Abstract
Background
Intralesional excision and en bloc resection are used to treat giant cell tumors (GCTs) of the distal radius. However, it is unclear whether one provides lower rates of recurrences and fewer complications, and whether the use of polymethylmethacrylate (PMMA) after curettage reduces the risk of recurrence.
Questions/Purposes
We examined whether curettage was associated with lower rates of recurrence and fewer major complications compared with en bloc excision, and whether PMMA resulted in lower rates of recurrence compared with a bone graft.
Methods
We systematically searched the literature using the criteria, “giant cell tumor” AND “curettage” OR “intralesional excision” OR “resection”. Six relevant articles were identified that reported data for 80 curettage cases (PMMA, n = 49; bone graft, n = 26; no PMMA or bone grafts, n = 5) and 59 involving en bloc excision. A meta-analysis was performed using these data.
Results
Overall, patients in the intralesional excision group had a higher recurrence rate (relative risk [RR], 2.80; 95% CI, 1.17–6.71), especially for Campanacci Grade 3 GCTs (RR, 4.90; 95% CI, 1.36–17.66), yet fewer major complications (RR, 0.21; 95% CI, 0.09–0.54) than the en bloc resection group. The use of PMMA versus bone graft did not affect the recurrence rate (RR, 0.98; 95% CI, 0.44–2.17).
Conclusions
Based on data obtained from the limited number of studies available, intralesional excision appears to be more appropriate for the treatment of local lesions (eg, Grades 1 and 2) than Grade 3 GCTs of the distal radius. Moreover, PMMA was not additionally effective as an adjuvant.
Level of Evidence
Level III, therapeutic study (systematic review). See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Giant cell tumors (GCTs) of bone are relatively rare, and in some cases are enigmatic. For example, although they typically are considered benign, some are aggressive and metastasize to the lungs. GCTs typically affect young adults between the ages of 20 and 40 years [16, 37, 50], and occur somewhat more commonly in women [2, 4, 7, 16, 28]. GCTs have occurred in the distal radius in approximately 10% of cases (range, 8%–13%) [7, 16, 19, 28, 38, 40], whereas more common sites have included the distal femur and proximal tibia. Local control and reconstruction of GCTs in the distal end of the radius have been challenging owing to the limited amount of surrounding soft tissue, the proximity of this region to adjacent neurovascular structures, and the juxtaarticular location.
Treatment options have included intralesional excision (curettage) with or without adjunctive modalities (eg, high-speed burring, cryotherapy, phenol, and hydrogen peroxide), en bloc resection followed by reconstruction, or arthrodesis. The best treatment approach for GCTs that occur in the distal radius is unclear. Recurrence rates have ranged from 25% to 89% for both procedures [1, 4, 29, 35, 41], although the recurrence rates for en bloc resection reportedly range from 0% to 33% [1, 3, 29, 41]. In addition, en bloc resection usually requires sacrifice of the articular surface, and secondary arthritis has occurred in 13% to 50% of patients [8, 13, 16]. Based on these considerations and the decreased function associated with the en bloc method, some surgeons have suggested that resection provides a more aggressive treatment for GCTs [8, 46]. In contrast, some surgeons have reported that intralesional excision, combined with various adjunct therapies, provides comparable recurrence rates ranging from 0% to 28% [8, 18, 19, 23, 46, 51]. Furthermore, if curettage fails, it does not preclude other forms of treatment, such as en bloc resection followed by reconstruction or arthrodesis. Moreover, repeated curettage has been used with recurrent GCTs in the distal radius, and local control has been achieved in 89% to 100% of cases [18, 19, 46].
After curettage, various filling materials have been used, including autografts [8, 16, 38], polymethylmethacrylate (PMMA) [16, 18, 37, 38, 46, 51], calcium phosphate [31], or no packing [19]. Furthermore, numerous authors have suggested PMMA extended the effective curettage area owing to toxicity mediated by the PMMA monomer and the exothermic reaction produced by curing polymer [5, 30, 32, 44]. However, the use of bone grafts still is considered a more physiologic method for reconstruction [38].
Given the inconsistent conclusions reported in various studies for surgical management of GCTs in the distal radius, we examined: (1) whether overall recurrence rates differed between curettage and en bloc excision, (2) whether recurrence rates differed between curettage and en bloc excision for Campanacci Grade 3 GCTs [7], (3) whether recurrence rates differed with the use of PMMA versus bone grafts for patients treated with curettage, and (4) whether major complication rates differed between curettage and en bloc excision.
Search Strategy and Criteria
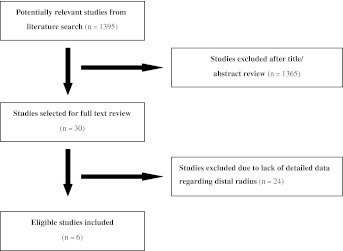
Two independent assessors (YPL, BHS) performed a review of the literature (using electronic searches of Google Scholar [1966 to May 2011], Medline [1966 to May 2011], EMBASE [1974 to May 2011], and Cochrane Controlled Trial Register databases [The Cochrane Library 2011]). Furthermore, they conducted searches without language restrictions to identify all studies that assessed recurrence rates and complications associated with GCTs in the distal radius. The keyword pairs searched for included: “giant cell tumor” AND “radius”, “giant cell tumor” AND “curettage”, “giant cell tumor” AND “intralesional excision”, “giant cell tumor” AND “resection”, “giant cell tumor” AND “bone graft”, “giant cell tumor” AND “cement”. Additional searches were made of the reference lists in the retrieved studies relevant to the distal radius and retrieved all potentially relevant studies. An initial search yielded 1395 titles (Fig. 1). The two authors (YPL, BHS) then independently reviewed titles and abstracts for the studies that reported the outcome of interest for curettage and resection. After this initial review, 1365 articles were excluded and 30 were retained for full text review. From these 30 articles, those that reported on patients from the same cohort who underwent intralesional excision and en bloc resection for treatment of GCTs of the distal radius were included, as were studies that reported local recurrence and complications as a primary outcome. In contrast, exclusion criteria included studies where: (1) the overall number of patients with GCTs of the distal radius did not exceed six, (2) an uncontrolled case series study was performed, and/or (3) it was impossible to extrapolate or calculate the dates of recurrence or complications from the published results. After application of these criteria, two reviewers (YPL, BHS) independently selected relevant studies, and discussed any discrepancies to reach a consensus. The reviewers selected a total of six studies, all of which were cohort studies published in English or Turkish between 1993 and 2010 (Table 1) [8, 16, 18, 37, 46, 51]. Moreover, none of these studies included Level I or II evidence, primarily because in musculoskeletal oncologic surgical trials, patients have differences in incision length and tumor size and cannot be randomly divided into two groups.
Fig. 1.
The flow diagram shows how the literature search was performed.
Table 1.
Characteristics of the trials examined in this study
| Study* | Total number of patients (ulna^) | Mean age, years (range) | Male/female | Mean followup months (range) | Adjuvant | Resection/reconstruction | Filling material |
|---|---|---|---|---|---|---|---|
| Kang et al. [18] (2010) | 15 | 38 (25–87) | 10/5 | 60 (22–99) | HSB, cryosurgery | Arthrodesis | PMMA |
| Ozalpt et al. [37] (2006) | 23 (5) | 32 (12–74) | 6/17 | 80 | Phenol | Arthrodesis, fibular | PMMA, bone graft |
| Harness and Mankin [16] (2004) | 49 (3) | 31 (15–54) | 20/29 | 168 (48–336) | HSB, phenol | Allograft | PMMA, bone graft |
| Cheng et al. [8] (2001) | 12 | 36 (16–72) | 4/8 | 72 (36–192) | HSB, phenol | Allograft, fibular | Bone graft |
| Sheth et al. [46] (1995) | 26 | 34 (17–81) | 12/14 | 108 (36–408) | Cryosurgery | Arthrodesis | PMMA, bone graft |
| Vander Griend and Funderburk [51] (1993) | 22 | 32 (16–74) | 5/17 | (24–228) | HSB, pulsating lavage | Arthrodesis, arthroplasty | PMMA |
* All studies used a cohort design; HSB = high-speed burr; PMMA = polymethylmethacrylate; ^ of the total number of patients, the subset that involved GCT of the ulna.
The same two reviewers (YPL, BHS) also independently evaluated the six studies using the Methodological Index for Nonrandomized Studies (MINORS) scale [47], and the Newcastle-Ottawa scale (NOS) [54]. These scales allocated a maximum of 24 points (MINORS) and nine points (NOS), respectively, for quality of selection, comparability, exposure, and outcome of study participants. For these six studies, both reviewers had median scores of 17 and 8 for the MINORS scale and NOS questionnaire, respectively. The two reviewers then independently extracted data on tumor recurrence and complications, resolving discrepancies through discussion.
In the six studies selected for examination, data for 139 patients were available. Of these patients, 80 underwent intralesional excision and 59 underwent en bloc resection, with 71 patients in five studies [8, 16, 18, 46, 51] treated for Grade 3 GCTs available for subgroup analysis. In addition, a total of 75 patients received PMMA (n = 49) versus bone grafts (n = 26). Major complications were reported in five studies [8, 18, 37, 46, 51] for 49 patients in the curettage group versus 44 patients in the en bloc resection group. These major complications included: nonunion at the graft-radius junction, fracture of graft, skin necrosis, death, fragmentation with carpal collapse, and subluxation or arthrosis which impaired joint function.
Results from the meta-analysis performed are reported in terms of relative risk (RR) with 95% confidence interval (CI). In addition, we evaluated heterogeneity among studies using the Cochrane Q test, with a p-value set at 0.1 for significance. Heterogeneity between trials was evaluated based on an assigned I2 value [17], and substantial heterogeneity was represented by an I2 value greater than 50%. In the presence of heterogeneity, we used a random effect model; otherwise, we used a fixed effect model. The level of significance for the combined estimates was 0.05, and all calculations were performed using RevMan 5.1 software (The Cochrane Collaboration, Copenhagen, Denmark).
Results
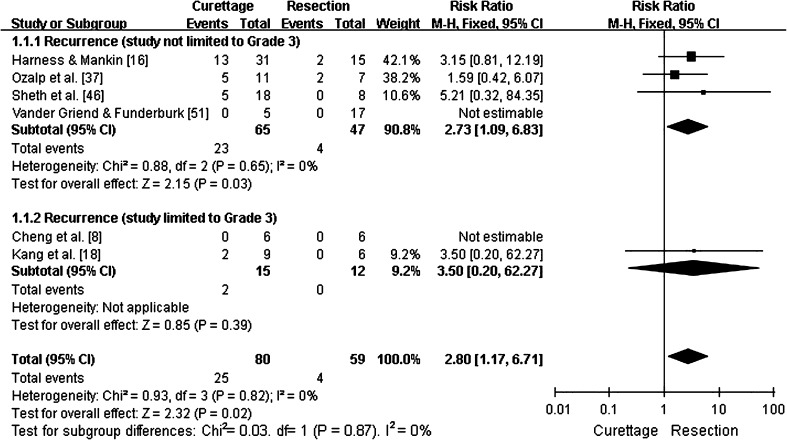
Overall recurrence rates for GCTs of the distal radius treated with intralesional excision versus en bloc resection was 31% (25 of 80) versus 7% (four of 59), respectively. Furthermore, in four studies that directly compared intralesional excision and en bloc resection [16, 18, 37, 46], the pooled RR for tumor recurrence was 2.8 (95% CI, 1.17–6.71; p = 0.02; homogeneity I2, 0%), suggesting that intralesional excision was associated with a higher rate of recurrence (Fig. 2).
Fig. 2.
This forest plot shows pooling of relative risks (RR) of overall recurrence by type of treatment (95% CI, 1.17–6.71; p = 0.02). Overall recurrence rates were higher for patients treated with curettage than for patients treated by resection, whether studies limited to Campanacci Grade 3 were included or excluded.
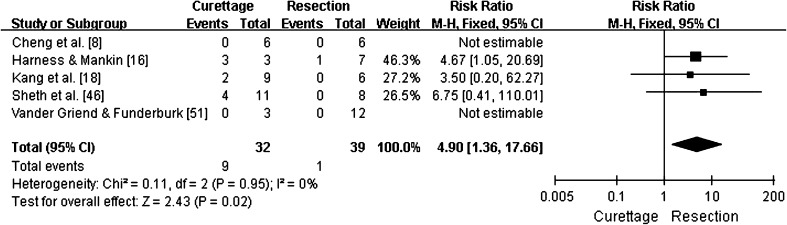
A subgroup analysis of tumor recurrence for Campanacci Grade 3 GCTs treated with intralesional excision versus en bloc resection was reported in 28% (nine of 32) versus 3% (one of 39) of cases, respectively. In three studies where these two groups were directly compared [16, 18, 46], the pooled RR for tumor recurrence was 4.9 (95% CI, 1.36–17.66; p = 0.02; homogeneity I2, 0%). Based on these results, the possibility of recurrence after intralesional excision was nearly fivefold greater than after en bloc resection (Fig. 3).
Fig. 3.
This forest plot shows pooling of relative risks (RR) of recurrence for Campanacci Grade 3 GCTs (95% CI, 1.36–17.66; p = 0.02). Recurrence rates were higher for patients treated with curettage than for patients treated by resection.
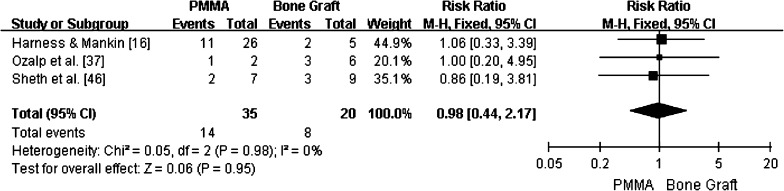
For cases in which PMMA was used to fill the resulting cavity versus bone graft, local recurrence occurred in 32.7% (16 of 49) versus 30.8% (eight of 26) of cases, respectively. Moreover, in three studies that directly compared patients who received PMMA versus bone grafts [16, 37, 46], the RR for local recurrence was 0.98 (95% CI, 0.44–2.17; p = 0.95; homogeneity I2, 0%) (Fig. 4), indicating there was no difference in recurrence rates.
Fig. 4.
Pooling of relative risks (RR) of overall recurrence according to the type of bone filling used is shown (95% CI, 0.44–2.17; p = 0.95). No differences in recurrence were found.
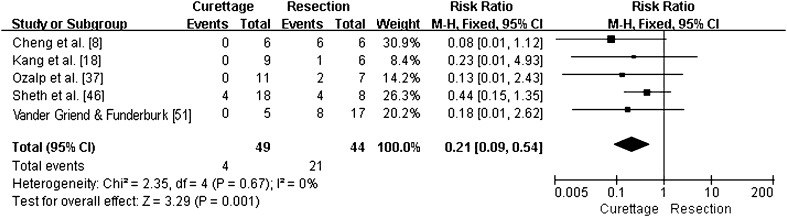
For major complications that were reported, the pooled RR for the intralesional excision group versus the en bloc resection group was 0.21 (95% CI, 0.09–0.54; p = 0.001; homogeneity I2, 0%) (Fig. 5). Therefore, a lower rate of major complications was associated with the intralesional excision group.
Fig. 5.
This forest plot shows pooling of relative risks (RR) of major complications according to the type of intervention (95% CI, 0.09–0.54; p = 0.001). Patients treated with en bloc resection reported more major complications than patients treated with curettage.
Discussion
Extension of curettage has been achieved using a high-speed burr combined with adjuvant to preserve joint function, and this method has been associated with a low rate of recurrence (4%–14%) [5, 6, 12, 13, 23, 25, 27, 36, 44, 48, 52, 53, 56]. Therefore, our objective was to identify cases of GCTs of the distal radius to determine whether curettage is associated with lower rates of recurrences and fewer major complications compared with en bloc excision, and whether recurrence rates differed with the use of PMMA versus bone graft in patients treated with curettage.
Our meta-analysis has some limitations. First, few studies met the inclusion criteria, perhaps because GCTs of the distal radius are relatively uncommon. As a result, a small number of cases was available for evaluation. Correspondingly, subgroup analyses according to each stage of disease and an analysis of the effect of previous treatment were unable to be performed. Second, there were an additional 24 articles (Table 2) that were relevant to our study, yet did not provide sufficient data to calculate or extrapolate recurrence or complications. For articles that had more than six cases of the distal radius, the authors were contacted to request the original data. However, these requests either were not addressed or were denied. Third, randomized controlled trials have not been conducted. Therefore, the retrospective cohort studies examined reflected the best evidence currently available. However, substantial selection bias is present as patients with less aggressive lesions likely would have been treated with intralesional excision. Fourth, only three studies reported patient function. Moreover, the data provided were limited by inconsistent reporting of outcome measures used, and grip strength and ROM data could not be pooled owing to the lack of standard deviation and individual patient data. Fifth, the followups for the studies examined were variable, with a minimum followup inconsistently established. In addition, delayed recurrence has been reported as much as 20 years after treatment [45]. However, 75% to 80% of patients have experienced recurrence of GCTs within 2 years [14, 19, 20, 26, 40]. Sixth, different adjuvants were used (eg, phenol, cryosurgery, high-speed burr) in the curettage group reviewed. When these data were pooled (I2 = 0%), no obvious heterogeneity was found, suggesting that a subgroup analysis to assess the role of adjuvants was not needed. Finally, there were neither uniform definitions nor established classifications available for reporting major complications. Consequently, we defined this category ourselves; however, given that a greater number of complications was associated with the en bloc resection group, our results were unlikely to have been affected.
Table 2.
Characteristics of the trials (n = 24) excluded in this study after full text review
| Study | Total number of patients (distal radius cases) | Followup range | Filling material (number of cases) | Resection (number of cases) | Overall recurrence rate |
|---|---|---|---|---|---|
| McDonald et al. [28] (1986)‡ | 221 (29) | 2–21 years | NR | NR | C 34% W 7% |
| Campanacci et al. [7] (1987)‡ | 280 (NR) | 2–44 years | NR | NR | I 27% M 8% W or RE 0% |
| Rooney et al. [43] (1993)* | 31 (3) | 2–13 years | NR | NR | I 25% M or W 9% |
| Gitelis et al. [13] (1993) * | 40 (5) | 32–204 months | None | Allograft (5) | I 5% R 0% |
| Renard et al. [42] (1994)* | 19 (1) | 4–31 years | None | Arthrodesis (1) | I 25% M or W 0% |
| Lausten et al. [24] (1996)* | 31 (2) | 8–406 months | NR (1) | NR (1) | I 56% W 0% |
| Oda et al. [34] (1998)* | 47 (5) | 60–371 months | NR (3) | Fibular (2) | I 75% E and C 50% W 0% |
| Labs et al. [22] (2001)* | 23 (1) | 31–89 months | NR | NR | I 13% W 0% |
| Turcotte et al. [50] (2002)‡ | 186 (19) | 24–192 months | NR | NR | C 18% R 16% |
| Ng et al. [33] (2002)* | 31 (4) | 20–121 months | None | Fibular (3), amputation (1) | C 29% W 6% |
| Ghert et al. [12] (2002)‡ | 75 (8) | 24–224 months | NR | NR | I 14% M or W 13% |
| Ward and Li [53] (2002)* | 30 (4) | 1–9.6 years | PMMA (1), bone graft (3) | Allograft (1) | C 8% W 0% |
| Su et al. [48] (2004)‡ | 87 (9) | 28–138 months | NR | NR | C18% W 3% |
| Wang et al. [52] (2005)* | 24 (2) | 2–20 years | PMMA (1) | Allograft (1) | C 14% W 0% |
| Lim et al. [26] (2005)* | 16 (0) | 30–132 months | None | None | C 29% W 50% |
| Guo et al. [14](2006)‡ | 146 (21) | 24–180 months | NR | NR | C 19% R 6% |
| Zhang et al. [55](2006)* | 38 (2) | 12–144 months | NR | NR | C 27% R 0% |
| Panchwagh et al. [38] (2007)‡ | 23 | 18–71 months | PMMA (5), bone graft (7) | Arthrodesis (9), fibular (6), ulnar (5) | Overall 32% |
| Gupta et al. [15] (2007)† | 93 (14) | 3–20 years | None | Fibular (14) | C 35% W 6% |
| Balke et al. [4] (2008)† | 214 (9) | 8–280 months | PMMA(5), NR (4) | None | C 34% W 0% |
| Muramatsu et al. [31] (2009)* | 23 (3) | 12–180 months | Calcium phosphate (2) | Fibular (1) | C 0% W 0% |
| Pietschmann et al. [39] (2010)* | 46 (2) | 1–289 months | NR | NR | C 38% R 17% |
| Errani et al. [11] (2010)‡ | 349 (37) | 36–204 months | NR | NR | C 16% R 12% |
| Klenke et al. [21] (2011)‡ | 118 (12) | 36–233 months | NR | NR | I 5% W 25% |
All studies used a cohort design; NR = not reported; C = curettage; R = resection, I = intralesional excision; M = marginal excision; W = wide excision; RE = radical excision; PMMA = polymethylmethacrylate; * overall number of patients with GCTs of distal radius was less than six; †an uncontrolled case series study was performed; ‡ impossible to extrapolate or calculate the dates of recurrence or complications from the published results.
The pooled data indicate that intralesional excision did not provide the same local control as en bloc resection, and recurrence rates remained high (31%) despite various adjuvants being used. This may be attributable to the close proximity of the distal radius to the distal ulna, carpal bones, and various tendons, nerves and vessels, in addition to limited surrounding soft tissues [46].
For Campanacci Grade 3 GCTs, the possibility of recurrence for the intralesional excision group was nearly fivefold greater than for the en bloc resection group. In contrast, other studies have reported that Grade 3 lesions were treated effectively with intralesional procedures [8, 18, 46]. As the latter involved the selection of patients with minimum cortical perforation and less tumor extension, the chance of recurrence would be reduced. These considerations, in combination with the results of our study, suggested that curettage should be used prudently with Grade 3 GCTs. This finding is consistent with other studies [16, 19] where curettage also was recommended for Grades 1 and 2 CGTs.
Some studies have reported a lower risk of local recurrence with the use of PMMA for curetted cavities [2, 4, 20, 21]. However, we found that tumor recurrence for patients who received PMMA did not differ from patients who underwent a bone graft. These data were consistent with those of other studies, leading to questions regarding the benefit of PMMA [12, 35, 50] versus the potential for cartilaginous degeneration [6, 49]. In our survey of 75 patients using PMMA or a bone graft, only one patient from each group reported having arthritis [16]. This may be attributable to the small amount of cement used in the distal radius [51] and the wrist not being a weightbearing joint.
Previously, en bloc resection was associated with a relatively high rate of major complications (range, 29%–100%) [8, 16, 37, 46, 51]. These complications included nonunion at the graft-radius junction (12%–38%) [16, 37, 46, 51], fracture of graft (13%–29%) [46, 51], subluxation (12%–67%) [8, 51], and arthritis (13%–50%) [8, 16], and one patient died of postsurgical pneumonia [18]. In contrast, major complications reported for intralesional excision only involved curettage and cryosurgery [46]. Correspondingly, in our meta-analysis, the rate of major complications was fivefold greater for patients who had en bloc resection versus those who had intralesional excision.
Regarding function, nearly all studies consistently showed that curettage provided the best preservation of wrist function [8, 18, 38, 46]. For example, when function data for 34 patients were pooled [8, 46] (despite the use of different scoring systems [9, 10]), the following categories for the curettage and resection cases could be identified: “excellent” function was reported by 41% and 24% of patients respectively; “good” function were reported for 47% in both groups; and “fair” function was reported by 6% and 24% of patients, respectively. In addition, for the curettage versus the resection group, mean grip strength compared with the contralateral side was 66% to 83% [18, 46] and 47% to 70% [8, 18, 46], respectively, and the mean ROM compared with the opposite wrist was 61% to 100% [8, 46] and 69% [8], respectively.
To facilitate better evaluation of the two methods for treatment of GCTs of the distal radius, a larger, multicenter prospective cohort study is needed. The availability of these data for future meta-analyses of individual patient data is important.
Acknowledgments
We thank Jie Ding MD and Mai Xu MD, who offered valuable support and help in the writing and revising this article.
Footnotes
This work was supported by the National 863 project of China (2011AA030101), the freedom explore program of Central South University (2012QNZT103), and National Clinical Key Department Construction Projects of China.
Each author certifies that he or she, or a member of their immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved or waived approval for the reporting of this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Aithal VK, Bhaskaranand K. Reconstruction of the distal radius by fibula following excision of giant cell tumor. Int Orthop. 2003;27:110–113. doi: 10.1007/s00264-002-0414-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arbeitsgemeinschaft K, Becker WT, Dohle J, Bernd L, Braun A, Cserhati M, Enderle A, Hovy L, Matejovsky Z, Szendroi M, Trieb K, Tunn PU. Local recurrence of giant cell tumor of bone after intralesional treatment with and without adjuvant therapy. J Bone Joint Surg Am. 2008;90:1060–1067. doi: 10.2106/JBJS.D.02771. [DOI] [PubMed] [Google Scholar]
- 3.Asavamongkolkul A, Waikakul S, Phimolsarnti R, Kiatisevi P. Functional outcome following excision of a tumour and reconstruction of the distal radius. Int Orthop. 2009;33:203–209. doi: 10.1007/s00264-007-0441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balke M, Schremper L, Gebert C, Ahrens H, Streitbuerger A, Koehler G, Hardes J, Gosheger G. Giant cell tumor of bone: treatment and outcome of 214 cases. J Cancer Res Clin Oncol. 2008;134:969–978. doi: 10.1007/s00432-008-0370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bini SA, Gill K, Johnston JO. Giant cell tumor of bone: curettage and cement reconstruction. Clin Orthop Relat Res. 1995;321:245–250. [PubMed] [Google Scholar]
- 6.Blackley HR, Wunder JS, Davis AM, White LM, Kandel R, Bell RS. Treatment of giant-cell tumors of long bones with curettage and bone-grafting. J Bone Joint Surg Am. 1999;81:811–820. doi: 10.1302/0301-620X.81B1.9001. [DOI] [PubMed] [Google Scholar]
- 7.Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69:106–114. [PubMed] [Google Scholar]
- 8.Cheng CY, Shih HN, Hsu KY, Hsu RW. Treatment of giant cell tumor of the distal radius. Clin Orthop Relat Res. 2001;383:221–228. doi: 10.1097/00003086-200102000-00026. [DOI] [PubMed] [Google Scholar]
- 9.Enneking WF. A system for the functional evaluation of the surgical management of musculoskeletal tumors. In: Enneking WF, editor. Limb Salvage in Musculoskeletal Tumors. New York, NY: Churchill Livingstone; 1987. pp. 5–16. [Google Scholar]
- 10.Enneking WF. Modification of the system for the functional evaluation of the surgical management of musculoskeletal tumors. In: Enneking WF, editor. Limb Salvage in Musculoskeletal Tumors. New York, NY: Churchill Livingstone; 1987. pp. 626–639. [Google Scholar]
- 11.Errani C, Ruggieri P, Asenzio MA, Toscano A, Colangeli S, Rimondi E, Rossi G, Longhi A, Mercuri M. Giant cell tumor of the extremity: a review of 349 cases from a single institution. Cancer Treat Rev. 2010;36:1–7. doi: 10.1016/j.ctrv.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Ghert MA, Rizzo M, Harrelson JM, Scully SP. Giant-cell tumor of the appendicular skeleton. Clin Orthop Relat Res. 2002;400:201–210. doi: 10.1097/00003086-200207000-00025. [DOI] [PubMed] [Google Scholar]
- 13.Gitelis S, Mallin BA, Piasecki P, Turner F. Intralesional excision compared with en bloc resection for giant-cell tumors of bone. J Bone Joint Surg Am. 1993;75:1648–1655. doi: 10.2106/00004623-199311000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Guo QC, Shen JN, Wang J, Huang G, Zou CY, Jin S, Yin JQ, Liao WM. Analysis of the factors affecting the recurrence of giant cell tumor of bone][in Chinese. Zhonghua Wai Ke Za Zhi. 2006;44:797–800. [PubMed] [Google Scholar]
- 15.Gupta A, Nath R, Mishra M. Giant cell tumor of bone: multimodal approach. Indian J Orthop. 2007;41:115–120. doi: 10.4103/0019-5413.32041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harness NG, Mankin HJ. Giant-cell tumor of the distal forearm. J Hand Surg Am. 2004;29:188–193. doi: 10.1016/j.jhsa.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang L, Manoso MW, Boland PJ, Healey JH, Athanasian EA. Features of grade 3 giant cell tumors of the distal radius associated with successful intralesional treatment. J Hand Surg Am. 2010;35:1850–1857. doi: 10.1016/j.jhsa.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Khan MT, Gray JM, Carter SR, Grimer RJ, Tillman RM. Management of the giant-cell tumours of the distal radius. Ann R Coll Surg Engl. 2004;86:18–24. doi: 10.1308/003588404772614632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kivioja AH, Blomqvist C, Hietaniemi K, Trovik C, Walloe A, Bauer HC, Jorgensen PH, Bergh P, Folleras G. Cement is recommended in intralesional surgery of giant cell tumors: a Scandinavian Sarcoma Group study of 294 patients followed for a median time of 5 years. Acta Orthop. 2008;79:86–93. doi: 10.1080/17453670710014815. [DOI] [PubMed] [Google Scholar]
- 21.Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Giant cell tumor of bone: risk factors for recurrence. Clin Orthop Relat Res. 2011;469:591–599. doi: 10.1007/s11999-010-1501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labs K, Perka C, Schmidt RG. Treatment of stages 2 and 3 giant-cell tumor. Arch Orthop Trauma Surg. 2001;121:83–86. doi: 10.1007/s004020000158. [DOI] [PubMed] [Google Scholar]
- 23.Lackman RD, Hosalkar HS, Ogilvie CM, Torbert JT, Fox EJ. Intralesional curettage for grades II and III giant cell tumors of bone. Clin Orthop Relat Res. 2005;438:123–127. doi: 10.1097/01.blo.0000180051.27961.c3. [DOI] [PubMed] [Google Scholar]
- 24.Lausten GS, Jensen PK, Schiodt T, Lund B. Local recurrences in giant cell tumour of bone: long-term follow up of 31 cases. Int Orthop. 1996;20:172–176. doi: 10.1007/s002640050057. [DOI] [PubMed] [Google Scholar]
- 25.Lewis VO, Wei A, Mendoza T, Primus F, Peabody T, Simon MA. Argon beam coagulation as an adjuvant for local control of giant cell tumor. Clin Orthop Relat Res. 2007;454:192–197. doi: 10.1097/01.blo.0000238784.98606.d4. [DOI] [PubMed] [Google Scholar]
- 26.Lim YW, Tan MH. Treatment of benign giant cell tumours of bone in Singapore. Ann Acad Med Singapore. 2005;34:235–237. [PubMed] [Google Scholar]
- 27.Malawer MM, Bickels J, Meller I, Buch RG, Henshaw RM, Kollender Y. Cryosurgery in the treatment of giant cell tumor: a long-term followup study. Clin Orthop Relat Res. 1999;359:176–188. doi: 10.1097/00003086-199902000-00019. [DOI] [PubMed] [Google Scholar]
- 28.McDonald DJ, Sim FH, McLeod RA, Dahlin DC. Giant-cell tumor of bone. J Bone Joint Surg Am. 1986;68:235–242. [PubMed] [Google Scholar]
- 29.Minami A, Kato H, Iwasaki N. Vascularized fibular graft after excision of giant-cell tumor of the distal radius: wrist arthroplasty versus partial wrist arthrodesis. Plast Reconstr Surg. 2002;110:112–117. doi: 10.1097/00006534-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 30.Mjoberg B, Pettersson H, Rosenqvist R, Rydholm A. Bone cement, thermal injury and the radiolucent zone. Acta Orthop Scand. 1984;55:597–600. doi: 10.3109/17453678408992403. [DOI] [PubMed] [Google Scholar]
- 31.Muramatsu K, Ihara K, Taguchi T. Treatment of giant cell tumor of long bones: clinical outcome and reconstructive strategy for lower and upper limbs. Orthopedics. 2009;32:491. doi: 10.3928/01477447-20090527-08. [DOI] [PubMed] [Google Scholar]
- 32.Nelson DA, Barker ME, Hamlin BH. Thermal effects of acrylic cementation at bone tumour sites. Int J Hyperthermia. 1997;13:287–306. doi: 10.3109/02656739709023537. [DOI] [PubMed] [Google Scholar]
- 33.Ng ES, Saw A, Sengupta S, Nazarina AR, Path M. Giant cell tumour of bone with late presentation: review of treatment and outcome. J Orthop Surg (Hong Kong). 2002;10:120–128. doi: 10.1177/230949900201000204. [DOI] [PubMed] [Google Scholar]
- 34.Oda Y, Miura H, Tsuneyoshi M, Iwamoto Y. Giant cell tumor of bone: oncological and functional results of long-term follow-up. Jpn J Clin Oncol. 1998;28:323–328. doi: 10.1093/jjco/28.5.323. [DOI] [PubMed] [Google Scholar]
- 35.O’Donnell RJ, Springfield DS, Motwani HK, Ready JE, Gebhardt MC, Mankin HJ. Recurrence of giant-cell tumors of the long bones after curettage and packing with cement. J Bone Joint Surg Am. 1994;76:1827–1833. doi: 10.2106/00004623-199412000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Ofluoglu O. Aggressive treatment of giant cell tumour with multiple local adjuvants. Acta Orthop Belg. 2008;74:831–836. [PubMed] [Google Scholar]
- 37.Ozalp T, Yercan H, Okcu G, Ozdemir O, Coskunol E. Giant cell tumor at the wrist: a review of 23 cases][in Turkish. Acta Orthop Traumatol Turc. 2006;40:144–150. [PubMed] [Google Scholar]
- 38.Panchwagh Y, Puri A, Agarwal M, Anchan C, Shah M. Giant cell tumor - distal end radius: do we know the answer? Indian J Orthop. 2007;41:139–145. doi: 10.4103/0019-5413.32046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pietschmann MF, Dietz RA, Utzschneider S, Baur-Melnyk A, Jansson V, Durr HR. The influence of adjuvants on local recurrence rate in giant cell tumour of the bone. Acta Chir Belg. 2010;110:584–589. [PubMed] [Google Scholar]
- 40.Prosser GH, Baloch KG, Tillman RM, Carter SR, Grimer RJ. Does curettage without adjuvant therapy provide low recurrence rates in giant-cell tumors of bone? Clin Orthop Relat Res. 2005;435:211–218. doi: 10.1097/01.blo.0000160024.06739.ff. [DOI] [PubMed] [Google Scholar]
- 41.Puri A, Gulia A, Agarwal MG, Reddy K. Ulnar translocation after excision of a Campanacci grade-3 giant-cell tumour of the distal radius: an effective method of reconstruction. J Bone Joint Surg Br. 2010;92:875–879. doi: 10.1302/0301-620X.92B6.23194. [DOI] [PubMed] [Google Scholar]
- 42.Renard AJ, Veth RP, Pruszczynski M, Wobbes T, Lemmens JA, Horn JR. Giant cell tumor of bone: oncologic and functional results. J Surg Oncol. 1994;57:243–251. doi: 10.1002/jso.2930570408. [DOI] [PubMed] [Google Scholar]
- 43.Rooney RJ, Asirvatham R, Lifeso RM, Ali MA, Parikh S. Giant cell tumour of bone: a surgical approach to grade III tumours. Int Orthop. 1993;17:87–92. doi: 10.1007/BF00183548. [DOI] [PubMed] [Google Scholar]
- 44.Saiz P, Virkus W, Piasecki P, Templeton A, Shott S, Gitelis S. Results of giant cell tumor of bone treated with intralesional excision. Clin Orthop Relat Res. 2004;424:221–226. doi: 10.1097/01.blo.0000128280.59965.e3. [DOI] [PubMed] [Google Scholar]
- 45.Scully SP, Mott MP, Temple HT, O’Keefe RJ, O’Donnell RJ, Mankin HJ. Late recurrence of giant-cell tumor of bone: a report of four cases. J Bone Joint Surg Am. 1994;76:1231–1233. doi: 10.2106/00004623-199408000-00013. [DOI] [PubMed] [Google Scholar]
- 46.Sheth DS, Healey JH, Sobel M, Lane JM, Marcove RC. Giant cell tumor of the distal radius. J Hand Surg Am. 1995;20:432–440. doi: 10.1016/S0363-5023(05)80102-9. [DOI] [PubMed] [Google Scholar]
- 47.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 48.Su YP, Chen WM, Chen TH. Giant-cell tumors of bone: an analysis of 87 cases. Int Orthop. 2004;28:239–243. doi: 10.1007/s00264-004-0564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szalay K, Antal I, Kiss J, Szendroi M. Comparison of the degenerative changes in weight-bearing joints following cementing or grafting techniques in giant cell tumour patients: medium-term results. Int Orthop. 2006;30:505–509. doi: 10.1007/s00264-006-0190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turcotte RE, Wunder JS, Isler MH, Bell RS, Schachar N, Masri BA, Moreau G. Canadian Sarcoma Group. Giant cell tumor of long bone: a Canadian Sarcoma Group study. Clin Orthop Relat Res. 2002;397:248–258. doi: 10.1097/00003086-200204000-00029. [DOI] [PubMed] [Google Scholar]
- 51.Vander Griend RA, Funderburk CH. The treatment of giant-cell tumors of the distal part of the radius. J Bone Joint Surg Am. 1993;75:899–908. doi: 10.2106/00004623-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 52.Wang HC, Chien SH, Lin GT. Management of grade III giant cell tumors of bones. J Surg Oncol. 2005;92:46–51. doi: 10.1002/jso.20338. [DOI] [PubMed] [Google Scholar]
- 53.Ward WG, Sr, Li G., 3rd Customized treatment algorithm for giant cell tumor of bone: report of a series. Clin Orthop Relat Res. 2002;397:259–270. doi: 10.1097/00003086-200204000-00030. [DOI] [PubMed] [Google Scholar]
- 54.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed October 30, 2011.
- 55.Zhang Z, Zhu B, Sun T. Case analysis on treatment and recurrence of giant cell tumor of bone] [in Chinese. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006;20:1007–1010. [PubMed] [Google Scholar]
- 56.Zhen W, Yaotian H, Songjian L, Ge L, Qingliang W. Giant-cell tumour of bone: the long-term results of treatment by curettage and bone graft. J Bone Joint Surg Br. 2004;86:212–216. doi: 10.1302/0301-620X.86B2.14362. [DOI] [PubMed] [Google Scholar]