Abstract
Background
Periprosthetic joint infection (PJI) is a devastating complication after total joint arthroplasty. Lack of confirmation of an infecting organism poses a challenge with regard to the selection of an appropriate antibiotic agent and surgical treatment. It is unclear whether patients with negative cultures presumed to have infections achieve similar rates of infection-free survival as those with positive cultures.
Questions/purposes
The purposes of this study were (1) to report the infection control rates using irrigation and débridement and two-stage exchange for treatment of culture-negative PJIs; and (2) to compare infection control rates in culture-negative cases with those in culture-positive cases.
Methods
We retrospectively reviewed 55 patients with culture-negative PJI treated between 2000 and 2007. We compared the infection-free survival rate in the culture-negative patients with that of 295 culture-positive cases of PJI.
Results
Overall infection control rate in culture-negative cases was 73% at minimum 1-year followup (mean, 47 months; range, 12–119 months). We found similar infection control rates in culture-negative and culture-positive PJI. Infection-free survival rates in both groups were highest after two-stage exchange arthroplasty and postoperative vancomycin therapy.
Conclusion
Our observations suggest aggressive two-stage exchange arthroplasty and postoperative parenteral vancomycin therapy in patients with culture-negative PJI achieves similar rates of infection-free survival as with patients having culture-positive PJI.
Level of Evidence
Level III, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Throughout many decades of total joint arthroplasty (TJA) procedures, periprosthetic joint infection (PJI) has remained one of the most devastating postoperative complications. Infection rates after primary hip and knee arthroplasty occur in approximately 0.3% to 2.0% after primary TJA [9, 11, 18, 20–22] and from 1% to 9% after revision arthroplasty [5, 8, 17]. Not only is PJI associated with high morbidity, but the economic burden is high [11].
Accurate diagnosis of PJI is essential and often involves withholding antibiotic therapy in hopes of isolating an organism from a preoperative joint aspiration or intraoperative tissue cultures. Determining the causative organism then allows tailored local and systemic antibiotic therapy. Despite extensive efforts, the cultures often have a high false-negative rate despite adequate clinical, radiographic, and surgical suspicion for PJI. Rates of culture-negative PJI reportedly occur in 7% to 9.5% of all PJIs [3, 13].
Treatment of PJI depends on various factors such as patient presentation, patient comorbidities, prosthetic factors, pathogen factors, and physician judgment. Two-stage revision with resection of the prosthetic joint combined with implantation of an antibiotic-impregnated polymethylmethacrylate cement spacer in the first stage, followed by later prosthesis reimplantation, controls the infection in culture-positive cases in 80% to 90% of patients [2, 4, 7]. However, in a culture-negative PJI, the effectiveness of a local intraarticular antimicrobial spacer is unclear. Furthermore, choosing appropriate antibiotic therapy is difficult. Therefore, it is important to understand the rates of infection control of various surgical interventions and determine whether the antibiotic choices in culture-negative PJIs control the infections.
The purposes of this study were (1) to report the infection control rates using irrigation and débridement (I&D) and two-stage exchange for treatment of culture-negative PJIs; and (2) to compare infection control rates in culture-negative cases with those in culture-positive cases.
Patients and Methods
We retrospectively reviewed 462 consecutive cases of suspected PJIs of the knee and hip at our institution between January 2000 and December 2007. Patients were identified using a prospectively maintained institutional database, which includes billing data, clinical microbiology data, and a TJA registry that included outcomes and complications. Criteria for defining PJI were based on the new diagnostic criteria proposed by the MSIS work group in 2011 [19]. Because intraoperative histopathology was not used at our institution, we considered cases infected if there was a pathogen isolated from at least two separate cultures (tissue or fluid) obtained from the infected joint, if there was presence of a sinus tract communicating with the prosthesis, or if three of the following five were present: (1) an abnormal preoperative C-reactive protein (CRP) of > 0.8 mg/dL or erythrocyte sedimentation rate (ESR) of > 30 mm/hr; (2) abnormal joint aspirate with a positive finding of > 10,700 white blood cell count (WBC)/μL in acute infections or > 1760 WBC/μL in chronic infections; (3) > 89% neutrophils in acute infections or > 73% neutrophils in chronic infections; (4) intraoperative purulence; or (5) at least one positive culture.
Fifty-five of the 462 total cases (11.9%) had a presumed culture-negative PJI, whereas 407 had positive cultures. Seven patients with culture-negative and 112 patients with culture-positive infections had < 1 year of followup available and were excluded from the study. Of the 48 remaining culture-negative patients, there were 19 males and 29 females (21 hips and 28 knees; one patient had bilateral knee infections). Mean age was 63.7 years (range, 39–85 years), and the average body mass index (BMI) was 31.8 kg/m2 (range, 18.6–58.7 kg/m2). Minimum followup was 12 months (average, 47 months; range, 12–119 months). The comparison group of culture-positive PJIs included 295 cases (250 patients) treated between January 2000 and December 2007 with a minimum 1-year followup. The mean of the 250 patients age was 66.7 years (range, 18–89 years), and the average BMI was 32.0 kg/m2. Minimum followup was 12 months (average, 33.2 months; range, 12–125.7 months). No patients were recalled specifically for this study; all data were obtained from medical records and followup telephone calls in 12 of the culture-negative patients and 38 of the culture-positive patients. We had prior institutional review board approval.
Age, sex, BMI, Charlson Comorbidity Index, and time between index surgery and initial treatment for infection were similar between the culture-negative and culture-positive patients (Table 1).
Table 1.
Comparison of demographic and treatment factors between culture-negative and -positive patients
| Variable | Culture-positive (n = 250 patients) | Culture-negative (n = 48 patients) | p value |
|---|---|---|---|
| Age (years) | 66.7 (95% CI; 65.3–68.2) | 63.7 (95% CI; 60.0–67.3) | 0.106 |
| Sex | 0.197 | ||
| Male | 122 | 19 | |
| Female | 128 | 29 | |
| Body mass index (kg/m2) | 32.0 (95% CI, 30.7–33.3) | 31.8 (95% CI, 28.9–34.6) | 0.887 |
| Charlson Comorbidity Index | 2.5 (95% CI, 2.3–2.7) | 2.1 (95% CI, 1.7–2.5) | 0.079 |
| Time to initial treatment (months) | 30.0 (95% CI, 24.7–35.4) | 30.8 (95% CI, 13.6– 47.7) | 0.957 |
CI = confidence interval.
We reviewed the culture results of all treated patients. The culture was considered positive if the pathogen was isolated on solid media by the microbiology laboratory; light growth or very light growth was defined as a positive culture finding in this study. According to standard practice at our institution, every specimen was sent for aerobic/anaerobic, fungal, and acid-fast bacilli culture. Both solid media and broth were used. In all treated cases of suspected infections, two or more intraoperative specimens (based on the practices of the individual surgeons) were obtained from areas representative of inflammation during each surgical procedure. In 10 samples, culture was positive in broth only. However, in each of these cases, a positive culture from a different sample isolated the same organism on solid media. Therefore, these broth cultures were considered positive.
In the 48 culture-negative patients, 33 patients initially underwent planned two-stage exchange, six of whom were never reimplanted. Twelve patients initially underwent I&D with component retention. The polyethylene was changed in 11 of 12 cases. In one case, the polyethylene was washed with Betadine and reinserted. Another three patients underwent one-stage exchange. Although a standardized protocol was not used to determine two-stage exchange versus I&D versus single-stage exchange, all surgeons were from a single institution and used similar principles in determining treatment of choice. In general, the decision to use I&D therapy was based on a combination of factors, including acute postoperative infections within 6 weeks after the index procedure, presence of nonresistant organisms, and a relatively healthy patient based on each clinician’s judgment. Two-stage exchange, which includes resection of the component, débridement of the joint, and implantation of an antibiotic-impregnated cement spacer followed by reimplantation surgery, was generally used for chronic infections. One-stage exchange was performed in three patients who had substantial medical comorbidities, suboptimal control of chronic systemic conditions, and the surgeon presumed they would not likely tolerate a two-stage procedure.
Data on postoperative antibiotic administration were recorded. Thirty-nine patients completed at least 4 weeks of intravenous (IV) vancomycin administration as suggested by an infectious disease consultant. Two of these patients received combination antibiotics; one received IV ciprofloxacin along with IV vancomycin and the other received IV doxycycline with IV vancomycin. Additionally, of the 39 patients who received IV vancomycin, four patients also received oral ciprofloxacin every 12 hours, one received oral rifampin, one received IV ceftriaxone, and one received oral vancomycin along with IV vancomycin therapy. Of the patients who did not receive vancomycin, four patients received ceftriaxone, one received ceftazidime, one received daptomycin and oral ciprofloxacin, one received IV nafcillin, and one patient did not receive IV antibiotics. This patient was suspected to have PJI and received two-stage exchange arthroplasty, but antibiotics were stopped when the patient’s cultures all came back negative per infectious disease doctors. Nevertheless, the patient had met our definition of infection based on serologic, synovial fluid, and intraoperative findings. This patient eventually failed 2 years later and required repeat two-stage exchange arthroplasty.
Patients were routinely followed up in the office at 6 weeks postoperatively, 3 months, 6 months, and annually thereafter. If there was concern for recurrent infection, patients would be seen back earlier. At each followup, surgeons evaluated serum markers of infection including ESR and CRP, and radiographs would be taken. Joints would be aspirated if there was concern for infection. Patient hospital and outpatient charts were reviewed to determine the outcome of treatment and any postoperative complications. Demographics and comorbidity data were also collected. Infection control was defined as the preservation of the prosthesis in the index joint without any further surgery related to infection.
Chi-square, descriptive, and Student’s t-test analyses were used to compare demographics and comorbidities between the culture-negative and culture-positive groups. Kaplan-Meier survival analyses were performed to compare infection-free survival rates of two-stage and I&D treatments between the culture-negative and culture-positive cases. Log-rank tests were used to determine differences in infection-free survival between the two groups. Statistical analyses were performed using SPSS Version 20 (IBM, Armonk, NY, USA).
Results
Overall infection control rate in culture-negative cases was 73% at minimum 1-year followup (mean, 47 months; range, 12–119 months). Six of the 12 patients who underwent I&D as their initial treatment required repeat surgery for infection an average of 4.8 months after I&D (range, 1–13 months) (Table 2). Of the 33 patients who underwent planned two-stage exchange, three had failed previous I&D. Six of these 33 patients never underwent reimplantation as a result of extensive comorbidities. In the remaining 27 patients (eight of 27 patients), the three patients who underwent one-stage resection were infection-free at an average of 73 months (range, 32–119 months). Of the six culture-negative patients who failed I&D treatment, three patients underwent an additional two-stage exchange, two patients had spacer implantation only, and one patient underwent above-knee amputation. Of the eight culture-negative patients who failed two-stage exchange treatment, five patients had repeat two-stage exchange, two patients underwent I&D, and one underwent one-stage exchange. Complications other than infection included a periprosthetic fracture treated with open reduction and internal fixation 6 months postoperatively in the two-stage exchange group. Antibiotic choices in the cement spacers included vancomycin and tobramycin in 28 of 33 patients who underwent spacer implantation. Information on the remaining five spacers was unavailable. Thirty-nine patients were treated with postoperative parenteral vancomycin. Eleven (28.2%) failed treatment and required repeat surgical intervention.
Table 2.
Culture-negative versus culture-positive treatment results.
| Treatment | Culture-positive (n = 295 cases) | Culture-negative (n = 48 cases) |
|---|---|---|
| I&D | 85 | 12 |
| Infection-free survival (%) | 38 (44.7%) | 6 (50%) |
| Reinfection (%) | 47 (55.3%) | 6 (50%) |
| Planned two‐stage exchange | 205 | 33 |
| After failed I&D (%) | 33 (16.1%) | 3 (9.1%) |
| Resection as first treatment (%) | 172 (83.9%) | 30 (90.9%) |
| Never reimplanted (percent of resected) | 54 (26.3%) | 6 (18.2%) |
| Reimplanted (percent of resected) | 151 (73.7%) | 27 (81.8%) |
| Infection-free survival (%) | 118 (78.1%) | 19 (70.4%) |
| Reinfection (%) | 33 (21.9%) | 8 (29.6%) |
| Other treatment | ||
| Fusion | 1 | 0 |
| One‐stage exchange | 2 | 3 |
| Amputation | 1 | 1 (after spacer) |
| Total femur Prostalac | 1 | 0 |
I&D = irrigation and débridement.
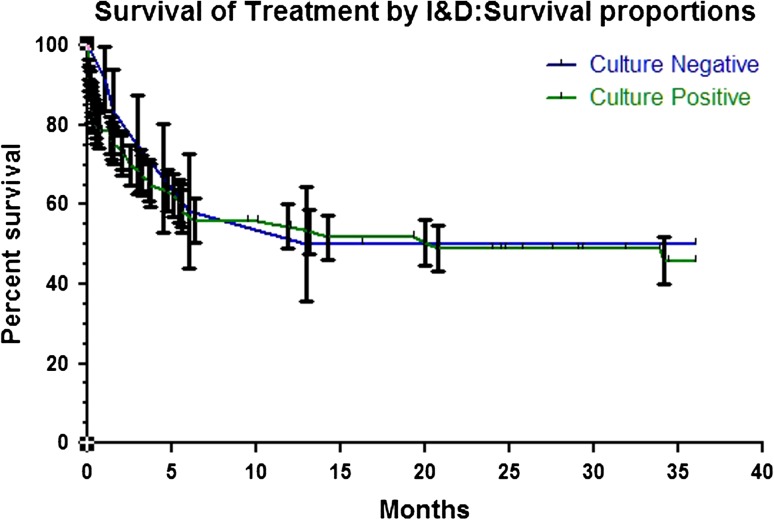
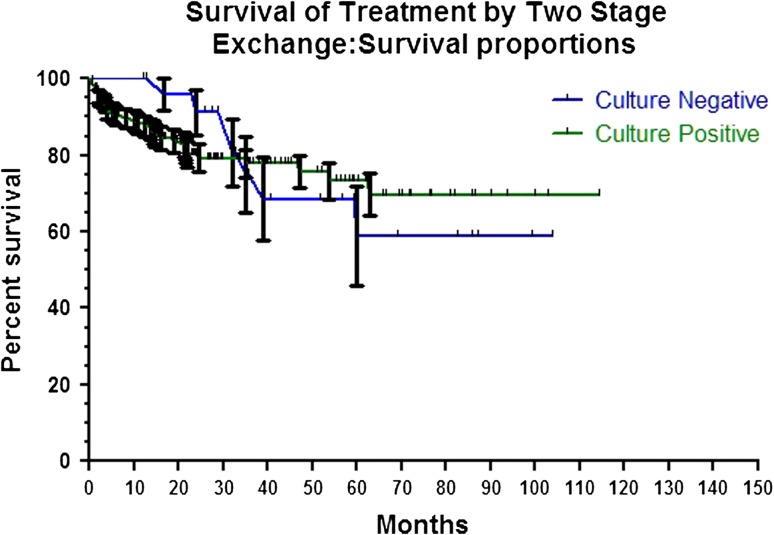
The overall infection control rate was similar (p = 1.00) in culture-negative and culture-positive cases, 73% versus 73%, respectively. We found no difference between culture-negative and -positive implant survival rates after initial I&D (p = 0.73) (Fig. 1) and two-stage exchange arthroplasty (p = 0.96) (Fig. 2).
Fig. 1.
Graph of Kaplan-Meier survival curve demonstrating similar (p = 0.73) infection-free survival rates of culture-negative and culture-positive cases after treatment by I&D.
Fig. 2.
Graph of Kaplan-Meier survival curve demonstrating similar (p = 0.96) infection-free survival rates of culture-negative and culture-positive cases after treatment by two-stage exchange arthroplasty.
Discussion
The diagnosis, risk factors, treatment options, and clinical outcomes of PJI have been extensively discussed in the past two decades. However, data on culture-negative PJIs are relatively rare in the literature. Malekzadeh et al. [13] reported the largest series of culture-negative PJI, which includes 135 cases (10.6% incidence) within 16 years. In their series, the free of treatment failure rate of culture-negative PJI at 5 years after I&D and two-stage exchange was 79% and 78%, respectively, compared with 74% and 51%, respectively, in culture-positive PJIs. Berbari et al. [3] reported on a cohort of 62 culture-negative PJIs (7% incidence), finding a 71% survival free of treatment failure after I&D and 94% after two-stage exchange. Bejon et al. [2] also described a cohort of 152 PJIs including 62 culture-negative PJIs that underwent two-stage exchange arthroplasty with an overall infection control rate of 83% at 5.75 years followup. They found no difference between treatment failure in culture-negative and -positive cases. We found an infection-free survival rate of 50% after I&D and 70% after two-stage exchange arthroplasty in a cohort of 48 culture-negative PJIs that was similar to 45% and 78%, respectively, in a comparison group of 295 culture-positive PJIs. Our study aims to report the outcomes of treatment for culture-negative PJI, compare infection control rates with those after similar treatments in culture-positive cases, and identify the antibiotic therapies used in culture-negative cases identified using a strict definition of infection.
Inevitably, there are few limitations in our study. First, the treatment methods were not standardized. Thus, some important intraoperative factors in PJI treatment such as intraarticular spacer drug choice/dose and tissue status could not be controlled. However, the two groups were similar in their demographic distribution, Charlson Comorbidity Index, and time to initial treatment. Second, we determined only the survival rate of the prosthetic joint and not patient satisfaction or function. This decision was made to stay within the realm of our study objective: to report the objective outcome of infection-free survival. Determining functional status would be an important but separate study. Third, seven of 55 patients (13%) were lost to followup. Nevertheless, the percentage of patients lost to followup was small and likely would not influence the findings of our study unless the majority of these lost cases had become reinfected. Fourth, intraoperative tissue sampling for cultures was not uniform among all surgeons. However, 90% of cases had documentation of at least two tissue samples taken from separate locations.
Regardless of a positive or negative culture, it is clear that the choice of surgical procedure greatly affects the treatment result of PJIs (Table 3). The 10- to 15-year infection-free survival rate of two-stage exchange arthroplasty was 96% in one series in culture-positive patients [4]. Other studies report an overall infection control rate can be high as 91% to 100% at followup, ranging from 19 to 76 months after two-stage exchange for culture-positive PJI [4, 7, 12]. In patients with culture-negative PJI, the estimated 5-year infection control rate after two-stage treatment is reportedly lower than that in culture-positive cases at 79% [2, 13]. Nevertheless, we found similar infection control rates can be achieved in culture-negative and culture-positive infections when treated with two-stage exchange arthroplasty.
Table 3.
Outcomes of culture-negative periprosthetic joint infection
| Study | Time period | Number of cases | Definition of PJI | I&D infection-free survival rate | Two-stage revision infection-free survival rate | Followup | Antibiotic therapy (culture-negative cases) |
|---|---|---|---|---|---|---|---|
| Berbari et al. [3] | January 1990 to December 1999 | 60 (7%) | Intraoperative purulence or Positive histopathology or sinus tract communicating with prosthesis | 71% | 94% | 5 years | Cephalosporins (82%) Vancomycin (12%) Other (6%) |
| Malekzadeh et al. [13] | January 1985 to December 2000 | 135 (10.6%) | Intraoperative purulence or positive histopathology or sinus tract communicating with prosthesis | 78% | 78% | Median 4.6 years (range, 1 day to 22 years) | Cefazolin (69%) Vancomycin (13%) None or other (18%) |
| Bejon et al. [2] | January 1999 to April 2003 | 62 (41%) | Persistent sinus tract or positive histology | Not Available | Overall success of 83% similar in culture positive and negative cases | Mean 5.75 years | Glycopeptides (100%) |
| Current study | January 2000 to December 2007 | 48 (11.9%) | Two positive cultures or sinus tract communicating with prosthesis or 4 of 5 of the following: (1) elevated ESR or CRP; (2) elevated Synovial fluid white cell count; (3) elevated synovial fluid PMN%; (4) one positive culture; (5) intraoperative purulence | 6/12 (50%) | 19/27 (70%) | Mean 3.9 years (range, 1.0–9.9 years) | Vancomycin (81%) Cephalosporins (10%) Other (9%) |
PJI = periprosthetic joint infection; I&D = irrigation and débridement; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein; PMN = polymorphonuclear cells.
I&D reportedly has lower infection control rates than two-stage exchange in treatment of PJI [6, 8, 10]. Although I&D is a relatively simple procedure with low morbidity, the infection control rate has been estimated as low as 44% to 60% after 2 to 5.7 years of followup [1, 15]. The decision to retain the prosthesis in situ or resect it as a result of PJI is still controversial in acute hematogenous infections or cases of PJI with well-fixed components. However, our data suggest that in both culture-negative and culture-positive PJI, there are higher infection control rates observed in patients undergoing two-stage treatment compared with those undergoing I&D.
Antimicrobial therapy plays another important role in the treatment of PJI. In the majority of PJIs, preoperative joint fluid or intraoperative tissue culture is able to isolate a responsible organism, allowing physicians to use directed intravenous antibiotic therapy. A majority of PJIs (65%–90%) is caused by common aerobic Gram-positive microorganisms [14, 16] with most identified as Staphylococcus species, which can be effectively treated by parenteral vancomycin. Aerobic Gram-negative bacilli are less frequently isolated, and anaerobic organisms are even rarer. Our higher infection control rates with vancomycin compared with other parenteral antibiotics suggest that vancomycin-sensitive Gram-positive organisms may still be the most common culprit in culture-negative infections.
Infection control rates of treatment for culture-negative PJIs appear to be similar to those in culture-positive infections. Although all possible efforts should be made to identify the culprit organism in a case of PJI to guide antibiotic therapy, our findings suggest appropriate procedure selection plays a large role in long-term infection control. Furthermore, with the high incidence of Gram-positive PJIs and the increased incidence of vancomycin-susceptible Staphylococcus aureus in recent years, vancomycin seems to be a logical first choice in culture-negative patients. Therefore, we recommend aggressive two-stage exchange arthroplasty and a trial of postoperative parenteral vancomycin therapy in patients with culture-negative PJI.
Footnotes
One of the authors (JP) certifies that he has or may receive payments or benefits, in any one year, an amount in excess of $10,000, from Stryker Orthopaedics (Mahwah, NJ, USA) and SmarTech (Philadelphia, PA, USA).
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Azzam KA, Seeley M, Ghanem E, Austin MS, Purtill JJ, Parvizi J. Irrigation and debridement in the management of prosthetic joint infection: traditional indications revisited. J Arthroplasty. 2010;25:1022–1027. doi: 10.1016/j.arth.2010.01.104. [DOI] [PubMed] [Google Scholar]
- 2.Bejon P, Berendt A, Atkins BL, Green N, Parry H, Masters S, McLardy-Smith P, Gundle R, Byren I. Two-stage revision for prosthetic joint infection: predictors of outcome and the role of reimplantation microbiology. J Antimicrob Chemother. 2010;65:569–575. doi: 10.1093/jac/dkp469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berbari EF, Marculescu C, Sia I, Lahr BD, Hanssen AD, Steckelberg JM, Gullerud R, Osmon DR. Culture-negative prosthetic joint infection. Clin Infect Dis. 2007;45:1113–1119. doi: 10.1086/522184. [DOI] [PubMed] [Google Scholar]
- 4.Biring GS, Kostamo T, Garbuz DS, Masri BA, Duncan CP. Two-stage revision arthroplasty of the hip for infection using an interim articulated Prostalac hip spacer: a 10- to 15-year follow-up study. J Bone Joint Surg Br. 2009;91:1431–1437. doi: 10.1302/0301-620X.91B11.22026. [DOI] [PubMed] [Google Scholar]
- 5.Blom AW, Brown J, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86:688–691. doi: 10.1302/0301-620X.86B5.14887. [DOI] [PubMed] [Google Scholar]
- 6.Deirmengian C, Greenbaum J, Lotke PA, Booth RE, Jr, Lonner JH. Limited success with open debridement and retention of components in the treatment of acute Staphylococcus aureus infections after total knee arthroplasty. J Arthroplasty. 2003;18:22–26. doi: 10.1016/S0883-5403(03)00288-2. [DOI] [PubMed] [Google Scholar]
- 7.Haleem AA, Berry DJ, Hanssen AD. Mid-term to long-term followup of two-stage reimplantation for infected total knee arthroplasty. Clin Orthop Relat Res. 2004;428:35–39. doi: 10.1097/01.blo.0000147713.64235.73. [DOI] [PubMed] [Google Scholar]
- 8.Hirakawa K, Stulberg BN, Wilde AH, Bauer TW, Secic M. Results of 2-stage reimplantation for infected total knee arthroplasty. J Arthroplasty. 1998;13:22–28. doi: 10.1016/S0883-5403(98)90071-7. [DOI] [PubMed] [Google Scholar]
- 9.Jamsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91:38–47. doi: 10.2106/JBJS.G.01686. [DOI] [PubMed] [Google Scholar]
- 10.Koyonos L, Zmistowski B. Della Valle CJ, Parvizi J. Infection control rate of irrigation and débridement for periprosthetic joint infection. Clin Orthop Relat Res. 2011;469:3043–3048. doi: 10.1007/s11999-011-1910-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23:984–991. doi: 10.1016/j.arth.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Lentino JR. Prosthetic joint infections: bane of orthopedists, challenge for infectious disease specialists. Clin Infect Dis. 2003;36:1157–1161. doi: 10.1086/374554. [DOI] [PubMed] [Google Scholar]
- 13.Malekzadeh D, Osmon DR, Lahr BD, Hanssen AD, Berbari EF. Prior use of antimicrobial therapy is a risk factor for culture-negative prosthetic joint infection. Clin Orthop Relat Res. 2010;468:2039–2045. doi: 10.1007/s11999-010-1338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marculescu CE, Berbari EF, Cockerill FR, 3rd, Osmon DR. Fungi, mycobacteria, zoonotic and other organisms in prosthetic joint infection. Clin Orthop Relat Res. 2006;451:64–72. doi: 10.1097/01.blo.0000229337.21653.f2. [DOI] [PubMed] [Google Scholar]
- 15.Marculescu CE, Berbari EF, Hanssen AD, Steckelberg JM, Harmsen SW, Mandrekar JN, Osmon DR. Outcome of prosthetic joint infections treated with debridement and retention of components. Clin Infect Dis. 2006;42:471–478. doi: 10.1086/499234. [DOI] [PubMed] [Google Scholar]
- 16.Moran E, Masters S, Berendt AR, McLardy-Smith P, Byren I, Atkins BL. Guiding empirical antibiotic therapy in orthopaedics: the microbiology of prosthetic joint infection managed by débridement, irrigation and prosthesis retention. J Infect. 2007;55:1–7. doi: 10.1016/j.jinf.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Mortazavi SM, Molligan J, Austin MS, Purtill JJ, Hozack WJ, Parvizi J. Failure following revision total knee arthroplasty: infection is the major cause. Int Orthop. 2011;35:1157–1164. doi: 10.1007/s00264-010-1134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ong KL, Kurtz SM, Lau E, Bozic KJ, Berry DJ, Parvizi J. Prosthetic joint infection risk after total hip arthroplasty in the Medicare population. J Arthroplasty. 2009;24:105–109. doi: 10.1016/j.arth.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 19.Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469:2992–2994. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001;392:15–23. doi: 10.1097/00003086-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Phillips JE, Crane TP, Noy M, Elliott TS, Grimer RJ. The incidence of deep prosthetic infections in a specialist orthopaedic hospital: a 15-year prospective survey. J Bone Joint Surg Br. 2006;88:943–948. doi: 10.1302/0301-620X.88B7.17150. [DOI] [PubMed] [Google Scholar]
- 22.Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466:1710–1715. doi: 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]