Abstract
Background
Osteoporotic vertebral compressed fractures (VCFs) are the most common osteoporotic fractures. Although percutaneous vertebroplasty (PVP) reportedly relieves pain and improves function, a recent pooled analysis from two multicenter randomized controlled trials concluded the improvement in pain and disability treated with PVP was similar to those with sham surgery.
Questions/Purpose
Using meta-analysis we therefore asked whether compared with either nonoperative therapy or a sham injection for patients with VCF, PVP would (1) better relieve pain, (2) provide greater improvement in pain-related disability, and (3) increase the recurrence of vertebral fractures.
Methods
We searched PubMed, EMBASE, Medline, and the Cochrane library using the keywords “vertebroplasty AND osteoporosis OR fracture”. We included nine of the 469 articles identified. Using a random effects model, we calculated the weighted mean differences to evaluate the pain reduction at different times as the primary outcome. Pain-related disability was assessed by a quality of life (QOL) measure. Improvement of QOL and recurrence of vertebral fractures were the secondary outcomes. We used subgroup analysis to reinvestigate pain relief and function improvement of PVP based on two different controls: nonoperative therapy and sham injection. The total number of patients was 886.
Results
Pain scoring was similar between the PVP group and the sham injection group at 1 to 29 days and 90 days. However, compared with nonoperative therapy, PVP reduced pain at all times studied. QOL in the PVP group was improved or tended to be improved compared with QOL for both control groups. The risk of new fractures was similar between the PVP groups and both control groups.
Conclusions
Different control groups may have accounted for the different conclusions in the literature regarding the ability of PVP to relieve pain and restore function recovery. Compared with nonoperative treatment PVP relieved pain better and improved QOL. PVP did not increase the risk of new fractures.
Level of Evidence
Level II, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Osteoporosis is an age-related progressive skeletal disease, with consequent loss in bone mass and considerable tendency to fracture [7, 38]. Vertebral compressed fractures (VCFs) are the most common type of osteoporotic fractures, resulting in severe back pain, spinal deformity, muscle atrophy, physical decline, prolonged hospitalization, and potential risk of increased mortality [6, 25]. Osteoporotic vertebral fractures occur in approximate 20% of people older than 70 years worldwide [12], with an estimated 1,400,000 new fractures occurring every year [24].
Traditional nonoperative treatment of painful osteoporotic fractures usually consists of bed rest, narcotic anesthesia, and bracing [35], leading to high mortality, impaired function, and severely compromised quality of life (QOL) [42]. Percutaneous vertebroplasty (PVP) is a minimally invasive technique in which polymethylmethacrylate (PMMA) is injected into the vertebral body to stabilize the fracture [16, 41]. The stability of the fracture and recovery of vertebral height after PVP using postural reduction have been considered to be the major causes of pain relief [11, 40]. PVP has been clinically justified by abundant retrospective evidence and used worldwide for treatment of painful osteoporotic VCFs [3, 5, 9, 11, 13, 18, 21, 36]. Since 2003, seven prospective controlled studies, including three nonrandomized trials [2, 14, 50] and four randomized trials [15, 27, 37, 47], have concluded PVP relieves pain better and improves function compared with nonoperative therapy. PVP has been recommended for treatment of osteoporotic VCFs that do not respond to nonoperative therapy [23, 32].
However, one uncontrolled study reported the short-term ability of PVP to relieve pain and improve function was unclear [46], and another reported that PVP could increase the risk of new fractures [44]. Moreover, serious complications have been reported, including pulmonary embolism [10], severe infection [1, 29], and paraplegia [28]. Two recent multicenter randomized trials [8, 26] found sham injection provided similar pain relief and restoration of function as PVP. The therapeutic effect of PVP for VCFs was questioned by these authors. Thus, there is controversy regarding whether PVP is beneficial for treating VCFs.
To address this controversy, we pooled available prospective evidence and differentiated the evidence based on differing controls. The purposes of our study were to compare PVP with either nonoperative therapy or sham injection regarding pain relief, QOL improvement, and recurrence of VCFs.
Search Strategy and Criteria
Electronic databases (PubMed, EMBASE,Medline, and the Cochrane library) were searched with a limit of “clinical trial” (“clinical trial” or “randomized controlled trial” in PubMed; “controlled study” in EMBASE; “controlled clinical trial”, “randomized controlled trial”, or “comparative study” in Medline; “clinical trials” in Cochrane library) by two independent investigators (MMS and TL). Results retrieved were last updated on August 25, 2011. The search used terms and Boolean operators as follows: “vertebroplasty AND (osteoporosis OR fracture)”. Reference lists of all the selected articles were hand-searched for any additional trials (Fig. 1).
Fig. 1.
A flowchart illustrates the selection of nine trials included in our meta-analysis.
The literature search initially yielded 786 relevant trials from PubMed (N = 104), EMBASE (N = 410), Medline (N = 227), and the Cochrane library (N = 45); of these there were 317 duplicates leaving 469 studies. Two of us (MMS and XCZ) reviewed the titles and abstracts of all 469 trials. We included those in which (1) the target population consisted of patients undergoing PVP, (2) the intervention was injection of PMMA, (3) the outcomes included at least VAS pain score, and (4) the trial was a randomized controlled trial (RCT) or prospective, nonrandomized, controlled trial. Trials were excluded if (1) they were Phase I or observational studies, case reports, or reviews, (2) pain score data were unavailable, and (3) the RCTs had a followup less than 2 weeks. Of the 469 studies, we excluded 453 because they did not fulfill the selection criteria. Of the 16 remaining articles, three were study protocols, two did not report data regarding pain relief, one was not a controlled trial, and one had a later report with intention-to-treat (ITT) analysis by the same authors. Ultimately, nine prospective controlled trials were included in our meta-analysis, of which three were nonrandomized trials [2, 14, 50] and six were randomized trials [8, 15, 26, 27, 37, 47].
For each eligible trial, two authors (MMS and XZC) independently extracted the relevant data and checked the accuracy. Specifically, they abstracted study design, sample size, demographic data (age, sex proportion), intervention protocol, duration of the trial, loss to followup, and trial outcomes. We used ITT data from the trials whenever possible. If not available, we used data from the analysis of the available data or data from the analysis of treatment received. If the data were not reported in the original article, we extrapolated them from the accompanying illustrations. We recorded the characteristics of the nine included trials (Table 1), and details of intervention and measurement (Table 2). All the trials were prospective controlled trials, of which sham injections were used as controls in two [8, 26], and the nonoperative therapy control was used in the other seven trials [2, 14, 15, 27, 37, 47, 50]. Only adults with a mean age older than 50 years with symptomatic back pain and radiographic fracture findings were included in all of the studies. Radiographic-guided injections were used in all of the studies. All the studies included data for pain relief for at least one time, four studies used the Roland-Morris Disability Questionnaire (RDQ), four used European Quality of Life–5 Dimensions (EQ-5D) (of which three trials were available), and three had Quality of Life Questionnaire of the European Foundation for Osteoporosis (QUALEFFO) results. The weighted kappa for agreement on eligibility between reviewers was 0.88 (95% CI, 0.78–0.99).
Table 1.
Study characteristics
| Study | Study design | Sample size (PVP/control) | Ages of patients (years) (mean ± SD) | Sex distribution (female/male) | Inclusion criteria |
|---|---|---|---|---|---|
| Buchbinder et al. [8] | Multicenter, randomized, double-blind, placebo-controlled trial | 78 (38/40) | 76.6 ± 11.8 | 16/62 | Age older than 50 years; back pain of no more than 12 months duration; two recent painful fractures; bone edema or other signs on MRI. |
| Kallmes et al. [26] | Multicenter, randomized, double-blind, controlled trial | 131 (68/63) | 73.8 ± 9.5 | 32/99 | Age older than 50 years; one to three painful fractures; a current rating for pain intensity of at least 3. |
| Voormolen et al. [47] | Randomized, controlled trial | 34 (18/16) | 73.0 ± 8.2 | 6/28 | Age older than 50 years; back pain at least 6 weeks and no longer than 6 months; focal tenderness; imaging signs. |
| Rousing et al. [37] | Randomized, controlled trial | 50 (26/24) | 80.0 ± 7.8 | 41/9 | Age older than 65 years; intractable pain because of either acute or subacute VCFs. |
| Farrokhi et al. [16] | Single-blinded, randomized, controlled trial | 82 (40/42) | 71 ± 8 | 22/60 | Severe back pain; focal tenderness; imaging signs; not responsive to the medical therapy. |
| Diamond et al. [14] | Prospective, nonrandomized, controlled trial | 126 (88/38) | 76.4 ± 9.4 | 87/39 | Acute vertebral fracture; imaging signs. |
| Klazen et al. [27] | Multicenter, randomized, double-blind, open-label, controlled trial | 202 (101/101) | 75.3 ± 9.1 | 140/62 | Age older than 50 years; VCF; VAS score of 5 or more; focal tenderness; imaging signs. |
| Wang et al. [50] | Prospective, nonrandomized, controlled trial | 55 (32/23) | 72.8 ± 10.7 | 8/47 | Age older than 50 years; acute vertebral fracture pain caused by osteoporosis, with more than 20% loss of height; focal tenderness; imaging signs. |
| Alvarez et al. [2] | Prospective, nonrandomized, controlled trial | 128 (101/27) | 72.5 ± 7.8 | 25/103 | Imaging signs; less than satisfactory response to conventional therapy during at least a 6-week period. |
PVP = percutaneous vertebroplasty groups; VCFs = vertebral compressed fractures.
Table 2.
Details of intervention and measurement of studies
| Study | Intervention (timing) | Followup (days) | Outcome measurement | Cofactors | Missing information (PVP/control) |
|---|---|---|---|---|---|
| Buchbinder et al. [8] | PVP (32% < 6 weeks) Sham PVP (32% < 6 weeks) |
180 | Pain score (VAS); QUALEFFO total score; AQoL score; RDQ score; EQ–5D score. | Standard care | (3/4) (7.9%/10%) |
| Kallmes et al. [26] | PVP (16 weeks) Sham PVP (20 weeks) |
90 | Pain score(VAS); RDQ score; Pain Frequency Index; Pain Bothersomeness Index; SOF–ADL scale; EQ–5D scale | 0.25% bupivacaine during injection; 35 underwent crossover intervention at < 3 months | (4/2) (5.9%/3.2%) |
| Voormolen et al. [47] | PVP (85 days) Conservative therapy (76 days) |
360 | Pain score (VAS); QUALEFFO; RDQ; decrease in analgesic dosage | Initial pain medication | (0/0) |
| Rousing et al. [37] | PVP conservative therapy (40 patients < 2 weeks, 10 patients between 2 and 8 weeks) | 90 | Pain score (VAS); Medical outcome SF-36; DPQ; EQ-5D; Barthel; MMSE; Tandem test; timed up and go | Pain medication and physiotherapy until discharged | (2/1) (7.7%/4.2%) |
| Farrokhi et al. [16] | PVP (27 weeks) Conservative therapy (30 weeks) |
1080 | Pain score (VAS); Oswestry LBP score for QOL; vertebral body height; sagittal index | Not mentioned | (3/3) (7.5%/7.15%) |
| Diamond et al. [14] | PVP; conservative therapy (1–6 weeks) | 720 | Pain score (PPI); physical function; new fractures and mortality | Antiosteoporotic therapy | (6/1) (6.8%/2.6%) |
| Klazen et al. [27] | PVP (29.3 days) Conservative therapy (26.8 days) |
360 | Pain score (VAS); EQ-5D; QUALEFFO; RDQ | Not mentioned | (15/24) (14.6%/23.8%) |
| Wang et al. [50] | PVP; conservative therapy (< 6 weeks) | 360 | Pain score (VAS); physical function; pain medication requirement; complications and new fractures | Pain medication after the procedure | (0/3) (0/13%) |
| Alvarez et al. [2] | PVP; conservative therapy (> 6 weeks) | 360 | Pain score (VAS); Oswestry function test; decrease in analgesic dosage; SF-36 | Standard care | (0/0) |
PVP = percutaneous vertebroplasty groups; QOL = quality of life; QUALEFFO = Quality of Life Questionnaire of the European Foundation for Osteoporosis; AQoL = Assessment of Quality of Life; RDQ = Roland-Morris Disability Questionnaire; DPQ = Dallas Pain Questionnaire; EQ-5D = European Quality of Life–5 Dimensions; SOF–ADL = Study of Osteoporotic Fractures–Activities of Daily Living; MMSE = modified mini-mental state examination; SF-36 = Short Form-36; LBP = low-back pain; PPI = present pain intensity.
Two reviewers (SGY and WW) independently assessed the methodologic quality of the included trials with a 12-item scale [17], assessing factors such as randomization, allocation concealment, similar baseline, blinding, selective reporting, patient’s compliance, loss to followup, similar timing, and ITT analysis, resolving disagreements through discussion (Table 3). We also used the criteria of the Grading of Recommendations Assessment, Development and Evaluation (GRADE) to evaluate the quality of evidence [4]. Because data for pain relief in one trial was not described using VAS, we extracted the data from a study [39] by the same authors in which all the participants were included in the later study. Three studies explicitly stated the allocation concealment [8, 26, 37], and seven reported inadequate details of blinding [2, 14, 15, 27, 37, 47, 50]. ITT analysis was used in five studies [8, 14, 26, 47, 50], and no outcomes were selectively reported in all studies. The weighted kappa for the agreement on the trial quality between reviewers was 0.86 (95% CI, 0.78–0.94).
Table 3.
Methodologic quality of included studies
| Study | Randomized adequately* | Allocation concealed | Similar baseline | Patient blinded | Care provider blinded | Outcome assessor blinded | Avoid selective reporting | Similar or avoided cofactors | Patients’ compliance† | Acceptable drop-out rate‡ | Similar timing | ITT analysis§ | Quality‖ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buchbinder et al. [8] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Kallmes et al. [26] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Voormolen et al. [47] | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | No | High |
| Rousing et al. [37] | Yes | No | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Farrokhi et al. [16] | Yes | No | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | No | High |
| Diamond et al. [14] | No | No | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Klazen et al. [27] | No | No | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Wang et al. [50] | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | No | Moderate |
| Alvarez et al. [2] | No | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | No | Moderate |
* Only if the method of sequence generated was explicitly described could get a “Yes”; sequence generated by “Dates of Admission” or “Patients Number” received a “No”; † intermittent treatment or therapy duration less than 6 months means “Yes”, otherwise “No”; ‡drop-out rate ≥ 20% means “No”, otherwise “Yes”; §ITT = intention-to-treat, only if all randomized patients are analyzed in the group they were allocated to could receive a “Yes”; ‖the frequences of “Yes” greater than 7 means “High”; greater than 4 but 7 or less means “Moderate”; 4 or less means “Low”.
The VAS pain scores [22, 34] evaluated with credible measurement at different times after treatment were our primary outcome. From 1 to 29 days, we used data at the earliest time. The data for 90 days refers to the data on the day closest to the 90th day from the 30th day to the 179th day. The data for more than 180 days refers to the data on the day closest to the 180th day, which should meet the following criteria: (1) more than 180 days after inclusion, and (2) closest to the 180th day. We combined the two sham injection controlled trials by Buchbinder et al. [8] and Kallmes et al. [26] and abstracted data from a meta-analysis of these two studies by Staples et al. [39]. The authors divided the patients in the two studies [8, 26] into two subgroups according to pain of recent onset (6 weeks or less, or greater than 6 weeks). We used the combined data in our subgroup analysis, and named them “combination less than or equal to 6 weeks” and “combination less than 6 weeks”.
We also evaluated the recurrence of vertebral fractures and improvement of QOL scored by the RDQ [45] (30 days and 90 days), EQ-5D [49] (30 days), and QUALEFFO [30] (30 days) as secondary outcomes. There were four studies reporting RDQ [8, 26, 27, 47], and three for EQ-5D [8, 26, 27] and QUALEFFO [8, 27, 47].
We converted all outcome measures to weighted mean differences (WMD) using Review Manager 5.1.3 software (The Cochrane Collaboration, Oxford, UK). We calculated the statistical heterogeneity using a chi-square test on N-1 degree of freedom (N = sample size). We also assessed the inconsistency I2 using the formula: (Q-df)/Q × 100% (Q = the chi-square statistic; df = degree of freedom) to describe the percentage of the variability in effect estimates attributable to the heterogeneity [20]. We considered I2 values of 25%, 50%, and 75% as low, medium, and high heterogeneity, respectively. A fixed effects model was used if there was no statistical heterogeneity among the studies; otherwise, we used the random effects model.
Funnel plots were used to assess publication bias among the included trials graphically. Sensitivity analyses were performed to evaluate whether specified factors (methodologic parameters: ITT analysis, adequate randomization; potential relevant modifiers: control design, duration of back pain before trials, bone edema of vertebral fracture observed on MRI in inclusion criteria) could influence the overall effects of pain scoring and recurrence of fractures. We performed such sensitivity analyses only if there were three or more trials included in the comparison.
Results
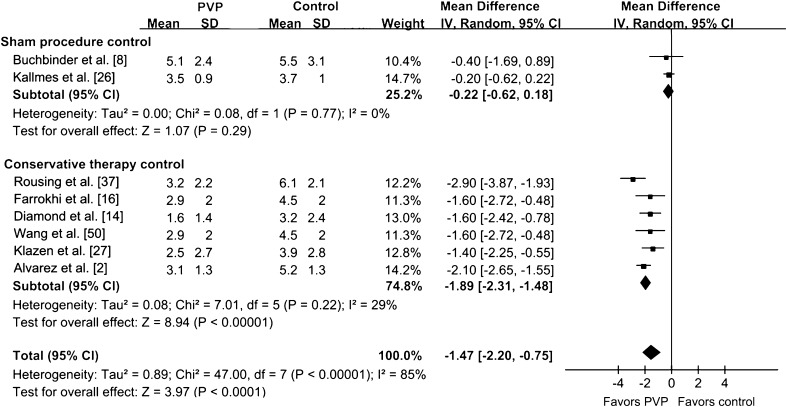
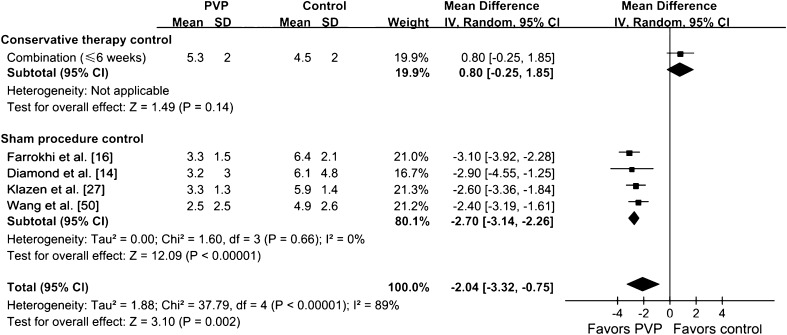
We found no difference in pain scoring between the PVP group and the sham injection group at 1 to 29 days (p = 0.68) (Fig. 2). There also was no difference in pain scoring between the PVP group and the sham injection group at 90 days (p = 0.29) (Fig. 3). However, compared with nonoperative therapy, PVP reduced pain (p < 0.001) at 1 to 29 days (Fig. 2). Pain relief in the PVP group also was greater than that of the conservative group at 90 days (Fig. 3) and 180 days (Fig. 4). The results of pain scoring at different times were heterogeneous in some degree. In the subgroup analysis, compared with nonoperative therapy, the efficacy of PVP on pain relief was greater than that of the controlled treatment at 1 to 29 days (p = 0.002) (Fig. 5), of which the recent onset of pain was 6 weeks or less. For the patients whose recent onset of pain was 6 weeks or less, pain relief in the PVP group was greater than that of the conservative therapy group at 90 days (p = 0.0005) (Fig. 6). The overall results of subgroup analysis were not changed by omitting two trials [37, 47] with sample sizes less than 50 (WMD difference [95% CIs] at 1 to 29 days, −2.25 [−3.29, −1.21], p < 0.0001; 90 days, −1.45 [−2.01, −0.89], p < 0.00001), or by omitting four trials [2, 16, 47, 50] without ITT analysis (WMD difference [95% CIs] at 1 to 29 days, −1.89 [−3.62, −0.15], p = 0.02; 90 days, −1.18 [−1.88, −0.48], p = 0.001). Pain relief at 90 days for PVP was greater (p = 0.002) in studies with bone edema of vertebral fractures observed on MRI as an inclusion criterion than in those without this inclusion criterion (Table 4). Pain relief at 90 days for PVP was greater (p = 0.03) in the adequately randomized studies than in the inadequately randomized studies (Table 4). Using primary subgroup analysis of the two control types, the global heterogeneity in effect for pain relief between these two groups were: 1 to 29 days, I2 = 96%; 90 days, I2 = 85%; and greater than 180 days, I2 = 38%. However, the heterogeneity of pain scoring analysis decreased at all times in both subgroups (sham injection controlled subgroup: 1–29 days, I2 = 54%; 90 days, I2 = 0%; greater than 180 days, not available; nonoperative therapy controlled subgroup: 1–29 days, I2 = 0%; 90 days, I2 = 29%; greater than 180 days, I2 = 0%). Potential publication bias was observed for both pain scores at 1 to 29 days and at 90 days in the funnel plots (Fig. 7).
Fig. 2.
The forest plot for pain relief at 1 to 29 days shows a difference between vertebroplasty and nonoperative therapy but no difference between vertebroplasty and sham injection. IV = inverse variance; PVP = percutaneous vertebroplasty.
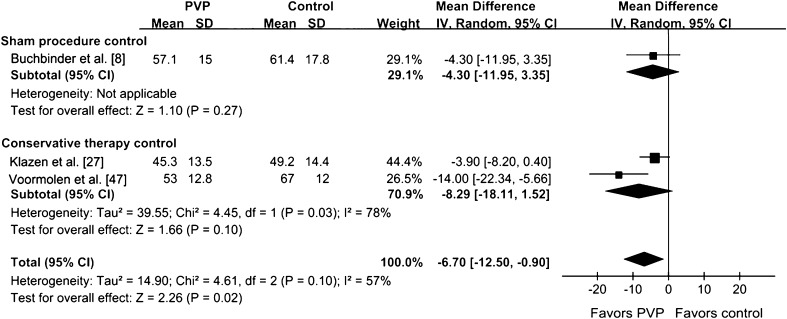
Fig. 3.
The forest plot for pain relief at 90 days shows a difference between vertebroplasty and nonoperative therapy but no difference between vertebroplasty and sham injection. IV = inverse variance; PVP = percutaneous vertebroplasty.
Fig. 4.
The forest plot for pain relief greater than 180 days shows a difference between vertebroplasty and nonoperative therapy but no difference between vertebroplasty and sham injection. IV = inverse variance; PVP = percutaneous vertebroplasty.
Fig. 5.
The forest plot shows pain relief in the subgroup for which pain of recent onset was 6 weeks or less compared with control treatment at 1 to 29 days. IV = inverse variance; PVP = percutaneous vertebroplasty.
Fig. 6.
The forest plot shows pain relief in the subgroup for which pain of recent onset was 6 weeks or less compared with control treatment at 30 to 90 days. IV = inverse variance; PVP = percutaneous vertebroplasty.
Table 4.
Subgroup analyses of the included studies at different times by different influential factors
| Factors | 1 to 29 days | 90 days | 180 days | Refractures | ||||
|---|---|---|---|---|---|---|---|---|
| Subgroups (number) | WMD* (95% CI) | Subgroups (number) | WMD† (95% CI) | Subgroups (number) | WMD (95% CI) | Subgroups (number) | WMD (95% CI) | |
| Recent onset pain‡ | Yes (4) | −2.70 (−3.14, −2.26) | Yes (3) | −2.10 (−2.65, −1.55) | Yes (4) | −1.06 (−1.58, −0.53) | Yes (4) | 0.73 (0.42, 2.53) |
| No (2) | −2.99 (−3.68, −2.26) | No (3) | −1.55 (−1.86, −1.25) | No (1) | −1.30 (−1.88, −0.72) | No (2) | 1.11 (0.48, 2.53) | |
| p = 0.48 | p = 0.09 | p = 0.88 | p = 0.42 | |||||
| Edema seen on MRI in inclusion criteria | Yes (4) | −3.07 (−3.47, −2.66) | Yes (3) | −2.12 (−2.53, −1.70) | Yes (3) | −1.35 (−1.68, −1.01) | Yes (4) | 0.79 (0.48, 2.62) |
| No (2) | −2.74 (−3.43, −2.05) | No (3) | −1.02 (−1.41, −0.63) | No (2) | −1.30 (−1.93, −0.66) | No (2) | 1.25 (0.34, 4.62) | |
| p = 0.42 | p = 0.002 | p = 0.88 | p = 0.51 | |||||
| ITT analysis | Yes (3) | −2.59 (−3.19, 0−1.99) | Yes (2) | −1.60 (−2.26, −0.94) | Yes (3) | −1.37 (−1.78, −0.96) | Yes (2) | 5.97 (1.01, 35.17) |
| No (3) | −2.95 (−3.43, −2.48) | No (4) | −1.52 (−1.84, −1.21) | No (3) | −1.30 (−1.73, −0.87) | No (4) | 0.65 (0.39, 1.07) | |
| p = 0.36 | p = 0.84 | p = 0.82 | p = 0.02 | |||||
| Randomized adequately§ | Yes (4) | −1.40 (−3.65, 0.86) | Yes (3) | −1.25 (−1.63, −0.87) | Yes (2) | −1.75 (−2.34, −1.16) | Yes (4) | 0.73 (0.36, 1.46) |
| No (3) | −2.54 (−3.06, −2.02) | No (3) | −1.89 (−2.32, −1.47) | No (3) | −1.20 (−1.54, −0.86) | No (3) | 0.94 (0.50, 1.78) | |
| p = 0.33 | p = 0.03 | p = 0.11 | p = 0.59 | |||||
PVP = percutaneous vertebroplasty; WMD = weighted mean differences; ITT = intention-to-treat; * a negative value of WMD means it favors experimental, and positive value means favoring control; †all positive values in the WMD of pain score and the values ≥ 1 in the WMD of refractures rate means favors control; otherwise favors PVP; ‡yes = all patients ≤ 6 weeks, otherwise “No”; §Yes = only if the methods of sequence generated were explicitly described, otherwise “No”.
Fig. 7A–D.
Asymmetry for the outcomes at different times shows moderate publication bias in pain score at (A) 1 to 29 days, (B) 90 days, (C) 180 days, and (D) reoccurrence of fracture. MD = mean difference; RR = risk ratio.
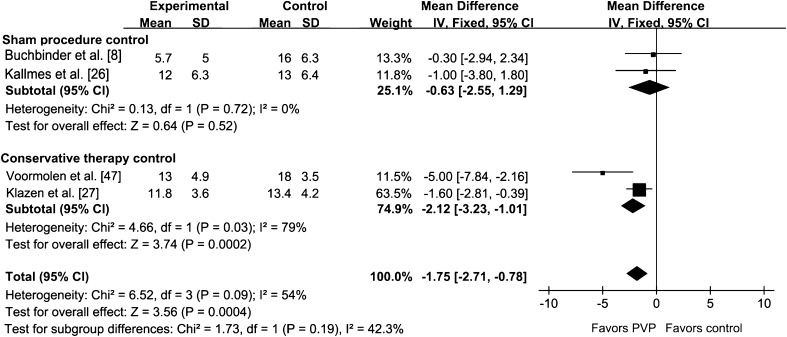
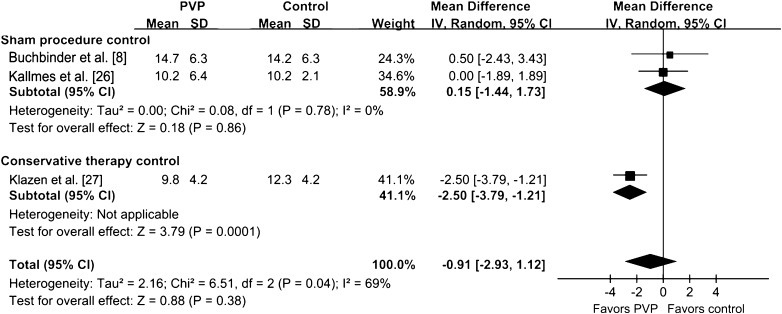
Improvement of QOL in the PVP group was assessed by RDQ, QUALEFFO, and EQ-5D in our analysis. The RDQ score (at 30 days) was greater than that of the control group (p < 0.001) (Fig. 8). The QUALEFFO score was greater than that of the control group (p = 0.02). The RDQ at 90 days for the PVP group was not greater than that of the control group (p = 0.38) (Fig. 9). There was no difference between the PVP group and control group in EQ-5D score (p = 0.06) (Fig. 10). The analysis results of the RDQ at 30 days (Fig. 8) and QUALEFFO (Fig. 11) showed acceptable heterogeneity (RDQ at 30 days, I2 = 54%; QUALEFFO, I2 = 57%), and the results of the RDQ score at 90 days and EQ-5D were heterogeneous (RDQ at 90 days, I2 = 69%; EQ-5D, I2 = 79%).
Fig. 8.
The forest plot shows improvement of QOL in the PVP group assessed by the RDQ score at 30 days. QOL = quality of life; PVP = percutaneous vertebroplasty; RDQ = Roland-Morris Disability Questionnaire; IV = inverse variance.
Fig. 9.
The forest plot shows improvement of QOL in the PVP group assessed by the RDQ score at 90 days. QOL = quality of life; PVP = percutaneous vertebroplasty; RDQ = Roland-Morris Disability Questionnaire; IV = inverse variance.
Fig. 10.

The forest plot shows improvement of QOL in the PVP group assessed by the EQ-5D score. EQ-5D = European Quality of Life-5 Dimensions; PVP = percutaneous vertebroplasty; IV = inverse variance.
Fig. 11.
The forest plot shows improvement of QOL in the PVP group assessed by QUALEFFO score. QOL = quality of life; PVP = percutaneous vertebroplasty; IV = inverse variance.
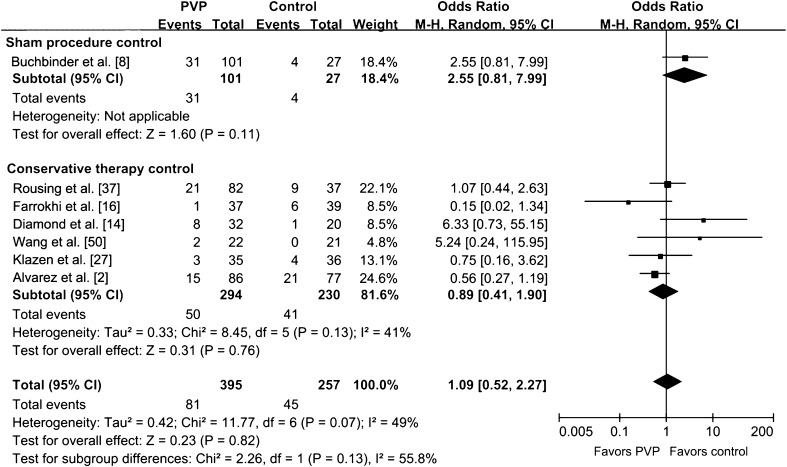
We saw no difference in the risk of new fractures between PVP and controlled treatment (p = 0.82) (Fig. 12). The overall difference in the risk of new fractures was not altered by omitting two trials with sample sizes less than 50 [37, 47] (WMD difference [95% CIs] 1.00 [0.62, 1.61], p = 0.99), or by omitting four trials without ITT analysis [2, 16, 47, 50] (WMD difference [95% CIs] 1.63 [0.90, 2.95], p = 0.11). The studies with ITT analysis obtained more benefit in reducing the risk of new fractures (p = 0.02) than those without ITT analysis. No publication bias was observed in the funnel plot (Fig. 7).
Fig. 12.
The forest plot for risk of new fractures shows no difference in the risk of new fractures was seen between PVP and controlled treatment. PVP = percutaneous vertebroplasty; M-H = Mantel-Haenszel.
Discussion
There has been controversy regarding the ability of PVP to relieve pain and restore function after VCFs. Although most clinical evidence supports the use of PVP [2, 14, 15, 27, 37, 47, 50], two more recent RCTs [8, 26] found no advantage of PVP over controls, thus challenging earlier literature. Instead of using nonoperative treatment as controls as in the earlier studies, the two more recent RCTs used sham surgery as controls. Furthermore, some retrospective evidence [44] suggests PVP might increase the risk of recurrent fractures. We therefore performed a meta-analysis based on different controls with the aims of determining whether PVP would better relieve pain, provide better QOL improvement, and reduce the recurrence of fractures, compared with either nonoperative therapy or sham injection.
Our meta-analysis has some limitations. First, owing to the limited number of included trials, we could not analyze the influence of other clinically relevant factors, for example, baseline severity of back pain, initial medication, and different methods of nonoperative therapy. Second, as the included trials did not separate the results according to their respective indications for PVP in detail, it was impossible to draw conclusions regarding the effect of PVP on other analogues of VCFs, for example, Kümmell’s spondylitis. Third, missing information such as loss to followup, declining participation, and crossover led to incomplete data and potentially biased results in two trials [26, 27], but one of them used ITT analysis, and presumably reduced the bias [26]. Fourth, the small sample sizes and moderate methodologic quality of some included studies (eg, failure to note the process of ITT analysis) might cause bias in our meta-analysis. However, by omitting these two trials [37, 47] or four trials without ITT analysis [2, 16, 47, 50], the results of overall difference analysis in pain relief and risk of new fractures were unchanged.
During the past few years PVP has been recommended for patients with VCFs that have no response to traditional therapy [23, 33]. However, its ability to relieve pain was questioned owing to two multicenter, randomized, placebo-controlled trials, reporting that pain and QOL with PVP were not improved compared with the sham injection of PMMA [8, 26]. Our meta-analysis showed a different control group might account for different assessments of pain relief and function recovery for PVP reported in the literature. Compared with the nonoperative therapy, PVP was more effective for pain relief and function improvement. The two trials using sham injections as a control group found pain relief and disability improvement were similar between the PVP group and sham group [8, 26]. Therefore, we asked why these two different control groups resulted in different assessments of pain relief and function recovery for PVP. Our interpretation included: (1) local analgesia of the sham procedure might contribute to a certain effect of pain relief. Local injection with bupivacaine reportedly acts as a nerve block to relieve pain for more than 3 months [31]; (2) The improvements in sham control subjects were likely attributable to being in the prone position during and after the injection, which might have been helpful in restoring vertebral height, relieving pain, and recovering function to a certain extent [43]; (3) Although these two trials questioned the benefits of PVP, there was still a trend toward a better outcome with PVP; (4) The two trials were subject to potential selection bias resulting from a high proportion of patients declining to enroll and a high crossover rate in these two trials, respectively; (5) The two studies included subacute and chronic fractures in 72.7% of all participants (even up to 1 year); additionally, bone edema of vertebral fractures observed on MR images was not a prerequisite inclusion criterion. These two factors might contribute to the impaired effect of PVP for some chronic fractures that probably were included [19, 48]; (6) The relatively small sample size of these two studies (n = 209) might have led to false negative differences between PVP and sham injection; (7) The main assessment of healing effect of PVP used in these trials, including pain scoring (VAS) and other questionnaires, was a comparatively subjective appraisement, which might be influenced by the placebo effect. Although the sham procedure might have a placebo effect, the two trials comparing PVP with a sham procedure [8, 26] eliminated bias of confounding treatments. In this sense the results might be closer to the truth than the trials that compare PVP with nonoperative treatment. For a more convincing conclusion, more RCTs with sham controls should be done.
A previous meta-analysis [39] evaluated the effect of PVP on vertebral fractures using data from two randomized placebo controlled trials [8, 26], however our review was the first attempt to include all the available prospective evidence, of which most was published during the past 5 years. We developed explicit inclusion and exclusion criteria. In addition, the nine included trials were prospective controlled studies, of which six were RCTs. Our review quadrupled the sample size compared with the previous meta-analysis (886 versus 209). Thus, the summarized trials in this meta-analysis may constitute the totality of the available evidence on the topic. We performed a comprehensive set of subgroup analyses and a sensitivity analysis not only to explain the heterogeneity, but also to provide additional insights into the potential influential factors of the healing effect on pain relief and function improvement of PVP for VCFs, especially different controls which accounted for different assessments of the efficacy of PVP.
One systematic review [44] including all the retrospective and prospective evidence concluded PVP might increase the risk of new fractures. However, in our analysis only including prospective evidence, there was no elevated risk of new fractures after PVP. The most common adverse event of PVP was leakage of cement, which could have led to paralysis of the spinal cord, but the data regarding complications or adverse events of PVP in all the studies were limited, which needs further investigation.
According to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system of rating quality of evidence [4], each trial reviewed in this meta-analysis began with high-quality evidence, but was downgraded by five categories of limitations (Table 5). Insufficient participants in some studies might cause limitations, and adequate sequence generation, allocation concealment, and blinding were described explicitly, which could make the limitation not serious. The main reasons to downgrade the level of evidence was that the imprecision probably resulted from the small sample sizes and two comparisons were considered serious. Thus, the present conclusion regarding the efficacy of PVP for disability improvement should be considered with caution.
Table 5.
GRADE evidence profile of RCTs for effect of vertebroplasty for osteoporotic vertebral fractures
| Outcome | Summary of findings | Quality assessment | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Time (months) | Number (treated/control) | WMD (95% CI, g/cm2) | Risk of bias* | Inconsistency† | Indirectness | Imprecision‡ | Others§ | Quality | |
| Pain score | 1–29 | 8 (449/285) | −2.06 (−3.39, −0.74) | No serious | Not serious | Not serious | Not serious | None | High |
| 90 | 8 463/289) | −1.47 (−2.20, −0.75) | Not serious | Not serious | Not serious | Not serious | None | High | |
| Greater than 180 | 6 (379/237) | −1.25 (−1.64, −0.86) | Not serious | Not serious | Not serious | Not serious | None | High | |
| RDQ score | 30 | 4 (191/161) | −1.75 (−2.71, −0.78) | Not serious | Not serious | Not serious | Serious | None | Moderate |
| 90 | 3 (173/145) | −1.44 (−2.45, −0.44) | Not serious | Not serious | Not serious | Serious | None | Moderate | |
| EQ-5D score | 30–90 | 3 (103/85) | −0.11 (−0.13, −0.08) | Not serious | Not serious | Not serious | Serious | None | Moderate |
| QUALEFFO | 1–29 | 3 (139/129) | −5.68 (−9.10, −2.26) | Not serious | Not serious | Not serious | Serious | None | Moderate |
| Refracture | 7 (81/45) | −1.44 (−2.45, −0.44) | Not serious | Not serious | Not serious | Not serious | None | High | |
GRADE = Grading of Recommendations Assessment, Development and Evaluation; WMD = weighted mean difference; RDQ = Roland-Morris Disability Questionnaire; EQ-5D = European Quality of Life–5 Dimensions; QUALEFFO = Quality of Life Questionnaire of the European Foundation for Osteoporosis; * inadequate blinding, lack of allocation concealed in some trials may increase risk of bias; †inconsistent report of outcomes and significant heterogeneity existed across the trials, but all were well explained by the subgroup analysis; ‡if a study has a wide confidence interval around the estimate of the effect, or included patients less than 400, it may cause imprecision; §“Other” consisted of publication bias and upgraded quality of evidence (large effect, plausible residual confounding and dose-response gradient).
A different control group may account for the different assessment of pain relief, improvement of function, and risk of refracture for PVP reported in the literature. Compared with nonoperative therapy, PVP was more effective in relieving pain and in improving the QOL for patients with VCFs. No matter what the control group—either nonoperative therapy or sham injection, PVP never raised the reoccurrence of fractures. The above argument should be further confirmed with future large sample-sized RCTs using nonoperative therapy and sham injection as controls. In addition, the efficacy of PVP for diseases similar to osteoporosis, like Kümmell’s disease, needs further investigation.
Acknowledgments
We thank all the corresponding authors from the included trials for their kind assistance in obtaining additional data that contributed to our meta-analysis.
Footnotes
The institution of the authors has received funding from the National Natural Science Foundation (81101345); Zhejiang Natural Science Foundation (Y2110239); Zhejiang Key Program Science and Technology (2011C13033); and Zhejiang Medical Research Foundation (2010KYA105); National Clinical Key Specialism Construction Program.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
The first two authors contributed equally to this work and should be considered as co-first authors.
References
- 1.Alfonso Olmos M, Silva Gonzalez A, Duart Clemente J, Villas Tome C. Infected vertebroplasty due to uncommon bacteria solved surgically: a rare and threatening life complication of a common procedure: report of a case and a review of the literature. Spine (Phila Pa 1976) 2006;31:E770–E773. doi: 10.1097/01.brs.0000240202.91336.99. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez L, Alcaraz M, Perez-Higueras A, Granizo JJ, Miguel I, Rossi RE, Quinones D. Percutaneous vertebroplasty: functional improvement in patients with osteoporotic compression fractures. Spine (Phila Pa 1976). 2006;31:1113–1118. doi: 10.1097/01.brs.0000216487.97965.38. [DOI] [PubMed] [Google Scholar]
- 3.Anselmetti GC, Manca A, Hirsch J, Montemurro F, Isaia G, Osella G, Chiara G, Iussich G, Debernardi F, Regge D. Percutaneous vertebroplasty in osteoporotic patients: an institutional experience of 1,634 patients with long-term follow-up. J Vasc Interv Radiol. 2011;22:1714–1720. doi: 10.1016/j.jvir.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Atkins D, Briss PA, Eccles M, Flottorp S, Guyatt GH, Harbour RT, Hill S, Jaeschke R, Liberati A, Magrini N, Mason J, O’Connell D, Oxman AD, Phillips B, Schünemann H, Edejer TT, Vist GE. GRADE Working Group. Systems for grading the quality of evidence and the strength of recommendations II: pilot study of a new system. BMC Health Serv Res. 2005;5:25. doi: 10.1186/1472-6963-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barr JD, Barr MS, Lemley TJ, McCann RM. Percutaneous vertebroplasty for pain relief and spinal stabilization. Spine (Phila Pa 1976). 2000;25:923–928. doi: 10.1097/00007632-200004150-00005. [DOI] [PubMed] [Google Scholar]
- 6.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513–521. doi: 10.1001/jama.2009.50. [DOI] [PubMed] [Google Scholar]
- 7.Boluki D. [Surgical therapy of osteoporotic vertebral body fractures] [in German]. Z Rheumatol. 2011;70:45–55; quiz 55. [DOI] [PubMed]
- 8.Buchbinder R, Osborne RH, Ebeling PR, Wark JD, Mitchell P, Wriedt C, Graves S, Staples MP, Murphy B. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361:557–568. doi: 10.1056/NEJMoa0900429. [DOI] [PubMed] [Google Scholar]
- 9.Chang CY, Teng MM, Wei CJ, Luo CB, Chang FC. Percutaneous vertebroplasty for patients with osteoporosis: a one-year follow-up. Acta Radiol. 2006;47:568–573. doi: 10.1080/02841850600690405. [DOI] [PubMed] [Google Scholar]
- 10.Chen HL, Wong CS, Ho ST, Chang FL, Hsu CH, Wu CT. A lethal pulmonary embolism during percutaneous vertebroplasty. Anesth Analg. 2002;95:1060–1062. doi: 10.1097/00000539-200210000-00049. [DOI] [PubMed] [Google Scholar]
- 11.Chin DK, Kim YS, Cho YE, Shin JJ. Efficacy of postural reduction in osteoporotic vertebral compression fractures followed by percutaneous vertebroplasty. Neurosurgery. 2006;58:695–700. doi: 10.1227/01.NEU.0000204313.36531.79. [DOI] [PubMed] [Google Scholar]
- 12.Cohen LD. Fractures of the osteoporotic spine. Orthop Clin North Am. 1990;21:143–150. [PubMed] [Google Scholar]
- 13.Cortet B, Cotten A, Boutry N, Flipo RM, Duquesnoy B, Chastanet P, Delcambre B. Percutaneous vertebroplasty in the treatment of osteoporotic vertebral compression fractures: an open prospective study. J Rheumatol. 1999;26:2222–2228. [PubMed] [Google Scholar]
- 14.Diamond TH, Champion B, Clark WA. Management of acute osteoporotic vertebral fractures: a nonrandomized trial comparing percutaneous vertebroplasty with conservative therapy. Am J Med. 2003;114:257–265. doi: 10.1016/S0002-9343(02)01524-3. [DOI] [PubMed] [Google Scholar]
- 15.Farrokhi MR, Alibai E, Maghami Z. Randomized controlled trial of percutaneous vertebroplasty versus optimal medical management for the relief of pain and disability in acute osteoporotic vertebral compression fractures. J Neurosurg Spine. 2011;14:561–569. doi: 10.3171/2010.12.SPINE10286. [DOI] [PubMed] [Google Scholar]
- 16.Farrokhi MR, Torabinezhad S, Ghajar KA. Pilot study of a new acrylic cage in a dog cervical spine fusion model. J Spinal Disord Tech. 2010;23:272–277. doi: 10.1097/BSD.0b013e3181b63da6. [DOI] [PubMed] [Google Scholar]
- 17.Furlan AD, Pennick V, Bombardier C, Tulder M. Editorial Board, Cochrane Back Review Group. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine (Phila Pa 1976). 2009;34:1929–1941. doi: 10.1097/BRS.0b013e3181b1c99f. [DOI] [PubMed] [Google Scholar]
- 18.Grados F, Depriester C, Cayrolle G, Hardy N, Deramond H, Fardellone P. Long-term observations of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Rheumatology (Oxford). 2000;39:1410–1414. doi: 10.1093/rheumatology/39.12.1410. [DOI] [PubMed] [Google Scholar]
- 19.Grafe IA, Noldge G, Weiss C, Libicher M, Baier M, Nawroth P, Meeder PJ, Wiedenhöfer B, Kasperk C. Prediction of immediate and long-term benefit after kyphoplasty of painful osteoporotic vertebral fractures by preoperative MRI. Eur J Trauma Emerg Surg. 2011;37:379–386. doi: 10.1007/s00068-010-0050-9. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 21.Hiwatashi A, Moritani T, Numaguchi Y, Westesson PL. Increase in vertebral body height after vertebroplasty. Am J Neuroradiol. 2003;24:185–189. [PMC free article] [PubMed] [Google Scholar]
- 22.Huskisson EC. Measurement of pain. J Rheumatol. 1982;9:768–769. [PubMed] [Google Scholar]
- 23.Jensen ME, McGraw JK, Cardella JF, Hirsch JA. Position statement on percutaneous vertebral augmentation: a consensus statement developed by the American Society of Interventional and Therapeutic Neuroradiology, Society of Interventional Radiology, American Association of Neurological Surgeons/Congress of Neurological Surgeons, and American Society of Spine Radiology. J Neurointerv Surg. 2009;1:181–185. doi: 10.1136/j.jvir.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 24.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 25.Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159:1215–1220. doi: 10.1001/archinte.159.11.1215. [DOI] [PubMed] [Google Scholar]
- 26.Kallmes DF, Comstock BA, Heagerty PJ, Turner JA, Wilson DJ, Diamond TH, Edwards R, Gray LA, Stout L, Owen S, Hollingworth W, Ghdoke B, Annesley-Williams DJ, Ralston SH, Jarvik JG. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361:569–579. doi: 10.1056/NEJMoa0900563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klazen CA, Lohle PN, Vries J, Jansen FH, Tielbeek AV, Blonk MC, Venmans A, Rooij WJ, Schoemaker MC, Juttmann JR, Lo TH, Verhaar HJ, Graaf Y, Everdingen KJ, Muller AF, Elgersma OE, Halkema DR, Fransen H, Janssens X, Buskens E, Mali WP. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II): an open-label randomised trial. Lancet. 2010;376:1085–1092. doi: 10.1016/S0140-6736(10)60954-3. [DOI] [PubMed] [Google Scholar]
- 28.Lee BJ, Lee SR, Yoo TY. Paraplegia as a complication of percutaneous vertebroplasty with polymethylmethacrylate: a case report. Spine (Phila Pa 1976). 2002;27:E419–E422. [DOI] [PubMed]
- 29.Lin WC, Lee CH, Chen SH, Lui CC. Unusual presentation of infected vertebroplasty with delayed cement dislodgment in an immunocompromised patient: case report and review of literature. Cardiovasc Intervent Radiol. 2008;31(suppl 2):231–235. doi: 10.1007/s00270-007-9234-z. [DOI] [PubMed] [Google Scholar]
- 30.Lips P, Cooper C, Agnusdei D, Caulin F, Egger P, Johnell O, Kanis JA, Kellingray S, Leplege A, Liberman UA, McCloskey E, Minne H, Reeve J, Reginster JY, Scholz M, Todd C, Vernejoul MC, Wiklund I. Quality of life in patients with vertebral fractures: validation of the Quality of Life Questionnaire of the European Foundation for Osteoporosis (QUALEFFO). Working Party for Quality of Life of the European Foundation for Osteoporosis. Osteoporos Int. 1999;10:150–160. doi: 10.1007/s001980050210. [DOI] [PubMed] [Google Scholar]
- 31.Manchikanti L, Singh V, Falco FJ, Cash KA, Pampati V. Effectiveness of thoracic medial branch blocks in managing chronic pain: a preliminary report of a randomized, double-blind controlled trial. Pain Physician. 2008;11:491–504. [PubMed] [Google Scholar]
- 32.McGraw JK, Cardella J, Barr JD, Mathis JM, Sanchez O, Schwartzberg MS, Swan TL, Sacks D. Society of Interventional Radiology Standards of Practice Committee. Society of Interventional Radiology quality improvement guidelines for percutaneous vertebroplasty. (Republished from J Vasc Interv Radiol. 2003;14:827-831.) J Vasc Interv Radiol. 2003;14:S311–S315. doi: 10.1097/01.RVI.0000082822.75926.4c. [DOI] [PubMed] [Google Scholar]
- 33.McGraw JK, Cardella J, Barr JD, Mathis JM, Sanchez O, Schwartzberg MS, Swan TL. Society of Interventional Radiology Standards of Practice Committee. Society of Interventional Radiology quality improvement guidelines for percutaneous vertebroplasty. J Vasc Interv Radiol. 2003;14:827–831. doi: 10.1016/S1051-0443(07)60242-5. [DOI] [PubMed] [Google Scholar]
- 34.Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 35.Phillips FM. Minimally invasive treatments of osteoporotic vertebral compression fractures. Spine (Phila Pa 1976). 2003;28(15 suppl):S45–S53. [DOI] [PubMed]
- 36.Ploeg WT, Veldhuizen AG, The B, Sietsma MS. Percutaneous vertebroplasty as a treatment for osteoporotic vertebral compression fractures: a systematic review. Eur Spine J. 2006;15:1749–1758. doi: 10.1007/s00586-006-0159-z. [DOI] [PubMed] [Google Scholar]
- 37.Rousing R, Andersen MO, Jespersen SM, Thomsen K, Lauritsen J. Percutaneous vertebroplasty compared to conservative treatment in patients with painful acute or subacute osteoporotic vertebral fractures: three-months follow-up in a clinical randomized study. Spine (Phila Pa 1976). 2009;34:1349–1354. [DOI] [PubMed]
- 38.Shih MS, Cook MA, Spence CA, Palnitkar S, McElroy H, Parfitt AM. Relationship between bone formation rate and osteoblast surface on different subdivisions of the endosteal envelope in aging & osteoporosis. Bone. 1993;14:519–521. doi: 10.1016/8756-3282(93)90189-H. [DOI] [PubMed] [Google Scholar]
- 39.Staples MP, Kallmes DF, Comstock BA, Jarvik JG, Osborne RH, Heagerty PJ, Buchbinder R. Effectiveness of vertebroplasty using individual patient data from two randomised placebo controlled trials: meta-analysis. BMJ. 2011;343:d3952. doi: 10.1136/bmj.d3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun G, Jin P, Li M, Liu XW, Li FD. Height restoration and wedge angle correction effects of percutaneous vertebroplasty: association with intraosseous clefts. Eur Radiol. 2011;21:2597–2603. doi: 10.1007/s00330-011-2218-z. [DOI] [PubMed] [Google Scholar]
- 41.Sun G, Jin P, Li M, Lu Y, Ding J, Liu X, Li F. Percutaneous cementoplasty for painful osteolytic humeral metastases: initial experience with an innovative technique. Skeletal Radiol. 2011;40:1345–1348. doi: 10.1007/s00256-011-1170-y. [DOI] [PubMed] [Google Scholar]
- 42.Tamayo-Orozco J, Arzac-Palumbo P, Peon-Vidales H, Mota-Bolfeta R, Fuentes F. Vertebral fractures associated with osteoporosis: patient management. Am J Med. 1997;103(2A):44S–50S; discussion 48S–50S. [DOI] [PubMed]
- 43.Tan BHM, Hee HT. Analysis of the effects of percutaneous vertebroplasty in osteoporotic compression fractures. J Orthopaedics. 2011;8(3):e9. Available at: http://www.jortho.org/2011/8/3/e9. Accessed April 26, 2012.
- 44.Trout AT, Kallmes DF. Does vertebroplasty cause incident vertebral fractures? A review of available data. AJNR Am J Neuroradiol. 2006;27:1397–1403. [PMC free article] [PubMed] [Google Scholar]
- 45.Trout AT, Kallmes DF, Gray LA, Goodnature BA, Everson SL, Comstock BA, Jarvik JG. Evaluation of vertebroplasty with a validated outcome measure: the Roland-Morris Disability Questionnaire. AJNR Am J Neuroradiol. 2005;26:2652–2657. [PMC free article] [PubMed] [Google Scholar]
- 46.Uebelhart B, Casez P, Rizzoli R, Louis-Simonet M. Prophylactic injection of methylmetacrylate in vertebrae located between two previously cemented levels does not prevent a subsequent compression fracture in a patient with bone fragility. Joint Bone Spine. 2008;75:322–324. doi: 10.1016/j.jbspin.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Voormolen MH, Mali WP, Lohle PN, Fransen H, Lampmann LE, Graaf Y, Juttmann JR, Jansssens X, Verhaar HJ. Percutaneous vertebroplasty compared with optimal pain medication treatment: short-term clinical outcome of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS study. AJNR Am J Neuroradiol. 2007;28:555–560. [PMC free article] [PubMed] [Google Scholar]
- 48.Voormolen MH, Rooij WJ, Sluzewski M, Graaf Y, Lampmann LE, Lohle PN, Juttmann JR. Pain response in the first trimester after percutaneous vertebroplasty in patients with osteoporotic vertebral compression fractures with or without bone marrow edema. AJNR Am J Neuroradiol. 2006;27:1579–1585. [PMC free article] [PubMed] [Google Scholar]
- 49.Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res. 2005;14:1523–1532. doi: 10.1007/s11136-004-7713-0. [DOI] [PubMed] [Google Scholar]
- 50.Wang HK, Lu K, Liang CL, Weng HC, Wang KW, Tsai YD, Hsieh CH, Liliang PC. Comparing clinical outcomes following percutaneous vertebroplasty with conservative therapy for acute osteoporotic vertebral compression fractures. Pain Med. 2010;11:1659–1665. doi: 10.1111/j.1526-4637.2010.00959.x. [DOI] [PubMed] [Google Scholar]