Abstract
ADHD, sleep, and ADHD treatments are highly interrelated. In this review, we describe the effects of stimulants and non stimulant medications on sleep in children, adolescents, and adults with ADHD. Clinical predictors of sleep problems during pharmacotherapy include age, sleep problems prior to initiating treatment, and dose and dosing schedule. As yet, we have little understanding of the biological or genetic factors related to individual variation in drug response and sleep.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-012-0130-0) contains supplementary material, which is available to authorized users.
Keywords: ADHD, Sleep, Psychopharmacology
Evidence-Based Treatments for Attention-Deficit/Hyperactivity Disorder
Pharmacological treatment for attention-deficit/hyperactivity disorder (ADHD) includes both stimulant medications (methylphenidate [MPH] and amphetamine formulations) and nonstimulants, such as atomoxetine [1–3] and alpha-2 agonists [4, 5]. The efficacy of these medications has been well-established through numerous randomized, controlled trials and described in meta-analyses [6]. During the last decade, extended release medications have been developed to provide longer duration of ADHD symptom control [6, 7]. However, ADHD medications and their different formulations (e.g., stimulant and nonstimulant, short-acting and long acting) can have important and differential impacts on sleep in individuals with ADHD.
Evidence-based psychosocial treatments for ADHD include behavioral parent training, behavioral classroom management, and peer-based interventions, such as summer treatment programs, which can be combined with medication [8]. Relative to pharmacological treatment for ADHD, there has been little systematic study of the effects of psychosocial treatments on sleep. Sleep problems are also common in children treated with psychosocial treatments. A recent study of children attending a summer treatment program and receiving comprehensive behavioral therapy, parent training, and medication management demonstrated 61 % reported sleep onset difficulties, short duration of sleep was evident (i.e., mean sleep time of 6 hours, 58 minutes), and considerable night-to-night variability was displayed [9]. Thus, sleep problems are common in both untreated and pharmacologically and behaviorally treated children with ADHD. According to the National Sleep Foundation, improving sleep can lead to improved daytime behavior in children.
There are several noteworthy examples of children with sleep disorders and ADHD symptoms who displayed improvement in attention and overactivity with treatment of the sleep disorder. For example, studies of children with sleep-disordered breathing indicate that removing tonsils and adenoids often results in improved ADHD symptoms, possibly because of improvements in sleep quality after surgery [10]. Moreover, lack of sleep or sleep restriction can cause or exacerbate inattention and other cognitive symptoms in children with ADHD and non-ADHD controls [11].
In a study of children with ADHD and chronic sleep onset insomnia, 3-6 mg of melatonin reduced sleep onset latency and improved total sleep time. However,despite improvements in these sleep parameters there was no effect on ADHD symptoms, cognitive performance, or quality of life [12]. Thus, treating primary insomnia (not related to stimulant medication) may improve sleep onset latency, but it will not necessarily result in the remission of ADHD. It should be noted, however, that children with insomnia who are treated with stimulants, sleep hygiene and melatonin maintain the beneficial effects of the stimulants on ADHD symptoms, as well as improved sleep [13].
Although further investigation is warranted on the relationship of psychosocial treatment to sleep, in this review we will focus primarily on the impact of ADHD medications on sleep and sleep problems.
Prevalence and Types of Sleep Problems Associated with ADHD
ADHD and sleep problems are common, highly interrelated, heterogeneous, and both are impacted by stimulant and nonstimulant medications in different ways [14–16]. Previously, we reported that moderate-to-severe sleep problems occurred at least once a week in 19.3 % of children with ADHD, 13.3 % of the psychiatric controls, and 6.2 % of the pediatric controls, according to parents [17]. In addition, nearly one third (29 %) of stimulant-treated ADHD children displayed nightly insomnia (i.e., taking >30 minutes to fall asleep) compared to 10 % of untreated children.
To understand the effects of pharmacotherapy on sleep, it is first necessary to determine the base rate of sleep problems in untreated children. Insomnia is usually evaluated with sleep logs, with parent ratings of side effects on questionnaires, and with actigraphy monitoring. Actigraphy provides an objective assessment of several relevant sleep parameters, such as sleep onset latency (SOL), awakenings, and sleep duration.
Insomnia is the most studied sleep problem, although it is typical for a child with ADHD who has problems with sleep to have characteristics of several sleep disorders. A recent meta-analysis by Cortese et al. [18] addressed this question by examining 16 studies of children and adolescents with ADHD who were not medicated. Children with ADHD displayed higher rates of bedtime resistance, sleep onset difficulties, night awakenings, difficulties with morning awakenings, sleep disordered breathing, and daytime sleepiness than non-ADHD controls according to parent reports.
On objective sleep measures, children with ADHD displayed increased sleep onset latency, lower sleep efficiency on polysomnography, less true sleep time on actigraphy monitoring, and excessive daytime sleepiness as measured by a standard test (i.e., the Multiple Sleep Latency Test) in comparison to non-ADHD controls [18]. The most consistent findings using both subjective and objective measures are that ADHD is strongly associated with difficulty falling asleep, with achieving adequate sleep duration, and with tiredness during the day [19, 20]. The difference between subjective and objective measures of sleep in ADHD children may be explained, at least in part, by a high rate of night-to-night variability, which would be captured subjectively, but would not be evident on polysomnogram testing in a sleep laboratory.
Additional methodological issues, such as small sample size and how sleep problems and ADHD are measured, can have a significant impact on the results and may partially explain inconsistent findings between studies. For example, Choi et al. [21] used both polysomnographic (PSG) recordings and questionnaires to assess sleep habits in children with ADHD. Children with ADHD compared to controls displayed more sleep disturbances, such as sleep onset latency, night awakenings, and daytime sleepiness; however, the objective PSG recordings showed no difference in sleep quality and quantity between the 2 groups of children. Another study [22] examining PSG recordings showed that only increased periodic leg movements were more common in children with ADHD compared to controls. In contrast, Gruber et al. [20] reported a significant difference in sleep architecture, including shorted rapid eye movement and sleep duration, using PSG recordings.
Sample characteristics, including ADHD subtype, psychiatric comorbidity, age, gender, and medication status may all impact sleep findings. As reported by Mayes et al. [23], children with the inattentive subtype displayed fewer nighttime sleep problems than those with the combined subtype of ADHD. However, children with the inattentive subtype were reported to display more daytime sleepiness and increased duration of sleep. Recently, Accardo et al. [24] demonstrated that children with ADHD and anxiety displayed higher total sleep disturbance scores on the Children’s Sleep Habits Questionnaire, and increased bedtime resistance, sleep onset delay, and nighttime awakenings compared with children with ADHD alone [23, 24]. Children with ADHD and depression also displayed increased sleep onset latency and increased sleep duration. Furthermore, it should be noted that parent reports of sleep problems are highly correlated with both internalizing and externalizing psychopathology [25–27], and these occur with increased rates in other psychiatric and developmental disorders [28]. These studies show that objective measures, such as actigraphy or PSG recordings, and subjective assessment, such as parent and child questionnaires and sleep diaries, do not always concur. In combination, they provide a comprehensive picture of both the objective sleep problem and the subjective or perceived impairment.
In contrast to many studies in ADHD youth, there are only a handful of studies examining sleep disturbances in adults with ADHD [29]. Kooij et al. [30] reported that adults with ADHD experienced considerable subjective and objective impairment in sleep, including increased nocturnal motor activity in a small sample. Sobanski et al. [31] surveyed sleep problems in a larger sample of 182 adults with ADHD compared to 117 non-ADHD controls, and reported that adults with ADHD went to bed later, were more likely to take more than 1 hour to fall asleep, and reported daytime sleepiness. Similar findings were reported by Surman et al. [32].
Sleep Problems Associated with Stimulant Medications and Their Measurement
The most common and well-studied treatments for ADHD are immediate and extended-release formulations of methylphenidate and amphetamine-based stimulants. Among the many stimulant formulations, lisdexamphetamine and the methylphenidate patch stand out as the longest-acting amphetamine and methylphenidate formulations, respectively, with effects on ADHD symptoms that can exceed 12 h [33–35].
Insomnia or delayed SOL greater than 30 minutes is one of the most common adverse events associated with stimulant medications [12, 17, 35]. This should be distinguished from bedtime resistance, which is when the child refuses to go to bed.
A second common sleep problem, especially in adolescents, is the delayed sleep phase syndrome. This is a disturbance in circadian rhythm that involves a phase shift in the sleep–wake cycle such that the adolescent goes to bed late and sleeps late, sometimes to the point of day/night reversal. This can have a devastating effect on school performance. Delayed sleep phase syndrome is of particular importance in that it has become a cultural norm to stay out late on the weekends, sleep late into the afternoon on Saturday and Sunday, and then try to shift back to a normal school schedule by Monday. One can consider that delayed sleep phase syndrome is not only a physiological insult to the entrainment of circadian rhythm, but it has the effect of leaving these teens in a state of chronic jet lag.
Methylphenidate
Studies of the effects of stimulant medication on sleep have largely been focused on methylphenidate (MPH) [36]. Insomnia is a frequent side effect of all stimulant medications, based on parent report or side effects scales completed side effects scales by parents [37, 38]. Research on the impact of MPH on sleep will show different findings depending on the length of the trial, when MPH is administered, and when sleep is measured in the trial and dose, and whether a fixed or flexible dosing methodology is used [38, 39]. Studies of flexible dosing (in which the dose is individually titrated as in clinical practice) are less likely to detect a dose effect or acute disruptions in sleep, as the dose is not increased once a response is obtained or if tolerability is a problem [40]. In addition, the impact of MPH on sleep will vary depending on whether the child has just started medication, or whether they have been on medication for an extended period of time [41]. There are reports of greater difficulty falling asleep during rebound when medication is wearing off, as well as anecdotal reports of children who fall asleep more easily on a low dose of medication [42]. Therefore, the relationship between sleep and medication is complex. With this being said, it has been well-demonstrated that the more prominent initial side effects of stimulant medication is initial insomnia.
Stein et al. [43] utilized actigraphy to measure sleep onset latency (SOL) and duration of sleep in children treated with low to moderate doses of MPH administered twice or three times daily. SOL increased for both dosing conditions compared to placebo, however, there was not much difference in SOL between taking the last dose of MPH at noon or at 4 pm. Children took approximately 40 minutes to fall asleep when given a placebo, as compared to 60 to 70 minutes for those children who were given an MPH. Severe insomnia was reported for 4 % of those given the placebo with 3 times per day conditions and 0 % with 2 times per day conditions, respectively. It should be noted that the sample size for this study was quite small (n = 25). However, similar findings were reported by Kent et al. [44] in a study of psychiatric inpatients for which a 4 pm dose resulted in improved behavior with no significant difference in SOL, as determined by nursing logs. In this study, the average sleep latency was 50 to 51 minutes during MPH treatment, and the higher dose was associated with a markedly higher standard deviation. Thus, although group effects were minimal, individual variation appears to be quite high. Similar findings for 3 times of daily dosing of MPH and significantly increased SOL were reported by Sangal et al. [45] in a study comparing the sleep effects of MPH to atomoxetine.
There have also been several PSG studies of sleep in children with ADHD who were receiving MPH. In general, few consistent findings are reported [22, 46]. O’Brien et al. [29] compared 53 children taking stimulants, 34 children with ADHD who were not taking stimulants, and 53 controls, and found no differences in sleep architecture. The rates of insomnia were not reported. The methodology of this study is flawed in that the children were not randomized to treatment. Thus, any child who discontinued stimulant medication because of a sleep problem would not necessarily have been identified.
During the last decade, numerous stimulant formulations have been developed, including sustained release, biphasic, triphasic, and ascending delivery profiles. One study compared the efficacy and safety of 2 second-generation, long-acting MPH formulations with ascending delivery profiles [7]. In this study, there were no significant difference in adverse advents, including rates of insomnia, between the osmotic controlled-release oral stimulant OROS Methylphenidate, Concerta, McNeil Pharmaceuticals, USA and the extended-release Methylphenidate HCL, Metadate CD, UCB, USA.
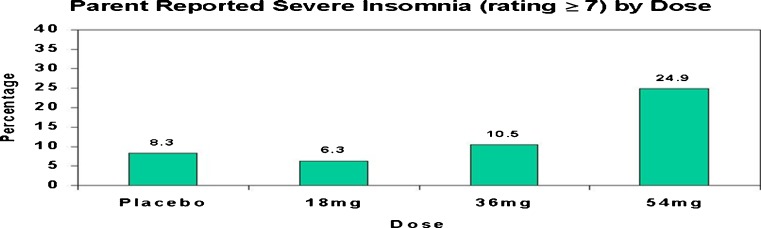
Stein et al. [39] examined the effects of 3 doses of OROS MPH and a placebo in 47 ADHD youth in a double-blind, crossover dose-response study. As seen in Fig. 1, parent ratings of severe insomnia were increased at the higher dose level, and were more likely to occur in younger and smaller patients at the 54 mg dose level [39].
Fig. 1.
Parent ratings of severe insomnia by dose condition
Recently, Kim et al. [47] examined the effects of open-label OROS MPH treatment on sleep use in 24 children with ADHD. Polysomnographic data indicated that the percentage of stage 2 sleep was increased, and the number of awakenings was decreased. Sleep onset latency was increased only for those (n = 6) who had sleep difficulties prior to beginning the trial, but not for the sample as a whole. In this study, the small sample size and flexible dosing may have contributed to the lack of effect on SOL for the overall sample.
In addition to oral MPH preparations, there is also a methylphenidate transdermal system (MTS) or skin patch, which results in approximately 12 hours of coverage if applied to the skin for 9 hours. Although there is additional MPH contained in the patch, the effects of longer wearing times have not been reported [48]. In a 5-week study, treatment emergent side effects and sleep questionnaires were obtained for 262 of the 6- to 12-year-old children with ADHD who were treated with MTS or OROS MPH [49]. There were no statistically significant dose-response effects on subjective sleep measures in this study. Sleep problems at baseline predicted sleep problems during treatment. Although total scores on the Children’s Sleep Questionnaire were similar for children taking placebo, OROS MPH, or the MTS system, 13 % of those wearing the MTS patch were reported to display insomnia, as compared to 7 % taking OROS MPH and 5 % taking a placebo [49].
Amphetamine Stimulants
Efron et al. [50] compared twice-daily, immediate-release MPH and dextroamphetamine in 125 ADHD youth in a crossover study. Using the parent-completed, Barkley Side Effect Scale, dextroamphetamine, but not MPH, was associated with higher ratings of severe insomnia relative to baseline.
McCracken et al. [51] conducted a double-blind, crossover analog classroom study of Extended Release Dexmethylphenidate Hydrochloride, Focalin XR, Novartis Pharmaceuticals, USA, Extended Release Mixed Amphetamine Salts, Adderal XR, Shire Pharmaceuticals, USA. In this parallel group study, 3 doses of ER-MAS were compared to 10 mg MAS and a placebo. Parent-rated adverse effects for insomnia did not show a clear dose response (i.e., placebo, 20 %; 10 mg MAS, 35 %; 10 mg ER-MAS, 12 %; 20 mg ER-MAS, 32 %; and 30 mg ER-MAS, 29 %). It is interesting that the highest rates of insomnia were for 10 mg MAS, which the duration of effect on attention and behavior is significantly shorter than that of ER-MAS. The sample predominantly consisted of subjects with previous stimulant experience, leaving the possibility open that higher rates of insomnia would have been identified if this had been a stimulant naïve clinical population, and also offering a potential explanation for the failure to demonstrate the expected dose to side-effect relationship.
Stein et al. [52] recently compared 3 dose levels of ER-MAS with ER dexmethylphenidate and a placebo, using a crossover design in 56 children (30 % stimulant naïve) and adolescents in a placebo, controlled, crossover 8-week trial. As seen in Fig. 2, parent ratings of severe insomnia were significantly higher for ER-MAS at the 10 mg dose level, but at higher dose levels there was no drug-related difference in percentage with severe insomnia. However, in this study, the dose was confounded with time, which may partially explain the lower rates of insomnia at the highest dose level, as participants receiving the highest dose level had more time to accommodate to the stimulant.
Fig. 2.
Severe Insomnia by Dose of Extended Release Dexmethylphenidate (ER-D-MPH) and Extended Release Mixed Amphetamine Salts (ER MAS)
Lisdexamphetamine dimesylate (LDX) is an amphetamine prodrug. Biederman et al. [53] conducted a double-blind, forced dose, parallel group study of 290 children in which they received the study drug for 4 weeks. More than half of the sample was stimulant naïve. Insomnia was the second most common, spontaneously reported adverse event after decreased appetite, and it occurred in approximately 2 % of those taking the placebo, 15 % of those taking 30 mg of LDX, 16 % of those taking 50 mg of LDX, and 25 % of those taking 70 mg of LDX. This study randomized children to different doses, and therefore the absence of a dose-response relationship at the lower dose levels for insomnia is a robust finding for this medication.
In another study, comparing LDX and mixed amphetamine salts [54], reports of insomnia were most common during the first week of treatment for both stimulants. Of 52 children and adolescents in a placebo-controlled, crossover study of 30, 50, and 70 mg LDX and 10, 20, and 30 mg MAS, 8 % of those who had been treated with stimulants sometime in the past year displayed insomnia while taking LDX as compared to 2 % taking ER-MAS, and 2 % on the placebo [54].
Examining sleep outcomes in more detail, Giblin and Strobel [40] used parent ratings, actigraphy, and polysomnography to evaluate the sleep effects of LDX in 24 children 6 to 12 years of age with ADHD. Latency to persistent sleep, as measured during polysomnography, was approximately 10 minutes longer for the LDX group compared to the placebo controls, but this was not statistically significant. Other than fewer nighttime awakenings in the LDX group, there were no significant differences between LDX and placebo. The small sample size, flexible dosing titration period, time to habituation to medication before assessment of sleep effects, and exclusion of subjects with a history of adverse or nonresponse to amphetamine are all aspects of the method that would lead to a bias toward failing to find an effect on sleep, if there was one.
Stimulant Effects on Sleep in Adults
Recently, several studies have examined the effects of stimulant medications on sleep of adults with ADHD. In a polysomnographic study of 8 adults who were stimulant-naïve, open-label treatment with MPH resulted in increased sleep efficiency (i.e., the ration of time spent asleep relative to the time in bed), as well as a subjective feeling of improved restorative value of sleep. The author suggests that MPH appears to have beneficial effects on sleep parameters in adults with ADHD [55].
Surman et al. [32] surveyed sleep problems in 182 adults with ADHD or ADHD not otherwise specified (NOS) compared to 117, non-ADHD controls. The ADHD adults reported a later bed time, more difficulty falling asleep, difficulty waking, and more daytime sleepiness relative to controls. All sleep problems were significantly associated with ADHD, independent of contributions to sleep disruption from ADHD pharmacotherapy, psychiatric comorbidities likely to contribute to sleep disturbance, and age of ADHD onset.
Surman and Roth [56] also reported the sleep effects of medication with secondary analysis using the Pittsburgh Sleep Questionnaire, which was administered to participants of 2 clinical trials of long-acting amphetamine formulations for adult ADHD, LDX, and mixed amphetamine salts triple bead. In a study of “triple bead” amphetamine, insomnia was the most common adverse event in adults with ADHD, but no differences were detected in reported sleep problems between baseline and the end of treatment. In this study, 8.3 % of those receiving LDX or MAS displayed impaired sleep versus 9.7 % of those receiving the placebo [56].
In reviewing the aforementioned studies, several unresolved issues should be highlighted that require further study. First, there is a high level of individual variability in whether stimulants cause insomnia, thus small studies are difficult to interpret. Second, studies vary in whether the insomnia is dose-related and this may depend on previous stimulant exposure, the medication studied, randomization, and the doses used. Third, none of the studies cited provide clear information on whether the dosing schedule for that medication would indicate if the child was either on medication, in rebound, or off medication for several hours when sleep onset was measured. The question as to whether children have as much difficulty falling asleep in rebound when medication is waning, as they do on low doses that allow them to go to bed, has not been adequately investigated.
Of interest, approximately one third of subjects had clinically meaningful sleep improvement in 2 adult ADHD trials [32, 56] emphasizing that change in sleep quality during treatment may be either positive or negative. As yet, we do not have dose response or fixed dose studies of sleep in larger, representative samples of adults with ADHD, which would help clarify sleep effects of the stimulant medications. Nonetheless, these early reports suggest that the effects of stimulants on sleep in adults and children may differ, and that children’s sleep may be more sensitive to stimulant effects. One possible consideration is that if an adult and a child both take a 12-hour medication in the morning, the adult will have more opportunity for stimulant effects to wear off prior to initiating sleep.
Nonstimulant Effects on Sleep: Atomoxetine and Alpha-2 Agonists
Atomoxetine is the first nonstimulant approved by the Food and Drug Administration for ADHD. Although there are reports of insomnia as an adverse event with atomoxetine [57], multiple trials in ADHD have noted somnolence to be much more common [58], especially during the early stages of a trial or if the titration is too rapid [59]. However, somnolence can be minimized with evening administration, although daytime efficacy may be decreased [59].
Sangal et al. [45] conducted a crossover study in children and adolescents with ADHD comparing twice-daily MPH with atomoxetine on sleep, as assessed with actigraphy and polysomnography. MPH was associated with a greater incidence of insomnia and increased SOL compared to atomoxetine (39.2 vs 12.1 minutes). Atomoxetine was associated with more frequent night awakenings. Although this was found to be a statistically significant difference in favor of atomoxetine, individual children may nonetheless experience insomnia as a side effect.
Clonidine and guanfacine had been used off-label to treat hyperactivity and sleep problems in children with ADHD and stimulant-induced insomnia [60]. Prince et al. [61] conducted a chart review and found that low-dose clonidine had a beneficial effect on sleep disturbance children and adolescents with ADHD at baseline, medicine-induced, or medicine-exacerbated sleep disturbance. Extended-release formulations of these alpha-2 agonists have recently been developed and approved for treating ADHD as either monotherapy or in combination with a stimulant [4]. All of these agents are associated with somnolence, sedation, and fatigue in 20 to 40 % of children when used as monotherapy [62]. These adverse events are significantly improved when co-administered with a stimulant, but still occur in 15 to 20 % of children, especially at the beginning of a trial [5, 63]. In contrast to immediate release clonidine, there has been no evidence of hypnotic effects or differences in sleep with morning (am) versus evening (pm) dosing.
Discussion
Stimulant medications are associated with increased difficulty in falling asleep, longer latency to sleep onset, and overall shorter duration of sleep, as demonstrated in numerous studies using both objective and subjective measures of sleep. Dose-response and fixed-dose studies have demonstrated that increasing dose and shorter duration of stimulant exposure are associated with more frequent reports of insomnia. Children who present with a diathesis to insomnia may worsen, especially when they start stimulant therapy, take higher doses of stimulants, or if they are young, small, or stimulant-naïve. Children will also be more vulnerable to adverse sleep effects if they have a pre-existing sleep disorder prior to stimulant treatment, or a comorbid condition also associated with sleep disorders, such as depression or anxiety.
It would appear that both ADHD and the treatment of ADHD are associated with dysregulation of sleep through the life cycle from infancy to adulthood, although for a subset of adults, stimulant treatment may be associated with improvements in sleep. Clearly, ADHD, sleep, and ADHD treatment are closely intertwined. Considerable evidence suggests that ADHD is in fact a 24-hour disorder, with an increased tendency for delayed sleep phase, restless legs, periodic limb movement disorders, and paradoxically excessive daytime sleepiness.
One might ask how can a disorder characterized by hyperactivity is also said to be associated with sleepiness, yet the evidence to date is that the ADHD phenotype is not unlike what we see in overtired children. These patients are “on the go,” but given the chance, they have features of chronic sleep deprivation and poor sustained vigilance [64]. Inadequate duration of sleep will contribute to “sleep debt,” making it more difficult to wake up in the morning and increasing reports of tiredness during the day. Although the cumulative impact of significant night-to-night variability that occurs when medication is not administered consistently is concerning, this is not systematically evaluated in most medication studies. Thus, optimal treatment of ADHD needs to balance daytime functioning while minimizing effects on sleep duration and variability.
How are ADHD, Sleep, and Sleep Problems Related?
The mechanisms explaining the relationship between sleep and ADHD are complex and there are several potential etiologies that are not mutually exclusive. As noted by Owens [65], sleep problems may mimic ADHD symptomatology, may exacerbate underlying ADHD symptoms, and may be associated with or exacerbated by ADHD, and psychotropic medications used to treat ADHD may result in sleep problems. Furthermore, in any individual, the relationship between ADHD treatment may be direct (i.e., between improved, worse, or variable sleep), may be an artifactual or indirect effect (i.e., ADHD medications or treatment improve comorbid condition or functioning, and sleep subsequently improves); or may be a moderator of response (e.g., sleep problems may limit dosing necessary to achieve an optimal response). Examining the relationship between sleep and ADHD is further complicated by the differing time course of ADHD treatments and their effects on ADHD symptoms and sleep. For example, some sleep problems, such as increased sleep onset latency may be more prevalent during titration and may decrease with time, as ADHD symptoms improve, or as the child adjusts to the medication. On the other hand, if ADHD medications have an effect on circadian rhythm or result in chronic sleep deprivation, sleep effects (e.g., difficulty waking, somnolence) will be subtler and will occur later [66]. Thus, short-term studies or long-term studies of responders to ADHD medications may not capture all sleep effects of ADHD treatment that are observed in clinical settings. Furthermore, rates of sleep problems and other side effects are likely affected by both sample selection and methodological issues (i.e., length of trial, titration duration, method for increasing or decreasing, and how drop-outs are handled).
Clinical Implications
Before starting a treatment, patients should first be screened for primary sleep disorders that require a different treatment. The clinician should ask the patient questions regarding problems going to bed, falling asleep, maintaining a sleep schedule, waking or unusual behaviors during the night, snoring, difficulty getting up in the morning, or tiredness during the day. Sleep studies may be warranted in those with suspicion of obstructive sleep apnea or symptoms of restless leg syndrome (urge to move, worse at rest, worse at night better with movement). In addition, sleep onset latency, night-to-night variability, and total sleep duration should all be assessed prior to beginning treatment to track changes in sleep associated with treatment. This information can be used to educate the family on sleep hygiene and the importance of consistent sleep. In developing a treatment plan, impairments for that individual and relationship to nighttime functioning, which emphasize the 24-h phenotype of ADHD should be considered. As needed or PRN use of immediate release stimulants during evenings and weekends should be discussed with the recognition that many adolescents and adults will require more than once a day dosing.
Much more is known about the average effects of ADHD medications on groups than on individuals. Clinical predictors of sleep problems during pharmacotherapy include age, sleep problems prior to initiating treatment, dose, and dosing schedule. Individual variability in the dose-response effects of ADHD medications, duration of effect, and perhaps withdrawal effects or rebound, as stimulant concentrations decline. A stimulant dose that necessary to reduce hyperactivity or impulsivity may be associated with a detrimental effect on sleep in some individuals, whereas others may display no noticeable effect on sleep or even improved sleep.
Future Direction
ADHD should not just be viewed as a daytime disorder. Optimal treatment for ADHD should include promoting sleep hygiene and pharmacological adjustments to minimize adverse effects on sleep. As yet, we have little understanding of the biological or genetic factors related to individual drug response and sleep. Future studies with adaptive designs are needed to determine optimal, personalized treatment strategies for treating sleep problems in ADHD patients through their lifespan. In the meanwhile, the clinician needs to take the individual patient’s complaint at face value, and for many patients with ADHD, sleep is the primary complaint. Similarly, for many patients who present with sleep difficulty, ADHD is the primary complaint. Specialists in ADHD and sleep disorders are going to need skills in managing both disorders to obtain an optimal response in many patients.
Electronic supplementary material
(PDF 374 kb)
Acknowledgments
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Dulcan MK, Benson RS. AACAP Official Action. Summary of the practice parameters for the assessment and treatment of children, adolescents, and adults with ADHD. J Am Acad Child Adolesc Physchiatry. 1997;36:1311–1317. doi: 10.1097/00004583-199709000-00033. [DOI] [PubMed] [Google Scholar]
- 2.Adler LA. Pharmacotherapy for adult ADHD. J Clin Psychiatry. 2009;70:e12. doi: 10.4088/JCP.7129br5c. [DOI] [PubMed] [Google Scholar]
- 3.Wilens TE. Pharmacotherapy of ADHD in adults. CNS Spectr. 2008;13(5 suppl 8):11–13. doi: 10.1017/s1092852900002960. [DOI] [PubMed] [Google Scholar]
- 4.Sallee FR, McGough J, Wigal T, Donahue J, Lyne A, Biederman J. Guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Physchiatry. 2009;48:155–165. doi: 10.1097/CHI.0b013e318191769e. [DOI] [PubMed] [Google Scholar]
- 5.Kollins SH, Jain R, Brams M, et al. Clonidine extended-release tablets as add-on therapy to psychostimulants in children and adolescents with ADHD. Pediatrics. 2011;127:e1406–e1413. doi: 10.1542/peds.2010-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolraich M, Brown L, Brown RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128:1007–1022. doi: 10.1542/peds.2011-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swanson JM, Wigal SB, Wigal T, et al. A comparison of once-daily extended-release methylphenidate formulations in children with attention-deficit/hyperactivity disorder in the laboratory school (the Comacs Study) Pediatrics. 2004;113(3 pt 1):e206–e216. doi: 10.1542/peds.113.3.e206. [DOI] [PubMed] [Google Scholar]
- 8.Pelham WE, Jr, Fabiano GA. Evidence-based psychosocial treatments for attention-deficit/hyperactivity disorder. J Clin Child Adolesc Psychol. 2008;37:184–214. doi: 10.1080/15374410701818681. [DOI] [PubMed] [Google Scholar]
- 9.Spruyt K, Raubuck D, Grogan K, Gozal D, Stein M. Variable sleep schedules and outcomes n chiuldren with psychopathological problems: preliminary observations. Nat Sci Sleep. 2012;4:9–17. doi: 10.2147/NSS.S29299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chervin RD, Ruzicka DL, Giordani BJ, et al. Sleep-disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics. 2006;117:e769–e778. doi: 10.1542/peds.2005-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gruber R, Wiebe S, Montecalvo L, Brunetti B, Amsel R, Carrier J. Impact of sleep restriction on neurobehavioral functioning of children with attention deficit hyperactivity disorder. Sleep. 2011;34:315–323. doi: 10.1093/sleep/34.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heijden KB, Smits MG, Someren EJ, Ridderinkhof KR, Gunning WB. Effect of melatonin on sleep, behavior, and cognition in ADHD and chronic sleep-onset insomnia. J Am Acad Child Adolesc Physchiatry. 2007;46:233–241. doi: 10.1097/01.chi.0000246055.76167.0d. [DOI] [PubMed] [Google Scholar]
- 13.Weiss MD, Wasdell MB, Bomben MM, Rea KJ, Freeman RD. Sleep hygiene and melatonin treatment for children and adolescents with ADHD and initial insomnia. J Am Acad Child Adolesc Physchiatry. 2006;45:512–519. [PubMed] [Google Scholar]
- 14.Owens JA. A clinical overview of sleep and attention-deficit/hyperactivity disorder in children and adolescents. J Can Acad Child Adolesc Psychiatry. 2009;18:92–102. [PMC free article] [PubMed] [Google Scholar]
- 15.Heijden KB, Smits MG, Gunning WB. Sleep-related disorders in ADHD: a review. Clin Pediatr. 2005;44:201–210. doi: 10.1177/000992280504400303. [DOI] [PubMed] [Google Scholar]
- 16.Huang YS, Tsai MH, Guilleminault C. Pharmacological treatment of ADHD and the short and long term effects on sleep. Curr Pharm Des. 2011;17:1450–1458. doi: 10.2174/138161211796197179. [DOI] [PubMed] [Google Scholar]
- 17.Stein MA. Unravelling sleep problems in treated and untreated children with ADHD. J Child Adolesc Psychopharmacol. 1999;9:157–168. doi: 10.1089/cap.1999.9.157. [DOI] [PubMed] [Google Scholar]
- 18.Cortese S, Faraone SV, Konofal E, Lecendreux M. Sleep in children with attention-deficit/hyperactivity disorder: meta-analysis of subjective and objective studies. J Am Acad Child Adolesc Physchiatry. 2009;48:894–908. doi: 10.1097/CHI.0b013e3181ac09c9. [DOI] [PubMed] [Google Scholar]
- 19.Corkum P, Tannock R, Moldofsky H. Sleep disturbances in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Physchiatry. 1998;37:637–646. doi: 10.1097/00004583-199806000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Gruber R, Xi T, Frenette S, Robert M, Vannasinh P, Carrier J. Sleep disturbances in prepubertal children with attention deficit hyperactivity disorder: a home polysomnography study. Sleep. 2009;32:343–350. doi: 10.1093/sleep/32.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi J, Yoon IY, Kim HW, Chung S, Yoo HJ. Differences between objective and subjective sleep measures in children with attention deficit hyperactivity disorder. J Clin Sleep Med. 2010;6:589–595. [PMC free article] [PubMed] [Google Scholar]
- 22.Sadeh A, Pergamin L, Bar-Haim Y. Sleep in children with attention-deficit hyperactivity disorder: A meta-analysis of polysomnographic studies. Sleep Med Rev. 2006;10:381–398. doi: 10.1016/j.smrv.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Mayes SD, Calhoun SL, Bixler EO, et al. ADHD subtypes and comorbid anxiety, depression, and oppositional-defiant disorder: differences in sleep problems. J Pediatr Psychol. 2009;34:328–337. doi: 10.1093/jpepsy/jsn083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Accardo JA, Marcus CL, Leonard MB, Shults J, Meltzer LJ, Elia J. Associations between psychiatric comorbidities and sleep disturbances in children with attention-deficit/hyperactivity disorder. J Dev Behav Pediatr 2012;33:97-105. [DOI] [PMC free article] [PubMed]
- 25.Willoughby MT, Angold A, Egger HL. Parent-reported attention-deficit/hyperactivity disorder symptomatology and sleep problems in a preschool-age pediatric clinic sample. J Am Acad Child Adolesc Physchiatry. 2008;47:1086–1094. doi: 10.1097/CHI.0b013e31817eed1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein MA, Mendelsohn J, Obermeyer WH, Amromin J, Benca R. Sleep and behavior problems in school-aged children. Pediatrics. 2001;107:E60. doi: 10.1542/peds.107.4.e60. [DOI] [PubMed] [Google Scholar]
- 27.Cortese S, Konofal E, Yateman N, Mouren MC, Lecendreux M. Sleep and alertness in children with attention-deficit/hyperactivity disorder: a systematic review of the literature. Sleep. 2006;29:504–511. [PubMed] [Google Scholar]
- 28.Tsai MH, Huang YS. Attention-deficit/hyperactivity disorder and sleep disorders in children. Med Clin North Am. 2010;94:615–632. doi: 10.1016/j.mcna.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 29.O’Brien LM, Ivanenko A, Crabtree VM, et al. The effect of stimulants on sleep characteristics in children with attention deficit/hyperactivity disorder. Sleep Med. 2003;4:309–316. doi: 10.1016/S1389-9457(03)00071-6. [DOI] [PubMed] [Google Scholar]
- 30.Kooij JJ, Middelkoop HA, Gils K, Buitelaar JK. The effect of stimulants on nocturnal motor activity and sleep quality in adults with ADHD: an open-label case-control study. J Clin Psychiatry. 2001;62:952–956. doi: 10.4088/JCP.v62n1206. [DOI] [PubMed] [Google Scholar]
- 31.Sobanski E, Schredl M, Kettler N, Alm B. Sleep in adults with attention deficit hyperactivity disorder (ADHD) before and during treatment with methylphenidate: a controlled polysomnographic study. Sleep. 2008;31:375–381. doi: 10.1093/sleep/31.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Surman CB, Adamson JJ, Petty C, et al. Associations between attention-deficit/hyperactivity disorder and sleep impairment in adulthood: evidence from a large controlled study. J Clin Psychiatry. 2009;70:1523–1529. doi: 10.4088/JCP.08m04514. [DOI] [PubMed] [Google Scholar]
- 33.Wigal SB, Kollins SH, Childress AC, Squires L. A 13-hour laboratory school study of lisdexamfetamine dimesylate in school-aged children with attention-deficit/hyperactivity disorder. Child Adolesc Psychiatry Ment Health. 2009;3:17. doi: 10.1186/1753-2000-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Findling RL, Turnbow J, Burnside J, Melmed R, Civil R, Li Y. A randomized, double-blind, multicenter, parallel-group, placebo-controlled, dose-optimization study of the methylphenidate transdermal system for the treatment of ADHD in adolescents. CNS Spectr. 2010;15:419–430. doi: 10.1017/s1092852900000353. [DOI] [PubMed] [Google Scholar]
- 35.Rechtschaffen A, Maron L. The effect of amphetamine on the sleep cycle. Electroencephalogr Clin Neurophysiol. 1964;16:438–445. doi: 10.1016/0013-4694(64)90086-0. [DOI] [PubMed] [Google Scholar]
- 36.Stein M, Pao M. Is sleep delayed, disrupted, or disturbed? In: Greenhill L, Osman B, editors. Ritalin: theory and patient management: New York: Liebert, 1999:287-299.
- 37.Ahmann PA, Waltonen SJ, Olson KA, Theye FW, Erem AJ, LaPlant RJ. Placebo-controlled evaluation of Ritalin side effects. Pediatrics. 1993;91:1101–1106. [PubMed] [Google Scholar]
- 38.Barkley RA, McMurray MB, Edelbrock CS, Robbins K. Side effects of methylphenidate in children with attention deficit hyperactivity disorder: a systemic, placebo-controlled evaluation. Pediatrics. 1990;86:184–192. [PubMed] [Google Scholar]
- 39.Stein MA, Sarampote CS, Waldman ID, et al. A dose-response study of OROS methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics. 2003;112:e404. doi: 10.1542/peds.112.5.e404. [DOI] [PubMed] [Google Scholar]
- 40.Giblin JM, Strobel AL. Effect of lisdexamfetamine dimesylate on sleep in children with ADHD. J Atten Disord. 2011;15:491–498. doi: 10.1177/1087054710371195. [DOI] [PubMed] [Google Scholar]
- 41.Wigal SB, Wong AA, Jun A, Stehli A, Steinberg-Epstein R, Lerner MA. Adverse events in medication treatment naive children with attention-deficit/hyperactivity disorder: results from a small, controlled trial of lisdexamfetamine dimesylate. J Child Adolesc Psychopharmacol 2012;22:149-156. [DOI] [PubMed]
- 42.Chatoor I, Wells KC, Conners CK, Seidel WT, Shaw D. The effects of nocturnally administered stimulant medication on EEG sleep and behavior in hyperactive children. J Am Acad Child Psychiatry. 1983;22:337–342. doi: 10.1016/S0002-7138(09)60668-3. [DOI] [PubMed] [Google Scholar]
- 43.Stein MA, Blondis TA, Schnitzler ER, et al. Methylphenidate dosing: twice daily versus three times daily. Pediatrics. 1996;98(4 pt 1):748–756. [PubMed] [Google Scholar]
- 44.Kent JD, Blader JC, Koplewicz HS, Abikoff H, Foley CA. Effects of late-afternoon methylphenidate administration on behavior and sleep in attention-deficit hyperactivity disorder. Pediatrics. 1995;96(2 pt 1):320–325. [PubMed] [Google Scholar]
- 45.Sangal RB, Owens J, Allen AJ, Sutton V, Schuh K, Kelsey D. Effects of atomoxetine and methylphenidate on sleep in children with ADHD. Sleep. 2006;29:1573–1585. doi: 10.1093/sleep/29.12.1573. [DOI] [PubMed] [Google Scholar]
- 46.Greenhill L, Puig-Antich J, Goetz R, Hanlon C, Davies M. Sleep architecture and rapid eye movement sleep measures in prepubertal children with attention deficit disorder with hyperactivity. Sleep. 1983;6:91–101. doi: 10.1093/sleep/6.2.91. [DOI] [PubMed] [Google Scholar]
- 47.Kim HW, Yoon IY, Cho SC, et al. The effect of OROS methylphenidate on the sleep of children with attention-deficit/hyperactivity disorder. Int Clin Psychopharmacol. 2010;25:107–115. doi: 10.1097/YIC.0b013e3283364411. [DOI] [PubMed] [Google Scholar]
- 48.Wilens TE, Boellner SW, Lopez FA, et al. Varying the wear time of the methylphenidate transdermal system in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Physchiatry. 2008;47:700–708. doi: 10.1097/CHI.0b013e31816bffdf. [DOI] [PubMed] [Google Scholar]
- 49.Faraone SV, Glatt SJ, Bukstein OG, Lopez FA, Arnold LE, Findling RL. Effects of once-daily oral and transdermal methylphenidate on sleep behavior of children with ADHD. J Atten Disord. 2009;12:308–315. doi: 10.1177/1087054708314844. [DOI] [PubMed] [Google Scholar]
- 50.Efron D, Jarman F, Barker M. Side effects of methylphenidate and dexamphetamine in children with attention deficit hyperactivity disorder: a double-blind, crossover trial. Pediatrics. 1997;100:662–666. doi: 10.1542/peds.100.4.662. [DOI] [PubMed] [Google Scholar]
- 51.McCracken JT, Biederman J, Greenhill LL, et al. Analog classroom assessment of a once-daily mixed amphetamine formulation, SLI381 (Adderall XR), in children with ADHD. J Am Acad Child Adolesc Physchiatry. 2003;42:673–683. doi: 10.1097/01.CHI.0000046863.56865.FE. [DOI] [PubMed] [Google Scholar]
- 52.Stein MA, Waldman ID, Charney E, et al. Dose effects and comparative effectiveness of extended release dexmethylphenidate and mixed amphetamine salts. J Child Adolesc Psychopharmacol. 2011;21:581–588. doi: 10.1089/cap.2011.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Biederman J, Krishnan S, Zhang Y, McGough JJ, Findling RL. Efficacy and tolerability of lisdexamfetamine dimesylate (NRP-104) in children with attention-deficit/hyperactivity disorder: a phase III, multicenter, randomized, double-blind, forced-dose, parallel-group study. Clin Ther. 2007;29:450–463. doi: 10.1016/S0149-2918(07)80083-X. [DOI] [PubMed] [Google Scholar]
- 54.Biederman J, Boellner SW, Childress A, Lopez FA, Krishnan S, Zhang Y. Lisdexamfetamine dimesylate and mixed amphetamine salts extended-release in children with ADHD: a double-blind, placebo-controlled, crossover analog classroom study. Biol Psychiatry. 2007;62:970–976. doi: 10.1016/j.biopsych.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 55.Brams M, Giblin J, Gasior M, Gao J, Wigal T. Effects of open-label lisdexamfetamine dimesylate on self-reported quality of life in adults with ADHD. Postgrad Med. 2011;123:99–108. doi: 10.3810/pgm.2011.07.2309. [DOI] [PubMed] [Google Scholar]
- 56.Surman CB, Roth T. Impact of stimulant pharmacotherapy on sleep quality: post hoc analyses of 2 large, double-blind, randomized, placebo-controlled trials. J Clin Psychiatry. 2011;72:903–908. doi: 10.4088/JCP.11m06838. [DOI] [PubMed] [Google Scholar]
- 57.Adler LA, Liebowitz M, Kronenberger W, et al. Atomoxetine treatment in adults with attention-deficit/hyperactivity disorder and comorbid social anxiety disorder. Depress Anxiety. 2009;26:212–221. doi: 10.1002/da.20549. [DOI] [PubMed] [Google Scholar]
- 58.Yildiz O, Sismanlar SG, Memik NC, Karakaya I, Agaoglu B. Atomoxetine and methylphenidate treatment in children with ADHD: the efficacy, tolerability and effects on executive functions. Child Psychiatry Hum Dev. 2011;42:257–269. doi: 10.1007/s10578-010-0212-3. [DOI] [PubMed] [Google Scholar]
- 59.Block S, Kelsey D, Coury D, et al. Once-daily atomoxetine for treating pediatric attention-deficit/hyperactivity disorder: comparison of morning and evening dosing. Clin Pediatr 2010;48:723-733. [DOI] [PubMed]
- 60.Wilens TE, Biederman J, Spencer T. Clonidine for sleep disturbances associated with attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Physchiatry. 1994;33:424–426. doi: 10.1097/00004583-199403000-00018. [DOI] [PubMed] [Google Scholar]
- 61.Prince JB, Wilens TE, Biederman J, Spencer TJ, Wozniak JR. Clonidine for sleep disturbances associated with attention-deficit hyperactivity disorder: a systematic chart review of 62 cases. J Am Acad Child Adolesc Physchiatry. 1996;35:599–605. doi: 10.1097/00004583-199605000-00014. [DOI] [PubMed] [Google Scholar]
- 62.Daviss WB, Patel NC, Robb AS, et al. Clonidine for attention-deficit/hyperactivity disorder: II. ECG changes and adverse events analysis. J Am Acad Child Adolesc Physchiatry. 2008;47:189–198. doi: 10.1097/chi.0b013e31815d9ae4. [DOI] [PubMed] [Google Scholar]
- 63.Faraone SV, Glatt SJ. Effects of extended-release guanfacine on ADHD symptoms and sedation-related adverse events in children with ADHD. J Atten Disord. 2010;13:532–538. doi: 10.1177/1087054709332472. [DOI] [PubMed] [Google Scholar]
- 64.Weinberg WA, Brumback RA. Primary disorder of vigilance: a novel explanation of inattentiveness, daydreaming, boredom, restlessness, and sleepiness. J Pediatr. 1990;116:720–725. doi: 10.1016/S0022-3476(05)82654-X. [DOI] [PubMed] [Google Scholar]
- 65.Owens JA. The ADHD and sleep conundrum: a review. J Dev Behav Pediatr. 2005;26:312–322. doi: 10.1097/00004703-200508000-00011. [DOI] [PubMed] [Google Scholar]
- 66.Spruyt K, Gozal D. Sleep disturbances in children with attention-deficit/hyperactivity disorder. Expert Rev Neurother. 2011;11:565–577. doi: 10.1586/ern.11.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 374 kb)