Abstract
The sensory systems of the New Zealand kiwi appear to be uniquely adapted to occupy a nocturnal ground-dwelling niche. In addition to well-developed tactile and olfactory systems, the auditory system shows specializations of the ear, which are maintained along the central nervous system. Here, we provide a detailed description of the auditory nerve, hair cells, and stereovillar bundle orientation of the hair cells in the North Island brown kiwi. The auditory nerve of the kiwi contained about 8,000 fibers. Using the number of hair cells and innervating nerve fibers to calculate a ratio of average innervation density showed that the afferent innervation ratio in kiwi was denser than in most other birds examined. The average diameters of cochlear afferent axons in kiwi showed the typical gradient across the tonotopic axis. The kiwi basilar papilla showed a clear differentiation of tall and short hair cells. The proportion of short hair cells was higher than in the emu and likely reflects a bias towards higher frequencies represented on the kiwi basilar papilla. The orientation of the stereovillar bundles in the kiwi basilar papilla showed a pattern similar to that in most other birds but was most similar to that of the emu. Overall, many features of the auditory nerve, hair cells, and stereovilli bundle orientation in the kiwi are typical of most birds examined. Some features of the kiwi auditory system do, however, support a high-frequency specialization, specifically the innervation density and generally small size of hair-cell somata, whereas others showed the presumed ancestral condition similar to that found in the emu.
Keywords: hair cell, basilar papilla, auditory nerve, Paleognathae
Introduction
The New Zealand kiwi (Apteryx spp.) is unique in many respects, having well-developed olfactory and tactile systems and a reduction to the visual system (Bang and Cobb 1968; Cobb 1960; Cunningham et al. 2007, 2009; Martin et al. 2007; Wenzel 1968, 1971). These adaptations are likely associated with their nocturnal, ground-dwelling niche. In addition, North Island brown kiwi (Apteryx mantelli) appears to have specializations of the auditory system, both in the ear and brain (Corfield et al. 2011). Specifically, the mechanosensitive hair bundles of the sensory hair cells in the basilar papilla (BP) showed little change in the basal, high frequency half of the epithelium, similar to that described for barn owls (Tyto alba). In addition, the dendritic lengths in the brainstem nucleus laminaris (NL) also showed a similar feature, with a less pronounced change in dendritic lengths in the presumed high-frequency regions. Together, these morphological features suggested a fovea-like overrepresentation of a narrow high-frequency band, approximately 4–6 kHz, in kiwi (Corfield et al. 2011). The kiwi BP also shows other interesting features, such as a low total hair-cell number with about 4,000 hair cells, which is much lower than would be expected given its length (4 mm). For instance, the chicken has a similar BP length to that of kiwi but has over twice as many hair cells and the emu has 17,000 hair cells for a BP length that is only slightly longer than that of kiwi (Köppl et al. 1998; Manley et al. 1996). The kiwi BP is also, correspondingly, much narrower than the BP of all other birds examined (Corfield et al. 2011), and only barn owls have fewer hair cells across the width of its basal half (Fischer et al. 1988). It is clear that the kiwi auditory system is unlike that of other birds and in particular unlike that of the closely related emu, which has been suggested to represent the ancestral condition of the avian inner ear.
The morphology of the BP and associated hair cells has been evaluated in detail in a number of avian species, ranging from those that have high or low frequency hearing, and those that are auditory generalists or specialists (chicken, Gallus gallus (Manley et al. 1996; Tilney and Saunders 1983), seagull, Larus marinus (Counter and Tsao 1986), starling, Sturnus vulgaris and pigeon, Columba livia (Gleich and Manley 1988), barn owl, Tyto alba (Fischer et al. 1988), rhea, Rhea americana (Jorgensen and Christensen 1989), budgerigar, Melopsittacus undulatus (Manley et al. 1993), canary, Serinus canaria and zebra finch, Taeniopygia guttata (Gleich et al. 1994), duck, Aythya fuligula (Manley et al. 1996), and emu, Dromaius novaehollandiae (Köppl et al. 1998)). Kiwi are unique in that they appear to be a high frequency specialist (Corfield et al. 2011), like barn owls, despite belonging to an ancestral group of birds, the Paleognathae. This feature is unlike that of the kiwi's closest relatives, which are all characterized by low-frequency vocalizations (Davies 2002) and those that have been examined show some low-frequency specializations to the ear and brain (Köppl et al. 1998; MacLeod et al. 2006).
The auditory nerve forms the link between the cochlear receptors and the brain. In birds, it carries the afferent and efferent fibers of both the BP and the lagenar macula, a vestibular endorgan situated at the apical end of the cochlear duct. In birds, the afferent component of the cochlear nerve contains nearly exclusively myelinated fibers (Fischer et al. 1994; Köppl 1997; Köppl et al. 2000). Beginning at both ends of the papilla, exiting afferent fibers travel parallel to the papilla, successively joining other fibers, and finally merging with the fiber stream originating from the opposite end to exit the inner ear through the internal auditory meatus. Birds show a differentiation of auditory hair cells into two types, these being the tall hair cells (THC) and the short hair cells (SHC) (Fischer 1992, 1994b; Takasaka and Smith 1971). Hair cells on the avian papilla show a gradual change from the THC extreme found at the apical-neural edge of the BP to the SHC extreme found at the basal-abneural edge of the BP (Fischer 1994a). Avian hair cells are therefore commonly classified using a shape factor, which is the ratio of the hair cell's height to its width: hair cells with a shape factor of less than 1 are classified as SHC and hair cells with a shape factor greater than 1 are classified as THC (Takasaka and Smith 1971). The innervation pattern provides an alternative, more functional basis for distinction between the two types of avian hair cells. Tall hair cells have large afferent terminals and receive small efferent contacts, whereas SHC receive only large efferent and no afferent contacts (Fischer 1992, 1994a). This innervation pattern gradually changes from the neural to the abneural side of the papilla.
In the present study, we add to our current knowledge of the kiwi ear by providing a detailed morphological analysis of the auditory nerve, hair cells, and stereovillar bundle orientation and determine if any features are present that further support auditory specializations in the kiwi. The results show that many features of the auditory system of kiwi were typical of other birds that have been examined. However, some features, such as innervation density and hair-cell soma size, suggested a high frequency specialization, whereas others showed the presumed ancestral condition similar to that found in the emu.
Materials and methods
The data reported here are derived from two North Island brown kiwi (Apteryx mantelli) specimens (NB20 and NB21, 6–8 months old and 1 week old, respectively). It is likely that the morphology of the ear and nerve in juvenile kiwi is in most respects adult-like, and the data reported here reflect the mature condition. Studies in the emu, a close relative of the kiwi, support this. Using very similar techniques to the present study, the morphology of the basilar papilla in hatchling and adult emus showed only very minor differences due to continued overall growth (Köppl et al. 1998, 2000). Experiments were carried out under research permits from the New Zealand Department of Conservation # NO-16732-FAU, NO-16732-RES, NO-18095-DOA. Both specimens were provided to us dead by conservation authorities and wildlife veterinarians and thus no further ethics approvals were required to undertake this research. One of the birds was immediately fixed by transcardial perfusion using 4 % paraformaldehyde (PFA) in 0.1 M phosphate buffer. The other individual was euthanized by a veterinarian, its head removed, transported under cooling, and immersion-fixed in 4 % PFA within 2 h of death. All tissue was stored in 4 % PFA until processed further.
Auditory nerve
The results obtained from the left auditory nerve of one juvenile kiwi are reported (NB20). The auditory nerve, including the auditory-vestibular ganglion and cochlea, were dissected out. The nerve was transected at the level of its entrance to the cochlea using fine iris scissors, washed in PBS buffer, and cryoprotected in 30 % sucrose in PBS. The nerve with the ganglion was then embedded in albumin-gelatine and serial cryosections 100 μm in thickness were cut on a sliding microtome. The sections were collected floating in PBS and examined under low magnification to select sections at the level of the central auditory meatus. Nerve sections at this level contain both the afferent and efferent fibers that supply the basilar papilla and the lagenar macula (Köppl et al. 2000). Sections were then post-fixed in 1 % OsO4 in PBS for 2 h, washed, and dehydrated in a graded series of alcohols. To prevent distortion of the sections, they were restrained in a flat position with a custom-built holder used during the dehydration process. Sections were then taken through three steps of increasing araldite concentration in propylene oxide and finally embedded in araldite (Durcupan by Sigma-Aldrich) using BEEM embedding capsule moulds and pre-hardened araldite cylinders to hold sections at the bottom of the mould. The resin was prepared as a conventional hard variant (components A/M:B:C:D at 22.7:20:0.7:0.3 parts by weight). Sections of 1–2 μm thickness were cut, mounted on superfrost slides, and flattened by waving a chloroform soaked cotton bud over the section on a hot plate. Sections were stained with a 1 % aqueous solution of toluidine blue and coverslipped with DePeX (Serva) from xylene.
The best nerve section (judged by the quality of staining and by whether it was sitting entirely flat on the slide) was imaged under a Leica DMR upright microscope, a Nikon Digital Sight cooled color camera, and a 100× oil immersion objective. The resulting images were merged using the Photomerge function of Adobe Photoshop (v 10.0, Adobe System Incorporated). Axons were counted manually using ImageJ (v1.34s, National Institutes of Health, USA). For analysis, each axon profile (enclosed by, but excluding, the myelin sheath) was identified using contrast thresholding in Photoshop to binarize the image and was colored white while everything else was colored black, resulting in an image with only white axons on a black background. This image was then subjected to particle analysis by analySIS FIVE (Soft Imaging Software, Münster, Germany). Two parameters were extracted for each particle = axon: the equivalent circle diameter (ECD) and the minimum diameter. To exclude fibers that were sectioned at an oblique angle, only axons with a shape factor (a measure of roundness) of less than 2 were included in the analysis (Köppl 1997). An independent count of fibers from the lagenar macula in the same specimen was obtained from semithin cochlear sections by counting the number of fibers that extended beyond the apical end of the basilar papilla.
Hair cell morphology
The left cochlea obtained from the 1 week old kiwi chick (NB21) of unknown sex was dissected free of bone and postfixed in 1 % OsO4 in PBS. The cochlea was then dehydrated in a graded series of ethanol concentrations and embedded in epoxy resin (“Durcupan” by Sigma-Aldrich, NSW 2154, Australia) via ascending concentrations in propylene oxide. The resin was prepared as a soft variant for semi-thin sectioning (components A/M:B:C:D at 24.5:17.8:1:3.8 parts by weight). The specimen was completely and serially cut into 5 μm-thick cross sections and stained with toluidine blue. The angle of sectioning was adjusted in areas of pronounced papilla curvature to maintain a section plane orthogonal to the long axis of the basilar papilla throughout. At 10 % intervals along the basilar papilla, three neighboring sections were selected for detailed analysis. The sections were digitized using a Nikon Digital Sight cooled color camera attached to a Leica DMR upright microscope and an oil immersion 100× objective (objective N.A. = 1.4; condenser N.A. = 0.9). For each section, the width of the BP was obtained along with the distance of each hair cell from the neural edge of the BP. Only hair cells with a complete nucleus were measured. The height of a hair cell was measured as the distance between the middle of the cell's apical surface and the lowest point on the cell's basal pole. The width of a hair cell was measured at the papillar surface, and this was usually the widest point of a hair cell in cross-section. All measurements were obtained from images using ImageJ (http://rsbweb.nih.gov/ij/). A ratio of height to length was calculated for each hair cell. This is the most commonly used criterion to classify tall and short hair cells in the avian basilar papilla (e.g., (Köppl et al. 1998)). Tall hair cells (THC) have a shape factor greater than 1, short hair cells (SHC) less than 1.
Stereovillar bundle orientation
The left cochlea from NB20 was dissected free of bone and lightly stained with Janus Green to visualize the tectorial membrane. The tegmentum vasculosum, tectorial membrane, lagenar otolith, and overhanging parts of the lagena were then dissected away with fine forceps. The specimen was post-fixed in 1 % OsO4 in phosphate-buffered saline (PBS), dehydrated, critical-point dried in carbon dioxide (Baltec CPD 030), mounted on a stub and sputter-coated with a thin layer of gold. The specimen was viewed using a Zeiss ULTRA plus (University of Sydney, Australia) scanning electron microscope at 5 and 20 kV acceleration voltage, respectively. The entire basilar papilla was first documented as a series of photographs while tilting the SEM stage to maintain a perpendicular view of the papilla's surface. These provided a virtually flattened photomontage of the entire structure (final print magnification of 800×) that was used to determine standard positions at regular 10 % intervals along the papilla. At each 10 % interval, a strip of the BP was imaged from the neural to abneural edge. The orientation of stereovillar bundles was determined as the angle of the long axis of the bundle relative to the neural edge of the basilar papilla at the same longitudinal position. Near-zero angles thus indicate an orientation parallel to the neural edge, with the tallest stereovilli facing abneurally. Positive angles indicate a deviation by which the tallest stereovilli are facing towards the papillar apex. Cells with negative angles have their tallest stereovilli facing the papillar base.
Results
Auditory nerve
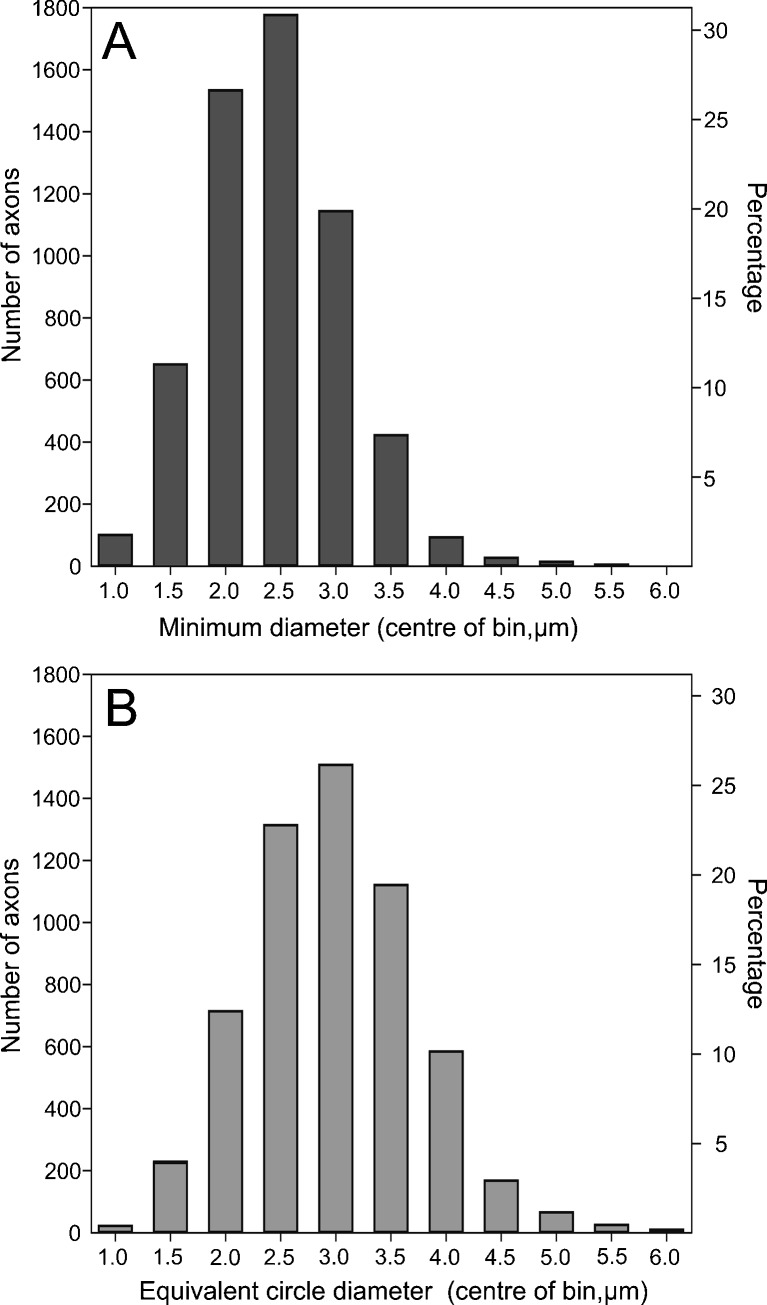
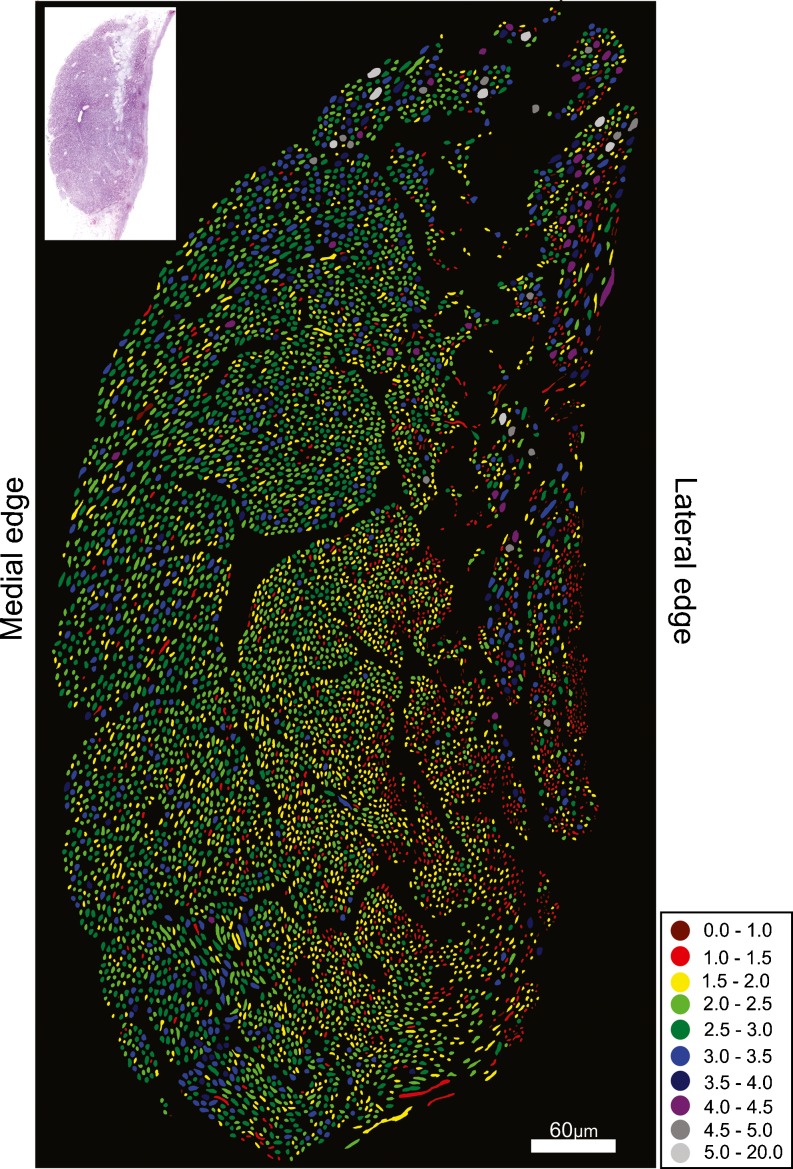
The auditory nerve of the investigated kiwi specimen contained 8,185 fibers, which included 1,249 lagenar fibers. Subtracting the number of lagenar fibers from the total number of fibers obtained from the cross sections of the auditory nerve gives an estimate of 6,936 papillar fibers. Axons had an average equivalent circle diameter (ECD) of 2.7 μm and an average minimum diameter (MinD) of 2.2 μm (ECD min = 0.3 μm, max = 7.7 μm, MinD min = 0.2 μm, max = 6.5 μm, n = 5,768). The majority of axon diameters for both measurements were between 2 and 3.5 μm (Fig. 1). The kiwi auditory-nerve section clearly showed a differential distribution of axon sizes (Fig. 2). Fibers with MinD values of 2.5 μm and above were predominately found along the medial edge, with the smallest axons occurring on the opposite edge of the nerve where fiber size decreased to a MinD value of below 1. There was also an area of the nerve along the lateral edge where nerve fiber size varied substantially, with a mixture of both small and large fibers. This area likely contains the lagenar fibers because, like other vestibular nerves, they are characterized by a larger range of fiber sizes (Köppl 1997; Köppl et al. 2000). Similar to the emu (Köppl et al. 2000), there was no clearly recognizable, separate bundle of efferent fibers.
FIG. 1.
Distribution of axon sizes in a section of the auditory nerve of the North Island brown kiwi, classified into 0.5 μm bins. A minimal diameter B equivalent circle diameter (ECD). The second y axis shows the percentage of axons in each bin.
FIG. 2.
Cross sections of the auditory nerve of the kiwi. Colors represent the minimal axonal diameter (micrometer) as indicated in the legend. The nerve section used for analysis is shown in the top left hand corner.
Hair cell morphology
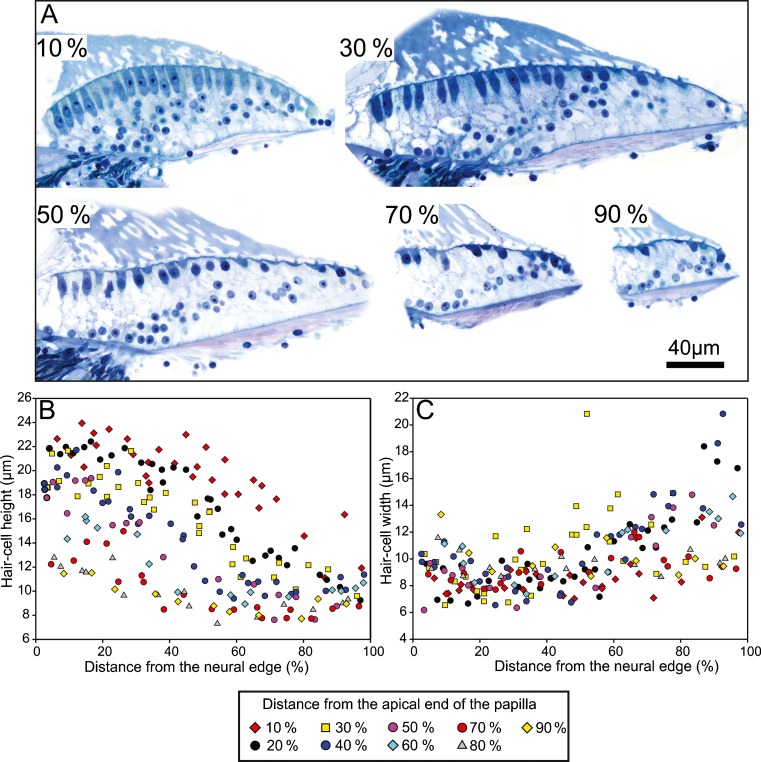
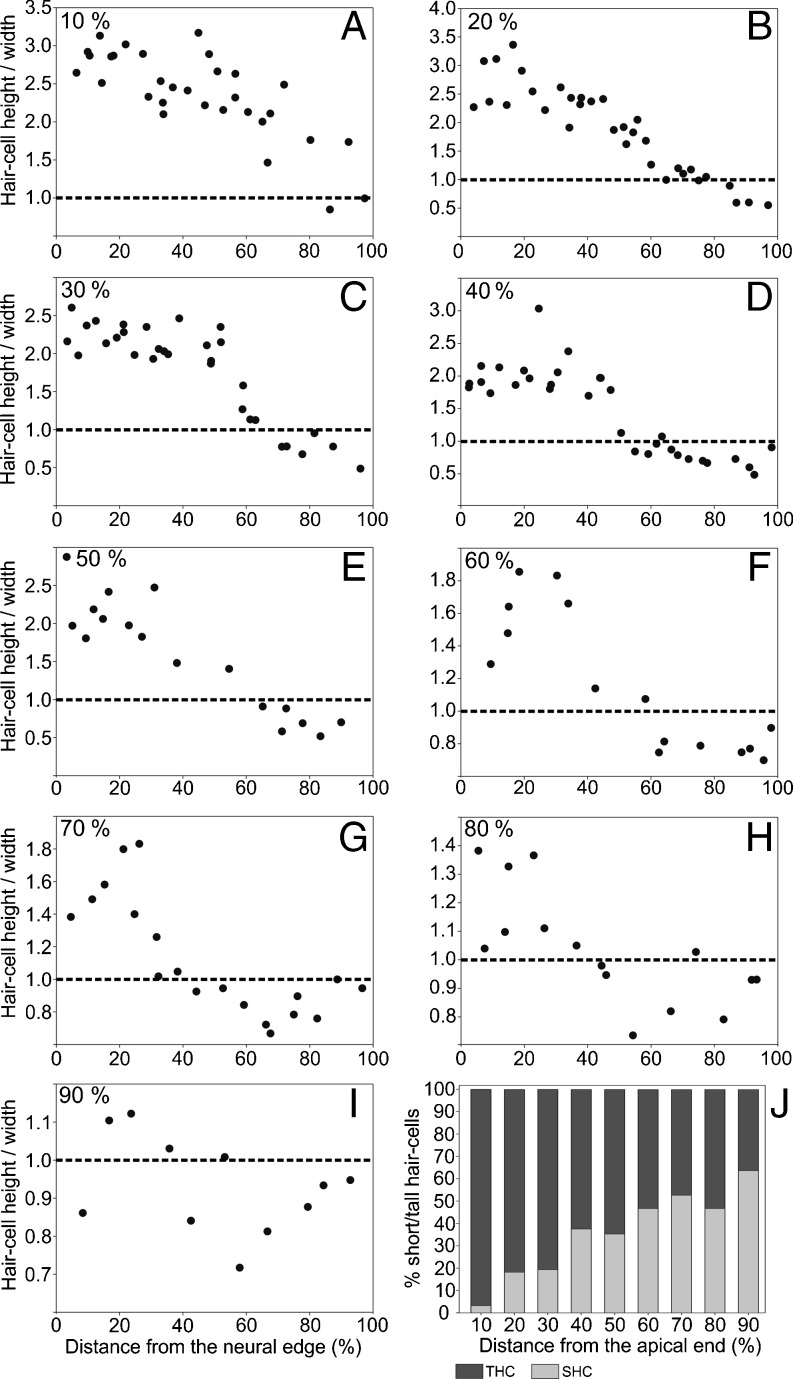
In the basilar papilla of the investigated kiwi specimen, hair cells generally became taller from the abneural to the neural edge, as well as from the basal to the apical end (Fig. 3A, B). At a position, 10 % from the apical end, neurally lying hair cells were the tallest, at 20–24 μm high, which decreased to 11–15 μm at the abneural edge (Fig. 3B). At the basal end (90 % from apex), hair cells did not vary much in height, with heights ranging between 8 and 12 μm (Fig. 3B). Hair-cell width did not show a clear trend either along the length or across the width of the papilla (Fig. 3A, C). However, near the abneural edge of the papilla, there were some hair cells that were much wider than elsewhere on the papilla, with hair-cell widths ranging from 16 to 21 μm (Fig. 3C). Hair-cell widths in other regions of the papilla ranged from ~6 to 15 μm. The ratio of hair-cell height to width (shape factor) generally decreased from the neural to the abneural side in any one cross section (Fig. 4). Shape factors below 1, i.e. SHC, were typically only observed close to the abneural edge of the papilla. Out of a total of 204 hair cells measured on the BP according to the shape-factor criterion, 62 (30.4 %) were SHC and 142 (69.6 %) were THC. The largest shape factor values were observed at the apical end of the papilla (3.3) and smallest at the basal end (0.4). The proportion of SHC at each 10 % interval long the BP increased from the apical to basal end (Fig. 4J). At 10 % from the apical end, only 3.2 % of all hair cells classified as SHC, whereas at 90 % from the apical end, 63.6 % of hair-cells were SHC.
Fig. 3.
Hair cell morphology in the kiwi basilar papilla. A High magnification images of the basilar papilla at 10 %, 30 %, 50 %, 70 %, and 90 % positions from the apical end of the papilla. Height (B) and width (C) of hair cells in the kiwi basilar papilla as a function of the normalized distance from the neural edge of the papilla. Symbols represent the position of the hair cells along the length of the basilar papilla as indicated in the legend.
Fig. 4.
The ratio of hair cell height to width as a function of normalized distance from the neural papillar edge, for nine different positions along the papilla, as indicated (A–I). The dashed line at a ratio of 1 indicates the commonly used criterion for distinguishing tall and short hair cells (tall above and short below). J Percentage of tall (dark graybar) and short (light graybar) hair cells as a function of normalized distance from the apical end of the papilla. THC tall hair cell; SHC short hair cell.
Orientation of hair cell bundles
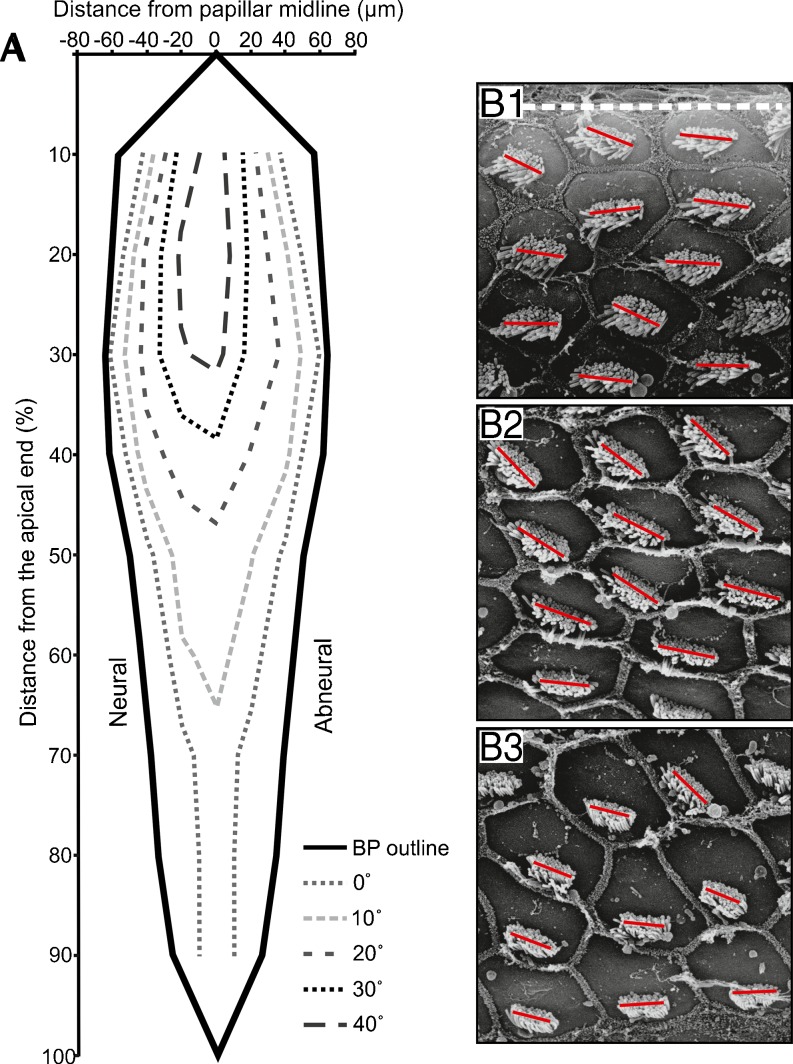
In the investigated kiwi specimen, the orientation of the hair-cell stereovillar bundles showed a consistent pattern of change across the width of the basilar papilla (Fig. 5). Bundles of hair cells near the edges of the papilla were oriented near 0 °, i.e., their mechanosensitive axis was in the radial dimension, and hair cells at increasing distances from either edge had their bundles increasingly rotated to positive angles (Fig. 5). Maximal deviations from radial orientation were observed in the apical 20 % of the papilla's length and near the midline, where peak rotation was 60–70 ° towards the papilla's apex. Towards the basal half of the papilla, the change in bundle orientation gradually got smaller and was not observed in the basal-most 30–40 %.
Fig. 5.
Stereovillar bundle orientations on the kiwi basilar papilla. A The schematic represents the average stereovillar bundle orientation in one kiwi papilla, derived from SEM measurements. The papillar outline is drawn in gray. Each colored contour encloses an area towards the midline of the papilla, where orientation angles greater than or equal to the respective value (as indicated in the legend) occurred. B1–3: Sample electron micrographs to illustrate the change in hair-bundle orientation across the basilar papilla. The panels show a group of hair cells each, in neural (B1), middle (B2), and abneural (B3) positions, at a location about 40 % from the apical end of the papilla. The dashed whiteline in (B1) marks the papilla's neural border and individual blacklines indicate the long axes of the stereovillar bundles.
Discussion
Fiber numbers in the auditory nerve suggest a cochlear specialization
The auditory nerve of the investigated kiwi specimen contained 8,185 fibers. We assume that most, if not all, of those were afferent fibers. In the kiwi, like in the emu (Köppl et al. 2000), a distinct bundle of efferent fibers could not be recognized. It is therefore likely that, as in the emu (Köppl 2001), the great majority of efferent fibers are unmyelinated axons of too small a diameter to be resolved light-microscopically.
Assuming ~8,185 afferent fibers for the kiwi, this number is low compared to that reported in other species (barn owl (T. alba): 32,500, chicken (G. gallus): 14,250, emu (D. novaehollandiae): 11,220, starling (S. vulgaris): 10,120, duck (A. fuligula): 11,000, budgerigar (Melopsittacus undulates): 10,890 canary (S. canaria): 6,880), with only the canary containing fewer fibers (Manley et al. 1993; Gleich et al. 1994; Köppl 1997; Köppl et al. 2000). Subtracting the number of lagenar fibers from the total number of afferent fibers gave an estimate of 6,936 auditory afferent fibers in the kiwi. In barn owls, emus, starlings, tufted ducks, and budgerigars the number of auditory fibers was again much higher than in kiwi (31,140, 12,400, 10,000, 8,770, 9,740, 9,760, 9,800, respectively), with, again, only the canary having fewer auditory fibers (6,080). However, this result was not unexpected, considering the small number of only around 4,000 hair cells found on the kiwi's basilar papilla (Corfield et al. 2011).
Knowledge of both the hair-cell number and the number of innervating nerve fibers permits the calculation of a ratio that may serve as an indicator of average innervation density. In kiwi, the ratio of afferent fiber number to hair cell number was 1.7, meaning that for every hair cell, there are 1.7 nerve fibers. This is near the upper end of the scale determined for a range of different bird species (Köppl et al. 2000); Table 1). However, in birds, a significant proportion of hair cells (the short hair cells) typically do not receive any afferent innervation (Fischer 1994a), so these non-innervated hair cells should ideally be excluded from the calculations. Although the number of hair cells that do not receive any afferent innervation is not known for the kiwi, in most other species, including the closely related emu, it is between 20 % and 25 % (Köppl et al. 2000, Table 1). Assuming the same for kiwi, this yielded a corrected fiber to hair cell ratio of 2.1–2.3. Again, this ratio falls at the upper end of the scale (Table 1). Although these values provide only a rough estimate of innervation ratio in kiwi, the results contradict the commonly held belief that only phylogenetically advanced species have a dense afferent innervation ratio (Gleich and Manley 2000). Furthermore, a high innervation ratio suggests a relative overrepresentation of certain frequency bands in terms of auditory-nerve fiber numbers (Köppl et al. 2000). This, in turn, is consistent with the prediction of a high-frequency fovea in the kiwi basilar papilla and auditory system (Corfield et al. 2011). A more in-depth study of innervation patterns and regional innervation densities in the cochlea of kiwi would be highly interesting.
TABLE 1.
Average innervation density and range of best hearing across avian species
| Species | Hair-cell number | Sources | No. of papillar afferent fibers | Sources | Ratio of affs./HC | Prop. of aff.-innervated HC (Köppl et al. 2000) | Ratio of affs./HC innervated | Frequency range (Hz) | Sources |
|---|---|---|---|---|---|---|---|---|---|
| Chicken | 11,000 | (Tilney and Tilney 1986) (Manley et al. 1996) | 12,406 | (Köppl et al. 2000) | 1.13 | 80 % | 1.41 | 10–5,000 | (Gleich et al. 2004) |
| Pigeon | 9,610 | (Gleich and Manley 1988) | 5,136 or ~10,000 | (Boord 1969) (Winter 1963) | 0.5–1 | Assume 80 % | 0.6–1.2 | 50–5,000 | (Smolders et al. 1995) |
| Emu | 17,564 | (Köppl et al. 1998) | 10,038 | (Köppl et al. 2000) | 0.57 | 80 % | 0.71 | 50–5,000 | (Köppl and Manley 1997) |
| Starling | 5,830 | (Gleich and Manley 1988) | 8,775 | (Köppl et al. 2000) | 1.51 | 75 % | 2.01 | 30–6,000 | (Gleich et al. 2004) |
| Canary | 3,000 | (Gleich et al. 1994) | 6,080 | (Gleich et al. 2001) | 2.03 | Assume 75 % | 2.70 | 400–7,500 | (Gleich et al. 2004) |
| Budgerigar | 5,372 | (Manley et al. 1993) | 9,766 | (Manley et al. 1993) | 1.82 | Assume 75 % | 2.42 | 200–6,000 | (Gleich et al. 2004) |
| Barn owl | 16,300 | (Fischer et al. 1988) | 31,142 | (Köppl 1997) | 1.91 | 64 % | 2.99 | 100–10,000 | (Köppl et al. 1993) |
| Kiwi | 4,000 | (Corfield et al. 2011) | 6,936 | This study | 1.7 | Assume 75–80 % | 2.1–2.3 | 400–6,000 | (Corfield et al. 2011) |
For a total of 8 avian species, the table lists published counts of basilar-papilla hair cells and auditory afferent axons from the sources given in adjacent columns. From these numbers, the average ratio of afferent fibers to hair cells was derived in column 6. For the chicken, emu, starling, and barn owl, where quantitative data on innervation are available, Köppl et al (2000) calculated the proportion of afferently innervated hair cells (column 7). For the remaining species, an estimate was added, based on the most closely related species with actual data. This enabled the calculation of a corrected ratio of afferent fibers to hair cells, taking only those hair cells into account which actually receive afferents (column 8). The last two columns list the range of characteristic frequencies represented on the basilar papilla. For the canary, budgerigar, and kiwi, these are estimates based on a multitude of data detailed in the respective sources. Note the clear trend for species with a higher average afferent innervation density to also have a more restricted and higher-frequency-biased range of best hearing
The range of auditory-nerve axon diameters was similar to that of other birds
The average diameter of cochlear afferents in birds that have been examined ranges from about 2–3 μm (Köppl 1997). The emu and the barn owl, however, tend to have larger axons (closer to 3 μm) than other species examined. The average diameter of cochlear afferent axons in kiwi were of an intermediate size, being around 2.5 μm. Axon diameter also showed a gradient across the nerve section in kiwi. The largest fibers were located towards the medial edge of the nerve and the smallest towards the lateral edge. This gradient pattern has been found in most birds examined (barn owls, chickens, emus, ducks, but not in starlings) (Köppl 1997; Köppl et al. 2000). This feature of the auditory nerve is thought to mirror the map of fibers innervating different regions along the basilar papilla, i.e., representing different frequency ranges. This was tested by Köppl (1997) and Köppl et al. (2000) who found a decline in axon diameter from the basal-most, high-frequency region of the papilla to the middle regions of the papilla where axons were slightly larger than more apical ones. The findings suggested that axon diameter may not be related to the absolute frequency range but instead related to the spatial point of origin in the cochlea. Alternatively, it was suggested that the variation in axonal size reflects the behavioral importance of specific frequencies, i.e., regions of the basilar papilla representing the behaviorally most important frequency range are innervated by the largest afferent axons. Therefore, in kiwi, it is difficult to determine the meaning of this fiber gradient at this stage; the gradient is most similar to that in emu, less so to that in chicken and duck.
Kiwi hair-cell populations most resemble those of the emu but show a high-frequency bias
Avian THC and SHC are clearly defined in their extremes but grade into each other in nearly every aspect that has been studied (review in (Gleich and Manley 2000). Any criterion applied to define relative proportions of THC and SHC is thus necessarily arbitrary. Importantly, different criteria may lead to different results (Köppl et al. 1998) for a given species. In the emu, a particularly striking dissociation was observed: according to the commonly used shape factor (ratio of hair-cell height to width), the emu had a comparatively small number of SHC and those did not extend into the apical 40 % of its basilar papilla (Köppl et al. 1998). However, hair cells without afferent innervation, an alternative definition of SHC associated with more obvious functional relevance (Fischer 1994a), were found all along the abneural edge (Fischer 1998). In neognathous birds, in contrast, the number of shape-factor defined SHC tends to be equal or higher than the number of hair cells without afferent innervation (Köppl et al. 1998). This was interpreted to indicate that the differential innervation pattern of avian THC and SHC evolved early and before any further differentiating characteristics.
The kiwi data now suggest that the hair-cell shape factor might be complicated by an additional dependence on the preferred frequencies of the hair cells. According to the shape-factor criterion, the region of SHC in the kiwi extended far more apically than in the emu and, consequently, kiwi had a higher overall proportion of SHC. However, this difference may be explained entirely by different tonotopic gradients. The emu is a known low-frequency specialist, devoting a full 65 % of its basilar papilla to frequencies below 1 kHz (Köppl and Manley 1997), while several lines of evidence suggest a high-frequency bias for the kiwi, with only an estimated apical 30 % of its papilla representing similarly low frequencies (Corfield et al. 2011). In both species, the tonotopic position where SHC according to the shape-factor criterion first appear is thus estimated to fall at about 500 Hz (between 10 % and 20 % from the apical end in kiwi, about 40 % in emu). It is well known that all avian hair cells, both THC and SHC, are shorter in basal, higher-frequency regions compared to apical, lower-frequency regions of the papilla (Gleich and Manley 2000). The fact that this will bias the definition by shape factor towards fewer SHC in low-frequency regions and more SHC in high-frequency regions is thus not surprising but has not been recognized so far. It alone might explain the observed discrepancy in the emu between SHC defined by shape factor and SHC defined by innervation. Unfortunately, the popular hair-cell shape factor has no known functional relevance. Therefore, data on the innervation pattern of kiwi hair cells and/or their complement of ion channels (Fuchs et al. 1998) are needed to judge the degree of functional THC-SHC differentiation. Intriguingly, and similar to the emu, no distinct bundle of efferent fibers could be identified in the kiwi auditory nerve. This suggests an early stage of differentiation of the efferent cochlear supply, whereby the majority of efferent fibers is still of an unmyelinated, small-diameter type (Köppl 2001, 2011).
Stereovillar bundle orientation is very typical of the avian basilar papilla
The orientation of the stereovillar bundles in kiwi shows a pattern similar to that in most other birds (Fischer et al. 1988; Gleich and Manley 1988; Gleich et al. 1994; Köppl et al. 1998; Manley et al. 1993, 1996; Tilney et al. 1987). Hair cells at both the neural and abneural edges of the papilla all have their bundles oriented nearly perpendicular to the edge, i.e., radially across the papilla, with bundles towards the papillar midline increasingly orientated towards the apex. As in other birds, this change in bundle orientation is greatest in apical areas and very small or uniform at the papillar base. This general pattern of hair-cell polarities is very typical and well characterized for the avian basilar papilla. Intriguingly, its functional significance remains unknown.
Conclusions
Overall, the auditory nerve, hair cells, and stereovillar bundle orientation in the kiwi showed some features that are typical of others birds that have been examined, whereas other features showed the presumed ancestral condition similar to the emu. There was also some evidence to support a fovea-like overrepresentation of a narrow high-frequency band in the BP of the kiwi, namely the innervation density and hair-cell size. Although it can only be speculated as to the function of this auditory fovea, it is likely to be associated with either their vocal communication system or an adaptation for prey detection and localization (Corfield et al. 2011). In the first instance, kiwi hearing could be tuned to a specific high frequency component of their call which, for example, could provide an important cue for individual recognition. Alternatively and akin to the barn owl, this auditory fovea may give the kiwi an enhanced ability to locate the many large invertebrates that are found in New Zealand forests at night. Nonetheless, it is likely that the presence of an auditory fovea in the kiwi is an adaptation to the nocturnal and ground dwelling ecological niche that kiwi occupy.
Acknowledgments
We wish to first thank C. Gardner and the New Zealand Department of Conservation and B. Gartrell at the Massey University College of Veterinary Medicine for their assistance in procuring specimens. We would also like to thank Hillary Holloway and the staff at the Biomedical Imaging Research Unit, the Electron and Light Microscopy Unit, and the Research Centre for Surface and Material Sciences at the University of Auckland, and the Electron Microscopy Unit at the University of Sydney for help with the Scanning Electron Microscopy.
Contributor Information
Jeremy R. Corfield, Phone: +1-403-3325211, FAX: +1-403-3292775, Email: jr.corfield@gmail.com
M. Fabiana Kubke, Email: f.kubke@auckland.ac.nz.
Stuart Parsons, Email: s.parsons@auckland.ac.nz.
Christine Köppl, Email: christine.koeppl@uni-oldenburg.de.
References
- Bang BG, Cobb S. Size of olfactory bulb in 108 species of birds. Auk. 1968;85:55–61. [Google Scholar]
- Boord RL. The anatomy of the avian auditory system. Ann NY Acad Sci. 1969;169:186–198. doi: 10.1111/j.1749-6632.1969.tb20444.x. [DOI] [Google Scholar]
- Cobb S. A note on the size of the avian olfactory bulb. Epilepsia. 1960;1:394–402. doi: 10.1111/j.1528-1157.1959.tb04276.x. [DOI] [PubMed] [Google Scholar]
- Corfield JR, Kubke MF, Parsons S, Wild JM, Köppl C. Evidence for an auditory fovea in the New Zealand kiwi (Apteryx mantelli) PLoS One. 2011;6(8):e23771. doi: 10.1371/journal.pone.0023771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counter SA, Tsao P. Morphology of the seagull's inner ear. Acta Otolaryngol. 1986;101:34–42. doi: 10.3109/00016488609108605. [DOI] [PubMed] [Google Scholar]
- Cunningham SJ, Castro I, Alley M. A new prey-detection mechanism for kiwi (Apteryx spp.) suggests convergent evolution between paleognathous and neognathous birds. J Anat. 2007;211:493–502. doi: 10.1111/j.1469-7580.2007.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham SJ, Castro I, Potter MA. The relative importance of olfaction and remote touch in prey detection by North Island brown kiwis. Anim Behav. 2009;78:899–905. doi: 10.1016/j.anbehav.2009.07.015. [DOI] [Google Scholar]
- Davies S. Ratites and tinamous. New York: Oxford University Press Inc.; 2002. [Google Scholar]
- Fischer FP. Quantitative analysis of the innervation of the chicken basilar papilla. Hear Res. 1992;61:167–178. doi: 10.1016/0378-5955(92)90048-R. [DOI] [PubMed] [Google Scholar]
- Fischer FP. General pattern and morphological specializations of the avian cochlea. Scanning Microsc. 1994;8:351–363. [PubMed] [Google Scholar]
- Fischer FP. Quantitative TEM analysis of the barn owl basilar papilla. Hear Res. 1994;73:1–15. doi: 10.1016/0378-5955(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Fischer FP. Hair cell morphology and innervation in the basilar papilla of the emu (Dromaius novaehollandiae) Hear Res. 1998;121:112–124. doi: 10.1016/S0378-5955(98)00072-0. [DOI] [PubMed] [Google Scholar]
- Fischer FP, Köppl C, Manley GA. The basilar papilla of the barn owl Tyto alba: a quantitative morphological SEM analysis. Hear Res. 1988;34:87–101. doi: 10.1016/0378-5955(88)90053-6. [DOI] [PubMed] [Google Scholar]
- Fischer FP, Eisensamer B, Manley GA. Cochlear and lagenar ganglia of the chicken. J Morphol. 1994;220:71–83. doi: 10.1002/jmor.1052200107. [DOI] [PubMed] [Google Scholar]
- Fuchs P, Zidanic M, Michaels R, Yuhas W, Jiang GJ. Ion channels and synaptic function in chick cochlear hair cells. In: Palmer AR, Rees A, Summerfield AQ, Meddis R, editors. Psychophysical and psychological advances in hearing. London: Whurr Published Ltd; 1998. pp. 97–104. [Google Scholar]
- Gleich O, Manley GA. Quantitative morphological analysis of the sensory epithelium of the starling and pigeon basilar papilla. Hear Res. 1988;34:69–85. doi: 10.1016/0378-5955(88)90052-4. [DOI] [PubMed] [Google Scholar]
- Gleich O, Manley GA. The hearing organ of birds and crocodilia. In: Dooling RJ, Fay R, Popper A, editors. Comparative hearing: birds and reptiles. New York: Springer-Verlag; 2000. [Google Scholar]
- Gleich O, Manley GA, Mandl A, Dooling RJ. Basilar papilla of the canary and zebra finch: a quantitative scanning electron microscopical description. J Morphol. 1994;221:1–24. doi: 10.1002/jmor.1052210102. [DOI] [PubMed] [Google Scholar]
- Gleich O, Dooling RJ, Ryals BM. A quantitative analysis of the nerve fibers in the VIIIth nerve of Belgian Waterslager canaries with a hereditary sensorineural hearing loss. Hear Res. 2001;151:141–148. doi: 10.1016/S0378-5955(00)00221-5. [DOI] [PubMed] [Google Scholar]
- Gleich O, Fischer FP, Köppl C, Manley GA. Hearing organ evolution and specialization: Archosaurs. In: Manley GA, Popper A, Fay RR, editors. Evolution of the vertebrate auditory system. New York: Springer Verlag; 2004. pp. 224–255. [Google Scholar]
- Jorgensen JM, Christensen JT. The inner ear of the common rhea (Rhea americana L.) Brain Behav Evol. 1989;34:273–280. doi: 10.1159/000116512. [DOI] [PubMed] [Google Scholar]
- Köppl C. Number and axon calibres of cochlear afferents in the barn owl. Aud Neurosci. 1997;3:313–344. [Google Scholar]
- Köppl C. Efferent axons in the avian auditory nerve. Eur J Neurosci. 2001;13:1889–1901. doi: 10.1046/j.0953-816x.2001.01567.x. [DOI] [PubMed] [Google Scholar]
- Köppl C. Evolution of the octavolateral efferent system. In: Ryugo D, Fay RR, Popper AN, editors. Auditory and vestibular efferents. New York: Springer Science + Business Media, LLC; 2011. pp. 217–259. [Google Scholar]
- Köppl C, Manley GA (1997) Frequency representation in the emu basilar papilla. J Acoust Soc Am 101:1574–1584
- Köppl C, Gleich O, Manley GA. An auditory fovea in the barn owl cochlea. J Comp Physiol A. 1993;171:695–704. doi: 10.1007/BF00213066. [DOI] [Google Scholar]
- Köppl C, Gleich O, Schwabedissen G, Siegl E, Manley GA. Fine structure of the basilar papilla of the emu: implications for the evolution of avian hair-cell types. Hear Res. 1998;126:99–112. doi: 10.1016/S0378-5955(98)00156-7. [DOI] [PubMed] [Google Scholar]
- Köppl C, Wegscheider A, Gleich O, Manley GA. A quantitative study of cochlear afferent axons in birds. Hear Res. 2000;139:123–143. doi: 10.1016/S0378-5955(99)00178-1. [DOI] [PubMed] [Google Scholar]
- MacLeod KM, Soares D, Carr CE. Interaural timing difference circuits in the auditory brainstem of the emu (Dromaius novaehollandiae) J Comp Neurol. 2006;495:185–201. doi: 10.1002/cne.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GA, Schwabedissen G, Gleich O. Morphology of the basilar papilla of the Budgerigar, Melopsittacus undulatus. J Morphol. 1993;218:153–165. doi: 10.1002/jmor.1052180205. [DOI] [PubMed] [Google Scholar]
- Manley GA, Meyer B, Fischer FP, Schwabedissen G, Gleich O. Surface morphology of basilar papilla of the tufted duck Aythya fuligula, and domestic chicken Gallus gallus domesticus. J Morphol. 1996;227:197–212. doi: 10.1002/(SICI)1097-4687(199602)227:2<197::AID-JMOR6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Martin GR, Wilson KJ, Martin Wild J, Parsons S, Fabiana Kubke M, Corfield J. Kiwi forego vision in the guidance of their nocturnal activities. PLoS One. 2007;2(2):e198. doi: 10.1371/journal.pone.0000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders JW, Ding-Pfennigdorff D, Klinke R. A functional map of the pigeon basilar papilla: correlation of the properties of single auditory nerve fibres and their peripheral origin. Hear Res. 1995;92:151–169. doi: 10.1016/0378-5955(95)00214-6. [DOI] [PubMed] [Google Scholar]
- Takasaka T, Smith CA. The structure and innervation of the pigeon's basilar papilla. J Ultrastruct Res. 1971;35:20–65. doi: 10.1016/S0022-5320(71)80141-7. [DOI] [PubMed] [Google Scholar]
- Tilney LG, Saunders JC. Actin filaments, stereocilia, and hair cells of the bird cochlea. I. Length, number, width, and distribution of stereocilia of each hair cell are related to the position of the hair cell on the cochlea. J Cell Biol. 1983;96:807–821. doi: 10.1083/jcb.96.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, Tilney MS. Functional organization of the cytoskeleton. Hear Res. 1986;22:55–77. doi: 10.1016/0378-5955(86)90077-8. [DOI] [PubMed] [Google Scholar]
- Tilney M, Tilney L, DeRosier D. The distribution of hair cell bundle lengths and orientations suggest an unexpected pattern of hair cell stimulation in the chick cochlea. Hear Res. 1987;25:141–151. doi: 10.1016/0378-5955(87)90087-6. [DOI] [PubMed] [Google Scholar]
- Wenzel BM. Olfactory prowess of the kiwi. Nature. 1968;220:1133–1134. doi: 10.1038/2201133a0. [DOI] [PubMed] [Google Scholar]
- Wenzel BM. Olfactory sensation in the kiwi and other birds. Ann N Y Acad Sci. 1971;188:183–193. doi: 10.1111/j.1749-6632.1971.tb13097.x. [DOI] [PubMed] [Google Scholar]
- Winter P. Vergleichende qualitative und quantitative Untersuchungen an der Hörbahn von Vögeln. Z Morphol Ökol Tiere. 1963;52:365–400. doi: 10.1007/BF00408568. [DOI] [Google Scholar]