Abstract
The effect of artificial food colors (AFCs) on child behavior has been studied for more than 35 years, with accumulating evidence from imperfect studies. This article summarizes the history of this controversial topic and testimony to the 2011 Food and Drug Administration Food Advisory Committee convened to evaluate the current status of evidence regarding attention-deficit/hyperactivity disorder (ADHD). Features of ADHD relevant to understanding the AFC literature are explained: ADHD is a quantitative diagnosis, like hypertension, and some individuals near the threshold may be pushed over it by a small symptom increment. The chronicity and pervasiveness make caregiver ratings the most valid measure, albeit subjective. Flaws in many studies include nonstandardized diagnosis, questionable sample selection, imperfect blinding, and nonstandardized outcome measures. Recent data suggest a small but significant deleterious effect of AFCs on children’s behavior that is not confined to those with diagnosable ADHD. AFCs appear to be more of a public health problem than an ADHD problem. AFCs are not a major cause of ADHD per se, but seem to affect children regardless of whether or not they have ADHD, and they may have an aggregated effect on classroom climate if most children in the class suffer a small behavioral decrement with additive or synergistic effects. Possible biological mechanisms with published evidence include the effects on nutrient levels, genetic vulnerability, and changes in electroencephalographic beta-band power. A table clarifying the Food and Drug Administration and international naming systems for AFCs, with cross-referencing, is provided.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-012-0133-x) contains supplementary material, which is available to authorized users.
Keywords: ADHD, Food dyes, Artificial food colors, Hyperactivity, Child behavior, FDA
In March 2011, the United States (U.S.) Food and Drug Administration (FDA) Food Advisory Committee held a hearing on the behavioral effects of synthetic food dyes, technically known as artificial food colors (AFCs). The focus of the meeting was on attention-deficit/hyperactivity disorder (ADHD), so discussion of that disorder was requested as background for understanding the data on AFCs. The controversial committee decision (8-6 vote) was not to recommend banning AFCs or requiring a warning label. This article summarizes relevant background information, further needs for research, and interim conclusions.1
AFC Classification Systems
To make them more identifiable, AFCs have both a common name and an official number that may differ from country to country. The International Numbering System (INS) is the world standard for classifying everything associated with food and uses the numbers 100 to 199 for color additives (approved for use or not) from the Codex Alimentarius (“Book of Food”) established in 1963 by the Food and Agriculture Organization (FAO) and World Health Organization (WHO). The safety of AFCs and other food additives is regulated by the Joint FAO/WHO Expert Committee on Food Additives ([JECFA] established in 1955.) The European Union (EU) uses the INS and adds an “E” prefix (“E” for “Europe”) for both natural and synthetic food colors approved by the European Food Safety Authority ([EFSA] established in 2002). Unlike the EU, in the U.S. the FDA separates synthetically produced colors or AFCs, which are batch-tested for safety (“certified colors,” N = 9) from those derived from natural sources in which individual batch testing is not required (“exempt from certification,” N = 29). There are 7 certified food colors (“FD&C colors”; FD&C = Food, Drugs & Cosmetics) approved for widespread use in the U.S.: brilliant blue (Blue No. 1b), indigotine (Blue No. 2), fast green (Green No. 3), tartrazine (Yellow No. 5b), sunset yellow (Yellow No. 6b), erythrosine (Red No. 3) and allura red (Red No. 40b). Two other AFCs are approved for specific limited use: citrus red (Citrus Red No. 2) (to color orange rinds) and Orange B (to color weiner/sausage casings). Table 1 lists these 9 AFCs with FD&C, INS, and EU “E” numbers and approval status for each classification and JECFA acceptable daily intakes (ADIs).
Table 1.
Artificial Food Coloring (AFC) classifications and approvals
| FD & C# | Common Name | Hue/Shade | INS# | E# | ADIs (mg/kg) |
|---|---|---|---|---|---|
| Permitted in U.S. | |||||
| Blue #1 A | Brilliant Blue FCF | Bright Blue | 133 | E133 A | 0-12.5 |
| Blue #2 A | Indigotine | Royal Blue/Indigo | 132 | E132 A | 0-5 |
| Green #3 A | Fast Green FCF | Sea Green/Turquoise | 143 | E143 | 0-25 |
| Red #3A | Erythrosine | Cherry Pink | 127 | E127 A | 0-0.1 |
| Red #40 A | Allura Red | Orange Red | 129 | E129 A | 0-7 |
| Yellow #5 A | Tartrazine | Lemon Yellow | 102 | E102 A | 0-7.5 |
| Yellow #6 A | Sunset Yellow FCF | Orange | 110 | E110 A | 0-4 |
| Citrus Red #2 RU | Citrus Red | Red | --- | --- | <2 PPM |
| Orange B RU | --- | Orange | --- | --- | <150 PPM |
| Not Permitted in U.S. | |||||
| --- | Quinoline Yellow | Greenish/Yellow | 104 | E104 | 0-5 |
| --- | Ponceau 4R | Scarlet | 124 | E124 | 0-4 |
| --- | Patent Blue V | Dark Blue | 131 | E131 | NA |
| --- | Fast Green FCF | Green | 142 | E142 | 0-25 |
| --- | Brilliant Black BN | Brown-Black | 151 | E151 | 0-1 |
| --- | Brown FK | Brown-Black | 154 | E154 | NA |
| --- | Lithol Rubine BK | Red | 180 | E180 | NA |
Note: FD&C: Food, Drugs & Cosmetics (U.S. Food & Drug Administration), INS: International Numbering System (global classification by Food & Agriculture Organization/World Health Organization), E# (European Union), A = Approved, RU: restricted use (Citrus Red 2 for orange peels, Orange B for weiner/sausage casings), ADI: Acceptable Daily Intake - estimate of amount, listed in units of mg per kg of body weight (based on (standard human = 60 kg) that can be ingested daily over a lifetime “without appreciable risk” from the OnLine Edition of the JECFA Compendium of Food Additive Specifications; FCF = For Food Coloring; PPM = Parts per million based on weight of product; NA = Not allocated.
History of the Food-Dye/AFC-ADHD Controversy
Food coloring with natural substances has been used since approximately 1500 B.C. in Ancient Egypt, and has been regulated from the time of England’s King Edward I, in the 13th century, to control their unsafe and fraudulent use (for more detail see Burrows [1] for a fascinating review of the history of food coloring and regulation). The 1856 development of synthetic dyes from petroleum or coal (so-called “coal-tar colors,” most of which are still in use today) by the English chemist Sir William Henry Perkin ushered in a series of acts in Europe and the U.S. regulating food colors. The FDA was established in1938 and the Joint FAO/WHO in 1955. Since then, the role of the FDA has been to ensure AFCs safety and prevent its fraudulent use in making food appear better or of greater value than it really is. Although not without criticism, the FDA has dramatically revolutionized AFCs from a time when highly toxic color additives were indiscriminately and widely used to the present when a “food colorant with a 1 in 19 billion chance of causing cancer is legally considered too dangerous” [1, p.405].
One of the more current controversies in the field of AFCs is concerned with their effect on children’s behavior. Although the idea that food allergies or hypersensitivities lead to behavior and learning problems dates back to the 1920s [2], a specific hypothesis regarding this relationship was not developed until the 1970s. In 1973, Dr. Benjamin Feingold [3] presented an article at the annual meeting of the American Medical Association, proposing that pediatric hyperactivity and learning problems were due to certain foods and food additives. Based on his own clinical observations, he believed that his patients were often sensitive to foods containing natural salicylates, AFCs, and flavors, and he devised a diet (the “Kaiser Permanente” or “K-P” diet) free of these substances [4, 5]. By 1977, [6, 7] Feingold also eliminated 2 preservatives (butylated hydroxytoluene and butylated hydroxyanisole, which he thought also led to hyperactivity [6] and claimed that 60 to 70 % of the children he treated improved [7].
Feingold’s [8] initial presentation generated a great deal of attention from the media, public, and professionals, and led to his best-selling book, Why Your Child is Hyperactive. However, his work was generally criticized by the medical and pharmaceutical fields [9, 10], and in 1975, the food industry, represented by the National Advisory Committee of the Nutrition Foundation, declared, “No controlled studies have demonstrated that hyperkinesis is related to the ingestion of food additives” [11]. However, Feingold’s work was accepted by many parents, who formed the still-existing Feingold Association of the United States (FAUS) (established in 1976). The first study on AFCs was conducted in 1976 by Conners et al. [12] at the University of Pittsburgh. After several additional studies, the National Institutes of Health held a conference on defined diets and hyperactivity in 1982 and concluded that further studies were needed [13]. One year later a meta-analysis by Kavale and Forness [14] (of 23 controlled group studies) concluded that the overall effect size (ES, 0.11) was too small to be important and the results did not support the K-P diet as a treatment for hyperactivity. This led to a decrease in interest in the K-P diet and the effect of AFCs until 2004 when Schab and Trinh [15] published their meta-analysis of 15 double-blind, placebo-controlled studies and found an ES of 0.28 between AFCs and the placebo on ADHD symptoms, in parent ratings, but not teacher or observer ratings, and only when children were preselected as diet responders; however, this pertained to all of the subjects, regardless of whether they were initially hyperactive or not.
Typical Elimination Studies and Examples
Besides a few studies that actually single-blinded an elimination and the “placebo” regular diet, there are many others following a different model, similar in some ways to a drug discontinuation study. Modern studies often start with an oligoantigenic or “few foods” diet for everyone. If there is improvement, then foods or components are openly added back 1 at a time to find the offenders. Then what follows is a double-blind challenge with offenders that can be blinded. Fortunately, AFCs are 1 of the easier things to blind; they are tasteless and can be camouflaged by dark food or drink. Preservatives are also easy to blind, which unfortunately makes it tempting to challenge blindly with mixes of the 2 classes of ingredients. In any event, the ease of blinding makes AFCs (and preservatives) better studied than other dietary components, but they should not be considered the whole problem. For details, see Stevens et al. [16].
A few exemplary highlights are these: Egger et al. [17], in a sample of 76 hyperactive children, found 48 foods with evidence of deterioration on being added back. Colors and preservatives were the most frequent offenders, but were not the sole problem in most children. In a sample of 200 selected from 800 hyperactive children, 150 openly improved with the elimination of AFCs and deteriorated on their resumption; there were 34 of these children who entered a double-blind challenge with 6 doses of tartrazine and placebo. Twenty-two of the 34 children clearly reacted with irritability, restlessness, and sleep disturbance [18]. This study illustrates the denominator problem in trying to determine the prevalence of AFC sensitivity in ADHD: were the 22 proven reactors 60 % of 34, or 15 % of 150, or 11 % of 200, or 2.5 % of 800? If we take 11 % as the most plausible, it highlights another interesting finding in this study: 20 nonhyperactive controls were also challenged at the same time, and 2 of those, or 10 %, also clearly reacted [16]. Thus, the controls showed a similar rate of reactivity as the hyperactive children. This presages the findings of the Southampton studies.
Southampton Studies
In 2004 and 2007, there were 3 landmark studies published from Stevenson & colleagues at Southampton University, as reported in 2 articles [19, 20]. Two are in preschool [19, 20], and 1 in the 8- to 9-year-olds [20]. All 3 samples included more than 100 participants each (N = 277, 153, and 144). The samples were nonclinical, acquired in an epidemiological manner (the first invited all the preschoolers on the Isle of Wight). The children were classified as hyperactive or not based on a rating scale and, in the first study [19], as atopic or not by a skin prick test. No other diagnostic assessment was done. However, severity assessments were done on double-blind challenges of an AFC mix and placebo using several measures. What was called “hyperactivity” included all 18 Diagnostic and Statistical Manual, fourth edition (DSM-IV) [21] ADHD symptoms, not just the hyperactive symptoms. The authors refrained from calling it an ADHD measure because they had not formally diagnosed any of the children.
All children were given an elimination diet free of AFCs and preservatives for 2 weeks, then challenged with mixed fruit juice with or without a mix of AFCs (sunset yellow, tartrazine, carmoisine, ponceau 4R, quinoline yellow, and/or allura red AC) and 45 mg Na benzoate. The first preschool study [19] used 5 mg each of sunset yellow, tartrazine, carmoisine, and ponceau 4R (20 mg total). The second preschool study [20] used 2 mixes and 2 doses besides the placebo. Mix A had the same AFCs and total dose as the first study, but different proportions (2.5 mg carmoisine, 7.5 mg tartrazine, 5 mg each of SY, and ponceau 4R). Mix B had 7.5 mg each of sunset yellow, carmoisine, quinoline yellow, and allura red AC, totaling 30 mg. The 2 doses for the 8- to 9-year-olds [20] were: mix A was the same as the preschool mix A, but 1.25 times as large, totaling 25 mg of AFC. Mix B was the same as preschool mix B, but 2.08 times as large, totaling 62 mg AFC, approximately the 2010 per capita daily consumption of AFCs in the U.S. There was a 1-week placebo washout between challenges with the placebo or AFC/benzoate mix, which were administered in random order.
The blinding was excellent. Preliminary adult tasting panels could not distinguish challenge from the placebo drink after inspecting, opening the sealed bottles, and tasting. Parent guesses of order (placebo-AFC vs AFC-placebo) in the first preschool study [19] reflected chance: half guessed correctly, exactly what would be expected by chance for a binary guess.
The main outcome measure for the first study [19] was “hyperactivity,” a composite of overactivity, inattention, and impulsiveness, rated by parents in all studies and by teachers in the second and third studies [20]. The first study [19] also had an aggregated test of hyperactivity, composited from psychologist observation and task performance. The second and third studies [20] also had classroom observation, and for the 8- to 9-year-olds the Conners Continuous Performance Test. For the second and third studies, a global hyperactivity score was derived from all the measures and used as the primary outcome measure.
Results of the First Study
In the first study [19], parent ratings, but not the aggregated psychologist’s score, showed a significantly greater increase in “hyperactivity” on active challenge than on placebo by a small-medium difference. Importantly, there was no association with ADHD (defined by rating scale score) or atopy (defined by skin prick). Thus the deleterious effect applied to all children.
Results of 2nd preschool study
Mix A but not Mix B showed a significantly greater increase than placebo in the primary outcome, the Global Hyperactivity score (d = 0.2). Again, there was no interaction with designation of child as hyperactive. Thus this study replicated the first study in a different sample [20].
Results for 8-9 year-olds
On intent-to-treat analysis, Mix B (higher dose) but not Mix A showed a significantly greater increase than placebo in Global Hyperactivity score. On analysis of those with 85 % compliance, both mixes showed a significant effect. The effect size was small, d = 0.12-0.2. Again, there was no interaction with baseline hyperactivity; the results of all 3 studies showed a small significant effect for all children, not just those meeting criterion A of DSM-IV ADHD [20]. This suggests that food AFCs are more of a public health problem than an ADHD problem.
The results of these studies led to some significant changes in the field of public health, with the United Kingdom government requesting that food manufacturers avoid these additives in favor of natural food colors and flavors, and the EU asking manufacturers to voluntarily remove several AFCs from foods and beverages or list the following warning on the label: “[this AFC] may have an adverse effect on activity and attention in children" [22]. In the U.S., the Southampton studies inspired a petition to the FDA from the Center for Science in the Public Interest (CSPI) [23] and, along with media interest and congressional support, led the FDA Food Advisory Committee to review the evidence on AFCs and ADHD and have a public hearing on March 30–31, 2011. This committee was given three documents prior to this meeting. One described the charge: “to consider available relevant data on the possible association between consumption of certified color additives in food and hyperactivity in children, and to advise FDA as to what action, if any, is warranted to ensure consumer safety” [24]. Another described the FDA’s history of food color regulation [25], and the third was a literature review of publications on AFCs and ADHD [26]. During the hearing the committee heard two days of testimony from several reviewers, experts on ADHD and food colors, members of the public, and representatives of advocacy groups and industry. The committee was given 5 questions. On question #2, “Do the current relevant data support FDA's conclusion, as set forth in the September 1, 2010 Interim Toxicology Review Memorandum, that a causal relationship between consumption of certified color additives in food and hyperactivity or other adverse effects on behavior in children in the general population has not been established?” the committee members voted 79 % yes; 21 % no [27].” On question #4, “Should additional information be disclosed on the product label of food containing certified color additives to ensure their safe use? The Committee members voted 43 % yes; 57 % no.” [27]. Finally, on question #5, the need for additional studies, “The Committee members voted 93 % yes; 7 % no” [27].
In 2012 Weiss [28] reviewed the FDA's decision [27] and noted four flaws in the process. 1). The FDA review confined itself to the relationship between AFCs and the clinical diagnosis of ADHD rather than broader behavioral problems. Weiss stated this was important because most children, not just those with ADHD, consume AFCs; few of the studies investigated a DSM-IV [21] diagnosis of ADHD; nearly all of the studies examined short-term rather than long-term effects expected of a chronic disease like ADHD; and narrow-band measures of ADHD would not identify non-ADHD symptoms caused by AFCs (e.g., irritability & sleep problems). 2). The FDA looked for large numbers of children to be affected by AFCs rather than recognize the importance of smaller but still vulnerable subpopulations. 3). The FDA judged McCann et al.’s [20] ES of 0.18 (in the range of many studies on AFCs & ADHD) as of "low magnitude.” Weiss [28] estimated such an ES as equivalent to a loss of three IQ points, and concluded “Most observers would not consider this to be a value of “rather low magnitude” (p. 3). 4). The FDA committee’s conclusion that further research was needed before taking preventive action did not consider the implications for institutional review board (IRB) approval for studies with documented risk and the cost of studies examining each of the certified AFCs.
Since the FDA hearing two more reviews have been published: Stevens, Kuczwk, Burgess, Hurt and Arnold [16] and Nigg et al. [2]. In their review of "35 years of research," Stevens et al. [16] noted scientists have examined Feingold’s hypotheses using 3 types of diets: (1) the K-P diet, (2) an elimination diet followed by AFC challenges, and (3) an oligoantigenic or few-foods diet followed by AFC and natural food challenges. From their review of four K-P diet studies, Stevens et al [164] concluded there are a small proportion (11 %-33 %) of children with hyperactivity whose functioning at home and school is improved by the K-P diet. From their review of 11 elimination diet-AFC challenge studies with children and with animals, Stevens et al [16] concluded most studies suggest that AFC challenges (mixed or with just tartrazine), compared with placebo, cause significant behavioral changes in ADHD subpopulations, the general pediatric population and in laboratory animals.. From their review of seven oligoantigenic/few-foods elimination diet-AFC/natural food challenges, Stevens et al [16] concluded all studies reported high response rates to various elimination diets (>70 %) and most parents reported more hyperactivity when challenged with offending foods/AFCs than placebo, with AFCs and preservatives the most likely to cause reactions, but no child responded only to AFCs.
Most recently, Nigg et al. [2] published a meta-analysis examining studies of dietary restriction and AFCs. For restriction diets they identified 14 open-label trials with a random-effects–weighted response rate of 47.4 %, which would set the upper limit on response rates for such diets (the rate could be lower considering that some of the response could be nonspecific effects). Five double-blind randomized placebo-challenge studies were also identified for restricted diets with an ES = 0.29, which Nigg et al. [2] noted was 1/3 the ES for medication and equivalent to a clinically meaningful change from the 50th to 62nd percentile. For AFCs 24 double-blind, placebo-controlled crossover studies were included and produced an ES for parent reports of 0.18, reduced to 0.12 after adjustment for potential publication bias. This effect was reliable in studies of food colors without preservatives (ES = .21) but not for studies of only FDA-approved food colors. Teacher/observer reports had a nonsignificant ES of 0.07 for studies in general, but when restricted to AFCs, it rose to 0.22 and survived correction. Psychometric tests of attention had an ES of 0.27, which also survived correction. Nigg and colleagues [2] concluded that "Although the evidence is too weak to justify action recommendations absent a strong precautionary stance, it is too substantial to dismiss" (p. 96) and "Although these average effect sizes are small in clinical terms, they could be quite substantial from the perspective of population-wide prevention efforts" (p. 96.).
Summary of Prevalent Flaws in Published Research on Dietary Sensitivities Relevant to ADHD
Although there were many studies with various controls, all were flawed in some way. First, diagnosis often does not follow DSM criteria. This is partly because many studies predated the current DSM-IV operational criteria [21]. But even some studies conducted after DSM-IV criteria [21] were available do not follow them. Frequently the “diagnosis” is a rating scale threshold or clinical impression. Another problem is that blinding is frequently imperfect, sometimes nonexistent, and its validity rarely examined in the studies of dietary sensitivities in which AFCs have been examined along with other suspected dietary components. However, the blinding is usually better for AFCs than other dietary components tested (e.g., the blinding was excellent in the Southampton studies [19, 20]). When AFCs are tested, they are often done as a mix rather than a single AFC (or possibly even mixed with a preservative), so that often it is difficult to attribute effects accurately to particular AFCs.
Admixture of preservatives in challenges is especially vexing because they require a different standard of evidence for removing them from the food supply. Removal of preservatives would carry an economic and public health cost in terms of food spoilage and possible food poisoning, whereas AFCs are purely cosmetic, so that removal would not seem to have an economic or public health cost. Thus it would be important to distinguish AFC effects from preservative effects. Other limitations of studies include the wide variety of AFC doses, duration of exposure to AFCs, and the timing of post-challenge testing.
Clarifications about ADHD Relevant to Evaluating the AFC Behavioral Literature
There are several aspects of ADHD diagnosis and evaluation relevant to evaluating the impact of AFCs and, relatedly, to the FDA preliminary review of materials. First, ADHD is a phenomenological, not causal, diagnosis [29]. Syndromes that meet the diagnostic criteria may be considered ADHD regardless of cause. In fact, there are probably many causes for a common phenotype.
Moreover, the phenotype itself is pleomorphic with four main variations recognized in the Diagnostic and Statistical Manual-Fourth Edition-Text Revision [29]: predominantly inattentive type, predominantly hyperactive-impulsive type, combined type, and “not otherwise specified.” Although the literature is not conclusive, there appears to be some variation in pharmacologic and other treatment response by type (e.g., [30]) and it would not be surprising if there were some variation in etiology. Nevertheless, the distinctions among types, despite being operationally/definitionally clear, are both fuzzy and arbitrary: Of the 9 inattentive and 9 hyperactive-impulsive symptoms, it is possible to have 6 inattentive symptoms and 5 hyperactive-impulsive symptoms and be classified as inattentive type, only one symptom short of being combined type with 6 of each. In fact, persons with 9 inattentive symptoms and 6 hyperactive-impulsive symptoms would be diagnosed as combined type even though they would have a greater preponderance of inattentive symptoms than the person with 6 and 5 respectively. Finally, two people could theoretically have the inattentive type with only 3 symptoms in common and up to 7 symptoms different (3 inattentive and 4 hyperactive-impulsive). Further, the phenotype defined by ICD-10 [31] hyperkinetic disorder and hyperkinetic conduct disorder is different yet; only 145 of the 579 children in the Multimodal Treatment Study of ADHD (the MTA) with combined-type ADHD met the ICD-10 criteria [32].
Diagnostic Procedure
The diagnosis requires 5 criteria, several of which are relevant to understanding the effect of AFCs: symptom count and severity; impairment; pervasiveness across settings; chronicity (6 months or more, starting young); and not better explained by another mental disorder [29].
Analogy to Hypertension
Because of the first criterion (symptom count and severity), ADHD is a dimensional diagnosis, like hypertension. Although everyone has some blood pressure (BP), too much is a problem. Cardiologists have struggled with how to set the threshold for problematic BP and have changed the benchmarks or thresholds from time to time, as psychiatrists have done for ADHD (DSM-IIIR [33] required 8 of 14 symptoms rather than the current 6 of 9). The current threshold for pre-hypertension is ≥80 or more diastolic BP and for hypertension is ≥90 or more diastolic [34]. Wherever it is set, some people will be on the cusp, such that a few mm change in BP will nudge them into a different category (e.g., going from 78 to 82 diastolic gives one pre-hypertension and from 88 to 92 gives one hypertension). Risk factors like stress, excess salt, or obesity could nudge some people over the BP threshold. As a corollary, one does not need diagnosable hypertension to be harmed by stress, excess salt, or obesity, which makes the prevention of those problems applicable to the general population. Similarly, a cause with only a small effect (as found for AFC in group averages) can nudge someone into the diagnosable range for ADHD (e.g., from 5 to 6 symptoms), and one does not need to have diagnosable ADHD to be harmed by a cause with a small deleterious effect, making the preventive implications widely applicable.
Chronicity and Pervasiveness Criteria Affect Measurement
One criticism of the studies of AFC effects on behavior is that they mainly show an effect on parent ratings, not so much on objective tests or on clinic observations. This criticism appears vacuous when we consider the diagnostic criteria of chronicity and pervasiveness, which require a consistent pattern of behavior over time, in more than one setting. Therefore severity and change (improvement or deterioration) cannot be adequately measured or appreciated in short time fragments in an artificial setting. They depend on caregiver ratings, or at least caregiver informants, usually parent and teacher, who know the child well and in various settings. These are subjective, but the most valid [35]. In recognition of this fact, the FDA has approved indications for ADHD drugs on the basis of parental information as the primary outcome.
Multifactorial Causes of ADHD
Genetics and epigenetics are perhaps the best-documented causes. Numerous family studies, including twin studies, have shown heritability up to 80 %, and this is backed up by replicated candidate genes such as the 7-repeat allele of DRD4 and the 10-repeat allele of DAT1 [36]. However, this should not translate to genetic determinism, because genes are expressed by interaction with the environment, including diet. A good example is phenylketonuria (PKU), a well-established 100 % genetic disorder, lack of the gene for the enzyme to metabolize phenylalanine to tyrosine, resulting in a toxic alternative metabolic pathway for phenylalanine. Because the disease develops only in the presence of phenylalanine in the diet, it is 100 % environmental as well as 100 % heritable [37]. Thus the fact that ADHD is 80 % heritable means that it is between 20 % and 100 % environmental. We can see that heritability could partly be genes for vulnerability to specific environmental factors, such as AFCs, insecticides, environmental chemicals, infections, parasites, trauma, poor diet, etc.
First Suspected Cause: MBD
Minimal brain damage or minimal brain dysfunction (MBD) was posited as a cause from the beginning because the syndrome was noted to be a sequela of infections (von Economo’s encephalitis, intrauterine rubella, childhood infections), perinatal “reproductive casualty” (e.g., kernicterus), head trauma, and lead poisoning [38]. More modern additions to the list of suspects might include insecticide residues, industrial, construction, and consumer product chemical pollutants, and AFCs.
Increased Prevalence and Putative Causes
The estimated prevalence of ADHD has increased since 1970, when it was 3-5 %, a figure that was still quoted as late as the 1994 DSM-IV [21]. More recent estimates have ranged up to 10-12 % [39]. Part of this, increase, of course, is increased recognition and more liberal diagnostic practice. Nevertheless, there may be an actual increase beyond diagnostic inflation. Many possible causes have been suspected. Those with genes for vulnerability may be exposed to more stresses, insults, or lack of developmental opportunities, which are likely to bring out the problems. The past century has seen multiple environmental changes. The educational setting has changed from one-room schools and self-contained classrooms with individualized instruction and close school-home cooperation to group classes with less discipline and home cooperation at the same time that the information age has ratcheted up the amount and difficulty of material to be learned. Parents working outside the home reduce the amount of one-to-one task-oriented adult mentorship that was available on a farm or in a small family business. Social breakdown of neighborhoods, families, and mores deprive children of the structure that is so helpful to youngsters vulnerable to developing ADHD. Electronic pastimes have reduced the amount of cerebellar exercise, which some suspect may be necessary for optimal cognition. Finally, there are new chemicals in the environment that could stress neurophysiology.
Environmental Contaminants and ADHD Symptoms
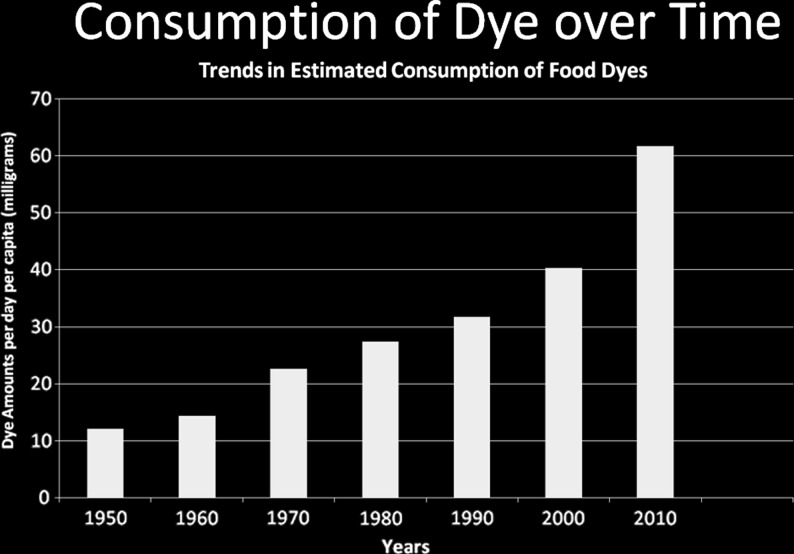
A good bit of research, especially in the 1960s and 70s, established the role of subclinical lead burden in cognitive and behavioral problems [40], to the extent that lead was eventually removed from gasoline Interestingly, the inverse correlation of intelligence quotient with subclinical blood lead levels [41] was of the same magnitude as the effect on hyperactivity recently reported for AFCs + Na benzoate in the Southampton studies [19, 20]). Lead contamination had been around for decades (albeit perhaps increasing Post-World War II because of increasing use of leaded gasoline), but there are other environmental contaminants of more recent origin. For example, insecticides have been associated with ADHD symptom severity: In one study, organophosphate insecticide residues and metabolites in children’s urine doubled the risk of ADHD [42]. The source was suspected to be residues on fruits and vegetables. In another study, maternal gestational serum insecticide level had an association with the child’s later behavior at age 5 [43]. Other modern sources of possible toxicity are industrial, construction, and consumer product chemicals. For instance, children whose cord blood had a PCB level in the top 25 % had 1.76 times the risk of ADHD symptoms as those whose cord blood had the bottom 25 % of PCB levels [44]. In another study polyfluoralkyl levels in adolescents age 12-15 were associated with ADHD symptoms [45]. These chemicals, of course, have come into common use in the past 50-60 years. Another environmental chemical class recently prevalent in children’s food is artificial coloring. Figure 1 shows the increase in per capita consumption of AFCs since 1950, taken from FDA data.
Fig. 1.
Daily per capita Consumption of Food AFC 1950-2010 (compiled by Laura J. Stevens, M.S., Purdue, used with permission)
AFC Interaction with Nutrient
Other changes have occurred in the foods available to children. Mineral content of fruits and vegetables has decreased since the 1930s [46] with intensive agriculture that mainly fertilizes with nitrogen, phosphorus, and potassium, largely neglecting other essential elements. Further, food is processed much more extensively, which changes its nutrient content in known and unknown ways, and such processed foods may be associated with vulnerability to depression [47]. One of the known changes in children’s diets, of course, is addition of artificial AFCs. There have been repeated reports of low blood and other tissue levels of iron, zinc, magnesium, and omega-3 fatty acids. A couple of interesting studies by Ward [48, 49] may tie some of this together.
The first study [48] was done with 10 hyperactive children whose parents said their behavior was sensitive to food AFCs and 10 healthy control children. At baseline, the hyperactive children had lower serum, urine, and nail zinc than the controls. After a 3-day additive-free diet, half of each group consumed a tartrazine challenge consisting of a commercial drink containing tartrazine, and half drank a similar orange drink without tartrazine. The serum and saliva Zn went down nonsignificantly and urine zinc went up significantly in the hyperactive children who drank tartrazine, but not in controls. Behavioral observations correlated with the amount of zinc change. In 1997, Ward [49] replicated these findings in 47 more children that parents reported to react to food AFC, again with age- and sex-matched controls. This time 23 children were given 50 mg tartrazine (slightly less than the 60 mg per capita daily consumption of AFC in 2010), 12 were given amaranth, and 12 sunset yellow. Five controls were given each of the 3 AFCs (N = 15 controls). Again serum zinc went down nonsignificantly, urine Zn went up significantly in hyperactive children given tartrazine and sunset yellow (not amaranth), but not in controls. Again, zinc changes from AFC challenge were associated with behavioral deterioration. Both studies suggest zinc wasting (excessive excretion) from tartrazine (and sunset yellow), possibly by chelation. This raises a question of what other nutrients may be wasted or otherwise interfered with by various AFCs. It also shows a possible mechanism for AFC to affect the brain without crossing the blood-brain barrier, given that Zn is essential for normal brain function [50],. This is relevant to the argument that AFC should not affect behavior because only Blue #1 crosses a mature blood-brain barrier in appreciable amounts.
Genetic Basis of AFC Sensitivity
Genetic tests were done in the second and third Southampton studies [20] on 6 candidate genetic variants, 2 histamine and 4 dopamine-relevant polymorphisms [51]. Three gene polymorphisms moderated the effect of AFC mixes on the Global Hyperactivity score. Both histamine polymorphisms were significant: HNMT Thr105Ile for both ages and HNMT T939C for the 8-9 year olds. There was one significant Dopamine polymorphism, DAT1 in 8-9 year-olds. COMT val108met, ADRA C1291G, and DRD4 rs740373 were not significant moderators. For the moderating effect of HNMT T939C, the C allele is protective against AFC effect, and absence of the C allele, the marker for vulnerability to the AFC effect, occurred in about 60 % of the children, supporting the public health import of the findings.
Other Biological Evidence: Brain Topographical Mapping
In another study [52], Brain Electrical Activity Mapping was done with and without a provoking food (including AFCs) during the preceding weeks and the same day in a crossover design, with blind interpretation of the EEG. With the provoking food, but not without it, there was an increase in frontotemporal Beta-1 band activity and behavioral symptoms. Unfortunately, the challenges were not blind, so one could not rule out the possibility that the parents’ knowing might have influenced the child’s EEG.
Additional Laboratory Evidence of Biological Effect
Selected examples of laboratory results, mainly in animals, include erythrosine-induced inhibition of serotonergic activity in rats [53] , corticosterone effects of erythrosine in rats [54] (another way in which AFCs could affect brain function without crossing the blood-brain barrier), changes in liver function tests from AFC mixtures in rats [55], and human mast cell degranulation with tartrazine, releasing histamine [56]. Further details may be found in Stevens et al [16].
Conclusions
Additional research should attend to the following points: Sample selection and characterization should consider specialty clinics vs. general mental health clinics vs. normal “controls” to address the denominator problem, and there should be careful diagnosis by DSM criteria, including both patients with ADHD and controls without, or controls with other diagnoses, to confirm the public health breadth of the risk. Age effects should be examined: Do adolescents and adults outgrow the sensitivity? Challenges should be “unbundled” to examine individual AFCs as well as various mixes, and especially examine AFCs separate from preservatives. Dose effects need more systematic exploration, especially in light of the ascending curve of daily consumption. How much is too much? How much is consumed by the highest-ingesting children? Careful blinding should be incorporated as much as possible. This should include double-blinding to prevent unwitting telegraphing of expectation by the research staff and testing of blinding validity for all those who should be blinded. Standard scales and observations should be used for comparison across studies and with studies of other interventions.
Three kinds of interactions should be systematically examined: interaction with nutrients, interaction with medications, and interactions between/among AFCs. A special question in light of the Southampton demonstration of a widespread effect on the general population [19, 20] is the effects on a whole classroom as well as individual children. If most of the children in a classroom deteriorate behaviorally by a small amount every day, is the aggregate and cumulative effect on the classroom climate greater than the sum of its parts?
Finally, samples need to be large enough to detect the small population effect sizes demonstrated in the Southampton studies [19, 20]. The 3-figure Southampton samples were barely sufficient to detect the statistically small but clinically important effects noted. An analogy is the subclinical-lead-burden literature, where it apparently took a sample size of about a thousand to demonstrate the effect convincingly. There is great danger of Type II error here, and statistical expertise needs to be involved in the early stages of planning any such study. Such large samples raise an interesting question about cost and who should pay for it. Some would argue that industry should sponsor studies to prove safety, similar to what is needed for drug approval. Others might argue that these AFCs have already been approved and are in use, and it is up to public health advocates to test whether they are unsafe. Perhaps the cost should be shared between industry and public health agencies.
Interim Working Conclusions
While awaiting the results of such further research, the following conclusions seem reasonable:
AFCs are not a main cause of ADHD, but they may contribute significantly to some cases, and in some cases may additively push a youngster over the diagnostic threshold.
There are several threads of evidence for a biological mechanism.
Although there is probably not an immune-mediated reaction, a direct release of histamine may be involved.
By affecting nutrients and other metabolism in the periphery, AFCs could affect the brain without crossing the blood-brain barrier.
The deleterious effect does not appear to be confined to ADHD (a general effect has been replicated). Therefore AFCs may be more a general public health problem than an ADHD problem
A small deleterious effect regardless of diagnosis was replicated and a possible mechanism (related to histamine genes) identified
The magnitude of reported effect is reminiscent of subclinical lead poisoning (<10 mcg/dL): r ~ 0.11 after correction for social factors [41], which led to the eventual removal of lead from gasoline.
- Per capita daily consumption of AFCs quadrupled in the last 50 yrs.“The dose alone makes the poison” --Paracelsus
There may conceivably be a possible deleterious effect on classroom climate from most children deteriorating slightly, thus additively or even synergistically impairing the learning atmosphere.
The current status of evidence is inconclusive “but too substantial to dismiss.”(2) Until safety can be better determined, we suggest minimizing children’s exposure to AFCs. With the current concerns about childhood obesity, there appears to be no need to make food look more attractive than its natural color.
Electronic supplementary material
(PDF 510 kb)
Acknowledgments
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
This article developed from the first author’s testimony to the 2011 FDA Food Advisory Committee on the behavioral effects of food AFCs, which is incorporated in updated summary.
References
- 1.Burrows A. Palette of our palates: a brief history of food coloring and its regulation. Compr Rev Food Sci Food Saf. 2009;8:394–408. doi: 10.1111/j.1541-4337.2009.00089.x. [DOI] [Google Scholar]
- 2.Nigg JT, Lewis K, Edinger T, Falk M. Meta-analysis of attention-deficit/hyperactivity disorder or attention-deficit/hyperactivity disorder symptoms, restriction diet, and synthetic food color additives. J Am Acad Child Adolesc Psychiatry. 2012;51:86–97. doi: 10.1016/j.jaac.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feingold BF. Adverse reactions to food additives. Presented at: The American Medical Association Annual Meeting; June 24–28, 1973; Chicago, IL.
- 4.Feingold BF. Hyperkinesis and learning disabilities linked to artificial food flavors and colors. Am J Nurs. 1975;75:797–803. [PubMed] [Google Scholar]
- 5.Feingold BF. Hyperkinesis and learning disabilities linked to the ingestion of artificial food colors and flavors. J Learn Disabil. 1976;9:551–559. doi: 10.1177/002221947600900902. [DOI] [Google Scholar]
- 6.Feingold BF. The role of diet in behaviour. Ecol Dis. 1982;1:153–165. [PubMed] [Google Scholar]
- 7.Feingold BF. Hyperkinesis and learning disabilities linked to the ingestion of artificial food colors and flavors. Speech to: American Academy of Pediatrics; November 8, 1977; New York, NY.
- 8.Feingold BF. Why Your Child is Hyperactive. New York: Random House; 1975. [Google Scholar]
- 9.Lipton MA, Mayo JP. Diet and hyperkinesis: an update. J Am Diet Assoc. 1983;83:132–134. [PubMed] [Google Scholar]
- 10.Mattes JA. The Feingold diet: a current reappraisal. J Learn Disabil. 1983;16:319–323. doi: 10.1177/002221948301600602. [DOI] [PubMed] [Google Scholar]
- 11.Feingold BF. A view from the other side. Speech to: Newspaper Food Editors and Writers Association; June 8, 1977; Milwaukee, WI.
- 12.Conners CK, Goyette CH, Southwick DA. Food additives and hyperkinesis: preliminary report of a double-blind crossover experiment. Psychopharmacol Bull. 1976;12:10–11. [PubMed] [Google Scholar]
- 13.Defined diets and childhood hyperactivity. NIH Consens Statement, 1982:4:1-11.
- 14.Kavale KA, Forness SR. Hyperactivity and diet treatment: a meta-analysis of the Feingold hypothesis. J Learn Disabil. 1983;16:324–330. doi: 10.1177/002221948301600604. [DOI] [PubMed] [Google Scholar]
- 15.Schab DW, Trinh NH. Do artificial food colors promote hyperactivity in children with hyperactive syndromes? A meta-analysis of double-blind placebo-controlled trials. J Dev Behav Pediatr. 2004;25:423–434. doi: 10.1097/00004703-200412000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Stevens L, Kuczek T, Burgess JR, Hurt EA, Arnold LE. Dietary sensitivities and ADHD: 35 years of research. Clin Pediatr. 2011;50:279–293. doi: 10.1177/0009922810384728. [DOI] [PubMed] [Google Scholar]
- 17.Egger J, Carter CM, Graham PJ, Gumley D, Soothill JF. Controlled trial of oligoantigenic treatment in the hyperkinetic syndrome. Lancet. 1985;1:540–545. doi: 10.1016/S0140-6736(85)91206-1. [DOI] [PubMed] [Google Scholar]
- 18.Rowe KS, Rowe KJ. Synthetic food coloring and behavior: a dose response effect in a double-blind, placebo-controlled, repeated-measures study. J Pediatr. 1994;125:691–698. doi: 10.1016/S0022-3476(06)80164-2. [DOI] [PubMed] [Google Scholar]
- 19.Bateman B, Warner JO, Hutchinson E, et al. The effects of a double blind, placebo controlled, artificial food colourings and benzoate preservative challenge on hyperactivity in a general population sample of preschool children. Ach Dis Child. 2004;89:506–511. doi: 10.1136/adc.2003.031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCann D, Barrett A, Cooper A, et al. Food additives and hyperactive behavior in 3-year-old and 8/9-year-old children in the community: a randomised, double-blinded, placebo-controlled trial. Lancet. 2007;370:1560–1567. doi: 10.1016/S0140-6736(07)61306-3. [DOI] [PubMed] [Google Scholar]
- 21.Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Association, 2000.
- 22.Regulation (EC) No 1333/2008 of the European Parliament and of the Council. In: Official Journal of the European Union. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:354:0016:0033:en:PDF.
- 23.Center for Science in the Public Interest, 2008. Petition to Ban the Use of Yellow 5 and Other Food Dyes, in the Interim to Require a Warning on Foods Containing These Dyes, to Correct the Information the Food and Drug Administration Gives to Consumers on the Impact of These Dyes on the Behavior of Some Children, and to Require Neurotoxicity Testing of New Food Additives and Food Colors. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/FoodAdvisoryCommittee/UCM248005.pdf. Accessed April 6, 2012.
- 24.FDA/CFSAN Food Advisory Committee, 2011. Center for Food Safety and Applied Nutrition, March 30-31, 2011, Food Advisory Committee Meeting
- 25.Certified Color Additives and Childhood Hyperactivity, Food Advisory Charge and Questions. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/FoodAdvisoryCommittee/UCM247999.pdf. Accessed May 19, 2012.25. FDA/CFSAN Food Advisory Committee, 2011. Background Document for the Food Advisory Committee: Certified Color Additives in Food and Possible Association with Attention Deficit Hyperactivity Disorder in Children March 30-31, 2011. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/FoodAdvisoryCommittee/UCM248549.pdf. Accessed May 19, 2012.
- 26.FDA/CFSAN Food Advisory Committee, 2011. Overview and Evaluation of Proposed Association between Artificial Food Colors and Attention Deficit Hyperactivity Disorders (ADHD) and Problem Behaviors in Children. Interim Toxicology Review Memorandum, September 1, 2010, Attachment 4. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/FoodAdvisoryCommittee/UCM248113.pdf. Accessed May 19, 2012.
- 27.FDA Food Advisory Committee, 2011. Quick Minutes: Food Advisory Committee Meeting March 30-31, 2011. Available at: http://www.fda.gov/advisorycommittees/committeesmeetingmaterials/foodadvisorycommittee/ucm250901.htm. Accessed 19 May, 2012.
- 28.Weiss B. Synthetic food colors and neurobehavioral hazards: the view from environmental health research. Environ Health Perspect. 2012;120:1–5. doi: 10.1289/ehp.1103827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diagnostic and statistical manual of mental disorders, 4th ed., text revision. Washington, DC: American Psychiatric Association, 2000.
- 30.Arnold LE, Bozzolo H, Amato A, et al. Acetyl-l-carnitine (ALC) in attention-deficit/hyperactivity disorder (ADHD): a multi-site placebo-controlled pilot trial. J Child Adolesc Psychopharmacol. 1997;17:791–801. doi: 10.1089/cap.2007.018. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization. ICD-10: International statistical classification of diseases and related health problems, 10th revised ed. New York, NY, 2008.
- 32.Santosh PJ, Taylor E, Swanson J, et al. Reanalysis of the multimodal treatment study of attention-deficit/hyperactivity disorder (ADHD) based on ICD-10 criteria for hyperkinetic disorder (HD) Clin Neurosci Res. 2005;5:307–314. doi: 10.1016/j.cnr.2005.09.010. [DOI] [Google Scholar]
- 33.Diagnostic and statistical manual of mental disorders, 3rd ed. Washington, DC: American Psychiatric Association, 1980.
- 34.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 35.Pelham WE, Fabiano GA, Massetti Evidence-based assessment of attention deficit hyperactivity disorder in children and adolescents. J. Clin Child Adol Psychol. 2005;34:449–476. doi: 10.1207/s15374424jccp3403_5. [DOI] [PubMed] [Google Scholar]
- 36.Farone SV, Perlis RH, Doyle AE, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 37.Pearce N. Epidemiology in a changing world: variation, causation, and ubiquitous risk factors. I J Epidemiol. 2011;40:503–512. doi: 10.1093/ije/dyq257. [DOI] [PubMed] [Google Scholar]
- 38.Wender PH. The minimal brain dysfunction syndrome. Ann Rev Med. 1975;26:45–62. doi: 10.1146/annurev.me.26.020175.000401. [DOI] [PubMed] [Google Scholar]
- 39.Akinbami LJ, Xiang L, Pastor PN, Reuben CA. Attention deficit hyperactivity disorder among children aged 5-17 years in the United States, 1998-2009. NCHS Data Brief 2011;70:xx. [PubMed]
- 40.Burde B, Choate MS. Early asymptomatic lead exposure and development at school age. Pediatrics. 1975;87:638–642. doi: 10.1016/S0022-3476(75)80845-6. [DOI] [PubMed] [Google Scholar]
- 41.Fergusson DM, Horwood LJ, Lynskey MT. Early dentine lead levels and subsequent cognitive and behavioural development. J Child Psychol Psychiat. 1993;34:215–227. doi: 10.1111/j.1469-7610.1993.tb00980.x. [DOI] [PubMed] [Google Scholar]
- 42.Bouchard MF, Bellinger DC, Wright RO, Weisskopf MG. Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrics. 2010;125:1270–1277. doi: 10.1542/peds.2009-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marks AR, Harley K, Bradman A, et al. Organiophosphate pesticide exposure and attention in young Mexican-American children: The CHAMACOS study. Environ Health Perspect. 2010;118:1768–1774. doi: 10.1289/ehp.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sagiv SK, Thurston SW, Bellinger DC, et al. Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. Am J Epidemiol. 2010;171:593–601. doi: 10.1093/aje/kwp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoffman K, Webster TF, Weisskopf MG, Weinberg J, Vieira VM. Exposure to polyfluoroalkyl chemicals and attention deficit/hyperactivity disorder in US children 12-15 years of age. Environ Health Perspect. 2010;118:1762–1767. doi: 10.1289/ehp.1001898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayer AM. Historical changes in the mineral content of fruits and vegetables. British Food Journal. 1997;99:207–211. doi: 10.1108/00070709710181540. [DOI] [Google Scholar]
- 47.Sánchez-Villegas A, Toledo E, Irala J, Ruiz-Canela M, Pla-Vidal J, Martínez-González MA. Fast-food and commercial baked goods consumption and the risk of depression. Public Health Nutr. 2012;15:424–432. doi: 10.1017/S1368980011001856. [DOI] [PubMed] [Google Scholar]
- 48.Ward N, Soulsbury K, Zettel V, et al. The influence of the chemical additive tartrazine on the zinc status of hyperactive children — a double-blind placebo-controlled study. J Nutr Med. 1990;1:51–58. doi: 10.3109/13590849009003134. [DOI] [Google Scholar]
- 49.Ward NI. Assessment of chemical factors in relation to child hyperactivity. J Nutr Med. 1997;7:333–342. doi: 10.1080/13590849762466. [DOI] [Google Scholar]
- 50.Arnold LE, DiSilvestro R. Zinc in attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2005;15:619–627. doi: 10.1089/cap.2005.15.619. [DOI] [PubMed] [Google Scholar]
- 51.Stevenson J, Sonuga-Barke E, McCann, et al. The role of histamine degradation geen polymorphisms in moderating the effects of food additives on children’s ADHD symptoms. Am J Psychiatry. 2010;167:108–1115. doi: 10.1176/appi.ajp.2010.09101529. [DOI] [PubMed] [Google Scholar]
- 52.Uhlig T, Merkenschlager A, Brandmaier R, Egger J. Topographic mapping of brain electrical activity in children with food-induced attention deficit hyperkinetic disorder. Eur J Pediatr. 1997;156:557–561. doi: 10.1007/s004310050662. [DOI] [PubMed] [Google Scholar]
- 53.Dalel A, Poddar MK. Short-term erythrosine B-induced inhibition of the brain regional serotonergic activity suppresses motor activity (exploratory behavior) of young adult mammals. Pharmacol Biochem Behav. 2009;92:574–582. doi: 10.1016/j.pbb.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 54.Dalal A, Poddar MK. Corticosterone effects of erythrosine in rats. Toxicology Mec Methods. 2010;20:287–297. doi: 10.3109/15376516.2010.483070. [DOI] [PubMed] [Google Scholar]
- 55.Aboel-Zahab H, el-Khyat Z, Sidhom G, et al. Physiological effects of some synthetic food colouring on rats. Boll Chim Farm. 1997;136:615–627. [PubMed] [Google Scholar]
- 56.Schaubschlager WW, Zabel P, Sclaak M. Tartrazine-induced histamine release from gastric mucosa. Lancet. 1987;2:800–801. doi: 10.1016/S0140-6736(87)92534-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 510 kb)