Abstract
Age-related hearing loss, or presbyacusis, is a major public health problem that causes communication difficulties and is associated with diminished quality of life. Limited satisfaction with hearing aids, particularly in noisy listening conditions, suggests that central nervous system declines occur with presbyacusis and may limit the efficacy of interventions focused solely on improving audibility. This study of 49 older adults (M = 69.58, SD = 8.22 years; 29 female) was designed to examine the extent to which low and/or high frequency hearing loss was related to auditory cortex morphology. Low and high frequency hearing constructs were obtained from a factor analysis of audiograms from these older adults and 1,704 audiograms from an independent sample of older adults. Significant region of interest and voxel-wise gray matter volume associations were observed for the high frequency hearing construct. These effects occurred most robustly in a primary auditory cortex region (Te1.0) where there was also elevated cerebrospinal fluid with high frequency hearing loss, suggesting that auditory cortex atrophies with high frequency hearing loss. These results indicate that Te1.0 is particularly affected by high frequency hearing loss and may be a target for evaluating the efficacy of interventions for hearing loss.
Keywords: presbyacusis, age-related hearing loss, auditory cortex
Introduction
Age-related hearing loss, or presbyacusis, is a chronic health condition that affects nearly everyone over 70 years of age with more rapid and pronounced effects occurring in the higher frequencies and in the oldest adults (Brant and Fozard 1990; Matthews et al. 1997; Lee et al. 2005; Wiley et al. 2008; Echt et al. 2010; Lin et al. 2011). This chronic and progressive hearing loss affects communication as the high to low frequency progression of presbyacusis encroaches on frequencies important for speech recognition (Gates and Mills 2005; Dubno et al. 2008; Humes and Dubno 2010). Current interventions for hearing loss provide communication benefit for some people, but well-fit hearing aids provide limited satisfaction (Bertoli et al. 2009). Declines in the central auditory system, independently or as a result of peripheral auditory system decline, could contribute to limited success for interventions focused solely on improving speech audibility.
Peripheral auditory system declines have pronounced effects on central auditory system structure and function. For example, noise exposure that causes cochlear damage is associated with structural changes in the cochlear nuclei (Coordes et al. 2012) and inferior colliculus (Coordes et al. 2012). Similarly, presbyacusis-related changes have been observed structurally in spiral ganglion cells that were collected from human temporal bones (Francis et al. 2011; Hinojosa and Nelson 2011) and functionally in spiral ganglion cells recorded using an animal model of presbyacusis (Lang et al. 2010). Age-related changes also have been observed in the rat inferior colliculus and auditory cortex that are associated with increased excitability in auditory cortex neurons (Caspary et al. 2008).
The consequences of peripheral hearing loss appear to extend to auditory cortex morphology. Lower gray matter volume estimates in auditory cortex (Husain et al. 2011; Peelle et al. 2011) and changes in extra-temporal gray matter and white matter (Husain et al. 2011) have been observed in middle-aged and older adults with mild to moderate hearing loss. It is not clear from these studies the extent to which (1) low and/or high frequency hearing loss contributes to the findings or (2) there is an indirect influence of age on hearing and brain morphology. It is also possible that (3) hearing associations with brain morphology reflect normal variation in anatomy that is associated with risk for hearing loss and/or gender effects given evidence that men are more likely to exhibit high frequency hearing loss than women (Raynor et al. 2009; Lin et al. 2011) and that men may exhibit relatively less auditory cortex volume than women (Rademacher et al. 2001), even in early adulthood (Brun et al. 2009). Clarifying these questions is important for determining the causal mechanism(s) for central auditory system declines that may influence treatment for a disorder that is growing as a major public health problem (Kalayam et al. 1995; Cruickshanks et al. 2003).
Materials and methods
Participants
Forty-nine older adults with a mean age of 69.58 years [SD = 8.22; range = 54.14–88.41 years; 29 female; Edinburgh handedness = 81.84, SD = 7.10, (Oldfield 1971)] participated in this study. All participants reported American English to be their native language, and they did not report fluency in a second language. Only one participant who had a career as a music teacher reported expertise as an organist and so it is unlikely that musical expertise influenced results of this study (Schneider et al. 2002; Gaser and Schlaug 2003; Schneider et al. 2005). Only one participant reported wearing a hearing aid. Exclusionary criteria included a history of head trauma, seizures, self-reported central nervous system disorders, conductive hearing loss or otologic disease, and contraindications for safe MRI scanning. Participants provided written informed consent before participating in this Medical University of South Carolina Institutional Review Board approved study.
Audiometric evaluation
Cerumen management was performed prior to audiometric evaluation for participants whose otoscopic evaluation demonstrated excessive cerumen. Pure-tone thresholds at conventional frequencies (250, 500, 1,000, 2,000, 3,000, 4,000, 6,000, and 8,000 Hz) were obtained with a Madsen OB922 clinical audiometer calibrated to appropriate ANSI standards (ANSI 2004) and equipped with TDH-39 headphones. Bone conduction testing (500, 1,000, 2,000, 3,000, and 4,000 Hz) was performed for all subjects to exclude the possibility that elevated thresholds were due to conductive hearing loss. The pure-tone audiometric thresholds for each ear and mean of those thresholds across the sample are shown in Figure 1A. Factor analysis was performed using the pure tone threshold data from both ears for all 49 participants with the same variables for 852 older adults (mean = 69.92, SD = 7.24 years; range = 50.35–97.46 years; 474/55.6 % female) who had participated in a study on presbyacusis and who were recruited with exclusionary criteria for conductive hearing loss or otologic disease (Lee et al. 2005; Dubno et al. 2008). This larger sample was used to obtain stable estimates of pure tone threshold components and standardize the factor variance relative to the larger population of older adults.
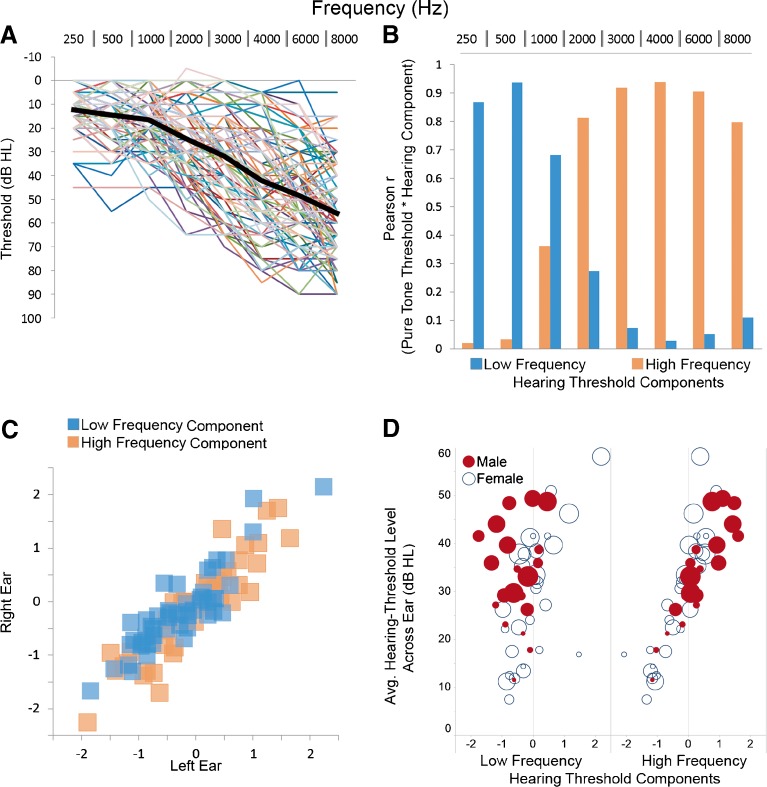
FIG. 1.
Thresholds and factor analysis components. A Left and right ear pure-tone thresholds (dB HL) are plotted for each participant’s left and right ear as a function of frequency (Hz) with the mean thresholds across participants and ears (large black line). B Low frequency (blue) and high frequency (orange) hearing threshold components from the factor analysis were correlated with the pure tone thresholds to demonstrate the frequencies that load with each hearing threshold component. C Low frequency [blue squares: r(49) = 0.89, p < 0.001] and high frequency [orange squares: r(49) = 0.91, p < 0.001] components were strongly correlated for the left and right ears. D The high frequency component [r(49) = 0.87, p < 0.001; male mean = 0.30 (0.79), female mean = −0.32 (0.74)], but not the low frequency component [r(49) = −0.17, ns; male mean = −0.55 (0.57), female mean = −0.01 (0.73)], was significantly correlated with the average pure tone threshold across frequencies and ears. Note that the greater variance and poorer low frequency hearing in female (open blue circles) compared to male (filled red circles). The increasing size of the symbols represents increasing age across subjects.
MRI data acquisition and image pre-processing
A Siemens 3T Trio and 32 channel head coil were used to collect T1-weighted images with the following parameters: 160 slices with a 256 × 256 matrix, TR = 8.13 ms, TE = 3.7 ms, flip angle = 8 º, slice thickness = 1 mm, and no slice gap. Each participant’s T1-weighted image was rigidly aligned into AC-PC orientation and then bias-field corrected and segmented using the SPM8 unified segmentation algorithm. The segmented gray matter, white matter, and cerebrospinal fluid (CSF) images were normalized into a study-specific coordinate space using diffeomorphic image registration (DARTEL) in SPM8 (Ashburner and Friston 2005; Ashburner 2007) to preserve cortical topology using a membrane bending energy or Laplacian model (Ashburner 2007). This procedure created invertible and smooth deformations of each participant’s native space gray matter image into a common coordinate space, producing a template that is representative of the brain size and shape of all the participants (Eckert et al. 2010). The normalized images were modulated to adjust gray matter, white matter, and CSF voxel intensities for the degree of volumetric displacement that occurred during normalization. A Gaussian smoothing kernel of 8 mm was used for the voxel-wise analyses to ensure that the data were normally distributed and to limit false positive results. Jacobian determinant images also were created from the normalization deformation fields to examine the extent to which the modulated gray matter volume results could be attributed to volumetric displacement of the voxels during normalization.
Statistics
Low and high frequency hearing metrics were obtained by performing a factor analysis with principal components extraction and Varimax rotation using the pure tone threshold data for each ear from 49 participants in the current imaging study and from 852 participants in the longitudinal study. This analysis was performed with SPSS to characterize hearing threshold variance components in the data set using an eigenvalue threshold >1. Regression scores for each participant indicated the relative contribution of each threshold to the low and high frequency hearing components across all 1,802 ears. The resulting low and high frequency regression scores were used in subsequent analyses with demographic and anatomical measures for the 49 participants. Multiple regression and correlation analyses were performed to characterize the extent to which gray matter volume was associated with the low and high frequency hearing threshold components. Region of interest (ROI) and voxel-based analyses included a covariate for total gray matter volume to examine locally specific associations with hearing thresholds that were independent of global variance in brain volume. The total gray matter volume estimate was obtained by summing gray matter volume across voxels.
ROI analyses
Primary auditory cortex (PAC) masks were used to obtain gray matter volume estimates within probabilistic cytoarchitectonic regions of the unsmoothed modulated gray matter images [medial to lateral: Te1.1, Te1.0, and Te1.2 (Morosan et al. 2005)]. These probabilistic maps [SPM Anatomy Toolbox; (Eickhoff et al. 2005)], which are in Montreal Neurological Institute (MNI) space, were normalized into study-specific DARTEL space by normalizing the ICBM a priori gray matter image to the DARTEL gray matter template and applying the normalization parameters to the masks. The masks were thresholded at 50 % probability (i.e., each cytoarchitectonic region was overlapping in at least 50 % of post-mortem brains after being normalized to MNI space) to limit the spatial overlap between masks in each hemisphere and obtain gray matter volume estimates with sufficient voxels to limit the possibility of outlier bias of the mean across all voxels in the masks. In addition, voxels were limited to those with >20 % gray matter probabilities in the DARTEL gray matter template to prevent white matter regions from influencing the mean gray matter volume estimates. The gray matter volume estimates were then standardized relative to total gray matter volume to control for potential age and gender effects by saving the unexplained variance in the measures after accounting for total gray matter volume. This standardized estimate of locally specific gray matter volume was then correlated with the low and high frequency hearing threshold components. Control analyses were performed similarly for primary visual cortex ROI [hOC1 from the Anatomy Toolbox (Eickhoff et al. 2005)] because of functional associations observed between auditory and visual cortex (Eckert et al. 2008). Non-significant associations with the hearing measures were observed (not shown).
Voxel-based analyses
Voxel-wise analyses were performed to provide local specificity within the PAC Te subfields and characterize the extent to which gray matter effects could be attributed to atrophy as represented by elevated CSF in auditory cortex. While the ROI analyses provide information about average volume of gray matter across a large region in unsmoothed data, the voxel-based analyses provide locally specific information about where effects would be most prominent within a brain region. We predicted that lower gray matter volume in auditory cortex would be associated with hearing loss, and for that reason, we restricted our analyses to auditory cortex regions within the left and right PAC Te (1.1, 1.0, and 1.2) cytoarchitectonic maps with the goal of limiting the number of comparisons and therefore used a corresponding family wise error correction (FWE corrected at p < 0.05; peak voxel-wise) for the number of comparisons within the combined left and right Te masks. The low and high frequency components, as well as a total gray matter volume covariate, were included in the regression model. Follow-up voxel-wise control analyses were performed to determine the extent to which (1) volumetric displacement and possible shape effects influenced the gray matter results (Jacobian determinant image correlations with the threshold components) and (2) gray matter effects could be attributed to atrophy based on CSF associations with low and high frequency hearing loss. Finally, exploratory whole brain gray matter, white matter, and CSF analyses were performed to determine the extent to which hearing loss was associated with individual differences in brain morphology outside the auditory system (FWE corrected at p < 0.05; peak voxel-wise).
Results
Low and high frequency hearing loss
Consistent with previous factor analysis results in older adults (Jerger and Chmiel 1997), two components were identified that accounted for 87 % of the variance in pure tone thresholds using an eigenvalue threshold >1. Component 1 accounted for 69 % of the variance and was most positively associated with variance in the higher frequency pure tone thresholds. Component 2 accounted for 18 % of the variance and was most positively associated with variance in the lower frequency pure tone thresholds (Fig. 1B). The component estimates for the left and right ears were strongly associated in the 49 subjects [Fig. 1C: component 1 left and right ear: r = 0.91; component 2 left and right ear: r = 0.89] and averaged across right ears to examine demographic and anatomical associations with the high and low frequency hearing threshold components.
The oldest adults [r = 0.44, p < 0.01] and men [t(47) = 2.70, p < 0.01] exhibited the most high frequency hearing loss. While age was not related to low frequency hearing loss [r = 0.12, ns], women were more likely to exhibit the most low frequency hearing loss [t(47) = −2.72, p < 0.01; Fig. 1D]. For comparison to previous studies using an average pure tone threshold variable composed of the above frequencies [e.g., Peelle et al. (2011)], the high frequency component [r = 0.90, p < 0.001], and to a lesser degree the low frequency component [r =0.32, p < 0.05], were related to the average pure tone threshold of the 49 participants (Fig. 1D). Table 1 presents threshold means, SDs, and the factor analysis component coefficients for each frequency so that the low and high frequency hearing threshold components can be created for data from new samples that are used to replicate this study.
TABLE 1.
Descriptive statistics and factor analysis coefficients that can be used to estimate low and high frequency hearing threshold components
| Frequency (Hz) | Mean | SD | Component score coefficient matrix | |
|---|---|---|---|---|
| Low frequency component | High frequency component | |||
| 250 | 17.37 | 12.06 | 0.405 | −0.139 |
| 500 | 17.70 | 13.29 | 0.420 | −0.137 |
| 1,000 | 20.05 | 15.48 | 0.316 | −0.039 |
| 2,000 | 29.44 | 19.97 | 0.096 | 0.137 |
| 3,000 | 38.63 | 22.81 | −0.065 | 0.253 |
| 4,000 | 47.14 | 23.98 | −0.112 | 0.280 |
| 6,000 | 55.31 | 24.19 | −0.101 | 0.273 |
| 8,000 | 58.57 | 23.69 | −0.081 | 0.248 |
The low and high frequency threshold variables used in this study were obtained from a principal components factor analysis (see Materials and methods). These variables were obtained using 1,704 left and right ears from a sample of older adults with a mean age = 69.92 years (SD = 7.24) that was 56 % female and the 92 left and right ears from the older adults in this imaging study. These variables can be obtained in a new sample of participants using the data from these 1796 ears by: (1) standardizing each pure tone threshold to the respective mean and SD in the table below; (2) multiplying the standardized pure tone score by the respective pure tone frequency component coefficient; and (3) summing the values from step 2 for each component across pure tone variables
Cytoarchitectonic region of interest analyses
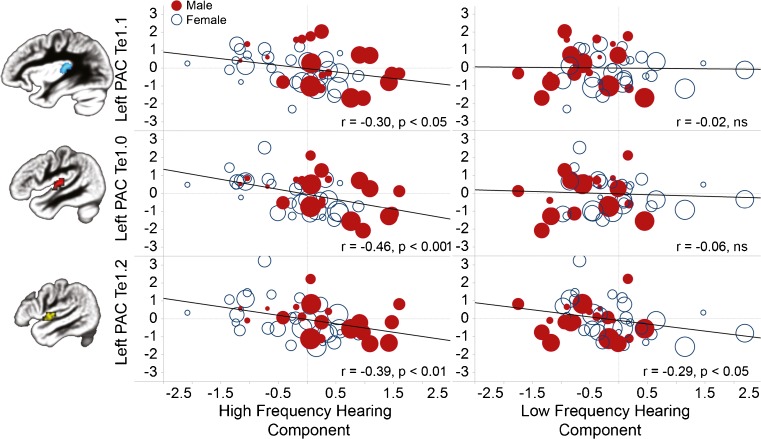
Older adults with more high frequency hearing loss exhibited lower gray matter volume across the PAC Te cytoarchitectonic ROIs of each hemisphere, with the relation most pronounced in the PAC Te1.0 ROI (Table 2, Fig. 2). In contrast, low frequency hearing was weakly correlated with gray matter volume only in the left PAC Te1.2 ROI. The associations between the PAC Te gray matter volume estimates and the low and high frequency hearing components were not affected by covarying out age and gender (Table 2), aside from the loss in degrees of freedom. The left Te1.0 association with high frequency hearing was present within males (r = −0.45, p < 0.05) and females (r = −0.55, p < 0.001). The left Te1.0 association was also present after controlling for age in a partial correlation (r = −0.35, p < 0.05). The left Te1.2 association with low frequency hearing, however, was largely driven by females (r = −0.46, p < 0.05) compared to males (r = −0.06, ns), which is consistent with the greater low frequency hearing loss observed in the females (Fig. 1D).
TABLE 2.
Pearson r values demonstrating the strength of associations between the PAC Te gray matter variables and the hearing variables
| Left PAC Te1.1 | Left PAC Te1.0 | Left PAC Te1.2 | Right PAC Te1.1 | Right PAC Te1.0 | Right PAC Te1.2 | |
|---|---|---|---|---|---|---|
| Low frequency threshold component | −0.02 | −0.06 | −0.29* | −0.05 | −0.11 | −0.18 |
| Low frequency threshold component (controlling for age and gender; df = 45) | 0.07 | −0.01 | −0.30* | −0.01 | −0.10 | −0.20 |
| High frequency threshold component | −0.29* | −0.45***a | −0.40** | −0.21 | −0.40** | −0.33* |
| High frequency threshold component (controlling for age and gender; df = 45) | −0.25† | −0.40** | −0.34* | −0.26† | −0.31* | −0.21 |
A similar pattern of results was observed when gray matter data were collected from masks with a 75 % probability of cytoarchitectural overlap in the PAC Te probability images. In partial support of controlling for total gray matter volume, total gray matter volume was related to low (r = −0.30, p < 0.05) and high frequency hearing (r = 0.27, p < 0.10), but was not related to low (partial r = −0.17, ns) and high frequency hearing (partial r = 0.13, ns) after controlling for gender
†p < 0.10; *p < 0.05; **p < 0.01; ***p < 0.001
aSurvives Bonferroni correction (p = 0.05/24 comparisons)
FIG. 2.
Variation in auditory cortex gray matter (Te1.0 unsmoothed gray matter volume average) was associated with the high frequency hearing threshold component (left), but not the low frequency hearing threshold component (right). While men (filled red circles) had more high frequency hearing loss than women (open blue circles), an association between auditory cortex gray matter and high frequency hearing threshold was present across the sample. Presented with each plot is an image of the associated primary auditory cortex (PAC) cytoarchitectonic mask (50 % probability). std gm vol volume relative to total gray matter volume. Increasing age, represented by symbol size, did not substantially impact the left Te1.0 findings.
Voxel-based analyses
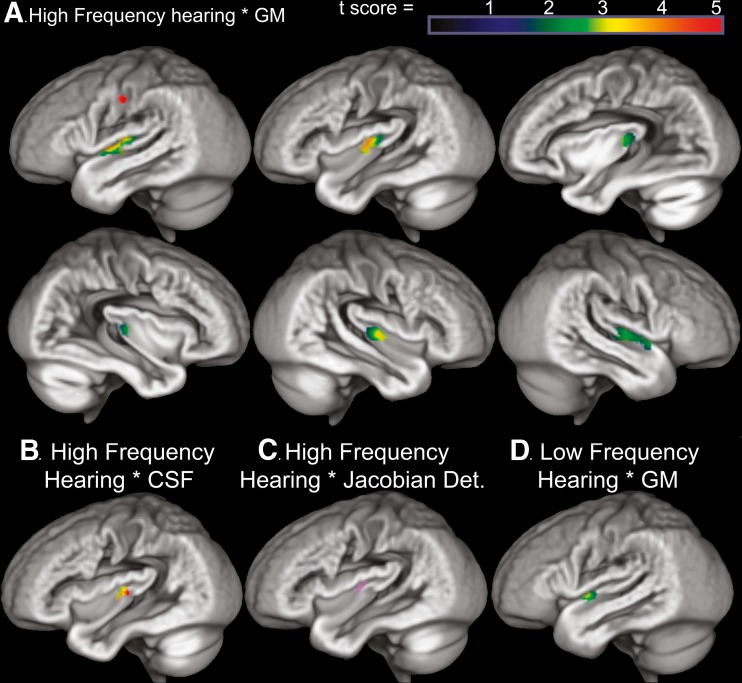
Consistent with the Te1.0 ROI results, variation in high frequency hearing was significantly correlated with left hemisphere auditory cortex gray matter volume within the Te masks (MNI peak voxel coordinate: −47, −16, 6; Z = 3.89, p < 0.05 FWE) and to a lesser extent lower right hemisphere auditory cortex volume. These effects were most prominent along the anterior half of Heschl’s gyrus and along the anterior superior temporal gyrus, corresponding to the Te1.0 and 1.2 regions (Fig. 3A). Importantly, lower gray matter volume appeared to reflect gray matter atrophy as the same auditory cortex voxels exhibited significantly increased CSF volume with increased high frequency hearing loss (Fig. 3B; MNI peak voxel coordinate: −42, −20, 10; Z = 3.22, p < 0.05 FWE).
FIG. 3.
A High frequency hearing loss was related to lower voxel-wise gray matter volume (smoothed 8 mm) in bilateral temporal lobe regions. After correcting for the number of comparisons within the combined left and right Te masks, significant [p < 0.05 FWE, t(45) = 3.44] high frequency hearing loss effects were predominantly in the left PAC Te1.0 region (50 % probability) on the anterior crown of Heschl’s gyrus (orange region in A) and extended laterally into the left PAC Te1.2 region (50 % probability) along the superior temporal gyrus. Each medial to lateral sagittal image corresponds spatially to the location of the Te1.1, 1.0, and 1.2 regions. The red voxels in somatosensory cortex exhibited a significant association between gray matter volume and high frequency hearing loss after correcting for whole brain exploratory comparisons thresholded [p < 0.05 FWE, t(45) = 5.25]. B Auditory cortex regions where gray matter volume was associated with high frequency hearing loss were also regions exhibiting elevated CSF [green: gray matter volume and high frequency hearing association (thresholded p < 0.05 FWE, t(45) = 3.44); red: CSF volume and high frequency hearing association (thresholded p < 0.05 FWE, t(45) = 3.11); yellow: overlap of the gray matter and CSF volume results]. These results suggest that the gray matter findings more likely reflect hearing loss related atrophy than individual differences in morphology. C Auditory cortex also required increased volumetric displacement (Jacobian determinant) in older adults with the most high frequency hearing loss (p < 0.01 uncorrected). The mean volumetric displacement was obtained from the space of the Jacobian determinant result and used as a covariate in the gray matter and hearing analysis, which demonstrated that voxels in the Te1.0 region remained significant after controlling for these age influenced displacement effects. D The lateral auditory cortex region where gray matter was weakly associated with low frequency hearing loss (p < 0.01 uncorrected). These effects are unique and overlap with the high frequency hearing threshold effects. Increased CSF was weakly related to low frequency hearing loss in this same region (p < 0.05 uncorrected). See color scale in A. for t-score in D.
To further confirm that the relation between high frequency hearing and auditory cortex was due to locally specific changes in gray matter volume, control analyses were performed to examine the extent to which the gray matter results could be attributed to gross volumetric displacement effects. An analysis of the Jacobian determinant images suggested that increased volumetric displacement occurred in the auditory cortex of people with high frequency hearing loss (p < 0.01, uncorrected; Fig. 3C). The mean volumetric displacement from this left auditory cortex region was collected and observed to exhibit a strong association with age [r = 0.43, p < .005], but the association between auditory cortex gray matter volume and high frequency hearing loss remained significant (p < 0.05 FWE) when the estimate of auditory cortex volumetric displacement was included as a covariate in voxel-wise analyses. Thus, the auditory cortex gray matter volume findings appear to reflect pathology associated with the influences of high frequency hearing loss. This result further suggests that associations between gray matter and hearing loss were not driven by indirect effects of aging that is reflected in global changes in gray matter volume.
Lateral superior temporal gyrus gray matter volume in the left hemisphere (Te1.2) was associated with low frequency hearing (p < 0.01, uncorrected; Fig. 3D), but these effects did not survive correction for multiple comparisons across the voxels in Te1.1, 1.0, and 1.2 space across males and females (p < 0.05 FWE). In addition, low and high frequency hearing threshold components were not significantly related to white matter or CSF voxels across the brain (p < 0.05 FWE). Finally, there was significantly reduced gray matter volume in left primary somatosensory cortex with increasing high frequency hearing loss (MNI peak voxel coordinate: −52, −16, 42; Z = 4.97, p < 0.05 FWE; Fig. 3A).
Discussion
The results of this study are consistent with the premise that high frequency hearing loss has cascading effects throughout the auditory system in older adults. High frequency hearing loss was associated with lower auditory cortex gray matter volume and increased CSF in the same region, suggesting that auditory cortex is atrophying with hearing loss. PAC Te1.0 was particularly affected by high frequency hearing loss. These effects were present even after controlling for age and gender effects, thereby providing additional support for direct effects of hearing loss on auditory cortex morphology.
The location of hearing loss effects in bilateral PAC Te1.0 appears to be consistent in location with the bilateral results observed by Peelle et al. (2011). The effect sizes in Peelle et al. (2011) were relatively weak compared to the current results, suggesting that their inclusion of low frequency thresholds in an average pure tone threshold across frequencies may have limited the strength of association between gray matter and hearing levels. Importantly, the high frequency hearing threshold component used in this study was strongly associated with the average pure tone threshold (Fig. 1D), suggesting that the Peelle results also were largely driven by high frequency hearing loss.
We were able to examine specific effects of low and high frequency hearing loss by leveraging 1,802 audiograms to establish stable high and low frequency hearing threshold metrics. Indeed, there appeared to be unique effects of low and high frequency hearing loss on PAC Te1.2 gray matter. The relatively greater high frequency hearing loss effects in Te1.0 could reflect statistical effects of having a broader range of high frequency thresholds. Our data reflect, however, the typical variance in pure tone thresholds that is found in older adults samples (Jerger and Chmiel 1997). For that reason, studies designed to examine the effects of low and high frequency hearing loss can be performed using the hearing threshold metrics in Table 1.
Differences in cytoarchitecture across the PAC Te regions that correspond to Brodmann’s area 41 (1909) could provide a basis for the more pronounced effects in Te1.0 compared to Te1.1 and Te1.2. Layer IV is thicker in the Te1.0 region compared to the Te1.1 and Te1.2 regions (Morosan et al. 2005), suggesting that Te1.0 receives the greatest thalamic input across these regions. High frequency hearing loss-mediated changes in thalamic innervation of auditory cortex could explain why the gray matter volume effects were most pronounced in Te1.0. The integration of postmortem imaging and histological data from older adults with well-characterized auditory function has the potential to test this hypothesis.
Hearing threshold associations with auditory cortex gray matter have been interpreted as reflecting causal effects of hearing loss on cortex (Peelle et al. 2011). Noise and lesion induced cochlear damage produces downstream changes in morphology throughout the rat and cat auditory systems (Powell and Erulkar 1962; Groschel et al. 2010; Coordes et al. 2012) and causes changes in tonotopic representation in macaque cortex (Rajan and Irvine 1998; Kakigi et al. 2000). This type of damage is similar to transsynaptic degeneration effects observed in the visual system (Minkowski 1913; Johnson and Cowey 2000; Zikou et al. 2012). The elevated CSF in auditory cortex among people with high frequency hearing loss (Fig. 3B) is supportive evidence of cortical atrophy as a result of hearing loss damage in lower levels of the auditory system. Longitudinal studies incorporating auditory brainstem responses, subcortical anatomical measures, and cortical anatomical measures could be used to evaluate the extent to which declining spiral ganglion integrity (Landry et al. 2011; Sly et al. 2012) occurs with declines throughout the aging auditory system.
An intriguing explanation for the unique low and high frequency hearing effects observed in this study is that they stem from different subtypes of presbyacusis. There are several causes for presbyacusis, which have unique patterns of associated hearing loss, including metabolic and sensory presbyacusis (Schmiedt 2010). Metabolic or strial presbyacusis involves endocochlear potential decline due to atrophy of the stria vascularis across apical and basal turns of the cochlea (Johnsson and Hawkins 1972; Pauler et al. 1988; Schulte and Schmiedt 1992; Gratton et al. 1996; Suzuki et al. 2006) and is associated with a relatively flat loss in the low frequencies coupled to a gradually sloping loss in the high frequencies. In sharp contrast, sensory presbyacusis involves the loss of outer hair cells (Schuknecht 1974) that are particularly affected in the basal turn of the cochlea (Makary et al. 2011) and is associated with relatively better low frequency hearing and more steeply sloping high frequency loss than metabolic presbyacusis (Schmiedt 2010). This pattern of anatomical and functional changes is consistent with evidence for low and high frequency hearing loss factors or components that were observed in this study and by Jerger and Chmiel (1997). Our results suggest that metabolic presbyacusis and increasing low frequency hearing loss is associated with changes in lateral superior temporal gyrus morphology (Te1.2) in females, while sensory presbyacusis and increasing high frequency hearing loss is associated with changes in medial auditory cortex (Te1.0) morphology in males and females. These predictions will be testable in the future when the subtypes of presbyacusis can be determined with audiometric data (Schmiedt 2010), which would help overcome the limited evidence for the causes or duration of hearing loss in our sample.
The location of the Te1.0 high frequency hearing loss result is in close proximity, but lateral to a Heschl’s gyrus region where gray matter volume was associated with word recognition in a difficult listening condition (Harris et al. 2009). The word recognition effects appeared to be due in part to normal variation in auditory cortex morphology because the association was present within groups of younger and older adults who had clinically normal hearing. These results are consistent with evidence that normal variation Heschl’s gyrus morphology is associated with language and music expertise (Golestani et al. 2002; Schneider et al. 2002; Gaser and Schlaug 2003; Schneider et al. 2005; Golestani et al. 2007; Wong et al. 2008). The Harris et al. (2009) effects could have been amplified by subclinical high frequency hearing loss, however. Central auditory system declines precede pure tone threshold evidence of peripheral auditory system decline (Makary et al. 2011), perhaps because of redundant afferent innervation of spiral ganglion neurons (Liberman et al. 1990). Future studies of older adults will be performed to evaluate the extent to which auditory cortex morphology relates to word recognition independently of hearing loss.
One question stemming from the current study is how to interpret the exploratory whole brain result demonstrating reduced primary somatosensory gray matter with increasing high frequency hearing loss. A search of coordinates reported within 8 mm of this effect yielded findings from experiments that included tongue movement (Hesselmann et al. 2004) and articulation (Pulvermuller et al. 2006), oral stimulation (Miyamoto et al. 2006), listening to non-verbal emotional stimuli (Warren et al. 2006), and listening passively to speech (Davis et al. 2007). If primary auditory and somatosensory cortices are part of a functional network (van de Ven et al. 2009), then perhaps anatomical changes in one region could impact another region in the network (Oh et al. 2011). In addition, extratemporal and temporal lobe findings may reflect comorbid factors such as vascular disease that have widespread aging effects on the peripheral auditory system (Gates et al. 1993; Thomopoulos et al. 1997; Torre et al. 2005; Liew et al. 2007; Helzner et al. 2011; although see Parving et al. 1993; Karamitsos et al. 1996) and the brain (Eckert 2011). Influences of vessel disease on hearing in females (Gates et al. 1993; Helzner et al. 2011) could also explain the association between gray matter in the left Te1.2 region and low frequency hearing loss. Regardless of direct or indirect hearing loss effects on cortical morphology, extratemporal and temporal changes that occur with age are likely to further limit the efficacy of hearing loss interventions focused solely on improving audibility.
In summary, the results of this study replicate findings from previous hearing loss studies and further demonstrate that: (1) high frequency hearing loss is related to robust changes in auditory cortex (PAC Te1.0) in males and females, thereby clarifying the hearing loss effects in previous studies and together with (2) CSF evidence of cortical atrophy with hearing loss, support the premise that high frequency hearing loss has downstream effects on auditory cortex. While this study was focused on older adults, the limited effects of age and the prevalence of high frequency noise exposure in our society raises important questions about the degree to which hearing loss effects on cortex are specific to presbyacusis or also occur for noise exposure (Kujawa and Liberman 2009). PAC Te1.0 therefore appears to be an important target for (1) understanding the causes of auditory cortex changes in people with hearing loss, (2) understanding the significance of hearing loss to the speech recognition difficulties that older adults experience, and 3) developing a biomarker to evaluate the effects of hearing aid and speech training intervention on neurobiology and behavioral outcome.
Acknowledgments
This work was supported by the National Institutes of Health/National Institute on Deafness and Other Communication Disorders (P50 DC 00422), the Deafness Research Foundation Centurion Clinical Research Award, South Carolina Clinical and Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, National Institutes of Health/National Center for Research Resources (UL1 RR029882), and the MUSC Center for Biomedical Imaging. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program (C06 RR14516) from the National Center for Research Resources, National Institutes of Health. We thank the study participants and members of the MUSC Hearing Research Program, including Lois Matthews.
References
- ANSI (2004) Specification for audiometrics. American National Standards Institute, New York
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bertoli S, Staehelin K, Zemp E, Schindler C, Bodmer D, Probst R. Survey on hearing aid use and satisfaction in Switzerland and their determinants. Int J Audiol. 2009;48:183–195. doi: 10.1080/14992020802572627. [DOI] [PubMed] [Google Scholar]
- Brant LJ, Fozard JL. Age changes in pure-tone hearing thresholds in a longitudinal study of normal human aging. J Acoust Soc Am. 1990;88:813–820. doi: 10.1121/1.399731. [DOI] [PubMed] [Google Scholar]
- Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in Ihren Prinzipien Dargestellt auf Grund des Zellaufbaus. Leipzig: Barth; 1909. [Google Scholar]
- Brun CC, Lepore N, Luders E, Chou YY, Madsen SK, Toga AW, Thompson PM. Sex differences in brain structure in auditory and cingulate regions. Neuroreport. 2009;20:930–935. doi: 10.1097/WNR.0b013e32832c5e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol. 2008;211:1781–1791. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coordes A, Groschel M, Ernst A, Basta D (2012) Apoptotic cascades in the central auditory pathway after noise exposure. J Neurotrauma 29:1249–1254 [DOI] [PubMed]
- Cruickshanks KJ, Tweed TS, Wiley TL, Klein BE, Klein R, Chappell R, Nondahl DM, Dalton DS. The 5-year incidence and progression of hearing loss: the epidemiology of hearing loss study. Arch Otolaryngol Head Neck Surg. 2003;129:1041–1046. doi: 10.1001/archotol.129.10.1041. [DOI] [PubMed] [Google Scholar]
- Davis MH, Coleman MR, Absalom AR, Rodd JM, Johnsrude IS, Matta BF, Owen AM, Menon DK. Dissociating speech perception and comprehension at reduced levels of awareness. Proc Natl Acad Sci USA. 2007;104:16032–16037. doi: 10.1073/pnas.0701309104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubno JR, Lee FS, Matthews LJ, Ahlstrom JB, Horwitz AR, Mills JH. Longitudinal changes in speech recognition in older persons. J Acoust Soc Am. 2008;123:462–475. doi: 10.1121/1.2817362. [DOI] [PubMed] [Google Scholar]
- Echt KV, Smith SL, Burridge AB, Spiro A., 3rd Longitudinal changes in hearing sensitivity among men: the Veterans Affairs Normative Aging Study. J Acoust Soc Am. 2010;128:1992–2002. doi: 10.1121/1.3466878. [DOI] [PubMed] [Google Scholar]
- Eckert MA. Slowing down: age-related neurobiological predictors of processing speed. Front Neurosci. 2011;5:25. doi: 10.3389/fnins.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Kamdar NV, Chang CE, Beckmann CF, Greicius MD, Menon V. A cross-modal system linking primary auditory and visual cortices: evidence from intrinsic fMRI connectivity analysis. Hum Brain Mapp. 2008;29:848–857. doi: 10.1002/hbm.20560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Keren NI, Roberts DR, Calhoun VD, Harris KC. Age-related changes in processing speed: unique contributions of cerebellar and prefrontal cortex. Front Hum Neurosci. 2010;4:10–21. doi: 10.3389/neuro.09.010.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Francis HW, Makary C, Halpin C, Crane BT, Merchant SN. Temporal bone findings in a case of Susac’s syndrome. Otol Neurotol. 2011;32:1198–1204. doi: 10.1097/MAO.0b013e31822e9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaser C, Schlaug G. Gray matter differences between musicians and nonmusicians. Ann N Y Acad Sci. 2003;999:514–517. doi: 10.1196/annals.1284.062. [DOI] [PubMed] [Google Scholar]
- Gates GA, Mills JH. Presbycusis. Lancet. 2005;366:1111–1120. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- Gates GA, Cobb JL, D’Agostino RB, Wolf PA. The relation of hearing in the elderly to the presence of cardiovascular disease and cardiovascular risk factors. Arch Otolaryngol Head Neck Surg. 1993;119:156–161. doi: 10.1001/archotol.1993.01880140038006. [DOI] [PubMed] [Google Scholar]
- Golestani N, Paus T, Zatorre RJ. Anatomical correlates of learning novel speech sounds. Neuron. 2002;35:997–1010. doi: 10.1016/S0896-6273(02)00862-0. [DOI] [PubMed] [Google Scholar]
- Golestani N, Molko N, Dehaene S, Lebihan D, Pallier C. Brain structure predicts the learning of foreign speech sounds. Cereb Cortex. 2007;17:575–582. doi: 10.1093/cercor/bhk001. [DOI] [PubMed] [Google Scholar]
- Gratton MA, Schmiedt RA, Schulte BA. Age-related decreases in endocochlear potential are associated with vascular abnormalities in the stria vascularis. Hear Res. 1996;102:181–190. doi: 10.1016/S0378-5955(96)90017-9. [DOI] [PubMed] [Google Scholar]
- Groschel M, Gotze R, Ernst A, Basta D. Differential impact of temporary and permanent noise-induced hearing loss on neuronal cell density in the mouse central auditory pathway. J Neurotrauma. 2010;27:1499–1507. doi: 10.1089/neu.2009.1246. [DOI] [PubMed] [Google Scholar]
- Harris KC, Dubno JR, Keren NI, Ahlstrom JB, Eckert MA. Speech recognition in younger and older adults: a dependency on low-level auditory cortex. J Neurosci. 2009;29:6078–6087. doi: 10.1523/JNEUROSCI.0412-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helzner EP, Patel AS, Pratt S, Sutton-Tyrrell K, Cauley JA, Talbott E, Kenyon E, Harris TB, Satterfield S, Ding J, Newman AB. Hearing sensitivity in older adults: associations with cardiovascular risk factors in the health, aging and body composition study. J Am Geriatr Soc. 2011;59:972–979. doi: 10.1111/j.1532-5415.2011.03444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselmann V, Sorger B, Lasek K, Guntinas-Lichius O, Krug B, Sturm V, Goebel R, Lackner K. Discriminating the cortical representation sites of tongue and up movement by functional MRI. Brain Topogr. 2004;16:159–167. doi: 10.1023/B:BRAT.0000019184.63249.e8. [DOI] [PubMed] [Google Scholar]
- Hinojosa R, Nelson EG. Cochlear nucleus neuron analysis in individuals with presbycusis. Laryngoscope. 2011;121:2641–2648. doi: 10.1002/lary.22383. [DOI] [PubMed] [Google Scholar]
- Humes LE, Dubno JR. Factors affecting speech understanding in older adults. New York: Springer; 2010. [Google Scholar]
- Husain FT, Medina RE, Davis CW, Szymko-Bennett Y, Simonyan K, Pajor NM, Horwitz B. Neuroanatomical changes due to hearing loss and chronic tinnitus: a combined VBM and DTI study. Brain Res. 2011;1369:74–88. doi: 10.1016/j.brainres.2010.10.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerger J, Chmiel R. Factor analytic structure of auditory impairment in elderly persons. J Am Acad Audiol. 1997;8:269–276. [PubMed] [Google Scholar]
- Johnson H, Cowey A. Transneuronal retrograde degeneration of retinal ganglion cells following restricted lesions of striate cortex in the monkey. Exp Brain Res. 2000;132:269–275. doi: 10.1007/s002210000384. [DOI] [PubMed] [Google Scholar]
- Johnsson LG, Hawkins JE., Jr Strial atrophy in clinical and experimental deafness. Laryngoscope. 1972;82:1105–1125. doi: 10.1288/00005537-197207000-00002. [DOI] [PubMed] [Google Scholar]
- Kakigi A, Hirakawa H, Harel N, Mount RJ, Harrison RV. Tonotopic mapping in auditory cortex of the adult chinchilla with amikacin-induced cochlear lesions. Audiology. 2000;39:153–160. doi: 10.3109/00206090009073068. [DOI] [PubMed] [Google Scholar]
- Kalayam B, Meyers BS, Kakuma T, Alexopoulos GS, Young RC, Solomon S, Shotland R, Nambudiri D, Goldsmith D. Age at onset of geriatric depression and sensorineural hearing deficits. Biol Psychiatry. 1995;38:649–658. doi: 10.1016/0006-3223(95)00175-1. [DOI] [PubMed] [Google Scholar]
- Karamitsos DG, Kounis NG, Zavras GM, Kitrou MP, Goudevenos JA, Papadaki PJ, Koutsojannis CM. Brainstem auditory evoked potentials in patients with ischemic heart disease. Laryngoscope. 1996;106:54–57. doi: 10.1097/00005537-199601000-00011. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry TG, Wise AK, Fallon JB, Shepherd RK. Spiral ganglion neuron survival and function in the deafened cochlea following chronic neurotrophic treatment. Hear Res. 2011;282:303–313. doi: 10.1016/j.heares.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang H, Jyothi V, Smythe NM, Dubno JR, Schulte BA, Schmiedt RA. Chronic reduction of endocochlear potential reduces auditory nerve activity: further confirmation of an animal model of metabolic presbyacusis. J Assoc Res Otolaryngol. 2010;11:419–434. doi: 10.1007/s10162-010-0214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FS, Matthews LJ, Dubno JR, Mills JH. Longitudinal study of pure-tone thresholds in older persons. Ear Hear. 2005;26:1–11. doi: 10.1097/00003446-200502000-00001. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Dodds LW, Pierce S. Afferent and efferent innervation of the cat cochlea: quantitative analysis with light and electron microscopy. J Comp Neurol. 1990;301:443–460. doi: 10.1002/cne.903010309. [DOI] [PubMed] [Google Scholar]
- Liew G, Wong TY, Mitchell P, Newall P, Smith W, Wang JJ. Retinal microvascular abnormalities and age-related hearing loss: the Blue Mountains hearing study. Ear Hear. 2007;28:394–401. doi: 10.1097/AUD.0b013e3180479388. [DOI] [PubMed] [Google Scholar]
- Lin FR, Thorpe R, Gordon-Salant S, Ferrucci L. Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011;66:582–590. doi: 10.1093/gerona/glr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makary CA, Shin J, Kujawa SG, Liberman MC, Merchant SN. Age-related primary cochlear neuronal degeneration in human temporal bones. J Assoc Res Otolaryngol. 2011;12:711–717. doi: 10.1007/s10162-011-0283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews LJ, Lee FS, Mills JH, Dubno JR. Extended high-frequency thresholds in older adults. J Speech Lang Hear Res. 1997;40:208–214. doi: 10.1044/jslhr.4001.208. [DOI] [PubMed] [Google Scholar]
- Minkowski M. Experimentelle untersuchungen uber die beziehungen der grosshirnrinde und der netzhaut zu den primaren optischen zentren besonders zum corpus geniculatum externum. Arb Hirnanat Inst Zurich. 1913;7:255. [Google Scholar]
- Miyamoto JJ, Honda M, Saito DN, Okada T, Ono T, Ohyama K, Sadato N. The representation of the human oral area in the somatosensory cortex: a functional MRI study. Cereb Cortex. 2006;16:669–675. doi: 10.1093/cercor/bhj012. [DOI] [PubMed] [Google Scholar]
- Morosan P, Schleicher A, Amunts K, Zilles K. Multimodal architectonic mapping of human superior temporal gyrus. Anat Embryol (Berl) 2005;210:401–406. doi: 10.1007/s00429-005-0029-1. [DOI] [PubMed] [Google Scholar]
- Oh H, Mormino EC, Madison C, Hayenga A, Smiljic A, Jagust WJ. Beta-amyloid affects frontal and posterior brain networks in normal aging. Neuroimage. 2011;54:1887–1895. doi: 10.1016/j.neuroimage.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Parving A, Hein HO, Suadicani P, Ostri B, Gyntelberg F. Epidemiology of hearing disorders. Some factors affecting hearing. The Copenhagen Male Study. Scand Audiol. 1993;22:101–107. doi: 10.3109/01050399309046025. [DOI] [PubMed] [Google Scholar]
- Pauler M, Schuknecht HF, White JA. Atrophy of the stria vascularis as a cause of sensorineural hearing loss. Laryngoscope. 1988;98:754–759. doi: 10.1288/00005537-198807000-00014. [DOI] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Grossman M, Wingfield A. Hearing loss in older adults affects neural systems supporting speech comprehension. J Neurosci. 2011;31:12638–12643. doi: 10.1523/JNEUROSCI.2559-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell TPS, Erulkar SD. Transneuronal cell degeneration in the auditory relay nuclei of the cat. J Anat. 1962;96:249–268. [PMC free article] [PubMed] [Google Scholar]
- Pulvermuller F, Huss M, Kherif F, Prado M, Martin F, Hauk O, Shtyrov Y. Motor cortex maps articulatory features of speech sounds. Proc Natl Acad Sci USA. 2006;103:7865–7870. doi: 10.1073/pnas.0509989103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher J, Morosan P, Schleicher A, Freund HJ, Zilles K. Human primary auditory cortex in women and men. Neuroreport. 2001;12:1561–1565. doi: 10.1097/00001756-200106130-00010. [DOI] [PubMed] [Google Scholar]
- Rajan R, Irvine DR. Neuronal responses across cortical field A1 in plasticity induced by peripheral auditory organ damage. Audiol Neurootol. 1998;3:123–144. doi: 10.1159/000013786. [DOI] [PubMed] [Google Scholar]
- Raynor LA, Pankow JS, Miller MB, Huang GH, Dalton D, Klein R, Klein BE, Cruickshanks KJ. Familial aggregation of age-related hearing loss in an epidemiological study of older adults. Am J Audiol. 2009;18:114–118. doi: 10.1044/1059-0889(2009/08-0035). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt RA. The physiology of cochlear presbyacusis. New York: Springer; 2010. [Google Scholar]
- Schneider P, Scherg M, Dosch HG, Specht HJ, Gutschalk A, Rupp A. Morphology of Heschl’s gyrus reflects enhanced activation in the auditory cortex of musicians. Nat Neurosci. 2002;5:688–694. doi: 10.1038/nn871. [DOI] [PubMed] [Google Scholar]
- Schneider P, Sluming V, Roberts N, Bleeck S, Rupp A. Structural, functional, and perceptual differences in Heschl’s gyrus and musical instrument preference. Ann N Y Acad Sci. 2005;1060:387–394. doi: 10.1196/annals.1360.033. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF. Presbyacusis. Cambridge: Harvard University Press; 1974. [Google Scholar]
- Schulte BA, Schmiedt RA. Lateral wall Na, K-ATPase and endocochlear potentials decline with age in quiet-reared gerbils. Hear Res. 1992;61:35–46. doi: 10.1016/0378-5955(92)90034-K. [DOI] [PubMed] [Google Scholar]
- Sly DJ, Hampson AJ, Minter RL, Heffer LF, Li J, Millard RE, Winata L, Niasari A, O’Leary SJ (2012) Brain-derived neurotrophic factor modulates auditory function in the hearing cochlea. J Assoc Res Otolaryngol 13:1–16 [DOI] [PMC free article] [PubMed]
- Suzuki T, Nomoto Y, Nakagawa T, Kuwahata N, Ogawa H, Suzuki Y, Ito J, Omori K. Age-dependent degeneration of the stria vascularis in human cochleae. Laryngoscope. 2006;116:1846–1850. doi: 10.1097/01.mlg.0000234940.33569.39. [DOI] [PubMed] [Google Scholar]
- Thomopoulos GN, Spicer SS, Gratton MA, Schulte BA. Age-related thickening of basement membrane in stria vascularis capillaries. Hear Res. 1997;111:31–41. doi: 10.1016/S0378-5955(97)00080-4. [DOI] [PubMed] [Google Scholar]
- Torre P, 3rd, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. The association between cardiovascular disease and cochlear function in older adults. J Speech Lang Hear Res. 2005;48:473–481. doi: 10.1044/1092-4388(2005/032). [DOI] [PubMed] [Google Scholar]
- Ven V, Esposito F, Christoffels IK. Neural network of speech monitoring overlaps with overt speech production and comprehension networks: a sequential spatial and temporal ICA study. Neuroimage. 2009;47:1982–1991. doi: 10.1016/j.neuroimage.2009.05.057. [DOI] [PubMed] [Google Scholar]
- Warren JE, Sauter DA, Eisner F, Wiland J, Dresner MA, Wise RJ, Rosen S, Scott SK. Positive emotions preferentially engage an auditory-motor “mirror” system. J Neurosci. 2006;26:13067–13075. doi: 10.1523/JNEUROSCI.3907-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley TL, Chappell R, Carmichael L, Nondahl DM, Cruickshanks KJ. Changes in hearing thresholds over 10 years in older adults. J Am Acad Audiol. 2008;19:281–292. doi: 10.3766/jaaa.19.4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PC, Warrier CM, Penhune VB, Roy AK, Sadehh A, Parrish TB, Zatorre RJ. Volume of left Heschl’s gyrus and linguistic pitch learning. Cereb Cortex. 2008;18:828–836. doi: 10.1093/cercor/bhm115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikou AK, Kitsos G, Tzarouchi LC, Astrakas L, Alexiou GA, Argyropoulou MI (2012) Voxel-based morphometry and diffusion tensor imaging of the optic pathway in primary open-angle glaucoma: a preliminary study. AJNR Am J Neuroradiol 33: 128–134 [DOI] [PMC free article] [PubMed]