Abstract
Background
Cis-2 decenoic acid (C2DA) disperses biofilm in many strains of microorganisms. However, whether C2DA inhibits bacterial growth or has potential to boost the actions of antibiotics is unknown.
Questions/purposes
We asked whether (1) C2DA inhibited MRSA growth and biofilm, (2) antibiotics increased inhibitory effects, (3) inhibitory concentrations of C2DA were cytotoxic to human cells, and (4) effective concentrations could be delivered from a chitosan sponge drug delivery device.
Methods
Broth containing seven concentrations of C2DA and six concentrations of either daptomycin, vancomycin, or linezolid was inoculated with a clinical isolate of MRSA and added to a total of 504 coated microtiter plate wells in triplicate (n = 3) for turbidity bacterial growth and crystal violet biofilm mass quantification. We used fibroblast cell viability assays of six C2DA concentrations (n = 4) to evaluate preliminary biocompatibility. We measured the elution of C2DA from a chitosan sponge drug delivery device with two representative loading concentrations (n = 3).
Results
C2DA at concentrations of 500 μg/mL and above inhibited growth, while 125 μg/mL C2DA inhibited biofilm. Combination with antibiotics increased these effects. At concentrations up to 500 μg/mL, there were no cytotoxic effects on fibroblasts. Chitosan sponges loaded with 100 mg of C2DA eluted concentrations at or above biofilm-inhibitory concentrations for 5 days.
Conclusions
C2DA inhibited biofilm formation by MRSA at biocompatible concentrations, with increasing biofilm reduction with added antibiotics. Elution of C2DA from a chitosan sponge can be modified through adjusting loading concentration.
Clinical Relevance
By inhibiting biofilm formation on implant surfaces, C2DA may reduce the number of infections in musculoskeletal trauma.
Introduction
Treatment of musculoskeletal trauma has often involved fixation or replacement of tissues with implanted biomaterials. These materials provided a surface for bacterial attachment and biofilm formation, leading to severe infection [8, 9]. In orthopaedics, a large number of these infections have been due to methicillin-resistant Staphylococcus aureus (MRSA) [38, 39], many strains of which have biofilm-forming tendencies [6]. Prevention of bacterial biofilm formation could reduce the need for interventional treatments, such as systemic and local antibiotic administration, debridement, reconstruction, or amputation, in musculoskeletal trauma. This successful prevention of biofilm-associated infections would improve patient outcomes and reduce the costs associated with infection treatment.
Cis-2 decenoic acid (C2DA) is a medium-chain fatty acid chemical messenger produced by bacteria to signal dispersion in biofilms for multiple types of bacteria [12]. In addition to dispersing already formed biofilms, C2DA reportedly prevents biofilm formation [12]. This potential biofilm-preventative characteristic could make it useful as an adjunctive therapy for infection prevention. As in reports of several other medium- and long-chain fatty acids, studies have indicated that C2DA may also have growth inhibitory or bactericidal effects [14, 29]. Further, since antibiotics have been less effective against biofilm-associated bacteria [40], C2DA could improve the efficacy of antibiotics in preventing or treating biofilm-associated infections.
Therapeutic interventions with C2DA could be in the form of local delivery of the fatty acid directly to potentially contaminated tissue. Local drug delivery carriers, such as calcium sulfate, collagen, or chitosan, have been effective in targeted, local delivery of C2DA [22, 26, 41]. For these initial pilot studies, the chitosan sponge was chosen for evaluation because it was a biocompatible delivery device that released antimicrobial compounds at therapeutic levels [36, 41]. To establish the preliminary feasibility of local delivery of C2DA, the effects of inhibitory concentrations of C2DA on cell survival and proliferation should be evaluated to determine potential cytotoxic effects and to optimize dosing strategies.
Our primary goals were to address whether (1) C2DA inhibited growth and biofilm formation in a biofilm-forming clinical isolate of MRSA and (2) C2DA affected the efficacy of antibiotics in growth or biofilm inhibition. We also asked (3) whether or not C2DA had negative effects on cell survival or proliferation of normal human cells and (4) whether this fatty acid could be delivered locally to tissue using a chitosan sponge.
Materials and Methods
We evaluated varying concentrations of C2DA (Grupo Nitrile, San Ysidro, CA, USA) for inhibitory effects on growth and biofilm formation of UAMS-1 (a MRSA strain) (n = 18) (Fig. 1). Each of these C2DA concentrations was also combined with twofold dilutions of three different antibiotics to which UAMS-1 was susceptible, daptomycin, vancomycin, and linezolid [46], and the dependent variables turbidity and biofilm mass were measured (n = 3). We then added C2DA, dissolved in the same ethanol concentration as bacteriological studies, to a culture of normal human dermal fibroblasts to evaluate cell number after 24 and 48 hours of exposure to C2DA (n = 4). We loaded C2DA at two different concentrations into chitosan drug delivery sponges and tested eluates by high performance liquid chromatography (HPLC) over a 5-day period to determine the amount of C2DA in the eluate (n = 3).
Fig. 1.
Diagram shows the experimental design.
Our primary research question was if C2DA inhibited biofilm. In studies of biofilm-reducing compounds, approximate 40% to 80% reductions in biofilm mass were observed [25, 27]. With six concentrations of C2DA studied, given the approximate 20% standard deviation (SD), we could detect a difference of 75% of control values with 80% power using ANOVA analysis with three wells for the biofilm inhibition studies. Because each plate included a standard curve of C2DA alone, there were 18 wells for C2DA without added antibiotics. We used three wells for all combinations of C2DA with antibiotics, and four wells for biocompatibility tests. In studies of antimicrobial effects on toxicity, 50% to 100% reductions in viability were observed [5, 11, 24]. Given the approximate SD of 500 cells per well, we could detect a difference of 1500 cells per well (around 20% of control values) with 80% power using ANOVA analysis. For the pilot elution analyses, we used three replicates for each loading concentration.
For microbiological evaluations, we diluted C2DA at varying concentrations in 50% ethanol in deionized water. These concentrations of C2DA were diluted in tryptic soy broth (TSB) (BD Bacto™, BD Biosciences, Franklin Lakes, NJ, USA) with calcium chloride added to a concentration of 50 μg/mL calcium in sterile 5-mL polystyrene tubes (BD Falcon™, BD Biosciences, Franklin Lakes, NJ, USA). The dilution resulted in a final ethanol concentration of 1.25%. We also prepared controls with and without ethanol to observe the effects of this ethanol concentration on biofilm formation. Daptomycin (Cubist Pharmaceuticals, Lexington, MA, USA), vancomycin (MP Biomedicals, Solon, OH, USA), and linezolid (Waterstone Technology, Carmel, IN, USA) at varying concentrations were added. We inoculated each well with approximately 1 × 105 colony forming units of UAMS-1 (a clinical isolate of MRSA having well-characterized biofilm formation obtained from the University of Arkansas for Medical Sciences) [2, 3, 21]. We also prepared controls without bacteria as blanks. All assays were done in triplicate. Microtiter plates (Corning Inc., Corning, NY, USA) were prepared by coating with 1 mg/mL gelatin (Fisher Scientific, Pittsburgh, PA, USA) for 1 hour, then adding horse serum (Gibco, Grand Island, NY, USA) and incubating for 3 hours at 37°C and then at 4°C overnight. After removing the serum and washing with phosphate buffered saline (PBS), we added inoculated broth solutions in triplicate 100 μL aliquots to microtiter plates and placed them in an incubator at 37°C within a humidified chamber. After incubation for 24 hours, we read absorbance at 540 nm to determine turbidity of the solutions and assess bacterial growth. Broth and nonadherent bacteria were then gently aspirated from the wells. Each well was washed two times by gently adding 300 μL of sterile PBS and aspirating. Adherent biofilm was heat-fixed by placing in an oven at 60°C for 1 hour. We stained each well with 100 μL of crystal violet for 2 minutes, and then rinsed them with tap water until rinse water was clear. After removing residual fluid, we photographed each plate. We then added 100 μL of destain solution (10% methanol, 7.5% acetic acid in water) to each well. Absorbance was read at 540 nm in a microplate reader. We subtracted readings of blank wells containing broth without bacteria from the data for each plate. Data was normalized to positive controls without ethanol on each plate.
For cell culture experiments, normal human dermal fibroblasts (Lonza, Allendale, NJ, USA) at passage 11 were plated at 104 cells per cm2 in 48 individual wells of a 96 well plate in a medium of Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum. After attachment overnight, we added 100 μL of C2DA dissolved at six concentrations from 0 to 1000 μg/ml in DMEM containing 2.5% ethanol to each well (n = 4). We investigated each of the six concentrations in culture after 24 and 48 hours. The final concentration of ethanol in each well was 1.25% and the final concentration of serum was 10%. We also included controls with DMEM alone and ethanol in DMEM alone. After 24 and 48 hours, cell number was determined using Cell-titer Glo (Promega, Madison, WI, USA) and a standard curve of known concentrations of cells.
To develop methods to deliver C2DA locally to tissue, we incorporated into chitosan sponges two concentrations of C2DA, which bracketed the expected release profile to be within concentration ranges evaluated in microbiological and biocompatibility studies. Chitosan sponges were fabricated by mixing 1.5 weight percent of chitosan (Primex, Siglufjordur, Iceland) with a blend of 3:1, 1% lactic to 1% acetic acid. Solutions were frozen at −80°C and lyophilized for 48 hours. We neutralized sponges by immersing them in 1 molar of sodium hydroxide, followed by thorough washing in deionized water until rinse water was neutral in pH. Sponges were then refrozen and relyophilized. We loaded six sponges with 100 mg or 10 mg of C2DA by immersing in 1 mL of 100 mg/mL or 10 mg/mL solution of C2DA in 10% ethanol. Immediately after loading, we placed sponges into individual glass vials in 15 mL of PBS, which completely covered the sponges. Sampling and complete refreshment of media occurred each day for 5 days. Concentration of C2DA in the eluate was determined with HPLC, using a C18 column and a mobile phase of acetonitrile to tetrahydrofuran to deionized water (50.4:21.6:28 v/v/v), adjusted to a pH of 2.5 with phosphoric acid with a flow rate of 1.5 mL per minute [20].
The quantity of bacteria attached to the surface of the microtiter plate after washing was correlated to the amount of crystal violet stain taken up and the corresponding absorbance of the destained wells. The CellTiter-Glo® assay (Promega Corp., Madison, WI, USA) measured adenosine triphosphate (ATP) production by cells, which is assumed to be a uniform amount per cell [10]. The luminescence from the CellTiter-Glo® assay was normalized to a standard dilution of a known concentration of cells in order to obtain cell number.
We used one-way Kruskal-Wallis ANOVA with Tukey post-hoc analysis to determine statistical differences in biofilm formation, turbidity, and cellular activity between different concentrations of C2DA. Analysis was performed with SigmaStat 3.1 software (Systat, Chicago, IL, USA).
Results
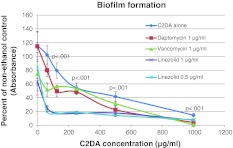
C2DA at concentrations at or above 500 μg/mL inhibited bacterial growth, while concentrations from 62.5 to 125 μg/mL resulted in bacterial growth and increased turbidity readings for both C2DA and C2DA with antibiotics compared to controls with ethanol alone (Fig. 2). C2DA at concentrations greater than or equal to 125 μg/mL inhibited biofilm formation in microtiter plates (Fig. 3). Visual inspection of biofilm staining indicated even coverage of wells in control groups, with reduction in staining and coverage as concentration of C2DA increases (Fig. 4). The addition of ethanol alone at 1.25% increased biofilm formation compared to nonethanol controls. For daptomycin, vancomycin, and linezolid, concentrations of 2 μg/mL were inhibitory to growth. At the subinhibitory concentration of 1 μg/mL, when combined with C2DA, there was an additive effect for growth inhibition and biofilm inhibition for daptomycin and vancomycin. The combination of linezolid at 1 μg/mL and 0.5 μg/mL reduced biofilm formation at all C2DA concentrations investigated.
Fig. 2.
Graph shows the turbidity of solutions before washing plates, indicating bacterial growth, represented as mean percentage of absorbance values at 540 nm of positive controls ± SD.
Fig. 3.
Graph shows the biofilm formation in microtiter plates in varying concentrations of C2DA, represented as mean percentage of absorbance values at 540 nm of positive controls ± SD.
Fig. 4.
Representative photographs of crystal violet stained wells at various concentrations of C2DA and antibiotics.
After exposure to C2DA, fibroblasts were still attached and appeared healthy at all concentrations of C2DA (Fig. 5). There was a decrease in cell number at 24 hours for concentrations of C2DA of 250 μg/mL and above. Although the controls increased in cell number from 24 hours to 48 hours, for all wells containing C2DA, there were no detectable increases in overall cell number from 24 to 48 hours.
Fig. 5A–B.
(A) Graph shows normal human dermal fibroblast cell number per well 24 and 48 hours after addition of varying concentrations of C2DA, represented as mean ± SD. (B) Representative phase contrast micrographs of fibroblasts at various concentrations of C2DA at 24 and 48 hours at magnification 4×.
C2DA was released from chitosan sponges (Fig. 6). The release pattern for 10 mg loading followed a burst-release pattern. Loading with 100 mg resulted in concentrations at growth-inhibitory concentrations for the first day, with extended elution at levels above biofilm-inhibitory concentrations on days 2 through 5.
Fig. 6.
Graph shows the concentration of C2DA in PBS eluate of chitosan sponges loaded with either 10 mg or 100 mg of C2DA over 5 days of elution.
Discussion
Because the presence of C2DA disperses already formed biofilms [12], we examined the action of this fatty acid alone and when combined with antibiotics in inhibiting growth and biofilm formation in a clinical isolate of MRSA. Clinical applications of C2DA would likely include local delivery or coatings, which could result in high local concentrations in the tissues. Therefore, we also evaluated various C2DA concentrations added to cultures of normal human fibroblast cells to check for any cytotoxic or stimulatory effects. Finally, we also incorporated this fatty acid into a local drug-delivery sponge and investigated elution for two different loading concentrations.
We noted some limitations of our study. First, we explored a limited number of in vitro methods to identify and evaluate potential clinical use against MRSA infections, but we did not confirm that these results were applicable in vivo. Second, in these preliminary investigations, we used limited but focused groups using one model organism to demonstrate feasibility. Our cell culture model was a contained system with one cell type, whereas an injury site would have multiple cell types, including inflammatory cells, with varying responses to fatty acids [17, 19, 45], as well as diffusion and metabolism of fatty acids. For this preliminary work, we aimed to identify basic cellular responses to C2DA and establish thresholds for potential clinical use. In vivo studies are required for more comprehensive evaluation of cell and tissue response prior to clinical use of C2DA in preventative therapies. Another limitation was that we used a single bacterial strain to model biofilm formation in vivo, whereas clinical biofilms had a polymicrobial nature and were likely to be populated with Gram-positive, Gram-negative, and fungal microorganisms [15, 42]. We chose to study MRSA biofilm first, since they have been a primary organism in many orthopaedic infection cases and presented substantial clinical and financial burdens [28, 38, 39]. In a study of C2DA [12], it was reported to induce dispersion of biofilms in multiple bacterial strains, including Gram-positive, Gram-negative, and yeast strains, although biofilm inhibition was only demonstrated in Pseudomonas aeruginosa. Although we did not evaluate these different strains, we believed that the preventative action of C2DA may have extended across multiple strains of microorganisms. We based this assertion on a study showing that the dispersion-inducing ability of C2DA was effective against multiple different types microorganisms, including yeast [12], and the demonstration of biofilm-preventative properties applicable to a clinical strain of MRSA in our study. For clinical use in preventing biofilm on indwelling medical devices, this preventative property is critical. A further limitation of our study was that polystyrene microtiter plate assays, while commonly used to study bacterial biofilms, may not have accurately modeled biofilm formation on implant surfaces, which could consist of metallic, ceramic, or polymeric components. Immediately upon implantation, biomaterials interact with blood and tissue components and adsorbed serum proteins, forming a conditioning film [47]. Because staphylococci and other contaminating bacteria interacted with these proteins more than the implant surface itself, we used serum-coated wells to model a general biomaterial surface. Future studies will evaluate the interactions of C2DA with biomaterials, including metals, ceramics, and commonly implanted polymers. Another limitation was that the poor solubility of C2DA in water and aqueous solutions, such as bacterial broth or cell culture media, required the use of a carrier, ethanol [12]. For enhanced local delivery of C2DA, we are currently investigating more biocompatible solvent systems in our laboratories.
Some free fatty acids have been reported to have antimicrobial properties [13, 14] and serve an important role in maintaining the microbial flora of the skin [29, 43]. We demonstrated that C2DA inhibited bacterial growth at higher concentrations, but not at lower concentrations. At some lower concentrations there was an increase in bacterial growth (turbidity), which could be the result of increased growth or an increased amount of bacteria in the planktonic state. This lack of growth inhibition at lower concentrations was not surprising since bacteria produced this fatty acid and used it as a signaling molecule [12, 37, 44]. The mechanism of this growth-inhibitory effect at higher concentrations of C2DA was unknown.
The advantage of C2DA was its ability to control biofilms at very low concentrations, acting as a diffusible signal factor [12, 16, 37]. A further advantage of C2DA for infection prevention was that, contrary to the microorganism-specific nature of most diffusible signal factors, it appeared to have a broad spectrum of action against multiple types of microorganisms [12, 16]. We confirmed that, in addition to dispersing already-formed biofilm, C2DA inhibited biofilm formation by MRSA. This antibiofilm activity could prove useful in prophylactic measures for preventing infection, especially when biomaterials have been implanted.
C2DA did not completely eliminate biofilm formation in this study, unless antibiotics were also included. Since antibiotics have greater bactericidal efficacy against planktonic bacteria than biofilm [1, 7, 40], the combination of C2DA with traditional antibiotics could have improved bactericidal efficacy and infection prevention therapies [16]. The combination of daptomycin or vancomycin with C2DA slightly enhanced the antibiofilm effects, but the combination of linezolid resulted in biofilm inhibition at concentrations two to 16 times lower than for either antimicrobial alone. Of the three antibiotics investigated, linezolid was the only one that acted intracellularly, with daptomycin and vancomycin acting at the cell wall to exert their bactericidal effects. A proposed mechanism for the enhancement of antibiofilm activity when combined with linezolid was that C2DA, as a medium chain fatty acid resembling a phospholipid, may easily be incorporated into the plasma membrane of bacterial cells, and in the cis-conformation would increase membrane permeability. This increased membrane permeability may allow for increased entry of linezolid into the bacterial cells.
To be useful as a clinical therapeutic, C2DA must be delivered locally to tissue or implanted biomaterials in an active form. Systemic delivery of fatty acids through injection or oral administration has been limited due to metabolism, limited diffusion to contaminated tissue, and cytotoxicity at high doses [16, 18]. Local delivery overcomes some of these obstacles, but diffusion-controlled release of C2DA may require high initial loading concentrations within a drug delivery device, which may cause damage to tissue. Chitosan has lipid binding properties [48], which may give this biomaterial an advantage over other drug delivery devices for controlled delivery of C2DA. The primary disadvantage of many local drug delivery systems has been initial burst release and limited extended release characteristics [32, 35]. We showed that by increasing the loading concentration, elution duration of C2DA can be extended. At high concentrations, the chitosan sponge may reversibly bind the lipid portion of C2DA and release it at therapeutic concentrations for clinically-relevant durations.
We found that, at intermediate biofilm-inhibitory concentrations, there was no observable cytotoxicity. Although there was no observable cytotoxic response, the lag in cell increase from 24 to 48 hours suggested that C2DA may slow proliferation. A fatty acid similar in structure and size, 10-hydroxy-2-decenoic acid, is a component of royal jelly, which stimulates cell growth, migration, and production of matrix and growth factors [23, 30, 31, 34]. Royal jelly also appears to have antimicrobial characteristics as well, perhaps owing to decenoic and decanoic acid content [4, 33]. The possible effects of C2DA in stimulating other cellular activities, such as inflammatory responses, should be evaluated further.
In conclusion, our pilot study confirmed that C2DA could potentially control initiation of biofilm formation in addition to dispersion of existing biofilms. Similar to a study of biofilm dispersion [12], C2DA inhibited MRSA biofilm in the UAMS-1 strain, but did not completely eliminate it. We also reported that higher concentrations of C2DA could inhibit bacterial growth, and that the combination of C2DA with antibiotics may have had additive or synergistic effects on biofilm inhibition. We confirmed that biofilm-inhibitory concentrations could be delivered from chitosan sponges with clinically relevant loading concentrations. The biocompatibility of C2DA at these concentrations was in agreement with previous studies of the effects of fatty acids on tissues [23, 30, 31, 34] and suggested that delivery of C2DA to injured tissue could potentially prevent biofilm formation clinically. Together, the growth- and biofilm-inhibitory properties of C2DA, as well as the capability of delivering C2DA from a biocompatible local delivery system, demonstrated the potential for clinical investigation and use. Future studies will investigate different solvents and delivery systems to enhance local delivery of the water-insoluble fatty acid.
Acknowledgments
We thank Mark Smeltzer PhD, of the University of Arkansas for Medical Sciences, for providing the UAMS-1 bacterial strain used in this research.
Footnotes
The institution of one or more of the authors (JAJ, HSC, WOH) has received funding from the Department of Defense, Peer Reviewed Orthopaedic Research Program Award # OR090687.
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
The work was performed at both the University of Memphis and the Veteran’s Affairs Medical Center, Memphis, TN, USA.
References
- 1.Aaron SD, Ferris W, Ramotar K, Vandemheen K, Chan F, Saginur R. Single and combination antibiotic susceptibilities of planktonic, adherent, and biofilm-grown Pseudomonas aeruginosa isolates cultured from sputa of adults with cystic fibrosis. J Clin Microbiol. 2002;40:4172–4179. doi: 10.1128/JCM.40.11.4172-4179.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beenken KE, Blevins JS, Smeltzer MS. Mutation of sarA in Staphylococcus aureus limits biofilm formation. Infect Immun. 2003;71:4206–4211. doi: 10.1128/IAI.71.7.4206-4211.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beenken KE, Dunman PM, McAleese F, Macapagal D, Murphy E, Projan SJ, Blevins JS, Smeltzer MS. Global gene expression in Staphylococcus aureus biofilms. J Bacteriol. 2004;186:4665–4684. doi: 10.1128/JB.186.14.4665-4684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blum MS, Novak AF, Taber S., 3rd 10-Hydroxy-delta 2-decenoic acid, an antibiotic found in royal jelly. Science. 1959;130:452–453. doi: 10.1126/science.130.3373.452. [DOI] [PubMed] [Google Scholar]
- 5.Boyce ST, Warden GD, Holder IA. Cytotoxicity testing of topical antimicrobial agents on human keratinocytes and fibroblasts for cultured skin grafts. J Burn Care Rehabil. 1995;16:97–103. doi: 10.1097/00004630-199503000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Cassat JE, Lee CY, Smeltzer MS. Investigation of biofilm formation in clinical isolates of Staphylococcus aureus. Methods Mol Biol. 2007;391:127–144. doi: 10.1007/978-1-59745-468-1_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerca N, Martins S, Cerca F, Jefferson KK, Pier GB, Oliveira R, Azeredo J. Comparative assessment of antibiotic susceptibility of coagulase-negative staphylococci in biofilm versus planktonic culture as assessed by bacterial enumeration or rapid XTT colorimetry. J Antimicrob Chemother. 2005;56:331–336. doi: 10.1093/jac/dki217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costerton JW. Overview of microbial biofilms. J Ind Microbiol. 1995;15:137–140. doi: 10.1007/BF01569816. [DOI] [PubMed] [Google Scholar]
- 9.Costerton JW, Montanaro L, Arciola CR. Biofilm in implant infections: its production and regulation. Int J Artif Organs. 2005;28:1062–1068. doi: 10.1177/039139880502801103. [DOI] [PubMed] [Google Scholar]
- 10.Crouch SP. Biocompatibility testing using ATP bioluminescence. Med Device Technol. 2000;11:12–15. [PubMed] [Google Scholar]
- 11.Damour O, Hua SZ, Lasne F, Villain M, Rousselle P, Collombel C. Cytotoxicity evaluation of antiseptics and antibiotics on cultured human fibroblasts and keratinocytes. Burns. 1992;18:479–485. doi: 10.1016/0305-4179(92)90180-3. [DOI] [PubMed] [Google Scholar]
- 12.Davies DG, Marques CN. A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J Bacteriol. 2009;191:1393–1403. doi: 10.1128/JB.01214-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desbois AP, Lebl T, Yan L, Smith VJ. Isolation and structural characterisation of two antibacterial free fatty acids from the marine diatom, Phaeodactylum tricornutum. Appl Microbiol Biotechnol. 2008;81:755–764. doi: 10.1007/s00253-008-1714-9. [DOI] [PubMed] [Google Scholar]
- 14.Desbois AP, Smith VJ. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl Microbiol Biotechnol. 2010;85:1629–1642. doi: 10.1007/s00253-009-2355-3. [DOI] [PubMed] [Google Scholar]
- 15.Dowd SE, Wolcott RD, Sun Y, McKeehan T, Smith E, Rhoads D. Polymicrobial nature of chronic diabetic foot ulcer biofilm infections determined using bacterial tag encoded FLX amplicon pyrosequencing (bTEFAP) PLoS One. 2008;3:e3326. doi: 10.1371/journal.pone.0003326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Estrela AB, Abraham W-R. Combining biofilm-controlling compounds and antibiotics as a promising new way to control biofilm infections. Pharmaceuticals. 2010;3:1374–1393. doi: 10.3390/ph3051374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fritsche K. Fatty acids as modulators of the immune response. Annu Rev Nutr. 2006;26:45–73. doi: 10.1146/annurev.nutr.25.050304.092610. [DOI] [PubMed] [Google Scholar]
- 18.Fu M, Koulman A, Rijssel M, Lutzen A, Boer MK, Tyl MR, Liebezeit G. Chemical characterisation of three haemolytic compounds from the microalgal species Fibrocapsa japonica (Raphidophyceae) Toxicon. 2004;43:355–363. doi: 10.1016/j.toxicon.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Garland SH. Short chain fatty acids may elicit an innate immune response from preadipocytes: a potential link between bacterial infection and inflammatory diseases. Med Hypotheses. 2011;76:881–883. doi: 10.1016/j.mehy.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 20.Genc M, Aslan A. Determination of trans-10-hydroxy-2-decenoic acid content in pure royal jelly and royal jelly products by column liquid chromatography. J Chromatogr A. 1999;839:265–268. doi: 10.1016/S0021-9673(99)00151-X. [DOI] [PubMed] [Google Scholar]
- 21.Gillaspy AF, Hickmon SG, Skinner RA, Thomas JR, Nelson CL, Smeltzer MS. Role of the accessory gene regulator (agr) in pathogenesis of staphylococcal osteomyelitis. Infect Immun. 1995;63:3373–3380. doi: 10.1128/iai.63.9.3373-3380.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanssen A. Local Antibiotic Delivery Vehicles in the Treatment of Musculoskeletal Infection. Clin Orthop Relat Res. 2005;437:91–96. doi: 10.1097/01.blo.0000175713.30506.77. [DOI] [PubMed] [Google Scholar]
- 23.Hattori N, Nomoto H, Fukumitsu H, Mishima S, Furukawa S. Royal jelly and its unique fatty acid, 10-hydroxy-trans-2-decenoic acid, promote neurogenesis by neural stem/progenitor cells in vitro. Biomed Res. 2007;28:261–266. doi: 10.2220/biomedres.28.261. [DOI] [PubMed] [Google Scholar]
- 24.Hidalgo E, Bartolome R, Barroso C, Moreno A, Dominguez C. Silver nitrate: antimicrobial activity related to cytotoxicity in cultured human fibroblasts. Skin Pharmacol Appl Skin Physiol. 1998;11:140–151. doi: 10.1159/000029820. [DOI] [PubMed] [Google Scholar]
- 25.Hochbaum AI, Kolodkin-Gal I, Foulston L, Kolter R, Aizenberg J, Losick R. Inhibitory effects of D-amino acids on Staphylococcus aureus biofilm development. J Bacteriol. 2011;193:5616–5622. doi: 10.1128/JB.05534-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanellakopoulou K, Giamarellos-Bourboulis EJ. Carrier systems for the local delivery of antibiotics in bone infections. Drugs. 2000;59:1223–1232. doi: 10.2165/00003495-200059060-00003. [DOI] [PubMed] [Google Scholar]
- 27.Karaolis DK, Rashid MH, Chythanya R, Luo W, Hyodo M, Hayakawa Y. c-di-GMP (3’-5’-cyclic diguanylic acid) inhibits Staphylococcus aureus cell-cell interactions and biofilm formation. Antimicrob Agents Chemother. 2005;49:1029–1038. doi: 10.1128/AAC.49.3.1029-1038.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaye KS, Anderson DJ, Choi Y, Link K, Thacker P, Sexton DJ. The deadly toll of invasive methicillin-resistant Staphylococcus aureus infection in community hospitals. Clin Infect Dis. 2008;46:1568–1577. doi: 10.1086/587673. [DOI] [PubMed] [Google Scholar]
- 29.Kenny JG, Ward D, Josefsson E, Jonsson IM, Hinds J, Rees HH, Lindsay JA, Tarkowski A, Horsburgh MJ. The Staphylococcus aureus response to unsaturated long chain free fatty acids: survival mechanisms and virulence implications. PLoS One. 2009;4:e4344. doi: 10.1371/journal.pone.0004344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J, Kim Y, Yun H, Park H, Kim SY, Lee KG, Han SM, Cho Y. Royal jelly enhances migration of human dermal fibroblasts and alters the levels of cholesterol and sphinganine in an in vitro wound healing model. Nutr Res Pract. 2010;4:362–368. doi: 10.4162/nrp.2010.4.5.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koya-Miyata S, Okamoto I, Ushio S, Iwaki K, Ikeda M, Kurimoto M. Identification of a collagen production-promoting factor from an extract of royal jelly and its possible mechanism. Biosci Biotechnol Biochem. 2004;68:767–773. doi: 10.1271/bbb.68.767. [DOI] [PubMed] [Google Scholar]
- 32.McLaren AC. Alternative materials to acrylic bone cement for delivery of depot antibiotics in orthopaedic infections. Clin Orthop Relat Res. 2004;427:101–106. doi: 10.1097/01.blo.0000143554.56897.26. [DOI] [PubMed] [Google Scholar]
- 33.Melliou E, Chinou I. Chemistry and bioactivity of royal jelly from Greece. J Agric Food Chem. 2005;53:8987–8992. doi: 10.1021/jf051550p. [DOI] [PubMed] [Google Scholar]
- 34.Narita Y, Nomura J, Ohta S, Inoh Y, Suzuki KM, Araki Y, Okada S, Matsumoto I, Isohama Y, Abe K, Miyata T, Mishima S. Royal jelly stimulates bone formation: physiologic and nutrigenomic studies with mice and cell lines. Biosci Biotechnol Biochem. 2006;70:2508–2514. doi: 10.1271/bbb.60240. [DOI] [PubMed] [Google Scholar]
- 35.Nelson CL. The current status of material used for depot delivery of drugs. Clin Orthop Relat Res. 2004:72–78. [DOI] [PubMed]
- 36.Noel SP, Courtney HS, Bumgardner JD, Haggard WO. Chitosan sponges to locally deliver amikacin and vancomycin: a pilot in vitro evaluation. Clin Orthop Relat Res. 2010;468:2074–2080. doi: 10.1007/s11999-010-1324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryan RP, Dow JM. Communication with a growing family: diffusible signal factor (DSF) signaling in bacteria. Trends Microbiol. 2011;19:145–152. doi: 10.1016/j.tim.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Sanderson PJ. The role of methicillin-resistant Staphylococcus aureus in orthopaedic implant surgery. J Chemother. 2001;13(Spec No 1):89–95. doi: 10.1179/joc.2001.13.Supplement-2.89. [DOI] [PubMed] [Google Scholar]
- 39.Shams WE, Rapp RP. Methicillin-resistant staphylococcal infections: an important consideration for orthopedic surgeons. Orthopedics. 2004;27:565–568. doi: 10.3928/0147-7447-20040601-12. [DOI] [PubMed] [Google Scholar]
- 40.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–138. doi: 10.1016/S0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 41.Stinner DJ, Noel SP, Haggard WO, Watson JT, Wenke JC. Local antibiotic delivery using tailorable chitosan sponges: the future of infection control? J Orthop Trauma. 2010;24:592–597. doi: 10.1097/BOT.0b013e3181ed296c. [DOI] [PubMed] [Google Scholar]
- 42.Stoodley P, Conti SF, DeMeo PJ, Nistico L, Melton-Kreft R, Johnson S, Darabi A, Ehrlich GD, Costerton JW, Kathju S. Characterization of a mixed MRSA/MRSE biofilm in an explanted total ankle arthroplasty. FEMS Immunol Med Microbiol. 2011;62:66–74. doi: 10.1111/j.1574-695X.2011.00793.x. [DOI] [PubMed] [Google Scholar]
- 43.Takigawa H, Nakagawa H, Kuzukawa M, Mori H, Imokawa G. Deficient production of hexadecenoic acid in the skin is associated in part with the vulnerability of atopic dermatitis patients to colonization by Staphylococcus aureus. Dermatology. 2005;211:240–248. doi: 10.1159/000087018. [DOI] [PubMed] [Google Scholar]
- 44.Vilchez R, Lemme A, Ballhausen B, Thiel V, Schulz S, Jansen R, Sztajer H, Wagner-Dobler I. Streptococcus mutans inhibits Candida albicans hyphal formation by the fatty acid signaling molecule trans-2-decenoic acid (SDSF) Chembiochem. 2010;11:1552–1562. doi: 10.1002/cbic.201000086. [DOI] [PubMed] [Google Scholar]
- 45.Vucevic D, Melliou E, Vasilijic S, Gasic S, Ivanovski P, Chinou I, Colic M. Fatty acids isolated from royal jelly modulate dendritic cell-mediated immune response in vitro. Int Immunopharmacol. 2007;7:1211–1220. doi: 10.1016/j.intimp.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Weiss EC, Spencer HJ, Daily SJ, Weiss BD, Smeltzer MS. Impact of sarA on antibiotic susceptibility of Staphylococcus aureus in a catheter-associated in vitro model of biofilm formation. Antimicrob Agents Chemother. 2009;53:2475–2482. doi: 10.1128/AAC.01432-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. Mediation of biomaterial-cell interactions by adsorbed proteins: a review. Tissue Eng. 2005;11:1–18. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- 48.Wydro P, Krajewska B, Hac-Wydro K. Chitosan as a lipid binder: a langmuir monolayer study of chitosan-lipid interactions. Biomacromolecules. 2007;8:2611–2617. doi: 10.1021/bm700453x. [DOI] [PubMed] [Google Scholar]