Abstract
Background
High-dose antimicrobial-loaded bone cement (ALBC) is used to treat orthopaedic infections. High-dose ALBC is not commercially available and requires surgeon directed formulation, and there are several different methods used to mix high-dose ALBC.
Questions/purposes
We asked whether the mixing method affected antimicrobial elution and mechanical properties of high-dose ALBC.
Methods
ALBC was formulated with Simplex® P bone cement and 10 g of vancomycin per batch using one of three mixing methods: (1) hand-stirred using a standard bowl and spatula, (2) bowl-mixed using a mechanical mixing bowl, and (3) dough-phase mixing where the vancomycin was left in chunks (1–5 mm) and folded into the cement during the dough phase after adding the monomer. We eluted 45 standardized test cylinders (15 per mixing technique) for 30 days under infinite sink conditions. We tested 135 (45 per mixing method) similarly eluted cylinders in axial compression to failure.
Results
Dough-phase mixing lead to greater antimicrobial delivery, but lower compressive strength than the hand-stirred or bowl-mixed methods. Dough-phase cement released 18,570 lg of vancomycin versus 11,731 for hand-stirred and 7700 μg for bowl mixed. Compressive strength for dough-phase mixing after 30 days of elution was 36 MPa, while both hand-stirred and bowl mixed cements were 56 MPa.
Conclusions
Performance of high-dose ALBC was affected by mixing method. Dough-phase mixing led to greater antimicrobial delivery, but caused greater loss in compressive strength.
Introduction
Local delivery of antimicrobials is an established modality in the treatment of orthopaedic infections [9], and high antimicrobial concentrations are necessary to control established orthopaedic infections because of the presence of biofilm [5, 17]. Local delivery achieves high local antimicrobial levels while minimizing systemic toxicity [8]. Acrylic bone cement has been a common delivery vehicle for local antimicrobials in the form of antimicrobial-loaded bone cement (ALBC) [9]. Mechanical performance of ALBC decreases with increasing antimicrobial load, limiting the amount that can be loaded in cement used for implant fixation. Low-dose preparations of ALBC (up to 3% by volume) have acceptable mechanical integrity (70 MPa, as defined by ISO 5833 [10]). Low-dose preparations achieve sufficient antimicrobial delivery for prophylaxis [6] and second stage reconstructions, but not for the treatment of established infections [9], which are usually treated with high-dose ALCB. High-dose preparations (greater than 10% by volume) generally require a load of 10 g or more of commonly used antibacterials per batch of acrylic bone cement, [16] have interconnecting porosity capable of delivering higher amounts of antimicrobial, and have been considered appropriate for delivering antimicrobials to the surgical wound following complete resection of established infections [9]. While low-dose preparations (1–2 g per 40 g batch) have been commercially available [15], high-dose preparations (greater than 10 g per 40 g batch) have not.
Surgeon directed formulations of high-dose ALBC have been mixed in the operating room. While some surgeons have attempted to mix the powder components homogeneously by hand-stirring the antimicrobial powder into the polymethylmethacrylate (PMMA) powder using a simple bowl and spatula before polymerization, some have used commercially available mechanical mixing bowls, and others have folded large antimicrobial chunks into the cement during the dough phase of polymerization.
Homogenous mixing of the powders has been aimed at uniform, continuous release over the entire ALBC surface. Polymerizing ALBC with higher volumes of antimicrobial powder is difficult to mix. Folding the antimicrobial powder into the polymerizing cement after it is in the dough phase has the advantage of easier mixing. Keeping the antimicrobial powder in large chunks rather than breaking them up into a fine powder for homogenous mixing has the potential advantage of higher local delivery from the larger chunks in the ALBC. Previous investigations have determined the effect of mixing method on low-dose ALBC, and demonstrated no appreciable difference in antimicrobial release or compressive strength of ALBC made using four mixing methods: no mixing, suspension of antimicrobial powder in monomer, hand-stirred, and bowl-mixed [13, 15]. However, the effects on high-dose ALBC are unknown.
We therefore determined the effects of three mixing methods, hand-stirred, bowl-mixed, and dough-phase, on (1) the release of vancomycin from high-dose ALBC and (2) the compressive strength of ALBC.
Materials and Methods
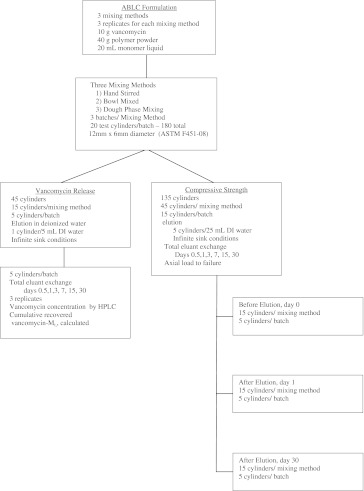
This study used high-dose ALBC mixed with three different mixing techniques. We mixed three batches of high-dose ALBC for each mixing method, resulting in a total of nine batches of cement. We evaluated the cumulative antimicrobial release and compressive strength before and after elution for each of the mixing methods (Fig. 1). Since this was a pilot study, we did not perform a power calculation.
Fig. 1.
The diagram shows the experimental design for mixing method as a determinate for antimicrobial release and compressive strength of high-dose ALBC.
All batches of ALBC in this study were mixed with 10 g of vancomycin hydrochloride powder per batch of Simplex® P acrylic bone cement (Stryker®, Mahwah NJ, USA) using one of three [15] mixing methods, producing high-dose ALBC with a vancomycin volume fraction of approximately 13% [9]. We determined the vancomycin release by elution in deionized water under infinite sink conditions for 30 days. Compressive strength was determined by loading standardized test cylinders (ASTM F451-08) in axial compression to failure before elution and after elution in deionized water under infinite sink conditions for 1 and 30 days.
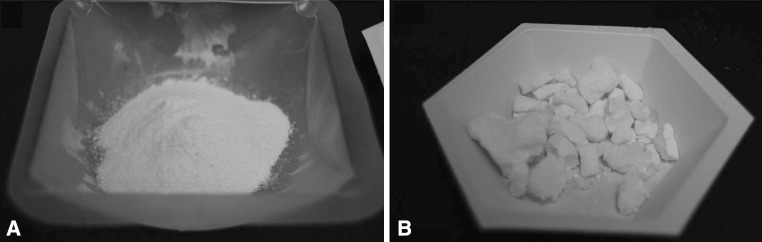
Each of the three mixing methods was replicated three times (totaling nine batches of ALBC) by mixing 10 g of vancomycin hydrochloride (Hospira Inc., Lake Forest, IL, USA) per batch of Simplex® P acrylic cement. The three mixing methods were hand-stirred, bowl-mixed, and dough-phase. Hand-stirred described a method where vancomycin was gently ground to a fine powder with a mortar and pestle then mixed homogeneously with 40 g of PMMA powder by hand-stirring with a spatula in a bowl. Then, 20 mL of monomer were added and the cement polymerized by hand-stirring without vacuum. Bowl-mixed was when vancomycin was gently ground to a fine powder with a mortar and pestle then mixed homogeneously with the PMMA powder in a mechanical mixing bowl. Then, 20 mL of monomer were added and the cement polymerized by hand-stirring without vacuum. Dough-phase mixing was when 40 g of PMMA powder and 20 mL of monomer were polymerized by hand-stirring in a bowl without vacuum. When in the dough phase, 10 g of vancomycin was folded into the polymerizing cement without breaking up the chunks that were present in the bottle. More than half of each gram of vancomycin was in chunks up to 5 mm in cross section, as estimated on visual observation (Fig. 2).
Fig. 2A–B.
The photographs show (A) ground vancomycin powder prior to mixing with PMMA powder, and (B) vancomycin chunks prior to addition to cement. This figure was provided to document the qualitative differences in antimicrobial preparation prior to their use in the different techniques.
Standardized test cylinders, measuring 12 mm long by 6 mm diameter (ASTM F451-08), were fabricated from each batch while it was in the dough phase using a polytetrafluoroethylene mold. The ends of the cylinders were machined flat and square for mechanical testing and to ensure accurate length. We used 20 test cylinders from each of the nine batches, totaling 180 cylinders. From each batch, five of the 20 cylinders were eluted for 30 days, and 15 cylinders were loaded in axial compression to failure, five before elution and 10 following elution, five after 1 day and five after 30 days (Fig. 1).
Five cylinders from each batch were individually eluted in 5 mL of deionized water at 37°C, maintaining infinite sink conditions. Infinite sink conditions exist when the eluant can take on more of the released drug without limitation. High concentrations of the drug decrease the concentration gradient between the delivery vehicle and the eluant, thereby limiting release by diffusion. To avoid limitation of release caused by high concentrations of drug, we performed total eluant exchange before the concentration increased too high, often not more than 10% of the saturation concentration. We confirmed low drug concentration by the level measured at the time of the eluant exchange. Total eluant exchange was performed on Days 0.5, 1, 3, 7, and 15. We eluted a total of 45 cylinders (five from each of the three batches, for each of three mixing methods) for vancomycin release. Vancomycin concentration in the eluate was assayed using isocratic HPLC on a Beckman Gold system (Beckman Coulter, Brea, CA, USA). A Phenomenex Prodigy (Phenomenex, Torrance, CA, USA) 5μ ODS, 100 Å pore diameter, 250 mm by 4.6 mm column was used at a flow rate of 2 mL per minute. Mobile phase was 8:92 acetonitrile to buffer. The buffer was 0.2% triethylamine in water, titrated to a pH of 3 by the addition of phosphoric acid [19]. Detection was performed at 220 nm. We produced a standard curve for standards of known vancomycin concentration. We determined vancomycin concentration in the eluate by interpolation on the standard curve using MATLAB (Mathworks Inc, Natick, MA, USA). We calculated cumulative recovered vancomycin (Mt) at Days 0.5, 1, 3, 7, 15, and 30.
We tested the compressive strength for each mixing method at three time points, one before elution and two after elution on Days 1 and 30. A total of 135 cylinders, 45 per mixing method, (five for each of the three batches for each of the three time intervals) for the three mixing methods were tested for compressive strength. Elution of each five-cylinder group was carried out in 25 mL of deionized water to achieve equivalent elution conditions to one cylinder in 5 mL used for the release studies, maintaining infinite sink conditions at 37°C. Total eluant exchange was performed on Days 0.5, 1, 3, 7, and 15. For each of the three mixing methods, 15 test cylinders were loaded to failure in axial compression at 24 mm per minute (ASTM F451-08) using an MTS Sintech 1/S mechanical testing machine (MTS Systems, Eden Prairie, MN, USA), five before elution and 10 after elution: five after 1 day and five after 30 days of elution. We analyzed load-displacement data using a custom MATLAB algorithm to determine compressive strength in accordance with American Society for Testing And Materials standard F451-08 [2] .
Differences in release of vancomycin and compressive strength of ALBC formulations were determined with repeated-measures analysis of variance (RM-ANOVA) using time in elution, batch, and mixing method as the factors. We confirmed the appropriateness of the ANOVA model results through the use of standard normal plots of residuals, and performed all statistical analyses using Minitab® (Minitab Inc, State College, PA, USA).
Results
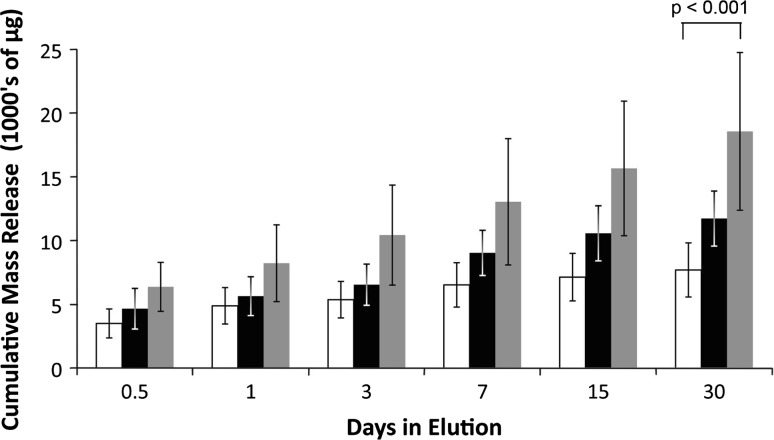
Cumulative vancomycin release over 30 days from dough-phase ALBC was greater (p < 0.001) than that from either hand-stirred or bowl-mixed ALBC (Fig. 3). Recovered vancomycin (Mt) at 30 days was 7700 μg per cylinder (18% of the contained vancomycin) for the hand-stirred method, 11,731 μg per cylinder (26% of the contained vancomycin) for the bowl-mixed method, and 18,570 μg per cylinder (39% of the contained vancomycin) for the dough-phase mixing method. The coefficient of variance (CoV) for vancomycin release was 7% for hand-stirred, 11% for bowl-mix, and 32% for the dough-phase method.
Fig. 3.
The graph shows the cumulative elution of high-dose cement formulated with 10 g of vancomycin. Error bars show standard deviation. White bars indicate the elution from the hand-stirred ALBC. Black bars indicate the elution from the bowl-mixed ALBC. Grey bars indicate the elution of the dough-phase mixed ALBC. Dough-phase mixed ALBC delivered more vancomycin than the other two mixing methods.
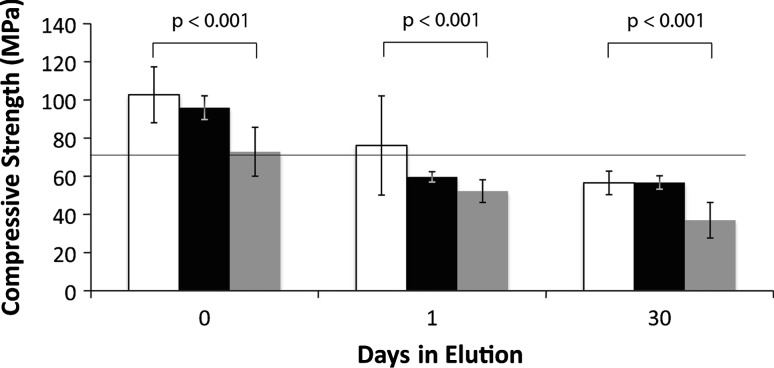
The compressive strength of dough-phase mixed ALBC was lower (p < 0.001) than the compressive strength of ALBC made by either the hand-stirred or bowl-mixed methods (Fig. 4). Compressive strength before elution was 102 MPa for the hand-stirred method, 96 MPa for the bowl-mixed method, and 72 MPa for the dough-phase mixing method. After 30 days in elution, compressive strength was 56 MPa for the hand-stirred method, 56 MPa for the bowl-mixed method, and 36 MPa for the dough-phase mixing method. Additionally, dough-phase mixed ALBC sustained catastrophic fracture into several fragments during compression, unlike the hand-stirred or bowl-mixed ALBC, which underwent plastic deformation.
Fig. 4.
The graph shows compressive strength for high-dose ALBC formulated with 10 g of vancomycin per batch of Simplex® P cement. White bars indicate the strength of the hand-stirred ALBC. Black bars indicate the strength of the bowl-mixed ALBC. Grey bars indicate the strength of the doughphase mixed ALBC. Dough phase mixing produced ALBC that was weaker than the other two mixing methods. Compressive strength decreased over time in elution for all three mixing methods. Error bars indicate standard deviation.
Discussion
Orthopaedic surgeons use high-dose ALBC for local antimicrobial delivery to surgical sites following resection for established orthopaedic infections. High-dose ALBC is a surgeon-directed formulation. Several mixing methods have been used in preparation of high-dose ALBC, but the effect that the mixing method had on the performance of high-dose ALBC was unknown. We determined the effect that three commonly used mixing methods: hand-stirred, bowl-mixed, and dough-phase, had on antimicrobial delivery and compressive strength of high-dose ALBC.
There were little data in the literature that provided direct comparison of surgeon-directed mixing methods for high-dose ALBC. This study provided antimicrobial release and compressive strength data of ALBC prepared with three separate mixing techniques applicable to the clinical preparation of ALBC [9]. While Adams et al. [1] and Keuchle et al. [11] established that antimicrobials were released from high-dose cement over extended periods and increased porosity lead to increased delivery, the effect of mixing method on delivery and strength has not been studied. This study was markedly different from previous studies of low-dose ALBC mixing [13, 15] because the increased volume of antimicrobial powder added to the cement likely changed the pore structure and behavior of the resultant ALBC. Our study did not include vacuum mixing because the goal was to create porosity. Askew et al. [3] reported vacuum mixing ALBC did not increase its mechanical properties due to the effects of the poragen. Antimicrobials are not soluble in monomer. Suspending antimicrobial in the monomer has been shown to be an ineffective mixing method [15]. Finally, our study did not include a control group. Two controls for this study would be possible: high-dose ALBC with no elution, and cement without drug. Cement without drug has been characterized and did not change significantly in elution [14]. The compressive strength of high-dose ALBC stored in a dry area does not represent the environment encountered in clinical use.
Our study had several limitations. First, while our in vitro data were appropriate for comparisons of release and mechanical characteristics of delivery vehicles, these data did not quantitatively represent antimicrobial concentrations that would occur from release in vivo or mechanical durability that could occur in clinical use. In vivo performance is dependent on unknown local conditions, including volume of distribution and fluid dynamics, which affects the magnitude of the response by many multiples. Although the relative performance seen in vitro (more or less release and greater or lower strength) could be expected to exist in vivo, we could not determine the magnitude of the differences from in these in vitro data. Second, we only studied one type of cement and one antimicrobial. Based on our experience with other commonly used antimicrobials and acrylic bone cements, the magnitude of the findings would likely differ, but not enough to draw different conclusions. Further study is necessary to evaluate any differences that may exist with other antimicrobials and other cements. Third, we did not measure the fatigue properties of these ALBC formulations. Fatigue limit was a better mechanical property related to cyclic loading in clinical use; however, compressive strength has been generally accepted as an indication of acrylic cement mechanical properties [10].
Our data on release performance were generally consistent with the reported data [1, 11]. Antimicrobial release for the three mixing methods was in the range expected from high-dose ALBC [16]. Dough-phase ALBC provided more antimicrobial release than bowl-mixed and hand-stirred ALBC. We anticipated increased release from the dough-phase mixing due to the large antimicrobial clumps; however, the clumps were fragile and they did not survive mixing into the dough-phase cement despite our efforts to fold them in gently to preserve them. We observed no intact clumps on inspection of the cement as it was being introduced into the mold or in the cylinders after fabrication, but they may have caused sufficient confluence of antimicrobial to regionally function as a better poragen. The large variation in release performance (CoV, dough-phase, 32%) indicated less homogeneity [15] than the other two mixing methods (bowl-mixed, 11%; hand-stirred, 7%). The increased variability in release was likely related to the antimicrobial clumps causing inhomogeneous antimicrobial distribution within the ALBC. Variation from operator technique was less likely the cause of the large release variation because all batches were mixed by the same investigator, using a standardized technique for each mixing method, and all three batches for each mixing method were mixed one after the other. The higher antimicrobial release during elution from dough-phase ALBC could be associated with increased drug delivery in vivo, although we can not predict the magnitude of that difference. Other studies reporting data on the effect of mixing method were on low-dose formulations [4, 7, 18] and were summarized in Lewis and Bhattaram [13] as part of their study. Lewis and Bhattaram reported no effect on drug delivery or compressive strength for low-dose cement.
Our data on compressive strength were generally consistent with one study that reported compressive strength data on ALBC formulated with up to 10 g of gentamicin per batch of Simplex® P cement [12] (Table 1). The increased drug delivery from dough-phase ALBC came at the cost of decreased mechanical strength. As elution proceeded, areas with higher confluence of antimicrobials left areas of relatively greater porosity in the ALBC, making it weaker. Hand-stirred and bowl-mixed ALBC were stronger than dough-phase mixed ALBC. The variability in compressive strength was greatest for the dough-phase ALBC (CoV, 26%), again indicating less homogeneity than the other two mixing methods (bowl-mixed, 6%; hand-stirred, 11%). Dough-phase mixing was the only mixing method that produced ALBC that was below the 70 MPa limit for implant fixation before elution, and, over time, it lost strength faster than ALBC made with the other two methods. However, all three mixing methods produced ALBC that was far below the 70 MPa recommendation for implant fixation after 1 month of elution. Hand-stirred and bowl-mixed ALBC underwent plastic deformation during compression testing, whereas the dough-phase ALBC sustained catastrophic fracture into multiple pieces. Fragmentation at failure and lower compressive strength made this formulation less desirable for structural applications, such as load-bearing spacers. However, our use of small test cylinders in this study may have overestimated the loss in mechanical properties caused by dough-phase mixing. The standardized test cylinders were relatively small (6 mm) compared to the size of the vancomycin clumps (5 mm). Although the clumps appeared to have broken up during the mixing, with no visible clumps after cylinder fabrication, there was likely a greater regionalization in vancomycin distribution in the resultant ALBC, as shown by the greater coefficient of variance. This variation in vancomycin distribution may have been relatively more important for a 6-mm cylinder than it would be for a spacer that could be several centimeters in cross section.
Table 1.
Relevant literature
| Study | Elution | Compression |
|---|---|---|
| Adams et al. [1] | High-dose cement delivers over 4 weeks for a variety of antimicrobials | Not studied |
| Kuechle et al. [11] | Increased porosity leads to increased delivery for high-dose cement | Not studied |
| Lautenschlager et al. [12] | Not studied | The addition of up to 10 g of gentamicin sulfate to Simplex P caused gradual loss in mechanical strength as dose increased from low to high-dose with a single mixing method |
| Lewis and Bhattaram [13] | Not studied | Low-dose ALBC commercially premixed vs hand mixed no difference in compressive strength, fatigue, or saline uptake |
| McLaren et al. [15] | Mixing method did not change the elution properties of low-dose cement | Unpublished data, mixing method did not affect compressive strength for low-dose cement |
In conclusion, the dough-phase method of mixing high-dose ALBC released more vancomycin, but had weaker compressive strength than hand-stirred and bowl-mixed high-dose ALBC. The higher vancomycin release would be expected to deliver more vancomycin in vivo, making it more desirable for local delivery, but would be expected to fail at lower loads, making it less desirable for structural applications.
Acknowledgments
The authors thank Francis Calara BSE (HPLC assays and data analysis) and Mary Martin PharmD at Banner Good Samaritan Medical Center, and John Lopez and Zachary Laughrey at ASU (use of the Proteomics Laboratory and technical support for HPLC) for their contributions to this study.
Footnotes
One or more of the authors received funding from the Southwest Orthopaedic Trauma Association (SWOTA) (RBM), the Herbert Louis Fund at the OREF (ACM), and Banner Good Samaritan Medical Center (RM).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
This work was performed at Arizona State University and Banner Good Samaritan Medical Center, Phoenix, AZ, USA.
References
- 1.Adams K, Couch L, Cierny G, Calhoun J, Mader JT. In vitro and in vivo evaluation of antibiotic diffusion from antibiotic-impregnated polymethylmethacrylate beads. Clin Orthop Relat Res. 1992;278:244–252. [PubMed]
- 2.American Society for Testing And Materials, Subcommittee F04.11. ASTM F451-08 Standard Specification for Acrylic Bone Cement. 2008.13.01. Available at: http://www.astm.org/Standards/F451.htm. Accessed December 19, 2011.
- 3.Askew MJ, Kufel MF, Fleissner PR, Jr, Gradisar IA, Jr, Salstrom S, Tan JS. Effect of vacuum mixing on the mechanical properties of antibiotic‐impregnated polymethylmethacrylate bone cement. J Biomed Mater Res. 1990;24:573–580. doi: 10.1002/jbm.820240504. [DOI] [PubMed] [Google Scholar]
- 4.Davies JP, O’Connor DO, Burke DW, Harris WH. Influence of antibiotic impregnation on the fatigue life of simplex P and palacos R acrylic bone cements, with and without centrifugation. J Biomed Mater Res. 1989;23:379–397. doi: 10.1002/jbm.820230402. [DOI] [PubMed] [Google Scholar]
- 5.Diefenbeck M, Mückley T, Hofmann GO. Prophylaxis and treatment of implant-related infections by local application of antibiotics. Injury. 2006;37:S95–S104. doi: 10.1016/j.injury.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Engesæter LB, Espehaug B, Lie SA, Furnes O, Havelin LI. Does cement increase the risk of infection in primary total hip arthroplasty? Revision rates in 56,275 cemented and uncemented primary THAs followed for 0–16 years in the Norwegian Arthroplasty Register. Acta Orthop. 2006;77:351–358. doi: 10.1080/17453670610046253. [DOI] [PubMed] [Google Scholar]
- 7.Hansen D, Jensen JS. Mixing does not improve mechanical properties of all bone cements. Manual and centrifugation-vacuum mixing compared for 10 cement brands. Acta Orthop Scand. 1992;63:13–18. doi: 10.3109/17453679209154841. [DOI] [PubMed] [Google Scholar]
- 8.Hanssen AD. Local antibiotic delivery vehicles in the treatment of musculoskeletal infection. Clin Orthop Relat Res. 2005;437:91–96. doi: 10.1097/01.blo.0000175713.30506.77. [DOI] [PubMed] [Google Scholar]
- 9.Hanssen AD, Spangehl MJ. Practical applications of antibiotic-loaded bone cement for treatment of infected joint replacements. Clin Orthop Relat Res. 2004;427:79–85. doi: 10.1097/01.blo.0000143806.72379.7d. [DOI] [PubMed] [Google Scholar]
- 10.International Organization for Standardization. ISO 5833. Implants for surgery-acrylic resin cements 2002. Available at: http://www.iso.org/iso/iso_catalogue/catalogue_tc/catalogue_detail.htm?csnumber=30980. Accessed December 19, 2011.
- 11.Kuechle DK, Landon GC, Musher DM, Noble PC. Elution of vancomycin, daptomycin, and amikacin from acrylic bone cement. Clin Orthop Relat Res. 1991;264:302–308. [PubMed] [Google Scholar]
- 12.Lautenschlager EP, Jacobs JJ, Marshall GW, Meyer PR. Mechanical properties of bone cements containing large doses of antibiotic powders. J Biomed Mater Res. 1976;10:929–938. doi: 10.1002/jbm.820100610. [DOI] [PubMed] [Google Scholar]
- 13.Lewis G, Bhattaram A. Influence of a pre-blended antibiotic (gentamicin sulfate powder) on various mechanical, thermal, and physical properties of three acrylic bone cements. J Biomater Appl. 2006;20:377–408. doi: 10.1177/0885328206055124. [DOI] [PubMed] [Google Scholar]
- 14.Lewis G. Fatigue testing and performance of acrylic bone‐cement materials: state‐of‐the‐art review. J Biomed Mater Res B Appl Biomater. 2003;66:457–486. doi: 10.1002/jbm.b.10018. [DOI] [PubMed] [Google Scholar]
- 15.McLaren A, Nugent M, Economopoulos K, Kaul H, Vernon B, McLemore R. Hand-mixed and premixed antibiotic-loaded bone cement have similar homogeneity. Clin Orthop Relat Res. 2009;467:1693–1698. doi: 10.1007/s11999-009-0847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nugent M, McLaren A, Vernon B, McLemore R. Strength of antimicrobial bone cement decreases with increased poragen fraction. Clin Orthop Relat Res. 2010;468:2101–2106. doi: 10.1007/s11999-010-1264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belt H, Neut D, Schenk W, Horn JR, Mei HC, Busscher HJ. Staphylococcus aureus biofilm formation on different gentamicin-loaded polymethylmethacrylate bone cements. Biomaterials. 2001;22:1607–1611. doi: 10.1016/S0142-9612(00)00313-6. [DOI] [PubMed] [Google Scholar]
- 18.Wright TM, Sullivan DJ, Arnoczky SP. The effect of antibiotic additions on the fracture properties of bone cements. Acta Orthop Scand. 1984;55:414–418. doi: 10.3109/17453678408992386. [DOI] [PubMed] [Google Scholar]
- 19.Xu Q, Trissel L. Stability-Indicating HPLC Methods for Drug Analysis. London, UK: Pharmaceutical Press; 2003. [Google Scholar]