Abstract
Background
The incidence of methicillin-resistant Staphylococcus aureus (MRSA) infection is increasing. However, the prevalence of MRSA colonization among patients undergoing spine surgery is unclear.
Questions/purposes
We therefore (1) determined the prevalence of MRSA colonization in a population of patients scheduled for elective spine surgery; and (2) evaluated whether MRSA screening and treatment reduce the rate of early wound complications.
Methods
We retrospectively reviewed prospectively collected data from 1002 patients undergoing elective spine surgery in 2010. There were 719 primary and 283 revision surgeries. Instrumentation was used in 72.0% cases and autologous iliac crest bone graft was taken in 65.1%. Twelve patients were lost to followup; of the remaining 990 patients, 503 were screened for MRSA and 487 were not. MRSA-colonized patients were treated with mupirocin and chlorhexidine. An early wound complication was defined as wound drainage or the presence of an abscess. Patients were followed for a minimum of 3 months (average, 7 months; range, 3–545 days).
Results
Of the patients undergoing elective spine surgery and screened for MRSA, 14 of 503 (2.8%) were colonized with MRSA. The rates of early wound complications were similar for patients who were screened and pretreated for MRSA (17 of 503 [3.4%]) compared with those who were not (17 of 487 [3.5%]).
Conclusions
The colonization rate for MRSA in our elective spine surgery population was comparable to that in the arthroplasty literature.
Level of Evidence
Level III, retrospective comparative study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Infections after elective spinal surgery can be devastating for patients. More than 650,000 spinal surgeries are performed annually in the United States and the reported rate of infection after instrumented spinal surgery ranges from 0.2% to 4.7% with an average infection rate of 2% [6, 13, 17, 18, 20]. The most common organisms causing infections are Gram-positive organisms found on skin flora, most notably Staphylococcus aureus and Staphylococcus epidermidis [9]. Although methicillin-sensitive S aureus (MSSA) is the predominant strain of S aureus infections, there have been increasing rates of methicillin-resistant S aureus (MRSA) infections [9]. MRSA nares colonization correlates with an increased rate of MRSA infection and bacteremia [7, 11, 19]. Thus, correctly diagnosing and treating MRSA colonization preoperatively is important. This is also one of the priority topics for comparative effectiveness research in the United States [2], in which the effectiveness of screening, prophylaxis, and treatment for MRSA eradication is being emphasized in the community, institution, and hospital settings.
Intranasal swabs are reportedly effective for detecting MRSA carriers [7]. One study [12] demonstrated that in an elective arthroplasty population, treating MRSA-colonized patients with intranasal mupirocin and topical chlorhexidine reduces surgical site infections (SSIs). Because of the substantial financial and emotional costs associated with revision surgery secondary to infection, it is desirable to investigate the efficacy of MRSA decolonization for reducing the rate of SSI in elective spine cases. A 10% reduction rate in revision spine surgery reportedly would cover the cost of screening for MRSA and preoperatively treating MRSA colonization [15]. Although Epstein [4] encouraged the use of nasal cultures to detect MRSA, the rates of MRSA colonization among patients undergoing elective spine surgery are unclear.
We therefore (1) determined the prevalence of MRSA colonization in a population of patients scheduled for elective spine surgery; and (2) evaluated whether MRSA screening and treatment could reduce the rate of early wound complications in patients undergoing spine surgery.
Patients and Methods
We retrospectively analyzed prospectively collected data in a cohort study from all 1002 patients undergoing elective spine surgery in 2010. All procedures were performed at a single institution and the study was conducted with Institutional Review Board approval. We included patients if surgery was performed for deformity and degenerative disease. Patients were excluded if they had anterior cervical procedures, preexisting infection (discitis, osteomyelitis), traumatic injuries, or were lost to followup. A power analysis was conducted and sample size was determined by assuming a Type I error rate set at 5% and the power of detecting a true difference set at 80%. The calculation was adjusted based on Bonferroni correction for two comparisons. Assuming that outcome measurements could be obtained on all patients and assuming that patients were equally allocated to each group, there needed to be a minimum of 437 patients in each group to detect a difference of a 2% reduction in early wound complications between the two groups.
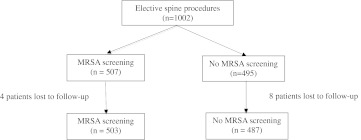
Of the 1002 patients identified, 12 were lost to followup, which was determined by the patient’s last clinic visit or if they had died. Patients who never appeared for a postoperative visit were considered lost to followup. Patients who were screened for MRSA (503 patients) were compared with those who were not screened for MRSA (487 patients) (Fig. 1). Patients were randomly screened for MRSA if they received preoperative medical testing within our hospital, whereas those who were not screened for MRSA received preoperative clearance from their primary care physicians. There were 507 males (51.2%) and 483 females (48.8%). The average age was 57.0 years ± 14.7 years and BMI was 30.0 ± 6.4 kg/m2. Surgery was performed in the cervical (255 [25.8%]), thoracic (26 [2.6%]), and lumbar (709 [71.6%]) regions. There were 708 (71.5%) primary and 282 (28.5%) revision surgeries. Instrumentation was used in 710 (71.7%) cases and iliac crest bone graft (ICBG) was taken in 644 (65.1%). The minimum followup was 3 days (average, 6.7 months; range, 3–545 days). No patients were recalled specifically for this study; all data were obtained from medical records.
Fig. 1.
This study compared patients who were screened and treated for MRSA with those who were not in an elective spine surgery population.
Patients were screened for MRSA 2 to 6 weeks before surgery, where an individual in the preoperative medical clearance clinic swabbed one nares using an unmoistened nasal swab (BBL™ CultureSwab™ Plus; BD Diagnostics, Sparks, MD, USA). Each swab was inoculated onto CHROMagar MRSA plates (BD Microbiology Systems, Sparks, MD, USA) and incubated for 20 to 28 hours at 35 to 37°C. Cultures that grow on CHROMagar MRSA plates were MRSA colonized. Negative cultures were further incubated for 24 hours. After 48 hours, S aureus-positive mauve cultures were verified by Gram stain and coagulase testing (Staphaurex; Remel, Lenexa, KS, USA). Those who were colonized for MRSA were treated with intranasal mupirocin twice a day for 5 days as well as chlorhexidine body washes for 5 days before surgery (including the morning of surgery).
We collected data including patient demographics, surgical, and clinical parameters. Patient demographics included age, gender, and BMI. Surgical data included the location of surgery, primary versus revision surgery, the use of instrumentation, and harvesting of autologous ICBG. The two populations were similar with regard to age, gender, and BMI (Table 1). Of the 503 patients screened for MRSA, there were 252 (50.1%) males and 251 (49.9%) females. The average age was 57.3 ± 14.0 years and average BMI was 29.9 ± 6.7 kg/m2. For those not screened for MRSA, there were 255 (52.4%) males and 232 (47.6%) females. The average age was 56.9 ± 15.3 years at the time of surgery, and the average BMI was 30.1 ± 6.1 kg/m2. The populations were also similar with regard to the location of surgery, primary versus revision surgery, instrumentation, ICBG, the levels of fusion, and the preoperative antibiotic administered. The average time to followup was longer in the MRSA screened population (7.2 months) compared with the population not screened for MRSA (6.2 months). All surgical techniques were standard posterior midline approaches to the spine; there were no unique approaches performed for the purpose of this study.
Table 1.
Demographic, surgical, and clinical data from elective spine patients screened for MRSA compared with those not screened for MRSA
| Data | Total | MRSA screened | MRSA screened 95% CI | Not MRSA screened | Not MRSA screened 95% CI |
|---|---|---|---|---|---|
| Number | 990 | 503 (50.8%) | (46.4%–55.2%) | 487 (49.2%) | (44.8%–53.6%) |
| Gender | |||||
| Male | 507 (51.2%) | 252 (50.1%) | (43.9%–56.3%) | 255 (52.4%) | (46.3%–58.5%) |
| Female | 483 (48.8%) | 251 (49.9%) | (43.7%–56.1%) | 232 (47.6%) | (41.2%–54.0%) |
| Age (years) | 57.0 ± 14.7 | 57.3 ± 14.0 | (56.1–58.5) | 56.9 ± 15.3 | (55.5–58.3) |
| BMI (kg/m2) | 30.0 ± 6.4 | 29.9 ± 6.7 | (29.3–30.5) | 30.1 ± 6.1 | (29.6–30.6) |
| Location | |||||
| Cervical | 255 (25.8%) | 145 (28.8%) | (21.4%–36.2%) | 110 (22.6%) | (14.8%–30.4%) |
| Thoracic | 26 (2.6%) | 12 (2.4%) | (−6.3% to 11.1%) | 14 (2.9%) | (−5.9% to 11.7%) |
| Lumbar | 709 (71.6%) | 346 (68.8%) | (63.9%–73.7%) | 363 (74.5%) | (70.0%–79.0%) |
| Primary | 708 (71.5%) | 350 (69.6%) | (64.8%–74.4%) | 358 (73.5%) | (68.9%–78.1%) |
| Revision | 282 (28.5%) | 153 (30.4%) | (23.1%–37.7%) | 129 (26.5%) | (18.9%–34.1%) |
| Instrumentation | 710 (71.7%) | 374 (74.4%) | (70.0%–78.8%) | 336 (69.0%) | (64.1%–73.9%) |
| Level of fusion | |||||
| 2 or less | 589 (59.5%) | 308 (61.2%) | (55.8%–66.6%) | 281 (57.7%) | (51.9%–63.5%) |
| 3 or more | 401 (40.5%) | 195 (38.8%) | (32.0%–45.6%) | 206 (42.3%) | (35.6%–49.0%) |
| Iliac crest bone autograft | 644 (65.1%) | 329 (65.4%) | (60.3%–70.5%) | 315 (64.7%) | (59.4%–70.0%) |
| Time to followup (days) | 201.5 (3–545) | 215.3 (3–545) | (206.0–224.6) | 187.3 (5–537) | (178.2–196.4) |
| Early wound complications | 34 (3.4%) | 17 (3.4%) | (−5.2% to 12.0%) | 17 (3.5%) | (−5.2% to 12.2%) |
| Wound drainage | 31 (3.1%) | 14 (2.8%) | (−5.8% to 11.4%) | 17 (3.5%) | (−5.2% to 12.2%) |
| Abscess | 3 (0.3%) | 3 (0.6%) | (−8.1% to 9.3%) | 0 (0%) | (0%–0%) |
| Perioperative antibiotics | |||||
| Cefazolin | 878 (88.7%) | 433 (86.1%) | (82.8%–89.4%) | 445 (91.4%) | (88.8%–94.0%) |
| Clindamycin | 35 (3.5%) | 18 (3.6%) | (−5.0% to 12.2%) | 17 (3.5%) | (−5.2% to 12.2%) |
| Vancomycin | 73 (7.4%) | 49 (9.7%) | (1.4%–18.0%) | 24 (4.9%) | (−3.7% to 13.5%) |
| Ciprofloxacin | 1 (0.1%) | 1 (0.2%) | (−8.6% to 9.0%) | 0 (0%) | (0%–0%) |
| Ceftriaxone | 2 (0.2%) | 2 (0.4%) | (−8.3% to 9.1%) | 0 (0%) | (0%–0%) |
CI = confidence interval, MRSA = methicillin-resistant Staphylococcus aureus; BMI = body mass index.
Once discharged, patients followed up in the clinic 2 weeks postoperatively, at 6 weeks, 3 months, 6 months, and at 1 year. AP and lateral radiographs of the surgical location were obtained at each postoperative visit. Ambulatory and neurological function was assessed at each clinic visit as well as the presence of potential complications. Major complications that resulted in long-term morbidity (Grade IV) or required operative management (Grade III) were assessed as well as minor complications (Grade I-II) [3, 14]. We focused on early wound complications (Grade III), which were defined as wounds with continuous drainage or the presence of an abscess. All patients determined to have early wound complications were irrigated and débrided in the operating room. Serological examinations for erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were obtained from these patients. The average ESR was 56.0 ± 32.0 mm/hr (normal value, 0–40 mm/hour) and the average CRP was 8.7 ± 9.5 mg/dL (normal, 0–0.7 mg/dL).
Descriptive statistics were calculated on the demographic, surgical, and clinical variables described previously. The prevalence of MRSA colonization in an elective spine population was determined by the frequency of MRSA-positive patients within those screened for MRSA. We determined differences in continuous demographic variables such as age, BMI, and time to followup between those screened for MRSA and those not screened for MRSA using the nonparametric Mann-Whitney U test. The differences in nominal variables such as gender, surgical variables, early wound complications, and perioperative antibiotic administration between MRSA-screened and non-MRSA-screened patients were determined using Fisher’s exact test. Ninety-five percent confidence intervals were calculated and compared for overlap to determine if there were any differences between populations. All statistical analysis was performed using Predictive Analytics SoftWare Statistics (PASW) Version 18.0 (SPSS, Chicago, IL, USA).
Results
The MRSA colonization rate in our elective spine patients screened for MRSA was 2.8% (14 of 503).
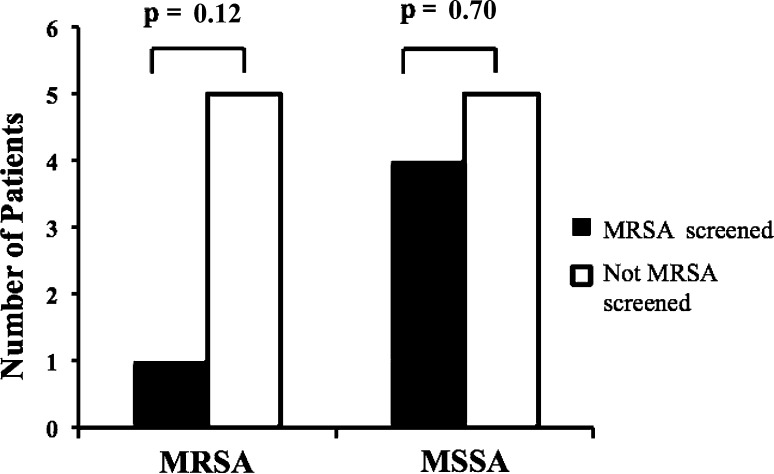
We identified a total of 34 wound complications for the entire study population (3.1%) with similar rates (p = 0.924) of infection between those screened for MRSA (17 of 503 [3.4%]) and those not screened for MRSA (17 of 487 [3.5%]) (Table 1). The infectious organisms found in the early wound complications were similar between those who were MRSA-screened and treated and those who were not screened for MRSA (Table 2). However, those who were tested and treated for MRSA showed a trend (p = 0.118) toward fewer MRSA wound complications (Fig. 2): one patient had a MRSA wound complication in the population of patients screened for MRSA compared with five patients who had MRSA wound complications in the group not screened for MRSA. The remaining infectious organisms included MSSA (n = 9), coagulase-negative Staphylococcus (n = 4), Escherichia coli (n = 6), Group G Streptococcus (n = 2), E coli and Pseudomonas aeruginosa (n = 1), Acinetobacter baumanii (n = 1), and Aspergillus fumigatus (n = 1).
Table 2.
Organisms from early wound complications in elective spine surgery patients
| Culture results | MRSA screened | Not MRSA screened | p value |
|---|---|---|---|
| Negative | 4 | 1 | 0.374 |
| MRSA | 1 | 5 | 0.118 |
| MSSA | 4 | 5 | 0.749 |
| CNS | 3 | 1 | 0.624 |
| Escherichia coli | 2 | 4 | 0.445 |
| E coli and Pseudomonas aeruginosa | 1 | 0 | 1.000 |
| Group G Streptococcus | 2 | 0 | 0.500 |
| Acinetobacter baumanii | 0 | 1 | 0.492 |
| Aspergillus fumigatus | 1 | 0 | 1.000 |
MRSA = methicillin-resistant Staphylococcus aureus; MSSA = methicillin-sensitive S aureus; CNS = coagulase-negative Staphylococcus.
Fig. 2.
The frequency of early wound complications resulting from MRSA or MSSA was compared in patients who were screened and treated for MRSA colonization with those who were not.
Discussion
Infections in elective spinal surgery cases are devastating complications. The average infection rate after instrumented spinal surgery is 2% [6, 13, 17, 18, 20] and an increasing number of these are the result of MRSA spine infections [9]. One study in elective arthroplasty populations [12] suggests screening and treating for MRSA and MSSA is associated with decreased rates of SSIs. We asked whether similar benefits would occur for patients undergoing elective spine surgery. Thus, the goals of our study were to (1) determine the prevalence of MRSA colonization in a population of patients scheduled for elective spine surgery; and (2) evaluate whether MRSA screening and treatment could reduce the rate of early wound complications in patients undergoing spine surgery.
There are several limitations to our study. First, patients were only screened for MRSA and not MSSA. Because there is a higher incidence of MSSA infections in orthopaedics [8], screening and treating MSSA-colonized patients may have a greater effect in reducing the rate of early wound complications. The rate of MRSA colonization is low, so treating MRSA alone may explain why there was no decrease in early wound complications in our limited population. Second, only intranasal MRSA swabs were obtained, which is the current standard of care. Future studies will examine the efficacy of swabbing additional sites. Third, the administration of swabs was not standardized. Only one nostril was swabbed and the swabs were not moistened before administration. This may have not captured all the patients who were MRSA-positive in our population. Fourth, we had no method for monitoring compliance to the treatment regimen of intranasal mupirocin and topical chlorhexidine in MRSA-colonized patients. SSI rates may have remained equal to those not screened and treated for MRSA if there was low patient compliance. Fifth, we had no measure of medical status in this study, including comorbidities and American Society of Anesthesia scores. These factors, along with an immunocompromised patient, affect the risk of SSI independently from MRSA colonization. Finally, this was a small retrospective analysis that could have been improved with a larger sample size and randomization of MRSA screening.
We found the MRSA colonization rate in an elective spine surgery population was 2.8%. This rate is comparable to that reported in the orthopaedic arthroplasty literature, which ranges from 0.5% to 4% [1, 10, 12]. With the increasing prevalence of MRSA, this number can be expected to rise with time. Thus, it is encouraged to take measures before, during, and after surgery to minimize the risk of surgical site infections, including MRSA nares swab screening [4].
The purpose of undergoing MRSA screening is to facilitate appropriate treatment of colonized patients with intranasal mupirocin and topical chlorhexidine; such treatment has demonstrated success in reducing the number of SSIs and subsequently reduce patient morbidity and hospital costs [10, 15, 16]. As a result of the limited followup available for patients in our cohort, our study was not able to address the rate of SSIs. At least one other study has suggested that a reduction in the rate of SSIs is facilitated by MRSA screening and treatment [5]. Although our study did not corroborate these findings irrefutably, closer inspection of our data demonstrates a trend toward decreased MRSA early wound complications in those patients who were screened for MRSA colonization. This trend may be of clinical importance, because decreasing the rate of infections can inherently reduce the high morbidity associated with infections. The demonstrated benefits of screening and decolonization in other patient populations, and a trend toward such outcomes in our own data, suggest that a larger patient sampling with longer followup may show that decolonization protocols do in fact reduce the postoperative development of SSIs.
Our study represents an important step in the direction of evaluating MRSA colonization in the elective spine population with the hopes of reducing MRSA SSIs. Before this study, the colonization rate for MRSA in an elective spine surgery population had not been determined. We found a rate of infection comparable to that for MRSA colonization rates in the arthroplasty literature [12]. However, our data trend toward the reduction of MRSA early wound complications in patients screened for MRSA, indicating there may be clinical usefulness for MRSA screening and treatment. Future prospective, randomized controlled trials with larger sample sizes and longer followup are necessary to evaluate the screening of both MRSA and MSSA to determine if treatment can reduce the rate of SSIs in patients undergoing spine surgery.
Acknowledgments
We thank Dr Nalini Rao for her infectious disease expertise and assistance with the MRSA/MSSA screening and decolonization program at our institution.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the University of Pittsburgh Medical Center, Pittsburgh, PA, USA.
References
- 1.Blom AW, Brown J, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86:688–691. doi: 10.1302/0301-620X.86B5.14887. [DOI] [PubMed] [Google Scholar]
- 2.Committee on Comparative Effectiveness Research Prioritization, Institute of Medicine. Initial National Priorities for Comparative Effectiveness Research. Washington, DC, USA: The National Academies Press; 2009.
- 3.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epstein NE. Preoperative, intraoperative, and postoperative measures to further reduce spinal infections. Surg Neurol Int. 2011;2:17. doi: 10.4103/2152-7806.76938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalmeijer MD, Coertjens H, Nieuwland-Bollen PM, Bogaers-Hofman D, Baere GA, Stuurman A, Belkum A, Kluytmans JA. Surgical site infections in orthopedic surgery: the effect of mupirocin nasal ointment in a double-blind, randomized, placebo-controlled study. Clin Infect Dis. 2002;35:353–358. doi: 10.1086/341025. [DOI] [PubMed] [Google Scholar]
- 6.Kang BU, Lee SH, Ahn Y, Choi WC, Choi YG. Surgical site infection in spinal surgery: detection and management based on serial C-reactive protein measurements. J Neurosurg Spine. 2010;13:158–164. doi: 10.3171/2010.3.SPINE09403. [DOI] [PubMed] [Google Scholar]
- 7.Kluytmans J, Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10:505–520. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindeque B, Rutigliano J, Williams A, McConnell J. Prevalence of methicillin-resistant Staphylococcus aureus among orthopedic patients at a large academic hospital. Orthopedics. 2008;31:363. doi: 10.3928/01477447-20080401-22. [DOI] [PubMed] [Google Scholar]
- 9.Nagashima H, Yamane K, Nishi T, Nanjo Y, Teshima R. Recent trends in spinal infections: retrospective analysis of patients treated during the past 50 years. Int Orthop. 2010;34:395–399. doi: 10.1007/s00264-009-0741-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001;392:15–23. doi: 10.1097/00003086-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Perl TM, Golub JE. New approaches to reduce Staphylococcus aureus nosocomial infection rates: treating S aureus nasal carriage. Ann Pharmacother. 1998;32:S7. doi: 10.1177/106002809803200104. [DOI] [PubMed] [Google Scholar]
- 12.Rao N, Cannella B, Crossett LS, Yates AJ, Jr, McGough R., 3rd A preoperative decolonization protocol for Staphylococcus aureus prevents orthopaedic infections. Clin Orthop Relat Res. 2008;466:1343–1348. doi: 10.1007/s11999-008-0225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuster JM, Rechtine G, Norvell DC, Dettori JR. The influence of perioperative risk factors and therapeutic interventions on infection rates after spine surgery: a systematic review. Spine(Phila Pa 1976). 2010;35(Suppl):S125–S137. [DOI] [PubMed]
- 14.Sink EL, Beaulé PE, Sucato D, Kim Y, Millis MB, Dayton M, Trousdale RT, Sierra RJ, Zaltz I, Schoenecker P, Monreal A, Clohisy J. Multicenter study of complications following surgical dislocation of the hip. J Bone Joint Surg Am. 2011;93:1132–1136. doi: 10.2106/JBJS.HS.K.00142. [DOI] [PubMed] [Google Scholar]
- 15.Slover J, Haas JP, Quirno M, Phillips MS, Bosco JA., 3rd Cost-effectiveness of a Staphylococcus aureus screening and decolonization program for high-risk orthopedic patients. J Arthroplasty. 2011;26:360–365. doi: 10.1016/j.arth.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Urban JA. Cost analysis of surgical site infections. Surg Infect (Larchmt). 2006;7(Suppl 1):S19–S22. doi: 10.1089/sur.2006.7.s1-19. [DOI] [PubMed] [Google Scholar]
- 17.Viola RW, King HA, Adler SM, Wilson CB. Delayed infection after elective spinal instrumentation and fusion. A retrospective analysis of eight cases. Spine(Phila Pa 1976). 1997;22:2444–2450. [DOI] [PubMed]
- 18.Weinstein MA, McCabe JP, Cammisa FP., Jr Postoperative spinal wound infection: a review of 2391 consecutive index procedures. J Spinal Disord. 2000;13:422–426. doi: 10.1097/00002517-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Wenzel RP, Perl TM. The significance of nasal carriage of Staphylococcus aureus and the incidence of postoperative wound infection. J Hosp Infect. 1995;31:13. doi: 10.1016/0195-6701(95)90079-9. [DOI] [PubMed] [Google Scholar]
- 20.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]