Abstract
Background
Sonication and scraping of infected prostheses usually are used to improve diagnosis of prosthetic infections, reducing false negatives. Chemical methods that reduce biofilms also may allow higher levels of detection.
Questions/Purposes
We therefore asked: (1) Do dithiothreitol (DTT) and N-acetylcysteine (NAC) remove bacteria from biofilm formed on prosthetic materials? (2) Is bacterial recovery affected by differing DTT and NAC concentrations and incubation times? (3) Do treatments with DTT and NAC detach the same amounts of bacteria from biofilm on prosthetic materials as sonication and scraping? (4) Are these methods reproducible?
Methods
We treated polyethylene and titanium discs covered by biofilm formed by Pseudomonas aeruginosa and Staphylococcus aureus with DTT or NAC solutions at different concentrations for different times. We compared colony counts of S aureus, P aeruginosa, Staphylococcus epidermidis and Escherichia coli after treatment with NAC, DTT, sonication and scraping. We determined colony counts after treatment of biofilm formed by one strain of S aureus and one of P aeruginosa on five discs of each material analyzed on the same day and on five discs analyzed on five consecutive days.
Results
Mean colony counts (LogCFU/mL) obtained after treatment with 1 g/L DTT for 15 minutes (5.3) were similar to those after sonication (4.9) and greater than those obtaining by scraping (3.4) and treatment with 2 g/L NAC for 30 minutes (1.9). DTT and sonication showed good reproducibility.
Clinical Relevance
Our data suggest that treatment of prostheses with DTT may be a reasonable alternative to sonication to improve detection of biofilm-associated bacteria and supplement conventional laboratory culturing techniques for diagnosing periprosthetic infections.
Introduction
Prosthetic joint infections (PJIs) are one of the major causes of implant failure leading to pain, major disruption to patient’s lives, repeated surgeries, and prolonged antibiotic therapies, occurring in 0.8% to 1.9% with an increase of as much as 20% for revision procedures [6, 14, 15, 21, 22, 24]. PJIs typically are caused by adherence of microorganisms onto the surface of a foreign body, which progresses to an aggregation forming a biofilm [9].
A major problem for microbiologic diagnosis of these infections is that bacteria in a biofilm attach well to the surface of foreign bodies and to each other and resist detection by conventional methods [8]. Given a frequency of false negatives of 7% to 20% and sensitivity of approximately 40% to 70% associated with tissue cultures [3, 13, 30], methods have been developed to dislodge the pathogens causing the infection from the biofilm [1, 3, 12, 13, 23, 30]. Sonication reportedly yields rates of successful bacterial recovery of 70% to 100% compared with 10% to 100% when scraping the prosthetic surface [3] and sensitivity of approximately 65% to 80% depending on prior antibiotic therapy [13, 30]. Combining vortexing and sonication has further improved an increase in sensitivity greater than 10% with respect to sonication only (78.5% versus 60.8%) [28, 29]. Routine diagnostic systems based on biochemical reactions may not accurately identify bacteria dislodged from implants by sonication. Escherichia coli, for example, reportedly forms microcolonies on agar plates characterized by different pigmentation and hemolysis and by inability to synthesize essential nutrients compared with planktonic isolates and reference strains [27]. Moreover, difficulties in managing large implants and the risk of their contamination during handling must be considered [29].
N-acetylcysteine (NAC) is a well-known antioxidant thiol with antimicrobial properties, and given its ability to dissolve mucus, it is widely used in treating respiratory tract infections. NAC application reportedly decreases either biofilm formation by different bacteria or the production of extracellular polysaccharide matrix along with the removal of mature biofilm [19]. Dithiothreitol (DTT) is a strong reducing agent commonly used in chemical laboratories to reduce disulfide bonds and to maintain monothiols in a reduced state. It also acts as a protein denaturant, cleaving disulfide linkages between cysteine groups in proteins and peptide. For these reasons, DTT is widely used in microbiology laboratories for liquefying specimens from the respiratory tract. Moreover, it reportedly reduces staphylococcal biofilm [32] and thus may enhance detachment of bacteria from biofilms on prosthetic materials. We therefore reasoned DTT and NAC might be used to remove bacteria from prosthetic implants.
We asked the following questions: (1) Do DTT and NAC remove bacteria from biofilm formed on prosthetic materials? (2) Is bacterial recovery affected by differing DTT and NAC concentrations and incubation times? (3) Do treatments with DTT and NAC detach the same amounts of bacteria from biofilm on prosthetic materials as sonication and scraping? (4) Are these methods reproducible?
Materials and Methods
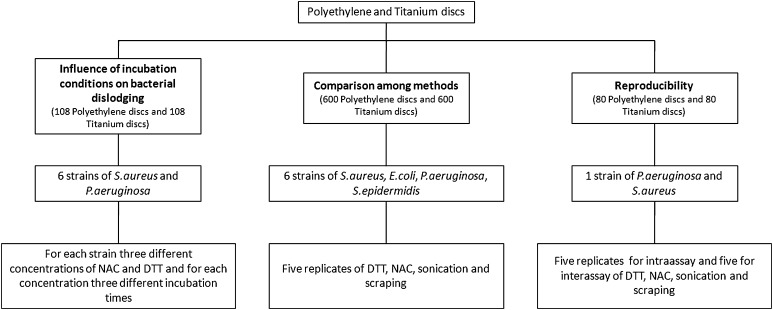
The experimental design of the study is summarized in Figure 1. For the first and second questions, the number of colony forming units (CFU)/mL was calculated after treatment of 108 polyethylene (PE) and 108 titanium (Ti) discs covered by biofilm formed by six isolates of Pseudomonas aeruginosa and six of Staphylococcus aureus, with DTT and NAC solutions at different concentrations for different times (Fig. 2). All the bacteria used were isolated from PJIs and characterized by a strong production of biofilm, which was evaluated using a previously described spectrophotometric method [7]. As materials used in orthopaedic surgery have different affinities for bacteria [18], we assessed discs with different compositions and surface roughnesses similar to those found in commonly used prostheses.
Fig. 1.
The diagram shows the experimental design and the scope of the study.
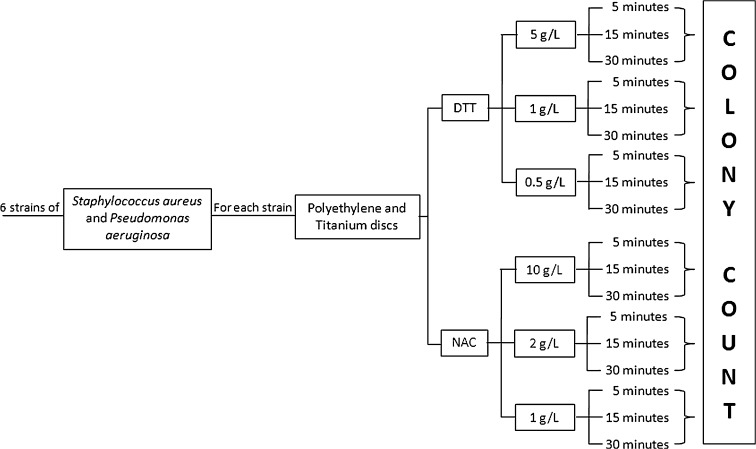
Fig. 2.
The flow chart shows the research design used to determine the optimal conditions of treatment with dithiothreitol (DTT) and N-acetylcysteine (NAC).
To obtain bacterial biofilms, PE and Ti discs, with a diameter of 20 mm and thickness of 6 mm (AdlerOrtho SpA, Milan, Italy), were placed in six-well polystyrene plates (CELLSTAR® Multiwell Plates; VWR International, Milan, Italy) containing 5 mL brain heart infusion broth and inoculated with approximately 1.2 × 108 CFU of bacteria per milliliter. After overnight incubation at 37°C, the medium was refreshed to remove nonadherent bacteria and plates were further incubated for 72 hours under the same conditions until a visible biofilm was formed. Then, each disc was placed in a sterile container with 5 mL sterile saline and gently mixed for 10 seconds. Discs then were transferred to another sterile container and the same procedure was repeated six times to remove the nonadherent bacteria. After washings, the discs were transferred to six-well plates and treated with 5 mL NAC or DTT solutions at different concentrations under agitation for 5, 15, and 30 minutes. NAC was tested at 1 g/L, 2 g/L, and 10 g/L, while DTT solutions were prepared at 0.5 g/L, 1 g/L and 5 g/L (Fig. 2). The total final count was performed by plating 0.01 mL and 0.1 mL from treated bacterial suspensions (after proper dilutions) on Mueller-Hinton agar plates (DASIT, Cornaredo, Italy) incubated aerobically at 37°C for 18 hours.
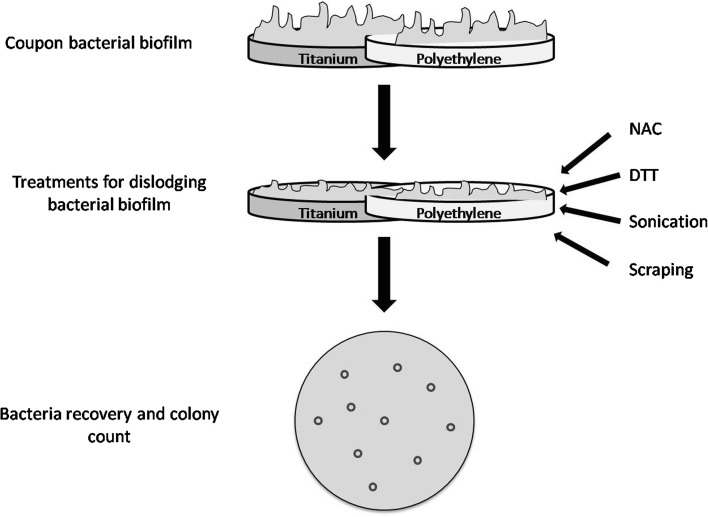
To compare removal of bacteria from prosthetic materials by different methods (Fig. 3), the number of CFU/mL was calculated after treatment with DTT, NAC, sonication, or scraping of PE (n = 600) and Ti (n = 600) discs covered by biofilm formed by six isolates of P aeruginosa, six of S aureus, six of Staphylococcus epidermidis, and six of E coli (Fig. 4). Once biofilm was established as described above, PE and Ti discs were washed to remove nonadherent bacteria. Treatment with DTT and NAC consisted, respectively, of incubation with 5 mL of 1 g/L DTT or 2 g/L NAC solutions for 15 minutes and 30 minutes under agitation. The total final count was performed by plating 0.01 mL and 0.1 mL treated bacterial suspension (after proper dilutions) on Mueller-Hinton agar plates (DASIT) incubated aerobically at 37°C for 18 hours. Sonication was performed by transferring coupons onto sterile plastic containers with 5 mL sterile saline. The container then was sealed and immersed in an ultrasonic bath (VWRTM Ultrasonic Cleaner; VWR International). Sonication at 30 kHz with a power output of 300 W was performed at 37°C for 5 minutes. The resulting sonicated fluid was plated in 0.1-mL aliquots on Mueller-Hinton agar plates incubated as described above. We used a sterile surgical blade to scrape the whole surface of the disc. The blade then was transferred into a sterile tube containing 5 mL sterile saline, and after vortexing for 30 seconds, the total colony count was performed as described above.
Fig. 3.
The figure shows the experimental plan of the study.
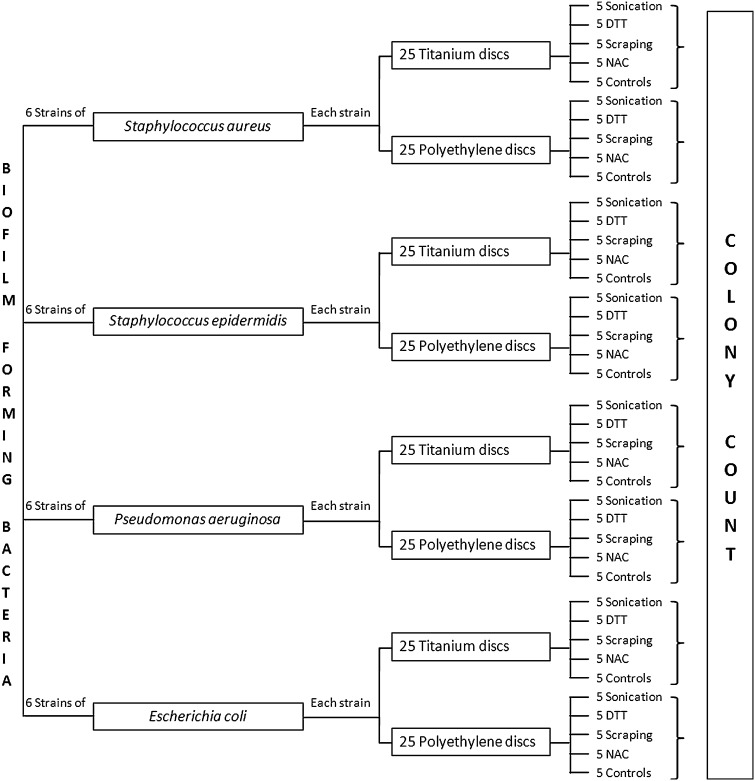
Fig. 4.
The flow chart shows the research design used to compare bacterial recovery from PE and Ti coupons by using different methods.
Experiments to compare removal of bacteria from prosthetic materials by the different methods were performed in quintuplicate for each isolate (Fig. 4).
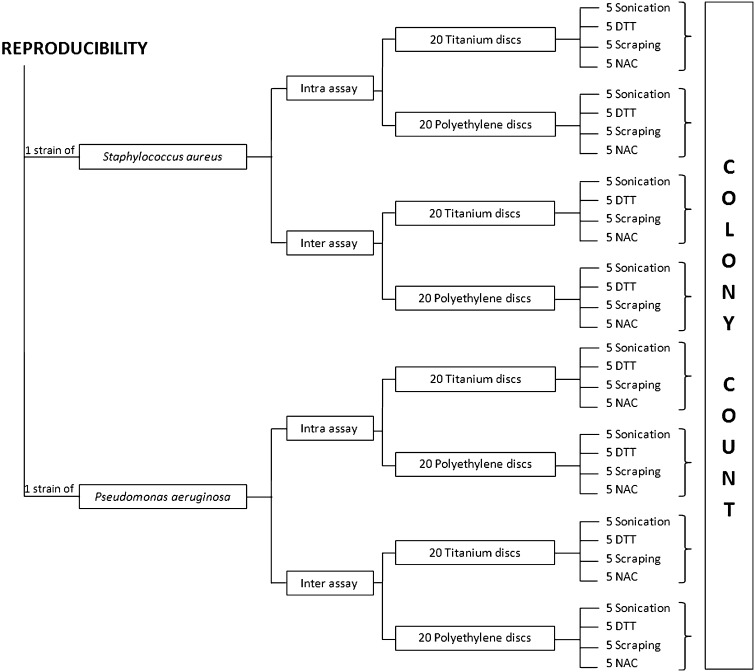
To evaluate the intraassay reproducibility of each method, variation in colonies counts, expressed as coefficient of variation (CV%), was calculated after treating as described above, on the same day, five Ti and five PE discs covered by biofilms formed by one isolate of S aureus and one of P aeruginosa (Fig. 5). Interassay reproducibility was determined by calculating CV% of colony counts obtained on five consecutive days after treatment of five Ti and five PE coupons covered by biofilm produced by one isolate of P aeruginosa and one of S aureus which were inoculated, as described above, on five consecutive days (Fig. 5).
Fig. 5.
The research design used to evaluate reproducibility of the tested methods is shown in this flow chart.
We used confocal laser scanning microscopy to confirm biofilm formation on PE and Ti discs and to evaluate removal of the adherent bacteria by the methods under comparison. Treated discs were stained with 0.4 mL SYTO® 9 green-fluorescent nucleic acid stain, according to the manufacturer’s description (LIVE/DEAD® BacLight™ Bacterial Viability Kits; Invitrogen, Milan, Italy), and 0.2 mL FilmTracerTM SYPRO® Ruby Biofilm Matrix Stain (Invitrogen) for 20 minutes in the dark. The excess stain was rinsed twice with 0.4 mL distilled water. In addition, untreated biofilms formed by P aeruginosa and S aureus on PE and Ti discs were used as controls and stained as described above. Images were captured using a Leica TCS SP5 confocal microscope equipped with an ArgonPlus™ Ar ion laser, a diode laser, and an HCX PL APO CS ×63 oil immersion lens (all from Leica Microsystems, Wetzlar, Germany). The excitation/emission wavelengths were set such that the live stain (SYTO® 9) appeared green at 488/500 nm and the extracellular polymeric substance-specific stain (SYPRO® Ruby) appeared red at 405/610 nm. We performed image analyses using ImageJ 1.43u (NIH, Bethesda, MD, USA) by adjusting output levels in the individual channels and assembling the images into image stacks for maximum projection. No other manipulation of the images was performed. Qualitative comparison of the images was performed by one of the authors (VS), blinded to the treatment used to remove bacteria, who randomly examined five microscopic fields per sample, comparing the presence of bacterial cells and biofilm matrix on treated discs with their respective controls.
Colony counts, expressed as log(CFU/mL), given by each method, were compared using one-way ANOVA, and multiple comparisons were performed by Bonferroni corrections. We used SAS® computer software (SAS Institute Inc, Cary, NC, USA) to perform the statistical analysis.
Results
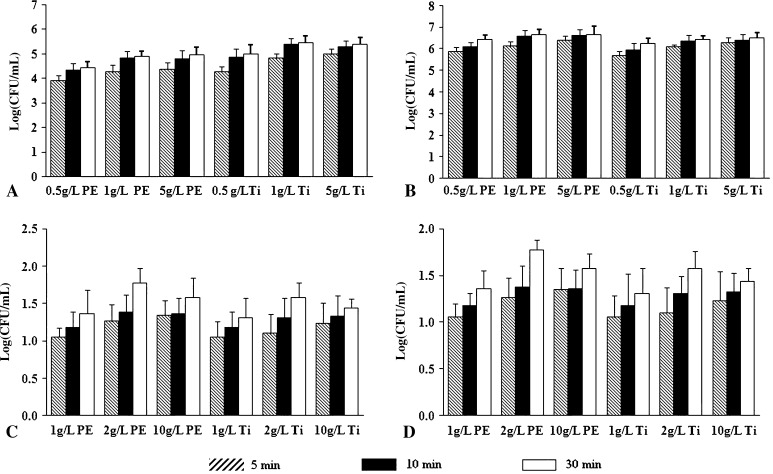
Among the tested NAC concentrations, the highest colony counts (log(CFU/mL)) were obtained when a 2 g/L solution was used (Fig. 6). Increasing incubation time from 5 to 30 minutes gradually led to an increment in bacteria removed (Fig. 6). For DTT, a marked increment in colony counts was observed by increasing DTT concentration from 0.5 to 1 g/L (p values ranging from 0.001 to 0.04), whereas only smooth increases in bacterial counts were obtained when DTT concentration was increased to 5 g/L (p values ranging from 0.19 to 0.76). In the same way, increment of incubation time from 5 minutes to 15 minutes led to an increase in colony counts, whereas minor changes were observed when incubation was prolonged to 30 minutes (Fig. 6).
Fig. 6A–D.
The graphs show the influence of concentrations of dithiothreitol (DTT) and N-acetylcysteine (NAC) and incubation lengths on bacterial retrieval (Log(CFU/mL)). (A) S aureus biofilm treated with DTT, (B) P aeruginosa biofilm treated with DTT, (C) S aureus biofilm treated with NAC, and (D) P aeruginosa biofilm treated with NAC are shown. CFU = colony-forming unit. For DTT, incubation at a concentration of 1 g/L for 15 minutes assured good recovery of bacteria from PE and Ti, whereas for NAC, incubation with a 2-g/L solution for 30 minutes yielded better recovery of bacteria.
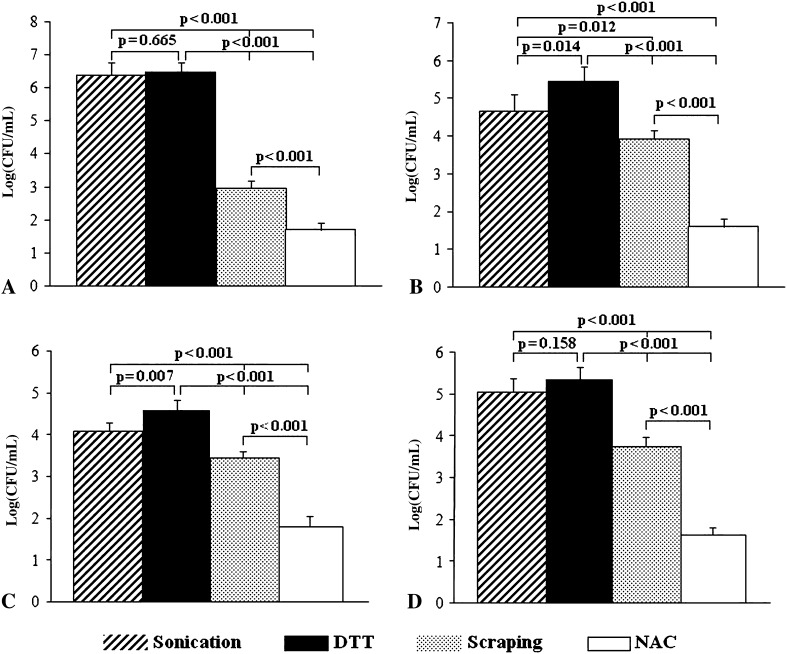
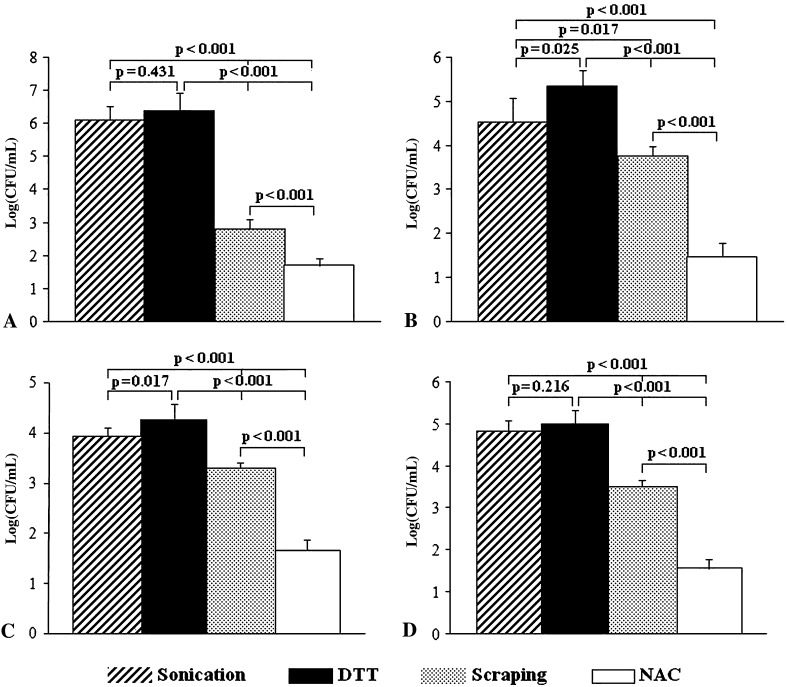
Colony counts obtained after treatment with DTT, sonication, scraping and NAC of PE and TI discs are shown in Figures 7 and 8, respectively. Dislodging of P aeruginosa from the biofilm formed on PE and Ti discs provided a similar yield for DTT and sonication (p = 0.665 and 0.431 for PE and Ti, respectively), whereas lower colony counts were observed after scraping and NAC treatment (p < 0.001 for DTT and sonication versus scraping and NAC, PE and Ti discs). Counts of S aureus (log (CFU/mL)) removed from biofilm by DTT were greater than those obtained after sonication (p = 0.014 for PE and 0.025 for Ti), scraping (p < 0.001 for PE and Ti), and NAC (p < 0.001 for PE and Ti). Similarly, colony counts for S epidermidis were greater after DTT treatment than after sonication (p = 0.002 and 0.017 for PE and Ti, respectively), scraping (p < 0.001 for PE and Ti) and NAC treatments (p < 0.001 for PE and Ti). The number of E coli colonies obtained after sonication and DTT were similar (p = 0.216 and 0.158 for PE and Ti, respectively) and greater than counts found with scraping and NAC (p < 0.001).
Fig. 7A–D.
The graphs show the bacterial retrieval from biofilm formed on PE discs for (A) P aeruginosa, (B) S aureus, (C) S epidermidis, and (D) E coli. DTT treatment is similar to sonication and more efficient than scraping and NAC treatment in dislodging adherent bacteria from S aureus and P aeruginosa biofilms. CFU = colony-forming unit.
Fig. 8A–D.
The graphs show the bacterial retrieval from biofilm formed on Ti discs for (A) P aeruginosa, (B) S aureus, (C) S epidermidis, and (D) E coli. DTT treatment is similar to sonication and more efficient than scraping and NAC treatment in dislodging adherent bacteria from S aureus and P aeruginosa biofilms. CFU = colony-forming unit.
The numbers of retrieved bacterial cells were similar (p = 0.991) with PE and Ti.
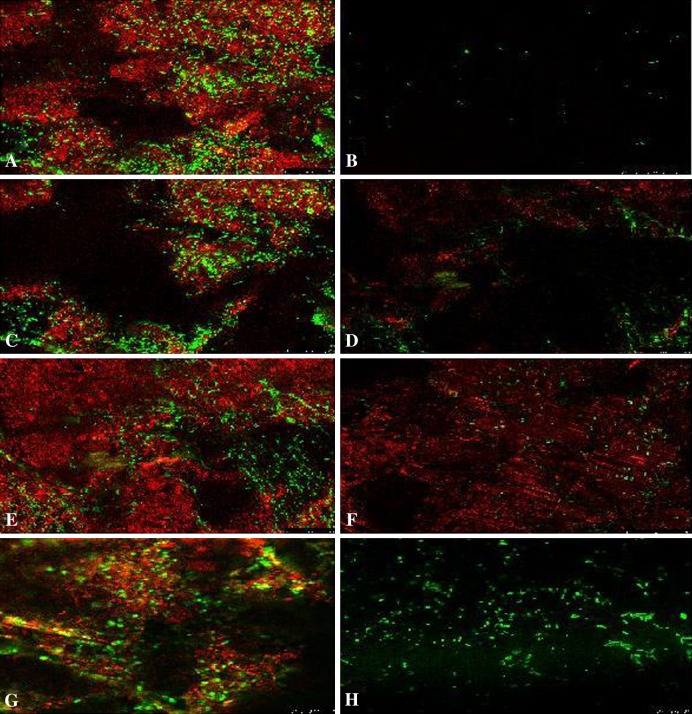
Confocal laser scanning microscopy images showed the presence of few bacterial cells (stained green) on DTT-treated discs compared with controls, while no appreciable biofilm matrix (stained red) was present (Fig. 9A–B). After sonication, biofilm matrix was still present, but fewer bacteria were present compared with controls (Fig. 9C–D). When disc surfaces were scraped, biofilm matrix and some bacteria were still evident (Fig. 9E–F). Discs treated with NAC did not show any biofilm matrix, whereas bacterial cells not removed by NAC were still present (Fig. 9G–H).
Fig. 9A–H.
Confocal laser scanning microscopy shows the formation and removal of biofilms on prosthetic materials for S aureus on PE discs (Stains: bacteria were stained green by Syto-9, the biofilm matrix was stained red by FilmTracerTM SYPRO Ruby Matrix Stain; original magnification, x 3000). Disc surfaces (A) before and (B) after DTT treatment, (C) before and (D) after sonication, (E) before and (F) after scraping, and (G) before and (H) after NAC treatment are shown. DTT was the most effective in removing live bacteria from PE discs compared with the other methods. Sonication removed most of the biofilm but not all live bacteria. Biofilm and bacteria removal were not efficient when disc surfaces were scraped. NAC was ineffective in bacterial removal but actively removed biofilm.
Treatment with NAC had highest intraassay and interassay variabilities with CV%s ranging from 1.2% to 7.4% (Table 1). DTT treatment and sonication had intraassay and interassay CV%s less than 4.0%.
Table 1.
Intraassay and interassay variabilities of the tested methods
| Microorganism | Polyethylene discs | Titanium | ||
|---|---|---|---|---|
| Interassays CV% | Intraassays CV% | Interassays CV% | Intraassays CV% | |
| S aureus | ||||
| Scraping | 4.9 | 1.5 | 3.5 | 1.8 |
| Sonication | 2.8 | 0.8 | 3.5 | 0.5 |
| DTT | 2.8 | 0.4 | 3.3 | 0.8 |
| NAC | 7.4 | 1.8 | 6.8 | 2.6 |
| P aeruginosa | ||||
| Scraping | 3.0 | 0.7 | 5.2 | 1.3 |
| Sonication | 1.9 | 0.7 | 1.6 | 0.3 |
| DTT | 2.5 | 0.6 | 2.1 | 0.9 |
| NAC | 4.7 | 1.2 | 5.0 | 4.9 |
CV = coefficient of variation; DTT = dithiothreitol; NAC = N-acetylcysteine.
Discussion
Recovery of the infectious microorganisms is essential to the selection of appropriate antimicrobial therapy to treat PJIs. Currently, isolation of a pathogen by culture from at least two separate tissue or fluid samples is considered one of the standard criteria proposed for defining a PJI [20]. However, the sensitivity of the culture method often is affected by previous use of antibiotics, sampling errors, inadequate amounts of vital bacteria retrieved, and inappropriate transport. Another problem the microbiologist faces is that the presence of bacteria in the biofilms makes them difficult to remove with traditional sampling techniques and subsequently to cultivate and identify. Sonication of explanted implants has notably improved sensitivity of microbiologic diagnosis of PJIs by dislodging bacteria from biofilm adhered to prosthetic surfaces, as documented in numerous studies [1, 4, 12, 13, 23, 28–30]. Since NAC exerts activity against bacterial biofilms [2, 10, 16, 26, 33], we presumed NAC and DTT could be used to remove bacteria from a prosthetic biofilm. We asked the following questions: (1) Do DTT and NAC remove bacteria from biofilm formed on prosthetic materials? (2) Is bacterial recovery affected by differing DTT and NAC concentrations and incubation times? (3) Do treatments with DTT and NAC detach the same amounts of bacteria from biofilm on prosthetic materials as sonication and scraping? (4) Are these methods reproducible?
We acknowledge limitations to our study. First, we evaluated four species, S aureus, P aeruginosa, S epidermidis, and E coli, and we did not include fastidious bacteria, such as the Haemophilus species, which has been reported as a frequent cause of PJIs [5], or anaerobic bacteria. S aureus and S epidermidis were chosen because they had been used in similar studies [4, 13], being the most frequent pathogens involved in PJIs and known biofilm producers, whereas P aeruginosa and E coli were chosen as representative of gram-negative bacteria able to produce biofilm. Second, we used an in vitro model of bacterial biofilm, which could not fully reflect conditions occurring on clinical samples. Third, we used only confocal laser scanning microscopy to determine whether the bacterial counts could be confirmed by imaging and we used no other quantitative analyses. Our choice mainly was because the primary issue of the study was to verify whether treatment with DTT or NAC could be used in a clinical laboratory for diagnosis of PJIs. Finally, we evaluated two materials (PE and Ti), not including bone cements loaded with antibiotics. The characteristics of bone cements, such as roughness and porosity, reportedly influence biofilm formation on antibiotic loaded cements and also influence antibiotic release [11, 25, 31]. Moreover, previous studies suggest sensitivity of methods used to remove bacteria from prosthetic implants decreased in the case of previous antibiotic therapy [12, 28]. Therefore it is possible that removal of bacteria from clinical specimens might be less than that observed in our study. However, this is a pilot comparative study to evaluate if treatment with sulfhydryl compounds may be useful to remove bacteria from prosthetic materials for diagnosis of PJIs and a clinical comparative trial obviously is needed.
We chose concentrations and length of treatment with DTT and NAC after preliminary studies in which different conditions were assessed to find those giving the highest yield, measured as bacterial colonies grown on agar plates after each treatment. Concentrations and length of treatment with DTT and NAC were chosen in the first phase of the study when different conditions were assessed to find those giving the highest yield, measured as bacterial colonies grown on agar plates after each treatment. We decided to assess two NAC concentrations lower and one similar to or even greater than the mean inhibitory concentration of the tested strains (6–48 g/L for P aeruginosa and S epidermidis, 12–48 g/L for S aureus and E coli). Increasing NAC from 1 to 2 g/L led to an improvement in bacterial recovery, whereas the highest concentration did not notably increase bacterial counts, probably because it affected viability of isolates with the lowest mean inhibitory concentrations. Instead, prolonging incubation until 30 minutes seemed to improve bacterial recovery, but we decided not to test longer incubations, as longer incubation times may represent a limitation for a method which should be used in clinical laboratories. In the same way, an increase in DTT concentration from 0.5 to 1 g/L led to improvement in bacterial recovery, which was not further ameliorated by the highest concentration. Therefore we decided to use 1 g/L to decrease the risk of toxicity of greater amounts of DTT. Moreover, this concentration (1 g/L) is used routinely for liquefying respiratory specimens for diagnosis of respiratory tract infections. As we did not observe notable increases in bacterial counts when we prolonged incubation to 30 minutes, we decided to use 15 minutes to save time.
We found that treatment with DTT provided a greater bacterial recovery rate than those obtained with NAC treatment and scraping and similar to that observed with sonication. Sonication was capable of detaching more bacteria than the NAC and scraping methods, thus confirming the usefulness of this method reported in clinical studies [4, 12, 23, 28–30]. Some studies [2, 10, 16, 26, 33] have shown that NAC alone or in combination with other compounds has strong activity against bacterial biofilm, and the mechanism involving the disruption of biofilm may induce the susceptibility of bacteria during antibiotic therapies [2]. The concentration of NAC we used was chosen to avoid any possible interference in bacterial growth by NAC and was less than the minimum inhibitory concentrations of the tested strains (6–48 mg/mL for P aeruginosa and 12–48 mg/mL for S aureus). NAC treatment produced a lower bacterial recovery in comparison with the other methods, whereas confocal laser scanning microscopy showed less biofilm than untreated samples, suggesting the activity of NAC may be limited to reduce biofilm matrix. Data regarding the effectiveness of scraping to dislodge bacteria from biofilm are scarce and contrasting. In one clinical study, scraping was better than conventional methods, such as culture of periprosthetic soft tissues, as a diagnostic tool [17]. In an in vitro study comparing scraping with sonication, the latter allowed the highest bacterial recovery [4]. Our data confirm this observation, although its technical simplicity makes it easily applicable for most laboratories. It has been reported biofilm cells recovered by scraping are not capable of growing on agar plates [9], which could explain the low amount of bacteria recovered in our study.
We found limited data regarding reproducibility of methods used to dislodge bacteria from prosthetic implants [4, 13]. Our data suggest that sonication and DTT treatment seem to provide reproducible bacterial counts, whereas high variability was observed for NAC and scraping. In the study of Bjerkan et al. [4] comparing sonication with three different scraping methods, groups of 10 discs of Ti and steel inoculated with the same strain were assessed and the rate of successful bacterial recovery was calculated. Sonication was associated with the highest rate, with 100% of positive cultures of S aureus, as observed in our study for all the methods tested. Bjerkan et al. reported only range of bacterial counts, not means and SDs, therefore it is not possible to compare their data with ours. Kobayashi et al. [13] reported means and SDs of bacterial counts after sonication and vortexing from which we extrapolated a CV% of approximately 2%. This value is less than the interassay variability we observed for S aureus, ranging from 2.8% to 3.5%, whereas it is greater than our intraassay CV% of 0.5% to 0.8%.
The major advantages of DTT treatment are the ease of use, the reduced risk of contamination as it does not require excessive manipulation of the specimen and the limited costs required, although this treatment requires better evaluation by assessing clinical samples. Although our work allowed insight into bacterial recovery from biofilm of some of the most common pathogens associated with PJIs, further evaluation of various other pathogens involved in orthopaedic infections is needed.
Treatment with DTT might provide a useful adjunct to the decision-making process when confronted with implant removal associated with PJI, and in terms of a clinical perspective, it may provide additional support to conventional laboratory culturing techniques for the diagnosis of PJIs. A large clinical trial is needed to determine whether DTT treatment can become a standard technique.
Footnotes
The institution of one or more of the authors (LD, VS, ED) has received, during the study period, funding from Italian Ministry of Health. Each author certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
This work was performed at Laboratory of Clinical Chemistry and Microbiology, IRCCS Galeazzi Institute, Milan, Italy.
References
- 1.Achermann Y, Vogt M, Leunig M, Wust J, Trampuz A. Improved diagnosis of periprosthetic joint infection by multiplex PCR of sonication fluid from removed implants. J Clin Microbiol. 2010;48:1208–1214. doi: 10.1128/JCM.00006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aslam S, Trautner BW, Ramanathan V, Darouiche RO. Combination of tigecycline and N-acetylcysteine reduces biofilm-embedded bacteria on vascular catheters. Antimicrob Agents Chemother. 2007;51:1556–1558. doi: 10.1128/AAC.00893-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berbari EF, Marculescu C, Sia I, Lahr BD, Hanssen AD, Steckelberg JM, Gullerud R, Osmon DR. Culture-negative prosthetic joint infection. Clin Infect Dis. 2007;45:1113–1119. doi: 10.1086/522184. [DOI] [PubMed] [Google Scholar]
- 4.Bjerkan G, Witso E, Bergh K. Sonication is superior to scraping for retrieval of bacteria in biofilm on titanium and steel surfaces in vitro. Acta Orthop. 2009;80:245–250. doi: 10.3109/17453670902947457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cataldo MA, Petrosillo N, Cipriani M, Cauda R, Tacconelli E. Prosthetic joint infection: recent developments in diagnosis and management. J Infect. 2010;61:443–448. doi: 10.1016/j.jinf.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 6.Choong PF, Dowsey MM, Carr D, Daffy J, Stanley P. Risk factors associated with acute hip prosthetic joint infections and outcome of treatment with a rifampin-based regimen. Acta Orthop. 2007;78:755–765. doi: 10.1080/17453670710014527. [DOI] [PubMed] [Google Scholar]
- 7.Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, Beachey EH. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical device. J Clin Microbiol. 1985;22:996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clauss M, Trampuz A, Borens O, Bohner M, Ilchmann T. Biofilm formation on bone grafts and bone graft substitutes: comparison of different materials by a standard in vitro test and microcalorimetry. Acta Biomater. 2010;6:3791–3797. doi: 10.1016/j.actbio.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 10.El-Feky MA, El-Rehewy MS, Hassan MA, Abolella HA, Abd El-Baky RM, Gad GF. Effect of ciprofloxacin and N-acetylcysteine on bacterial adherence and biofilm formation on ureteral stent surfaces. Pol J Microbiol. 2009;58:261–267. [PubMed] [Google Scholar]
- 11.Ensing GT, Horn JR, Mei HC, Busscher HJ, Neut D. Copal bone cement is more effective in preventing biofilm formation than Palacos R-G. Clin Orthop Relat Res. 2008;466:1492–1498. doi: 10.1007/s11999-008-0203-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holinka J, Bauer L, Hirschl AM, Graninger W, Windhager R, Presterl E. Sonication cultures of explanted components as an add-on test to routinely conducted microbiological diagnostics improve pathogen detection. J Orthop Res. 2011;29:617–622. doi: 10.1002/jor.21286. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi H, Oethinger M, Tuohy MJ, Procop GW, Bauer TW. Improved detection of biofilm-formative bacteria by vortexing and sonication: a pilot study. Clin Orthop Relat Res. 2009;467:1360–1364. doi: 10.1007/s11999-008-0609-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res. 2010;468:52–56. doi: 10.1007/s11999-009-1013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jämsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty: a register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91:38–47. doi: 10.2106/JBJS.G.01686. [DOI] [PubMed] [Google Scholar]
- 16.Naves P, Prado G, Huelves L, Rodríguez-Cerrato V, Ruiz V, Ponte MC, Soriano F. Effects of human serum albumin, ibuprofen and N-acetyl-L-cysteine against biofilm formation by pathogenic Escherichia coli strains. J Hosp Infect. 2010;76:165–170. doi: 10.1016/j.jhin.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Neut D, Mei HC, Bulstra SK, Busscher HJ. The role of small-colony variants in failure to diagnose and treat biofilm infections in orthopedics. Acta Orthop. 2007;78:299–308. doi: 10.1080/17453670710013843. [DOI] [PubMed] [Google Scholar]
- 18.Oga M, Arizono T, Sugioka Y. Bacterial adherence to bioinert and bioactive materials studied in vitro. Acta Orthop Scand. 1993;64:273–276. doi: 10.3109/17453679308993623. [DOI] [PubMed] [Google Scholar]
- 19.Olofsson AC, Hermansson M, Elwing H. N-acetyl-L-cysteine affects growth, extracellular polysaccharide production, and bacterial biofilm formation on solid surfaces. Appl Environ Microbiol. 2003;69:4814–4822. doi: 10.1128/AEM.69.8.4814-4822.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469:2992–2994. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001;392:15–23. doi: 10.1097/00003086-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Phillips JE, Crane TP, Noy M, Elliott TS, Grimer RJ. The incidence of deep prosthetic infections in a specialist orthopaedic hospital: a 15-year prospective survey. J Bone Joint Surg Br. 2006;88:943–948. doi: 10.1302/0301-620X.88B7.17150. [DOI] [PubMed] [Google Scholar]
- 23.Piper KE, Jacobson MJ, Cofield RH, Sperling JW, Sanchez-Sotelo J, Osmon DR, McDowell A, Patrick S, Steckelberg JM, Mandrekar JN, Fernandez Sampedro M, Patel R. Microbiologic diagnosis of prosthetic shoulder infection by use of implant sonication. J Clin Microbiol. 2009;47:1878–1884. doi: 10.1128/JCM.01686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466:1710–1715. doi: 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramage G, Tunney MM, Patrick S, Gorman SP, Nixon JR. Formation of Propionibacterium acnes biofilms on orthopaedic biomaterials and their susceptibility to antimicrobials. Biomaterials. 2003;24:3221–3227. doi: 10.1016/S0142-9612(03)00173-X. [DOI] [PubMed] [Google Scholar]
- 26.Schwandt LQ, Weissenbruch R, Stokroos I, Mei HC, Busscher HJ, Albers FW. Prevention of biofilm formation by dairy products and N-acetylcysteine on voice prostheses in an artificial throat. Acta Otolaryngol. 2004;124:726–731. doi: 10.1080/00016480410022516. [DOI] [PubMed] [Google Scholar]
- 27.Sendi P, Frei R, Maurer TB, Trampuz A, Zimmerli W, Graber P. Escherichia coli variants in periprosthetic joint infection: diagnostic challenges with sessile bacteria and sonication. J Clin Microbiol. 2010;48:1720–1725. doi: 10.1128/JCM.01562-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trampuz A, Osmon DR, Hanssen AD, Steckelberg JM, Patel R. Molecular and antibiofilm approaches to prosthetic joint infection. Clin Orthop Relat Res. 2003;414:69–88. doi: 10.1097/01.blo.0000087324.60612.93. [DOI] [PubMed] [Google Scholar]
- 29.Trampuz A, Piper KE, Hanssen AD, Osmon DR, Cockerill FR, Steckelberg JM, Patel R. Sonication of explanted prosthetic components in bags for diagnosis of prosthetic joint infection is associated with risk of contamination. J Clin Microbiol. 2006;44:628–631. doi: 10.1128/JCM.44.2.628-631.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357:654–663. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- 31.Belt H, Neut D, Schenk W, Horn JR, Mei HC, Busscher HJ. Staphylococcus aureus biofilm formation on different gentamicin-loaded polymethylmethacrylate bone cements. Biomaterials. 2001;22:1607–1611. doi: 10.1016/S0142-9612(00)00313-6. [DOI] [PubMed] [Google Scholar]
- 32.Wu X, Wang Yu, Tao L. Sulfhydryl compounds reduce Staphylococcus aureus biofilm formation by inhibiting PIA biosynthesis. FEMS Microbiol Lett. 2011;316:44–50. doi: 10.1111/j.1574-6968.2010.02190.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhao T, Liu Y. N-acetylcysteine inhibits biofilms produced by Pseudomonas aeruginosa. BMC Microbiol. 2010;10:140. doi: 10.1186/1471-2180-10-140. [DOI] [PMC free article] [PubMed] [Google Scholar]