation used in the study). Thus, more research is needed before the θ/β power ratio can be used clinically as a diagnostic tool for determining the presence of ADHD.
New Methods for ADHD Discrimination
There have been several recent studies that have attempted to use more advanced signal processing approaches to improve ADHD discrimination. One example is a study by Mueller et al. [41] who used machine-learning methods on independent component analysis (ICA)-resolved ERP features from samples of healthy controls and ADHD adults. A combination of 5 peak amplitude and latency measures associated with inhibition, monitoring and other executive operations were extracted to maximize group discrimination. High rates of classification accuracy for both the original sample (91 %) and a subsequent validation sample (94 %) were obtained [41]. This, and other studies, using a semi-supervised feature selection to define new features of the EEG signal [42], as well as studies using graph theory and community pattern analysis of EEG-derived functional connectivity [43] may provide new avenues to identify and test EEG measures, which can both tolerate sample heterogeneity and provide maximal discrimination between individuals with and without ADHD.
Neurofeedback Therapy for ADHD
As previously reviewed, there is a long history of EEG research findings documenting EEG abnormalities in ADHD, particularly increased frontocentral theta power, decreased beta activity, and increased theta/beta ratio. Attempts to correct these EEG abnormalities, coupled with the less than uniform positive response to stimulant medications form the rationale for EEG biofeedback, also known as neurotherapy or neurofeedback (NF). For the purposes of this review, all of these studies will be referred to as NF. This treatment is based on the experimental work of Sterman et al. [44] who first demonstrated that operant conditioning of the EEG was feasible in cats and that this training inoculated the cats against subsequent drug-induced seizure activity. Furthermore, when the cats were immobilized, they were observed to produce rhythms in the lower range of the beta frequency band (12-16 Hz), now often referred to as the sensorimotor rhythm (SMR) [45]. Because reduced motor activity is associated with increased SMR spectral power, this frequency range became a treatment target for NF training in cats. NF training was then generalized to humans with intractable epilepsy in whom NF appeared to be efficacious in reducing the frequency and severity of seizure activity [46]. NF has since become an accepted treatment for epilepsy, particularly in cases in which seizures are not well controlled by medications [47].
The first case studies using NF in ADHD were conducted by Shouse and Lubar [48] and Lubar and Shouse [49] and these studies demonstrated positive treatment effects on behavioral and cognitive functioning within a single-subject ABAB design. The treatment used was a combination of reinforcement to decrease theta band activity and increase beta band activity in the 12 to 20 Hz range. After the first Lubar studies [48, 49], a number of uncontrolled studies that reported positive effects of NF among children with ADHD were published [50–58]. In 2005, Loo and Barkley [37] reviewed the NF literature and concluded that the methodological problems (e.g., lack of treatment randomization, placebo control, treatment blind, small sample size, and inappropriate statistics) of the published studies greatly limited the strength of allowable conclusions regarding the efficacy of this mode of NF for ADHD.
Randomization, treatment blinds, and placebo controls are crucial methodological components because they serve to control for the expectations, motivations, and nonspecific treatment effects that affect investigators, parents, and children involved in the study. These factors in turn influence treatment outcome as rated by parents and study evaluators. There have since been several NF studies with substantially stronger scientific methodology, including randomization, blinding procedures, and active and sham control treatments; these studies are the focus of the following review.
Another group of studies, conducted primarily in Europe, have focused on the slow cortical potential (SCP), which is thought to index regulation of cortical excitability. The SCP reflects cortical shifts between positive and negative slow waves or trends lasting between several hundred milliseconds and several seconds [59]. SCP training is thought to activate specific attentional networks. These studies were not included in the previous 2005 review, but will also be reviewed in detail here.
Collectively, while there are many variations in reported and possible NF protocols, the 2 main types of NF reported to date have generally involved either: 1) decreasing theta activity and/or increasing SMR/beta activity, or 2) increasing control of the SCPs. Instead of discussing each study individually (as previously done), this review will summarize across the studies that are grouped according to experimental design, in which subjects were randomly assigned to wait-list control, placebo control/sham-feedback, or active comparison treatment. Furthermore, we focus here on methodologically rigorous, empirically sound studies published in peer-reviewed, English language journals; in our opinion, these studies contribute most strongly to scientifically informed conclusions as to the potential efficacy of NF for ADHD (see Table 2).
Table 2.
Summary of neurofeedback treatment studies for ADHD
| NF protocol | Study | Year | Total N (NF, control) | Age range | Experimental design | Control condition | Main findings |
|---|---|---|---|---|---|---|---|
| Active control studies | |||||||
| θ/β | Bakhshayesh et al. [69] | 2011 | 35 (18,17) | 6-14 years | 1, 2 | EMG biofeedback | NF > EMG on IN, CPT RT, and concentration |
| θ/β | Steiner et al. [72] | 2011 | 41 (13, 13 AT, 15) | 11-13 years | 1, 6 | Attention training (AT), WLC | Both txs result in reduced ADHD sxs. Feasible in school setting |
| θ/β and SCP | Gevensleben et al. [70] | 2009 | 94 (59, 35) | 9-12 years | 1, 5, 6 | Computerized attention training (AT) | NF = larger tx change than AT. NF responders 52 %, AT responders 29 % |
| reanalysis | Gevensleben et al. [67] | 2009b | 72 (46, 26) | 9-12 years | 1,5,6 | NF = reduced central and parietal theta activity. Different EEG mechanisms affected by NF protocol | |
| reanalysis | Gevensleben et al. [77] | 2010 | 61 (38, 23) | 9-12 years | 1,5,7 | At 6-mo FU, improvement maintained for both NF and AT groups | |
| reanalysis | Wangler et al. [74] | 2011 | 84 (56, 28) | 9-12 years | 1,5 | SCP training results in larger CNV amplitude compared to AT, but no difference in cognitive performance | |
| θ/β or SCP | Leins et al. [68] | 2007 | 38 (19, 19) | 8-13 years | 1,2,7 | SCP vs θ/β ratio NF | Both groups improved in ADHD behavior, IQ, and cortical regulation. Effects maintained at 6-mo FU |
| SCP | Drechsler et al. [71] | 2007 | 30 (17, 13) | 9-13 years | 3 | Group therapy (GT) | NF > GT on IN; all cognitive results NS. Parent support signif associated with outcome |
| reanalysis | Doehnert et al. [73] | 2008 | 26 (14, 12) | 9-12 years | 3 | NF = GT in resting EEG, CNV amp, cognitive performance. NF = incr in parietal alpha associated with improved impulsivity | |
| Sham/Placebo-controlled studies | |||||||
| θ/β | Arnold et al. [64] | 2012 | 39 (26, 13) | 6-12 years | 1,2 | Sham NF | Both groups improved, no signif differences betw active and sham NF |
| θ/β | Lansbergen et al. [24] | 2011 | 14 (8, 6) | 8-15 years | 1,2,4 | Sham NF | Both groups improved, no signif. differences betw active and sham NF |
| θ/β | Logemann et al. [60] | 2010 | 26 (14, 13) | College age | 1,2,4 | Sham NF | Both groups improved, no signif. differences betw active and sham NF |
| θ/β | Perreau-Linck et al. [65] | 2010 | 9 (5, 4) | 8-13 years | 1,2,6 | Sham NF | Both groups improved, neither group improved more than the other |
| Wait-list control studies | |||||||
| θ/β | Levesque et al. [62] | 2006 | 20 (15, 5) | 8-12 years | 1,6 | WLC | NF improved IN, cognitive performance, functional magnetic resonance activation. WLC no change |
| SCP | Heinrich et al. [61] | 2004 | 22 (13, 9) | 7-13 years | 1 | WLC | NF > WLC on ADHD behaviors, CPT impulsivity errors, incr CNV amp |
| θ/β | Linden et al. [63] | 1996 | 18 (9, 9) | 5-15 years | 1 | WLC | NF = WLC on ADHD behaviors, IQ |
θ/β = theta/beta (including SMR) ratio; 1 = randomized; 2 = singe/double blind; 3 = incomplete randomization; 4 = individualized NF; 5 = multi-site; 6 = no direct or inappropriate statistical comparison between groups; 7 = 6-month follow-up; ADHD = attention-deficit/hyperactivity disorder; btw = between; CNV = contingent negative variation; CPT = continuous performance test; FU = follow-up; impr = improved; IN = inattentive symptoms; incr = increased; IQ = intelligence; NF = neurofeedback; NS = not significant; RT = reaction time; SCP = slow cortical potential; signif = significant; SMR = sensorimotor rhythm; sxs = symptoms; txs = treatment; WLC = wait-list control
NF Therapy Procedures
To better describe the NF therapy process, first we briefly review the 2 types of NF protocols in which participants attempt to influence theta/beta ratio and SCP amplitude measures, respectively.
Theta/Beta Protocols
As previously stated, the original rationale for reducing theta band activity and increasing SMR/beta band activity during NF (subsequently referred to as θ/β) was to remediate observed diffuse EEG slowing that has been interpreted as expressing or supporting cortical underarousal in ADHD. In the studies reviewed, θ/β NF generally has been delivered in 1-to-1 sessions involving a child and a therapist. In these studies, 1 to 3 active electrodes are applied to the scalp using a single earlobe or linked-earlobes reference. A single electrode at the vertex (Cz) is most often used for NF, however, location of the dispositive electrode has varied by study, including frontal (FZ, F3, F4), central (Cz, C3, C4), parietal (PZ, P3, P4), and other midline (FCZ, CPZ) scalp sites. Therapy sessions in these studies generally occur 2 to 3 times per week and last 30 to 60 minutes. The total number of sessions per child in these studies ranges between 20 and 40 sessions. In all studies, positive reinforcement/reward is given by the controlling computer program for decreased theta power and increased SMR (13-16 Hz) or beta (16-20 Hz) band activity. Although individualized reinforcement thresholds in the θ/β frequency bands are set and then adjusted throughout the course of NF, the electrode locations and reinforced frequency bands are usually standard across all subjects in the study. Two studies [24, 60] have used individualized treatment protocols to target specific neurophysiologic deficits exhibited by the individual subject relative to a normative database.
SCP Protocols
With the exception of the first SCP study [61], the reviewed SCP studies have used a fairly uniform treatment procedure. In these studies, SCP NF involves 1 therapist and 1 to 2 children who receive training together. A single electrode at the vertex (Cz) is used in SCP NF. Treatment consists of 3 phases of 10 sessions each and delivered 5 days a week, each phase therefore lasting approximately 2 weeks. In these studies, each NF session includes both feedback and transfer trials. During the feedback trials, participants are given auditory and visual feedback regarding their ability to control the polarity (positive or negative) of the measured slow cortical shift, and are rewarded with a positive-valence audiovisual display when they produce a shift in the desired direction (positive or negative). During transfer trials, no immediate feedback on their cortical shift is given; here the child receives a reward when the desired cortical shift is produced. Children are not explicitly instructed on how to produce the desired cortical shift, but are told to attend to the feedback and attempt to find the most successful strategy, which they are later asked to describe verbally to the therapist. Children in these studies are also encouraged to engage in “transfer exercises” during the 2 breaks of 4- to 6-weeks between treatment phases, as well as during the last treatment phase. During these transfer exercises, the children are asked to practice their potential-shifting strategies at home and to record their daily practice. At the end of training, a memory aid is given to the children to use as a visual cue for self-regulation while doing homework and at other times when self-regulation is needed. The entire course of treatment in these studies lasts 14 to 18 weeks.
Wait-list Control Studies
Wait-list control (WLC) studies tend to be the first step in establishing whether a treatment is effective in ameliorating symptomatology compared to the absence of treatment while still controlling for maturation, practice effects, and the developmental course of the disorder, which is particularly important in studies involving children and adolescents. Because the WLC group does not receive any treatment, however, it is nearly impossible for parents and children to be blind to which group they are in, and difficult for experimenters (with the exception of independent evaluators) to be blind to treatment group status. In addition, the amount of nonspecific treatment effects is unequal among groups with the active treatment group having much more exposure to a therapeutic environment. This may lead to differences in motivation and expectation that are hard to control for in post-treatment outcome measures. Nonetheless, WLC studies are a first step in demonstrating treatment efficacy. Three such studies [61–63] have used a WLC experimental design; of these, 2 used a θ/β protocol, and the third a SCP protocol.
WLC Study Results
Overall, findings of the 3 studies suggest a positive effect of NF training on post-treatment measures relative to pre-treatment levels, with 1 study demonstrating significant improvement relative to the WLC group. Only the Heinrich et al. [61] study demonstrated a significant group by time interaction, which suggests that the active NF group differed significantly from the WLC group on the outcome measures of ADHD behavior, cognitive impulsivity, and EEG measures. The Heinrich et al. [61] study, however, involved a heavy NF training schedule of 25 SCP training sessions of 50 minutes each over the course of 3 weeks (21 days), suggesting double sessions occurred for some or all of the training. This intensive training may have resulted in a stronger treatment effect but might only be feasible for children who, from the outset of treatment, can engage in nearly 2 hours per day of NF treatment. The other 2 studies [62, 63] did not demonstrate a statistically significant improvement of the NF group in comparison to the WLC groups, with the Levesque et al. [62] study using statistics that demonstrated significant within-group change, but not between-group changes.
The inclusion of brain-based measures as outcome variables is a clear strength of 2 of the studies [61, 62]. The Heinrich et al. [61] study provides neurophysiological evidence for the effect of SCP treatment by applying ERP analysis to EEG recorded during a CPT task. The active NF group exhibited a larger amplitude contingent negative variation (CNV) to the cue stimulus compared to the WLC group (p = 0.02), but the 2 groups did not differ in mean P300 peak amplitude during the target response. Based on these findings, the authors suggest that SCP NF training may primarily affect self-regulatory capabilities (reflected by the enhanced CNV) rather than cognitive processing (represented by the unchanged P300 amplitude).
The Levesque et al. [62] study included pre- and post-treatment functional magnetic resonance imaging scans. After treatment, the NF group exhibited significant activation in the left substantia nigra, right anterior cingulate, and left caudate nucleus during Stroop interference, suggesting that NF may remediate core neurobiological mechanisms involved in ADHD. However, this finding should be considered preliminary until it has been replicated in additional studies with larger samples and proper controls. Furthermore, we note that post-treatment Stroop behavioral performance was low (<70 % accuracy) for both NF and WLC groups groups, demonstrating that remediation is not equivalent to normalization, nor is it the same as showing the mechanism by which the remediation is produced by the treatment. Both the Levesque et al. [62] and Heinrich [61] studies therefore contribute to our understanding of the potential biological mechanisms underlying NF treatment, and the important pathways that may be remediated in ADHD through NF training.
Methodological Weaknesses
Sample sizes for all 3 WLC studies are small and range from 18 to 22 participants, most of whom were boys (data not reported for Linden et al. [63]). Such small sample sizes have obvious limitations, such as low statistical power to detect treatment effects and potentially reduced generalizability of results. The limited statistical power is especially problematic for the Linden et al. [63] and Levesque et al. [62] studies in which inappropriate statistics were used to test treatment effects of NF.
In the Linden et al. [63] study, the omnibus multivariate analysis of variance that was used to test treatment effect (NF vs WLC) and time (pre- and post-treatment) on the dependent variables (intelligence scores and behavior ratings of inattention, hyperactivity, and aggression), was not significant for main or interaction effects. Because the multivariate analysis of variance was not significant, the subsequent univariate ANOVAs comparing pre- and post-treatment scores are inappropriate and increase the possibility of type 1 (false-positive) errors. Despite reporting adequate power (≥0.8) to detect group differences on all measures except hyperactivity, this study may have been underpowered due the use of multivariate versus univariate statistics. Similarly, in the Levesque et al. [62] study, the authors reported significant improvements on pre- and post-measurements within the NF group, but not in the WLC group, on working memory, sustained attention, and parent-rated inattention and hyperactive behaviors. These comparisons, however, were carried out within each group (NF and WLC) rather than between the groups. Thus, it is impossible to say whether the NF group improved more than the WLC group over time.
Similar analytic techniques were used with the functional magnetic resonance imaging data, in which the NF group reportedly demonstrated increased activation of the right anterior cingulate and left caudate nucleus after NF treatment relative to pre-treatment scans. Because the WLC group was very small (n = 5), the power to detect any increase or decrease in activation within this group is unacceptably low and confounds the conclusions based on these analyses. The same issue did not occur for the NF group, which had 15 children and was properly powered to detect differences between conditions (incongruent minus neutral) and time points (pre- and post-treatment). The statistical confounds of these 2 WLC studies limit the conclusions that can be made in regard to the efficacy of NF relative to no treatment.
Placebo Control/Sham Feedback Studies
Four recent NF studies have incorporated a placebo control in the form of sham-feedback [24, 60, 64, 65]. The placebo control studies constitute the strictest test of NF treatment effect because the participants experience the same treatment protocol but receive feedback based on brain signals other than their own. This design makes implementing a treatment blind (either single- or double-blind) more feasible because the children receive (at least superficially) the identical treatment experience. There are now NF applications available that can adjust reinforcement thresholds automatically during the NF sessions. This allows the therapist to be blind to active/placebo treatment, as well as the participants. All of the studies incorporated at least a single-blind in which children and parents did not know which treatment group they had been assigned and 3 of the 4 studies incorporated a double-blind in which therapists were also blind to treatment assignment. Post-treatment interviews in all studies support the fidelity of the treatment blind; in fact none of the participants (or their parents) was able to predict group assignment at levels higher than chance.
Sham NF Study Results
Overall, these studies do not support NF treatment as being more effective than the placebo. It is notable that these 4 studies, along with a fifth, uncontrolled study [66] are remarkably consistent in finding that both groups (active and sham feedback) demonstrated improvement in primary ADHD symptoms with no differential improvement for those who receive active treatment versus those in the placebo control group. After NF treatment, there were also no significant changes in secondary measures, such as resting EEG spectral power in the trained frequency bands [24, 60, 64], and no differences in the pattern of changes on neuropsychological tests measuring executive function [65].
These negative results for θ/β NF relative to placebo control are in contrast to the WLC studies previously reviewed, and they suggest that nonspecific treatment effects, such as investment of time, motivation, positive expectations, normal development, and/or exposure to a therapeutic environment may account for clinical improvement that has been attributed to NF training. In addition, these studies demonstrate that it is feasible to carry out placebo studies of NF and maintain an effective treatment blind for both participants and experimenters. There are, however, methodological limitations in this group of studies (to be discussed as follows) that constrain firm conclusions and necessitate further research.
Methodological Weaknesses
The primary limitation of the placebo control studies concerns the sample size, which was generally small for most of the studies. In the controlled studies to date, the sample sizes for NF active treatment (Ns range, 5-26) and for sham feedback (Ns range, 4-13) are quite small. Such small sample sizes make it difficult to have adequately powered statistical comparisons between groups. For example, in the Perreau-Linck et al. [65] study, the small sample size precluded direct statistical tests of the 2 groups. Instead, a reliable change index for each subject was used to analyze pre- and post-treatment outcome measures. Overall, both groups reportedly improved in parent-rated ADHD behaviors, on a computerized continuous performance test, and on other neuropsychological tests of executive function. The pattern of results did not suggest clear improvement for either group compared to the other on any of the outcome measures. It should be noted that the lack of statistically significant effects do not appear to be a result of low power because the group means for active and sham NF treatments did not differ and, in some cases, were actually higher at post-treatment for the placebo treatment on numerous measures [64].
All of the studies commented on methodological differences in the NF protocols that may also account, in part, for the contrasting results. Questions were raised in 3 of the 4 studies as to whether placebo feedback that is adjusted by the computer automatically and dynamically to maintain frequency thresholds for reinforcement (e.g., the θ/β ratio of the subject must be below the selected threshold 80 % of the time in a 30-second interval to receive a reward) is as effective as having a therapist manually adjust reinforcement thresholds. More data is needed on how often the threshold is, in fact, adjusted through the course of a NF session and whether having a moving target (i.e., changing threshold) versus a steady target changes one’s ability to exert control over their own brain activity. Individualized EEG protocols were used in 2 of 4 placebo-control studies [24, 60], which is in contrast with the standard NF format and electrode placement used in all other NF studies. Finally, the Perreau-Linck et al. [65] study screened children for EEG abnormality, creating a more homogeneous NF subject group, but differing from all other NF studies reviewed here. This limits the comparison of results to the other studies, although these procedures might also be expected to produce more consistent NF results.
None of the SCP studies have used a placebo-control methodology, usually due to ethical concerns about giving a placebo treatment for the length of NF treatment, which is often 3 to 4 months. The Lansbergen et al. [24] and Perreau-Linck et al. [65] study used a reasonable approach to this issue by allowing participants to remain on medication as long as they did not make changes during NF treatment. This raises other issues as to whether NF treatment differs in the presence of stimulant medication, however, until efficacy relative to placebo-control has been demonstrated, this appears to be a reasonable accommodation. Other concerns cited as prohibitive of using sham feedback entails the practice needed to gain mastery over cortical self-regulation and the possibility of demoralizing effects due to practicing a placebo strategy [67]. Because of these reasons, there have been relatively more active-treatment control studies for both θ/β and SCP training; these are reviewed next.
Active Treatment Control Studies
There are five recent studies involving the comparison of NF to other active treatments. Three of the studies use SCP training, with several subsequent articles providing additional analyses of the same sample, and the other 2 use θ/β training. This group of studies addresses the comparative effects of NF treatment to other treatments, including cognitive-behavioral based group therapy, computerized attention training, and electromyographic (EMG) biofeedback. There is also a study that provides a comparison between the effects of both types of NF protocols (θ/β and SCP) [68]. These studies provide another level of experimental control by comparing NF treatment to other active treatments, and these can thereby control the unequal amount of nonspecific treatment effects evident in WLC studies. It is also theoretically easier to implement a treatment blind because all of the participants experience an active treatment; however, treatment blinds were used in only 2 of these studies [69, 70]. This group of studies is also notable for other methodological strengths, such as the first multi-site study of NF with a large sample size [70], demonstration of feasibility of performing NF within a school setting [72], use of a control treatment also involving electrodes [69], assessment of parental support and satisfaction as mediating factors [68, 70, 71], and description of stability of effects at the 6-month follow-up [68, 70].
Active Treatment Control Results
Overall, these 5 active treatment control studies yield several conclusions. First, they demonstrate through direct statistical testing that NF treatment results in significantly fewer symptoms of parent-rated inattention when compared to group therapy [71] or EMG biofeedback [69]. Three studies used 1-tailed t tests to examine the amount of treatment-related change exhibited either within each treatment (e.g., through differences in pre- and post-treatment scores) [68, 72], or between treatments (e.g., through differences in change scores calculated by subtracting pre- and post-treatment scores) [70], but they did not directly compare post-treatment scores between treatments.
Thus, these statistics do not allow for conclusions as to whether the NF group had fewer ADHD symptoms at post-treatment relative to the comparison treatment, but are rather limited to the questions of whether significant change was achieved within each treatment or the relative degree of change between treatments. None of the studies demonstrated significant differences between NF and comparison treatments in teacher ratings of ADHD symptoms through direct testing, and 3 of the 4 studies (Bakhshayesh et al. [69] being the exception) failed to find significant differences in cognitive functioning (e.g., CPT, attention network test, test for attentional performance, trail-making test) at post-treatment assessment.
It is important to note here that comparing NF to an active treatment can constitute a more rigorous test of NF (vis a vis treatment alternatives), however, none of the comparison treatments have received empirical support as a treatment for ADHD. If NF demonstrates roughly comparable results to other treatments that have not been found to be effective in treating ADHD, then what can we conclude concerning the efficacy of NF in treating ADHD? The strongest conclusions that can be made are, first, that NF is superior to unproven treatments for ADHD, and second, that it remains to be tested whether NF can be as effective as first-line treatments for ADHD with demonstrated efficacy (i.e., stimulant medication).
A third conclusion that can be made from this group of studies concerns specific versus nonspecific effects of NF training. Here NF treatment, particularly SCP training, appears to produce specific and nonspecific treatment effects that are additive. The Doehnert et al. [73] and Gevensleben et al. [67] studies demonstrate changes in some EEG characteristics and CNV amplitude with NF training that are related to behavioral outcomes. These studies also suggest that nonspecific treatment effects, such as active practice and parental support may be an important part of the NF effect. A final conclusion is that there is variability in response to NF training, and that it is only for a subgroup of children that the positive behavioral effects emerge. The most methodologically sound study conducted thus far [70] provides support for the efficacy of NF for some (~50 %) but not all children with ADHD.
Specific versus Nonspecific Treatment Effects
The question raised by Loo and Barkley [37] regarding the neurophysiological mechanism through which NF achieves its effects was examined in a number of ways within this group of studies. One such approach is to examine whether there were specific EEG changes consistent with the respective training procedures (SCP or θ/β) over the course of treatment and whether the changes in the trained components were associated with behavioral and cognitive improvement. θ/β protocols are hypothesized to train tonic aspects of cortical arousal, whereas SCP training is thought to assist with phasic regulation of cortical excitability [70]. The design of the SCP studies lends itself to answering this question because the extent to which the participant can regulate positive and negative shifts as directed can be examined during transfer trials when no feedback is given.
In a direct comparison of the 2 training protocols, Leins et al. [68] adapted the θ/β training to include treatment blocks and transfer sessions similar to SCP training. Gevensleben et al. [70] also used a combined treatment in which each subject received both SCP and θ/β training, given in discrete blocks in counterbalanced order. These studies provided direct tests of the EEG changes that occur after both types of training. Because the specific EEG effects differ by training protocol, we review them separately below; however, we note that the behavioral outcomes were generally similar for both types of NF [68, 70].
Specific Effects of θ/β Ratio Training
Findings in these studies for NF training focusing on the θ/β ratio were mixed. NF resulted in a decreased theta/beta ratio with treatment in some [68, 69], but not all studies [67]. The decrease in θ/β ratio was most pronounced in the Leins et al. [68] study, where effect sizes ranged between 0.74 and 1.32 for different aspects of the θ/β ratio at the end of treatment. Despite these large effect sizes, participants were unable to decrease their θ/β ratio during transfer sessions in which feedback was not given [68]. Effect sizes of the change in θ/β ratio were much smaller (range from 0.13 to 0.39), and this ratio was not reliably decreased across different feedback conditions within the Bakyshayesh [69] study. In the Gevensleben et al. [67] study, θ/β training did not result in a reduced θ/β power ratio but instead a decrease of posterior-midline theta power at post-treatment. None of the studies reported significant associations between the theta/beta power ratio (or its changes over treatment) and ADHD symptomatology. That theta and beta amplitudes at the measured scalp channel may change independently over the course of treatment and not in lock step may underlie some of the variability in the θ/β changes reported by the studies reviewed above. Nonetheless, these findings suggest significant variability across subjects in the ability to decrease the θ/β ratio as a consequence of active NF training. The specific neural effect of θ/β NF training thus remains unclear.
SCP Treatment Effects
SCP training appears to increase the degree to which children are able to regulate their brain potentials and produce negative SCPs (i.e., CNVs) during both active feedback and transfer sessions [68, 71]. Positive electrical shifts were not reliably produced. Effect sizes ranged from 1.04 to 1.07 at treatment end, suggesting a robust change in CNV amplitude over the course of treatment [68]. SCP training also resulted in an increase of central-midline alpha band activity, which was associated with improvements on a German ADHD rating scale [74]. Even though the parents of children who received SCP training reported that their children exhibited improved cognitive regulation (inattention, metacognition), these effects are not significantly correlated with CNV amplitude [71]. Collectively, these studies suggest that SCP training results in larger amplitude CNVs and improved regulation of positive and negative cortical shifts, however, the correlations between CNV amplitude and measures of behavioral/cognitive functioning are modest.
Specific Treatment Effects of NF Training vs Comparison Treatment
Another approach to examining specific treatment effects is to test changes in EEG/ERPs between treatment groups (NF vs comparison treatment) and any subsequent association with behavioral or cognitive functioning. Two follow-up articles of studies by Doehnert et al. [73] and Gevensleben et al. [67] provide comparative analysis of EEG characteristics at pre- and post-training measurement between NF and comparison treatments.
Specific treatment-related changes in the EEG were mixed. Doehnert et al [73] reported no significant differences in resting EEG between children who received SCP NF versus those who received group therapy, as well as no significant change in CNV amplitude during CPT performance after SCP training. In contrast, Gevensleben et al. [67] reported a significant decrease in resting state central-parietal theta band power among children who received combined θ/β and SCP NF training compared to those who received attention training. However, during the attention network task, children who received NF training exhibited significantly larger CNV amplitude to the pre-stimulus cues (but not the target) than children in the attention training condition, suggesting improved phasic regulation of cortical excitability and cognitive resources [74]. Subsequent analyses suggest that the larger CNV was due specifically to SCP rather than θ/β training and was associated with improved ADHD symptomatology [74].
Thus, these studies collectively show that NF training can produce some specific changes in EEG/ERP characteristics, such as decreased theta band, increased alpha band power and larger CNV amplitude, which are associated with specific improvements in hyperactive-impulsive symptomatology. These data, therefore, suggest there may be specific effects of learned self-regulation of cortical activity, although subject variability in the presence and strength of the EEG changes, and their inconsistent relationship with outcome variables suggests that nonspecific effects may also play a role in producing positive clinical outcome.
Nonspecific Treatment Effects
Nonspecific treatment effects are a broad category of effects that contribute to clinical outcome but are not considered an active ingredient in the treatment being administered. Traditional nonspecific effects, such as motivation and expectation for improvement, may result from going to a therapy site, having contact with a therapist who gives unconditional positive regard, or performing in a supportive environment. Three of these studies [68, 70, 71] included measures of parental expectancies, satisfaction and support and 1 study [71] examined the role that these factors play in clinical outcome.
Overall, parental satisfaction with NF was high, [70], however, parents preferred SCP training to θ/βtraining when these protocols were compared directly [68]. Parental support was significantly correlated with degree of improvement in parent ratings of inattention and the teacher’s global rating on the Conner’s rating scale, and more strongly related to clinical outcome than the EEG changes [71]. These data suggest that parental support may play a larger role in improved ADHD symptomatology than changes in cortical regulation per se.
In a subsequent article [73], the same authors conclude that NF training should be regarded as a kind of behavioral psychotherapy in which positive expectations and the experience of self-efficacy are important nonspecific variables [73]. This is not surprising given that SCP training does not merely include control over electrophysiological processes, but it involves behavioral learning principles and active learning strategies that appear to be integral to the treatment effect. Future research should assess which nonspecific treatment effects contribute most strongly to behavioral outcome and attempt to determine the best way to maximize these aspects of the treatment.
Responder Status
A final conclusion that can be reached from this group of studies is that some, but not all children with ADHD appear to show a positive response to NF training. This is logical, given the marked heterogeneity of ADHD in nearly all domains: behavioral, cognitive, and pathophysiological. Although stimulant medications are the golden standard of treatment for ADHD, a significant minority of children (20-30 %) are not medication responders. Thus, it seems reasonable that children with ADHD would also show variability in their response to NF training. This has been examined using 2 different approaches: 1) by the percentage of children whose primary ADHD symptoms improve, and 2) by the percentage of children who are able to regulate their trained EEG measure after NF training.
Using a behavioral criterion of 25 % improvement in primary ADHD symptoms to define responders, Gevensleben et al. [70] found that 52 % of children with ADHD were responders to NF, a higher response rate than to attention training (29 %). Subsequent analyses suggested that children who had lower parietal alpha band power and larger amplitude CNV during cued continuous performance test at baseline benefited most from SCP training [74]. When looked at from the standpoint of EEG regulation, Drechsler et al [71] performed a median split in their sample and compared outcomes for children who were able to discriminate between positive and negative cortical shifts and produce larger amplitude CNV (“good” responders) versus those who could not (“poor” responders). Although most analyses were not statistically significant due to small sample size (N ~8-9 in each group), good responders exhibited positive correlations between CNV amplitude and both improved ADHD symptoms and cognition [71], whereas the poor responders did not.
Studies examining the effects of NF training on healthy volunteers have also reported that the percentage of individuals who are able to exert control over their EEG is approximately 50 % [75, 76]. If only half of children trained with NF are responders, this may account for \the inconsistent results across studies, depending on the composition of the subject group. Further studies are needed to determine the rate of response for θ/β and SCP protocols separately, as well as the behavioral, cognitive, and EEG predictors of positive response to NF training [61, 62].
Summary Conclusions on the Current State of ADHD NF Studies
The literature on NF has grown considerably and the scientific methodologies of the studies have improved, allowing more firm conclusions with regard to NF treatment for ADHD. We have reviewed several types of controlled studies on NF involving WLC, placebo control, and active treatment comparison. Across these studies, there are only a handful of studies that have directly compared the effects of NF to other control therapeutic approaches. Among the SCP studies, Heinrich et al. [61] and Drechsler et al. [71] demonstrated that primary ADHD symptoms were significantly improved with NF training compared to WLC or group therapy, respectively. The Gevensleben et al. [70] study did not directly compare NF and attention training post-treatment scores on ADHD symptomatology, which is unfortunate because theirs is methodologically the strongest study in the NF literature. Examination of the mean scores in the Gevensleben et al. [77] 6-month follow-up article suggests there were likely some significant post-treatment differences between NF and AT; this can be easily answered with direct statistical testing of pre- and post-treatment scores of ADHD symptomatology. Among the θ/β studies, only Bakhshayesh et al. [69] demonstrated a significant treatment-by-time interaction on parent-rated inattention symptoms versus EMG biofeedback; all other studies either did not directly test or did not demonstrate significant differences between θ/β training compared to WLC, placebo control, or active treatment control.
Thus, the findings of the studies reviewed herein do not support NF training as a first-line, stand-alone treatment for ADHD. Until NF training can demonstrate an effect that is either superior to placebo control or equivalent to other empirically supported treatments for ADHD (i.e., psychostimulant medication, behavior therapy), it simply cannot be considered a primary treatment modality. In fact, given the expense and time/labor intensive nature of the NF, one might be hard pressed to recommend NF training versus stimulant medication, even if comparable effect sizes were demonstrated, unless there are clear contraindications for medication, or unless NF demonstrates continued long-term benefits after completion of treatment that exceeds that of medication treatment.
Although NF treatment is not recommended as a first-line treatment, SCP training appears likely to be efficacious as an adjunct treatment for a subset (~50 %) of children with ADHD. Among positive responders, SCP training appears to have specific effects on enhanced cortical regulation that is associated with improved ADHD symptomatology. Because not all children with ADHD can be expected to improve with NF training, it should be used as an adjunct treatment or as part of a multimodal treatment package that includes medication, psychosocial, and educational accommodations. However, more research is needed on issues such as response rate, predictors of positive response, the role of specific and nonspecific treatment effects in outcome, and side effects of NF treatment. The state of the published, peer-reviewed literature on θ/β training, as it currently stands, does not support theta/beta NF training even as an adjunct treatment.
Future Directions in EEG Research for ADHD
Because of the very broad point-spread function from cortical source to scalp recording (Fig. 1), EEG has been fairly described for a long time as a temporally precise, but spatially blurred brain imaging modality. For nearly the last 2 decades, however, a new approach to EEG analysis has developed from the observation that under most realistic conditions, temporally distinct source activities can be separated from the signal mixture “blindly” based on their separate contributions of information (e.g., distinctive wave shapes) to the recorded data. In the ideal case, source signals from each locally synchronous cortical area can be said to each constitute an independent component source of the recorded data.
If so, with correct application, independent component analysis (ICA) methods can be used to separately identify these independent component processes blindly (i.e., with no particular a priori knowledge of their individual spectral or other properties) [78]. During the last 15 years, we [79–82] have established that ICA can separate high-density EEG data into as many as dozens of brain source processes whose approximate or potentially even exact origins in cortex can be identified [3, 83, 84], as well as many of dozens of functionally distinct nonbrain process contributions (from eye blinks, scalp muscles activities, electrocardiographic artifact, line noise, and so forth).
How does ICA work? Intuitively, ICA finds the most temporally distinctive (i.e., independent) waveforms whose sum are the recorded data. ICA algorithms use an iterative approach, separating the data into more and more independent source processes until a maximum possible level of source independence is achieved. Naturally, if the source processes generating the data are largely functionally independent of one another, then their individual potential waveforms will exhibit more distinctive differences from each other than any weighted sums of their activities (including the recorded scalp signals themselves). Because each such source mixture must contain distinctive features from all its contributing sources, the mixtures cannot be as distinct from one another as the source signals themselves are from each other. Thus ICA, when correctly applied to sufficient data of good enough quality, must arrive at identifying the actual source signals from various locations (e.g., eyes, muscles, heart, and the cortical areas in which locally-synchronous field activities are currently being produced and delivered by volume conduction to the scalp).
Thus, ICA provides a method for separating brain from nonbrain signals in EEG data, for imaging its cortical sources, and for examining, with relatively high precision, the dynamics of single sources or source networks, even in single trials or other continuous time periods without requiring response averaging [85, 86].
The potential advantages for using ICA to discover brain-based biomarkers of ADHD status are several: 1) The specific cortical areas involved in the measure can be estimated far better than using scalp channel data directly [87]; 2) the effective signal-to-noise ratio of the ICA-separated source activities is much higher than in the original scalp channel mixtures [34], making it possible for the development of more statistically specific, robust, and individualized biomarker measure; 3) the likelihood of this possibility is further enhanced by the ability of ICA to separate out and allow subsequent measures to ignore irrelevant nonbrain signals in the data [88–90]; 4) new methods are available to model independent component source activities, including log spectral ICA decomposition [3], directed transfer function estimates of effective connectivity [91], and nonstationary ICA [92], all of which can deliver more specific measures of specific cortical activity patterns than any single scalp channel measure.
In the first example of the application of ICA to EEG data from a clinical ADHD study (previously reviewed), Mueller et al. [41] applied ICA decomposition to matrices of 19-channel ERP averages from 297 participants in a visual Go/NoGo task and used peak features of the resulting component ERPs to select ERP window inputs to a support-vector machine subject group classifier. Applying ICA to matrices of ERPs has the advantage of efficiently separating spatiotemporally distinct information in the data, but the results cannot be as spatially distinct as ICA decomposition of the nonaveraged continuous EEG data, which can also benefit from extra degrees of freedom afforded by higher density recordings. High-density EEG systems are becoming ever more portable, with at least 1 system of an 80-channel dry electrode communicating wirelessly with an ordinary cell phone or tablet computer, and this is now under final development.
IC activity features can also form the basis for optimized real-time classifiers of cognitive state or response [93], and comprehensive software is now available for developing and testing a range of individualized, brain source-based measures for NF applications [94, 95] (see: http://sccn.ucsd.edu/wiki/BCILAB). Statistical machine-learning methods for building source-level feature models of EEG recorded during individual subject periods of better and worse performance in baseline attention tasks could be used to deliver, to the same subject, brain-based feedback directly estimating their current level of attentiveness or feedback combining contributing features that were found to play a major role in recorded shifts from attention to inattention in the pilot data. This NF strategy could have twin benefits, first, of training endogenous EEG phenomena in each subject that are, second, most directly associated with the behavioral and cognitive phenomena of primary therapeutic interest (e.g., frequent lapses of engagement during tasks requiring sustained attention). Finally, source-level analysis of the whole EEG record before, during, and after NF therapy could establish the neurophysiological processes and estimate the concomitant psychological processes underlying learning direct volitional control over brain processes [96].
Although ICA (and other machine-learning) methods have not yet been used much in psychiatric research or in ADHD research in particular, we believe that they represent an important future direction for the field to better grasp the neurophysiologic information in (and significance of) the collected data, with likely applications both for discovering robust diagnostic biomarkers, and for developing individualized and more brain area-specific NF measurements [93]. At the same time, the near-future availability of convenient, easily worn, and prepared high-density dry-electrode recording systems will make recording of detailed EEG brain imaging data straightforward in both diagnostic and NF modalities. It is likely that new computational methods applied to joint brain and behavioral data using body motion capture, eye gaze tracking, and other measures synchronized with EEG data collection will add further information as to the links between ADHD brain dynamics and behavior [97]. Through these advances in data collection and analysis, EEG, the first noninvasive functional brain imaging modality, may come to play a more salient role in ADHD research.
Electronic supplementary material
(PDF 216 kb)
Acknowledgments
This work was supported by a grant from the National Institute of Mental Health (1RO3MH92829 to S.K.L.), and a gift to University of California, San Diego from The Swartz Foundation (Old Field NY) to S.M., and by grants (1R01NS047293-01A1 and 1R01MH084819-01 to S.M.) from the U.S. National Institutes of Health. The authors wish to thank Marjorie Garvey of the National Institute of Mental Health (NIMH) for encouraging innovation in psychiatric brain research, and Zeynep Akalin Acar for use of her simulation figure. Full conflict of interest disclosures are available in the electronic supplementary material for this article.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Berger H. On the electroencephalogram of man. Electroencephalogr Clin Neurophysiol 1969;xx(suppl 28):37-73. [PubMed]
- 2.Jasper H, Solomon P, Bradley C. Electroencephalographic analyses of behavior problem children. Am J Psychiatry. 1938;95:641. [Google Scholar]
- 3.Onton J, Makeig S. High-frequency broadband modulations of electroencephalographic spectra. Front Hum Neurosci. 2009;3:61. doi: 10.3389/neuro.09.061.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monastra VJ, Lubar JF, Linden M, et al. Assessing attention deficit hyperactivity disorder via quantitative electroencephalography: an initial validation study. Neuropsychology. 1999;13:424–433. doi: 10.1037/0894-4105.13.3.424. [DOI] [PubMed] [Google Scholar]
- 5.Satterfield JH, Cantwell DP, Satterfield BT. Pathophysiology of the hyperactive child syndrome. Arch Gen Psychiatry. 1974;31:839–844. doi: 10.1001/archpsyc.1974.01760180079010. [DOI] [PubMed] [Google Scholar]
- 6.Satterfield J, Cantwell D, Saul R, Lesser L, Podosin R. Response to stimulant drug treatment in hyperactive children: prediction from EEG and neurological findings. Clin Neurophysiol. 1973;121:1511–1518. doi: 10.1007/BF01537553. [DOI] [PubMed] [Google Scholar]
- 7.Barry RJ, Johnstone SJ, Clarke AR. A review of electrophysiology in attention-deficit/hyperactivity disorder: II. Event-related potentials. Clin Neurophysiol. 2003;114:184–198. doi: 10.1016/S1388-2457(02)00363-2. [DOI] [PubMed] [Google Scholar]
- 8.Snyder SM, Hall JR. A meta-analysis of quantitative EEG power associated with attention-deficit hyperactivity disorder. J Clin Neurophysiol. 2006;23:440–455. doi: 10.1097/01.wnp.0000221363.12503.78. [DOI] [PubMed] [Google Scholar]
- 9.Chabot RJ, Serfontein G. Quantitative electroencephalographic profiles of children with attention deficit disorder. Biol Psychiatry. 1996;40:951–963. doi: 10.1016/0006-3223(95)00576-5. [DOI] [PubMed] [Google Scholar]
- 10.Clarke AR, Barry RJ, McCarthy R, Selikowitz M. EEG analysis in attention-deficit/hyperactivity disorder: a comparative study of two subtypes. Psychiatry Res. 1998;81:19–29. doi: 10.1016/S0165-1781(98)00072-9. [DOI] [PubMed] [Google Scholar]
- 11.Clarke AR, Barry RJ, McCarthy R, Selikowitz M. Electroencephalogram differences in two subtypes of attention-deficit/hyperactivity disorder. Psychophysiology. 2001;38:212–221. doi: 10.1111/1469-8986.3820212. [DOI] [PubMed] [Google Scholar]
- 12.El-Sayed E, Larsson JO, Persson HE, Rydelius PA. Altered cortical activity in children with attention-deficit/hyperactivity disorder during attentional load task. J Am Acad Child Adolesc Psychiatry. 2002;41:811–819. doi: 10.1097/00004583-200207000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Hobbs MJ, Clarke AR, Barry RJ, McCarthy R, Selikowitz M. EEG abnormalities in adolescent males with AD/HD. Clin Neurophysiol. 2007;118:363–371. doi: 10.1016/j.clinph.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Lazzaro I, Gordon E, Whitmont S, et al. Quantified EEG activity in adolescent attention deficit hyperactivity disorder. Clin Electroencephalogr. 1998;29:37–42. doi: 10.1177/155005949802900111. [DOI] [PubMed] [Google Scholar]
- 15.Matsuura M, Okubo Y, Toru M, et al. A cross-national EEG study of children with emotional and behavioral problems: a WHO collaborative study in the Western Pacific Region. Biol Psychiatry. 1993;34:59–65. doi: 10.1016/0006-3223(93)90257-E. [DOI] [PubMed] [Google Scholar]
- 16.Snyder SM, Quintana H, Sexson SB, et al. Blinded, multi-center validation of EEG and rating scales in identifying ADHD within a clinical sample. Psychiatry Res. 2008;159:346–358. doi: 10.1016/j.psychres.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Ogrim G, Kropotov JD, Hestad K. The QEEG theta/beta ratio in ADHD and normal controls: sensitivity, specificity, and behavioral correlates. Psychiatry Res 2012. doi:10.1016/j.psychres.2011.12.041 [DOI] [PubMed]
- 18.Loo SK, Hale TS, Hanada G, et al. Familial clustering and DRD4 effects on electroencephalogram measures in multiplex families with ADHD. J Am Acad Child Adolesc Psychiatry. 2010;29:368–377. [PMC free article] [PubMed] [Google Scholar]
- 19.Bresnahan SM, Barry RJ. Specificity of quantitative EEG analysis in adults with attention deficit hyperactivity disorder. Psychiatry Res. 2002;112:133–144. doi: 10.1016/S0165-1781(02)00190-7. [DOI] [PubMed] [Google Scholar]
- 20.Coutin-Churchman P, Anez Y, Uzcategui M, et al. Quantitative spectral analysis of EEG in psychiatry revisited: drawing signs out of numbers in a clinical setting. Clin Neurophysiol. 2003;114:2294–2306. doi: 10.1016/S1388-2457(03)00228-1. [DOI] [PubMed] [Google Scholar]
- 21.Monastra VJ, Lubar JF, Linden M. The development of a quantitative electroencephalographic scanning process for attention deficit-hyperactivity disorder: reliability and validity studies. Neuropsychology. 2001;15:136–144. doi: 10.1037/0894-4105.15.1.136. [DOI] [PubMed] [Google Scholar]
- 22.Koehler S, Lauer P, Schreppel T, et al. Increased EEG power density in alpha and theta bands in adult ADHD patients. J Neural Transm. 2009;116:97–104. doi: 10.1007/s00702-008-0157-x. [DOI] [PubMed] [Google Scholar]
- 23.Lansbergen MM, Arns M, Dongen-Boomsma M, Spronk D, Buitelaar JK. The increase in theta/beta ratio on resting-state EEG in boys with attention-deficit/hyperactivity disorder is mediated by slow alpha peak frequency. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:47–52. doi: 10.1016/j.pnpbp.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Lansbergen MM, Dongen-Boomsma M, Buitelaar JK, Slaats-Willemse D. ADHD and EEG-neurofeedback: a double-blind randomized placebo-controlled feasibility study. J Neural Transm. 2011;118:275–284. doi: 10.1007/s00702-010-0524-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nazari MA, Wallois F, Ardalan A, Berquin P. Dynamic changes in quantitative electroencephalogram during continuous performance test in children with attention-deficit/hyperactivity disorder. Int J Psychophysiol. 2011;81:230–236. doi: 10.1016/j.ijpsycho.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Magee CA, Clarke AR, Barry RJ, McCarthy R, Selikowitz M. Examining the diagnostic utility of EEG power measures in children with attention deficit/hyperactivity disorder. Clin Neurophysiol 2005;116(5):1033-1040. [DOI] [PubMed]
- 27.Dongen-Boomsma M, Lansbergen MM, Bekker EM, et al. Relation between resting EEG to cognitive performance and clinical symptoms in adults with attention-deficit/hyperactivity disorder. Neurosci Lett. 2010;469:102–106. doi: 10.1016/j.neulet.2009.11.053. [DOI] [PubMed] [Google Scholar]
- 28.Clarke AR, Barry RJ, McCarthy R, Selikowitz M. Age and sex effects in the EEG: differences in two subtypes of attention-deficit/hyperactivity disorder. Clin Neurophysiol. 2001;112:815–826. doi: 10.1016/S1388-2457(01)00487-4. [DOI] [PubMed] [Google Scholar]
- 29.Clarke AR, Barry RJ, McCarthy R, Selikowitz M. EEG-defined subtypes of children with attention-deficit/hyperactivity disorder. Clin Neurophysiol. 2001;112:2098–2105. doi: 10.1016/S1388-2457(01)00668-X. [DOI] [PubMed] [Google Scholar]
- 30.Loo SK, Hale TS, Macion J, et al. Cortical activity patterns in ADHD during arousal, activation and sustained attention. Neuropsychologia. 2009;47:2114–2119. doi: 10.1016/j.neuropsychologia.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clarke AR, Barry RJ, Dupuy FE, et al. Behavioural differences between EEG-defined subgroups of children with attention-deficit/hyperactivity disorder. Clin Neurophysiol. 2011;122:1333–1341. doi: 10.1016/j.clinph.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 32.Clarke AR, Barry RJ, McCarthy R, Selikowitz M, Brown CR. EEG evidence for a new conceptualisation of attention deficit hyperactivity disorder. Clin Neurophysiol. 2002;113:1036–1044. doi: 10.1016/S1388-2457(02)00115-3. [DOI] [PubMed] [Google Scholar]
- 33.Bresnahan SM, Anderson JW, Barry RJ. Age-related changes in quantitative EEG in attention-deficit/hyperactivity disorder. Biol Psychiatry. 1999;46:1690–1697. doi: 10.1016/S0006-3223(99)00042-6. [DOI] [PubMed] [Google Scholar]
- 34.Onton J, Delorme A, Makeig S. Frontal midline EEG dynamics during working memory. Neuroimage. 2005;27:341–356. doi: 10.1016/j.neuroimage.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 35.Arns M, Gunkelman J, Breteler M, Spronk D. EEG phenotypes predict treatment outcome to stimulants in children with ADHD. J Integr Neurosci. 2008;7:421–438. doi: 10.1142/S0219635208001897. [DOI] [PubMed] [Google Scholar]
- 36.Johnstone J, Gunkelman J, Lunt J. Clinical database development: characterization of EEG phenotypes. Clin EEG Neurosci. 2005;36:99–107. doi: 10.1177/155005940503600209. [DOI] [PubMed] [Google Scholar]
- 37.Loo SK, Barkley RA. Clinical utility of EEG in attention deficit hyperactivity disorder. Appl Neuropsychol. 2005;12:64–76. doi: 10.1207/s15324826an1202_2. [DOI] [PubMed] [Google Scholar]
- 38.Chabot RJ, Merkin H, Wood LM, Davenport TL, Serfontein G. Sensitivity and specificity of QEEG in children with attention deficit or specific developmental learning disorders. Clin Electroencephalogr. 1996;27:26–34. doi: 10.1177/155005949602700105. [DOI] [PubMed] [Google Scholar]
- 39.Quintana H, Snyder SM, Purnell W, Aponte C, Sita J. Comparison of a standard psychiatric evaluation to rating scales and EEG in the differential diagnosis of attention-deficit/hyperactivity disorder. Psychiatry Res. 2007;152:211–222. doi: 10.1016/j.psychres.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 40.Nazari MA, Wallois F, Aarabi A, Berquin P. Dynamic changes in quantitative electroencephalogram during continuous performance test in children with attention-deficit/hyperactivity disorder. Int J Psychophysiol. 2011;81:230–236. doi: 10.1016/j.ijpsycho.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 41.Mueller A, Candrian G, Grane VA, et al. Discriminating between ADHD adults and controls using independent ERP components and a support vector machine: a validation study. Nonlinear Biomed Phys. 2011;5:5. doi: 10.1186/1753-4631-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abibullaev B, An J. Decision support algorithm for diagnosis of ADHD using electroencephalograms. J Med Syst 2011. doi:10.1007/s10916-011-9742-x [DOI] [PubMed]
- 43.Ahmadlou M, Adeli H. Wavelet-synchronization methodology: a new approach for EEG-based diagnosis of ADHD. Clin EEG Neurosci. 2010;41:1–10. doi: 10.1177/155005941004100103. [DOI] [PubMed] [Google Scholar]
- 44.Sterman MB, Wyrwicka W, Roth S. Electrophysiological correlates and neural substrates of alimentary behavior in the cat. Ann N Y Acad Sci. 1969;157:723–739. doi: 10.1111/j.1749-6632.1969.tb12916.x. [DOI] [PubMed] [Google Scholar]
- 45.Roth SR, Sterman MB, Clemente CD. Comparison of EEG correlates of reinforcement, internal inhibition and sleep. Electroencephalogr Clin Neurophysiol. 1967;23:509–520. doi: 10.1016/0013-4694(67)90017-X. [DOI] [PubMed] [Google Scholar]
- 46.Sterman MB, Friar L. Suppression of seizures in an epileptic following sensorimotor EEG feedback training. Electroencephalogr Clin Neurophysiol. 1972;33:89–95. doi: 10.1016/0013-4694(72)90028-4. [DOI] [PubMed] [Google Scholar]
- 47.Sterman MB. Biofeedback in the treatment of epilepsy. Cleve Clin J Med. 2010;77(suppl 3):S60–S67. doi: 10.3949/ccjm.77.s3.11. [DOI] [PubMed] [Google Scholar]
- 48.Shouse MN, Lubar JF. Operant conditioning of EEG rhythms and ritalin in the treatment of hyperkinesis. Biofeedback Self Regul. 1979;4:299–312. doi: 10.1007/BF00998960. [DOI] [PubMed] [Google Scholar]
- 49.Lubar JF, Shouse MN. EEG and behavioral changes in a hyperkinetic child concurrent with training of the sensorimotor rhythm (SMR): a preliminary report. Biofeedback Self Regul. 1976;1:293–306. doi: 10.1007/BF01001170. [DOI] [PubMed] [Google Scholar]
- 50.Fuchs T, Birbaumer N, Lutzenberger W, Gruzelier JH, Kaiser J. Neurofeedback treatment for attention-deficit/hyperactivity disorder in children: a comparison with methylphenidate. Appl Psychophysiol Biofeedback. 2003;28:1–12. doi: 10.1023/A:1022353731579. [DOI] [PubMed] [Google Scholar]
- 51.Lubar JF, Swartwood MO, Swartwood JN, O'Donnell PH. Evaluation of the effectiveness of EEG neurofeedback training for ADHD in a clinical setting as measured by changes in T.O.V.A. scores, behavioral ratings, and WISC-R performance. Biofeedback and Self Regul. 1995;20:83–99. doi: 10.1007/BF01712768. [DOI] [PubMed] [Google Scholar]
- 52.Lubar JO, Lubar JF. Electroencephalographic biofeedback of SMR and beta for treatment of attention deficit disorders in a clinical setting. Biofeedback & Self Regul. 1984;9:1–23. doi: 10.1007/BF00998842. [DOI] [PubMed] [Google Scholar]
- 53.Monastra VJ, Monastra DM, George S. The effects of stimulant therapy, EEG biofeedback, and parenting style on the primary symptoms of attention-deficit/hyperactivity disorder. Appl Psychophysiol Biofeedback. 2002;27:231–249. doi: 10.1023/A:1021018700609. [DOI] [PubMed] [Google Scholar]
- 54.Ramirez PM, Desantis D, Opler LA. EEG biofeedback treatment of ADD. A viable alternative to traditional medical intervention? Ann N Y Acad Sci. 2001;931:342–358. doi: 10.1111/j.1749-6632.2001.tb05789.x. [DOI] [PubMed] [Google Scholar]
- 55.Rossiter TR, La Vaque TJ. A comparison of EEG biofeedback and psychostimulants in treating Attention-Deficit/Hyperactivity Disorders. J Neurother 1995;xx:48-59.
- 56.Tansey MA. Righting the rhythms of reason: EEG biofeedback training as a therapeutic modality in a clinical office setting. Medical Psychotherapy. 1990;3:57–68. [Google Scholar]
- 57.Tansey MA, Bruner RL. EMG and EEG biofeedback training in the treatment of a 10-year-old hyperactive boy with a developmental reading disorder. Biofeedback Self Regul. 1983;8:25–37. doi: 10.1007/BF01000534. [DOI] [PubMed] [Google Scholar]
- 58.Thompson L, Thompson M. Neurofeedback combined with training in metacognitive strategies: effectiveness in students with ADD. Appl Psychophysiol Biofeedback. 1998;23:243–263. doi: 10.1023/A:1022213731956. [DOI] [PubMed] [Google Scholar]
- 59.Birbaumer N, Elbert T, Canavan AG, Rockstroh B. Slow potentials of the cerebral cortex and behavior. Physiol Rev. 1990;70:1–41. doi: 10.1152/physrev.1990.70.1.1. [DOI] [PubMed] [Google Scholar]
- 60.Logemann HN, Lansbergen MM, Os TW, Bocker KB, Kenemans JL. The effectiveness of EEG-feedback on attention, impulsivity and EEG: a sham feedback controlled study. Neurosci Lett. 2010;479:49–53. doi: 10.1016/j.neulet.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 61.Heinrich H, Gevensleben H, Freisleder FJ, Moll GH, Rothenberger A. Training of slow cortical potentials in attention-deficit/hyperactivity disorder: evidence for positive behavioral and neurophysiological effects. Biol Psychiatry. 2004;55:772–775. doi: 10.1016/j.biopsych.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 62.Levesque J, Beauregard M, Mensour B. Effect of neurofeedback training on the neural substrates of selective attention in children with attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study. Neurosci Lett. 2006;394:216–221. doi: 10.1016/j.neulet.2005.10.100. [DOI] [PubMed] [Google Scholar]
- 63.Linden M, Habib T, Radojevic V. A controlled study of the effects of EEG biofeedback on cognition and behavior of children with attention deficit disorder and learning disabilities. Biofeedback Self Regul. 1996;21:35–49. doi: 10.1007/BF02214148. [DOI] [PubMed] [Google Scholar]
- 64.Arnold LE, Lofthouse N, Hersch S, et al., EEG Neurofeedback for attention-deficit/hyperactivity disorder: double-blind sham-controlled randomized pilot feasibility trial. J Atten Disord 2012. doi:10.1177/1087054712446173 [DOI] [PMC free article] [PubMed]
- 65.Perreau-Linck E, Lessard N, Levesque J, Beauregard M. Effects of neurofeedback training on inhibitory capacities in ADHD children: a single-blind, randomized, placebo-controlled study. J Neurotherapy. 2010;14:229–242. doi: 10.1080/10874208.2010.501514. [DOI] [Google Scholar]
- 66.Heywood C, Beale I. EEG biofeedback vs. placebo treatment for attention-deficit/hyperactivity disorder: a pilot study. J Atten Disord. 2003;7:43–55. doi: 10.1177/108705470300700105. [DOI] [PubMed] [Google Scholar]
- 67.Gevensleben H, Holl B, Albrecht B, et al. Distinct EEG effects related to neurofeedback training in children with ADHD: a randomized controlled trial. Int J Psychophysiol. 2009;74:149–157. doi: 10.1016/j.ijpsycho.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 68.Leins U, Goth G, Hinterberger T, Klinger C, Rumpf N, Strehl U. Neurofeedback for children with ADHD: a comparison of SCP and Theta/Beta protocols. Appl Psychophysiol Biofeedback. 2007;32:73–88. doi: 10.1007/s10484-007-9031-0. [DOI] [PubMed] [Google Scholar]
- 69.Bakhshayesh AR, Hansch S, Wyschkon A, Rezai MJ, Esser G. Neurofeedback in ADHD: a single-blind randomized controlled trial. Eur Child Adolesc Psychiatry. 2011;20:481–491. doi: 10.1007/s00787-011-0208-y. [DOI] [PubMed] [Google Scholar]
- 70.Gevensleben H, Holl B, Albrecht B, et al. Is neurofeedback an efficacious treatment for ADHD? A randomised controlled clinical trial. J Child Psychol Psychiatry. 2009;50:780–789. doi: 10.1111/j.1469-7610.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- 71.Drechsler R, Straub M, Doehnert M, Heinrich H, Steinhausen HC, Brandeis D. Controlled evaluation of a neurofeedback training of slow cortical potentials in children with attention deficit/hyperactivity disorder (ADHD) Behav Brain Funct. 2007;3:35. doi: 10.1186/1744-9081-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steiner NJ, Sheldrick RC, Gotthelf D, Perrin EC. Computer-based attention training in the schools for children with attention deficit/hyperactivity disorder: a preliminary trial. Clin Pediatr. 2011;50:615–622. doi: 10.1177/0009922810397887. [DOI] [PubMed] [Google Scholar]
- 73.Doehnert M, Brandeis D, Straub M, Steinhausen HC, Drechsler R. Slow cortical potential neurofeedback in attention deficit hyperactivity disorder: is there neurophysiological evidence for specific effects? J Neural Transm. 2008;115:1445–1456. doi: 10.1007/s00702-008-0104-x. [DOI] [PubMed] [Google Scholar]
- 74.Wangler S, Gevensleben H, Albrecht B, et al. Neurofeedback in children with ADHD: specific event-related potential findings of a randomized controlled trial. Clin Neurophysiol. 2011;122:942–950. doi: 10.1016/j.clinph.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 75.Hanslmayr S, Sauseng P, Doppelmayr M, Schabus M, Klimesch W. Increasing individual upper alpha power by neurofeedback improves cognitive performance in human subjects. Appl Psychophysiol Biofeedback. 2005;30:1–10. doi: 10.1007/s10484-005-2169-8. [DOI] [PubMed] [Google Scholar]
- 76.Weber E, Koberl A, Frank S, Doppelmayr M. Predicting successful learning of SMR neurofeedback in healthy participants: methodological considerations. Appl Psychophysiol Biofeedback. 2011;36:37–45. doi: 10.1007/s10484-010-9142-x. [DOI] [PubMed] [Google Scholar]
- 77.Gevensleben H, Holl B, Albrecht B, et al. Neurofeedback training in children with ADHD: 6-month follow-up of a randomised controlled trial. Eur Child Adolesc Psychiatry. 2010;19:715–724. doi: 10.1007/s00787-010-0109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7:1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- 79.Makeig S, Bell AJ, TP J, Sejnowski TJ. Independent component analysis of electroencephalographic data. In: Advances in Neural Information Processing Systems. Touretzky D, Mozer M, Hasselmo M, eds. 1996:145-151
- 80.Makeig S, Debener S, Onton J, Delorme A. Mining event-related brain dynamics. Trends Cogn Sci. 2004;8:204–210. doi: 10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 81.Makeig S, Delorme A, Westerfield M, et al. Electroencephalographic brain dynamics following manually responded visual targets. PLoS Biol. 2004;2:e176. doi: 10.1371/journal.pbio.0020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Makeig S, Westerfield M, Jung TP, et al. Dynamic brain sources of visual evoked responses. Science. 2002;295:690–694. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- 83.Akalin A, Worrell G, Makeig S. Patch-basis electrocortical source imaging in epilepsy. In: Engineering in Biology and Medicine Conference,j 2009. Minneapolis: IEEE Proceedings. [DOI] [PMC free article] [PubMed]
- 84.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 85.Delorme A, Makeig S. EEG changes accompanying learned regulation of 12-Hz EEG activity. IEEE Trans Neural Sys Rehab Eng. 2003;2:133–136. doi: 10.1109/TNSRE.2003.814428. [DOI] [PubMed] [Google Scholar]
- 86.Makeig S, Enghoff S, Jung T, Sejnowski TJ. A natural basis for efficient brain-actuated control. IEEE Trans Rehab Eng. 2000;8:208–211. doi: 10.1109/86.847818. [DOI] [PubMed] [Google Scholar]
- 87.Delorme A, Palmer J, Onton J, Oostenveld R, Makeig S. Independent EEG sources are dipolar. PLoS One 2012; 7(2):e30135. [DOI] [PMC free article] [PubMed]
- 88.Jung TP, Makeig S, Humphries C, et al. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000;37:163–178. doi: 10.1111/1469-8986.3720163. [DOI] [PubMed] [Google Scholar]
- 89.Jung TP, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski TJ. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clin Neurophysiol. 2000;111:1745–1758. doi: 10.1016/S1388-2457(00)00386-2. [DOI] [PubMed] [Google Scholar]
- 90.Delorme A, Sejnowski T, Makeig S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. Neuroimage. 2007;34:1443–1449. doi: 10.1016/j.neuroimage.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Delorme A, Mullen T, Kothe C, et al. EEGLAB, SIFT, NFT, BCILAB, and ERICA: new tools for advanced EEG processing. Comput Intell Neurosci 2011;xx:130714. [DOI] [PMC free article] [PubMed]
- 92.Palmer J, Kreutz-Delgado K, Rao B, Makeig S. Modeling and estimation of dependent subspaces with non-radially symmetric and skewed densities. In: Proceedings of the 7th International Conference on Independent Components Analysis and Signal Separation. 2007, London, UK.
- 93.Makeig S, Kothe C, Mullen T, Bigdely-Shamlo N, Zhang Z, Kreutz-Delgado K. Evolving signal processing for brain-computer interface. Proc IEEE xxxx;xx:xx-xx.
- 94.Brunner C, Mellinger J, Schalk G, et al. BCI Software Platforms. In: (B+H)CI: The Human in Brain-Computer Interfaces and the Brain in Human-Computer Interactions. Tan D, Nijholt A, eds. 2011, Springer: Verlag.
- 95.Kothe C, Makeig S. Estimation of task workload from EEG data: new and current methods and perspective. In: Engineering in Biology and Medicine Conference. 2011, Boston: IEEE. [DOI] [PubMed]
- 96.Onton J, Makeig S. EEG spectral modulations involved in self-regulation of independent component alpha power. In: Society for Neuroscience 2006.
- 97.Makeig S, Gramann K, Jung TP, Sejnowski TJ, Poizner H. Linking brain, mind and behavior. Int J Psychophysiol. 2009;73:95–100. doi: 10.1016/j.ijpsycho.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 216 kb)