Abstract
Background
Although several types of culture medium have been used for preservation of osteochondral allografts, the viability of chondrocytes decreases with increasing storage duration. We previously showed the University of Wisconsin solution is more suitable for graft preservation than culture medium.
Questions/purposes
We determined whether the addition of allogenic serum to University of Wisconsin solution increases chondrocyte survival during prolonged storage of osteochondral allografts.
Methods
Osteochondral tissue samples harvested from the distal femora of rats were preserved in University of Wisconsin solution supplemented with 0%, 1%, 10%, and 50% allogenic serum at 4°C for 14 days. Cell viability and chondrocyte degenerative changes of the samples then were assessed using a tetrazolium assay and histologic methods. We also evaluated time-dependent changes in cell viability and histologic findings of samples preserved for 7, 14, and 21 days in University of Wisconsin solution supplemented with or without 10% allogenic serum.
Results
After 14 days of preservation, osteochondral tissue samples maintained in University of Wisconsin solution containing 10% or greater allogenic serum exhibited the highest cell viability and lowest degenerative changes in chondrocytes. In the evaluation of time-dependent changes, we found the chondrocyte degenerative changes were greater in cartilage preserved in University of Wisconsin solution alone than in University of Wisconsin solution containing 10% allogenic serum after Day 7 or later.
Conclusions
Our results suggest the addition of 10% allogenic serum to University of Wisconsin solution enhances viability of osteochondral tissue samples.
Clinical Relevance
The use of allogenic serum-supplemented University of Wisconsin solution is expected to prolong the duration of osteochondral allograft storage and result in higher-quality grafts.
Introduction
Osteochondral allografting is one of the options for treating large cartilage defects secondary to osteonecrosis, osteochondritis dissecans, or traumatic injury [7, 9, 10, 23]. Although the use of fresh osteochondral allografts initially was limited to a small number of institutions in North America, in 1998, commercial cold-stored osteochondral allografts became available for public use [10]. Unlike traditional fresh allografts, cold-stored specimens are maintained in a hypothermic nutritive medium and must undergo a 14-day sterility testing protocol for donor bacteria and viruses [18]. Currently, improvements of outcomes and function after transplantation of osteochondral allografts stored from 15 to 48 days for treating cartilage defects of the knee have been reported during an average followup of 3 to 4 years [9, 23]. As articular cartilage matrix in grafted tissues is dependent on chondrocyte metabolism for long-term maintenance, metabolically viable chondrocytes are critical for the functional survival of allografts [6, 18]. Therefore, establishing improved solutions that prolong storage periods is needed to increase the availability of higher-quality osteochondral allografts.
Culture medium-based preservation solution is commonly used for cold preservation of osteochondral allografts. However, we previously compared the preservation abilities of Dulbecco’s modified Eagle’s medium (DMEM), saline, Euro-Collins (EC) solution, and University of Wisconsin (UW) solution and found rat osteochondral tissue (OCT) samples maintained in UW solution exhibited the highest cell viability [14]. In studies examining cold preservation of fresh osteochondral allografts, Williams et al. [22] reported canine osteochondral allografts preserved in minimal essential medium (MEM) containing 10% fetal calf serum (FCS) retained cartilage cell viability and function and histologic and biochemical integrity for 28 days. Additionally, Pennock et al. [16] suggested the viable chondrocyte density and proteoglycan synthesis of human osteochondral allografts are superior after storage in Eagle’s MEM containing fetal bovine serum (FBS) versus serum-free medium after 28 days of cold preservation. However, there are no data concerning the optimal serum concentration for cold preservation of allografts.
Here, we examined the efficacy of UW solution containing allogenic serum for extending chondrocyte survival in preserved osteochondral allografts using OCT samples obtained from rats. Although FBS is commonly used as a culture medium supplement to enhance chondrocyte viability during storage in the clinical setting, we believed allogenic serum would be superior from an immunologic standpoint. Our purposes were to (1) determine the optimal concentration of allogenic serum in UW solution and (2) evaluate time-dependent changes in cell viability of OCT samples and histologic findings, including chondrocyte morphology, assessment of the International Cartilage Repair Society (ICRS) Visual Histologic Assessment Scale, and safranin O staining of cartilage preserved in UW solution supplemented with or without 10% allogenic serum.
Materials and Methods
OCT samples and serum were harvested form Sprague-Dawley rats. UW solution supplemented with or without rat allogenic serum was prepared. OCT samples preserved in UW solution supplemented with or without allogenic serum at 4°C were assessed by tetrazolium assays and histologic methods. We first examined the relationship between allogenic serum concentration and cell viability and chondrocyte degenerative change of OCT samples to determine a suitable concentration of allogenic serum and then measured time-dependent changes in cell viability of OCT samples and histologic findings of OCT samples to evaluate the effects of allogenic serum addition to UW solution. The data were analyzed statistically to evaluate the effect of addition of allogenic serum to UW solution.
A total of 48 male 14-week-old Sprague-Dawley rats weighing 420 to 536 g were obtained from Charles River Japan, Inc (Yokohama, Japan). Rats were administered diethyl ether for anesthesia induction and then anesthetized with a mixture of medetomidine, mitazoram, and butorphanol tartrate by intramuscular injection. To excise distal femora, a skin incision was first made just above the knee. The patellar, cruciate, and collateral ligaments were cut to expose distal femoral condyles, and the distal parts of the knee extensor muscles then were removed from bone. Distal femora were cut at the metaphyseal region using a bone saw. The weight of OCT samples was adjusted to approximately 300 mg wet weight (mean ± standard error, 300 ± 3.0 mg; n = 88) by trimming soft tissue and bone tissue with a scalpel or rongeur. Two OCT samples were harvested from the bilateral knees of each Sprague-Dawley rat. UW solution was prepared from Viaspan® (Bristol-Myers Squibb, New York, NY, USA) containing dexamethasone sodium phosphate (16 mg/L) and regular insulin (40 IU/L) according to the package insert. An antibiotic-antimycotic solution then was added to both UW solutions with and without serum at a final concentration of 1% (v/v). Rat serum was collected using Venogect® II plastic centrifuge tubes (Terumo, Tokyo, Japan) from blood samples obtained from the rats used in this study. Approximately 4 mL serum per rat was collected. Complement in rat serum was inactivated by incubation for 30 minutes in a 56°C water bath.
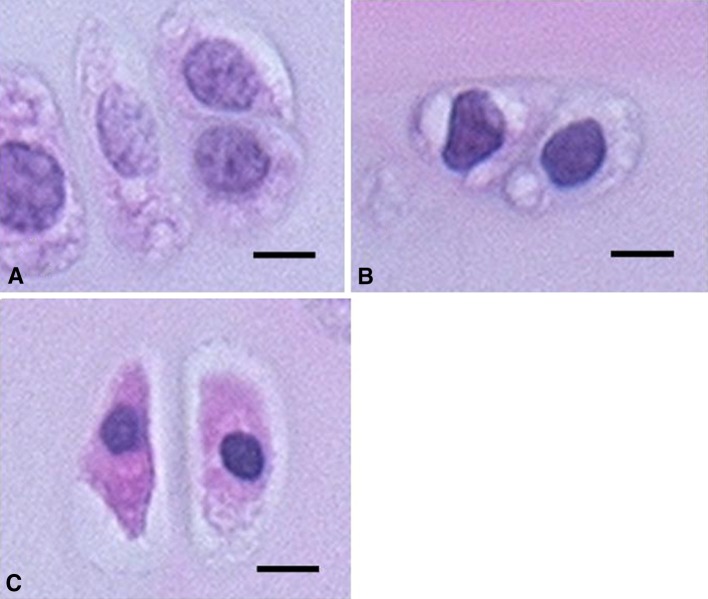
The first experiment was performed to determine the optimal allogenic serum concentration for preserving the cell viability of OCT samples. Forty OCT samples were obtained from 24 rats and separated into five groups (n = 8) (Table 1). A group of eight nonpreserved OCT samples (baseline group) were derived from the left knee of eight rats. The other four groups of 32 OCT samples were derived from the right and left knees of 16 rats. Collected rat serum from eight rats of the baseline group was pooled in a tube and stored at −30°C to be used in this experiment. Serum harvested from the other 16 rats also was pooled and stored at −30°C until used for the second experiment (described below). Each set of eight OCT samples was placed separately in 50-mL plastic centrifuge tubes filled with 8 mL UW solution supplemented with 0%, 1%, 10%, and 50% allogenic serum. After 14 days of preservation at 4°C, cell viability of OCT samples was estimated by the water-soluble tetrazolium (WST) assay using commercial WST kits (Cell Count Reagent SF; Nacalai Tesque, Kyoto, Japan), as previously described [14]. The WST assay, which was designed for quantification of cell proliferation in monolayer cultures, is based on the cleavage of the tetrazolium salt 4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate to formazan by cellular mitochondrial dehydrogenase. The formazan dye produced by viable cells can be quantified by measuring the absorbance of the solution and is directly proportional to the number of living cells. The usefulness of this assay for assessing cell viability of solid tissues has been shown [4, 8, 14]. Therefore, we used the WST assay for evaluating cell viability in place of the widely used live/dead cell assay. Here, nonpreserved (baseline group) and stored OCT samples in each condition were incubated at 37°C for 2 hours in 3 mL culture medium containing 10% WST assay reagent. After incubation, cell-free culture medium was transferred to 96-well plates, and the absorbance of each well was measured at 450 nm using a SpectraFluor Plus multiple plate reader (Tecan, Männedorf, Switzerland). The absorbance of each set of eight OCT samples was averaged, and cell viability then was calculated using a standard curve of dye absorbance versus quantity of OCT sample, which we previously established displays a linear relationship [14]. The cell viability was expressed as the absorbance of one nonpreserved OCT sample (100%) and nonOCT-containing control sample (0%). As the WST assay is unable to determine localization of viable and nonviable cells, the assessment of morphologic features of the cells in articular cartilage of OCT samples was performed after the WST assay. OCT samples were fixed in a 4% paraformaldehyde phosphate buffer solution for 48 hours at 4°C and then were decalcified in a 3-mol/L EDTA solution for 2 weeks at 4°C. After embedding the samples in paraffin, sagittal sections of the patellar groove of femoral condyles were prepared at 3-μm thickness and stained with hematoxylin and eosin. The thickest site of articular cartilage in the noncalcified zones, which had a mean depth of 170.4 ± 26.2 μm and width of 500 μm, was readily observed at a magnification of ×200. Each set of eight tissue specimens prepared from nonpreserved OCT samples (baseline group) and OCT samples preserved in UW solution with 0%, 1%, 10%, and 50% allogenic serum was assessed on an image filling system (Flovel, Tokyo, Japan). To determine the proportion of degenerative chondrocytes, an average of approximately 110 chondrocytes per slide in noncalcified zones were counted, and the number of chondrocytes with normal morphologic features (normal grade) (Fig. 1A), mild pyknotic and irregular nuclei (mild grade) (Fig. 1B), and severe pyknotic nuclei and eosinophilic shrunken cytoplasm (severe grade) (Fig. 1C) then were divided by the total number of chondrocytes.
Table 1.
Number of OCT samples analyzed by water-soluble tetrazolium assay and histologic evaluation at each serum concentration or time
| Experiment | Group | Preservation solution | Number of OCT samples |
|---|---|---|---|
| First | Baseline | 8 | |
| UW | UW + 0% serum | 8 | |
| 1% UWS | +1% serum | 8 | |
| 10% UWS | +10% serum | 8 | |
| 50% UWS | +50% serum | 8 | |
| Second | UW, Day 7 | UW | 8 |
| UWS, Day 7 | UW + 10% serum | 8 | |
| UW, Day 14 | UW | 8 | |
| UWS, Day 14 | UW + 10% serum | 8 | |
| UW, Day 21 | UW | 8 | |
| UWS, Day 21 | UW + 10% serum | 8 |
OCT = osteochondral tissue; UW = University of Wisconsin solution; UWS = University of Wisconsin solution with allogenic serum.
Fig. 1A–C.
( A) Normal chondrocytes (normal grade), (B) mild degenerative chondrocytes (mild grade), and (C) severe degenerative chondrocytes (severe grade) in osteochondral tissue samples are shown (Stain, hematoxylin and eosin; scale bar = 10 μm).
The second experiment was performed to observe time-dependent changes of cell viability of OCT samples and histologic degenerative findings. Forty-eight OCT samples were obtained from 24 Sprague-Dawley rats and separated into six groups (n = 8) (Table 1). Rat serum was prepared from Sprague-Dawley rats used in the first experiment. The cell viability of nonpreserved OCT samples (baseline) and OCT samples preserved in UW solution supplemented with or without 10% allogenic serum for 7, 14, and 21 days was estimated using the WST assay, as described above. After the WST assay, each set of eight tissue specimens prepared from OCT samples of each group was evaluated histologically by hematoxylin and eosin staining to determine the proportion of degenerative chondrocytes. The ICRS [12], cartilage surfaces, cartilage matrix, cell distribution, cell population viability, subchondral bone, and cartilage mineralization also were assessed. In addition, to evaluate matrix proteoglycans, each set of eight tissue specimens prepared from OCT samples of each group was stained with safranin O and then evaluated for loss of safranin O staining, which is one parameter of the histologic grading system [21, 22].
The StatMate III statistical software package (Atms, Tokyo, Japan) was used for statistical analysis. All data are expressed as the mean ± standard error. Statistical comparison between UW solutions with each serum concentration was performed by one-way ANOVA followed by Tukey’s test. Statistical comparison between UW solutions with and without allogenic serum at each time was performed using Student’s t-test. The level of statistical significance was set at p < 0.05.
Results
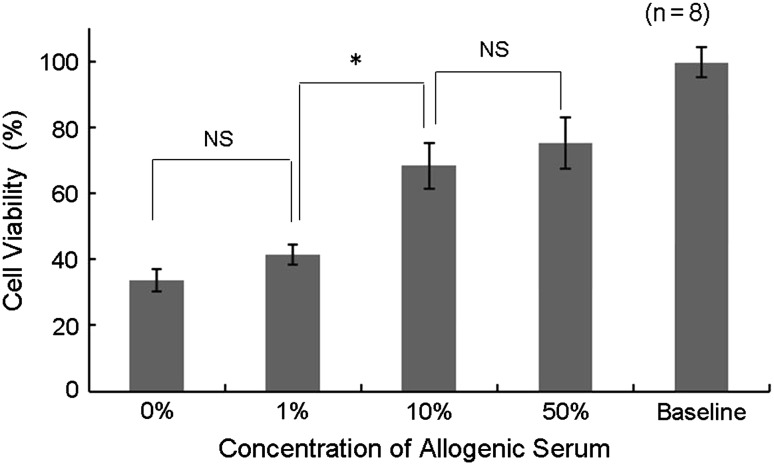
In the first experiment, OCT samples maintained in UW solution supplemented with 10% or 50% allogenic serum exhibited significantly greater cell viability than those preserved in UW solution supplemented with 0% or 1% allogenic serum (Fig. 2). In addition, the proportion of normal-grade chondrocytes was significantly higher while the proportion of severe-grade chondrocytes was significantly lower in OCT samples maintained in UW solution with 10% or 50% allogenic serum than in those preserved in UW solution supplemented with 0% or 1% allogenic serum (Table 2). No significant differences in either cell viability or the proportion of severe-grade chondrocytes were seen between the 0% UW and 1% UWS groups or the 10% and 50% UWS groups. After 14 days of cold preservation, the mean cell viability of OCT samples preserved in 0%, 1%, 10%, and 50% allogenic serum was 33.8%, 41.4%, 68.4%, and 75.1%, respectively. The mean proportion of severe-grade chondrocytes preserved in UW solution with 0%, 1%, 10%, and 50% allogenic serum was 24.3%, 27.2%, 13.4%, and 13.1%, respectively. From these findings, we determined the optimal concentration of allogenic serum was 10%.
Fig. 2.
A graph shows the relationship between cell viability and concentration of allogenic serum on Day 14 of preservation. OCT samples were preserved in UW solution with graded concentrations of allogenic serum (0%, 1%, 10%, and 50%), and cell viability was assayed by the water-soluble tetrazolium assay on Day 14 to determine the cell viability. OCT samples maintained in UW solution supplemented with 10% or greater allogenic serum exhibited higher cell viability than those preserved in UW solution supplemented with 1% or less allogenic serum. * p < 0.001; NS = not significant.
Table 2.
Relationship between serum concentration and proportion of each grade of chondrocytes in OCT samples preserved for 14 days (n = 8)
| Chondrocyte grade | Proportion of each chondrocyte grade (%) | ||||
|---|---|---|---|---|---|
| UW | 1% UWS | 10% UWS | 50% UWS | Baseline | |
| Normal | 28.7 ± 12.0 | 16.5 ± 8.0 | 47.3 ± 9.1† | 44.4 ± 11.0† | 95.8 ± 2.9 |
| Mild | 47.0 ± 8.0 | 56.3 ± 6.2 | 39.3 ± 7.0* | 42.4 ± 9.5† | 3.3 ± 2.5 |
| Severe | 24.3 ± 7.5 | 27.2 ± 7.3 | 13.4 ± 8.7* | 13.2 ± 8.0* | 0.9 ± 0.7 |
Values are expressed as mean ± standard error; * p < 0.01 and †p < 0.001 compared with 1% UWS; there were no significant differences between 0% UWS versus 1% UWS and 10% UWS versus 50% UWS for each grade of chondrocytes; OCT = osteochondral tissue; UW = University of Wisconsin solution; UWS = University of Wisconsin solution with allogenic serum.
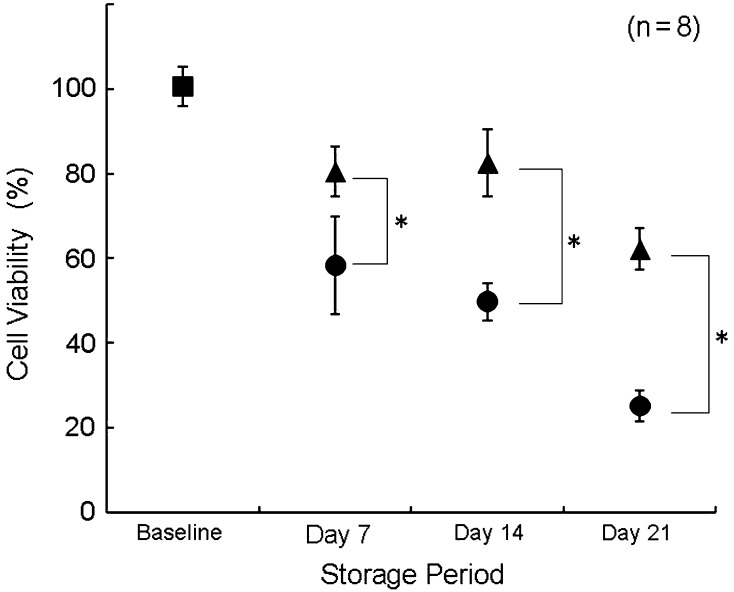
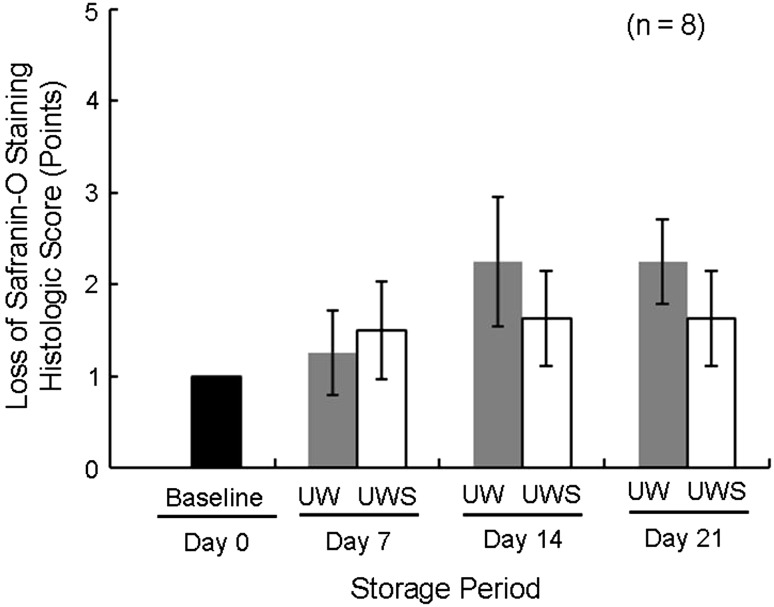
In the second experiment, as determined using the WST assay, OCT samples preserved in 10% allogenic-serum supplemented UW solution had significantly higher cell viability than those in UW solution alone at each examined time (Fig. 3). The mean percentages of cell viability of OCT samples maintained in UW solution without allogenic serum for 7, 14, and 21 days were 58.4%, 49.8%, and 25.2%, respectively, whereas those kept in UW solution supplemented with 10% allogenic serum were 80.5%, 82.5%, and 62.2%, respectively. OCT samples preserved in UW solution with 10% allogenic serum had significantly more normal-grade and fewer severe-grade chondrocytes compared with those in UW solution alone at all times (Figs. 4, 5; Table 3). The mean proportions of normal-grade chondrocytes preserved in UW solution alone on Days 7, 14, and 21 were 66.1%, 43.1%, and 34.7%, respectively, whereas those in UW solution with 10% allogenic serum were 89.6%, 80.3%, and 60.4%, respectively. The mean proportions of severe-grade chondrocytes preserved in UW solution alone on Days 7, 14, and 21 were 16.4%, 19.4%, and 47.3%, respectively, whereas the mean proportions of those in UW solution with 10% allogenic serum were 4.9%, 7.6%, and 16.0%, respectively. Based on the ICRS, the scores of cell population viability of OCT samples preserved in UW solution with 10% allogenic serum were greater than those in UW solution alone at each time (Table 4). The mean scores of the other evaluated items for both groups registered full scores (3.0 points). From the results of the histologic score by safranin O staining, loss of safranin O staining for OCT samples preserved in UW solution alone was greater than that for samples in UW solution with 10% allogenic serum on Days 14 and 21 (Figs. 6, 7).
Fig. 3.
A graph shows cell viability of OCT samples preserved in UW solution with or without 10% allogenic serum by water-soluble tetrazolium assay. OCT samples preserved in allogenic-serum supplemented UW solution had higher cell viability than those in UW solution alone after 21 days of storage. Filled box = baseline; filled circle = UW solution; filled triangle = UW solution with 10% allogenic serum; *p < 0.001.
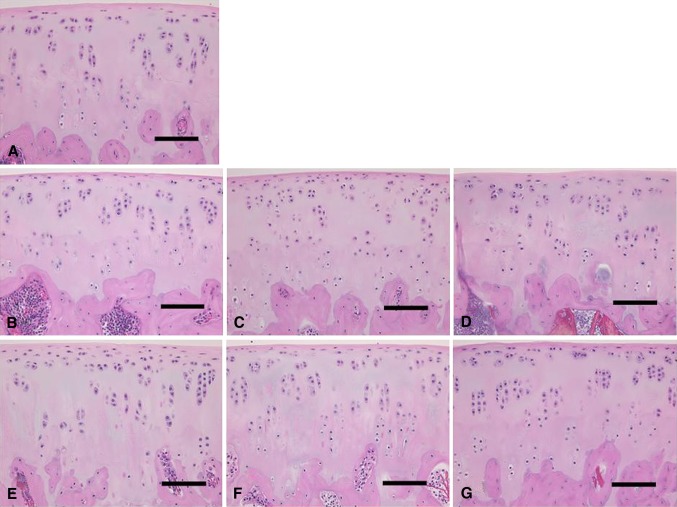
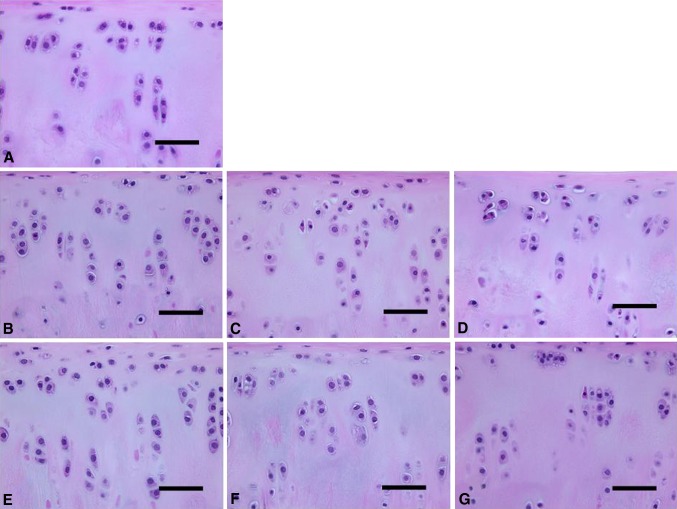
Fig. 4A–G.
Histologic assessments of (A) baseline and preserved articular cartilage in unsupplemented UW solution on (B) Day 7, (C) Day 14, and (D) Day 21 and UW solution with 10% allogenic serum on (E) Day 7, (F) Day 14, and (G) Day 21 are shown (Stain, hematoxylin and eosin; scale bar = 50 μm). Structures of the cartilage surface, cartilage matrix, and subchondral bone did not noticeably differ among the examined samples.
Fig. 5A–G.
Histologic assessments of magnification of the noncalcified zone in each specimen of Figure 4 are shown for (A) baseline and preserved articular cartilage in unsupplemented UW solution on (B) Day 7, (C) Day 14, and (D) Day 21 and UW solution with 10% allogenic serum on (E) Day 7, (F) Day 14, and (G) Day 21 (Stain, hematoxylin and eosin; scale bar = 100 μm). Increased numbers of chondrocytes with pyknotic nuclei and eosinophilic shrunken cytoplasm were observed with increasing length of storage in illustrations B to D compared with E to G.
Table 3.
Proportion of each grade of chondrocyte preserved in OCT samples in UW or UWS at each time (n = 8)
| Time | Normal grade (%) | Mild grade (%) | Severe grade (%) | |||
|---|---|---|---|---|---|---|
| UW | UWS | UW | UWS | UW | UWS | |
| Baseline | 95.8 ± 2.9 | 3.3 ± 2.5 | 0.9 ± 0.7 | |||
| Day 7 | 66.1 ± 14.5 | 89.6 ± 5.8* | 17.5 ± 8.7 | 5.5 ± 3.7* | 16.4 ± 7.8 | 4.9 ± 3.3* |
| Day 14 | 43.1 ± 15.9 | 80.3 ± 4.2† | 37.5 ± 12.5 | 12.1 ± 4.1† | 19.4 ± 10.7 | 7.6 ± 3.4* |
| Day 21 | 34.7 ± 7.7 | 60.4 ± 12.1† | 18.0 ± 10.9 | 23.6 ± 9.2* | 47.3 ± 10.2 | 16.0 ± 8.5† |
Values are expressed as mean ± standard error; * p < 0.01 and †p < 0.001 compared with UW; OCT = osteochondral tissue; UW = University of Wisconsin solution; UWS = University of Wisconsin solution with 10% allogenic serum.
Table 4.
ICRS scoring for baseline and OCT preserved in UW or UWS at each time (n = 8)
| Time/solution | I. Surface | II. Matrix | III. Cell distribution | IV. Cell population viability | V. Subchondral bone | VI. Cartilage mineralization |
|---|---|---|---|---|---|---|
| Baseline | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 |
| Day 7 | ||||||
| UW | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 | 2.0 ± 1.1 | 3.0 ± 0.0 | 3.0 ± 0.0 |
| UWS | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 |
| Day 14 | ||||||
| UW | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 | 1.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 |
| UWS | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 |
| Day 21 | ||||||
| UW | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 | 1.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 |
| UWS | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 | 1.3 ± 0.7 | 3.0 ± 0.0 | 3.0 ± 0.0 |
Values are expressed as mean ± standard error; OCT = osteochondral tissue; ICRS = International Cartilage Repair Society Visual Histologic Assessment Scale; UW = University of Wisconsin solution; UWS = University of Wisconsin solution with 10% allogenic serum.
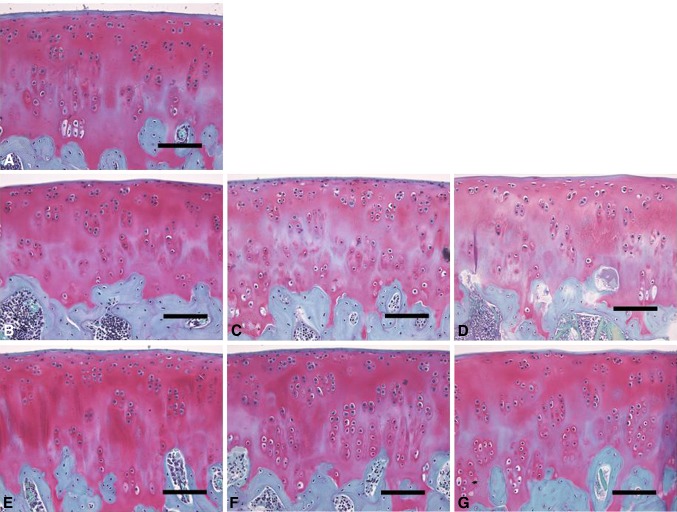
Fig. 6A–G.
Histochemical assessments of (A) baseline and preserved articular cartilage in unsupplemented UW solution on (B) Day 7, (C) Day 14, and (D) Day 21 and UW solution with 10% allogenic serum on (E) Day 7, (F) Day 14, and (G) Day 21 are shown (Stain, safranin O; scale bar = 50 μm). Greater loss of safranin O staining was observed in Illustrations C and D than in F and G.
Fig. 7.
A graph shows histologic scores of loss of safranin O staining of cartilage. Greater losses of safranin O staining were observed in cartilages preserved in UW solution alone than in those in UW solution containing 10% allogenic serum (UWS) on Days 14 and 21.
Discussion
We previously showed that UW solution was more suitable as a preservation solution for rat OCT samples than DMEM [14]. As FBS is commonly used to enhance chondrocyte viability during cold storage, we hypothesized allogenic serum, which is considered a better supplement in terms of immunologic aspect, would improve cold preservation of osteochondral allografts when added to UW solution. In our concept for clinical application, allogenic serum could be obtained from registered recipients and stored before the preservation of osteochondral allografts. To replicate this clinical situation in this study, we used allogenic serum, not FBS. To test this hypothesis, we investigated (1) the optimal concentration of allogenic serum for preservation of osteochondral allografts and (2) the effect of prolonged storage in UW solution containing serum on allografts. We found 10% allogenic serum markedly improved cold preservation of osteochondral tissues, with respect to cell viability and histologic findings.
A few limitations of our study warrant mention. First, although we performed WST assays to evaluate cell viability of whole OCT samples and histologically assessed articular cartilage, we did not evaluate chondrocyte viability using the widely used live/dead cell assay. However, we believe the WST assay combined with histologic examination is a reasonable approach to evaluate cell viability and determine viable and nonviable cell localization. Additionally, Pearsall et al. [15] histologically examined cold-preserved human osteochondral allografts and found severe pyknosis of chondrocytes and characteristic changes in cell shape reflected the response of nonviable chondrocytes. Therefore, we believe the proportion of degenerative chondrocytes accurately reflects the proportion of reduced or nonviable chondrocytes and supports the cell viability estimated by the WST assay. Second, for evaluation of the effects of preservation solution, we used OCT samples obtained from Sprague-Dawley rats, which have limited extrapolation to humans. As rat OCT samples have thinner articular cartilage compared with large animals or humans, our results for chondrocyte viability, which was determined from the proportion of normal-grade chondrocytes, are lower than those for large animals reported in previous studies evaluated at the same times [1, 16, 20, 22]. Finally, as the concentration of UW solution components decreased with increasing serum concentration, the effects of UW solution components and serum might have varied for the different storage conditions.
Our first experiment revealed UW solution containing 10% or greater allogenic serum maintained significantly higher cell viability of OCT samples and a greater proportion of normal-grade chondrocytes in articular cartilage than UW solution alone. These findings are also consistent with those in a study by Pennock et al. [16], who found addition of 10% FBS to serum-free media enhanced chondrocyte viability and metabolic activity of human osteochondral allografts. Therefore, a concentration of 10% allogenic serum was optimal for the storage and viability of osteochondral allografts.
The results of our second experiment showed the quality of OCT samples after storage in UW solution containing 10% allogenic serum, as measured by cell viability, proportion of degenerative chondrocytes, and histologic scoring, was superior to that in serum-free UW solution during 21 days of cold storage. Moreover, OCT samples preserved in serum-free UW solution on Day 7 had similar cell viability and proportion of normal- and severe-grade chondrocytes compared with those in UW solution containing 10% allogenic serum on Day 21. Teng et al. [20] reported osteochondral allografts preserved in serum-free DMEM after 1 week had lower cell viability compared with those in commercial Musculoskeletal Transplant Foundation medium containing FBS at Week 3. Williams et al. [22] reported canine osteochondral allografts preserved in MEM containing 10% FCS had approximately the same cell viability for up to 14 days compared with fresh controls and retained 78% cell viability after 28 days. These findings also are consistent with our results of prolonged storage. Taken together, the use of UW solution supplemented with 10% allogenic serum as a preservation solution appears suitable for prolonging the storage and viability of osteochondral allografts.
Although it is unclear what component of allogenic serum promotes chondrocyte survival, a few specific factors have been identified. For example, Teng et al. [20] reported addition of IGF-I to DMEM increased chondrocyte viability after 21 days of storage compared with DMEM alone. In addition, Bian et al. [2] reported the biochemical and biomechanical properties of osteochondral allografts might be enhanced by the addition of dexamethasone to culture media. Based on these findings, it is likely growth factors or hormones in allogenic serum improve chondrocyte survival during cold storage. Pylawka et al. [17] reported nitric oxide increases during rewarming of osteochondral allografts. Serum contains several antioxidants that are actively involved in the defense against reactive oxygen species and includes enzymes, such as superoxide dismutase, catalase, and glutathione peroxidase; macromolecules, such as albumin, ceruloplasmin, and ferritin; and an array of small molecules, including ascorbic acid, α-tocopherol, β-carotene, reduced glutathione, methionine, uric acid, and bilirubin [24]. Lee et al. [11] examined cold storage of oral keratinocytes and proposed the addition of albumin, which is the most abundant protein in serum, to preservation solution served an antioxidant role. Thus, it is likely antioxidants, particularly albumin, in allogenic serum improve chondrocyte survival during cold storage. Additionally, as osmotic agents, such as albumin, sucrose, dextrans, and hydroxyethyl starch, improve cold preservation of organs and tissues [3, 5, 13, 19], osmolality changes induced by albumin in allogenic serum might improve the viability of OCT samples.
We observed supplementation of UW solution with allogenic serum significantly enhances the viability of chondrocytes in OCT samples stored for up to 21 days at 4°C as compared with UW solution alone. Additional studies are needed to clarify the essential components in allogenic serum that promote graft preservation to allow the design of even more effective preservation solutions.
Acknowledgments
We thank Shi-Xu Jiang MD, PhD, a senior pathologist of Kitasato University, for helpful comments related to the histologic findings of this study, and Takeaki Yamamoto MD for help with assessing tissue specimens.
Footnotes
One or more of the authors (KO) have received funding from SRL Inc (Tokyo, Japan), without benefits from a commercial entity related to this work.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Kitasato University School of Medicine, Japan.
References
- 1.Ball ST, Amiel D, Williams SK, Tontz W, Chen AC, Sah RL, Bugbee WD. The effects of storage on fresh human osteochondral allografts. Clin Orthop Relat Res. 2004;418:246–252. doi: 10.1097/00003086-200401000-00043. [DOI] [PubMed] [Google Scholar]
- 2.Bian L, Stoker AM, Marberry KM, Ateshian GA, Cook JL, Hung CT. Effect of dexamethasone on the functional properties of cartilage explants during long-term culture. Am J Sports Med. 2010;38:78–85. doi: 10.1177/0363546509354197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coffey AK, Andrews PM. Ultrastructure of kidney preservation: varying the amount of an effective osmotic agent in isotonic and hypertonic preservation solutions. Transplantation. 1983;35:136–143. doi: 10.1097/00007890-198302000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Csönge L, Bravo D, Newman-Gage H, Rigley T, Conrad EU, Bakay A, Strong DM, Pellet S. Banking of osteochondral allografts: Part I. Viability assays adapted for osteochondral and cartilage studies. Cell Tissue Bank. 2002;3:151–159. doi: 10.1023/A:1023665418244. [DOI] [PubMed] [Google Scholar]
- 5.Dunphy G, Richter HW, Azodi M, Weigand J, Sadri F, Sellke F, Ely D. The effects of mannitol, albumin, and cardioplegia enhancers on 24-h rat heart preservation. Am J Physiol. 1999;276(5 Pt 2):H1591–H1598. [DOI] [PubMed]
- 6.Enneking WF, Campanacci DA. Retrieved human allografts: a clinicopathological study. J Bone Joint Surg Am. 2001;83:971–986. [PubMed] [Google Scholar]
- 7.Gross AE, Shasha N, Aubin P. Long-term followup of the use of fresh osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res. 2005;435:79–87. doi: 10.1097/01.blo.0000165845.21735.05. [DOI] [PubMed] [Google Scholar]
- 8.Jomha NM, Elliott JA, Law GK, McGann LE. Evaluation of chondrocyte survival in situ using WST-1 and membrane integrity stains. Cell Tissue Bank. 2007;8:179–186. doi: 10.1007/s10561-006-9028-6. [DOI] [PubMed] [Google Scholar]
- 9.LaPrade RF, Botker J, Herzog M, Agel J. Refrigerated osteoarticular allografts to treat articular cartilage defects of the femoral condyles: a prospective outcomes study. J Bone Joint Surg Am. 2009;91:805–811. doi: 10.2106/JBJS.H.00703. [DOI] [PubMed] [Google Scholar]
- 10.Lattermann C, Romine SE. Osteochondral allografts: state of the art. Clin Sports Med. 2009;28:285–301. doi: 10.1016/j.csm.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Lee EJ, Lee SA, Kim J. The effect of human serum albumin on the extended storage of human oral keratinocyte viability under mild hypothermia. Cryobiology. 2005;50:103–111. doi: 10.1016/j.cryobiol.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Mainil–Varlet P, Aigner T, Brittberg M, Bullough P, Hollander A, Hunziker E, Kandel R, Nehrer S, Pritzker K, Roberts S, Stauffer E. International Cartilage Repair Society. Histological assessment of cartilage repair: a report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS) J Bone Joint Surg Am. 2003;85(2):45–57. [PubMed] [Google Scholar]
- 13.Neveux N, Bandt JP, Charrueau C, Savier E, Chaumeil JC, Hannoun L, Giboudeau J, Cynober LA. Deletion of hydroxyethylstarch from University of Wisconsin solution induces cell shrinkage and proteolysis during and after cold storage of rat liver. Hepatology. 1997;25:678–682. doi: 10.1002/hep.510250331. [DOI] [PubMed] [Google Scholar]
- 14.Onuma K, Urabe K, Naruse K, Park HJ, Uchida K, Itoman M. Cold preservation of rat osteochondral tissues in two types of solid organ preservation solution, culture medium and saline. Cell Tissue Bank. 2009;10:1–9. doi: 10.1007/s10561-008-9108-x. [DOI] [PubMed] [Google Scholar]
- 15.Pearsall AW 4th, Tucker JA, Hester RB, Heitman RJ. Chondrocyte viability in refrigerated osteochondral allografts used for transplantation within the knee. Am J Sports Med. 2004;32:125–131. [DOI] [PubMed]
- 16.Pennock AT, Wagner F, Robertson CM, Harwood FL, Bugbee WD, Amiel D. Prolonged storage of osteochondral allografts: does the addition of fetal bovine serum improve chondrocyte viability? J Knee Surg. 2006;19:265–272. doi: 10.1055/s-0030-1248117. [DOI] [PubMed] [Google Scholar]
- 17.Pylawka TK, Virdi AS, Cole BJ, Williams JM. Reversal of suppressed metabolism in prolonged cold preserved cartilage. J Orthop Res. 2008;26:247–254. doi: 10.1002/jor.20487. [DOI] [PubMed] [Google Scholar]
- 18.Ranawat AS, Vidal AF, Chen CT, Zelken JA, Turner AS, Williams RJ., 3rd Material properties of fresh cold-stored allografts for osteochondral defects at 1 year. Clin Orthop Relat Res. 2008;466:1826–1836. doi: 10.1007/s11999-008-0311-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlachter K, Kokotilo MS, Carter J, Thiesen A, Ochs A, Khadaroo RG, Churchill TA. Redefining the properties of an osmotic agent in an intestinal-specific preservation solution. World J Gastroenterol. 2010;16:5701–5709. doi: 10.3748/wjg.v16.i45.5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teng MS, Yuen AS, Kim HT. Enhancing osteochondral allograft viability: effects of storage media composition. Clin Orthop Relat Res. 2008;466:1804–1809. doi: 10.1007/s11999-008-0302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams JM, Ongchi DR, Thonar EJ. Repair of articular cartilage injury following intra-articular chymopapain-induced matrix proteoglycan loss. J Orthop Res. 1993;11:705–716. doi: 10.1002/jor.1100110513. [DOI] [PubMed] [Google Scholar]
- 22.Williams JM, Virdi AS, Pylawka TK, Edwards RB, 3rd, Markel MD, Cole BJ. Prolonged-fresh preservation of intact whole canine femoral condyles for the potential use as osteochondral allografts. J Orthop Res. 2005;23:831–837. doi: 10.1016/j.orthres.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Williams RJ, 3rd, Ranawat AS, Potter HG, Carter T, Warren RF. Fresh stored allografts for the treatment of osteochondral defects of the knee. J Bone Joint Surg Am. 2007;89:718–726. doi: 10.2106/JBJS.F.00625. [DOI] [PubMed] [Google Scholar]
- 24.Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994;74:139–162. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]