Abstract
Background
Amphotericin is a highly toxic hydrophobic antifungal. Delivery of amphotericin from antifungal-loaded bone cement (ALBC) is much lower than would be expected for an equivalent load of water-soluble antibacterials. Lipid formulations have been developed to decrease amphotericin toxicity. It is unknown how lipid formulations affect amphotericin release and compressive strength of amphotericin ALBC.
Questions/purposes
We asked if amphotericin release from liposomal amphotericin ALBC (1) changed with amphotericin load; (2) differed from release from amphotericin deoxycholate ALBC; (3) was an active drug; and (4) if liposomal amphotericin affected the bone cement strength.
Methods
Forty-five standardized test cylinders were fabricated from three formulations of ALBC: Simplex™ P bone cement with 200 mg liposomal amphotericin, 800 mg liposomal amphotericin, or 800 mg amphotericin deoxycholate per batch. For each ALBC formulation, cumulative released amphotericin was determined from five cylinders, and compressive strength was measured for 10 cylinders, five before elution and five after. Activity of released amphotericin was determined by growth inhibition assay.
Results
Amphotericin release was greater for increased load of liposomal amphotericin: 770 μg for 800 mg versus 118 μg for 200 mg. Amphotericin release was greater from liposomal ALBC than from deoxycholate ALBC: 770 μg versus 23 μg over 7 days for 800 mg amphotericin. Released amphotericin was active. Compressive strength of liposomal ALBC is decreased, 67 MPa and 34 MPa by Day 7 in elution for the 200-mg and 800-mg formulations, respectively.
Conclusions
Liposomal amphotericin has greater amphotericin release from ALBC than amphotericin deoxycholate. Compressive strength of liposomal amphotericin ALBC decreases to less than recommended for implant fixation. Local toxicity data are needed before liposomal amphotericin ALBC can be used clinically.
Introduction
Amphotericin B is a highly toxic, hydrophobic antifungal commonly used in the management of fungal implant infections. It is commonly formulated with deoxycholate as a carrier to achieve solubility. Both amphotericin B and deoxycholate form micelles when solubilized for administration [11]. When loaded in acrylic bone cement for local delivery, amphotericin B does not behave like water-soluble antibacterials. Goss et al. [4] reported that no amphotericin B was released from antifungal-loaded bone cement (ALBC) and that amphotericin B increased the compressive strength of the ALBC. However, Marra et al. [8] reported measurable amphotericin B levels in drain fluid and clinical control of a Candida albicans implant infection when amphotericin B deoxycholate was locally delivered in acrylic bone cement. Kweon et al. [7] showed that small amounts of amphotericin B are released from ALBC containing 200 mg amphotericin B deoxycholate per batch of acrylic bone cement. Poragen increased the amount released, but it was still much less than the amount expected for water-soluble antibacterials in acrylic bone cement.
Lipid formulations of amphotericin B have been developed to decrease systemic toxicity from amphotericin B [2]. Liposomal amphotericin B (AmBisome®; Astellas Pharmaceuticals, Deerfield, IL, USA) is a lipid formulation of amphotericin B consisting of amphotericin B intercalated with phospholipid-forming liposomes [1]. It is supplied as a lyophilized yellow powder, which is 4% amphotericin B. It is unknown how lipid formulations of amphotericin B affect its release from ALBC.
Although liposomal amphotericin B is commonly used systemically for the management of fungal orthopaedic infections, local delivery from acrylic bone cement has not been reported or characterized. Limited release of amphotericin B deoxycholate from ALBC has stimulated efforts to develop higher local amphotericin B delivery. It is possible that the phospholipid used to form liposomes in liposomal amphotericin B will affect the release of amphotericin B from ALBC. As a result of the complex chemistry and potential physical interaction with acrylic bone cement, it is important that data exist to guide formulation and use of liposomal amphotericin B ALBC. The goal of this work is to provide data characterizing amphotericin B delivery and mechanical strength of ALBC formulated with liposomal amphotericin B.
We questioned whether (1) amphotericin B release from ALBC formulated with liposomal amphotericin B (liposomal ALBC) differed from release from ALBC formulated with amphotericin B deoxycholate (deoxycholate ALBC); (2) amphotericin B release depended on amphotericin B load; (3) released amphotericin B was active; and (4) whether liposomal amphotericin B affects the compressive strength of the ALBC.
Materials and Methods
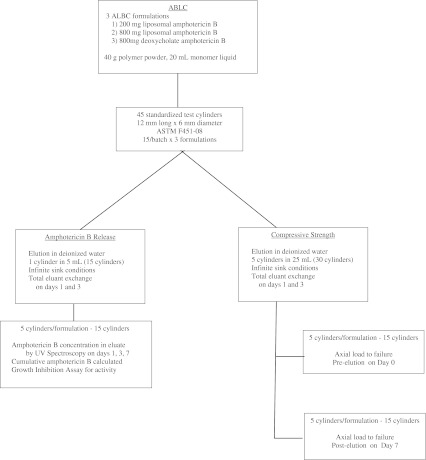
This study was designed to compare liposomal ALBC with deoxycholate ALBC by measuring (1) amphotericin B release; (2) activity of released amphotericin B; and (3) compressive strength (Fig. 1). ALBC was formulated with Simplex™ P (Stryker, Mahwah, NJ, USA) acrylic bone cement mixed with either liposomal amphotericin B or amphotericin B deoxycholate. Three formulations of ALBC were prepared by mixing one of the following loads of amphotericin B per batch of Simplex™ P acrylic bone cement: (1) 200 mg liposomal amphotericin B; (2) 800 mg liposomal amphotericin B; or (3) 800 mg amphotericin B deoxycholate. Liposomal amphotericin B is AmBisome® (Astellas Pharmaceuticals); and amphotericin B deoxycholate is generic (X-GEN Pharmaceuticals, Inc, Big Flats, NY, USA; Fig. 1). A total of 45 standardized test cylinders were made, 15 from each of the three ALBC formulations. The standardized test cylinders, measuring 12 mm × 6 mm in diameter (ASTM F451-08), were fabricated in a polytetrafluoroethylene (Teflon®) mold during the dough phase. The ends were machined flat and square for mechanical testing and to ensure accurate length.
Fig. 1.
The experimental layout for liposomal amphotericin B study is shown.
As a pilot study, a priori power calculation could not be performed because the effect size caused by the different amphotericin B formulations was unknown.
Amphotericin B release from each of the three ALBC formulations was determined by individually eluting five cylinders in 5 mL deionized water each at 37°C maintaining infinite sink conditions. Total eluent exchange was performed on Days 1 and 3. Amphotericin B concentration in the eluate was measured on Days 1, 3, and 7 using visual spectrum spectroscopy absorbance at 415 nm on a BMG FluoStar Omega (BMG Labtech, Ortenburg, Germany). Standard curves were generated from prepared samples of known concentrations of both liposomal amphotericin B and of amphotericin B deoxycholate. Amphotericin B was solubilized by mixing the eluate samples 50:50 with DMSO (Sigma-Aldrich, St Louis, MO, USA) to disperse the micelles. Amphotericin B concentration was determined by interpolation on the standard curves. Cumulative recovered amphotericin B (Mt) was calculated for Days 1, 3, and 7.
Activity of amphotericin B in all eluate samples was determined by growth inhibition of C albicans (ATCC No. 24433), a strain that is susceptible to amphotericin B and is suitable for assay of amphotericin B. Cultures were initiated from a fresh overnight culture diluted 1:100 in YM media (Beckton Dickinson, Franklin Lakes, NJ, USA). For each eluate sample, 1 mL of the diluted C albicans culture and 0.5 mL eluate were subcultured overnight at 25°C. One hundred microliters of each subculture were placed in a 96-well plate [3]. Absorbance at 600 ηm was measured on a BMG FluoStar Omega plate reader. Absorbance at 600 ηm was used to determine the relative number of C albicans in solution [9]. Absorbance measurements from eluate samples were compared with absorbance from subcultures with fresh media added in place of eluate and with media that had no C albicans.
Compressive strength was tested before elution and after 7 days of elution. Thirty test cylinders, five from each of the three ALBC formulations at each time point, were eluted in groups of five in 25 mL of deionized water at 37°C maintaining infinite sink conditions. Total eluent exchange was carried out on Days 1 and 3. All test cylinders were loaded to failure in axial compression at 24.0 mm/min (ASTM F451-08) using a Test Resources 830 AT mechanical testing machine (Test Resources, Shakopee, MN, USA). Load-displacement data were analyzed using Testbuilder® (Test Resources) to determine compressive strength in accordance with ASTM Standard F451-08.
We analyzed amphotericin B formulation and amphotericin B load as determinants for amphotericin B release and compressive strength using the Mann-Whitney U test. Changes in elution were analyzed using repeated-measures analysis of variance (RM ANOVA). Appropriateness of the ANOVA model results was confirmed through the use of standard normal plots of residuals. All statistical analyses were performed using Minitab (Minitab Inc, State College, PA, USA).
Results
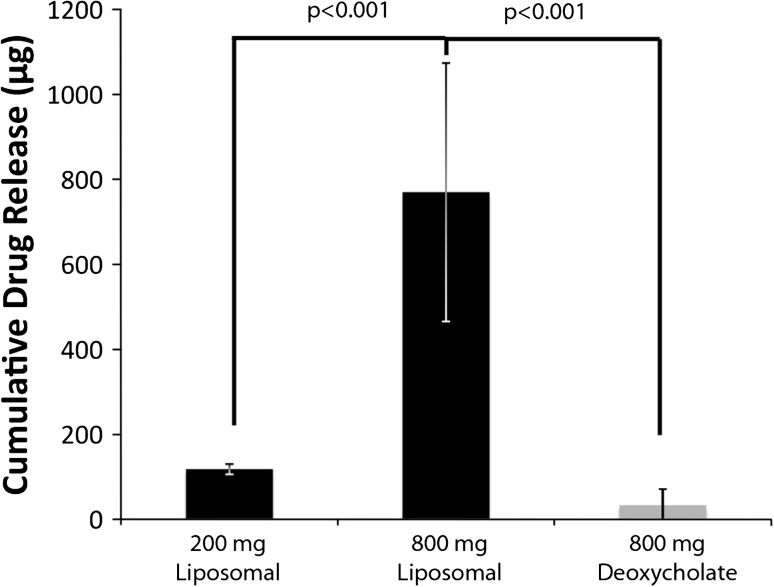
Amphotericin B release from liposomal ALBC was more (p < 0.001) than from deoxycholate ALBC for all time points: 770 μg for 800 mg liposomal ALBC versus 33 μg for 800 mg deoxycholate ALBC over 7 days (Fig. 2).
Fig. 2.
Day 7 cumulative recovered amphotericin B is shown for ALBC based on formulation and dose. The black bars are liposomal ALBC. The gray bar is deoxycholate ALBC.
Higher (p < 0.001) liposomal amphotericin B load released more amphotericin B: 770 μg for 800 mg liposomal ALBC versus 118 μg for 200 mg liposomal ALBC by Day 7 (Fig. 2).
Released amphotericin B was active. Subcultures with eluate from 800 mg liposomal ALBC on Days 1 and 3 had no absorbance at 600 ηm (no C albicans growth) indicating presence of antifungal activity. On Day 7, there was some absorbance at 600 ηm but this was less than the absorbance for the controls that had no eluate added indicating some C albicans growth consistent with the lower concentrations of amphotericin B in the Day 7 eluate. The eluate samples from 200 mg liposomal ALBC and the 800 mg deoxycholate ALBC had high absorbance at 600 ηm similar to controls with no eluate present, consistent with no inhibition of growth and the subtherapeutic levels of amphotericin B in those eluate samples.
The compressive strength of 200 mg and 800 mg liposomal ALBC decreased (p < 0.001) over 7 days in elution. The compressive strength of 200 mg liposomal ALBC was 82 MPa preelution and 67 MPa after 7 days in elution. Compressive strength of 800 mg liposomal ALBC was 43 MPa preelution and 36 MPa postelution. The compressive strength of 800 mg deoxycholate ALBC did not decrease (p = 1.0) over 7 days in elution. Eight hundred milligrams of deoxycholate ALBC had a compressive strength of 103 MPa before elution and 101 MPa after 7 days in elution (Fig. 3).
Fig. 3.
Compressive strength of ALBC is noted in MPa for amphotericin B ALBC in both formulations and both amphotericin B loads that were studied. Gray bars are the compressive strength of the deoxycholate ALBC pre- and postelution. Black bars are the compressive strength of the liposomal ALBC pre- and postelution.
Discussion
There is limited available literature to guide surgeon-directed formulation of antifungal-loaded bone cement. Previous authors have reported the release of amphotericin B deoxycholate from ALBC (Table 1), but the release of liposomal amphotericin B has not been studied. With previous work showing minimal amphotericin B release, this study was conducted to determine how a liposomal formulation affects amphotericin B release from ALBC. We questioned whether (1) amphotericin B release from ALBC formulated with liposomal amphotericin B (liposomal ALBC) differed from release from ALBC formulated with amphotericin B deoxycholate (deoxycholate ALBC); (2) amphotericin B release depended on amphotericin B load; (3) released amphotericin B was active; and (4) whether liposomal amphotericin B affects the compressive strength of the ALBC.
Table 1.
Amphotericin B ALBC literature
| Study | Elution | Compression |
|---|---|---|
| Kweon et al. [7] | Amphotericin B release is small but measurable Mt very low, < 1% of amphotericin B load |
Amphotericin B increased strength of 200 mg deoxycholate ALBC by 10% Added poragen caused loss of strength over time in elution |
| Sealy et al. [10] | Amphotericin B is released from 352 mg deoxycholate ALBC beads Mt not reported |
Not investigated |
| Goss et al. [4] | Amphotericin B is not released from 200 mg deoxycholate ALBC | Amphotericin B increases compressive strength of cement by 25% |
| Marra et al. [8] | Case report 187.5 mg deoxycholate ALBC beads produce measurable amphotericin B levels in wound fluid |
Not investigated |
This study has several limitations. First, ours was an in vitro investigation producing data that can be used to compare the performance of different delivery vehicles but cannot be used to quantitatively determine in vivo tissue, fluid concentrations, or mechanical durability. Second, only one type of cement was studied. It is possible other cements will perform at different magnitudes but the general relationships seen in our data can be expected to be similar for other cements. Third, we used a spectrophotometric assay to measure amphotericin B concentrations. We found this technique to be quantitatively accurate on known standards between 10 and 100 μg/mL. If greater sensitivity to detect lower levels or finer resolution is needed, a high-performance liquid chromatography technique similar to that used by Hong et al. [6] may be a consideration. Fourth, the activity testing performed provides only qualitative, not quantitative, results. The results are consistent with measured amounts of released drug in that all eluate samples containing sufficient amphotericin B to exceed usually fungicidal levels inhibited growth of C albicans. A strength of this study is that we studied amphotericin B release under infinite sink conditions. Diffusion-driven drug release decreases when drug concentrations in the eluent increase, thereby decreasing the diffusion gradient. Infinite sink refers to conditions in the eluent that do not limit drug release meaningfully, including the concentration of released drug in the eluate. Total eluate exchange, before the drug concentration increases too high, maintains infinite sink conditions. The concentration below which infinite sink conditions are maintained depends on multiple factors but generally might be approximately 10% of the saturation concentration or less. The concentrations recorded by assay of the eluate can be used to confirm acceptable levels. The formation of micelles or liposomes consumes free drug, lowering the drug concentration. It is unlikely that either of these phenomena compromise infinite sink conditions.
We found liposomal ALBC had greater amphotericin B release than deoxycholate ALBC. Liposomal amphotericin B has a lower formulation activity (4% by mass) than amphotericin B deoxycholate (44% by mass). For an equivalent amount of amphotericin B, the liposomal formulation has a larger volume of powder than does the deoxycholate formulation. This leads to a higher volume fraction and a much larger poragen effect. Our ALBC formulations lead to volume fractions of 18 vol% for 200 mg liposomal amphotericin B, 47 vol% for 800 mg liposomal amphotericin B, and 10% for 800 mg amphotericin B deoxycholate. Release from 800 mg deoxycholate ALBC was very low, less than expected for water-soluble antibacterials with similar volume fractions but consistent with Kweon et al. [7] who reported limited release of amphotericin B deoxycholate from ALBC (1.4 and 9.8 μg for 200 mg amphotericin B with and without poragen, respectively, at Day 7). The large range of release from the various ALBC formulations may be related to the release mechanism: diffusion from pores occupied by drug or diffusion from drug that is contained within the substance of the cement. Drug contained in the cement is termed partitioned. The diffusion of drugs from within the substance of the cement into surrounding fluid is called partition-controlled release. Hydrophilic antibacterials are extremely insoluble in methacrylate monomer leading to an infinitesimal amount of drug within the cement substance and essentially no partition-controlled release. However, amphotericin B is hydrophobic and slightly more soluble in the methacrylate monomer, potentially leading to some amphotericin B entering the cement partition during cement mixing. However, as the cement polymerizes, amphotericin B likely interacts directly with the polymerizing cement, bonding or entrapping the amphotericin B, preventing its release. This mechanism of limited amphotericin B release was suggested by Goss et al. [4] who based this on increases in compressive strength when amphotericin B deoxycholate was added to the cement. Our mechanical data with increased compressive strength observed in the 800-mg deoxycholate formulation support the data of Goss et al. The release of liposomal amphotericin B is therefore believed to primarily be related to diffusion from pores in the cement and not to partition-controlled release. In solution, amphotericin B deoxycholate forms micelles and liposomal amphotericin B forms liposomes; however, neither structure is present in the drugs provided as lyophilized powder before they are put in solution. ALBC is made by adding the powder drug to the powder poly(methyl methacrylate). It is unlikely that either micelle formation or liposome formation is affecting portioning of amphotericin B into the cement.
Release of amphotericin B increased with increased dose of liposomal ALBC. This further supports the argument that the liposomal formulation does not cause a meaningful amount of partition-controlled release.
The amphotericin B released from ALBC in this study is active, preventing growth of C albicans in growth inhibition testing. Our release data are consistent with amphotericin B release reported by Kweon et al. [7], but Kweon et al. did not quantify activity. In our study, the eluate from 800 mg liposomal ALBC inhibited growth of planktonic fungi, whereas eluate from other ALBC formulations that have amphotericin B concentrations below usually therapeutic levels showed no measurable growth inhibition. Toxicity is an unresolved issue. There have been no reports of systemic or local toxicity from locally delivered amphotericin B. Based on Marra et al. [8] in which toxicity, local or systemic, was not seen with wound drainage concentration of 3 μg/mL and data in Harmsen et al. [5] in which toxicity becomes important between 10 and 100 μg/mL, the 23-fold increase in release (1.4–33 μg) caused by increasing the amphotericin B deoxycholate load from 200 mg to 800 mg could cause in vivo levels in the range potentially important for local toxicity. Two hundred milligrams and 800 mg liposomal ALBC with 84-fold and 550-fold increase in release, respectively, have the potential to cause severe local toxicity in vivo. Liposomes can form spontaneously in the presence of phospholipids at specific concentrations in solution raising the possibility that the amphotericin B released from liposomal ALBC forms liposomes. This could markedly reduce the risk of toxicity from free amphotericin B. Toxicity studies are needed to determine if liposomal ALBC will cause local toxicity in vivo.
The compressive strength of the 800 mg deoxycholate ALBC is over 100 MPa consistent with Goss et al. [4] and Kweon et al. [7] who reported increased strength of ALBC made with amphotericin B deoxycholate. Increased compressive strength in these formulations supports the suggestion of Goss et al. [4] that amphotericin B has a direct interaction with the acrylic cement, possibly crosslinking with the cement during polymerization, thereby increasing its strength. The compressive strength of 200 mg liposomal ALBC (67 MPa) is below the strength recommended for implant fixation in ISO 5833 (70 MPa). However, 800 mg liposomal ALBC is much weaker (36 MPa), consistent with the mechanical effect from that volume of poragens. The magnitude of the loss in compressive strength raises a concern about using liposomal ALBC for structural applications.
In conclusion, ALBC formulated with liposomal amphotericin B has greater amphotericin B release than ALBC formulated with amphotericin B deoxycholate and can be expected to deliver more drug in vivo, but the compressive strength of ALBC formulated with higher-dose liposomal amphotericin B is very weak, raising concern about using it in structural applications. Local toxicity studies are needed before liposomal amphotericin B can be used clinically in ALBC.
Acknowledgments
We acknowledge Francis Calara BSE (mechanical testing) and Mary Martin PharmD at Banner Good Samaritan Medical Center for their contributions to this study and financial support from Banner Good Samaritan Medical Center and Astellas Pharma US, Inc.
Footnotes
One or more of the authors (ACM) received funding from the Astellas Pharmaceutical Corporation.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
This work was performed at Arizona State University and Banner Good Samaritan Medical Center, Phoenix, AZ, USA.
References
- 1.Alak A, Moy S, Bekersky I. A high-performance liquid chromatographic assay for the determination of amphotericin B serum concentrations after the administration of Am Bisome, a liposomal amphotericin B formulation. Ther Drug Monit. 1996;18:604–609. doi: 10.1097/00007691-199610000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Butler WT. Pharmacology, toxicity, and therapeutic usefulness of amphotericin B. JAMA. 1966;195:371–375. doi: 10.1001/jama.1966.03100050079024. [DOI] [PubMed] [Google Scholar]
- 3.CLSI. M27-A2: Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard—Second Edition. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2002:22.
- 4.Goss B, Lutton C, Weinrauch P, Jabur M, Gillett G, Crawford R. Elution and mechanical properties of antifungal bone cement. J Arthroplasty. 2007;22:902–908. doi: 10.1016/j.arth.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Harmsen S, McLaren AC, Pauken C, McLemore R. Amphotericin B is cytotoxic at locally delivered concentrations. Clin Orthop Relat Res. 2011;469:3016–3021. doi: 10.1007/s11999-011-1890-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong Y, Ramzan I, McLachlan AJ. Hepatobiliary disposition of liposomal amphotericin B in the isolated perfused rat liver. J Pharm Sci. 2005;94:169–176. doi: 10.1002/jps.20239. [DOI] [PubMed] [Google Scholar]
- 7.Kweon C, McLaren AC, Leon C, McLemore R. Amphotericin B delivery from bone cement increases with porosity but strength decreases. Clin Orthop Relat Res. 2011;469:3002–3007. doi: 10.1007/s11999-011-1928-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marra F, Robbins GM, Masri BA, Duncan C, Wasan KM, Kwong EH, Jewesson PJ. Amphotericin B-loaded bone cement to treat osteomyelitis caused by Candida albicans. Can J Surg. 2001;44:383–386. [PMC free article] [PubMed] [Google Scholar]
- 9.Morris S, Nicholls PJ. An evaluation of optical density to estimate fungal spore concentrations in water suspensions. Phytopathology. 1978;68:1240–1242. doi: 10.1094/Phyto-68-1240. [DOI] [Google Scholar]
- 10.Sealy PI, Nguyen C, Tucci M, Benghuzzi H, Cleary JD. Delivery of antifungal agents using bioactive and nonbioactive bone cements. Ann Pharmacother. 2009;43:1606–1615. doi: 10.1345/aph.1M143. [DOI] [PubMed] [Google Scholar]
- 11.Tancréde P, Barwicz J, Jutras S, Gruda I. The effect of surfactants on the aggregation state of amphotericin B. Biochim Biophys Acta. 1990;1030:289–295. doi: 10.1016/0005-2736(90)90305-8. [DOI] [PubMed] [Google Scholar]