Abstract
Exposure of mammalian cells to UV irradiation leads to activation of the c-Jun NH2-terminal protein kinase (JNK) pathway, which is associated with cell apoptosis. However, the molecular mechanism for JNK activation by UV exposure is not fully understood. We show here an essential role of a multisubstrate adapter, Gab1, in this signaling cascade. Gab1-deficient mouse fibroblast cells were defective in induction of JNK activity by UV exposure or heat shock, and this defect was rescued by reintroduction of Gab1 into Gab1−/− cells. Consistently, Gab1−/− cells displayed reduced caspase 3 induction and apoptotic cell death in response to UV irradiation. Gab1 was constitutively complexed with JNK and became tyrosine phosphorylated in UV-irradiated cells. Genetic and pharmaceutical analyses suggest the involvement of c-Met and the Src family tyrosine kinases in mediating UV-induced Gab1 phosphorylation as well as JNK activation. In aggregate, these observations identify a new function of Gab1 in the response of mammalian cells to UV light.
The human population will possibly face the exposure of increasing doses of short-wavelength UV irradiation due to progressive destruction of the ozone layer by industrial pollution, with the direct outcome of increasing rates of skin cancer. UV irradiation can have multiple effects on mammalian cells, such as DNA damage through formation of pyrimidine dimers and 6-4 photoproducts and induced expression of genes that are involved in DNA repair, cell growth, and apoptosis (8, 12). Exposure of cells to UV also rapidly induces the activation of c-Jun NH2-terminal kinases (JNK) that phosphorylate and activate transcription factors, including c-Jun and ATF2 (18).
JNK, also known as stress-activated protein kinase, is a group of mitogen-activated protein (MAP) kinases that include the extracellular signal regulated kinase (ERK) and p38 MAP kinase (32). JNK is activated during cellular responses to external stress, such as UV irradiation, heat shock, and inflammatory cytokines (41), and this group of kinases has been implicated in the activation of caspases and initiation of apoptotic process in a variety of cell types (40, 41). However, JNK activation is sometimes not associated with cell apoptosis (18). For example, suppression of the JNK pathway does not block Fas-induced apoptosis in Jurkat T cells (26). Characterization of fibroblast cells defective for c-Jun or c-Fos expression even suggested a protective role of JNK against cell apoptosis (30, 41). Apparently, whether JNK activates a proapoptotic or an antiapoptotic pathway might depend on the cellular context or the degree to which JNK is activated (23). It is also possible that the three members of JNK, JNK1, JNK2, and JNK3, have overlapping yet distinct functions in various cell types (18).
The molecular mechanism for activation of JNK by UV irradiation is not fully understood. However, it appears to involve signal relay of a MAP kinase signaling cascade from membrane-proximal events rather than a response to DNA damage in the nucleus (33). In particular, it has been proposed by several groups that UV irradiation induces ligand-independent activation of receptors for epidermal growth factor (EGF), tumor necrosis factor, and interleukin-1 that triggers the cytoplasmic signaling cascade leading to activation of JNK (16, 33, 38, 39). Cross-linking of these cell surface proteins induced by UV might be mediated by reactive oxygen intermediates (16). Involvement of c-Src, Ha-Ras, and Raf-1 in the cellular response to UV exposure has also been suggested by experiments using tyrosine kinase inhibitors and dominant negative mutants (6). However, these observations were disputed by a more recent report by Oksvold et al., arguing that UV exposure did not induce dimerization, tyrosine phosphorylation, or activation of EGF-R (31). Therefore, the initial events for signal relay in UV-induced JNK activation remain elusive and controversial.
The Grb2-associated binder 1 (Gab1) is a multisubstrate scaffold/adapter protein that contains a pleckstrin homology domain at the NH2 terminus and multiple tyrosyl residues and proline-rich motifs for association with src homology 2 (SH2)-containing and SH3-containing proteins (14). Gab1, as well as its close relatives Gab2 and Gab3, serves to couple signaling between cell surface receptors for growth factors and cytokines with a variety of downstream targets, including Shp2 tyrosine phosphatase, phosphatidylinositol 3-kinase (PI3K), and phospholipase C-γ (10, 11, 14, 29, 42, 44). Ablation of the Gab1 gene in mice resulted in embryonic death in homozygous mutants, with developmental defects in the placenta, heart, and skin (19, 34). Biochemical analysis of Gab1−/− fibroblast cells suggested a positive role of Gab1 in coupling the signal relay between cytokine receptors and the ERK pathway (19).
In this report, we describe a novel function of Gab1 in mediating the activation of the JNK pathway by UV light. Our data also suggest that the Src family kinases (SFK) play a role in mediating UV-induced tyrosine phosphorylation of Gab1 as well as activation of JNK.
MATERIALS AND METHODS
Cells, antibodies, and reagents.
Wild-type (+/+) and Gab1-deficient (−/−) mouse embryonic fibroblast cells were established from littermate embryos of Gab1 knockouts (19). Fibroblast cells devoid of Src, Yes, and Fyn kinases (SYF) and SYF cells expressing c-Src (SYF+ c-Src) were from ATCC (CRL-2459 and CRL-2498) (22). Cells were incubated in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS). For growth factor stimulation, cells grown at 80% confluency were starved in serum-free DMEM for 36 h prior addition of the factor. UV irradiation was done with a UV-B source at a cumulative dose rate of 100, 200, or 400 J/m2 (kindly provided by Robert Abraham's laboratory). Recombinant mouse EGF was purchased from Becton Dickinson Labware; hepatocyte growth factor (HGF) was from Sigma. Antibodies against phosphotyrosine (anti-PY; pY99), JNK1, JNK2, phospho-JNK (p-JNK), p38, and EGF-R were from Santa Cruz Biotechnology, Inc., or Promega. Anti-phospho-ERK (anti-p-ERK), anti-ERK, anti-phospho-p38 (anti-p-p38), and anti-phospho-MKK4 (anti-p-MKK4) were from Cell Signaling Tech. Anti-phospho-Met (Y1234 and Y1235) was from Upstate Biotechnology. Anti-HA (12CA5) and anti-Flag (M2) were from Sigma. Gab1 mouse monoclonal antibody was generated by Abgent Inc., La Jolla, Calif. The EGF-R-specific inhibitor AG1478 was obtained from Calibiochem, and the Src family kinase inhibitor PP2 was from Sigma. Caspase 3 substrate (Ac-DEVD-AFC) was from Biomol. Mammalian expression constructs for cDNAs encoding different Flag-tagged human Gab1 constructs, pGab1 (Gab1 wild type), pGab1ΔPI3K (Y447F/Y472F/Y589F, lacking PI3K binding sites), pGab1ΔPH (lacking the coding sequence for amino acids 10 to 177), and pGab1ΔShp2 (Y627F/Y659F, lacking Shp2 binding sites), were described previously (2, 3). cDNAs encoding JNK1 and JNK2 were generous gifts from Jiahuai Han at Scripps Research Institute.
Immunoprecipitation and immunoblotting.
Control or factor-stimulated cell lysates were made with cell lysis buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EGTA, 50 mM NaF, 5 mM NaPPi, 1 mM Na3VO4, 0.1 mM ZnCl2, 1 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin per ml, 10 μg of leupeptin per ml), and the protein concentration was determined using a Bio-Rad protein assay kit. For immunoprecipitation cell lysates were incubated with specific antibodies in the presence of protein G/A-Sepharose beads. Samples of immunoprecipitates or cell lysates were resolved on sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred to a nitrocellulose membrane. The membrane was then blotted with appropriate antibodies, and signals were detected by enhanced chemiluminescence (ECL kit; Perkin Elmer).
Cell transfection.
For transient transfection, Gab1−/− cells were plated into 60-mm dishes and transfected 1 day later (at ∼50% confluency) with expression constructs using the Lipofectamine method (Invitrogen) as specified by the supplier. Briefly, cells were incubated with DNA-Lipofectamine (8 μg total/12 μl) complexes in serum-free medium for 5 to 7 h. The DNA complexes were then removed from the medium, and the cells were incubated with DMEM plus 10% FBS. At 12 h after transfection, the cells were replated into 100-mm dishes, incubated for 24 h, and subsequently subjected to heat shock (42°C for 1 h) or UV-B irradiation (400 J/m2). Cell extracts were prepared for immunoprecipitation and immunoblotting. COS-1 cells were seeded in 100-mm dishes, and transfection was done 24 hr later (at 50% confluency) using FuGENE 6 (Roche), as specified by the supplier. Briefly, cells were incubated with DNA-FuGENE6 (4 μg total/12 μl) for 24 h and then used for experiments.
Cell apoptosis assay.
Cell apoptosis was measured by DNA laddering, as described previously (7, 21). Gab1+/+ and Gab1−/− fibroblasts were cultured in DMEM plus 10% FBS until subconfluency. The cells were treated with UV-B (400 J/m2), incubated for the indicated times, and collected by incubation with trypsin-EDTA and centrifugation. Harvested cells were washed twice with phosphate-buffered saline and then lysed for 20 min on ice in 0.33 ml of a buffer consisting of 5 mM Tris-HCl (pH 7.4), 20 mM EDTA, and 0.5% Triton X-100. After centrifugation at the top speed in a microcentrifuge for 10 min at 4°C, supernatants were transferred to new Eppendorf tubes and extracted with phenol-choloroform three times. Extracted DNA fragments were precipitated by ethanol, resuspended in Tris-EDTA (TE) buffer, and treated with 20 μg of RNase per ml for 30 min at 37°C. The DNA samples (20 μl) were subjected to electrophoresis on a 1.5% agarose gels and visualized by a UV illuminator. Cell apoptosis was also determined by YOPRO-1 staining as described previously (17, 27), and a Vybrant apoptosis assay kit was purchased from Molecular Probes, Inc.
Caspase 3 activity assay.
Cells were washed with cold phosphate-buffered saline and scraped into 100 μl of chilled lysis buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 0.2% Triton X-100, 1 mM dithiothreitol). The cell pellet was resuspended by pipetting and incubated on ice for 10 min. After centrifugation at the top speed in a microcentrifuge for 10 min at 4°C, supernatants were transferred to new Eppendorf tubes and the protein concentration was determined using a Bio-Rad protein assay kit. Caspase 3 activities were determined by measuring the release of 7-amino-4-trifluoromethylcoumarin (AFC) from the synthetic substrate Ac-DEVD-AFC. Lysates (20 μg of total proteins) were mixed with 100 μl of caspase buffer (20 mM PIPES, 100 mM NaCl, 1 mM EDTA, 0.1% 3-[3-(cholamidopropyl)-dimethylammonio]-1-propane sulfonate [CHAPS], 10% sucrose, 10 mM dithiothreitol [pH 7.2]) containing 0.1 mM AFC substrate. The amounts of AFC released into the buffer were measured at 37°C using a spectrofluorometric plate reader (Perkin-Elmer no. LS50B) in the kinetic mode with excitation and emission wavelengths of 400 and 505 nm, respectively. Values (Vmax) are reported as the rate of product formation obtained from the linear portion of the reaction curves within the first 10% of substrate depletion.
RESULTS
UV-induced JNK activation is defective in Gab1−/− fibroblast cells.
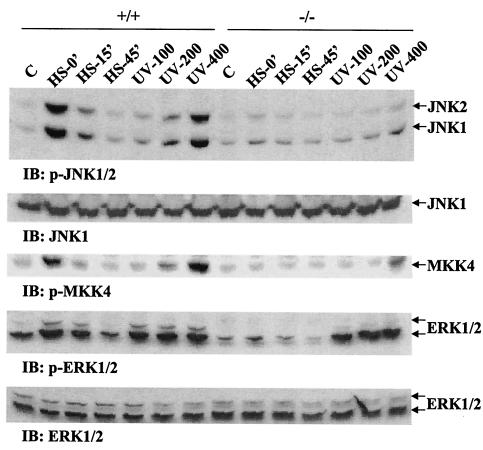
To directly assess the putative role of the adapter/scaffold protein Gab1 in mediating activation of the JNK pathway during cell response to external stress, we examined JNK activities in wild-type and Gab1−/− mouse embryonic fibroblasts after UV irradiation and heat shock. Previous experiments demonstrated a defect in growth factor-induced ERK activation in Gab1−/− cells (19). When wild-type cells were exposed to UV-B light at dosages of 100, 200, and 400 J/m2 (UV-100, UV-200, and UV-400), JNK activity was induced in a dose-dependent fashion, as measured by immunoblotting analysis with an antibody specific for phospho-JNK1/2, p-JNK1/2 (Fig. 1). Likewise, JNK activation was also detected in cells after heat shock at 42°C for 1 h, and the activity decayed very fast during subsequent cell incubation at 37°C for 15 and 45 min. Interestingly, JNK activation induced by either UV irradiation or heat shock treatment under the above conditions was almost abolished in Gab1−/− fibroblasts (Fig. 1). This provides direct evidence for Gab1 involvement in mediating activation of the JNK pathway, in the cellular response to stress, such as UV irradiation or heat shock. We then examined the activity of MKK4, the kinase that phosphorylates and activates JNK1/2, in wild-type and Gab−/− cells and obtained similar results to those obtained with JNK1/2, suggesting that Gab1 deficiency affected the JNK pathway upstream of MKK4 (Fig. 1).
FIG. 1.
Defective JNK activation induced by UV irradiation and heat shock in Gab1-deficient cells. Wild-type (+/+) and Gab1−/− (−/−) embryonic fibroblasts were treated with UV-B light (100, 200, and 400 J/m2) followed by incubation at 37°C for 1 h. For heat shock treatment, the cells were incubated at 42°C for 1 h and then allowed to recover at 37°C for 0, 15, and 45 min. Cell lysates were made, and JNK activation was detected by immunoblotting (IB) using an anti-p-JNK antibody. ERK activation was detected by immunoblotting using anti-p-ERK1/2 antibody. The activity of the JNK kinase MKK4 was also examined by immunoblotting with an anti-p-MKK4 antibody. Anti-JNK1 and anti-ERK1/2 immunoblotting indicated equal loading of cell lysates on each lane.
We also assessed ERK1/2 activation under cellular stress. As illustrated in Fig. 1, heat shock or UV irradiation induced ERK1/2 activation in wild-type cells. Although ERK activity was attenuated in Gab1−/− cells, the decrease was not as significant as the change of JNK activity in response to UV irradiation. Induction of p38 MAP kinase activity by UV or heat shock in wild-type and Gab1−/− cells was similar to that of ERK (data not shown).
Gab1 has a positive role in EGF-induced JNK and ERK pathways.
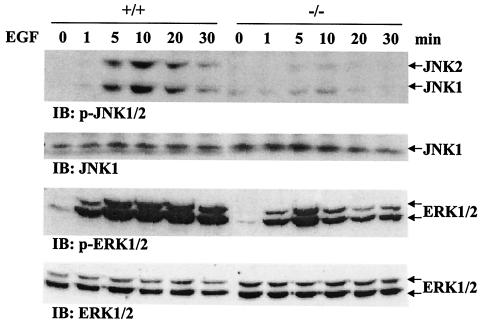
Following examination of JNK and ERK pathways under cellular stress, we assessed Gab1 participation in mediating EGF stimulation of these kinase activities. Wild-type and Gab1−/− fibroblasts were starved in serum-free medium for 36 h and then treated with EGF (100 ng/ml) for 1, 5, 10, 20, or 30 min. Cell lysates were subjected to immunoblot analysis with anti-p-JNK1/2 or anti-p-ERK1/2 antibodies. Figure 2 shows that EGF induced a transient activation of the JNK pathway in wild-type cells over the 30-min period, with the maximum levels detected at 10 min. Notably, JNK induction was almost undetectable in Gab1−/− cells (Fig. 2). EGF-induced ERK activities were sustained longer than JNK activity in wild-type fibroblasts, and this induction was significantly reduced in Gab1−/− cells (Fig. 2). Together, those data indicate a positive role for Gab1 in JNK and ERK pathways induced by a growth factor, such as EGF, as well as UV irradiation and heat shock. The requirement of Gab1 for the JNK pathway appears more stringent than for the ERK pathway.
FIG. 2.
Defective JNK and ERK induction by EGF in Gab1-deficient cells. Subconfluent fibroblasts were starved in serum-free DMEM for 36 h, stimulated with EGF (100 ng/ml) for 0, 1, 5, 10, 20, or 30 min, and lysed with lysis buffer. Equal amounts of cell lysates (50 μg of total proteins) were resolved on a SDS-polyacrylamide gel and immunoblotted (IB) by the indicated antibodies. +/+, wild-type cells; −/−, Gab1−/− cells.
Defect in JNK activation is rescued by reintroduction of Gab1.
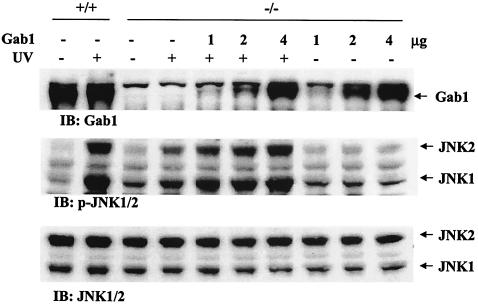
In a previous experiment, defective ERK activation induced by a cytokine, interleukin-6, in Gab1−/− cells was recovered by reconstitution of Gab1 expression (19). To define the function of Gab1 in induction of the JNK pathway by external stress, we transiently transfected Gab1−/− fibroblasts with a mammalian expression construct for Gab1 and reevaluated JNK activation after heat shock or UV irradiation. Notably, normal JNK activation was observed in Gab1−/− cells in which wild-type Gab1 protein was reexpressed (Fig. 3). Therefore, Gab1 is required for induction of JNK activity by UV irradiation or heat shock in mammalian fibroblast cells.
FIG. 3.
Rescue of the JNK pathway by expression of wild-type Gab1 in Gab1−/− cells. Gab1−/− cells were transiently transfected with 1, 2, or 4 μg of the expression construct for human Gab1 cDNA, using the GenePORTER 2 transfection reagent (Gene Therapy Systems Inc.). After incubation at 37°C for 72 h, the cells were irradiated with UV-B light at 400 J/m2 or left untreated. JNK activation was detected by immunoblotting (IB) using an anti-p-JNK antibody. The membrane was stripped and immunoblotted with an anti-JNK2 antibody as loading control. Exogenous expression of Gab1 in transfected cells was confirmed by anti-Gab1 immunoblot analysis with wild-type cells as positive control. +/+, wild-type cells; −/−, Gab1−/− cells.
Gab1 is complexed with JNK.
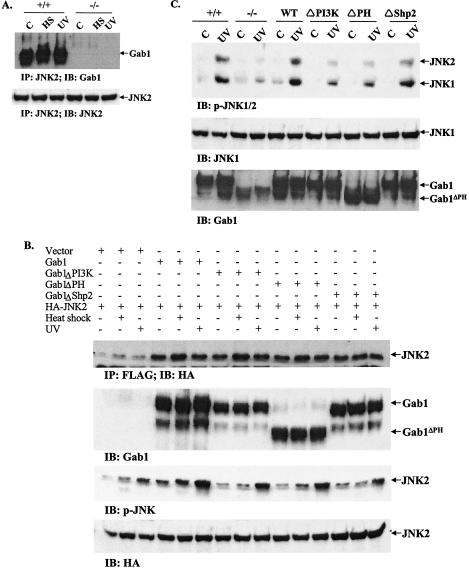
In exploring the molecular mechanism for Gab1 activity in the JNK pathway, coimmunoprecipitation was performed to investigate possible interaction between the two molecules, Gab1 and JNK, in cells. Lysates prepared from control cells and cells treated with UV or heat shock were subjected to immunoprecipitation with an anti-JNK2 antibody and then immunoblotted with an antibody against Gab1. The results in Fig. 4A demonstrate that Gab1 was constitutively complexed with JNK2 in fibroblast cells, since coimmunoprecipitation of the two proteins was detected not only in cells subjected to UV irradiation or heat shock but also in control cells without any treatment.
FIG. 4.
Signaling from Gab1 to JNK. (A). Association of Gab1 with JNK2 in fibroblast cells. Wild-type (+/+) and Gab1−/− (−/−) cells were heat shocked (HS) at 42°C for 1 h or irradiated with UV-B light (400 J/m2) and then incubated at 37°C for 1 h. Cell lysates were prepared and subjected to immunoprecipitation (IP) with an anti-JNK2 antibody. The immunoprecipitates were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblotted (IB) with an anti-Gab1 antibody. The membrane was then stripped and reprobed with an anti-JNK2 antibody. (B) Association of Gab1 mutants with JNK2. COS1 cells in 100-mm plates were cotransfected in triplicate with 2 μg of HA-JNK2 and 2 μg each of Gab1 constructs or pcDNA3.1 as indicated. Transfected cells were heat shocked at 42°C for 1 h or irradiated with UV-B light at 400 J/m2. Expression of JNK2 and Gab1 were detected by immunoblotting with anti-HA and anti-Gab1 antibodies, respectively. Association of Gab1 and JNK2 was demonstrated by immunoprecipitation of cell lystates with an anti-Flag antibody and immunoblotting with an anti-HA antibody. Activation of JNK was examined using a p-JNK antibody. (C) Rescue of defective JNK activation by wild-type and mutant Gab1. Gab1−/− fibroblast cells were transiently transfected with Flag tagged-human Gab1 and different mutants and then subjected to heat shock (42°C for 1 h) or UV-B irradiation at 400 J/m2. JNK activation was detected by immunoblotting using an anti-p-JNK antibody. Expression of exogenous Gab1 was shown by anti-Gab1 immunoblotting.
To further investigate the molecular interaction of Gab1 and JNK, we transiently cotransfected COS-1 cells with HA-JNK2 as well as wild-type Gab1 or various Gab1 mutants. These mutants include one with a deletion of the PH domain, one lacking PI3K binding sites, and one lacking Shp-2 tyrosine phosphatase binding sites. As demonstrated by the coimmunoprecipitation assay, these three Gab1 mutant proteins were also detected in association with JNK2 (Fig. 4B). Consistently, these mutants displayed modestly reduced activities with respect to wild-type Gab1 in promoting JNK activation induced by UV irradiation and a greater reduction in response to heat shock. The cotransfection experiment was also performed with Gab1−/− cells. The results in Fig. 4C show that the three mutant proteins were less active than wild-type Gab1 in rescuing the JNK activation induced by UV irradiation. Comparison of the experimental data presented in Fig. 4B and C revealed a difference in that the Gab1−/− cell mutants were not as potent as in COS1 cells for induction of the JNK pathway. This could be attributed to different cellular types, since endogenous wild-type Gab1 was expressed in COS1 cells.
Gab1 is tyrosine phosphorylated after UV irradiation.
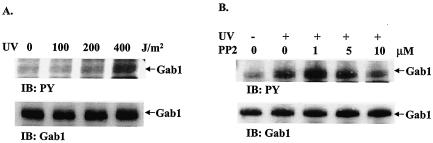
Gab1 has been shown to be highly phosphorylated on tyrosines after treatment of growth factors and cytokines, and this induced tyrosine phosphorylation of Gab1 is apparently associated with its scaffold-adapter function in promoting the ERK pathway (14, 35, 37). To understand the biochemical basis for Gab1 involvement in UV-induced JNK activation, we assessed its tyrosine phosphorylation levels. This was done by immunoprecipitation of Gab1 protein with its specific antibody followed by immunoblot analysis using an anti- PY antibody. UV irradiation of wild-type fibroblast-induced tyrosine phosphorylation of Gab1, in a dose-dependent fashion (Fig. 5A).
FIG. 5.
UV-induced tyrosine phosphorylation of Gab1 by SFK. (A) Wild-type fibroblasts were irradiated with UV-B light (0, 100, 200, or 400 J/m2) and incubated at 37°C for 20 min. Cell lysates (300 μg of total proteins) were immunoprecipitated with an anti-Gab1 antibody, resolved by SDS-PAGE, and immunoblotted (IB) with an anti-PY or an anti-Gab1 antibody. (B) Cells were pretreated with PP2 at the indicated doses for 30 min and then irradiated with UV-B light (400 J/m2). Cell lysates were immunoprecipitated and immunoblotted as above. (C) Wild-type (+/+) and Gab1−/− (−/−) cells were pretreated with PP2, exposed to UV-B light (400 J/m2), and incubated at 37°C for 1 h. Equal amounts of cell lysates (30 μg of total proteins) were immunoblotted with anti-p-JNK1/2 or anti-JNK2 antibodies. (D) SYF or SYF + c-Src cells were treated with UV-B (0, 100, 200, or 400 J/m2), and incubated at 37°C for 1 h. JNK activation was detected by immunoblotting using an anti-p-JNK antibody. The membrane was stripped and immunoblotted with anti-JNK antibody as loading control.
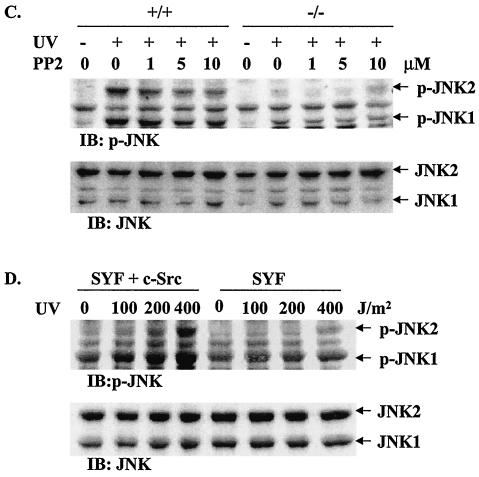
Induction of Gab1 tyrosine -phosphorylation by UV irradiation suggests an involvement of a tyrosine kinase(s) in the UV-responsive Gab1-JNK pathway. Previous experiments suggested a putative role of SFK in mediating UV-induced cellular responses (6). To explore this possibility, we pretreated fibroblasts with the selective SFK inhibitor PP2 before subjecting them to UV irradiation and then examined the tyrosine phosphorylation levels of Gab1 as well as JNK activation. PP2 at 1 to 10 μM significantly suppressed UV-induced Gab1 tyrosine phosphorylation (Fig. 5B) and also JNK activation (Fig. 5C). Consistently, significantly decreased levels of UV-induced JNK activation were detected in SYF fibroblasts devoid of the Src, Yes, and fyn kinases compared to that in SYF cells expressing the c-Src kinase (Fig. 5D).
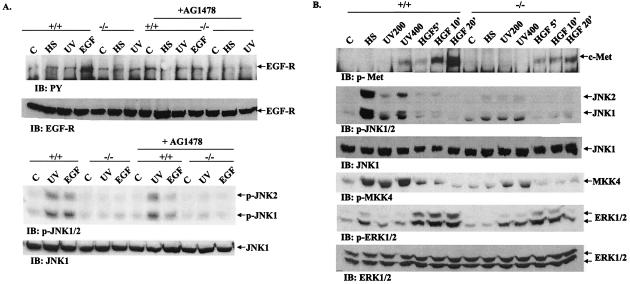
There are reports that UV exposure of fibroblasts and epithelial cells induced tyrosine phosphorylation and activation of EGFR (16, 33, 38, 39). Accordingly, we performed experiments to determine the possible role of EGFR in inducing tyrosine phosphorylation of Gab1 after UV irradiation (Fig. 6A). Although treatment of fibroblasts with EGF (50 ng/ml for 15 min) induced tyrosine phosphorylation of EGFR as detected by immunoblotting with an anti-PY antibody, neither UV exposure nor heat shock resulted in detectable EGFR phosphorylation on tyrosine (Fig. 6A). Furthermore, pretreatment of cells with a specific inhibitor of EGFR (AG1478), while significantly suppressing the activation of JNK by EGF, did not have a detectable effect on UV-induced JNK activation under otherwise identical conditions. Therefore, it is unlikely that EGF-R plays a role in JNK activation by UV irradiation in mouse embryonic fibroblasts under our experimental condition.
FIG. 6.
Possible involvement of receptor PTKs in UV-induced JNK activation. (A) To investigate the role of EGFR, cells were pretreated with the EGFR inhibitor AG1478 (200 nM) for 20 min or left untreated; they were then stimulated by heat shock (42°C for 1 h), UV-B irradiation (400 J/m2), or EGF (50 ng/ml, 15 min). Cell lystates were prepared and immunoblotted (IB) with an anti-PY antibody. The membrane was stripped and then reprobed with an anti-EGFR antibody. JNK activity was detected by immunoblotting with an antibody specific for p-JNK. Equal loading of samples on each lane was indicated by reprobing the same membrane with an anti-JNK1 antibody. (B) To explore the putative role of c-Met, wild-type (+/+) and Gab1−/− (−/−) cells were subjected to heat shock (42°C for 1 h), UV-B irradiation (200 or 400 J/m2), or HGF (50 ng/ml) for 5, 10, or 20 min. Cell lysates were prepared and subjected to immunoblotting with the indicated antibodies.
We also examined the putative role of c-Met, the receptor for HGF, in the UV-induced Gab1-JNK pathway. Previous reports showed that Gab1 is involved in JNK activation and cell transformation by an oncogenic form of c-Met, Tpr/Met (9, 24). In contrast to the results of with EGFR, UV irradiation induced tyrosine phosphorylation of c-Met in wild-type cells that was correlated to induction of JNK activity. Gab1−/− cells exhibited reduced JNK activation in response to UV irradiation, and no c-Met phosphorylation was detected (Fig. 6B). However, JNK activation by heat shock occurred in the absence of c-Met tyrosine phosphorylation (Fig. 6B), suggesting a distinct mechanism for signals elicited by UV and heat shock, consistent with a previous report (1). Tyrosine phosphorylation of c-Met is apparently not sufficient to induce the JNK pathway, since no JNK activation was detected in cells after HGF stimulation despite a significant activation of ERK under the same conditions (Fig. 6B). Therefore, it remains to be determined whether and how c-Met participates in UV-induced JNK pathway. Regardless, SFK clearly plays a role in UV-induced Gab1-JNK activation, and it will be interesting to determine whether SFK is directly activated by UV light or whether transmembrane protein tyrosine kinases (PTKs) are also involved in the process.
Gab1−/− cells display decreased sensitivity to UV-induced apoptotic effect.
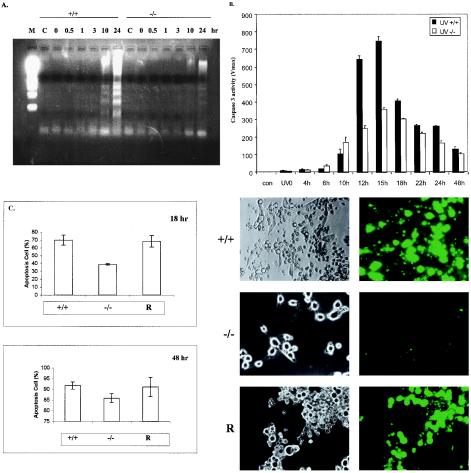
The experiments described above demonstrate a role for Gab1 in activating the JNK pathway induced by UV exposure, apparently involving SFKs. To explore the biological significance of JNK activation in mammalian fibroblasts, we compared wild-type and Gab1−/− cells in their response to apoptotic effect of UV irradiation. Cells were subjected to UV-B irradiation (400 J/m2) and then incubated for 0, 0.5, 1, 3, 10, or 24 h before DNA was extracted for an assay of fragmentation (laddering), which is indicative of cell apoptosis. Significant fragmentation of genomic DNA was detected after 10 and 24 h of incubation of wild-type cells following UV irradiation (Fig. 7A). However, the level of DNA fragmentation was significantly decreased in Gab1−/− cells. We also measured the induction of caspase 3 activity by UV irradiation. Cells were exposed to UV and incubated for different periods as indicated. Cell extracts were prepared for a caspase 3 activity assay. As illustrated in Fig. 7B, significantly higher levels of caspase 3 activity were detected in wild-type than in Gab1−/− cells, consistent with the DNA laddering results shown in Fig. 7A.
FIG. 7.
Decreased sensitivity of Gab1−/− cells to UV-induced apoptosis. Fibroblasts were stimulated by UV-B irradiation (400 J/m2) and incubated at 37°C for the indicated times. (A) Fragmented DNA was extracted and analyzed by electrophoresis on a 1.5% agarose gel. M, molecular marker; C, control. (B) Caspase 3 activity was determined as the rate of product formation obtained from the linear portion of the reaction curves within the first 10% of substrate depletion. Values represent the mean and standard deviation of three independent experiments. (C) Wild-type, Gab1−/−, and Gab1-transfected Gab1−/− cells (R) were irradiated with UV-B (400 J/m2). After incubation at 37°C for 18 or 48 h, cell apoptosis was evaluated using the YOPRO-1 staining kit. Apoptotic cells were counted under fluorescent optics, and the total cell number in the fields was counted under phase optics. The percentage of apoptotic cells was calculated, and the data were averaged from three samples (means and standard deviations are shown). Shown on the right are representative microscopic fields of cells at 48 h under phase optics (left) and fluorescent optics (right). +/+, wild-type cells; −/−, Gab1−/− cells.
To define the role of Gab1 in mediating UV-induced apoptosis, we transfected Gab1−/− cells with a Gab1 expression construct and then examined cell apoptosis after UV irradiation by YOPRO-1 staining (Fig. 7C). Using this assay kit, we obtained similar data for wild-type and Gab1−/− cells as the results of DNA laddering and caspase 3 activation. Notably, reintroduction of Gab1 into Gab1−/− cells resulted in a significant increase in cell death induced by UV irradiation (Fig. 7C). Therefore, Gab1 appears to be required to mediate UV-stimulated JNK activation and cell apoptosis.
DISCUSSION
JNK was identified almost 10 years ago as a group of MAP kinases that are activated by UV exposure and other external stress (5, 13) and has been implicated in a variety of cellular activities (18, 32). However, the molecular mechanism for UV activation of the JNK pathway remains to be elucidated. We present evidence in this report that Gab1, a scaffold protein previously known to be involved in transmitting signals from cytokine and growth factor receptors, plays an important role in mediating UV-induced JNK activation in mouse embryonic fibroblasts.
Without catalytic activity, Gab1 acts to organize cytoplasmic proteins and enzymes into specific signaling complexes downstream of a variety of cell surface receptors for growth factors, cytokines, antigens, and extracellular matrixes. In this regard, it is very interesting to detect a protein complex that contains both Gab1 and JNK2. It is not known at this stage whether Gab1 and JNK directly interact with each other. Presumably, Gab1 serves as a molecular link between upstream UV-activated SFK and the components in the JNK pathway. The detection of tyrosine phosphorylation of Gab1 in cells exposed to UV light suggests that Gab1 acts in the UV-responsive pathway in a simiar fashion to its action in signal relay for ligand-activated cell surface receptors. Removal of tyrosyl residues engaged in binding PI3K or Shp-2 had inhibitory effects on JNK activation by UV irradiation. This observation suggests possible participation of these Gab1-interacting proteins in the JNK pathway. Similarly, the decreased rescue capacity of the pleckstrin homology domain deletion mutant also indicate that the membrane localization of the Gab1 complex in this signaling scheme is significant. Based on the data presented in this paper, it seems unlikely that a single tyrosyl residue on Gab1 is solely responsible for activation of JNK; rather, several signals may be funneled from the Gab1-associated proteins into the JNK pathway.
In recognizing the significance of tyrosine phosphorylation induced on Gab1 by UV exposure, the critical issue was to determine which tyrosine kinase(s) is responsible for this phosphorylation event. In previous experiments, SFK was implicated in the cellular response to UV irradiation (6), and it has also been reported that SFK mediates the tyrosine phosphorylation of Gab1 elicited by the activated EGF receptor, cell adhesion as well as osmotic shock (4, 20, 36). We evaluated the putative role of SFK in mediating UV-induced Gab1-JNK activation by using a selective chemical inhibitor, PP2, and SYF cells lacking SFK expression (Fig. 5). This experiment demonstrates that SFKs do indeed participate in the tyrosine phosphorylation of Gab1 as well as in JNK activation after UV irradiation.
Since several groups have reported the UV-stimulated tyrosine phosphorylation of EGFR (16, 33, 38, 39), we reasonably thought about a model of as EGFR/Gab1/JNK signaling cascade and assessed the phosphorylation status of EGFR following UV irradiation, heat shock, or EGF stimulation. To our surprise, neither UV exposure nor heat shock induced tyrosine phosphorylation of EGFR, although EGF treatment did so efficiently in the same type of cells (Fig. 6). It should be pointed out that the UV irradiation dose (400 J/m2) used here induced a dramatic activation of JNK (Fig. 1) and tyrosine phosphorylation of Gab1 as well as c-Met in these cells (Fig. 5 and 6). Therefore it is unlikely that EGFR is responsible for the tyrosine phosphorylation of Gab1 and that EGFR could be involved in the UV-induced JNK activation observed in this study. Strong support of this proposal is provided by the observation that AG1478, a selective EGFR tyrosine kinase inhibitor, did not affect UV-induced JNK activation while clearly showing an inhibitory effect on EGF stimulation of JNK activity (Fig. 6A). Consistent with our results, Oksvold et al. reported recently that UV irradiation did not induce dimerization or tyrosine phosphorylation of EGFR but, rather, induced receptor internalization and endosome arrest (31). This is at odds with previous reports from several groups, and further investigation may be needed to clarify this controversial issue.
Although c-Met is normally expressed at low levels in normal fibroblasts, immortalization of fibroblast cell lines by simiar virus 40 large T antigen usually resulted in increased expression of c-Met. UV irradiation induced significant tyrosine phosphorylation of c-Met at doses and under conditions in which EGFR phosphorylation was not detectable. Treatment of embryonic fibroblasts with HGF also induced significant tyrosine phosphorylation of c-Met and activation of ERK. However, JNK activation was not detectable under the same conditions (Fig. 6B). Previous reports suggest an antiapoptotic effect of the HGF/c-Met pathway, and HGF treatment of NIH 3T3 cells or keratinocytes inhibited UV-induced apoptosis (28, 43). The data shown here may suggest that tyrosine phosphorylation and presumably activation of c-Met induced by UV or HGF lead to different consequences, particularly the activation of different MAP kinase pathways. HGF treatment induced dramatic activation of ERK with no effect on the JNK pathway, while UV irradiation stimulated the activity of JNK more than of ERK. It remains to be determined whether and how c-Met operates in activating the Gab1-JNK pathway during the cell response to UV light. The data reported here suggest a positive role of Gab1 in promoting JNK activation and also cell apoptosis in response to UV irradiation. Consistent with our results, Fan et al. showed that overexpression of Gab1 in MDCK cells down-regulated HGF/c-Met signaling for cell survival and DNA repair (7). Gab1 might act to redirect c-Met signals through PI3K away from a c-Akt/Pak1-regulated cell survival.
Murine fibroblasts devoid of all the functional JNK genes are resistant to UV-induced and cytochrome c-dependent cell death (40), suggesting a requirement of the JNK signaling pathway for stress-induced release of mitochondrial cytochrome c and apoptosis. A recent report indicates that proapoptotic members of the Bax protein subfamily act downstream of JNK in stress-induced apoptosis (25). Data reported in this paper identify Gab1 as an important player upstream of JNK in this pathway. While this paper was under revision, Holgado-Madruga and Wong reported a role of Gab1 in oxidative stress signaling by showing that Gab1 is required for H2O2-induced JNK activation (15).
Acknowledgments
We thank Robert Abraham, Morag Park, George Vande Woude, Masahiko Hibi, and Toshio Hirano for cell lines, reagents, or helpful discussion.
This work was supported by a postdoctoral fellowship award (5FB-0087) from the Breast Cancer Research Program of California (to Y.S.), by NIH grants R01GM53660 and R01HL66208 (to G.-S.F.), and by NNSFC (39928009).
REFERENCES
- 1.Adler, V., A. Schaffer, J. Kim, L. Dolan, and Z. Ronai. 1995. UV irradiation and heat shock mediate JNK activation via alternate pathways. J. Biol. Chem. 270:26071-26077. [DOI] [PubMed] [Google Scholar]
- 2.Cunnick, J. M., J. F. Dorsey, T. Munoz-Antonia, L. Mei, and J. Wu. 2000. Requirement of SHP2 binding to Grb2-associated binder-1 for mitogen-activated protein kinase activation in response to lysophosphatidic acid and epidermal growth factor. J. Biol. Chem. 275:13842-13848. [DOI] [PubMed] [Google Scholar]
- 3.Cunnick, J. M., L. Mei, C. A. Doupnik, and J. Wu. 2001. Phosphotyrosines 627 and 659 of Gab1 constitute a bisphosphoryl tyrosine-based activation motif (BTAM) conferring binding and activation of SHP2. J. Biol. Chem. 276:24380-24387. [DOI] [PubMed] [Google Scholar]
- 4.Daub, H., C. Wallasch, A. Lankenau, A. Herrlich, and A. Ullrich. 1997. Signal characteristics of G protein-transactivated EGF receptor. EMBO J. 16:7032-7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Derijard, B., M. Hibi, I. H. Wu, T. Barrett, B. Su, T. Deng, M. Karin, and R. J. Davis. 1994. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 76:1025-1037. [DOI] [PubMed] [Google Scholar]
- 6.Devary, Y., R. A. Gottlieb, T. Smeal, and M. Karin. 1992. The mammalian ultraviolet response is triggered by activation of Src tyrosine kinases. Cell 71:1081-1091. [DOI] [PubMed] [Google Scholar]
- 7.Fan, S., Y. X. Ma, M. Gao, R. Q. Yuan, Q. Meng, I. D. Goldberg, and E. M. Rosen. 2001. The multisubstrate adapter Gab1 regulates hepatocyte growth factor (scatter factor)-c-Met signaling for cell survival and DNA repair. Mol. Cell. Biol. 21:4968-4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedberg, E. C. (ed.). 1995. DNA repair and mutagenesis. ASM Press, Washington, D.C.
- 9.Garcia-Guzman, M., F. Dolfi, K. Zeh, and K. Vuori. 1999. Met-induced JNK activation is mediated by the adapter protein Crk and correlates with the Gab1-Crk signaling complex formation. Oncogene 18:7775-7786. [DOI] [PubMed] [Google Scholar]
- 10.Gu, H., and B. G. Neel. 2003. The ‘Gab’ in signal transduction. Trends Cell Biol. 13:122-130. [DOI] [PubMed] [Google Scholar]
- 11.Gu, H., J. C. Pratt, S. J. Burakoff, and B. G. Neel. 1998. Cloning of p97/Gab2, the major SHP2-binding protein in hematopoietic cells, reveals a novel pathway for cytokine-induced gene activation. Mol. Cell 2:729-740. [DOI] [PubMed] [Google Scholar]
- 12.Herrlich, P., H. Ponta, and H. J. Rahmsdorf. 1992. DNA damage-induced gene expression: signal transduction and relation to growth factor signaling. Rev. Physiol. Biochem. Pharmacol. 119:187-223. [DOI] [PubMed] [Google Scholar]
- 13.Hibi, M., A. Lin, T. Smeal, A. Minden, and M. Karin. 1993. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 7:2135-2148. [DOI] [PubMed] [Google Scholar]
- 14.Holgado-Madruga, M., D. R. Emlet, D. K. Moscatello, A. K. Godwin, and A. J. Wong. 1996. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature 379:560-564. [DOI] [PubMed] [Google Scholar]
- 15.Holgado-Madruga, M., and A. J. Wong. 2003. Gab1 is an integrator of cell death versus cell survival signals in oxidative stress. Mol. Cell. Biol. 23:4471-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang, R. P., J. X. Wu, Y. Fan, and E. D. Adamson. 1996. UV activates growth factor receptors via reactive oxygen intermediates. J. Cell. Biol. 133:211-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Idziorek, T., J. Estaquier, F. De Bels, and J. C. Ameisen. 1995. YOPRO-1 permits cytofluorometric analysis of programmed cell death (apoptosis) without interfering with cell viability. J. Immunol. Methods 185:249-258. [DOI] [PubMed] [Google Scholar]
- 18.Ip, Y. T., and R. J. Davis. 1998. Signal transduction by the c-Jun N-terminal kinase (JNK)—from inflammation to development. Curr. Opin. Cell Biol. 10:205-219. [DOI] [PubMed] [Google Scholar]
- 19.Itoh, M., Y. Yoshida, K. Nishida, M. Narimatsu, M. Hibi, and T. Hirano. 2000. Role of Gab1 in heart, placenta, and skin development and growth factor- and cytokine-induced extracellular signal-regulated kinase mitogen-activated protein kinase activation. Mol. Cell. Biol. 20:3695-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janez, A., D. S. Worrall, T. Imamura, P. M. Sharma, and J. M. Olefsky. 2000. The osmotic shock-induced glucose transport pathway in 3T3-L1 adipocytes is mediated by gab-1 and requires Gab-1-associated phosphatidylinositol 3-kinase activity for full activation. J. Biol. Chem. 275:26870-26876. [DOI] [PubMed] [Google Scholar]
- 21.Kitagawa, D., S. Tanemura, S. Ohata, N. Shimizu, J. Seo, G. Nishitai, T. Watanabe, K. Nakagawa, H. Kishimoto, T. Wada, T. Tezuka, T. Yamamoto, H. Nishina, and T. Katada. 2002. Activation of extracellular signal-regulated kinase by ultraviolet is mediated through Src-dependent epidermal growth factor receptor phosphorylation. Its implication in an anti-apoptotic function. J. Biol. Chem. 277:366-371. [DOI] [PubMed] [Google Scholar]
- 22.Klinghoffer, R. A., C. Sachsenmaier, J. A. Cooper, and P. Soriano. 1999. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 18:2459-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuan, C. Y., D. D. Yang, D. R. Samanta Roy, R. J. Davis, P. Rakic, and R. A. Flavell. 1999. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron 22:667-676. [DOI] [PubMed] [Google Scholar]
- 24.Lamorte, L., D. M. Kamikura, and M. Park. 2000. A switch from p130Cas/Crk to Gab1/Crk signaling correlates with anchorage independent growth and JNK activation in cells transformed by the Met receptor oncoprotein. Oncogene 19:5973-5981. [DOI] [PubMed] [Google Scholar]
- 25.Lei, K., A. Nimnual, W. X. Zong, N. J. Kennedy, R. A. Flavell, C. B. Thompson, D. Bar-Sagi, and R. J. Davis. 2002. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH2-terminal kinase. Mol. Cell. Biol. 22:4929-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lenczowski, J. M., L. Dominguez, A. M. Eder, L. B. King, C. M. Zacharchuk, and J. D. Ashwell. 1997. Lack of a role for Jun kinase and AP-1 in Fas-induced apoptosis. Mol. Cell. Biol. 17:170-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lynch, K., G. Fernandez, A. Pappalardo, and J. J. Peluso. 2000. Basic fibroblast growth factor inhibits apoptosis of spontaneously immortalized granulosa cells by regulating intracellular free calcium levels through a protein kinase Cδ-dependent pathway. Endocrinology 141:4209-4217. [DOI] [PubMed] [Google Scholar]
- 28.Mildner, M., L. Eckhart, B. Lengauer, and E. Tschachler. 2002. Hepatocyte growth factor/scatter factor inhibits UVB-induced apoptosis of human keratinocytes but not of keratinocyte-derived cell lines via the phosphatidylinositol 3-kinase/AKT pathway. J. Biol. Chem. 277:14146-14152. [DOI] [PubMed] [Google Scholar]
- 29.Nishida, K., Y. Yoshida, M. Itoh, T. Fukada, T. Ohtani, T. Shirogane, T. Atsumi, M. Takahashi-Tezuka, K. Ishihara, M. Hibi, and T. Hirano. 1999. Gab-family adapter proteins act downstream of cytokine and growth factor receptors and T- and B-cell antigen receptors. Blood 93:1809-1816. [PubMed] [Google Scholar]
- 30.Nishina, H., K. D. Fischer, L. Radvanyi, A. Shahinian, R. Hakem, E. A. Rubie, A. Bernstein, T. W. Mak, J. R. Woodgett, and J. M. Penninger. 1997. Stress-signalling kinase Sek1 protects thymocytes from apoptosis mediated by CD95 and CD3. Nature 385:350-353. [DOI] [PubMed] [Google Scholar]
- 31.Oksvold, M. P., H. S. Huitfeldt, A. C. Ostvold, and E. Skarpen. 2002. UV induces tyrosine kinase-independent internalisation and endosome arrest of the EGF receptor. J. Cell Sci. 115:793-803. [DOI] [PubMed] [Google Scholar]
- 32.Robinson, M. J., and M. H. Cobb. 1997. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 9:180-186. [DOI] [PubMed] [Google Scholar]
- 33.Rosette, C., and M. Karin. 1996. Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science 274:1194-1197. [DOI] [PubMed] [Google Scholar]
- 34.Sachs, M., H. Brohmann, D. Zechner, T. Muller, J. Hulsken, I. Walther, U. Schaeper, C. Birchmeier, and W. Birchmeier. 2000. Essential role of Gab1 for signaling by the c-Met receptor in vivo. J. Cell Biol. 150:1375-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi, Z. Q., D. H. Yu, M. Park, M. Marshall, and G. S. Feng. 2000. Molecular mechanism for the Shp-2 tyrosine phosphatase function in promoting growth factor stimulation of Erk activity. Mol. Cell Biol. 20:1526-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shinohara, M., A. Kodama, T. Matozaki, A. Fukuhara, K. Tachibana, H. Nakanishi, and Y. Takai. 2001. Roles of cell-cell adhesion-dependent tyrosine phosphorylation of Gab-1. J. Biol. Chem. 276:18941-18946. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi-Tezuka, M., Y. Yoshida, T. Fukada, T. Ohtani, Y. Yamanaka, K. Nishida, K. Nakajima, M. Hibi, and T. Hirano. 1998. Gab1 acts as an adapter molecule linking the cytokine receptor gp130 to ERK mitogen-activated protein kinase. Mol. Cell. Biol. 18:4109-4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wan, Y. S., Z. Q. Wang, J. Voorhees, and G. Fisher. 2001. EGF receptor crosstalks with cytokine receptors leading to the activation of c-Jun kinase in response to UV irradiation in human keratinocytes. Cell Signalling 13:139-144. [DOI] [PubMed] [Google Scholar]
- 39.Warmuth, I., Y. Harth, M. S. Matsui, N. Wang, and V. A. DeLeo. 1994. Ultraviolet radiation induces phosphorylation of the epidermal growth factor receptor. Cancer Res. 54:374-376. [PubMed] [Google Scholar]
- 40.Weston, C. R., and R. J. Davis. 2002. The JNK signal transduction pathway. Curr. Opin. Genet. Dev. 12:14-21. [DOI] [PubMed] [Google Scholar]
- 41.Whitmarsh, A. J., and R. J. Davis. 1996. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 74:589-607. [DOI] [PubMed] [Google Scholar]
- 42.Wolf, I., B. J. Jenkins, Y. Liu, M. Seiffert, J. M. Custodio, P. Young, and L. R. Rohrschneider. 2002. Gab3, a new DOS/Gab family member, facilitates macrophage differentiation. Mol. Cell. Biol. 22:231-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao, G. H., M. Jeffers, A. Bellacosa, Y. Mitsuuchi, G. F. Vande Woude, and J. R. Testa. 2001. Anti-apoptotic signaling by hepatocyte growth factor/Met via the phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase pathways. Proc. Natl. Acad. Sci. USA 98:247-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao, C., D. H. Yu, R. Shen, and G. S. Feng. 1999. Gab2, a new pleckstrin homology domain-containing adapter protein, acts to uncouple signaling from ERK kinase to Elk-1. J. Biol. Chem. 274:19649-19654. [DOI] [PubMed] [Google Scholar]