Abstract
Biosynthesis of the variable core domain of lipooligosaccharide (LOS) in Neisseria gonorrhoeae is mediated by glycosyl transferases encoded by lgtABCDE. Changes within homopolymeric runs within lgtA, lgtC, and lgtD affect the expression state of these genes, with the nature of the LOS expressed determined by the functionality of these genes. However, the mechanism for modulating the amount of multiple LOS chemotypes expressed in a single cell is not understood. Using mutants containing polar disruptions within the lgtABCDE locus, we determined that the expression of this locus is mediated by multiple promoters and that disruption of transcription from these promoters alters the relative levels of simultaneously expressed LOS chemotypes. Expression of the lgtABCDE locus was quantified by using xylE transcriptional fusions, and the data indicate that this locus is transcribed in trace amounts and that subtle changes in transcription result in phenotypic changes. By using rapid amplification of 5′ cDNA ends, transcriptional start sites and promoter sequences were identified within lgtABCDE. Most of these promoters possessed 50 to 67% homology with the consensus gearbox promoter sequence of Escherichia coli.
Neisseria gonorrhoeae is an obligate human pathogen that causes diseases of mucosal surfaces (see reference 32 for a review). Because the gonococcus is capable of proliferating in different physiological milieus, it has developed a variety of mechanisms for adapting to these environments. Lipooligosaccharide (LOS) is indispensable for disease pathogenesis. It is immunogenic, highly pyrogenic, and responsible for the localized inflammation and scarring characteristic of gonococcal infections and for the septic shock that results from disseminated disease (21-23, 31, 39, 45; H. M. Harper, A. L. Padmore, W. D. Smith, M. K. Taylor, and R. Demarco de Hormaeche, unpublished results [presented at the Tenth International Pathogenic Neisseria Conference, Baltimore, Md.]). In addition, specific chemotypes of LOS confer complement resistance (34) and facilitate intracellular invasion (47). LOS molecules are heterogeneous, with a single cell often simultaneously expressing two or more chemotypes in various proportions (2, 3, 11, 46). LOS also undergoes phase variation, and distinct LOS chemotypes are favored for survival of the gonococcus within different regions of the human body and at various points during the pathogenic process (15, 32, 49, 53, 55). We believe that the expression of the correct LOS chemotype(s) by the gonococcus at the correct time during infection is essential for establishing and maintaining infection.
Our understanding of the regulatory mechanisms that affect LOS biosynthesis and expression remains incomplete. Biosynthesis of the variable oligosaccharide portion of LOS is mediated by seven LOS glycosyl transferase (lgt) genes (7, 13, 20). Strand slippage in homopolymeric tracts within the coding sequence of lgtA, lgtC, lgtD, and lgtG during DNA replication leads to reading frameshifts in these genes. These changes result in the production of inactivate glycosyl transferases, thus altering the LOS chemotype(s) that is expressed by a given cell (7, 11, 13, 56). Gonococcal LOS expression can be further modified by specific environmental stimuli, suggesting that regulated gene expression occurs. Gonococci grown under anaerobic conditions or in the presence of lactate, which is normally present in the female genital tract, produce an altered LOS profile (16, 30). Gonococci grown under acidic and alkaline conditions also express different LOS profiles (35). Finally, gonococci grown at different growth rates express different LOS epitopes and possess different serum sensitivities (33). The present study investigates the transcriptional organization of the lgtABCDE genes.
MATERIALS AND METHODS
Bacterial strains, plasmids, oligonucleotides, and culture conditions.
All bacterial strains, plasmids, and oligonucleotide primers used in the present study are listed in Tables 1, 2, and 3. N. gonorrhoeae strains were grown in phosphate-buffered gonococcal medium (Difco) supplemented with 20 mM d-glucose and growth supplements (54) either in broth with the addition of 0.042% sodium bicarbonate or on agar where they were incubated in a 37° CO2 incubator. All Escherichia coli strains were grown in Luria-Bertani medium (43). Kanamycin was used in growth media at a concentration of 30 μg/ml, ampicillin was used at 60 μg/ml, spectinomycin was used at 50 μg/ml, and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) was used at 35 μg/ml. The optical densities of gonococcal and E. coli cultures were determined by using a Klett-Summerson colorimeter fitted with a green filter. One Klett unit corresponds to a culture density of ca. 107 CFU/ml. We used the DNA sequence numbering for the F62 lgtABCDE region, as described in the National Center for Biotechnology Information database under accession number U14554 to orient our constructs, promoter mapping, etc., to the literature.
TABLE 1.
Plasmids used in this study
| Plasmid | Relevant phenotype, genotype, or description | Source or reference |
|---|---|---|
| pGEM7Zf(−) | Cloning vector | Promegaa |
| pK18 | Cloning vector | 38 |
| pHP45Ω | Source of the Ω interposon | 37 |
| pXYLE20 | pK18 containing the xylE gene from Pseudomonas putida | 48 |
| pCLB2 | pGEM2 with a PstI-to-AgeI chromosomal DNA fragment containing lgtABCDE from N. gonorrhoeae FA19 | 11 |
| pCLB2Δ1 | The 5′ region between PstI and BsmI in pCLB2 is deleted | This study |
| pCLB2Δ1 lgtDfix#4 | pCLB2D1 containing an engineered NruI site within the polyguanine run of lgtD; primers LgtDfix-F and LgtDfix-R were used with a pCLB2Δ1 template in a PCR; the PCR amplicon was purified, digested with NruI, and self-ligated | This study |
| pCLB2Δ1 lgtDfix#4ΩNruI | The Ω interposon was liberated from pHP45Ω by SmaI digestion and inserted into the NruI site of pCLB2Δ1 | This study |
| pCLB2Δ1 lgtDfix#4ΩEcoRV | The Ω interposon was liberated from pHP45Ω by SmaI digestion and inserted into the EcoRV site of pCLB2Δ1 lgtDfix#4 | This study |
| pLgtDE(EcoRI) | Primers IgtDE-F and IgtDE-R were used to introduce an EcoRI site into pCLB2D1 lgtDfix#4; the PCR amplicon was purified, digested with EcoRI, and self-ligated | This study |
| pLgtDEΩEcoRI | pCLB2Δ1 lgtDfix#4 was digested with EcoRI and mixed with Ω interposon that had been liberated from pHP45Ω by EcoRI digestion and subsequently purified from an agarose gel; the mixture was ligated | This study |
| pDCB3 | A 5,243-bp amplicon containing the lgtABCDE locus was generated by Expand Long-Template PCR (Roche) with primers JL-50 (contains a 5′ EcoRI site) and JL-51 (contains a 5′ BamHI site) and was cloned into the EcoRI and BamHI sites of pK18 | This study |
| pDCB7 | The 5,243-bp fragment of pDCB3 containing the lgtABCDE region was cloned into the EcoRI and BamHI sites of pGEM7Zf(−) | This study |
| pDCB7ΩClaI | Primer ABC was used to amplify the Ω interposon (adds AscI, BsrGI, and ClaI sites onto the ends of the Ω interposon), and this fragment was cloned into the ClaI site of pDCB7 | This study |
| pDCB7ΩAscI | Primer ABC was used to amplify the Ω interposon (adds AscI, BsrGI, and ClaI sites onto the ends of the Ω interposon), and this fragment was cloned into the AscI site of pDCB7 | This study |
| pDCB9 | A 1,487-bp amplicon corresponding to bp 4316 to 5803 of the lgtABCDE region as described earlier (20) was amplified by using primers LgtDE-F (containing a 5′ EcoRI) and Got 5803-R (containing a 5′ BamHI) and cloned into the EcoRI and BamHI sites of pGEM7Zf(−) | This study |
| pDCB10 | Primers PostLgtE-F (containing a 5′ KpnI and HindIII sequence) and PostLgtE-R (containing a 5′ KpnI sequence) were used with a pDCB9 template in a PCR to introduce a KpnI site in pDCB9 | This study |
| pDCB10xylE | Primers XylE-F and XylE-R were used to amplify the xylE gene from pXYLE20 and cloned into the KpnI and HindIII sites of pDCB10 | This study |
| pDCB17 | Primers DCB17b-F (containing a 5′ KpnI-HindIII sequence) and DCB17b-R (containing a 5′ KpnI sequence) were used to introduce a KpnI site in pDCB7 | This study |
| pDCB17xylE | Primers XylE-F and XylE-R were used to amplify the xylE gene from pXYLE20 and cloned into the KpnI and HindIII sites of pDCB17 | This study |
| pDCB19 | A ≈4,000-bp amplicon amplicon corresponding to bp 1650 to 2364 of the lgtABCDE region as described earlier (20) was amplified using primers DCB19b-F (contains a 5′ EcoRI) and DCB19b-R (contains a 5′BamHI) and cloned into the EcoRI and BamHI sites of pGEM7Zf(−) | This study |
| pDCB19ΩBsrGI | Primer ABC was used to amplify the Ω interposon (adds AscI, BsrGI, and ClaI sites onto the ends of the Ω interposon), and this fragment was cloned into the BsrGI site of pDCB19 | This study |
| pDCB19xylE | Primers XylE-F and XylE-R were used to amplify the xylE gene from pXYLE20 and cloned into the KpnI and HindIII sites of pDCB19ΩBsrGI | This study |
Promega, Madison, Wis.
TABLE 2.
Bacterial strains used in this study
| Strain | Relevant phenotype, genotype, or description | Source or reference |
|---|---|---|
| E. coli DH5αMCR | F−mcrA Δ(mrr-hsdRMS-mcrBC) endA1 supE44 thi-1 recA1 gyrA relA1 Δ(lacIZYA-argF)U169 deoRφ80dlacΔ(lacZ)M15 | BRLa |
| N. gonorrhoeae | ||
| F62 | P. F. Sparlingb | |
| F62ΔlgtA | 239-bp ApoI deletion in lgtA; this strain produces L8LOS | 47 |
| F62ΔlgtAΔlgtE | 622-bp BspEI-to-AgeI deletion in lgtE of the parent strain F62ΔlgtA | 47 |
| F62ΩBsrGI | F62 containing the Ω interposon at the BsrGI site between the stop codon of glySβ and the start codon of lgtA | This study |
| F62ΩAscI | F62 containing the Ω interposon within the AscI site of lgtB | This study |
| F62ΩClaI | F62 containing the Ω interposon within the ClaI site of lgtC | This study |
| F62ΩNruI | F62 containing the Ω interposon within an engineered NruI site in the polyguanine region of lgtD | This study |
| F62ΩEcoRV | F62 containing the Ω interposon within the EcoRV site of lgtD | This study |
| F62ΩEcoRI | F62 containing the Ω interposon within an engineered EcoRI site between lgtD and lgtE; the termination codon of lgtD contains part of the EcoRI recognition sequence, and the last base of the recognition sequence is 18 bp from the lgtE start codon | This study |
| F62LgtA(xylE) | F62 with the xylE reporter gene inserted at the BsrGI site 58 bases before the start codon of lgtA | This study |
| F62LgtE(xylE) | F62 with the xylE inserted into lgtE | This study |
| F62LgtE(xylE)ΩBsrGI | F62LgtE(xylE) containing the Ω interposon at the BsrGI site 58 bases before the start codon of lgtA | This study |
| F62LgtE(xylE)ΩAscI | F62LgtE(xylE) containing the Ω interposon within the AscI site of lgtB | This study |
| F62LgtE(xylE)ΩClaI | F62LgtE(xylE) containing the Ω interposon within the ClaI site of lgtC | This study |
| F62LgtE(xylE)ΩNruI | F62LgtE(xylE) containing the Ω interposon within an engineered NruI site in the polyguanine region of lgtD | This study |
| F62LgtE(xylE)ΩEcoRV | F62LgtE(xylE) containing the Ω interposon within the EcoRV site of lgtD | This study |
| F62LgtE(xylE)ΩEcoRI | F62LgtE(xylE) containing the Ω interposon within an engineered EcoRI site between lgtD and lgtE | This study |
BRL, Bethesda Research Laboratories.
University of North Carolina, Chapel Hill.
TABLE 3.
Oligonucleotide primers used in this study
| Primer | Site(s) | Source or reference |
|---|---|---|
| Anchor | GACCACGCGTGAATTCEcoRIGTCGAC | This study |
| CB-4 | TATTGCGCGCACCGATGCCGACGA | 11 |
| CB-5 | GCCGGCATCGAGGACGTGGAACCTGA | This study |
| d(T) | GACCACGCGTGAATTCEcoRIGTCGAC[T]16V | This study |
| DA-1 | CAATCATTGGCCGCCGTAGTGGGGCAGACTTGGCGCA | 50 |
| DA-5 | GCCGTAAACTTTCTCAAGCTCCGCCT | This study |
| DCB13-F | GATC GGTACCKpnIGATC AAGCTTHindIIIGGGAGAGTAAATTGCAGCCTT | This study |
| DCB13-R | TCGA GGTACCKpnI GATAATTTGATGCCGCCTGAAGGC | This study |
| DCB14-F2 | GATC GAATTCEcoRI AGGATAATTTCCAATCCCCGC | This study |
| DCB15-F | GATC GAATTCEcoRI AGCGGCCCATCCCGATACGGA | This study |
| DCB15-R | TCGA GGATCCBamHI AGCTGATAACGTGGTTTTGCA | This study |
| DCB16-F | GATC GGTACCKpnI GATC AAGCTTHindIII GAACAGGATAAATCATGCAAAACC | This study |
| DCB16-R | TCGA GGTACCKpnI GGTTTCAATAGCTGCGGTATTTCC | This study |
| DCB17b-F | GATC GGTACCKpnI GATC AAGCTTHindIII GTATCGGAAAGGAGAAACGGATTG | This study |
| DCB17b-R | TCGA GGTACCKpnI CCGTCAATAAATCTTGCGTAAGAA | This study |
| DCB19b-F | GATC GAATTCEcoRI CTCCTTACCGAAGAACTCCCG | This study |
| DCB19b-R | ATGC GGATCCBamHI GCCGCAAATACGATGTCCATC | This study |
| Got (−180) | GATTCAGACGGCATTCGACA | This study |
| Got 310-R | AGGCGGTTCAGCAGGTTCAGGCGG | This study |
| Got 660-R | CGGAATTTTGAGCTTGTGCA | This study |
| Got 1020 | ACACCGAGCGGGATTGGGCGGAAG | This study |
| Got 1576-R | GATC GGATCCBamHI GCCAAGCTGATAACGTGGTTTTGC | This study |
| Got 3240-R | TGCGCCATCTTTGAAGCATACA | This study |
| Got 3742-R | CAATGGCGGCAAGCACGCTT | This study |
| Got 3869-F | GTTCGATCCAGCCTATATCCAC | This study |
| Got 3891-R | GTGGATATAGGCTGGATCGAAC | This study |
| Got 3997-R | TGGAAGAAGTCTGGTCTTGA | This study |
| Got 4232-R | AGCAAATCGGTCAAAGAATAC | This study |
| Got 5803-R | AGCCCG GGATCCBamHI GCGAACGCTGCATCGTCC | This study |
| Got F | AGCT GAATTCEcoRI CTGCAGGCCGTCGCCGTATTCAAACAACTG | This study |
| JL-9 | ACCACGTTATCAGCTTGGCT | This study |
| JL-12 | AGCGGCCCATCCCGATAC | 50 |
| JL-50 | CT GAATTCEcoRI GGCCGACATCGCGCTTTTGGGCG | 47 |
| JL-51 | AT GGATCCBamHI GGG GCGATTTTACCTAGCAGATGAA | 47 |
| LgtDE-F | GGAAATACCGCAGCTATTGAATTCEcoRI CGA | 50 |
| LgtDE-R | GCATGATTTATCCTGTTCGAATTCEcoRI AAT | 50 |
| LgtDfix-F | GGA TCGCGANruI GAACCGATGCCGACGATATTGCCT | This study |
| LgtDfix-R | GGTTC TCGCGANruI TATATTCTCCACCTCCGCCA CCCGACTTTGCCATTCGTCCAGCCCGAT | This study |
| OmegaABC | TCAGAT GGCGCGCCAscITGTACABsrGITCGATClaI GGTGA TTGATTGACGAAGCTTTATGC | This study |
| PostLgtE-F | GATC GGTACCKpnIAAGCTTHindIII CAGAAATGGACACACTGTCATTCC | This study |
| PostLgtE-R | TCGA GGTACCKpnI TGATTTTAATCCCCTATATTTTACAC | This study |
| XylE-BSR-F | GATC TGTACABsrGIAGGAGGARBS TGACGTCATGAAC | This study |
| XylE-BSR-R | TCGA TGTACABsrGI TCAGGTCAGCACGGTCAT | This study |
| XylE-F | GATC GGTACCKpnIAGGAGGARBS TGACGTCATGAAC | This study |
| XylE-R | TCGA AAGCTTHindIII TCAGGTCAGCACGGTCAT | This study |
Chemicals, reagents, and enzymes.
Restriction enzymes and T4 DNA ligase were purchased from New England Biolabs (Beverly, Mass.). PCR buffers and enzymes and buffers for 5′ rapid amplification of cDNA ends (5′-RACE) were purchased from Invitrogen (Carlsbad, Calif.) and Roche Molecular Biochemicals (St. Louis, Mo.). All chemicals used for the present study were reagent grade or better and purchased from Sigma Chemical Co. (St. Louis, Mo.) unless otherwise specified. The monoclonal antibodies (MAbs) 2-1-L8 and 17-1-L1 were generously provided by Wendell Zollinger of the Walter Reed Army Institute of Research, Washington, D.C. MAb 1B2 was a gift from J. McLeod Griffiss, University of California, San Francisco.
DNA and RNA isolation procedures.
Chromosomal DNA was isolated as described by Rodriguez and Tait (41). Plasmid DNA was isolated by the method of Birnboim and Doly (8). RNA was purified chromatographically from gonococci grown to a Klett reading of 100, by using the High-Pure RNA isolation kit (Roche).
PCR.
The PCR was generally performed by using Platinum PCR Supermix (Invitrogen) or the Expand Long-Template PCR kit (Roche) according to the manufacturers' directions. Primers were purchased from Bioserve Biotechnologies (Laurel, Md.) or from Integrated DNA Technologies (Coralville, Iowa). PCRs were resolved on agarose gels containing 500 μg of ethidium bromide/ml in Tris-borate-EDTA running buffer (43).
Transformation.
Competent cells of E. coli DH5α-MCR were prepared by the method of Inoue et al. (27). Recombinant DNA transformation of E. coli was done according to the standard heat shock protocol (43). Transformants were verified by digestion of recovered plasmid DNA with the appropriate restriction enzymes. Recombinant DNA transformation into N. gonorrhoeae F62 was done by either the tube or the spot transformation method of Gunn and Stein (24).
Purification of LOS and SDS-PAGE.
Gonococcal LOS was prepared from plated cultures as described by Hitchcock and Brown (25) and diluted 1:25 in lysing buffer. The suspension was boiled for 10 min immediately before 5 μl was loaded onto a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel. After electrophoresis, the gel was fixed overnight in a solution of 40% ethanol-5% acetic acid and then oxidized in 0.83% periodic acid for 5 min. The gel was washed for 2 h in multiple changes of H2O every 20 min, stained for 5 min in silver staining solution (22.5 mM NaOH, 0.42% NH4OH, 47 mM AgNO3), and rewashed for 2 h in multiple changes of H2O every 20 min. The gel was developed (100 ml of 0.005% citric acid-0.007% formaldehyde) until the bands became sufficiently visible and then photographed.
Immunological methods.
Colony blots were performed by transferring cells grown overnight on GCK agar onto nitrocellulose filters. The filters were air dried for 10 min and blocked with filler solution (20 mM Tris, 150 mM NaCl, 2% nonfat dry milk, 0.2% NaN3, 0.002% phenol red [pH 7.4]) for 30 min. Filters were blotted with Whatman 3MM paper to remove cellular debris prior to incubation in primary antibody (MAb 2-1-L8 or 17-1-L1) with gentle shaking for 2 h or longer. Filters were washed with Tris-buffered saline (TBS; pH 7.4) three times for 10 min each time. Membranes were incubated in filler solution containing goat anti-mouse immunoglobulin G conjugated with horseradish peroxidase for 2 h. Filters were washed three times for 10 min each time in TBS, and the membranes were developed by incubating them in 50 ml of 50 mM Tris-HCl-1% 4-chloro-1-naphthol-0.86% H2O2 (pH 8.0).
XylE assay.
Qualitative screening for XylE+ colonies was performed by spraying plates with 50 mM catechol, followed by incubation at 37°C for as short as 1 min to as long as 1 h to allow for color development. The presence of a yellow color indicated XylE activity. Quantitative assays were performed by inoculating cells into 20 ml of gonococcal broth plus supplements, with growth to a Klett reading of 100 (indicating an approximate density of 109 CFU/ml). Cells were harvested by centrifugation, washed once in 10 ml of 50 mM potassium phosphate (pH 7.5), and resuspended in 2.5 ml of 100 mM potassium phosphate-20 mM EDTA-10% (vol/vol) acetone (pH 7.2). Cells were then disintegrated with six 10-s pulses from a sonicator (Heat Systems, Inc.) set at 40% output; during this process, the tube containing the sample was kept submerged in ice water. The resulting crude lysate was clarified of cell debris with two rounds of centrifugation, first in a swinging bucket centrifuge at 4,000 × g for 5 min and then in a microfuge at 10,000 × g for 10 min. The clarified lysate was stored at −80°C. Assays were performed by diluting cell extracts in assay buffer (100 mM potassium phosphate, 0.2 mM catechol) such that a linear change in absorbance at 375 nm was seen over time. XylE activity was calculated by linear regression of the slope over six time points. One microunit of XylE activity corresponds to the formation of 1 nmol of 2-hydroxymuconic semialdehyde per min at 22°C. XylE activity was normalized against total protein concentration, as determined by the method of Bradford et al. (10), with bovine serum albumin (New England Biolabs, Beverly, Mass.) as the standard.
5′-RACE.
RACE analyses were performed essentially as described by Frohman et al. (18). Synthesis of cDNA was performed with 2 μg of total RNA template, 12 pmol of antisense primer, and 1 U of avian myeloblastosis virus reverse transcriptase (Roche) in reaction buffer supplied by the manufacturer. The reaction was carried out for 1 h at 55°C, followed by 10 min of incubation at 65°C. cDNA was purified on a Qiagen spin column according to the manufacturer's directions, eluted in 50 μl of 10 mM Tris-Cl (pH 8.0), and polyadenylated with 0.5 U of terminal transferase (Roche)/μl using the manufacturer's reaction buffer supplemented with 1.5 mM CoCl2 and 6.25 μM dATP. Three microliters of polyadenylated cDNA was used in a PCR with the d(T) forward primer (see Table 3) and a gene-specific antisense primer. The resulting products were subjected to a second round of PCR with the anchor primer and a nested reverse primer and then resolved on 3% low-melting-temperature agarose gels. The transcriptional start site was determined by DNA sequence analysis of the RACE products.
RESULTS
Analysis of transcriptional linkage by interposon mutagenesis.
The organization of the genes found in the lgt gene cluster suggested that they would be transcribed as an operon. Analysis of the DNA sequence (up to 500 bp) upstream of the putative lgtA start codon failed to identify any sequences with homology to known F 70-like promoters. This analysis did identify a DNA sequence that might form a rho-dependent termination sequence in the intergenic region between glyS stop codon and the putative lgtA start codon (see Fig. 1). However, if this stem-loop structure (26 bp upstream of the putative lgtA start codon) was functioning as a transcriptional stop signal, it was unclear what DNA sequence could be promoting the expression of the lgt gene cluster.
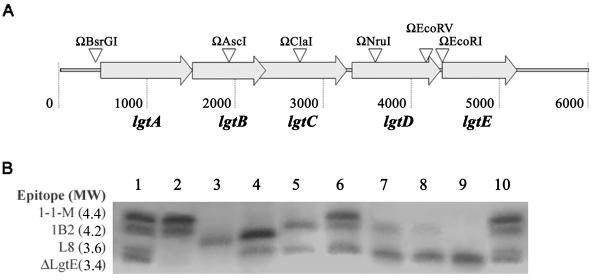
FIG. 1.
Genomic organization of the lgt gene region. This diagram is derived from the DNA sequence of the lgt gene cluster as originally published by Gotschlich (20), with the NCBI accession number U14554. The sequence numbers given in the figure correspond to those described in this accession. The features identified in this figure are indicated as follows: a1, glyS stop codon; a2, lgtC stop codon; b, BsrGI restriction site; c, potential stem-loop structure than could function as a transcriptional terminator; d, putative ribosome-binding site; e1, putative lgtA start codon; and e2, putative lgtD start codon.
In order to determine whether the stem-loop was functioning as a transcription termination signal, we introduced a strong transcriptional stop site 6 bp upstream from the start of the stem-loop structure by inserting the Ω interposon into a BsrGI site and analyzed the effect of its insertion on LOS expression. The location of the insertion event in each transformant was verified by PCR amplification of the appropriate region and restriction digestion of the PCR products. The data indicate that all transformants analyzed incorporated the Ω interposon sequence at the appropriate chromosomal location (data not shown). LOS was purified from 10 individual transformants and analyzed by SDS-PAGE, and the bands visualized by silver staining the gels. Each of the Ω interposon-derived mutants exhibited an altered LOS phenotype that possessed the same SDS-PAGE profile. A representative of the SDS-PAGE profile of one of these mutants (F62ΩBsrGI) is shown in Fig. 2, lane 3.
FIG. 2.
Phenotypic analysis of LOS produced by Ω mutants. (A) lgtABCDE gene locus. Inverted triangles represent Ω insertion points. The names above the triangles refer to the names of the F62 insertion mutants. (B) Silver-stained SDS-PAGE gel of LOS isolated from various mutants. Lanes 1, 6, and 10 are an LOS ladder derived from LOS isolated from F62, F62ΔlgtA, and F62ΔlgtE. The four bands show the mobilities of the MAb 1-1-M, 1B2, and L8 reactive LOSs and the ΔLgtE LOS chemotype. The remaining lanes represent LOS species from strains F62 (lane 2), F62ΩBsrGI (lane 3), F62ΩAscI (lane 4), F62ΩClaI (lane 5), F62ΩNruI (lane 7), F62ΩEcoRV (lane 8), and F62ΩEcoRI (lane 9).
The lgtE gene is the most downstream gene in the lgtABCDE locus, and yet it mediates the first biochemical step in the assembly of a growing LOS molecule. If the lgtABCDE locus is an operon driven by a single promoter, then an Ω interposon insertion at the BsrGI site should produce a strain whose LOS phenotype resembles the LOS of strains deleted in lgtE (produces LOS that will be referred to as ΔLgtE LOS). The data presented in Fig. 2 indicate that F62ΩBsrGI produces an LOS that is larger than the ΔLgtE LOS chemotype, suggesting that this strain possesses LgtE activity.
Western blot analysis of LOS expressed by F62ΩBsrGI indicated that the single band seen in Fig. 2, lane 3, reacted with MAb L1-1-17 (data not shown), an MAb that binds to an alternate LOS chemotype in neisserial LOS, one that is produced when LgtA is nonfunctional and LgtC is functional. From the data obtained in the analysis of LOS expressed by F62ΩBsrGI, we concluded that transcription of lgtA must initiate 5′ of the BsrGI. These data also indicate that the potential rho-dependent termination sequence still allows for the transcription of sufficient message such that the amount of LgtA present in in vitro-grown cells is not limiting. Because F62ΩBsrGI was able to produce an LOS that requires transcription of lgtC, we concluded that a promoter must exist 3′ of the BsrGI site. These data indicate that the overall transcriptional organization of the genes contained within this gene cluster must be driven from multiple promoters.
In order to localize promoter containing regions, we created a family of isogenic strains of N. gonorrhoeae F62 containing the Ω interposon inserted at different points within the lgtABCDE locus and observed the resulting LOS phenotypes. The plasmids used to make the various gonococcal constructs are described in Table 2. LOS was purified from 10 to 12 individual transformants for each construct and visualized after separation on SDS-PAGE gels (data not shown). Each of the Ω interposon mutants exhibited an altered LOS phenotype that was consistent across all of the individual transformants of each mutant that was analyzed, indicating that the change in LOS phenotype did not occur due to phase variation. We verified that the Ω interposon had inserted into the correct region by PCR amplification of the lgtABCDE locus of each of these mutants, with subsequent restriction digestion analysis of the amplicons. The expected restriction pattern was generated on an agarose gel from the amplicons generated from each of the mutants, indicating that the Ω interposon had been incorporated into the correct sites (data not shown).
The data presented in Fig. 2 indicate that all strains containing Ω interposon insertions within lgtABCDE continued to produce LOS chemotypes that require LgtE activity, except when the S interposon was inserted into an engineered EcoRI site immediately upstream of the lgtE ribosome-binding site (F62ΩEcoRI; Fig. 2, lane 9). Analysis of these results in detail allowed us to map the location of potential promoters. Strain F62ΩAscI (lane 4) produced two LOS components: one that possesses a mobility consistent with a ΔLgtB LOS (which corresponds to an LOS structure made when LgtB is nonfunctional) and one that possesses a mobility that corresponded to ΔLgtE LOS. The LOSs expressed by this strain failed to bind MAbs 1B2, 1-17-L1, and 2-1-L8. That this strain does not produce L1 LOS suggests that lgtC transcription has been inhibited. By combining these two results (data shown in Fig. 2, lanes 2 to 4), we could map a promoter region to between the BsrGI and AscI sites.
Simultaneous expression of at least two LOS components is seen in four mutant strains (see Fig. 2, lanes 5 and 7 to 9). In these strains, a mixture of the ΔLgtE LOS and a second LOS chemotype that binds MAb 1B2 (Western blot data are not shown) is produced. Since the Ω interposon is placed more proximal to the start codon of lgtE, a proportionally greater amount of ΔLgtE LOS is produced. In fact, the shift in the proportions between these two LOS components occurs in a stepwise fashion, suggesting the presence of multiple promoters.
Additional promoter regions were mapped by analyzing changes in the proportion of the two chemotypes that are produced. F62ΩClaI (Fig. 2, lane 5) produces a roughly 60:40 proportion of 1B2 LOS (LOS chemotype made when strain expresses a functional LgtA, and a nonfunctional LgtC and LgtD) and ΔLgtE LOS chemotypes, indicating inhibition of lgtD transcription and decreased transcription of lgtE versus that of the parental strain. Strain F62ΩNruI (Fig. 2, lane 7) produces a roughly 40:60 proportion of 1B2 LOS and ΔLgtE LOS. Strain F62ΩEcoRV (Fig. 2, lane 8) expresses less 1B2 LOS and more ΔLgtE LOS than strain F62ΩNruI. Strain F62ΩEcoRI mutant (Fig. 2, lane 9) only expresses ΔLgtE LOS. Altogether, the introduction of the Ω interposon in successive sites between ClaI and EcoRI results in stepwise increases in the amount of ΔLgtE LOS that is produced, suggesting a corresponding decrease in LgtE production. These data suggest the existence of promoters between each of the Ω interposon insertion sites, except between AscI and ClaI, and support a model of multiple, weak promoters existing throughout the lgtABCDE locus. This further suggests that the cumulative contribution of each of these promoters defines the nature of the LOS that is expressed.
Quantitation of gene expression.
In order to quantitate the level of gene expression within the lgtABCDE locus, we constructed three xylE transcriptional fusions. The xylE gene was inserted immediately upstream of lgtA [F62LgtA(xylE)], at the beginning of lgtD [F62LgtC(xylE)], and immediately downstream of lgtE [F62LgtE(xylE)]. Analysis of the LOS profiles of these strains by SDS-PAGE and colony blotting (data not shown) showed that the transformants expressed the expected wild-type LOS profile when the xylE gene was inserted before or after the lgtABCDE locus and the ΔlgtD LOS when the xylE gene was inserted into lgtD (Fig. 3).
FIG. 3.
(A) Phenotypic analysis of LOSs produced by xylE fusion strains. The dark triangles represent xylE insertion points within the lgtABCDE gene cluster. (B) Silver-stained SDS-PAGE gel of LOSs isolated from these strains. Lanes 1 and 5 represent an LOS ladder derived from LOS that has been isolated from strains F62, F62ΔlgtA, and F62ΔlgtE. The four bands show the mobility of the MAb 1-1-M, 1B2, and L8 reactive LOSs and the ΔLgtE LOS chemotype. Lane 2 represents LOS isolated from F62LgtA(xylE), lane 3 represents LOS isolated from F62 lgtC (xylE), and lane 4 represents LOS isolated from F62 lgtE (xylE).
We assayed these xylE fusion strains for catechol-2,3-dioxygenase (XylE) activity (Table 4). The assays showed a difference in XylE activity among the three strains. XylE activity was sevenfold higher in F62LgtA(xylE) than in F62LgtE(xylE) and F62LgtC(xylE). These data indicate that the different genes within the lgtABCDE gene locus can be expressed at different levels due to different mRNA concentrations. They further indicate that most transcription through this region terminates before it extends through the entire region.
TABLE 4.
XylE activity of selected mutants
| Strain | XylE activitya (μU) |
|---|---|
| F62 | 0.2 |
| F62LgtA(xylE) | 220 |
| F62LgtC(xylE) | 31.6 |
| F62LgtE(xylE) | 32.8 |
| F62RfaF(xylE)b | 15,400 |
One microunit of XylE activity corresponds to the formation of 1 nmol of 2-hydroxymuconic semialdehyde per min at 22°C.
This strain possesses a xylE insertion at the start of the gonococcal rfaF gene (14).
Positional effects of the Ω interposon insertion on XylE activity in F62LgtE(xylE).
The data presented above suggest the presence of multiple promoter sequences located within the lgtABCDE locus. In order to quantify the relative contribution that these sequences might play in overall gene expression, we introduced the Ω interposon into various chromosomal locations in F62LgtE(xylE). Each transformant was analyzed by PCR amplification of the region of interest, with subsequent restriction digestion analysis to verify that the insertion had incorporated into the correct chromosomal location. In addition, the SDS-PAGE profiles of each isogenic pair [i.e., F62LgtE(xylE)::ΩBsrGI compared to F62ΩBsrGI] were identical, indicating that the interposon incorporation was exerting the same phenotypic modulation in each pair of strains. The data presented in Fig. 4 indicate that insertion of the Ω interposon at the BsrGI site did not result in a change in XylE expression. This further supports the data presented above that indicated that transcription terminates within the lgtABCDE region. Insertions that occurred within the coding sequence reduced but did not eliminate XylE expression compared to the isogenic parent strain lacking the interposon insertion [F62LgtE(xylE)]. As the insertion site of the Ω interposon neared the lgtE coding sequence, the level of transcription decreased. Insertion of the Ω interposon in the engineered EcoRI site allowed for XylE activity, suggesting that weak promoter sequences were located within the lgtE coding sequence. Overall, these data suggest that multiple promoter sequences must occur within the lgtABCDE coding sequence.
FIG. 4.
Catechol-2,3-dioxygenase activity of xylE fusion strains. Enzymatic activity was detected biochemically by a spectrophotometric procedure that detects the appearance of 2-hydroxymuconic semialdehyde. Activity is measured in microunits (nanomoles per minute per milligram). Error bars indicate the standard error between triplicate assays.
Identification of transcriptional start sites.
Initial attempts to identify the size of transcripts produced from the lgtABCDE region by Northern hybridization experiments were unsuccessful. However, the XylE expression data described above suggest that the reason for this failure is due to the low level of mRNA that would correspond to this region. Therefore, we used RACE, a more sensitive approach, to identify putative transcriptional start sites.
RACE products were analyzed on an agarose gel (Fig. 5). In most of the lanes, multiple DNA fragments were generated by the RACE reactions. These fragments represent transcriptional start sites (TS) within the lgtDE region or RNA polymerase pause sites. The sequence of the RACE products was determined, and the upstream DNA sequences were analyzed for homology to known promoter sequences. Six of the seven sites identified by RACE corresponded to upstream sequences that showed between 50 and 79% homology with the consensus gearbox promoter sequence of E. coli (1, 9, 29). The sequence upstream of the seventh fragment (TS-3626) showed overlapping sequences with 58 and 75% homology to the σ70 consensus of Neisseria spp. and the σ28 consensus of E. coli, respectively (Fig. 6) (4, 6). In light of the fact that promoters with less homology have frequently been shown to have high expression levels, these results indicate that the identification of these sites as promoters is realistic.
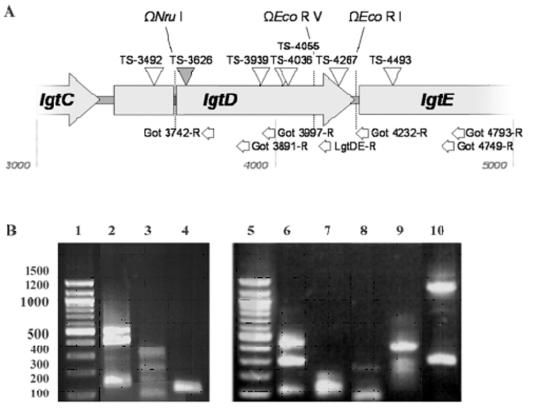
FIG. 5.
RACE products and associated transcriptional start sites. (A) Diagram showing elements of the 5′-RACE experiment. Vertical arrows represent the locations of putative promoters, named according to the relative position of the transcriptional start sites. Open arrows represent putative gearbox promoters, and the closed arrow represents the single putative σ70 promoter. Horizontal arrows represent the location of the reverse primers which were used. (B) Agarose gel electrophoresis of RACE products. DNA was resolved on an agarose gel. Lanes 1 and 5, 100-bp ladder (New England Biolabs). Major bands, from top to bottom, represent PCR products that initiate at cDNA 5′ ends (transcriptional start sites), as follows: lanes 2 and 3, TS-4267, TS-4055, and TS-3939; lane 4, TS-3939; lane 6, TS-4267, TS-4493 and unknown; lane 7, TS-4267; lane 8, TS-4055; lane 9, TS-3626; lane 10, TS-3492 and unknown.
FIG. 6.
Putative promoters identified by sequence homology. Putative transcriptional start sites were examined for potential homology to known consensus E. coli and Neisseria promoter sequences by calculating the percent identity between sequence upstream of TS and known consensus promoter sequences. The spacing between the −10 and −35 sequences was required to be within ±3 nucleotides of the consensus spacing; this requirement is not reflected in the homology percentages. Nucleotides in boldface represent putative TS identified by RACE.
Due to the extensive homology between lgtA and lgtD and between lgtB and lgtE, we hypothesized that promoters found in lgtDE may have homologous counterparts in lgtAB. We examined the lgtAB sequence for homology within the identified regions. Of the seven promoter sequences identified within lgtDE, only TS-3626 had an identical counterpart within lgtAB. Two other sequences showed homology with a two-base mismatch (corresponding to TS-4267 and TS-4493). The remaining regions had counterparts in which three or more bases mismatched in a manner which gave them less homology to the consensus promoter sequence.
DISCUSSION
The lgtABCDE gene cluster encodes most of the genes needed to make the gonococcal α-oligosaccharide (20). Our data indicate that a complex transcriptional control mechanism is responsible for regulating the expression of lgtABCDE. First, we demonstrate that multiple promoter regions exist throughout this cluster. Second, we show that the lgtABCDE genes are transcribed at minute levels relative to the transcriptional expression of lsi-1 (rfaF) glycosyl transferase (see Table 4). Subtle differences in the level of transcription appear to modulate the LOS phenotype in terms of the identity and relative amounts of each of the chemotypes that are simultaneously surface expressed.
The Ω interposon is a spectinomycin cassette (aadA) flanked by rho-independent transcriptional terminators derived from bacteriophage T4. The introduction of the Ω interposon in either orientation results in at least a 1,000-fold reduction in transcription, causing polar mutations (17, 18). This interposon cassette has been used to determine linkage relationships and to map the location of promoters in a number of organisms, including N. gonorrhoeae (12, 17, 37, 42). Using Ω interposon insertions within the lgtABCDE region, we were able to generate evidence that supports the presence of multiple promoters.
As the Ω interposon is placed more proximal to lgtE, a greater number of transcripts are terminated at the Ω interposon insertion site and overall transcription of lgtE is reduced, hence also the lower amounts of LgtE. When lgtE is transcribed in limiting amounts, not enough LgtE is produced to process all of the LOS precursors before they are surface expressed. Using xylE as a reporter gene for measuring lgtE transcription indicated that the overall level of transcription of this gene cluster is quite low. The level of XylE expression at all insertion points within this region is very low. This can be seen when we compared the level of expression of XylE to that seen when this gene was linked to another LOS biosynthetic gene, rfaF (14). Expression of this gene is almost 1,000 times greater than what is seen for the two lgt (xylE) insertions. Our data show that lgtE transcription (Fig. 4) and LgtE activity (Fig. 2) decreases if the Ω interposon is inserted within lgtABCDE. The amount of the decrease is more if the Ω interposon is inserted closer to the start codon of lgtE. This decrease in LgtE activity is seen as an increase in the proportion of ΔLgtE LOS between any two Ω interposon insertion mutants, which occurs between five of the six mutants examined. Therefore, promoter activity originates between each pair of adjacent Ω interposon insertion sites (see Fig. 2).
Seven potential transcriptional start sites were identified between the ClaI and EcoRI Ω interposon insertion sites by using RACE analysis. Each of these transcriptional start sites contained −10 and −35 upstream regions that possessed between 50 and 79% homology to consensus E. coli and Neisseria promoter sequences (see Fig. 5 and 6). Three of these TS had homologous promoter sequences within the lgtAB region. The location of these putative promoters identified by RACE to be within lgtDE is consistent with the phenotypes and reporter gene activities of the F62 lgtE (xylE) Ω interposon insertion mutants. The identification of a transcriptional start site within the lgtE coding sequence (TS-4493) indicates a promoter which, without further rationalization, appears to serve no biological function in this strain. However, the existence of this promoter within lgtE is logical in terms of evolutionary descent because extensive homology exists found between lgtB and lgtE. Any existing promoters are likely to be shared between these two genes. It has been demonstrated that intergenic recombination occurs between lgt genes, giving rise to recombinant lgt loci (5, 28). Therefore, the putative promoter upstream of TS-4493 may have had biological function in the context of lgtB transcription.
The identification of six out of seven putative promoter regions as gearbox promoters is consistent with recent studies and with data from the present study. In N. gonorrhoeae, the gearbox promoter has been implicated in the expression of two virulence factors, AniA (26) and Tpc (19). Gearbox promoters generally express at a strength that is inversely proportional to growth rate (52), a finding consistent with our observations that transcription of lgtE (xylE) increases fourfold between the mid-logarithmic and early stationary phase (data not shown).
Most significantly, we demonstrated here that limiting the transcription of lgtE leads to simultaneous production of two LOS chemotypes in a balance that is contingent upon the level of transcription. Assays of the xylE fusion strains allowed us to correlate visible proportions of LgtE+ and ΔLgtE LOSs with measured levels of transcriptional expression (Fig. 2 and 4). In addition, these results show that the lgtABCDE genes are transcribed at very low levels, demonstrating that subtle changes in transcription are likely to incur significant phenotypic changes.
The transcriptional fusion data also support an earlier study that demonstrated that a strain of N. gonorrhoeae FA19 with lgtA frameshifted to the “off” position, continued to produce trace amounts of LgtA+ LOS (11). Burch et al. speculated that transcriptional and/or translational strand slippage was occurring at a frequency just high enough to allow for visible amounts of LgtA+ LOS. Transcriptional strand slippage by the RNA polymerase holoenzyme would result in a small amount of in-frame lgtA transcript, whereas translational strand slippage of out-of-frame mRNA by the ribosomes would allow for the production of in-frame LgtA protein. Since the present study demonstrates that small changes in the amount of LgtE dramatically impact the nature of the expressed LOS, it is feasible that a low level of transcriptional or translational strand slippage of lgtA mRNA could result in sufficient LgtA to account for the LgtA+-LgtA− mixed phenotype of these strains.
From our data, we are able to formulate a logical explanation for why N. gonorrhoeae F62 normally produces a mixture of the 1-1-M and 1B2 LOS chemotypes. The LgtD glycosyl transferase facilitates the addition of GalNAc to the terminal Gal of the α chain, converting the α chain from the 1B2 chemotype to the 1-1-M chemotype. Our data show that the lgtD gene is transcribed at a level ∼1,000-fold less than that of lsi-1 (rfaF) (14). The low level of lgtD transcriptional expression is such that a distribution of both LgtD+ (1-1-M) and LgtD− (1B2) LOS chemotypes are produced. If transcriptional expression of lgtD were to be increased, then we would predict a phenotypic shift toward producing a greater proportion of the 1-1-M chemotype.
Control of LOS phenotype by limiting transcription is the likely mechanism in spontaneous L1-reactive phase variants of N. gonorrhoeae F62. These variants typically express a mixture of immunotype L1 (LgtC+) and other (LgtC−) LOS chemotypes (D. C. Stein, unpublished data). The data from the present study suggests that in this strain, lgtC is transcribed in amounts such that insufficient LgtC is being produced to catalyze all its available substrate. It is also possible that lgtC is transcribed and translated in strains that fail to make the L1 LOS but that the enzyme is outcompeted for substrate by LgtA.
Sequence analysis of the lgtABCDE region using the Neural Network algorithm (40) revealed 29 sequences that share significant homology with the σ70 promoter consensus of E. coli and Neisseria spp. Remarkably, the polyguanine regions of the lgtA, lgtC, and lgtD genes were among the highest scorers, suggesting that the polyguanine tracts may affect promoter functions. These regions all had clearly identifiable −35 and −10 regions, but the spacing between these regions was too long to suggest that these sequences were functional in these genes. There are other examples of promoter regions spanning homopolymeric runs in the pathogenic neisseriae. The porA and opc genes of N. meningitidis, respectively, contain a polyguanine and polycytosine run between the −35 and −10 consensus s70 sequence (44, 51). Frameshifting in these runs alters the spacing between the −35 and −10 sequences and leads to phase shifting between high, low, and intermediate transcriptional levels. It is feasible that this same mechanism may be occurring within the lgtABCDE locus and that these promoters are up- or downregulated based upon heritable changes to their sequence.
Our model is consistent with observations made by researchers who have shown that LOS expression is modulated by these environmental stimuli: growth rate, pH, aerobic versus anaerobic conditions, and carbon source (glucose versus lactate versus pyruvate) (16, 30, 33, 35). At present, the genetic mechanism that mediates this variation in expression is unknown. Most of these promoters identified by the present study are gearbox promoters, which exhibit expression at levels inversely proportional to growth rate. Since environmental changes can either increase or decrease the growth rate, changes in the environment would influence the level of expression from these promoters. Alternatively, additional epistatic factors may interact with these promoters and contribute to LOS expression in response to environmental conditions.
Our cumulative understanding of LOS phase variation in N. gonorrhoeae can be summarized as follows: N. gonorrhoeae regulates LOS biosynthesis and phase variation via a variety of disparate mechanisms. The specific LOS components produced by a particular strain are defined by on-off strand slippage of homopolymeric tracts within the lgtA, lgtC, lgtD, and lgtG genes (7, 13). Simultaneous production of multiple LOS epitopes is mediated by production of limiting amounts of the LgtA, LgtD, or LgtE. This occurs via transcriptional or translational strand slippage (11), via regulation of transcriptional expression (the present study), and via the low kinetic efficiencies of specific glycosyl transferases (36). In addition, recombination between glycosyl transferases is seen in some strains of Neisseria and these recombinant loci may consequently invoke one or more of the above mechanisms of phase variation (5, 36).
Acknowledgments
We thank Peijun He and Anne Corriveau for excellent technical assistance.
This study was supported by a grant from the National Institutes of Health to D.C.S. (AI24452) and an F31 fellowship to D.C.B. (AI09636).
REFERENCES
- 1.Aldea, M., T. Garrido, J. Pla, and M. Vicente. 1990. Division genes in Escherichia coli are expressed coordinately to cell septum requirements by gearbox promoters. EMBO J. 9:3787-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apicella, M. A., M. Ketterer, F. K. Lee, D. Zhou, P. A. Rice, and M. S. Blake. 1996. The pathogenesis of gonococcal urethritis in men: confocal and immunoelectron microscopic analysis of urethral exudates from men infected with Neisseria gonorrhoeae. J. Infect. Dis. 173:636-646. [DOI] [PubMed] [Google Scholar]
- 3.Apicella, M. A., M. Shero, G. A. Jarvis, J. M. Griffiss, R. E. Mandrell, and H. Schneider. 1987. Phenotypic variation in epitope expression of the Neisseria gonorrhoeae lipooligosaccharide. Infect. Immun. 55:1755-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arhin, F. F., F. Moreau, J. W. Coulton, and E. L. Mills. 1998. Sequencing of porA from clinical isolates of Neisseria meningitidis defines a subtyping scheme and its genetic regulation. Can. J. Microbiol. 44:56-63. [PubMed] [Google Scholar]
- 5.Arking, D., Y. Tong, and D. C. Stein. 2001. Analysis of lipooligosaccharide biosynthesis in the Neisseriaceae. J. Bacteriol. 183:934-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnosti, D. N., and M. J. Chamberlin. 1989. Secondary sigma factor controls transcription of flagellar and chemotaxis genes in Escherichia coli. Proc. Natl. Acad. Sci. USA 86:830-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerjee, A., R. Wang, S. Uljohn, P. A. Rice, E. C. Gotschlich, and D. C. Stein. 1998. Identification of the gene (lgtG) encoding the lipooligosaccharide β chain synthesizing glucosyl transferase from Neisseria gonorrhoeae. Proc. Natl. Acad. Sci. USA 95:10872-10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohannon, D. E., N. Connell, J. Keener, A. Tormo, M. Espinosa-Urgel, M. M. Zambrano, and R. Kolter. 1991. Stationary-phase-inducible “gearbox” promoters: differential effects of katF mutations and role of sigma 70. J. Bacteriol. 173:4482-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 11.Burch, C. L., R. J. Danaher, and D. C. Stein. 1997. Antigenic variation in Neisseria gonorrhoeae: production of multiple lipooligosaccharides. J. Bacteriol. 179:982-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carson, S. D., P. E. Klebba, S. M. Newton, and P. F. Sparling. 1999. Ferric enterobactin binding and utilization by Neisseria gonorrhoeae. J. Bacteriol. 181:2895-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danaher, R. J., J. C. Levin, D. Arking, C. L. Burch, R. Sandlin, and D. C. Stein. 1995. Genetic basis of Neisseria gonorrhoeae lipooligosaccharide antigenic variation. J. Bacteriol. 177:7275-7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danaher, R. J., E. F. Petricoin III, and D. C. Stein. 1994. Use of xylE fusions to demonstrate that lsi-1, a Neisseria gonorrhoeae lipooligosaccharide biosynthetic gene, and lsi-3 are not transcriptionally linked. J. Bacteriol. 176:3428-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Vries, F. P., A. van Der Ende, J. P. van Putten, and J. Dankert. 1996. Invasion of primary nasopharyngeal epithelial cells by Neisseria meningitidis is controlled by phase variation of multiple surface antigens. Infect. Immun. 64:2998-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frangipane, J. V., and R. F. Rest. 1993. Anaerobic growth and cytidine 5′-monophospho-N-acetylneuraminic acid act synergistically to induce high-level serum resistance in Neisseria gonorrhoeae. Infect. Immun. 61:1657-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frey, J., and H. M. Krisch. 1985. Omega mutagenesis in gram-negative bacteria: a selectable interposon which is strongly polar in a wide range of bacterial species. Gene 36:143-150. [DOI] [PubMed] [Google Scholar]
- 18.Frohman, M. A. 1994. On beyond classic RACE (rapid amplification of cDNA ends). PCR Methods Appl. 4:S40-S58. [DOI] [PubMed] [Google Scholar]
- 19.Fussenegger, M., A. F. Kahrs, D. Facius, and T. F. Meyer. 1996. Tetrapac (tpc), a novel genotype of Neisseria gonorrhoeae affecting epithelial cell invasion, natural transformation competence, and cell separation. Mol. Microbiol. 19:1357-1372. [DOI] [PubMed] [Google Scholar]
- 20.Gotschlich, E. C. 1994. Genetic locus for the biosynthesis of the variable portion of Neisseria gonorrhoeae lipooligosaccharide. J. Exp. Med. 180:2181-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregg, C. R., M. A. Melly, C. G. Hellerquist, J. G. Coniglio, and Z. A. McGee. 1981. Toxic activity of purified lipopolysaccharide of Neisseria gonorhoeae for human fallopian tube mucosa. J. Infect. Dis. 143:432-439. [DOI] [PubMed] [Google Scholar]
- 22.Gregg, C. R., M. A. Melly, and Z. A. McGee. 1980. Gonococcal lipopolysaccharide: a toxin for human fallopian tube mucosa. Am. J. Obstet. Gynecol. 138:981-984. [DOI] [PubMed] [Google Scholar]
- 23.Griffiss, J. M. 1995. The role of bacterial lipooligosaccharides in the pathogenesis of human disease. Trends Glycosci. Glycotechnol. 7:461-478. [Google Scholar]
- 24.Gunn, J. S., and D. C. Stein. 1996. Use of a non-selectable transformation technique to construct a multiple restriction modification deficient mutant of Neisseria gonorrhoeae. Mol. Gen. Genet. 251:509-517. [DOI] [PubMed] [Google Scholar]
- 25.Hitchcock, P. J., and T. M. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver stained polyacrylamide gels. J. Bacteriol. 154:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Householder, T. C., W. A. Belli, S. Lissenden, J. A. Cole, and V. L. Clark. 1999. cis- and trans-acting elements involved in regulation of aniA, the gene encoding the major anaerobically induced outer membrane protein in Neisseria gonorrhoeae. J. Bacteriol. 181:541-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inoue, H., H. Nojima, and H. Okayama. 1990. High efficiency transformation of Escherichia coli with plasmids. Gene 96:23-28. [DOI] [PubMed] [Google Scholar]
- 28.Jennings, M. P., Y. N. Srikhanta, E. R. Moxon, M. Kramer, J. T. Poolman, B. Kuipers, and P. van der Ley. 1999. The genetic basis of the phase variation repertoire of lipopolysaccharide immunotypes in Neisseria meningitidis. Microbiol. 145:3013-3021. [DOI] [PubMed] [Google Scholar]
- 29.Lange, R., and R. Hengge-Aronis. 1991. Growth phase-regulated expression of bolA and morphology of stationary-phase Escherichia coli cells are controlled by the novel sigma factor sigma S. J. Bacteriol. 173:4474-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGee, D. J., and R. F. Rest. 1996. Regulation of gonococcal sialyltransferase, lipooligosaccharide, and serum resistance by glucose, pyruvate, and lactate. Infect. Immun. 64:4630-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGee, Z. A., C. M. Clemens, R. L. Jensen, J. J. Klein, L. R. Barley, and G. L. Gorby. 1992. Local induction of tumor necrosis factor as a molecular mechanism of mucosal damage by gonococci. Microb. Pathog. 12:333-341. [DOI] [PubMed] [Google Scholar]
- 32.Merz, A. J., and M. So. 2000. Interactions of pathogenic neisseriae with epithelial cell membranes. Annu. Rev. Cell Dev. Biol. 16:423-457. [DOI] [PubMed] [Google Scholar]
- 33.Morse, S. A., C. S. Mintz, S. K. Sarafian, L. Bartenstein, M. Bertram, and M. A. Apicella. 1983. Effect of dilution rate on lipopolysaccharide and serum resistance of Neisseria gonorrhoeae grown in continuous culture. Infect. Immun. 41:74-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mühlecker, W., S. Gulati, D. P. McQuillen, S. Ram, P. A. Rice, and V. N. Reinhold. 1999. An essential saccharide binding domain for the mAb 2C7 established for Neisseria gonorrhoeae LOS by ES-MS and MSn. Glycobiology 9:157-171. [DOI] [PubMed] [Google Scholar]
- 35.Pettit, R. K., E. S. Martin, S. M. Wagner, and V. J. Bertolino. 1995. Phenotypic modulation of gonococcal lipooligosaccharide in acidic and alkaline culture. Infect. Immun. 63:2773-2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piekarowicz, A., and D. C. Stein. 2002. Biochemical properties of Neisseria gonorrhoeae LgtE. J. Bacteriol. 184:6410-6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prentki, P., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303-313. [DOI] [PubMed] [Google Scholar]
- 38.Pridmore, R. D. 1987. New and versatile cloning vectors with kanamycin-resistance marker. Gene 56:309-312. [DOI] [PubMed] [Google Scholar]
- 39.Ramsey, K. H., H. Schneider, R. A. Kuschner, A. F. Trofa, A. S. Cross, and C. D. Deal. 1994. Inflammatory cytokine response to experimental human infection with Neisseria gonorrhoeae. Ann. N. Y. Acad. Sci. 730:322-325. [DOI] [PubMed] [Google Scholar]
- 40.Reese, M. G., F. H. Eeckman, D. Kulp, and D. Haussler. 1997. Improved splice site detection in Genie. J. Comput. Biol. 4:311-323. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez, R. L., and R. C. Tait. 1983. Isolation of chromosomal DNA recombinant DNA techniques: an introduction. Addison-Wesley Publishing Company, Reading, Mass.
- 42.Ronpirin, C., A. E. Jerse, and C. N. Cornelissen. 2001. Gonococcal genes encoding transferrin-binding proteins A and B are arranged in a bicistronic operon but are subject to differential expression. Infect. Immun. 69:6336-6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 44.Sarkari, J., N. Pandit, E. R. Moxon, and M. Achtman. 1994. Variable expression of the Opc outer membrane protein in Neisseria meningitidis is caused by size variation of a promoter containing poly-cytidine. Mol. Microbiol. 13:207-217. [DOI] [PubMed] [Google Scholar]
- 45.Schneider, H., J. M. Griffiss, J. W. Boslego, P. J. Hitchcock, K. O. McJunkin, and M. A. Apicella. 1991. Expression of paragloboside-like lipooligosaccharide may be a necessary component of gonococcal pathogenesis in men. J. Exp. Med. 174:1601-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneider, H., T. L. Hale, W. D. Zollinger, R. C. Seid, C. A. Hammack, and J. M. Griffiss. 1984. Heterogeneity of molecular size and antigenic expression within lipooligosaccharides of individual strains of Neisseria gonorrhoeae and Neisseria meningitidis. Infect. Immun. 45:544-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song, W., L. Ma, W. Chen, and D. C. Stein. 2000. Role of lipooligosaccharide in opa-independent invasion of Neisseria gonorrhoeae into human epithelial cells. J. Exp. Med. 191:949-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stein, D. C. 1992. Plasmids with easily excisable xylE cassettes. Gene 117:157-158. [DOI] [PubMed] [Google Scholar]
- 49.Stein, D. C., J. S. Gunn, and A. Piekarowicz. 1998. Sequence similarities between the genes encoding the S.NgoI and HaeII restriction/modification systems. Biol. Chem. 379:575-579. [PubMed]
- 50.Tong, Y., B. Reinhold, V. Reinhold, B. Brandt, and D. C. Stein. 2001. Structural and immunochemical characterization of the lipooligosaccharides expressed by Neisseria subflava 44. J. Bacteriol. 183:942-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Ende, A., C. T. Hopman, S. Zaat, B. B. Essink, B. Berkhout, and J. Dankert. 1995. Variable expression of class 1 outer membrane protein in Neisseria meningitidis is caused by variation in the spacing between the −10 and −35 regions of the promoter. J. Bacteriol. 177:2475-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vicente, M., S. R. Kushner, T. Garrido, and M. Aldea. 1991. The role of the “gearbox” in the transcription of essential genes. Mol. Microbiol. 5:2085-2091. [DOI] [PubMed] [Google Scholar]
- 53.Virji, M., K. Makepeace, I. R. Peak, D. J. Ferguson, M. P. Jennings, and E. R. Moxon. 1995. Opc- and pilus-dependent interactions of meningococci with human endothelial cells: molecular mechanisms and modulation by surface polysaccharides. Mol. Microbiol. 18:741-754. [DOI] [PubMed] [Google Scholar]
- 54.White, L. A., and D. S. Kellogg, Jr. 1965. Neisseria gonorrhoeae identification in direct smears by a fluorescent antibody counterstain method. Appl. Microbiol. 13:171-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamasaki, R., D. E. Kerwood, H. Schneider, K. P. Quinn, J. M. Griffiss, and R. E. Mandrell. 1994. The structure of lipooligosaccharide produced by Neisseria gonorrhoeae, strain 15253, isolated from a patient with disseminated infection: evidence for a new glycosylation pathway of the gonococcal lipooligosaccharide. J. Biol. Chem. 269:30345-30351. [PubMed] [Google Scholar]
- 56.Yang, Q. L., and E. C. Gotschlich. 1996. Variation of gonococcal lipooligosaccharide structures is due to alterations in poly-G tracts in lgtgenes encoding glycosyl transferases. J. Exp. Med. 183:323-327. [DOI] [PMC free article] [PubMed] [Google Scholar]