Abstract
Otitis media with effusion (OME) is a pathologic condition of the middle ear that leads to a mild to moderate conductive hearing loss as a result of fluid in the middle ear. Recurring OME in children during the first few years of life has been shown to be associated with poor detection and recognition of sounds in noisy environments, hypothesized to result due to altered sound localization cues. To explore this hypothesis, we simulated a middle ear effusion by filling the middle ear space of chinchillas with different viscosities and volumes of silicone oil to simulate varying degrees of OME. While the effects of middle ear effusions on the interaural level difference (ILD) cue to location are known, little is known about whether and how middle ear effusions affect interaural time differences (ITDs). Cochlear microphonic amplitudes and phases were measured in response to sounds delivered from several locations in azimuth before and after filling the middle ear with fluid. Significant attenuations (20–40 dB) of sound were observed when the middle ear was filled with at least 1.0 ml of fluid with a viscosity of 3.5 Poise (P) or greater. As expected, ILDs were altered by ~30 dB. Additionally, ITDs were shifted by ~600 μs for low frequency stimuli (<4 kHz) due to a delay in the transmission of sound to the inner ear. The data show that in an experimental model of OME, ILDs and ITDs are shifted in the spatial direction of the ear without the experimental effusion.
Keywords: otitis media with effusion, conductive hearing loss, sound localization, cochlear microphonic
Introduction
Conductive hearing loss (CHL) results from a mechanical interference with the acoustic inputs to the inner ear. CHLs reduce (i.e., attenuate), but do not eliminate, sound-evoked activity in the auditory system (Wever and Lawrence 1954). Otitis media with effusion (OME) is a highly prevalent pathologic condition leading to a CHL resulting from a middle ear effusion (MEE; i.e., fluid in the middle ear). OME may present with variable effusion viscosity (serous vs. mucoid) and volume, which can produce conductive losses on the order of 30 dB or greater (Kokko 1974; Bluestone and Klein 1995). CHLs due to recurring OME in early childhood are of particular interest because they are associated with measurable speech and language deficits later in life (Bluestone et al. 1973; Bennett et al. 2001; Tollin 2010; Whitton and Polley 2011).
Chronic CHL during development has been shown to affect auditory system structure and function (Yoshinaga-Itano et al. 1998; Tucci et al. 2002). Early life exposure to CHL, particularly in cases of unilateral or asymmetrical losses, leads to impairments in psychophysical binaural hearing tasks (Hall and Derlacki 1986; Pillsbury et al. 1991; Moore et al. 1991, 2003; Hogan and Moore 2003; Hall et al. 1998) that often persist even after resolution of the CHL and after normal hearing sensitivity in both ears has been restored (Hall et al. 1995; Hogan and Moore 2003). The impaired binaural hearing abilities that result from CHL typically recover, but this can take months or even years (Hall et al. 1995; Hogan and Moore 2003). During recovery, a child may present as audiologically normal, yet speech perception in noisy, reverberant environments may continue to be compromised.
The binaural hearing impairments due to CHL have been hypothesized to arise from altered acoustical cues to sound source location, including interaural time (ITD) and level (ILD) differences (Hartley and Moore 2003; Knudsen et al. 1984; Slattery and Middlebrooks 1994; Lupo et al. 2011). Previous studies have tended to focus predominantly on the long-term effects of CHL in terms of the amount of attenuation produced by the CHL (the ILD cue), while fewer studies have examined whether CHL alters the temporal properties of sound input to the inner ear by specifically focusing on the ITD cue to sound location (Hartley and Moore 2003; Lupo et al. 2011). As low-frequency ITD cues are of particular importance for human binaural hearing capabilities (Wightman and Kistler 1992), it is of upmost interest to determine whether and how CHL alters the ITD cue to location.
The physiologic effects of CHL on binaural hearing due to MEEs are difficult to systematically study in children. Thus, animal models are necessary to understand how the MEEs that produce CHLs affect the binaural acoustic cues to sound localization and persistently alter the anatomy and physiology of the binaural auditory system (Tollin 2010). Toward this goal, the present study examines how the viscosity and volume of fluid in the middle ear jointly affect the binaural cues to location measured for sound source locations in azimuth. The chinchilla (Chinchilla lanigera) was used here due to its “human-like” auditory system. The chinchilla has a comparable audiogram to that of humans (hearing range of ~50 Hz to 33 kHz) in addition to similar physiological and anatomical characteristics (tympanic membrane size, cochlea size, etc.) as compared to the human auditory system (Miller 1970; Vrettakos et al. 1988; Heffner and Heffner 1991; Niemiec et al. 1992).
Methods
Animal Preparation
All experiments and methods were approved by the University of Colorado School of Medicine Institutional Animal Care and Use Committee. Thirteen ears were studied in seven adult male chinchillas (C. lanigera) weighing 0.5 to 0.7 kg (mean 0.58; standard deviation [SD], 0.07 kg) and were obtained from a licensed vendor (Moulton Chinchilla Ranch, Rochester, MN, USA). Chinchillas were anesthetized with an initial intramuscular (IM) dose of ketamine hydrochloride (KetaVed; 30 mg/kg IM) and xylazine hydrochloride (TranquiVed; 5 mg/kg IM). Anesthesia was maintained with supplemental doses of ketamine (15 mg/kg, IM) and xylazine (2.5 mg/kg, IM) given every 1.5–2.0 h and/or based on reflex response to a paw pinch and changes in physiological parameters (heart rate, respiration rate, etc.). Each chinchilla received a tracheotomy tube to help facilitate respiration. Heart rate, blood oxygen levels (SpO2), respiratory rate, and end-tidal CO2 were measured continuously via a capnograph (Surgivet V90040, Waukesha, WI, USA) and displayed graphically in real time on a PC monitor. Body temperature was continuously monitored with a rectal probe and carefully maintained with a heating pad at 37 °C (model TC 100; CWE, Inc., Ardmore, PA, USA).
The methods used here were adapted from those described in prior reports from our laboratory (Lupo et al. 2011; Jones et al. 2011). Briefly, the pinnae were removed bilaterally, exposing the bony portion of the external auditory canal. The skin and fascia were removed from the bulla bilaterally, and a small hole (~2–3 mm diameter) was bored through the bony wall of each posterior bulla to gain access to the middle ear space and expose the round window of the cochlea. Teflon-insulated, silver electrodes (bare wire diameter, 0.005 in.) were stripped of insulation at both ends. Under microscopic visualization, the electrode was advanced through the hole, placed on the round window, and then fixed in place with dental acrylic. The cochlear microphonic (CM) was differentially amplified (~10,000, DAM ISO-50, WPI, Sarasota, FL, USA), filtered (10–20,000 Hz), and verified by an oscilloscope. This process was done for both ears. For CM measurements, the differential electrode was placed on the posterior musculature of the neck and the ground electrode was placed on one paw.
Experimental Model of Middle Ear Effusion
In order to model a MEE, a ~6–7-mm diameter hole was carefully drilled into the lateral aspect of the inferior bulla as illustrated in Figure 1. A polythene tube (inner diameter, 0.125 in.) was inserted into the inferior bullae holes bilaterally and sealed firmly in place with dental acrylic and superglue. A small vent hole was drilled into the superior portion of the bulla bilaterally to equalize middle ear pressure. A 3-ml syringe containing silicone oil was fitted with a 14-gauge needle that was advanced through the inferior polythene filling tube until the opening of the syringe was flush with the inferior opening of the bulla. Silicone oil (polydimethylsiloxane fluid, Sigma-Aldrich Co. LTD) of different viscosities was used to implement the experimental model of a MEE (Fig. 1). The viscosities of the oil ranged from low (0.5 and 3.5 P) and medium (100 P) to high, or “glue-like” (600 P) to simulate the viscosities encountered clinically in OME. Silicone oil has a low electrical conductance (Miyahara et al. 2006) and, therefore, is not expected to interfere with CM measurements. The filling tubes, syringes, and needles were put into place before the baseline CM measurements were taken.
FIG. 1.
Experimental setup for instilling silicone oil into the middle ear space. A coated silver wire electrode is placed on the round window (RW) to record the cochlear microphonic (CM) and compound action potential (CAP) waveforms. Different viscosities of silicone oil are injected into the middle ear space through an inferior filling tube located in the posterior portion of the bulla. A vent hole is drilled in the superior portion of the bulla to let air escape as the fluid is instilled from the bottom of the bulla. The indicated fluid volumes are based on anatomical examination and are thus approximately anatomically correct.
Baseline CM measurements were taken from each animal before any silicone oil was injected into the middle ear space. After the baseline measurements, 0.5 ml of silicone oil was very slowly injected into the middle ear space. The polythene tubing (see above) of known volume was pre-filled with oil so that the desired volumes of oil (i.e., 0.5 ml) could be accurately delivered into the bullae. Moreover, as the tip of the injection needle was flush with the end of the filling tube, errors in the estimation of the injected fluid volume were expected to be negligible. Fluid injection into the bullae was jointly facilitated by very slow injection rates and by allowing air to vent through the superior vent hole in the bulla. Additional silicone oil was injected into the bulla in 0.5 ml increments, with evoked potential measurements taken after each addition of fluid. Experiments were conducted with 0.5, 1.0, and 1.5 ml volumes of oil and a last measurement taken when the bulla was completely filled with silicone oil as evidenced by oil just beginning to come out of the superior vent hole. Typically, both ears of each animal were studied simultaneously with a different viscosity of fluid being injected into the bullae of each ear. This method of MEE simulation was adapted, in part, from the methods used by Goodhill and Holcomb (1958) and more recently by Hartley and Moore (2003). As a confirmation that the bullae were completely filled with fluid via this method, in some animals, the silicone oil was mixed with food coloring. At the end of these experiments, the specimens were frozen and the bullae sectioned. Results confirmed complete filling of the chambers of the bullae.
Acoustic Stimulation
Methods for free-field acoustic stimulation were adapted from Tollin and Koka (2009) and Lupo et al. (2011). Experiments were performed in a double-walled, sound-attenuated room (Industrial Acoustics Company, Bronx, NY, USA). All walls and equipment were lined with 4 and 2 in. acoustic foam (Sonex Classic, Minneapolis, MN, USA), respectively. Stimuli were presented from loudspeakers (Morel MDT-20, Elmont, NY, USA) attached to a custom-built horizontally oriented (i.e., poles at the sides) semicircular boom (1 m radius). Twnety-five speakers spaced at 7.5 ° apart were attached to the boom, starting from −90 ° (left) to +90 ° (right). In these experiments, the boom was always positioned at 0 ° elevation so that all speakers were in the horizontal plane (azimuth). The interaural axis of each subject was aligned in the center of the sphere using three lasers attached to the two poles as well as the 0 ° azimuth position of the boom. The spatial alignment of the animal was also confirmed acoustically by an ~0-μs ITD when measured at the center position using broadband noise stimuli.
After alignment of the animal, 10-ms duration (2.5 ms rise/fall phase and a 5-ms plateau) sinusoidal stimuli were presented repeatedly (~50 times) with a 40-ms inter-stimulus period to measure the baseline recordings. Stimulus frequencies were 0.25, 0.5, 1, 2, 4, 8, 10, and 12 kHz. Stimuli were generated in MATLAB® (v7.1, The Mathworks Inc., Natick, MA, USA) and presented using Tucker Davis Technologies (TDT, Alachua, FL, USA) System III hardware (TDT, RP 2.1) with a sampling rate of 97,656.25 Hz at full 24-bit resolution. All stimulus presentation, acquisition, and processing was done using custom software in MATLAB®.
The acquired acoustic and CM signals were averaged in the time domain based on the number of presented repetitions. Both the time and frequency domain representations of these averaged signals were displayed on a PC monitor in real time for inspection. Initially, stimuli were presented from the midline where for each of the eight different stimulus frequencies the attenuation of the stimuli was varied in 10-dB steps from 100 dB (nearly completely attenuated) to 0 dB with attenuation levels set by a TDT PA5 digital attenuator. Because the results of these experiments depend only upon the relative differences in the CM amplitudes and phases with different volumes and viscosities of fluid, there was no need to convert the attenuation levels of the stimuli to decibel sound pressure level (SPL). Additionally, these baseline recordings were also used to measure the CM thresholds. Based on the initial measurements at the midline, an attenuation level for each stimulus frequency was chosen to ensure that the CM responses would remain in the linear portion of CM vs. stimulus amplitude curve. The average (±SD) linear CM amplitude vs. stimulus level dynamic range across frequencies in six ears was 56 ± 3 dB with a range of 53–60 dB. After the baseline recordings, silicone oil was instilled into the middle ear space as described above. After each fluid instillation, CM vs. stimulus level responses were again measured at the midline to obtain CM thresholds as well as the attenuation caused by the silicone oil. The CM responses were then re-measured as a function of sound source location in azimuth.
Data Analysis
The baseline CM thresholds were determined from baseline CM vs. dB attenuation curves. The CM thresholds were computed as described in Jones et al. (2011). Briefly, CM threshold was defined as the attenuation level at which CM amplitude exceeded 2 standard deviations above the mean noise level measured for attenuation levels well below thresholds. The baseline (i.e., normal unfilled bullae with no injected fluid) ITDs were measured by first assuming acoustic symmetry of the head, and then computing the ITD by comparing the CMs evoked in each ear individually but at complementary spatial locations in the frontal hemisphere (e.g., comparing CM waveforms evoked at +45 ° and at −45 °). This method of computing ITD, as well as the assumption of head symmetry, was necessary so that each ear could serve as its own baseline as fluid was instilled into the middle ear space. As described in Lupo et al. (2011), ITDs were computed by comparing the peaks of the cross-correlation of each individual ear’s CM after the signals were filtered (fifth order, low-pass Butterworth filters with a cutoff frequency placed ~1.5 octaves above the stimulus frequency); the low-pass filtering reduces the potentially confounding influence of harmonic distortion that can occur in above-threshold CM measurements (Wever and Lawrence 1954). Note that Lupo et al. (2011) also measured the acoustic ITDs in chinchilla via probe tube microphones placed near the tympanic membranes (TM) of each ear and demonstrated that the ITDs estimated from the CMs at each ear were virtually identical to those measured acoustically at the TM. The CM-based ITDs were also verified at each location by using the method of Roth et al. (1980) where the timings of the zero crossings of the raw CM waveforms were compared between the responses at the two ears.
In Lupo et al. (2011), ILDs were measured by first comparing the measured CM amplitude to calibrated SPL measured with probe tube microphones placed at the tympanic membrane and then computing the effective CM-derived SPL difference between the two ears. For the experiments in the current study, measurement of SPL via a probe tube microphone at the level of the tympanic membrane cannot determine the CHL that results due to the presence of fluid in the middle ear space. To estimate the effects of a MEE-induced CHL on the ILDs, the thresholds of the CM responses were compared before and after middle ear fluid instillation. Here, as with the computation of ITD above, each ear served as its own baseline for the computation of the resulting ILD due to a MEE.
Once all the baseline measurements were obtained, the attenuation of inputs to the inner ear was determined by comparing the CM thresholds measured in the baseline condition to the CM thresholds after silicone oil instillation. The temporal delay (which would be expected to affect the ITDs) due to the silicone oil was measured at each ear individually by comparing the baseline CM waveforms to those CM waveforms that resulted after silicone oil administration in the bullae of the same ear. The temporal delays due to silicone oil in the middle ear were determined by cross-correlating the baseline CM waveforms (i.e., no fluid) with the CM waveforms recorded after injection of the silicone oil into the middle ear. Temporal delays were computed at each ear individually (i.e., the baseline condition for the left ear is compared to the experimental condition for the same ear). To estimate the effect of these MEE-induced delays on the ITD cue to sound location, the computed delays were simply added to the baseline ITDs to arrive at the ITD shifts that would be expected to be caused by a MEE simulated by silicone oil in the middle ear.
The two-way ANOVA test was performed to determine the effect of silicone oil volume and viscosity on the inputs to the inner ear as assessed via the CMs. Post hoc pair-wise comparisons were conducted using the Scheffé HSD test. Statistical analyses were conducted using SYSTAT (v9.0, Systat Software, Inc, Chicago, IL, USA). Statistical significance was defined as p < 0.05.
Results
CM amplitudes and thresholds shift in the presence of middle ear fluid
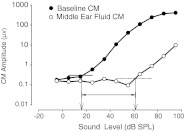
To test the hypothesis that the magnitude of the stimulus attenuation resulting from the MEE-induced CHL was influenced by the volume and viscosity of fluid in the middle ear space, the amplitudes and thresholds of CM recordings with silicone oil in the middle ear were compared to baseline CM measurements where no middle ear fluid was present. Figure 2 demonstrates the effects of silicone oil in the middle ear on CM amplitudes and thresholds for sounds presented at 0 ° azimuth. As expected, CM amplitudes were reduced and thresholds increased in the presence of silicone oil in the middle ear for viscosities of 3.5 P and above (as reference, the viscosity of water measures to 1.0 P, while 3.5 P is the approximate viscosity of fingernail polish). In both the baseline measurements and simulated effusion measurements, the CM amplitudes for tonal stimuli presented at stimulus levels above threshold increased linearly with an increase in stimulus level. CM thresholds shifted from ~20 dB (re: arbitrary reference) in baseline conditions to ~60 dB in experimental conditions (Fig. 2) indicating a MEE-induced CHL of 40 dB for this frequency. The average attenuation across animals demonstrates that fluid in the middle ear attenuates vibratory inputs to the inner ear in the range of ~40 dB, which is consistent with the mild to moderate conductive hearing loss seen clinically in OME (Bluestone and Klein 1995). These amplitude and threshold shifts were seen in all animals at all stimulus frequencies tested.
FIG. 2.
Example of CM amplitude and threshold measurements for baseline condition (closed circles, no fluid) and middle ear fluid condition (1.5 ml of 100 P silicone oil, open circles) in one animal with a 4-kHz tone stimulus. The CM amplitude is substantially reduced in the presence of middle ear fluid. Thresholds of CM responses are shifted towards higher stimulus levels by as much as ~40 dB SPL. Comparable amplitude and thresholds shifts were seen at all frequencies tested (250 Hz to 12 kHz).
The determinants of the attenuation of sound caused by an experimentally induced middle ear effusion
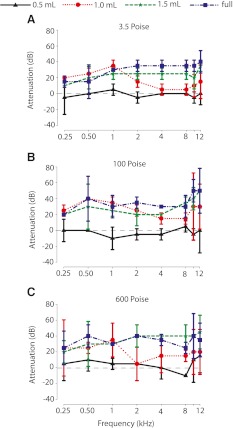
Figure 3 shows the attenuation in CM signals due to different volumes and viscosities of silicone oil across different frequencies measured at the midline (0 ° azimuth). Overall, across all ears studied (n = 13), the attenuation of sound increased with an increase in the volume of fluid present in the middle ear space. To test for the effect of fluid viscosity and volume on sound attenuation, data were pooled across animals (Fig. 3). First, to determine the fluid volume at which significant attenuation first occurred relative to baseline, regardless of viscosity, we performed a protected two-way ANOVA with fluid volume and frequency as independent variables. The results indicated a significant effect of oil volume (F4, 7 = 67.2, p < 0.001), but not frequency (F4, 7 = 1.6, p = 0.15); the interaction of oil volume and stimulus frequency was also not significant (F4, 7 = 0.8, p = 0.74). The Scheffe post hoc test revealed that there was no significant attenuation of the CM between baseline measurements (i.e., no fluid in the middle ear corresponding to 0 dB in Fig. 3) and 0.5 ml of fluid (p = 0.99); however, volumes of 1.0 ml and greater produced significant increased levels of attenuation relative to baseline and 0.5 ml (p < 0.001). Next, to determine the minimal viscosity at which significant attenuation first occurred regardless of fluid volume, a protected two-way ANOVA with viscosity (baseline condition removed) and frequency as independent variables was performed. The results indicated a significant main effect of oil viscosity (F3, 7 = 10.8, p < 0.001), but not frequency (F3, 7 = 0.82, p = 0.57); the interaction of viscosity and frequency was not significant (F3, 7 = 0.33, p = 0.99). The Scheffe post hoc test revealed that silicone oil of 0.5 P produced less attenuation than oil with higher viscosities (p = 0.9), but there were no pair-wise differences in attenuation produced by fluid viscosities of 3.5, 100, and 600 P. Finally, to examine the potential interaction effects of middle ear fluid volume and viscosity on CM attenuation, a two-way ANOVA was performed on the data after removing the baseline condition, as viscosity is undefined in that situation. The results indicate significant main effects for both fluid level (F3, 3 = 102.8, p < 0.001) and viscosity (F3, 3 = 43.7, p < 0.001); the interaction was also significant (F3, 3 = 2.1, p < 0.04). Post hoc examination confirmed that the main effects of viscosity and volume were due, as expected from the analyses above, to the 0.5 ml and 0.5 P conditions, respectively. For all viscosities greater than 0.5 P, there were no significant differences in attenuation (p < 0.05). However, there continued to be graded increases in additional attenuation as fluid volume increased above 0.5 ml. These latter two effects led to the significant interaction. Thus, the MEE-induced CHL was due almost entirely from the volume of fluid in the middle ear, with larger volumes producing larger CHLs.
FIG. 3.
Across-animal mean (n = 13 ears) attenuation of sounds presented at 0 ° azimuth using four different viscosities of silicone oil at four different volumes (0.5, 1.0, 1.5 ml, and bulla full). Positive attenuation values indicate a larger CHL. Attenuation remained nearly consistent across viscosities above 3.5 P (A, 3.5 P; B, 100 P; C, 600 P). There was an effect of fluid volume on sound attenuation for all three viscosities tested, with concomitant significant increases in CM attenuation for all fluid volumes >0.5 ml. Symbols and error bars indicate the mean attenuation and ±1 SD, respectively.
Baseline interaural time and level difference measurements via the cochlear microphonic waveforms
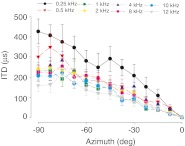
Figures 4 and 5 summarize the baseline control measurements for the interaural level and time differences, respectively. Baseline ILDs were taken from CM measurements in ears without any silicone oil in the middle ear space. To calculate the baseline ILDs, CM amplitudes were converted to corresponding attenuation levels (re: arbitrary decibels). Figure 4 shows the baseline ILDs calculated across azimuth (−90 ° to +90 °) for frequencies 0.25–12 kHz (n = 13 ears). Sounds presented from the midline resulted in ILDs of ~0 dB in the baseline condition, which was expected based on the method by which ILD was computed from the CMs. Furthermore, ILDs were generally larger for higher stimulus frequencies than for lower stimulus frequencies. For example, at the poles (±90 °), ILDs approached ~12 dB for 12 kHz sounds, but remained close to 0 dB for 0.25–1 kHz sounds. The ILDs measured here based on electrophysiologically measured CMs in the chinchilla are similar to the ILDs measured previously via microphones placed near the tympanic membrane (Koka et al. 2011; Lupo et al. 2011).
FIG. 4.
Baseline interaural level differences for eight different frequencies (0.25–12 kHz) as a function of sound source azimuth (−90 ° = left side) in the baseline condition without fluid in the middle ear space as measured by the cochlear microphonic. Due to the method by which ILD was computed, the ILDs were 0 dB at the midline and symmetrical across azimuth; therefore, data are only shown for one half of the azimuthal plane. Symbols and error bars indicate the across-animal mean ILD (n = 13 ears) and ±1 SD, respectively.
FIG. 5.
Baseline interaural time differences measured from cochlear microphonic measurements in the baseline condition without any fluid in the middle ear space for eight different frequencies (0.25–12 kHz). Due to the method by which ITDs were computed, the ITDs were 0 μs at the midline and were symmetrical across azimuth; therefore, data are only shown for half of the azimuthal plane. Symbols and error bars indicate the across-animal mean ITDs (n = 13 ears) and ±1 SD, respectively.
Figure 5 illustrates that CM-derived ITDs were larger for lower frequency (<1 kHz) than for higher frequency sounds. ITDs approached ~430 μs for 0.25-kHz tones presented from the pole positions (±90 °; Fig. 5). In contrast, a 12-kHz stimulus presented from the pole positions produced maximum ITDs of ~160 μs, nearly half of the ITD magnitude produced at low frequencies. ITD magnitudes, which were highly dependent on source azimuth, were largest at the poles (±90 °) and larger than the mathematically predicted ITDs at all frequencies. Predicted ITDs were calculated using two different, but commonly used, spherical head model equations based on an adult chinchilla head diameter of ~36 mm (Jones et al. 2011): Kuhn’s model (1977) of low-frequency ITDs predicts maximum ITDs of ~160 μs and the classic Woodworth (1938) model predicts maximum ITDs of ~136 μs.
Effects of silicone oil in the middle ear space on interaural level differences
ILDs for the silicone oil conditions were calculated at each sound source azimuth by comparing the stimulus level required to achieve a constant CM amplitude with that necessary to achieve the same CM amplitudes in the same ear (e.g., Fig. 2). Since we saw no significant difference in attenuation with viscosities >3.5 P and volumes >1.0 ml, we have used 1.5 ml of 100 P silicone oil (a viscosity similar to chocolate syrup at room temperature) to show graphically the representative effect of a simulated middle ear effusion on the spatial location dependence of the ILD cue to sound location in three animals.
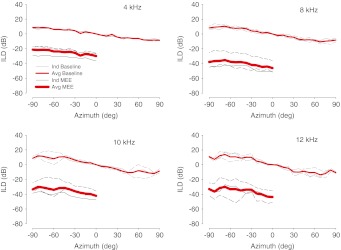
Figure 6 shows the representative large ILD shifts due to the silicone oil across frequencies 4–12 kHz. The MEE-induced ILD shifts are shown only for higher frequencies, although a similar trend in shifts was observed at low frequencies (<4 kHz). Figure 6 shows that ILD shifts ranged from 30 dB (±5.6 dB across three ears) at 4 kHz to 44 dB (±8 dB) at 12 kHz. The MEE-induced shifts in ILDs are shown only for source locations in the hemisphere ipsilateral to the ear with the MEE as the signal-to-noise ratios were prohibitively small for source locations contralateral to the MEE due to the additional attenuation produced by the head. As expected, due to the resulting MEE-induced CHL, these ILD shifts were correlated with the threshold shifts (~30–40 dB) that were measured using CM signals. The ILD shifts as a result of middle ear fluid were frequency dependent, with larger shifts occurring at higher than for lower frequencies. As might be expected, and consistent with a linear system, the ILD shifts due to the CHL for a given stimulus frequency and silicone oil volume were consistent across different sound source azimuths.
FIG. 6.
Across-animal mean and individual animal baseline ILDs (thin red = mean across animals, thin gray = individual animals) and experimental (thick red = mean across animals, thin black = individual animals) 1.5 ml of 100 P silicone oil) as a function of sound source azimuth (n = 3 ears). The simulated middle ear effusion due to silicone oil causes an ILD shift of ~30 dB across frequencies with a larger shift evident at higher frequencies both for individual animals and across animals.
Effects of silicone oil in the middle ear space on interaural time differences
The ITDs in low-frequency stimuli (500 Hz to 4 kHz) were calculated the same way as in the baseline condition. Figure 7 shows an example CM waveform in response to a 500-Hz tone pip presented at 0 ° azimuth both in the baseline condition without fluid in the middle ear (left panel) and after 1.5 ml of 100 P fluid was instilled into the middle ear (right panel). In the baseline condition, the CM signal overlapped perfectly, as a result of our assumption of head symmetry, thus producing an ITD of exactly 0 μs. After filling the middle ear, the evoked CM waveform was delayed by ~500 μs relative to the unfilled condition. In each ear, for each increment of middle ear fluid volume and viscosity, we computed the amount of delay induced by the fluid relative to the baseline condition without fluid.
FIG. 7.
Example cochlear microphonic (CM) waveform in response to a 500-Hz tone pip stimulus presented at 0 ° azimuth. Left panel CM waveform in the baseline condition (without fluid). In the baseline condition, the waveforms overlap due to the assumption of head symmetry, resulting in a 0-μs ITD. Right panel CM waveform after the middle ear is filled with 1.5 ml of 100 P silicone oil. Addition of the silicone oil results in a ~500-μs delay in the CM waveform.
We found that the volume of the middle ear fluid, as opposed to the fluid’s viscosity, had a significant effect on the ITD cue to sound location (Fig. 8). The two-way ANOVA with middle ear fluid volume and viscosity as factors (the baseline condition removed because viscosity is undefined) revealed a main effect on delay of fluid volume (F3, 2 = 44.22, p < 0.001) but not viscosity (F3, 2 = 1.7, p = 0.18); the interaction of volume and viscosity was not significant (F3, 2 = 0.25, p = 0.96). With the knowledge that fluid viscosity was not a factor in producing MEE-induced delays, a protected two-way ANOVA with fluid volume and stimulus frequency as factors was conducted; baseline was included in this analysis. Results indicated, as expected, a significant main effect of fluid volume (F4, 3 = 78.6, p < 0.001) and frequency (F4, 3 = 21.5, p < 0.001); the interaction between volume and frequency was also significant (F4, 3 = 3.3, p < 0.001). The Scheffe post hoc examination revealed that there was a significant increase in delay for all fluid volumes above baseline (p < 0.05) and for all volumes greater than 0.5 ml (p < 0.05) but no significant pair-wise differences for the volumes greater than 0.5 ml. Moreover, there was a general increase in delay for lower stimulus frequencies. Thus, the results reveal that middle ear fluid volume, but not viscosity, was the main determinant of the MEE-induced delays of sound transmission to the inner ear.
FIG. 8.
Simulated middle ear effusion induced delays (in microseconds) in transmission of sound to the inner ear across four frequencies (0.25–2 kHz) for different volumes (0.5, 1.0, 1.5 ml, and bulla full) and viscosities of silicone oil (n = 13 ears); negative value indicate delays in the CM waveforms re: baseline. The induced delays remained nearly consistent for fluid viscosities above 3.5 P (A, 3.5 P; B, 100 P; C, 600 P). There was an effect of fluid volume on sound transmission delay for all three viscosities tested, with significant increases in delay for all fluid volumes >0.5 ml. Symbols and error bars indicate the across-animal mean delay and ±1 SD, respectively.
Due to the fact that we saw no significant difference in attenuation with viscosities >3.5 P and volumes >1.0 ml, we chose to present data using 100 P silicone oil at a volume of 1.5 ml in the middle ear as a representative condition. With 1.5 ml of fluid in the middle ear, 100 % of the tympanic membrane’s medial area is covered with fluid (see Fig. 1). In three animals, fluid in the middle ear caused time delays that were frequency dependent. For example, Figure 9 shows the CM-derived ITDs in baseline and experimental conditions for four different low frequencies (0.25, 0.5, 1, and 2 kHz). Across frequencies, the ITDs ranged from 859 μs (±84 μs; n = 3 animals) at 250 Hz to 372 μs (±18 μs) at 2 kHz. As was the case for attenuation due to MEE, the magnitude of the MEE-induced delays was not dependent on the spatial location of the sound sources. Thus, the MEE introduced effective shifts in the spatial location dependence of the ITD cue due to an increased delay in sound transmission to the inner ear.
FIG. 9.
Across-animal mean and individual animal baseline (thin red = mean across animals, thin gray = individual animals) and experimental (thick red = mean across animals, thin black = individual animals; 1.5 ml of 100 P silicone oil) interaural time differences for four frequencies (250 Hz to 2 kHz; indicated in upper left of panels) across sound source azimuth (n = 3 ears). The simulated middle ear effusion due to silicone oil causes a shift in the spatial dependence of the ITD cue.
The temporal delays due to MEE are not due to confounds from the compound action potential waveform
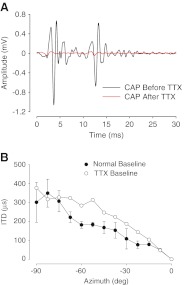
Recording sound-evoked potentials from the round window yields several different evoked potential waveforms including the summating potential, the cochlear microphonic (which is the waveform of interest here), and the compound action potential (CAP). The CAP represents a summation of action potentials from individual auditory nerve fibers. To rule out the possibility that the sound-evoked CAP waveforms, for example the little wiggles in the early phases of the waveforms shown in Figure 7 (see also Fig. 1 of Jones et al. 2011), which ride atop the CM “carrier”, could adversely influence the middle ear fluid dependent time delays computed from the CM component of this waveform, we conducted a control experiment. The CAP waveform confound could potentially arise due to the fact that the latencies of the CAP are highly dependent upon the stimulus input levels to the inner ear, whereas the CM latencies, upon which the effective ITDs are computed, are not (e.g., Fig. 3 of Lupo et al. 2011; Knudsen et al. 1984; Dallos and Cheatham 1971). To examine whether the CAP responses affected the CM-derived time delays, we used tetrodotoxin (TTX) to abolish the CAP response. TTX, a voltage-gated sodium channel blocker, has been shown in several studies to selectively eliminate the CAP response while leaving the CM waveform intact (Voss et al. 1996; Pasic and Rubel 1989; Kaplan et al. 1983). Following Voss et al. (1996), we used a 0.5-mM TTX (Sigma Chem. Co., St. Louis, MO, USA) solution (50 μl total comprising 1 mM TTX mixed with 50 μl artificial perilymph solution) of which 20 μl was placed onto the round window and allowed to diffuse through the cochlea over a 2–3-h period. In these control experiments, CM and CAP waveforms were evoked every ~10 min to assess the affect of the TTX on these waveforms. For tonal stimuli, TTX ultimately abolished the CAP response (Fig. 10), leaving only the CM response intact. In the example shown in Figure 10A, the stimulus was 4 kHz, which is a good stimulus frequency to easily elicit CAP responses. Baseline (i.e., no fluid in middle ear space) ITDs measured at 500 Hz in the presence of TTX were similar to the baseline ITDs measured when TTX was not present (Fig. 10B), except for three azimuthal locations around 60 °. At these azimuths, the CM-derived ITD was larger when the CAP waveform was abolished—this difference mostly occurred because the baseline ITDs for these three azimuths were smaller than expected (i.e., note the “sag” in the ITD vs. azimuth function that occurs around 60 °). Other stimulus frequencies produced the same negative result. Along with the prior publications demonstrating that the phase of the CM waveform is invariant with its amplitude (Lupo et al. 2011; Hartley and Moore 2003; Knudsen et al. 1984; Dallos and Cheatham 1971), this control experiment confirms that the MEE-induced timing delays derived from CM waveforms observed here were not materially affected by the latency of the CAP. More importantly, the estimated delays were not expected to be larger than those measured in the presence of TTX.
FIG. 10.
Compound action potential responses pre- and post-TTX administration to the round window membrane. Post-TTX measurements were taken ~3 h after TTX administration. A CAP amplitudes after TTX administration (red line) are significantly reduced compared to CAP amplitudes before TTX administration (black line). B Normal baseline (closed circles) and TTX-treated baseline (open circles) ITD measurements as a function of azimuth (−90 ° = left side) for a 500-Hz stimulus. Data are only shown for one hemisphere in the azimuthal plane.
Discussion
Here, we used evoked CM measurements to examine the effects of CHL due to an experimentally induced MEE on the binaural cues to sound location (i.e., ITD and ILD). Confirming earlier observations (Wever and Lawrence 1954; Knudsen et al. 1984; Hartley and Moore 2003; Lupo et al. 2011), the CM recordings demonstrate linearity over a wide range of sound intensities. The linearity enables accurate calibration to relate the CM amplitude measured at baseline to the hearing loss induced by the MEE. Through analysis of the CM, the effects of manipulations of the outer or middle ear on the transformation of sound to the inner ear are therefore quantifiable (Knudsen et al. 1984; Hartley and Moore 2003).
The volume of the middle ear effusion, not its viscosity, is the determinant of the resulting CHL
Attenuation of sound caused by fluid in the middle ear
The data in this paper demonstrate that the volume, but not viscosity, of fluid in the middle ear has a pronounced effect on the resulting CHL caused by a MEE. In the present study, there was no significant difference in attenuation of sound observed between different viscous fluids once the viscosity was greater than 3.5 P. Our results confirm those of several previous studies that have also shown that MEE viscosity is not an important determinant of the magnitude of a CHL due to experimentally induced MEEs (Brown et al. 1983; Weiderhold et al. 1980; Marsh et al. 1985; Ravicz et al. 2004; Jeselsohn et al. 2005). For example, Brown et al. (1983) instilled dextran solutions of low (7.5 cP) and medium (2.2 P) viscosities in the middle ears of adult guinea pigs. They found that the resulting CHL was due to middle ear compliance (related to volume of fluid in the middle ear) and was independent of fluid viscosity. Additionally, Weiderhold et al. (1980) found that viscosity was not related to the degree of CHL due to ligation of the Eustachian tube in cats. In an extensive and comprehensive study, Ravicz et al. (2004) also demonstrated that middle ear fluid volume, and not viscosity, was the primary determinant of the magnitude of the resulting CHL in human cadaveric temporal bones. In contrast, Hartley and Moore (2003) found in gerbils that higher viscosity fluids produced larger hearing losses, with 36 dB attenuation for high viscosity effusions, and 25 and 20 dB attenuations for medium and low viscosity effusions, respectively. However, Hartley and Moore (2003) acknowledged that there was considerable across-animal variability and thus overlap in attenuations of sound with different viscosities of fluid.
Delays in sound transmission to the inner ear due to a MEE-induced CHL
Delays caused by fluid in the middle ear
In the current study, the time delays resulting from middle ear fluid are similar, albeit smaller, to the earplug-induced delays observed in Lupo et al. (2011), and thus the effects on the ITD cues to location. Other animal studies have also examined how ITDs change as a result of a MEE. Hartley and Moore (2003) studied the effects of low (0.5 P), medium (3.4 P), and high (97.4 P) viscosity effusions on ITD shifts. Similar to the findings of the current study, they observed that, in general, larger time delays occurred at lower frequencies (1–6 kHz) than at higher frequencies. However, unlike the present study, they also found that these time delays were dependent on the viscosity of the effusion (Fig. 8). For example, at lower frequencies, low viscosity effusions produced across different animals a mean 82-μs time delay (but up to 230 μs delays in some animals), while medium viscosity effusions produced delays of 65 μs (and also larger delays in some animals) at lower frequencies. Again, they acknowledged that there was a quite large variability in the results across animals.
That fluid in the middle ear can cause increased delay of sound transmission to the inner ear is not new and has much experimental precedent. Using human temporal bone as a model, Ravicz et al. (2004) found that, after filling the middle ear space with saline, mechanical time delays in the transmission of sound to the inner ear were as large as 500 μs at 500 Hz. Recall that the volume of the human middle ear is comparable to that in the chinchilla. The magnitude of the delays of sound transmission to the inner ear of the chinchilla due to fluid in the middle ear in the present study was comparable to that observed in the human temporal bone by Ravicz et al. (2004). More recently, Guan and Gan (2011) demonstrated that filling the middle ear of the guinea pig completely with saline results in large temporal delays. These MEE-induced delays ranged from ~450 μs (with some animals producing delays of 800 μs or more) at low frequencies (<2 kHz) to 100–200 μs at higher (>2 kHz) frequencies (Guan and Gan 2011), indicating that there is a substantial effect of MEE on the temporal properties of sound input at the level of the inner ear that would be expected to alter the effective ITD cues to location.
Delays of sound transmission to the inner ear caused by earplugs and ossicular manipulations
Several studies have used earplugs as a method to experimentally induce a CHL in animal models (Knudsen et al. 1984; Hyson et al. 1994; Hartley and Moore 2003; Lupo et al. 2011). Knudsen et al. (1984) observed frequency-dependent delays of ~50 μs (but exceeding 100 μs in some animals) in the barn owl, but the effectiveness of the earplug was variable between animals. Hartley and Moore (2003) also examined the effect of earplugs on sound transmission and found frequency-dependent delays ranging from as large as ~250 μs at lower frequencies (1–6 kHz) to ~20 μs (but with considerable across-animal variability) at higher frequencies (8–16 kHz). However, Hartley and Moore (2003) only presented sounds from one position in azimuth (midline), so they were not able to get an accurate representation of how ITDs were changing at various locations across the horizontal plane. In humans, earplugs have been shown to create time delays up to ~500 μs at lower frequencies around 500 Hz (Hogan et al. 1995; Kumpik et al. 2010). Consistent with each of the former results, Lupo et al. (2011) recently used earplugs as a means of inducing CHL in the chinchilla and found the delays caused by the earplug to be quite substantial, on the order of several hundred microseconds, and that the magnitude of the delays were proportional to the volume of the earplug material used. Finally, Portmann et al. (1966) showed in the guinea pig that certain manipulations of the malleus which led to CHLs of 15–30 dB (as assessed via CMs) were also accompanied by frequency-dependent sound transmission delays in the CMs of ~500 μs at 500 Hz down to ~80 μs at 4 kHz (computed from Table 4 in Portmann et al. 1966). Thus, CHLs induced in the laboratory by methods other than MEE can also produce sound transmission delays to the inner ear that are comparable in magnitude to the delays produced by a simulated MEE.
In the current study, the effect of the MEE-induced temporal delays that we observed produced effective ITDs (i.e., ITDs that would be expected to be experienced by the observer) which were considerably larger than the physiological range of ITDs that an animal would actually experience, which is in accordance with previous studies (Knudsen et al. 1984; Hartley and Moore 2003; Hogan et al. 1995; Koka et al. 2011; Lupo et al. 2011). For example, an adult chinchilla experiences maximum ITDs of ~350 μs or more (Koka et al. 2011; Jones et al. 2011), and we observed MEE-induced ITD shifts across all animals and conditions of approximately the same magnitude. The temporal delays found in the Lupo et al. (2011) study using earplugs to induce CHL were similar in magnitude to the temporal delays that we observe in this study as a result of filling the middle ear with fluid. Together, these results suggest that earplug-induced CHL, at least in the chinchilla, alters the spectral and temporal properties of sound similarly to that observed in the more clinically relevant MEE-induced CHL. To this end, earplugs remain a viable method to experimentally implement a clinically relevant laboratory model of chronic yet reversible CHL that might be expected to result from OME because the results from the current study are wholly consistent with the results from prior studies using earplugs to induce a CHL (Lupo et al. 2011).
CHL due to MEE—implications for sound localization and development
Low-frequency sound localization is crucial for speech comprehension, especially in environments with multiple sound sources (Blauert 1997). Altered ITD processing as a result of CHL can negatively affect the ability to segregate speech sounds out of noisy background environments (“cocktail party effect”, Moore et al. 1991). Indeed, both children and adults with CHL performed poorly on masking level difference tasks compared to normal controls even after the CHL was compensated for (Hall et al. 1995, 1998; Hogan and Moore 2003), implying that altered binaural localization cues, specifically ITDs, affect the ability of subjects with CHL to spatially segregate sounds of interest using binaural acoustical cues.
In children, binaural localization deficits can persist long after the CHL has cleared and hearing sensitivity has returned to normal (Hall et al. 1995; Hogan and Moore 2003). Thus, models of CHL during developmental sensitive periods are especially important in determining the long-lasting and residual effects of CHL on the different cues to sound localization. Toward this end, experimental models of chronic CHL have been achieved in the laboratory through a variety of means, including ear plugging (Knudsen et al. 1984; Hartley and Moore 2003; Hyson et al. 1994; Clements and Kelly 1978; Lupo et al. 2011), ear canal ligation (Clopton and Silverman 1977; Brugge et al. 1985; Moore and Irvine 1981; Popescu and Polley 2010), ossicle disarticulation (Xu et al. 2007; Cook et al. 2002; Paterson and Hosea 1993), and instilled fluid in the middle ear space (Hartley and Moore 2003; Brown et al. 1983; Jeselsohn et al. 2005; Takeuchi et al. 1989). This previous work has shown that CHL, regardless of etiology, can have detrimental effects on the spectral and temporal properties of sound localization, especially if the CHL occurs during a sensitive developmental period (Knudsen et al. 1984; Clopton and Silverman 1977; Popescu and Polley 2010).
Conclusions
While several studies have produced convergent ideas about how CHL during development can affect the binaural cues to sound localization during development, there is more work to be done on characterizing the long-term changes to central auditory system function that persist after the CHL has been cleared. The current study demonstrates that while CHL can affect the ILD cues to sound location, the ITD cues are also affected in large and significant ways. Combined with the results from previous studies (Hartley and Moore 2003; Lupo et al. 2011), and given the clear importance of low-frequency ITDs for spatial hearing, it is clear that the alteration of ITDs in the presence of CHL may play a large, but heretofore unappreciated (and completely unstudied), role in the long-term central auditory processing deficits that are seen in children with chronic otitis media with effusion.
Acknowledgments
This work was supported by the National Institutes of Deafness and Other Communicative Disorders (NIDCD) Grant F31-DC011198-01, T32-NS007083, and T32-HD041697 to JLT and NIDCD R01-DC011555 to DJT. Support for the initial phases of this work was provided by the National Organization for Hearing Research (NOHR) Evie & Ron Krancer Grant in Auditory Science to DJT. Support was also provided by an American Academy of Otolaryngology-Head and Neck Surgery Foundation (AAO-HNSF) resident research grant to JEL. We thank Dr. Sukumar Vijayaraghavan for the help with the TTX.
References
- Bennett KE, Haggard MP, Silva PA, Stewart IA. Behaviour and developmental effects of otitis media with effusion into the teens. Arch Dis Child. 2001;85:91–95. doi: 10.1136/adc.85.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauert J (1997) Spatial hearing: the psychophysics of human sound localization. MIT, Cambridge pp. 237–271
- Bluestone CD, Beery QC, Paradise JL. Audiometry and tympanometry in relation to middle ear effusions in children. Laryngoscope. 1973;83:594–604. doi: 10.1288/00005537-197304000-00015. [DOI] [PubMed] [Google Scholar]
- Bluestone CD, Klein JO (eds) (1995) Definitions, terminology, and classification. In: Otitis media in infants and children (2nd Edn.). W.B. Saunders, Philadelphia, pp.1-3
- Brown DT, Marsh RR, Potsic WP. Hearing loss induced by viscous fluids in the middle ear. Int J Pediatr Otorhinolaryngol. 1983;5:39–46. doi: 10.1016/S0165-5876(83)80006-8. [DOI] [PubMed] [Google Scholar]
- Brugge JF, Orman SS, Coleman JR, Chan JCK, Phillips DP. Binaural interactions in cortical area AI of cats reared with unilateral atresia of the external ear canal. Hear Res. 1985;20:275–287. doi: 10.1016/0378-5955(85)90032-2. [DOI] [PubMed] [Google Scholar]
- Clements M, Kelly JB. Auditory spatial responses of young guinea pigs (Cavia porcellus) during and after ear blocking. J Comp Physiol Psychol. 1978;92:34–44. doi: 10.1037/h0077424. [DOI] [PubMed] [Google Scholar]
- Clopton BM, Silverman MS. Changes in latency and duration of neural responding following developmental auditory deprivation. Exp Brain Res. 1977;32:39–47. doi: 10.1007/BF00237388. [DOI] [PubMed] [Google Scholar]
- Cook RD, Hung TY, Miller RL, Smith DW, Tucci DL. Effects of conductive hearing loss on auditory nerve activity in gerbil. Hear Res. 2002;164:127–137. doi: 10.1016/S0378-5955(01)00424-5. [DOI] [PubMed] [Google Scholar]
- Dallos P, Cheatham MA. Travel time in the cochlea and its determination from cochlear–microphonic data. J Acoust Soc Am. 1971;49:1140–1143. doi: 10.1121/1.1912475. [DOI] [PubMed] [Google Scholar]
- Goodhill V, Holcomb AL. The relation of auditory response to the viscosity of tympanic fluids. Acta Otolaryngol. 1958;49:38–46. doi: 10.3109/00016485809134725. [DOI] [PubMed] [Google Scholar]
- Guan X, Gan RZ. Effect of middle ear fluid on sound transmission and auditory brainstem response in guinea pigs. Hear Res. 2011;277:96–106. doi: 10.1016/j.heares.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JW, Derlacki EL. Effect of conductive hearing loss and middle ear surgery on binaural hearing. Ann Otol Rhinol Laryngol. 1986;95:525–530. doi: 10.1177/000348948609500516. [DOI] [PubMed] [Google Scholar]
- Hall JW, Grose JH, Mendoza LL. Masker interaural phase and the MLD: effects of conductive hearing loss. Hear Res. 1995;84:91–98. doi: 10.1016/0378-5955(95)00016-W. [DOI] [PubMed] [Google Scholar]
- Hall JW, Grose JH, Dev MB, Ghiassi S. The effect of masker interaural time delay on the masking level difference in children with history of normal hearing or history of otitis media with effusion. Ear Hear. 1998;19:220–229. doi: 10.1097/00003446-199812000-00004. [DOI] [PubMed] [Google Scholar]
- Hartley DEH, Moore DR. Effects of conductive hearing loss on temporal aspects of sound transmission through the ear. Hear Res. 2003;177:53–60. doi: 10.1016/S0378-5955(02)00797-9. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Heffner HE. Behavioral hearing range of the chinchilla. Hear Res. 1991;52:13–16. doi: 10.1016/0378-5955(91)90183-A. [DOI] [PubMed] [Google Scholar]
- Hogan SC, Pralong D, Moore DR. Effects of unilateral ear-plugging in humans on binaural unmasking. Br J Audiol. 1995;29:56–57. [Google Scholar]
- Hogan SC, Moore DR. Impaired binaural hearing in children produces by a threshold level of middle ear disease. J Assoc Res Otolaryngol. 2003;4(2):123–129. doi: 10.1007/s10162-002-3007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyson RL, Overholt EM, Lippe WR. Cochlear microphonic measurements of interaural time differences in the chick. Hear Res. 1994;81:109–118. doi: 10.1016/0378-5955(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Jeselsohn Y, Freeman S, Segal N, Sohmer H. Quantitative experimental assessment of the factors contributing to hearing loss in serous otitis media. Otol Neurotol. 2005;26:1011–1015. doi: 10.1097/01.mao.0000185051.69394.01. [DOI] [PubMed] [Google Scholar]
- Jones HG, Koka K, Tollin DJ. Postnatal development of cochlear microphonic and compound action potentials in a precocious species, Chinchilla lanigera. J Acoust Soc Am. 2011;130:EL38–EL43. doi: 10.1121/1.3601881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MS, Szaro BG, Weiss TF. Components of the cochlear electric responses in the alligator lizard. Hear Res. 1983;12:323–351. doi: 10.1016/0378-5955(83)90004-7. [DOI] [PubMed] [Google Scholar]
- Knudsen EI, Esterly SD, Knudsen PF. Monaural occlusion alters sound localization during a sensitive period in the barn owl. J Neurosci. 1984;4:1001–1011. doi: 10.1523/JNEUROSCI.04-04-01001.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koka K, Jones HG, Thornton JL, Lupo JE, Tollin DJ. Sound pressure transformation by the head and pinnae of the adult chinchilla (Chinchilla lanigera) Hear Res. 2011;272:135–147. doi: 10.1016/j.heares.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokko E. Chronic secretory otitis media in children: a clinical study. Acta Otolaryngol Suppl. 1974;327:1–44. [PubMed] [Google Scholar]
- Kuhn GF. Model for the interaural time differences in the azimuthal plane. J Acoust Soc Am. 1977;62:157–167. doi: 10.1121/1.381498. [DOI] [Google Scholar]
- Kumpik DP, Kacelnik O, King AJ. Adaptive reweighting of auditory localization cues in response to chronic unilateral earplugging in humans. J Neurosci. 2010;30:4883–4894. doi: 10.1523/JNEUROSCI.5488-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupo JE, Koka K, Thornton JL, Tollin DJ. The effects of experimentally induced conductive hearing loss on spectral and temporal aspects of sound transmission through the ear. Hear Res. 2011;272:30–41. doi: 10.1016/j.heares.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh RR, Baranak CC, Potsic WP. Hearing loss and the visco-elasticity of middle ear fluid. Int J Pediatr Otorhinolaryngol. 1985;9:115–120. doi: 10.1016/S0165-5876(85)80011-2. [DOI] [PubMed] [Google Scholar]
- Miller JD. Audibility curve of the chinchilla. J Acoust Soc Am. 1970;48:513–523. doi: 10.1121/1.1912166. [DOI] [PubMed] [Google Scholar]
- Miyahara H, Nakajima A, Wada J, Yanabu S (2006) Breakdown characteristics of combined insulation in silicone oil for electric power apparatus. "2006 IEEE 8th International Conference on Properties and applications of Dielectric Materials". Properties and applications of Dielectric Materials, 2006. 8th International Conference, IEEE Conference Publications, Bali, pp. 661-664
- Moore DR, Irvine DRF. Plasticity of binaural interaction in the cat inferior colliculus. Brain Res. 1981;208:198–202. doi: 10.1016/0006-8993(81)90632-6. [DOI] [PubMed] [Google Scholar]
- Moore DR, Hutchings ME, Meyer SE. Binaural masking level differences in children with a history of otitis media. Audiology. 1991;30:91–101. doi: 10.3109/00206099109072874. [DOI] [PubMed] [Google Scholar]
- Moore DR, Hartley DE, Hogan SC. Effects of otitis media with effusion (OME) on central auditory function. Int J Pediatr Otorhinolaryngol. 2003;67(Suppl 1):S63–S67. doi: 10.1016/j.ijporl.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Niemiec AJ, Yost WA, Shofner WP. Behavioral measures of frequency selectivity in the chinchilla. J Acoust Soc Am. 1992;92:2636–2649. doi: 10.1121/1.404380. [DOI] [PubMed] [Google Scholar]
- Pasic TR, Rubel EW. Rapid changes in cochlear nucleus cell size following blockade of auditory nerve electrical activity in gerbils. J Comp Neurol. 1989;283:474–480. doi: 10.1002/cne.902830403. [DOI] [PubMed] [Google Scholar]
- Paterson JA, Hosea EW. Auditory behaviour and brainstem histochemistry in adult rats with characterized ear damage after neonatal ossicle ablation or cochlear disruption. Behav Brain Res. 1993;53:73–89. doi: 10.1016/S0166-4328(05)80267-0. [DOI] [PubMed] [Google Scholar]
- Pillsbury HC, Grose JH, Hall JW. Otitis media with effusion in children: binaural hearing before and after corrective surgery. Arch Otolaryngol Head Neck Surg. 1991;117:718–723. doi: 10.1001/archotol.1991.01870190030008. [DOI] [PubMed] [Google Scholar]
- Popescu MV, Polley DB. Monaural deprivation disrupts development of binaural selectivity in auditory midbrain and cortex. Neuron. 2010;65(5):718–731. doi: 10.1016/j.neuron.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portmann M, Meira LM, Gonzalez Alfaro D, Aran JM. Influences of the ossicular mass on the vibratory transmission, Experimental study. Int J Audiol. 1966;5:97–102. doi: 10.3109/05384916609074150. [DOI] [Google Scholar]
- Ravicz ME, Rosowski JJ, Merchant SN. Mechanisms of hearing loss resulting from middle-ear fluid. Hear Res. 2004;195:103–130. doi: 10.1016/j.heares.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Roth GL, Kochhar RK, Hind JE. Interaural time differences: implications regarding the neurophysiology of sound localization. J Acoust Soc Am. 1980;68:1643–1651. doi: 10.1121/1.385196. [DOI] [PubMed] [Google Scholar]
- Slattery WH, Middlebrooks JC. Monaural sound localization: acute versus chronic unilateral impairment. Hear Res. 1994;75:38–46. doi: 10.1016/0378-5955(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Majima Y, Hirata K, Morishita A, Hattori M, Sakakura Y. Prognosis of secretory otitis media in relation to viscoelasticity of effusions in children. Ann Otol Rhinol Laryngol. 1989;98:443–446. doi: 10.1177/000348948909800609. [DOI] [PubMed] [Google Scholar]
- Tollin DJ, Koka K. Postnatal development of sound pressure transformations by the head and pinnae of the cat: monaural characteristics. J Acoust Soc Am. 2009;125:980–994. doi: 10.1121/1.3058630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollin DJ. The development of sound localization mechanisms. In: Blumberg MS, Freeman JH, Robinson SR, editors. Oxford handbook of developmental behavioral neuroscience. Oxford: Oxford University Press; 2010. pp. 262–282. [Google Scholar]
- Tucci D, Cant NB, Durham D. Conductive hearing loss results in changes in cytochrome oxidase activity in gerbil central auditory system. J Assoc Res Otolaryngol. 2002;3:89–106. doi: 10.1007/s101620010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss SE, Rosowski JJ, Peake WT. Is the pressure difference between the oval and round windows the effective stimulus for the cochlea? J Acoust Soc Am. 1996;100:1602–1616. doi: 10.1121/1.416062. [DOI] [PubMed] [Google Scholar]
- Vrettakos PA, Sp D, Saunders JC. Middle ear structure in the chinchilla: a quantitative study. Am J Otolaryngol. 1988;9:58–67. doi: 10.1016/S0196-0709(88)80009-7. [DOI] [PubMed] [Google Scholar]
- Weiderhold ML, Zajtchuk JT, Vap JG, Paggi RE. Hearing loss in relation to physical properties of middle ear effusions. Ann Otol Rhinol Laryngol. 1980;89:185–189. doi: 10.1177/00034894800890s343. [DOI] [PubMed] [Google Scholar]
- Wever EG, Lawrence M (1954) Physiological acoustics. Princeton University Press, Princeton pp. 245–294
- Whitton JP, Polley DB. Evaluating the perceptual and pathophysiological consequences of auditory deprivation in early postnatal life: a comparison of basic and clinical studies. J Assoc Res Otolaryngol. 2011;12:535–546. doi: 10.1007/s10162-011-0271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman FL, Kistler DJ. The dominant role of low-frequency interaural time differences in sound localization. J Acoust Soc Am. 1992;91:1648–1661. doi: 10.1121/1.402445. [DOI] [PubMed] [Google Scholar]
- Woodworth RS. Experimental psychology. New York: H Holt and Company; 1938. [Google Scholar]
- Xu H, Kotak VC, Sanes DH. Conductive hearing loss disrupts synaptic and spike adaptation in developing auditory cortex. J Neurosci. 2007;27:9417–9426. doi: 10.1523/JNEUROSCI.1992-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga-Itano C, Sedey AL, Coulter DL, Mehl AL. Language of early- and later-identified children with hearing loss. Pediatrics. 1998;102:1161–1171. doi: 10.1542/peds.102.5.1161. [DOI] [PubMed] [Google Scholar]