Abstract
Background
Management of orthopaedic infections relies on débridement and local delivery of antimicrobials; however, the distribution and concentration of locally delivered antimicrobials in postdébridement surgical sites is unknown. Gadolinium-DTPA (Gd-DTPA) has been proposed as an imaging surrogate for antimicrobials because it is similar in size and diffusion coefficient to gentamicin.
Questions/purposes
Is in vivo distribution of locally delivered Gd-DTPA (1) visible on MRI; (2) reliably visualized by different observers; (3) affected by the anatomic delivery site; and (4) affected by the in vitro release rate from the delivery vehicle?
Methods
Twenty-four local delivery depots were imaged in nine rabbits using two anatomic sites (intramedullary canal, quadriceps) with Gd-DTPA in intermediate-porosity polymethylmethacrylate (PMMA) or high-porosity PMMA; six of the nine rabbits also had Gd-DTPA delivered in collagen at a third site (hamstring). A total of 45,000 fat-suppressed T1-weighted RARE scans were acquired using a 7-T Bruker Biospec MRI: nine rabbits, 2-mm slices over 10 cm, four TR values, 25 time periods (pre, every 15 minutes for 6 hours). T1 maps were constructed at every time period. Gd-DTPA distribution was observed qualitatively on the T1 maps. Interobserver reliability was determined.
Results
Locally delivered Gd-DTPA was visible. Interobserver agreement was excellent. Intramuscular delivery followed intermuscular planes; intramedullary delivery was contained within the canal by bone. Distribution from collagen decreased after 1 hour but from PMMA increased over 6 hours.
Conclusions
Locally delivered Gd-DTPA can be visualized on MRI; distribution is affected by anatomical location and delivery vehicle.
Clinical Relevance
Contrast-based imaging using locally delivered Gd-DTPA may be useful as an antibiotic surrogate to determine antibiotic distribution in surgical sites.
Introduction
Local antimicrobial delivery after complete débridement is an established modality in the management of orthopaedic infections [1, 29]. Typically antimicrobials are delivered to the surgical wound in a controlled-release vehicle. High local concentrations, up to 1000× minimum inhibitory concentration [8], are essential to the kill biofilm-based microbes associated with orthopaedic infections. High levels need to be distributed throughout the wound wherever retained debris or tissue with microbes in biofilm might be present. Failures in treatment are often attributed to inadequate débridement; however, it is not possible to know if adequate antimicrobial levels are achieved throughout the postdébridement wound in these failures. Clinically used delivery vehicles include acrylic bone cement (PMMA) and CaSO4, although many other vehicles, including collagen, have been investigated. Antimicrobial release from these vehicles ranges in vitro from 100% of contained antimicrobial over 24 hours for collagen [17] to 3–5% over 1 month for low-porosity PMMA [25]. Although there are considerable data characterizing in vitro release [1, 2, 7, 9, 11, 14–16, 23, 29], there are few in vivo data reporting antimicrobial distribution after local delivery. Antimicrobial concentration has been measured in bone and soft tissue adjacent to a local delivery in animals [1, 22, 24]. Adams et al. [1] measured antimicrobial concentration in fluid, soft tissue, and bone adjacent to antimicrobial PMMA beads in mongrel dogs. Nelson et al. [22] measured antimicrobial levels in soft tissue and in bone adjacent to local antimicrobial-loaded biodegradable polymer over 6 weeks. Further study by Nelson et al. [22] documented the distribution of antimicrobial in avascular bone of rabbit femurs. However, these assay techniques require tissue specimens limiting clinical application. Knowledge of antimicrobial distribution after local delivery could lead to improvement in location, volume, and release characteristics for local delivery depots.
One possible method for acquiring information on spatial distribution of locally delivered antimicrobials is imaging. Local delivery of chemotherapy agents and gadolinium to the brain has been imaged with MRI [4, 26, 28] and then mathematically modeled to improve catheter placement for local delivery [28]. Although antimicrobials cannot be visualized on MRI, Gd-DTPA, a T1 contrast agent, may function as an imaging surrogate for gentamicin. Gentamicin and Gd-DTPA have similar molecular size and diffusion coefficients (Table 1) [6, 12] and likely similar drug transport properties. Gd-DTPA concentration has been determined in agar gel using MRI [27]. Gd-DTPA distribution after local delivery from PMMA in agarose gel has been imaged with MRI [10]. MRI has the potential to determine antimicrobial concentrations in vivo after local delivery. Until antimicrobials can be made visible on MRI, possibly by conjugation with gadolinium, imaging of antimicrobial distribution on MRI after local delivery must be inferred using an imaging surrogate.
Table 1.
Physical properties
| Physical property | Gentamicin | Gd-DTPA |
|---|---|---|
| Molecular weight | 478 g/mol | 938 g/mol |
| Diffusion coefficient | 2.08 × 10−6 cm2/sec | 4.0 × 10−6 cm2/sec |
| Released from cement? | Yes | Yes |
Our research questions are: Is in vivo distribution of locally delivered Gd-DTPA (1) visible on MRI; (2) reliably visualized by different observers; (3) affected by the anatomic delivery site; and (4) affected by the in vitro release rate from the delivery vehicle?
Materials and Methods
Twenty-four local delivery depots were imaged in nine rabbits using two anatomic sites (intramedullary canal, quadriceps) with Gd-DTPA in intermediate-porosity PMMA or high-porosity PMMA; six of the nine rabbits also had Gd-DTPA delivered in collagen at a third site (hamstring). We imaged Gd-DTPA distribution in rabbits after local delivery at two anatomic sites, one contained within bone (intramedullary canal) and one contained in soft tissue (muscle), from three delivery vehicles with different in vitro release rates (collagen, high-porosity PMMA, and intermediate-porosity PMMA) [11, 23]. Our primary outcome was area of distribution as visible on MRI and interobserver reliability for reading the MR images was assessed.
Gd-DTPA collagen was prepared by mixing liquid Gd-DTPA (Magnavist; Sigma-Aldrich, St Louis, MO, USA) with 150 mM PBS. Powdered porcine collagen (Sigma-Aldrich) was added, and the solution was heated to 60°C. On cooling, a 17 wt% collagen hydrogel was created with a final concentration of 100 mM Gd-DTPA. Negative control collagen was prepared as 17 wt% porcine collagen without Gd-DTPA. Gd-DTPA PMMA was prepared by homogeneously mixing Gd-DTPA powder (Sigma-Aldrich), 1 g for intramedullary delivery or 2 g for intramuscular delivery, in 40 g of polymethylmethacrylate powder (Simplex P bone cement; Stryker, Kalamazoo, MI, USA) and enough xylitol powder (Xlear, Orem, UT, USA) to create total poragen of either 5 vol% (intermediate porosity) [23] consistent with intermediate-dose antimicrobial-loaded bone cement (ALBC) [15, 20] or 10 vol% (high-porosity) [23] consistent with high-dose ALBC that is used clinically [23], then polymerized with 20 mL of methylmethacrylate monomer. The Gd-DTPA powder was the same compound that was in the liquid Gd-DTPA. The particulate xylitol was used as a poragen. It was sieved to include only 250- to 425-μm particle size using ASTM E-11 sieves. Gd-DTPA PMMA rods, 4 mm in diameter and 9 cm in length with a curvature similar to a rabbit’s femoral bow, were fabricated using red rubber catheters (Covidien, Mansfield, MA, USA) as molds. Negative control PMMA rods were made using PMMA with no xylitol and no Gd-DTPA.
Nine 2.5-kg female New Zealand White rabbits were used for these experiments. Rabbits were sedated with intramuscular ketamine (35 mg/kg), xylazine (5 mg/kg), and butrophanol (0.1 mg/kg) before surgery, and anesthesia was maintained on 2% isoflurane. A heated water blanket was used to maintain the rabbit’s temperature at 37°C. Electrocardiogram, respiration, and temperature were monitored during surgery and the duration of the imaging. All procedures were approved by the Institutional Animal Care and Use Committee. National Institutes of Health guidelines for the care and use of laboratory animals were observed (Publication 85-23, revised 1985).
Gd-DTPA PMMA rods were inserted into the intramedullary canal of the left femur through a stab incision lateral to the patellar tendon. A 0.62-mm Kirschner wire was introduced into the femoral canal in a retrograde fashion through an entry point in the intercondylar notch. The entry path through the distal metaphysis was expanded to 4 mm with drill bits on a hand chuck. The rods were advanced to span the entire intramedullary canal from the subtrochanteric region to the distal metaphysis. Excess length was trimmed at the subchondral level. Gd-DTPA PMMA rods were also inserted into the right rectus femoris muscle through a stab incision made anteriorly proximal to the patella. The intramuscular rods were advanced the full length of the thigh within the substance of the rectus femoris. The excess length was trimmed proximal to the patella. The surgical wounds were closed with a single suture. The Gd-DTPA collagen depot (0.2 mL) was injected intramuscularly into the midsubstance of the left hamstring muscles using a 20-g needle.
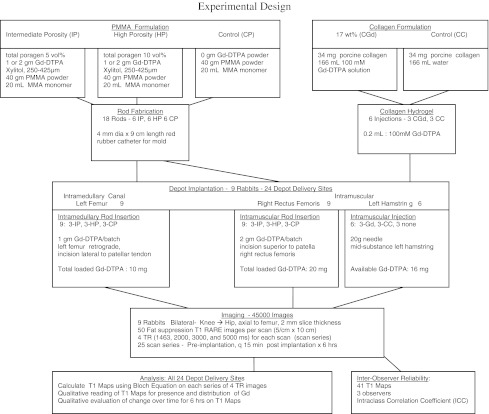
A total of 24 local delivery depots were implanted in nine rabbits (Fig. 1). All nine rabbits were implanted at two anatomic sites, the left femur and the right rectus femoris, with one of three delivery vehicles (high-porosity Gd-DTPA PMMA rods, intermediate-porosity Gd-DTPA PMMA rods, or negative control rods) replicated in triplicate in random order (18 depots: two sites × three vehicles × three replicates). Six of the nine rabbits had a third site, the left hamstring muscles, injected with Gd-DTPA collagen or negative control collagen, replicated in triplicate in random order (six depots: one site × two vehicles × three replicates).
Fig. 1.
A diagram of the experimental setup is shown.
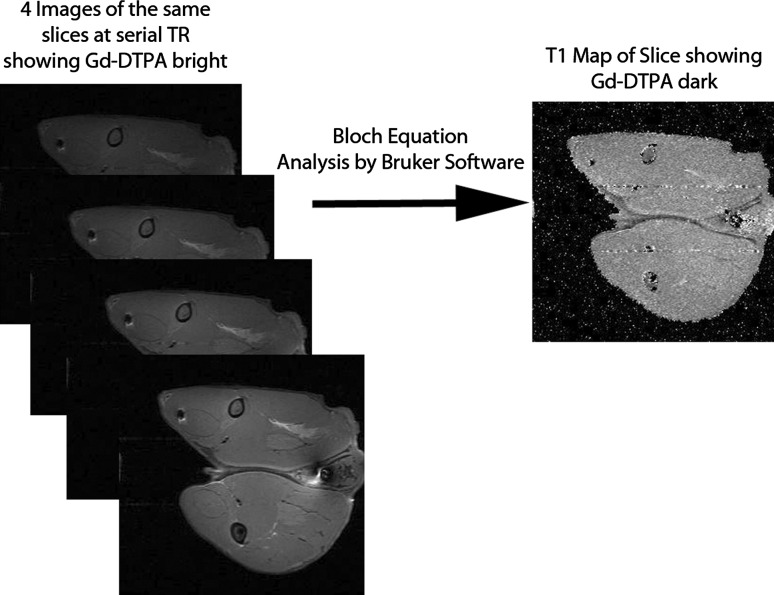
Imaging was performed on a 7-T Bruker Biospec MRI (Bruker Biospin; Billerica, MA, USA) using a rabbit coil (Bruker Biospin; Billerica). A total of 45,000 images were acquired. For each of nine rabbits, 50 2-mm slices over the 10-cm region of interest (ROI) (5 cm × 10 cm) with a series of four TR values for each slice were all repeated at 25 time periods (preimplantation, every 15 × 6 hours) (nine × 50 × four × 25 = 45,000). Fat-suppressed T1-weighted, rapid acquisition with relaxation enhancement (RARE) scans were acquired with a series of four TRs (repetition time 1463, 2000, 3000, and 5000 ms) for each rabbit at every time point. The ROIs that contained the local delivery depots within the thighs, from the knee to the hip, were scanned with 2-mm slices perpendicular to the long axis of the femur. Pixel resolution measured 0.3 mm × 0.3 mm. Total scan time was approximately 13 minutes per series. A control scan was taken of each rabbit before implantation of the delivery vehicles. Animals were scanned at 15-minute intervals for 6 hours after implantation of the delivery vehicles, acquiring 24 scan series per rabbit. Animals were euthanized with Beuthanasia D solution (120 mg/kg) intravenously after 6 hours of imaging. Each series of RARE images (four different TRs) were used to construct a T1 map of the ROI by solving the Bloch Equation for T1 given various intensities at various TR [13] using Bruker® software (Bruker Biospin; Billerica). The Block Equation uses an exponential relationship to calculate the T1 value of the tissue from the measured pixel intensity in each image generated by the four different TR values. There is an important difference between T1-weighted images and T1 maps: although Gd-DTPA causes brighter signal on T1-weighted images, it causes a decrease in the T1 values on a T1 map leading to a darker appearance on the T1 map. Locations with low water content such as acrylic bone cement and bone have low T1 values and also appear darker. Locations with high water content such as edema appear bright on a T1 map. The T1 maps were visually read to qualitatively determine the distribution of the locally delivered Gd-DTPA over both time and location (Fig. 2). Initial reading of all images, grouped by animal and delivery site, sequenced over time, was by MBG and RM. The findings were then reviewed by all authors. Quantitative measurements were made during interobserver assessment. Area of distribution from the PMMA rods or from collagen was compared with controls using the t-test.
Fig. 2.
Multiple RARE scans of the same slice are assembled into a single T1 map.
To determine interobserver reliability, three blinded observers (JA, JF, RBM) each outlined the perimeter of the area where they visualized Gd-DTPA on 41 images using MATLAB (Mathworks, Natick, MA, USA). The 41 images included three representative images from both PMMA and from collagen across the 6-hour period plus controls reviewed in random order. The observers were junior orthopaedic residents who were familiar with MRI but not familiar with the images generated in this project. One of the authors (CSE) trained all three observers by describing the project and demonstrating the technique to outline the area of visualized contrast on three sample images. The observers were then asked to outline the area where they could visualize Gd-DTPA on each image. Interobserver reliability was determined by calculating intraclass correlation coefficient (ICC) [3, 5, 18] including ICC average and examination of pairwise Bland-Altman plots. Differences in area of distribution between intramedullary and intramuscular sites were qualitatively compared.
The effect of different in vitro release rates of the vehicle on distribution of Gd-DTPA was compared using quantitative data from the interobserver analysis by comparing rates of change of distribution area. As a result of the inability to distinguish gadolinium in collagen from gadolinium in adjacent tissue, distribution area in this study is not an ideal metric for comparison of vehicles. The rate of change of area was calculated and normalized to the first postinjection image for each vehicle to determine if distribution of delivered Gd-DTPA was affected by delivery vehicle. The rates of change between vehicles were compared using regression (α = 0.05). Statistical analysis was performed in Minitab 15 (Minitab Inc., State College, PA, USA), MATLAB 7.9 (Mathworks), and Excel (Microsoft, Redmond, WA, USA).
Results
Locally delivered Gd-DTPA was visualized spreading radially from all three vehicles that contained Gd-DTPA and none was visualized from the controls. For the PMMA rods, the visible area measured during the interobserver reliability assessment was 27.5 ± 21.5 mm2, which included visible Gd-DTPA released from the rod plus the cross-sectional area of the rod. The area of low-intensity signal for controls was 10.5 ± 11.2 mm2, equal to the cross-sectional area of the rod. The area of the controls (10.5 mm2) is less (p < 0.001) than the area of the rods with visible release (27.5 mm2).
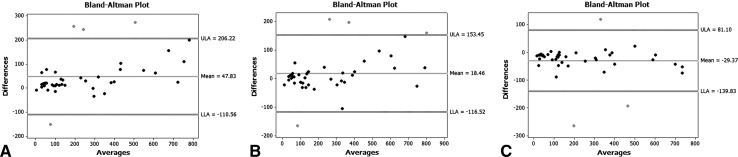
All combinations of pairs of blinded observers produced an average ICC of 0.95 (range, 0.92–0.96). Bland-Altman plots were consistent with strong agreement (Fig. 3).
Fig. 3A–C.
Bland-Altman plots comparing Observers 1, 2, and 3 in pairs. A shows Reviewers 1 and 2 compared, B shows Reviewers 2 and 3 compared, and C shows reviewers 1 and 3 compared. Plots show grouping of area measurements near zero difference with little dependence on size.
In the intramedullary canal of the femur, the distribution of Gd-DTPA was contained by the cortical bone and no penetration of bone could be detected visually (Fig. 4). For the intramuscular sites, the locally delivered Gd-DTPA penetrated muscle tissue until it reached the boundary of the muscle and then spread along the tissue plane between muscles (Fig. 5). Penetration into adjacent muscles was not seen.
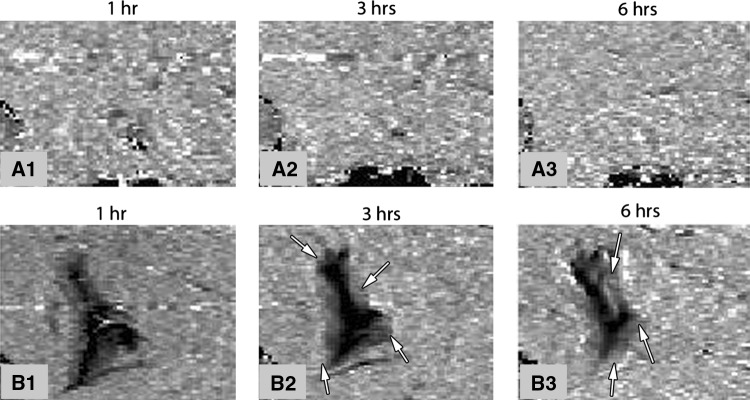
Fig. 4A–B.

Intramedullary Gd-DTPA PMMA rods. (A) Control: no Gd-DTPA; and (B) 1 g Gd-DTPA and 5 vol% total poragen is shown. Images are cross-sections perpendicular to the femur. The location of the implant is shown with a circle. The areas of low signal (white arrows) indicate drug release. B1-B3 show an increasing area of Gd-DPTA progressing with time. Images are from a similar location in the midthigh for both the (A) control and the 1 g (A) Gd-DTPA. The image series A 1–3 and B 1–3 are the same slice for the respective delivery site and animal at progressive time intervals to show change over time.
Fig. 5A–B.
Intramuscular PMMA Gd-DTPA with (A) control: no Gd-DTPA and (A) 1 g Gd-DTPA and total poragen of 10 vol% is shown. Images are cross-sections perpendicular to the femur. The location of the implant is shown with a white circle. Low signal adjacent to the rod (white arrows) indicates drug release. We believe that the bright signal surrounding the contrast is related to progressive edema secondary to the trauma of inserting the rod. B1–B3 show increasing area of Gd-DPTA progression with time. Images are from a similar location in the midthigh for both the (A) control and the (B) 1 g Gd-DTPA. The image series A 1–3 and B 1–3 are the same slice for the respective delivery site and animal at progressive time intervals to show change over time.
Gadolinium distribution was affected by in vitro release rate. For the intramuscular location, the visualized area of distribution of Gd-DTPA from the collagen was maximum at 1 hour and then decreased (p = 0.007) over time. Release from the PMMA rods tended to increase (p = 0.417) with time on the images selected for interobserver reliability assessment. We identified no difference in the distribution of delivered GD-DTPA from PMMA of differing porosities. We visualized no released Gd-DPTA from the control rods over 6 hours (Fig. 5). Collagen without Gd-DTPA was indistinguishable from the surrounding tissue (Fig. 6).
Fig. 6A–B.
Images of collagen Gd-DTPA injections over 6 hours are shown. (A) No contrast and (B) 100 mM Gd-DTPA. Images are cross-sections perpendicular to the femur from a similar location in the midthigh for both the (A) control and the (B) 1 g Gd-DTPA. The image series A 1–3 and B 1–3 are the same slice for the respective delivery site and animal at progressive time intervals to show change over time. Arrows indicate released Gd-DTPA. B1-B3 show decreasing area of Gd-DPTA progression with time.
Discussion
High levels of antimicrobial need to be distributed throughout surgical wounds wherever retained debris or tissue with microbes in biofilm might be present. Failures in treatment are often attributed to inadequate débridement; however, it is not possible to know if adequate antimicrobial levels are achieved throughout the postdébridement wound in these failures. If antimicrobial distribution in these wounds could be managed over time, improvement in local antimicrobial delivery may be possible. Gd-DTPA is selected as an imaging antimicrobial surrogate. This study was performed to determine if the distribution of locally delivered Gd-DTPA (1) can be visualized using MRI; (2) is reliably visualized by different observers; (3) is affected by the anatomic delivery site; and (4) is affected by the release rate from the delivery vehicle.
There are several limitations of this study. First, the study duration was short, 6 hours, and the number of study animals was small. The purpose of this study was confirmation of concept and determination of experimental parameters such as dose, delivery vehicle, and scan sequence. Thus, we judged the short time and small number of animals sufficient for the preliminary nature of this study. The greatest in vitro delivery of antimicrobials from the vehicles used in this study occurs early in elution studies, consistent with visualized Gd-DTPA seen in the images obtained during the first hour of this study. A longer duration will be necessary to fully characterize in vivo release, distribution, and accumulation throughout surgical wounds for clinically targeted durations of days to weeks [19]. Second, we were able to visualize the area to which Gd-DTPA had distributed, but we have not been successful in converting T1 values for each pixel to concentration values. Calculation of Gd-DTPA concentration requires measurement of T1 value for each pixel before and after the contrast agent is introduced [30]. Because the rabbit is moved and tissues are distorted during implantation of the local depots, precisely identifying the same pixels before and after local delivery of Gd-DTPA has not yet been achieved. We continue to develop techniques to resolve this technical challenge. However, for this pilot study in normal tissues, qualitative analysis has provided information on dose, delivery vehicle, scan sequence, and pattern of distribution from local delivery that can be used to design future studies. We expect to be able to perform quantitative analysis of the volume of distribution and of the concentration in future studies. Although quantitative data in normal tissues have value, we expect that there will be a considerable difference in the distribution in postdébridement surgical wounds with dead space and disruption of normal tissue planes. Third, Gd-DTPA is a surrogate for gentamicin sulfate with an unproven correlation between the distribution of Gd-DTPA and gentamicin sulfate. Gd-DTPA was chosen for these pilot studies to provide baseline transport data because it is available, inexpensive, its imaging properties are well understood, and its physical properties are similar to gentamicin. The intent for future work is to perform similar experiments with specific antimicrobials conjugated to a gadolinium contrast agent. Fourth, although we attempted to use a consistent surgical technique, the degree of tissue injury between animals cannot be ruled out as a cause of variation in Gd-DTPA distribution. Fifth, local drug delivery is a volumetric phenomenon, which will require three-dimensional reconstruction to quantify. Finally, we did not study distribution into pathological tissue (osteomyelitis) or postdébridement wounds.
Locally delivered contrast (Gd-DTPA) can be visualized over time and location within tissue on MRI. The concentration above which it is visible from in vitro data is approximately 100 μg/mL. This is consistent with other reports of Gd contrast sensitivity [21], although the sensitivity is likely dependent on the strength of the external magnetic field used. This sensitivity is also consistent with expected levels that should be achievable with Gd-conjugated antimicrobials but will be insensitive to systemic concentrations. Locally delivered Gd-DTPA was visualized from all three vehicles but absent from all control depots at all time points, as expected based on in vitro elution data [23]. Our data for distribution of locally delivered Gd-DTPA in soft tissue are consistent with in vivo tissue delivery measured by Adams et al. [1], Nelson et al. [22], and Owen et al. [24]; with MRI data for drug distribution from a local infusion in vitro by Raghavan et al. [26]; and with the local distribution after infusion into brain tissue in vivo [4] (Table 2). Our data showing visualization of Gd-DTPA over time with an MRI are consistent with Astary et al. [4] and Raghavan et al. [26], who also showed that gadolinium distribution can be seen to change with time.
Table 2.
Relevant literature
| Study | Use of MRI | Depot material | Depot placement |
|---|---|---|---|
| Adams et al. [1] | Not used | PMMA 28 days Cefazolin, ciprofloxacin, clindamycin Bone and soft tissue levels |
Canine tibia |
| Astary et al. [4] | Gd-labeled albumin Effectiveness of brain injection |
Local drug infusion | Rat hippocampus |
| Raghavan et al. [26] | Gd to track injections in the brain | Local drug infusion | In vitro gels |
| Owen et al. [24] | Not used | Tetracycline from bone cement penetrates canaliculi | Rabbit femur |
| Nelson et al. [22] | Not used | Gentamicin is distributed around implant site | Rabbit radius |
Gd = gadolinium; PMMA = polymethylmethacrylate.
Interobserver reliability is excellent even when the skill level of those interpreting the MR images is at the fundamental level of junior orthopaedic residents. The observers in this study were able to reliably identify the distribution of locally delivered Gd-DTPA.
Distribution of locally delivered Gd-DTPA differed by site of local delivery. For intramedullary sites, Gd-DTPA distributed throughout the marrow space contained by bone, whereas distribution of Gd-DTPA in intramuscular sites progressed away from the drug delivery depot to intermuscular tissue planes and then along intermuscular planes rather than penetrate the substance of adjacent muscles. These results have not been shown in other drug distribution studies.
The distribution is affected by delivery vehicle. The difference in in vitro release rate between high- and intermediate-porosity PMMA was not sufficient to cause measurable differences in the area of distribution seen on our images. Collagen releases essentially all of its drug load within 24 hours in vivo [17]. The decrease in area of distribution from Gd-DTPA collagen after 1 hour may represent a decrease in the amount of Gd-DTPA available for release over time. Typically in antimicrobial elution studies, all three delivery vehicles decrease their release over time after initial burst release as their contained load depletes, collagen the fastest. Based on the limited volume of distribution that we observed on the images in this study and the rate at which the Gd-DTPA disperses into that volume, we did not expect a receding volume of distribution during the first 6 hours, reinforcing the need to investigate the in vivo distribution of locally delivered antimicrobials. We studied bone cement with two amounts of added poragen corresponding to high-porosity and intermediate-porosity ALBC [23] but were not able to qualitatively visualize a difference in distribution of locally delivered drug between these two vehicles.
In conclusion, locally delivered Gd-DTPA can be visualized on MRI, supporting the concept of imaging locally delivered antimicrobials. The pattern of distribution is affected by anatomic structures and the release rate from the delivery vehicle.
Acknowledgments
We acknowledge the assistance of G. Turner PhD, and Q. Liu at the Keller Center for Preclinical Imaging at St Joseph’s Hospital in Phoenix. We further acknowledge the assistance of J. Abilgaard MD, R. Miller MD, and J. Fraser MD, who served as the blinded reviewers for the study.
Footnotes
One of the authors (MBG) receives funding from the Ira A. Fulton Schools of Engineering, and one or more of the authors (ACM, MBG, RM, MRC) receive funding from the Arizona Biomedical Research Commission (Grant 1116). One or more of the authors (RM, ACM) receive funding from Banner Good Samaritan Medical Center, and the authors received seed funding for this project from the Herbert Louis fund at OREF.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at St Joseph’s Hospital, Banner Good Samaritan Medical Center, Phoenix, AZ, USA, and Arizona State University, Tempe, AZ, USA.
References
- 1.Adams K, Couch L, Cierny G, Calhoun J, Mader JT. In vitro and in vivo evaluation of antibiotic diffusion from antibiotic-impregnated polymethylmethacrylate beads. Clin Orthop Relat Res. 1992;278:244–252. [PubMed] [Google Scholar]
- 2.Anagnostakos K, Fürst O, Kelm J. Antibiotic-impregnated PMMA hip spacers: current status. Acta Orthop. 2006;77:628–637. doi: 10.1080/17453670610012719. [DOI] [PubMed] [Google Scholar]
- 3.Armitage P, Berry G, Matthews JNS. Statistical Methods in Medical Research. Hoboken, NJ, USA: Wiley-Blackwell; 2001. [Google Scholar]
- 4.Astary GW, Kantorovich S, Carney PR, Mareci TH, Sarntinoranont M. Regional convection-enhanced delivery of gadolinium-labeled albumin in the rat hippocampus in vivo. J Neurosci Methods. 2010;187:129–137. doi: 10.1016/j.jneumeth.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 6.Bleicher K, Lin M, Shapiro MJ, Wareing JR. Diffusion edited NMR: screening compound mixtures by affinity NMR to detect binding ligands to vancomycin. J Org Chem. 1998;63:8486–8490. doi: 10.1021/jo9817366. [DOI] [Google Scholar]
- 7.Buchholz HW, Elson RA, Engelbrecht E, Lodenkämper H, Röttger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981;63:342–353. doi: 10.1302/0301-620X.63B3.7021561. [DOI] [PubMed] [Google Scholar]
- 8.Diefenbeck M, Mückley T, Hofmann GO. Prophylaxis and treatment of implant-related infections by local application of antibiotics. Injury. 2006;37(Suppl 1):S95–S104. doi: 10.1016/j.injury.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Dunbar MJ. Antibiotic bone cements: their use in routine primary total joint arthroplasty is justified. Orthopedics. 2009;32:660. doi: 10.3928/01477447-20090728-20. [DOI] [PubMed] [Google Scholar]
- 10.Estes C, McLaren A, Clavijo-Jordan V, McLemore R. MRI Determination of antimicrobial concentration distribution. In: Annual Meeting of the Musuculoskeletal Infection Society, Los Angeles, CA; 2010. Available at: www.msis-na.org/id260.htm. Accessed January 12, 2012.
- 11.Geiger M, Li RH, Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003;55:1613–1629. doi: 10.1016/j.addr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Gordon CA, Hodges NA, Marriott C. Use of slime dispersants to promote antibiotic penetration through the extracellular polysaccharide of mucoid Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991;35:1258–1260. doi: 10.1128/AAC.35.6.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haacke EM, Brown RW, Thompson MR, Venkatesan R. Magnetic Resonance Imaging: Physical Principles and Sequence Design. New York, NY, USA: Wiley-Liss; 1999. [Google Scholar]
- 14.Hanssen AD. Local antibiotic delivery vehicles in the treatment of musculoskeletal infection. Clin Orthop Relat Res. 2005;437:91–96. doi: 10.1097/01.blo.0000175713.30506.77. [DOI] [PubMed] [Google Scholar]
- 15.Hanssen AD, Spangehl MJ. Practical applications of antibiotic-loaded bone cement for treatment of infected joint replacements. Clin Orthop Relat Res. 2004;427:79–85. doi: 10.1097/01.blo.0000143806.72379.7d. [DOI] [PubMed] [Google Scholar]
- 16.Hsu C-S, Hsu C-C, Wang J-W, Lin P-C. Two-stage revision of infected total knee arthroplasty using an antibiotic-impregnated static cement-spacer. Chang Gung Med J. 2008;31:583–591. [PubMed] [Google Scholar]
- 17.Jørgensen LG, Sørensen TS, Lorentzen JE. Clinical and pharmacokinetic evaluation of gentamycin containing collagen in groin wound infections after vascular reconstruction. Eur J Vasc Surg. 1991;5:87–91. doi: 10.1016/S0950-821X(05)80933-8. [DOI] [PubMed] [Google Scholar]
- 18.Lin LI-K. Assay validation using the concordance correlation coefficient. Biometrics. 1992;48:599–604. doi: 10.2307/2532314. [DOI] [Google Scholar]
- 19.McLaren A, McLemore R, Gutierrez F, Martin M. Musculoskeletal infection. In: Cierney G III, McLaren A, Wongworowat M, editors. Orthopaedic Knowledge Update. Rosemont, IL: AAOS; 2009. pp. 95–117. [Google Scholar]
- 20.McLaren AC, McLaren SG, McLemore R, Vernon BL. Particle size of fillers affects permeability of polymethylmethacrylate. Clin Orthop Relat Res. 2007;461:64–67. doi: 10.1097/BLO.0b013e31811f350d. [DOI] [PubMed] [Google Scholar]
- 21.Mørkenborg J, Pedersen M, Jensen FT, Stødkilde-Jørgensen H, Djurhuus JC, Frøkiaer J. Quantitative assessment of Gd-DTPA contrast agent from signal enhancement: an in-vitro study. Magn Reson Imaging. 2003;21:637–643. doi: 10.1016/S0730-725X(03)00096-1. [DOI] [PubMed] [Google Scholar]
- 22.Nelson CL, Hickmon SG, Skinner RA. Treatment of experimental osteomyelitis by surgical débridement and the implantation of bioerodable, polyanhydride–gentamicin beads. J Orthop Res. 1997;15:249–255. doi: 10.1002/jor.1100150214. [DOI] [PubMed] [Google Scholar]
- 23.Nugent M, McLaren A, Vernon B, McLemore R. Strength of antimicrobial bone cement decreases with increased poragen fraction. Clin Orthop Relat Res. 2010;468:2101–2106. doi: 10.1007/s11999-010-1264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owen K, Nicholas R, Hickmon S, Skinner R, Stewart C, Nelson C. Antibiotic penetration of avascular bone. Orthop Trans. 1994;18:599. [Google Scholar]
- 25.Penner MJ, Duncan CP, Masri BA. The in vitro elution characteristics of antibiotic-loaded CMW and Palacos-R bone cements. J Arthroplasty. 1999;14:209–214. doi: 10.1016/S0883-5403(99)90128-6. [DOI] [PubMed] [Google Scholar]
- 26.Raghavan R, Brady ML, Rodriguez-Ponce MI, Hartlep A, Pedain C, Sampson JH. Convection-enhanced delivery of therapeutics for brain disease, and its optimization. Neurosurg Focus. 2006;20:E12. doi: 10.3171/foc.2006.20.4.7. [DOI] [PubMed] [Google Scholar]
- 27.Ramanan B, Holmes WM, Sloan WT, Phoenix VR. Application of paramagnetically tagged molecules for magnetic resonance imaging of biofilm mass transport processes. Appl Environ Microbiol. 2010;76:4027–4036. doi: 10.1128/AEM.03016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sampson JH, Raghavan R, Brady ML., Provenzale JM, Herndon JE, Croteau D, Friedman AH, Reardon DA, Coleman RE, Wong T, Bigner DD, Pastan I, Rodr¡guez-Ponce MI, Tanner P, Puri R, Pedain C. Clinical utility of a patient-specific algorithm for simulating intracerebral drug infusions. Neurooncology. 2007;9:343–353. [DOI] [PMC free article] [PubMed]
- 29.Sterling GJ, Crawford S, Potter JH, Koerbin G, Crawford R. The pharmacokinetics of Simplex-tobramycin bone cement. J Bone Joint Surg Br. 2003;85:646–649. [PubMed] [Google Scholar]
- 30.Zhu XP, Li KL, Kamaly-Asl ID, Checkley DR, Tessier JJL, Waterton JC, Jackson A. Quantification of endothelial permeability, leakage space, and blood volume in brain tumors using combined T1 and T2* contrast-enhanced dynamic MR imaging. J Magn Reson Imaging. 2000;11:575–585. doi: 10.1002/1522-2586(200006)11:6<575::AID-JMRI2>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]